

Implementation of the American- College of Physicians
Guideline for Low Back Pain (IMPACt-LBP): Protocol
for a Healthcare Systems Embedded Multisite
Pragmatic Cluster-randomised TrialThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: BMJ Open 2025 (Mar 26); 15 (3): e097133 ~ FULL TEXT
OPEN ACCESS Adam P Goode • Christine Goertz • Hrishikesh Chakraborty • Stacie A Salsbury • Samuel Broderick
Barcey T Levy • Kelley Ryan • Sharon Settles • Shoshana Hort • Rowena J Dolor, et al.
Duke University School of Medicine,
Durham, North Carolina, USA
Introduction: Low back pain (LBP) is a key source of medical costs and disability, impacting over 31 million Americans at any given time and resulting in US$100-US$200 billion per year in total healthcare costs. LBP is one of the leading causes of ambulatory care visits to US physicians; problematically, these visits often result in treatments such as opioids, surgery or advanced imaging that can lead to more harm than benefit. The American College of Physicians (ACP) Guideline for Low Back Pain recommends patients receive non-pharmacological interventions as a first-line treatment. Roadmaps exist for multidisciplinary collaborative care that include well-trained primary contact clinicians with specific expertise in the treatment of musculoskeletal conditions, such as physical therapists and doctors of chiropractic, as first-line providers for LBP. These clinicians, sometimes referred to as primary spine practitioners (PSPs) routinely employ many of the non-pharmacological approaches recommended by the ACP guideline, including spinal manipulation and exercise. Important foundational work has demonstrated that such care is feasible and safe, and results in improved physical function, less pain, fewer opioid prescriptions and reduced utilisation of healthcare services. However, this treatment approach for LBP has yet to be widely implemented or tested in a multisite clinical trial in real-world practice.
Methods and analysis: The Implementation of the American College of Physicians Guideline for Low Back Pain trial is a health system-embedded pragmatic cluster-randomised trial that will examine the effect of offering initial contact with a PSP compared with usual primary care for LBP. Twenty-six primary care clinics within three healthcare systems were randomised 1:1 to PSP intervention or usual primary care.
Primary outcomes are pain interference and physical function using the Patient-Reported Outcomes Measurement Information System Short Forms collected via patient self-report among a planned sample of 1800 participants at baseline, 1, 3 (primary end point), 6 and 12 months. A subset of participants enrolled early in the trial will also receive a 24-month assessment. An economic analysis and analysis of healthcare utilisation will be conducted as well as an evaluation of the patient, provider and policy-level barriers and facilitators to implementing the PSP model using a mixed-methods process evaluation approach.
Ethics and dissemination: The study received ethics approval from Advarra, Duke University, Dartmouth Health and the University of Iowa Institutional Review Boards. Study data will be made available on completion, in compliance with National Institutes of Health data sharing policies.
Trial registration number: ClinicalTrials.gov ID: NCT05626049.
Keywords: back pain; pragmatic clinical trial; primary health care.
From the FULL TEXT Article:
Introduction
Low back pain (LBP) is the leading musculoskeletal pain condition and a key source of medical costs and disability worldwide. [1–6] Up to 39% of US adults have LBP in the past 3 months, with 50%–80% reporting a significant episode in their lifetime. [7, 8] LBP impacts over 619 million people globally, [9] has increased threefold in prevalence in a 10-year period [5] and results in up to US$200 billion per year in total healthcare costs. [10, 11] Given the significance, it is no surprise that LBP is one of the leading causes of ambulatory care visits to US physicians. [12]
In 2018, 62% of US adults who reported seeing a healthcare provider for spine pain within the past year said they had seen a medical doctor. [13] Over half of LBP visits are made to primary care physicians (PCPs). [14] Unfortunately, it is becoming increasingly clear that the usual medical care for LBP, which commonly includes prescribed medications such as non-steroidal anti-inflammatory drugs (NSAIDs) and opioids, as well as invasive therapies like spinal fusions and epidural injections, is often ineffective or of questionable benefit. [15–17]
Furthermore, many of these standard treatments impose the risk of significant harm to patients. [18–24] Over 60% of opioid-related deaths are linked to chronic pain, and consistent opioid use is found in a majority (61%) of those with chronic LBP. [25] In 2012, a prescription for opioids was received by 20% of patients who visited a medical doctor for acute or chronic pain, [26] a number that is still unacceptably high. [27] Almost half of all opioid prescriptions are written by PCPs. [28]
In 2017, the American College of Physicians (ACP) guideline for LBP recommended patients receive non-pharmacological interventions as a first-line treatment. [29] To assist in the implementation of the guideline, roadmaps have been created for multidisciplinary collaborative care that place primary spine providers (PSPs) at the forefront of patient care for those with spine-related disorders, including LBP. [30–33]
The PSP model emphasises the use of doctors of chiropractic (DCs) and physical therapists (PTs) as first-line providers for LBP. These clinicians have specific expertise in treating musculoskeletal conditions and routinely employ many of the non-pharmacological approaches recommended by the ACP guideline, including spinal manipulation and exercise. Mounting evidence indicates that early treatment by DCs or PTs improves pain and functional outcomes in referral and direct-access settings. [34, 35] Important foundational work has demonstrated that PSP model care is feasible, safeand results in improved physical function, less pain, fewer opioid prescriptions and reduced utilisation of healthcare services. [34, 36, 37]
DCs and PTs are licensed as direct-access clinicians in all 50 states. [38, 39] Serious adverse events from these non-pharmacological treatments are rare, [40–42] with typically minimal side effects, such as mild muscle stiffness or soreness, commonly reported. [43–50] Such care is considerably safer than taking NSAIDs over time. [51]
DCs and PTs are well trained to perform a history and evidence-based examination to arrive at a diagnosis and then manage a patient’s care or make an appropriate referral for co-management or specialty care. Evidence supports that both DCs and PTs effectively screen and differentially diagnose musculoskeletal disorders from other systemic conditions like cancer. [52, 53] However, these studies are largely based on the use of large administrative or electronic health record (EHR) data or are observational designs. There is a need to test such models in real-world settings using rigorous randomised clinical trial designs.
The Implementation of the American College of Physicians Guideline for Low Back Pain (IMPACt-LBP) trial addresses gaps in prior studies of LBP care by implementing the PSP model of care for LBP in three health systems. The overall goal of this study is to compare usual primary care with the PSP model, which includes co-management between a DC or PT and a PCP. Patients seeking an LBP appointment at a clinic that has been randomised to PSP model care are asked if they would consider seeing a DC or PT first.
The primary objective is to determine if patients with LBP in intervention will have improved outcomes when compared with usual care based on the change in Patient-Reported Outcomes Measurement Information System (PROMIS) Pain Interference and Physical Function [54] scores from baseline to 3 months.
The secondary objective is to compare the effects of PSP model care with usual care for LBP at 3, 6 and 12 months on the Pain Catastrophising Scale-4 item short form, PROMIS Global-10 (V.1.2), opioid use and LBP-associated procedures and treatments including: imaging and diagnostic testing, provider visits, surgical procedures, medication prescriptions, hospital admissions and emergency room visits.
Our hypotheses are that patients with LBP asked to initiate contact with PSP clinicians will experience improved physical function, decreased pain, decreased opioid and other LBP-related medication prescriptions, improved patient satisfaction and decreased costs and utilisation of healthcare services when compared with patients with LBP who are not asked to initiate contact with a PSP first.
Methods and analysis
Trial overview and rationale

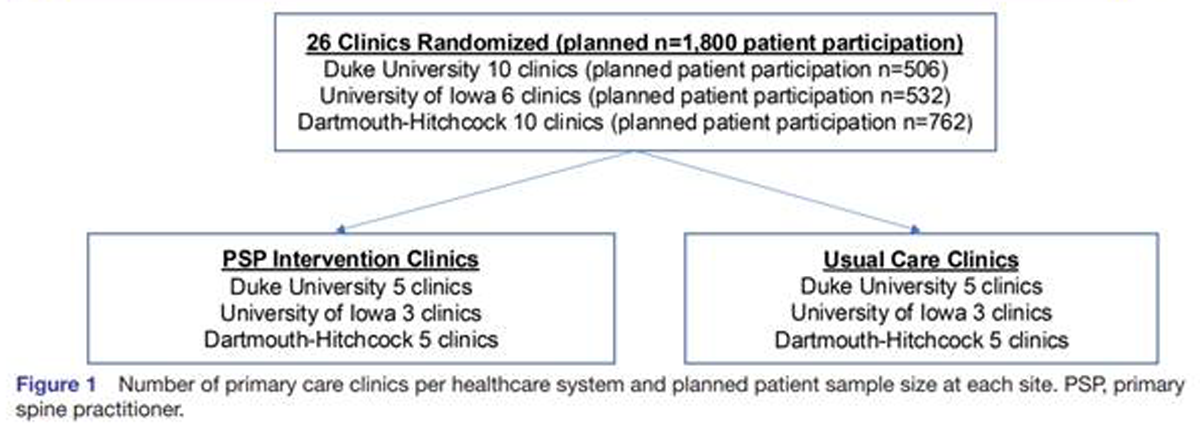
Figure 1 IMPACt-LBP is a pragmatic, multisite, two-arm, cluster-randomised trial with the unit of randomisation at the primary care clinic level initiated between February and April 2023. Twenty-six family medicine, primary care and general internal medicine clinics at three academic healthcare systems (Duke University, University of Iowa Hospitals and Clinics and Dartmouth Health) will be included in the study (Figure 1). The study plans to enrol 1800 patients, at least 18 years of age, who seek care at a participating primary care clinic with a complaint of LBP.
Patients presenting for care at primary care clinics in both study arms will have full access to usual medical care as needed throughout the study. All medical care is at the discretion of the clinical care teams. Data will be acquired through direct-to-patient surveys and via the EHR from each site. Patient-reported outcomes will be collected via questionnaires using a method of the patient’s choice, such as a centralised data capture system, email, text, telephone interviews or paper questionnaires. All study-related data will be coordinated and analysed by investigators affiliated with the Duke Clinical Research Institute at Duke University, which serves as the Data Coordinating Centre. This study will also include a process evaluation of patient, clinician and clinic administrator perceptions of non-specific treatment factors and the effectiveness of the PSP model across the three sites to assess adoption, implementation challenges and programme acceptability.
PSP interventions
Patients seeking care at intervention clinics are asked if they are willing to see either a DC or PT as their first contact clinician for LBP care. Each participating PCP clinic has a preferred set of DCs/PTs to which patients will be offered referral; these DCs/PTs have been identified by study investigators and approved by the site PIs, agree to receive educational materials (which may include the use of existing clinical care pathways, evidence-based treatment approaches and a standardised diagnostic classification system) and agree to send clinical notes to the referring PCP clinicians.
PSP educational materials recommend that if the patient is not improving as expected, the PSP refers the patient to their PCP for further evaluation. DC/PT care is presented to patients within the context of making an informed choice based on patient preference. Patients with an existing relationship with a DC or PT are encouraged to schedule with that provider or receive a referral to a study-identified PSP practice, whichever they prefer.
Any patient can decline the PSP model approach, stop seeing a PSP provider at any time and all patients will have access to usual medical care throughout the study. In addition to an evaluation and treatment plan, PSPs typically provide several evidence-based treatments, including: education and counselling about the patient’s condition to provide reassurance and reduce catastrophising and fear-avoidance behaviour; the application of a load (force) in the form of spinal manipulation to specific body tissues with therapeutic intent; myofascial therapies designed to stimulate sensory tissue, reduce pain, increase blood flow, relax muscle tone and break fibrous adhesions between neighbouring body tissues; therapeutic exercise, which can improve symptoms, prevent injury and encourage a team-based active treatment approach that enhances self-efficacy; proprioceptive neuromuscular facilitation, which involves specific stretching and assisted/unassisted exercise focusing on producing controlled movement to reduce abnormal muscle or joint strain; neural mobilisation, designed to improve nerve mobility and reduce tension on spinal nerves impaired by adhesions, inflammation or vascular compromise and nutritional/lifestyle and self-care strategies to reduce stress and/or change unhealthy behaviours, such as smoking.
The use of these interventions is supported by numerous national guidelines. [29, 55–59] It is important to note that the PSP model does not preclude additional treatments by these or other clinicians, such as psychological interventions, which are delivered as part of a multidisciplinary care approach.
Usual care
Usual care is defined as any care designated by a PCP. In most cases, this care is a combination of treatments or referrals that could consist of:(1) education and counselling,
(2) systemic medications (eg, NSAIDs, muscle relaxants, opioids, etc),
(3) referrals for non-pharmacological interventions such as PT, DC or massage or
(4) specialty care for invasive procedures such as nerve blocks, spinal injections or surgery.
Eligibility criteria—cluster and patientClinic (cluster) inclusion criteria
Primary care clinics: primary care clinics are eligible for study participation if they are:
affiliated with one of our three participating academic healthcare systems;
designated as a primary care, family medicine or general internal medicine clinic;
willing to participate in the PSP model;
provide a signed-site participation agreement;
had at least 250 unique patient visits with LBP assessed in the planning year.
Primary care clinics are not eligible for participation if they meet any of the following exclusion criteria
Any ongoing clinic-level study/project (research or quality improvement project) focused on LBP.
Designated as an urgent care or quick care clinic.
Patient inclusion criteria
Given the pragmatic approach to this study, the eligibility criteria are intentionally broad with minimal exclusion criteria to maximise the generalisability of the employed PSP model and the trial results. Patients are included if they are 18 years or older and are seeking an appointment at a participating study clinic with a complaint of LBP and agree to participate and complete the baseline questionnaire.
Patient exclusion criteria
Inability to provide consent or complete baseline questionnaires prior to the first PCP or PSP appointment.
Positive screening for cauda equina symptoms (total loss of control of bowel/bladder associated with this episode of LBP). Study personnel will screen for cauda equina symptoms and refer any patients screening positive to study personnel with a clinic background for further evaluation to consider immediate emergency department referral.
Patient eligibility
All identified patients 18 years and older seeking care at a participating clinic for a primary complaint of LBP will be offered participation. All three healthcare systems use dedicated scheduling centres for patient appointments at primary care clinics. Each scheduling centre has agreed to transfer phone calls of patients seeking care for LBP to the local study scheduling assistants. These scheduling assistants will be located at each respective healthcare system.
The IMPACt-LBP scheduling assistant provides the patient with an overview of the study as detailed in the IRB-approved phone script and offers them participation in the study. If the patient agrees, they are sent an IRB-approved consent form (online supplemental appendix 1) and a baseline questionnaire to complete via the patient’s preferred communication pathway.

Figure 2
page 5If the patient is seeking care at a primary care clinic randomised to the PSP model, the scheduling assistant offers to assist the patient with scheduling an appointment with a DC or PT. If the patient is seeking care at a primary care clinic randomised to the usual care model, the scheduling assistant assists with scheduling an appointment with their PCP at that clinic (Figure 2).
In addition, the first contact provider (either the PCP or PSP) will conduct additional screening to ensure that the patient does not require specialty care and/or imaging. This is part of standard clinical practice for both PCP and PSP providers. Patients with red flags (ie, severe or progressive neurological deficits, fever, trauma, serious underlying medical conditions) [53] identified by the first contact provider will continue to be followed in the trial.
Randomisation procedures
PCP clinic randomisation was stratified by health system. Primary care clinics that satisfied the clinic inclusion criteria and agreed to participate in the study were allocated 1:1 to either the intervention or control group. DCC statisticians generated the primary care clinic randomisation tables and provided a report to inform the investigators of clinic randomisation assignments before the start of patient enrolment.
Primary outcomes and secondary outcomes

Table 1
page 6Co-primary end points are changes in PROMIS Pain Interference and Physical Function scores from baseline to 3 months (Table 1). The PROMIS Pain Interference instrument measures the self-reported consequences of pain across aspects of life including social, cognitive, emotional, physical and recreational activities in the past 7 days.
This validated scale has five response options:not at all (1),
a little bit (2),
somewhat (3),
quite a bit (4) or
very much (5). [54]It has been shown to be a valid and reliable instrument [54] that is responsive to change in LBP status with a meaningful improvement in the total score between 3.5 and 5.5 points. [60]
PROMIS Physical Function (Short Form 4a) is a valid and reliable measure of self-reported physical function that uses four items with five response options;without any difficulty (5),
a little difficulty (4),
some difficulty (3),
much difficulty (2),
unable to do (1).It performs well in multiple race-ethnicity and age groups. [61, 62] It has excellent reliability, minimal ceiling/floor effects, limited item bias and is sensitive to change among patients with LBP [63] and spinal disorders. [64]

Table 2
page 7We have chosen to use these co-primary end points as they represent two domains that are important factors related to patient improvement. Secondary end points collected at baseline, 3, 6 and 12 months include Pain Catastrophising Scale; 4-item short form, PROMIS Global-10 (V.1.2); opioid use; imaging and diagnostic testing; provider visits; LBP-associated procedures and treatments including: surgical procedures, medication prescriptions, hospital admissions and emergency room visits; and two items from the NIH Task Force for LBP questionnaire: how often LBP is a problem and how long it has been an ongoing problem. [65] The schedule of assessments for the primary and secondary outcomes is provided in Table 2.
Additional exploratory analyses among enrolled patients will assess whether PSP care leads to long-term improvement compared with usual care using the PROMIS Pain Interference and Physical Function at 6, 12 and 24 months.
Similarly, we will determine if PSP model care leads to lower healthcare utilisation and costs for LBP in enrolled patients receiving PSP versus usual care at 12 and 24 months.
Only patients who are enrolled within the first 12 months after initiating recruitment will be included in the 24-month exploratory analyses. Additional analyses will evaluate LBP-related utilisation within the academic healthcare systems among all patients seen in intervention versus control primary care clinics using de-identified clinic-level data extracted from the EHR and on direct to patient surveys.
Adherence
Adherence is measured in terms of the number of participants in the intervention group offered an initial visit with a PSP who see the PSP within 30 days of initial contact. Non-adherence may occur by the participant rejecting the PSP visit in favour of a visit with the PCP or by accepting the PSP appointment but then either cancelling or ‘no-showing’ the visit. In the usual care arm, non-adherence is similarly measured by the number of participants who cancel or do not attend their scheduled PCP visit within 30 days of initial contact.
Receiving some DC or PT care in the usual care arm is an expected part of usual care. Rates will be measured and reported, although this would not be considered non-adherence nor cross-over. The level of adherence is measured and reported for both groups during study execution and reviewed by the study team leadership and sponsor during monthly meetings with study team leadership and study sponsor with the Oversight Committee.
The level of adherence is not included in our outcome analysis because, from a pragmatic trial point of view, the level of adherence is considered a characteristic of the intervention under ‘real-world’ conditions.
A different aspect of adherence is clinic-level implementation fidelity—how often a patient who called with a complaint of LBP is appropriately referred to the LBP scheduling assistant and offered a PSP appointment in the intervention clinics or a PCP appointment in the usual care clinics. Clinic-level implementation fidelity is assessed by reviewing patients scheduled for LBP visits within the participating clinics and determining how many were routed through the LBP scheduling assistant. Implementation fidelity will be monitored closely, with feedback from the clinic/scheduling staff and refinement of the intervention workflow to maximise implementation fidelity.
Blinding
Given the pragmatic design, participants and treating providers are not blinded to study interventions. As this is a cluster randomised pragmatic trial, patients, clinicians delivering care and the LBP scheduler and research coordinators at the sites are not blinded to clinic intervention assignment. Our analysis team includes both blinded and unblinded statisticians.
Safety monitoring
This study involves clinically determined standard-of-care treatments, does not collect any sensitive study data and uses no investigational drug or device; it therefore was considered a no greater than minimal risk study.
The Data and Safety Monitoring Board (DSMB) reviews our enrolment, trial conduct, adverse events and outcome data per the terms of the DSMB charter. Currently, no formal interim analyses are planned for this study. Any participant deaths that occur during study participation will be evaluated to assess whether they might be related to the study and reported to relevant entities as required.
Healthcare utilisation in the form of emergency department (ED) visits and hospitalisations will be collected as secondary outcomes and reported across groups in DSMB reports but will not specifically be reported as serious adverse events. The DSMB meets at the frequency specified in the DSMB charter and may meet more frequently if circumstances warrant.
Analytical methods
Study population details will be described, including the number enrolled in each treatment arm, in each stratum and those lost to follow-up. Baseline participant characteristics will be summarised as means, SD, medians and 25th, 75th percentiles for continuous variables, and as counts and percentages for categorical variables. Model assumptions for the analysis of study data will be examined prior to analysis and transformations implemented, if necessary, to meet the assumptions more adequately.
Primary analysis
To test the primary hypothesis that participants in the PSP model care group will show greater improvements in pain interference and physical function at 3 months compared with usual care, we will compare the changes in PROMIS Pain Interference and PROMIS Physical Function scores, individually, from baseline to 3-month follow-up between usual care and intervention arms using linear mixed-effects models (LMM) with random effects to account for correlated patients within clinic using an unstructured covariance matrix.
This primary analysis is unadjusted for covariates and will be conducted according to the principle of intention-to-treat with participants analysed and end points attributed according to the treatment arm, regardless of postrandomisation treatments received.
Secondarily, we will repeat analyses for the primary end points to examine any effects for the adjustment of covariates known to influence functional outcomes, including, at minimum, those covariates easily obtained from the EHR, such as age, sex and race. Additional secondary analyses will assess using LMMs, similar to that described above:
Differences in the change in pain interference or physical function from baseline to 6 months and from baseline to 12 months between treatment arms.
Differences in the time trend of pain interference or physical function between treatment arms.
Differences in the change of pain interference or physical function scores from baseline to 6 months and from baseline to 12 months between treatment arms will be assessed with LMM using random effects using an unstructured covariance structure to account for repeated measurements. Differences in the time trend of pain interference or physical function between treatment arms will be assessed with repeated measures mixed-effects models accounting for clustering within clinic as well as repeated measures.
For these models, we will select the appropriate covariance matrix (eg, compound symmetry, autoregressive, unstructured, etc) based on the data. This model will include pain interference or physical function outcomes at 1, 3, 6 and 12 months follow-up. Models will be repeated with adjustment for baseline covariates known to influence pain interference or physical function including age, sex, race/ethnicity and health-related quality of life.
We will adjust for between-group differences in clinic-level characteristics if necessary. If we encounter high rates of non-adherence in the intervention group relative to control, we will evaluate outcomes in the adherent versus non-adherent groups separately compared with the usual care group.
This method will be more informative than excluding the non-adherent as typically done in a ‘per-protocol’ analysis. We plan to assess the extent of non-adherence across clinic sites and ensure that the non-adherence is due to true patient needs and preferences and not a failure of implementation at the site level. Lastly, we will examine our primary outcomes stratified by sex to examine biological variabilities of interest.
Secondary analyses
For continuous secondary measures collected at baseline, 3, 6 and 12 months (ie, pain catastrophising, health-related quality of life, medical resource use and costs and opioid prescriptions), we will compare the change from baseline to 3 months, change from baseline to 6 months and change from baseline to 12 months between treatment arms using the LMMs described above.
We will select the appropriate covariance matrix (eg, compound symmetry, autoregressive, unstructured, etc) based on the data. Opioid prescriptions will be captured through EHR at baseline (and in 12 months prior to baseline). We will compare total opioid dosage (morphine equivalents) in the 12 months after enrolment.
In order to be able to compare opioid doses across classes, we will use a standard formula to calculate morphine equivalents from the CDC Morphine Equivalent Factors. [66] We will also compare differences in time trends for these continuous secondary end points between treatment arms with repeated measures, mixed-effects models as described above using a data-driven covariance structure.
Sample size determination
The primary end points are the PROMIS Pain Interference and Physical Function scores at 3-month follow-up between the PSP model and usual care. Because randomisation will occur at the clinic level and the enrolled patients’ outcomes may be correlated within clinics, we must account for the correlated observations in sample size calculations and in analysis. We assumed intracluster correlation (ICC) of 0.03 for both of our primary outcomes. We assumed effect sizes (mean change over SD) of 0.4 based on data from the pilot study by Goode et al. [67]
The changes in the PROMIS Physical Function were more conservative than those of the PROMIS Pain Interference. For the sample size calculation, we used a two-sided type I error of 2.5% to account for two primary outcomes with 90% power. Also, the calculations account for an assumed maximum attrition rate of 20% during the first 3 months of the trial.

Table 3 Table 3 presents the sample size requirements for effect sizes between 0.3 and 0.5, ICCs between 0.01 and 0.05, 80% and 90% power, a two-sided 2.5% type I error rate and a 20% dropout rate. Based on these assumptions, 1,800 patients from 26 clinics (13 clinics randomised to each arm), with an average of 80 enrolled participants per clinic and an ICC of 0.03, we will have >90% power to detect a difference of differences in minimum effect sizes of 0.4 for both primary outcomes.
We initiated the trial with 22 clinics. Due to slow enrolment early on in one of the health systems, we added four additional clinics from a better enrolling system for a total of 26 clinics as described above. The addition of these clinics did not materially affect the estimated total sample size of 1,800 planned enrollees.
The main secondary outcomes are the PROMIS Pain Interference and PROMIS Physical Function scores at the 12-month follow-up visit between the intervention arm and usual care arm. With a total of 1800 patients in 26 clinics with an average cluster size of 80 and ICC of 0.03, a two-sided type I error of 2.5% and 30% dropout rate during 12 months follow-up, we will be able to detect a minimum effect size of 0.4 with >80% power.
Procedures for missing data
Significant effort will be made to reduce the amount of missing data for all end points. Despite these efforts, missing data for primary and secondary end points are likely. For primary and secondary analyses that examine the change from baseline (to 3, 6 or 12 months), missing data in either baseline or the follow-up time point will lead to missing end point data for the patient. For these analyses, multiple imputation may be used for sensitivity analysis if the amount of missingness is >10% for the primary and secondary outcome variables.
Additional analyses
Exploratory analyses will include subgroup analyses, covariate-adjusted analyses and as-treated analyses. First, if the data provide evidence of an overall difference in the primary outcomes between treatment groups in the intention-to-treat analysis, we will further examine whether the therapeutic effect is similar for all participants, or whether it varies according to race (African American vs white), sex and LBP acuity (acute vs chronic) based on the NIH Task Force questions. All subgroup analyses will use LMMs or GLMMs as described above to test for interactions between treatment and subgroup variables. Effect estimates for subgroups will be assessed with formal interaction tests and, if needed, we will adjust the p values for multiple comparisons. Second, we will repeat analyses for all secondary end points with adjustments for covariates known to influence functional outcomes, including, at minimum, those covariates easily obtained from the EHR, such as age, sex and race.
As an exploratory safety analysis, we will determine if there is a difference in time from enrolment to diagnosis of serious underlying medical causes of LBP (vertebral fractures, inflammatory spondyloarthropathies, malignancy, infections and aortic dissections) between the study arms by using a time-to-event analysis. Diagnoses will be identified via International Classification of Disease-10 codes in the EHR extracts related to provider visits, ED visits and hospitalisations among enrolled patients and the time between enrolment and initial diagnosis occurrence will be compared between patients from intervention versus control clinics. Finally, we will assess whether the PSP model care leads to long-term improvement compared with usual care in our primary and secondary outcomes at 24 months.
Patient and public involvement
Patient and public involvement in IMPACt-LBP first occurred during the UG3 planning phase (2021). Clinicians and healthcare administrators from all three healthcare systems, and patients from the Duke and Iowa sites were invited to take part in focused interviews designed to refine the PSP model for patients with LBP and to identify potential barriers and facilitators to implementation of the clinical trial at each site. Community-based PSP clinicians not affiliated with the three healthcare systems also were invited to complete interviews during their onboarding process to identify their specific concerns for trial implementation.
While the overall research questions were not changed based on public involvement, the interview responses did lead to adaptation of outcome measures, recruitment and study implementation parameters. For example, the secondary end point, patient experience, was added based on interview feedback. Patients, clinicians and administrators noted that patients’ experiences with PSPs might vary depending on their expectations for chiropractic care and physical therapy as well as contextual factors from the clinic in which care was received.
These selected measures will allow our team to explore the patient experience with their PSP, treatment sessions, communication with their clinician and organisational aspects of the clinic setting. Patients also identified their typical ways of seeking care within their healthcare system, such as through phone calls or the EHR, which informed trial recruitment efforts.
Patients with chronic LBP and complex comorbidities also identified their preferences for first contact with their PCP, especially when they had previously tried physical therapy or chiropractic care. This information allowed us to anticipate that this group may decline participation, as well as to tailor study recruitment methods to address their concerns, such as adding details to the scheduler scripts. Patients were specifically asked about the potential benefits, burdens and time constraints of planned scheduling procedures and online questionnaires, as well as concerns regarding the use of EHRs, with patients generally approving the proposed methods.
Discussion
LBP is a prevalent and complex condition commonly seen in primary care clinics. Although many evidence-based guidelines [68] 69 recommend conservative, non-pharmacological first-line treatments, implementing these interventions has lagged. [15, 16] New data support that DC and PT treatments are effective, especially when delivered early in the patient care-seeking process. [18–21, 24] Challenges around guideline concordant care for LBP have increased the emphasis on pragmatic trials to examine real-world strategies to increase the use of non-pharmacological interventions.
The IMPACt-LBP trial will address this high-priority musculoskeletal area as it is designed to be a multisite, healthcare system, embedded pragmatic, cluster randomised clinical trial with the unit of randomisation at the primary care clinic level. The cluster randomisation provides the IMPACt-LBP trial an opportunity to examine the early intervention of PSP care at the PCP clinic level as an effective way to address LBP within healthcare systems.
A key aspect of the choice of cluster randomisation is to evaluate the change in workflow for patients entering the primary care system for LBP. Our design changes how patients schedule and receive care for LBP by providing them the option for initial DC or PT care at intervention clinics. Therefore, this choice of design allows the examination of a change in process at the primary care clinic level, which is important for the longstanding adoption of the intervention model.
The IMPACt-LBP trial used a 1-year planning grant period to further develop and improve the original study design. To do so, the IMPACt-LBP team worked with the NIH Pragmatic Trials Collaboratory Working Groups, a Protocol Review Committee, an insurance advisory committee, and gained patient, administrative and clinical stakeholder input on the original and proposed changes to the design. This planning process resulted in several important changes.
First, our initial plan for primary outcome data collection was to rely on the EHR during the routine clinical encounter. During the assessment of the EHR at each of our three healthcare systems, we identified several inconsistencies in currently available patient-reported outcomes measures and challenges with the timely implementation of measures to ensure consistency of outcome collection across sites. In addition, several ongoing and recently completed large pragmatic trials had experienced common challenges, such as inadequate collection of measures, lack of structured data collection or standardised data and difficulties aggregating data across sites and accessing the EHR. [70]
As such, we chose to use a hybrid of patient-reported outcomes and healthcare utilisation collected by direct-to-patient surveys and healthcare utilisation and safety events documented in the EHR. Similarly, our planning phase identified some advantages and disadvantages of EHR collection across the three healthcare systems. One disadvantage is that although two of our healthcare systems participated in PCORnet’s common data model [71] for standardisation of EHR data, one did not.
To address this challenge, our data coordinating centre mapped the required data elements from that site’s EHR to the common data model to ensure consistent measures across study sites. This led to additional costs and delays in EHR data access for healthcare utilisation and safety events. In addition, delays of up to 6 months in obtaining EHR data from PCORnet resulted in challenges with scheduling and review of these data by the DSMB.
A few notable changes to the protocol also occurred following the initial start of the trial. While two of the healthcare systems implemented the protocol and enrolled close to expected numbers, one site had substantially lower-than-expected accrual. As a result, the NIH and our DSMB requested and approved a mitigation plan expected to increase enrolment at that site. One key component that influenced enrolment was efforts within this academic healthcare system to improve access to same-day/next-day primary care appointments, as this would influence the ability to complete the baseline questionnaires prior to PCP or PSP appointments.
The same-day/next-day appointments limited our ability to offer PSP care before the PCP appointment. To assist in providing access to PSP care for the intervention clinics, several changes were made, including allowing same-day/next-day appointments for PSP providers, allowing MyChart self-scheduling for PSP appointments, and providing the option for PSP care for anyone making an appointment at one of the intervention clinics and then offering the option to participate in the study after the appointment was scheduled but before the visit took place. Another important component of this mitigation plan was to increase the number of primary care clinics (clusters) at one of our current sites that were meeting enrolment goals in order to increase overall enrolment.
Finally, we identified that initial positive cauda equina syndrome screening was differentially higher at one site, leading to the exclusion of potentially eligible patients and high rates of referral to emergency care. We investigated those patients who screened positive for cauda equina syndrome with a chart review at all sites. None of those patients who screened positive were diagnosed with cauda equina syndrome, and the leading cause of this positive screening was due to an additional question related to progressive muscle weakness that we had initially employed. As such, we modified cauda equina syndrome screening to that described earlier in this protocol to reduce unnecessary referrals to emergency care and concern to patients.
The IMPACt-LBP study has several strengths including a pragmatic design across three different healthcare systems on an important and common condition (ie, LBP) seen in primary care but is not without limitations. Our trial includes either DC or PT care as the intervention based on patient preference. However, there is limited availability of DCs within the three healthcare systems.
To overcome this problem, we partnered with chiropractic clinics within the communities around the healthcare systems. In addition, the trial is structured with the assumption that there would be no systematic difference in outcome between PT and DC care. We also identified challenges related to insurance coverage, differential copays for DC versus PT care and the perceived need for PCP referrals for PT with some insurance plans.
To assist with these challenges, we formed an insurance advisory committee consisting of leaders in major payers across the country to discuss barriers and solutions to access due to insurance coverage. Patients attending academic health centres may not be representative of those who attend community-based clinics. Despite these limitations, IMPACt-LBP was designed to compare a model in which patients are offered care from a PSP before or in lieu of a PCP compared with usual primary care for LBP. As a healthcare system-embedded pragmatic clinical trial, IMPACt-LBP will provide evidence on the effectiveness of real-world care processes to inform best clinical practice for LBP.
Ethics and dissemination
This study received ethics approval from the following institutional review boards: Advarra, Duke University, Dartmouth Health and the University of Iowa. On completion, study data will be made available in compliance with NIH data sharing policies. Dissemination activities will include presentations at local, national and international conferences to communicate study results and prepare publications for open-access journals.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is a pragmatic cluster randomised trial with the unit of randomisation at the primary care clinic level, using 26 clinics embedded within three academic healthcare systems.
This trial will compare the effectiveness of offering non-pharmacological care options with physical therapists and doctors of chiropractic at the point of entry into the healthcare system versus usual primary care.
Patients in the intervention clinics can choose between physical therapy or chiropractic care.
Limitations include the limited number of chiropractic providers at academic health systems and potential financial and logistical barriers to implementing PSP models of care within health systems.
Supplementary Material
Informed Consent Form and Authorization
to Use and Disclose Protected Health Information
This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.Contributors
CG, APG and JDL conceived the study. CG, APG, JDL, HC and SS designed the trial and obtained funding for the study, and made substantial contributions to the design of the trial and the intervention protocols. SS, BTL, KR, RJD, SK, ZS, JES and JD were responsible for implementation of recruitment methods and recruitment of participants. HC and SB are responsible for designing the data analysis plan. CG, APG, JDL, HC, SB, SS, SH, EAC, CA, SDR, PW, DH, BTL, KR, RJD, SK, ZS, JES and JD provided critical review of the manuscript and approved the final manuscript. APG is the guarantor of the manuscript.
Funding
This work was supported within the National Institutes of Health (NIH) Pragmatic Trials Collaboratory by cooperative agreements UG3AT011187 and UH3AT011187 (Clinical Coordinating Center) and U24AT011189 (Data Coordinating Center) from the National Center for Complementary and Integrative Health (NCCIH), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). This work also received logistical and technical support from the NIH Pragmatic Trials Collaboratory Coordinating Center through cooperative agreement U24AT009676 from NCCIH, the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute (NCI), the National Institute on Aging (NIA), the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Nursing Research (NINR), the National Institute of Minority Health and Health Disparities (NIMHD), NIAMS, the NIH Office of Behavioral and Social Sciences Research (OBSSR), and the NIH Office of Disease Prevention (ODP). Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002537.
Competing interests
None declared.
References:
Deyo RA, Mirza SK, Martin BI.
Back pain prevalence and visit rates:
estimates from U.S. national surveys, 2002.
Spine (Phila Pa 1976) 2006;31:2724–7.
doi:10.1097/01.brs.0000244618.06877.cdLicciardone JC.
The epidemiology and medical management of low back pain
during ambulatory medical care visits in the United States.
Osteopath Med Prim Care 2008;2:11.
doi:10.1186/1750-4732-2-11Martin BI, Deyo RA, Mirza SK, et al.
Expenditures and Health Status Among Adults With Back and Neck Problems
JAMA 2008 (Feb 13); 299 (6): 656–664Davis MA, Onega T, Weeks WB, et al.
Where the United States Spends its Spine Dollars: Expenditures
on Different Ambulatory Services for the Management
of Back and Neck Conditions
Spine (Phila Pa 1976). 2012 (Sep 1); 37 (19): 1693–1701Freburger JK, Holmes GM, Agans RP, et al.
The rising prevalence of chronic low back pain.
Arch Intern Med 2009;169:251–8.
doi:10.1001/archinternmed.2008.543Mafi JN, McCarthy EP, Davis RB, et al.
Worsening Trends in the Management and Treatment of Back Pain
JAMA Internal Medicine 2013 (Sep 23); 173 (17): 1573–1581Parsons S, Breen A, Foster NE, et al.
Prevalence and comparative troublesomeness by age
of musculoskeletal pain in different body locations.
Fam Pract 2007;24:308–16.
doi:10.1093/fampra/cmm027Lucas JW, Connor EM, Bose J.
Back, Lower Limb, and Upper Limb Pain Among U.S. Adults, 2019.
NCHS Data Brief 2021;1–8.GBD 2021 Low Back Pain Collaborators.
Global, Regional, and National Burden of Low Back Pain, 1990–2020,
Its Attributable Risk Factors, and Projections to 2050:
A Systematic Analysis of the Global Burden
of Disease Study 2021
Lancet Rheumatology 2023 (May 23); 5 (6): E316-E329Katz JN.
Lumbar disc disorders and low-back pain:
socioeconomic factors and consequences.
J Bone Joint Surg Am 2006;88:21–4.
doi:10.2106/JBJS.E.01273Dieleman JL, Cao J, Chapin A, et al.
US Health Care Spending by Payer and Health Condition, 1996-2016
JAMA 2020 (Mar 3); 323 (9): 863–884Statistics CNCfh.
Ambulatory care use and physician office visits. 2017. Available:
https://www.cdc.gov/nchs/fastats/physician-visits.htm
[Accessed 21 Jan 2019].Gallup-Palmer College of Chiropractic Annual Report 2018
Managing Neck and Back Pain in AmericaAtlas SJ, Deyo RA.
Evaluating and managing acute low back pain in the primary care setting.
J Gen Intern Med 2001;16:120–31.
doi:10.1111/j.1525-1497.2001.91141.xStevans JM, Delitto A, Khoja SS, et al.
Risk Factors Associated With Transition From Acute to Chronic
Low Back Pain in US Patients Seeking Primary Care
JAMA Network Open 2021 (Feb 1); 4 (2): e2037371Arana E, Kovacs FM.
The evidence gap in low back pain management strategies.
Lancet 2021;398:1130–1.
doi:10.1016/S0140-6736(21)01820-1Buchbinder R, Underwood M, Hartvigsen J, et al.
The Lancet Series Call to Action to Reduce
Low Value Care for Low Back Pain: An Update
Pain. 2020 (Sep); 161 (1): S57–S64Saragiotto BT, Machado GC, Ferreira ML, et al.
Paracetamol for low back pain.
Cochrane Database Syst Rev 2016;2016:CD012230.
doi:10.1002/14651858.CD012230Machado GC, Maher CG, Ferreira PH, et al.
Efficacy and safety of paracetamol for spinal pain and osteoarthritis:
systematic review and meta-analysis of randomised placebo controlled trials.
BMJ 2015;350:h1225.
doi:10.1136/bmj.h1225Machado GC, Maher CG, Ferreira PH, et al.
Non-steroidal Anti-inflammatory Drugs for Spinal Pain:
A Systematic Review and Meta-analysis
Annals of the Rheumatic Diseases 2017 (Jul); 76 (7): 1269–1278Deyo RA, Mirza SK, Turner JA, et al.
Overtreating Chronic Back Pain: Time to Back Off?
J Am Board Fam Med. 2009 (Jan); 22 (1): 62–68Fineberg SJ, Nandyala SV, Kurd MF, et al.
Incidence and risk factors for postoperative ileus following
anterior, posterior, and circumferential lumbar fusion.
Spine J 2014;14:1680–5.
doi:10.1016/j.spinee.2013.10.015Marquez-Lara A, Nandyala SV, Fineberg SJ, et al.
Cerebral Vascular Accidents After Lumbar Spine Fusion.
Spine (Phila Pa 1986) 2014;39:673–7.
doi:10.1097/BRS.0000000000000197Martin BI, Mirza SK, Franklin GM, et al.
Hospital and surgeon variation in complications and repeat surgery
following incident lumbar fusion for common degenerative diagnoses.
Health Serv Res 2013;48:1–25.
doi:10.1111/j.1475-6773.2012.01434.xOnishi E, Kobayashi T, Dexter E, et al.
Comparison of Opioid Prescribing Patterns in the United States and Japan:
Primary Care Physicians’ Attitudes and Perceptions.
J Am Board Fam Med 2017;30:248–54.
doi:10.3122/jabfm.2017.02.160299Daubresse M, Chang H-Y, Yu Y, et al.
Ambulatory diagnosis and treatment of nonmalignant pain
in the United States, 2000-2010.
Med Care 2013;51:870–8.
doi:10.1097/MLR.0b013e3182a95d86Goertz CM, Long CR, English C, et al.
Patient-Reported Physician Treatment Recommendations
and Compliance Among U.S. Adults with Low Back Pain.
J Altern Complement Med 2021;27:S99–105.
doi:10.1089/acm.2020.0392Levy B, Paulozzi L, Mack KA, et al.
Trends in Opioid Analgesic-Prescribing Rates by Specialty, U.S., 2007-2012.
Am J Prev Med 2015;49:409–13.
doi:10.1016/j.amepre.2015.02.020Qaseem A, Wilt TJ, McLean RM, et al.
Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain:
A Clinical Practice Guideline From the American College of Physicians
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 514–530Johnson CD, Haldeman S, Nordin M, et al.
The Global Spine Care Initiative:
Care Pathway Methodology, Contributors, and Disclosures
European Spine Journal 2018 (Sep); 27 (Suppl 6): 786–795Goertz C, Lyons SS, Andresen A, et al.
Collaborative Care for Older Adults (COCOA),
Palmer College of Chiropractic.
J Allied Health 2010;39 Suppl 1:e135–6.Goertz CM, Salsbury SA, Vining RD, et al.
Collaborative Care for Older Adults with low back pain by family
medicine physicians and doctors of chiropractic (COCOA):
study protocol for a randomized controlled trial.
Trials 2013;14:18.
doi:10.1186/1745-6215-14-18Goertz CM, Weeks WB, Justice B, et al.
A Proposal to Improve Health-care Value in Spine Care Delivery:
The Primary Spine Practitioner
Spine J. 2017 (Oct); 17 (10): 1570–1574Kazis LE, Ameli O, Rothendler J, et al.
Observational Retrospective Study of the Association of
Initial Healthcare Provider for New-onset Low Back Pain
with Early and Long-term Opioid Use
BMJ Open. 2019 (Sep 20); 9 (9): e028633George SZ, Goode AP.
Physical therapy and opioid use for musculoskeletal
pain management: competitors or companions?
Pain Rep 2020;5:e827.
doi:10.1097/PR9.0000000000000827Corcoran KL, Bastian LA, Gunderson CG, et al.
Association Between Chiropractic Use and Opioid Receipt Among
Patients with Spinal Pain: A Systematic Review and Meta-analysis
Pain Medicine 2020 (Feb 1); 21 (2): e139–e145Zheng P, Kao MC, Karayannis NV, et al.
Stagnant Physical Therapy Referral Rates Alongside Rising Opioid Prescription
Rates in Patients With Low Back Pain in the United States 1997–2010.
Spine (Phila Pa 1986) 2017;42:670–4.
doi:10.1097/BRS.0000000000001875Association APT.
Direct access in practice. 2019. Available:
http://www.apta.org/DirectAccess/
[Accessed 2 Jul 2019].Meeker WC, Haldeman S.
Chiropractic: A Profession at the Crossroads
of Mainstream and Alternative Medicine
Annals of Internal Medicine 2002 (Feb 5); 136 (3): 216–227Hall AM, Scurrey SR, Pike AE, et al.
Physician-reported barriers to using evidence-based recommendations
for low back pain in clinical practice: a systematic review
and synthesis of qualitative studies using the
Theoretical Domains Framework.
Implementation Sci 2019;14:49.
doi:10.1186/s13012-019-0884-4Slade SC, Kent P, Patel S, et al.
Barriers to Primary Care Clinician Adherence to Clinical Guidelines
for the Management of Low Back Pain: A Systematic Review
and Metasynthesis of Qualitative Studies.
Clin J Pain 2016;32:800–16.
doi:10.1097/AJP.0000000000000324Suman A, Schaafsma FG, van de Ven PM, et al.
Effectiveness of a multifaceted implementation strategy compared to usual
care on low back pain guideline adherence among general practitioners.
BMC Health Serv Res 2018;18:358.
doi:10.1186/s12913-018-3166-yWalker BF, Hebert JJ, Stomski NJ, et al.
Outcomes of Usual Chiropractic. The OUCH
Randomized Controlled Trial of Adverse Events
Spine (Phila Pa 1976). 2013 (Sep 15); 38 (20): 1723–1729Rubinstein SM.
Adverse Events Following Chiropractic Care for Subjects with
Neck or Low-back pain: Do the Benefits Outweigh the Risks?
J Manipulative Physiol Ther. 2008 (Jul); 31 (6): 461–464Cagnie B, Vinck E, Beernaert A, et al.
How Common Are Side Effects of Spinal Manipulation
And Can These Side Effects Be Predicted?
Manual Therapy 2004 (Aug); 9 (3): 151–156Senstad O, Leboeuf-Yde C, Borchgrevink C.
Frequency and Characteristics of Side Effects
of Spinal Manipulative Therapy.
Spine (Phila Pa 1986) 1997;22:435–40.
doi:10.1097/00007632-199702150-00017Foster NE, Hartvigsen J, Croft PR.
Taking responsibility for the early assessment and treatment of patients
with musculoskeletal pain: a review and critical analysis.
Arthritis Res Ther 2012;14:205.
doi:10.1186/ar3743PubMedMoore JH, McMillian DJ, Rosenthal MD, et al.
Risk determination for patients with direct access to
physical therapy in military health care facilities.
J Orthop Sports Phys Ther 2005;35:674–8.
doi:10.2519/jospt.2005.35.10.674Ojha HA, Snyder RS, Davenport TE.
Direct access compared with referred physical therapy
episodes of care: a systematic review.
Phys Ther 2014;94:14–30.
doi:10.2522/ptj.20130096Mintken PE, Pascoe SC, Barsch AK, et al.
Direct Access to Physical Therapy Services Is Safe
in a University Student Health Center Setting.
J Allied Health 2015;44:164–8.Lanas A, Perez-Aisa MA, Feu F, et al.
A nationwide study of mortality associated with hospital admission
due to severe gastrointestinal events and those associated
with nonsteroidal antiinflammatory drug use.
Am J Gastroenterol 2005;100:1685–93.
doi:10.1111/j.1572-0241.2005.41833.xGrotle M, Vøllestad NK, Brox JI.
Screening for yellow flags in first-time acute low back pain:
reliability and validity of a Norwegian version of the
Acute Low Back Pain Screening Questionnaire.
Clin J Pain 2006;22:458–67.
doi:10.1097/01.ajp.0000208243.33498.cbLeerar PJ, Boissonnault W, Domholdt E, et al.
Documentation of red flags by physical therapists
for patients with low back pain.
J Man Manip Ther 2007;15:42–9.
doi:10.1179/106698107791090105Amtmann D, Cook KF, Jensen MP, et al.
Development of a PROMIS item bank to measure pain interference.
Pain 2010;150:173–82.
doi:10.1016/j.pain.2010.04.025Chou R, Deyo R, Friedly J, et al.
Noninvasive Treatments for Low Back Pain
Agency for Healthcare Research and Quality
Comparative Effectiveness Review Number 169
Rockville (MD), February 2016.Delitto A, George SZ, Van Dillen L, et al.
Low back pain.
J Orthop Sports Phys Ther 2012;42:A1–57.
doi:10.2519/jospt.2012.42.4.A1Globe G, Farabaugh RJ, Hawk C, et al.
Clinical Practice Guideline:
Chiropractic Care for Low Back Pain
J Manipulative Physiol Ther 2016 (Jan); 39 (1): 1–22Chou R, Loeser JD, Owens DK, et al.
Interventional Therapies, Surgery, and Interdisciplinary Rehabilitation
for Low Back Pain: An Evidence-based Clinical Practice Guideline
From the American Pain Society
Spine (Phila Pa 1976) 2009 (May 1); 34 (10): 1066–1077VA/DoD Clinical Practice Guideline
VA/DoD Clinical Practice Guideline for Diagnosis
and Treatment of Low Back Pain
Department of Veterans Affairs/Department of Defense
VA, Washington, DC: U.S. Army Medical Command, 2017.Amtmann D, Kim J, Chung H, et al.
Minimally Important Differences for Patient Reported Outcomes
Measurement Information System Pain Interference
for Individuals with Back Pain
Journal of Pain Research 2016 (Apr 27): 9: 251–255Jensen RE, Potosky AL, Reeve BB, et al.
Validation of the PROMIS physical function measures in
a diverse US population-based cohort of cancer patients.
Qual Life Res 2015;24:2333–44.
doi:10.1007/s11136-015-0992-9Cook KF, Jensen SE, Schalet BD, et al.
PROMIS measures of pain, fatigue, negative affect, physical function,
and social function demonstrated clinical validity
across a range of chronic conditions.
J Clin Epidemiol 2016;73:89–102.
doi:10.1016/j.jclinepi.2015.08.038Schalet BD, Hays RD, Jensen SE, et al.
Validity of PROMIS physical function measured in diverse clinical samples.
J Clin Epidemiol 2016;73:112–8.
doi:10.1016/j.jclinepi.2015.08.039Hung M, Hon SD, Franklin JD, et al.
Psychometric Properties of the PROMIS Physical Function
Item Bank in Patients With Spinal Disorders.
Spine (Phila Pa 1986) 2014;39:158–63.
doi:10.1097/BRS.0000000000000097Deyo RA, Dworkin SF, Amtmann D, et al.
Report of the NIH Task Force on Research Standards
for Chronic Low Back Pain
Journal of Pain 2014 (Jun); 15 (6): 569–585Calculating total daily dose of opioids for safer dosage.
Available: https://stacks.cdc.gov/view/cdc/38481
[Accessed 17 Sep 2024].Goode AP, Taylor SS, Hastings SN, et al.
Effects of a Home-Based Telephone-Supported Physical Activity Program
for Older Adult Veterans With Chronic Low Back Pain.
Phys Ther 2018;98:369–80.
doi:10.1093/ptj/pzy026Oliveira CB, Maher CG, Pinto RZ, et al.
Clinical practice guidelines for the management of non-specific
low back pain in primary care: an updated overview.
Eur Spine J 2018;27:2791–803.
doi:10.1007/s00586-018-5673-2Chou R, Qaseem A, Owens DK, et al.
Diagnostic imaging for low back pain: advice for high-value
health care from the American College of Physicians.
Ann Intern Med 2011;154:181–9.
doi:10.7326/0003-4819-154-3-201102010-00008Richesson RL, Marsolo KS, Douthit BJ, et al.
Enhancing the use of EHR systems for pragmatic embedded research:
lessons from the NIH Health Care Systems Research Collaboratory.
J Am Med Inform Assoc 2021;28:2626–40.
doi:10.1093/jamia/ocab202Fleurence RL, Curtis LH, Califf RM, et al.
Launching PCORnet, a national patient-centered clinical research network.
J Am Med Inform Assoc 2014;21:578–82.
doi:10.1136/amiajnl-2014-002747Cella D, Yount S, Rothrock N, et al.
The Patient-Reported Outcomes Measurement Information System (PROMIS):
progress of an NIH Roadmap cooperative group during its first two years.
Med Care 2007;45:S3–11.
doi:10.1097/01.mlr.0000258615.42478.55Reeve BB, Hays RD, Bjorner JB, et al.
Psychometric evaluation and calibration of health-related quality
of life item banks: plans for the Patient-Reported Outcomes
Measurement Information System (PROMIS).
Med Care 2007;45:S22–31.
doi:10.1097/01.mlr.0000250483.85507.04Weinstein JN, Lurie JD, Tosteson TD, et al.
Surgical vs nonoperative treatment for lumbar disk herniation:
the Spine Patient Outcomes Research Trial (SPORT) observational cohort.
JAMA 2006;296:2451–9.
doi:10.1001/jama.296.20.2451Hills R, Kitchen S.
Satisfaction with outpatient physiotherapy: a survey comparing the views
of patients with acute and chronic musculoskeletal conditions.
Physiother Theory Pract 2007;23:21–36.
doi:10.1080/09593980601147876Pak SS, Miller MJ, Cheuy VA.
Use of the PROMIS-10 global health in patients with chronic low back pain
in outpatient physical therapy: a retrospective cohort study.
J Patient Rep Outcomes 2021;5:81.
doi:10.1186/s41687-021-00360-8
Return to LOW BACK PAIN
Since 3-28-2025


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |