

|
Basic Types of Sensory Perception Sensory Neuron Levels Receptors: General Considerations Major Pathways Clinical Considerations Basic Types of Pain The Motor System Basic Sensory and Motor Segmental Mechanisms Functional Anatomy: An Overview The Final Common Pathway Typical Motor Reflexes The Direct Activation Pathway Clinical Significance The Indirect Activation Pathway The Control Circuits Basal Ganglia Influences Cerebellar Influences Clinical Considerations Upper Motor Neuron Lesions: Summary Review Lower Motor Neuron Lesions: Summary Review Evaluation of the Motor System The Reticular Activating System Functional Anatomy: An Overview Arousal and Sleep States Attention Clinical Observations The Visceral System Functional Anatomy: An Overview Activation and Inhibition Mechanisms Viscerosensory Pathways Viscerosensory Receptors Central Visceral Control Visceromotor Pathways Clinical Considerations The Vascular System Functional Anatomy: An Overview Clinical Considerations Other Common Considerations The Consciousness System Central Neural Control Mechanisms Memory Cortical Hemispheric Dominance Cerebral Asymmetry Brain Waves Disorders of Consciousness The Cerebrospinal Fluid System Functional Anatomy: An Overview Clinical Considerations BibliographyChapter 3
The Longitudinal Neurologic Systems
From R. C. Schafer, DC, PhD, FICC's best-selling book:
Basic Principles of Chiropractic Neuroscience
Second Edition
The following materials are provided as a service to our profession. There is no charge for individuals to copy and file these materials. However, they cannot be sold or used in any group or commercial venture without written permission from ACAPress.
All of Dr. Schafer's books are now available on CDs, with all proceeds being donated
to chiropractic research. Please review the complete list of available books.
Overview The Sensory System
Chapter 3: The Longitudinal Neurologic Systems
The human nervous system is a marvel in organizing and adapting to internal and external environmental changes:
(1) The receptors and afferent neurons of the visceral and somatic input systems are necessary to detect internal and external environmental changes.
The visceral (autonomic, vegetative) system is organized in a manner that is similar to the somatic system, but there are some important differences. Both visceral and somatic systems have longitudinal afferent sensory and efferent motor components. These fibers interact at each horizontal level of the axial nervous system to provide the various reflex mechanisms necessary to maintain homeostasis. See Table 3.1.
This chapter succinctly describes the basic structure and function of the six major longitudinal systems; viz, the sensory, motor, visceral, vascular, consciousness, and cerebrospinal fluid systems.
As we begin this chapter, it might be well for the reader to subjectively grasp the significance of the motor and sensory systems as far as possible. One exercise in this is to imagine that you had become unconscious and someone has placed you in a remote dark empty cellar, far beyond any source of environmental sound. The first thing you realize is that you are a total sensory and motor paralytic from the neck caudad. You are unable to move even a fingertip because your motor system is not functioning. Because there is no feeling, you do not know whether you are recumbent or tied in a chair. Your vision is normal, but there is no light. Your hearing is normal, but there is no sound. Your taste buds are functional, but there is nothing to eat or drink. Your olfactory organs are functional, but there are no detectable odors. There is little left except thought and memory.
After a time in this predicament, thoughts undoubtedly arise such as, "I wish I had really looked at the beauty of the world when I had a chance. I wish I had listened to the music of the masters and even the birds in my backyard when I had a chance. I gulped down so many delicious meals. I had a beautiful garden, but I rarely took time to appreciate its design and fragrance. I even failed to take time to appreciate the texture of my own clothes. I was in such a hurry to go nowhere that was more important. I missed so much."
OVERVIEW
(2) The visceral efferent neurons and the muscles of the motor output system must be stimulated if action is to be taken.
(3) The integrative system serves as intermediary stations via a complex arrangement of interneurons whose synapses control impulse strength and signal direction from the sensory system to the motor system.
Table 3.1. Levels of the Autonomic Nervous System and Major Structures
Level Major Structures Involved
Peripheral Receptors, ganglia, effectors.
Spinal Preganglionic sympathetic and parasympathetic
neurons.
Posterior fossa Reticular formation and preganglionic parasympathetic
neurons; eg, respiration, cardiovascular control.
Supratentorial Hypothalamus and limbic system:
anterior hypothalamus, parasympathetic;
posterior hypothalamus, sympathetic.
Data are also transmitted to the thalamus and cerebral cortex.
The visceral system is far more independent from consciousness than is the somatic system. Three mechanisms especially provide this independence:
(1) All somatic reflexes are mediated in the CNS; visceral reflexes can also occur in the periphery.
(2) Many viscera are directly regulated by circulating hormones, but their respective glands are under neural control.
(3) Visceral receptors do not require external stimuli; eg, glands function without external control or conscious awareness.
Disorders within a longitudinal system may occur at any one or more of the horizontal levels; viz, the supratentorial, posterior fossa, spinal, and peripheral levels. When dysfunction occurs, symptoms usually arise as pain, pressure, tingling, numbness, weakness, paralysis, incoordination, nausea, dizziness, or altered states of consciousness.
The CNS derives information to determine its functions in voluntary and involuntary behavior by way of data from the body's internal and external media. The following review of the sensory system is designed to underscore how such environmental information is detected and translated by receptors, transmitted to the CNS, integrated, and perceived by conscious and subconscious faculties.
Superficial sensation includes such afferent signals as touch, pain, warmth, cold, two-point discrimination, weight differentiation (barognosis), and graphesthesia. This group would also include the special sensations of vision, smell, hearing, and taste.
Deep sensation includes the afferent signals of deep pressure, joint position and movement, equilibrium, deep pain, and vibration (pallesthesia). This group would also include the unconscious perception of such physiologic sensory input as muscle length and tension, arterial blood pressure, central venous pressure, lung inflation, cranial blood temperature, blood and CSF pH, blood oxygen and carbon dioxide levels, and plasma osmotic pressure.
Combined sensations include
(a) stereognosis, the ability to recognize familiar objects placed on the hand; and Protopathic. These essentially comprise the crude, poorly localized sensations perceived at the thalamic level such as simple touch, pain, and severe changes in temperature. Following nerve injury, protopathic sensations return rapidly (710 weeks) during regeneration.
Epicritic. These include sensations requiring cerebral participation for fine discrimination such as light touch, mildmoderate temperature changes, two-point discrimination, barognosis, and graphesthesia. Epicritic sensations return slowly (12 years) during regeneration or do not return at all.
Secondary Neurons
Mechanoreceptors, which detect mechanical deformation of the receptors or cells adjacent to the receptors.
Chemoreceptors, which detect tastes, odors, arterial oxygen levels, osmolarity of body fluids, and other factors that make up body chemistry.
Thermoreceptors, which detect changes in temperature, with some receptors detecting cold and others warmth.
Electromagnetic receptors (visual), which detect light on the retina.
The nociceptors, which detect painful damage in the tissues, whether it be of a physical or chemical nature.
Three systems of innervation are described by Rossi:
(1) a coarse system in the loose areolar tissues;
Clinicians realize that joint pain that is not associated with what is commonly thought of as acute sprain or strain often presents a history that contains mention of working in unusual postures just prior to the onset of pain. Painting a ceiling, lifting an object in an awkward position, prolonged ironing, and the chores of spring gardening are typical examples. Until recently, it was thought that the origins of such syndromes were primarily myogenic. Recent studies, however, have added a distinctly neurogenic component in such postural stresses.
Pain and Temperature Mechanisms. In contrast to other sensory systems, there is no central pain center that serves as a localized receiving center for nociceptive impulses. Peripheral nociceptive information is complexly modified in the CNS by neurotransmitters, synaptic relays, motor and sympathetic reflex linkage, and antinociceptive modulating and integrating mechanisms. All pain is mediated by the nervous system, yet only some pain originates from neuropathology.
(1) skin and subcutaneous tissues,
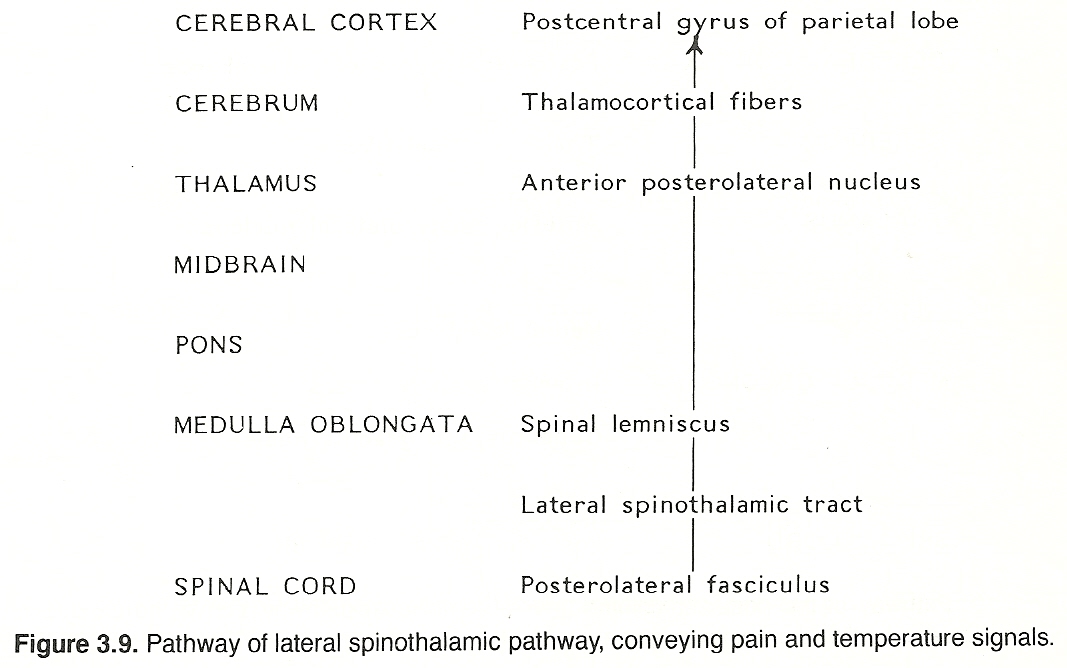
The parenchyma of the heart, lungs, abdominal and pelvic organs and the brain itself does not contain receptors for pain. The same is true for osseous tissue. However, the walls of associated arteries, the dura and visceral capsules, surrounding peritoneal and pleural membranes, and periosteum do. It is for this reason that almost any parenchymal lesion that produces inflammation, swelling, or severe contraction will cause pain if nearby bare nerve endings are stimulated. Proximal axons from the posterior root ganglia enter the spinal cord at the lateral aspect of the posterior root entry zone (refer to Figure 3.2) into the substantia gelatinosa, divide into branches that ascend and descend longitudinally in the posterolateral fasciculus (Lissauer's tract) for 13 segments, and then leave the fasciculus to synapse with cells of secondary neurons in the posterior gray horn of the spinal cord.
These secondary neurons either synapse locally or cross through the anterior white commissure to the contralateral side and ascend in the lateral spinothalamic tract (refer to Figure 3.2) in a route that is determined by fiber origin (ie, sacral, lumbar, thoracic, cervical). These axons extend up the cord and through the medulla, pons, and midbrain (spinal lemniscus) to synapse with cells of the anterior (ventral) posterolateral nucleus of the thalamus.
Third-order axons arising from the thalamus (thalamocortical fibers) extend to their respective terminations in the postcentral gyrus of the parietal lobe of the cerebral cortex. The conscious perception of pain apparently results when spinothalamic stimuli activate certain anterior posterolateral thalamic or cortical neurons, or both. Although the crude perception of pain occurs at the thalamic level, the localization of pain and associated psychic elements of somatic pain require functionally active thalamocortical fibers.
Peripheral level. Lesions of the peripheral neuraxis can cause either an increase or decrease in local pain/temperature perception, depending on whether the involved neurons or their synapses are in a state of hypersensitivity or if transmission is blocked.
Spinal level. Lesions involving the lateral spinothalamic tract produce contralateral pain/temperature loss at and below the level of the lesion.
Posterior fossa level. Lesions in the posterior fossa produce contralateral pain/temperature loss at and below the level of the lesion and possibly an ipsilateral deficit in the face.
Supratentorial level. Lesions of the higher CNS seldom cause painful syndromes unless pain-sensitive structures (eg, the dura, arteries, periosteum) are involved; rather, they frequently exhibit poor contralateral pain-localization and temperature-discrimination deficits.
Peripheral neurons conveying conscious proprioception data have their cell bodies in the posterior ganglia. The distal processes extend to receptors in striated muscle, tendons, and joints. The proximal axons enter the medial division of the posterior root entry zone of the spinal cord, and ascend in a posterior column without synapsing. In the posterior white matter, fibers from the upper extremity and trunk constitute the fasciculus cuneatus; thus, this tract is prominent in the cervical and upper thoracic cord but not found in the lower cord; while fibers from the pelvis and lower extremity comprise the fasciculus gracilis. The first-order neurons ascending in the fasciculi cuneatus and gracilis end in the cuneatus and gracilis nuclei of the lower medulla oblongata and synapse with second-order neurons.
These secondary neurons cross to the opposite side as internal arcuate fibers, ascend to the thalamus as the medial lemniscus, terminate in the anterior posterolateral nucleus, and synapse with third-order neurons.
Third-order thalamocortical axons arise from the posterolateral nucleus, extend via the posterior limb of the internal capsule, and then terminate in the somesthetic area of the postcentral gyrus of the parietal lobe in a homunculus-like manner.
Lesions within this pathway manifest as a loss of two-point discrimination and conscious joint position, atopognosia, asterognosis, and possibly as ataxia if the lesion is bilateral. Peripheral neurons conveying unconscious proprioception data have their first-order neuron cell bodies in the posterior root ganglia. The distal processes extend peripherally to receptors located primarily in muscle spindles and Golgi tendon organs.
(a) Some of the fibers from muscle spindles pass directly through the posterior gray matter to the anterior gray matter, comprising the afferent limb of the stretch reflex, which will be described later in this chapter.
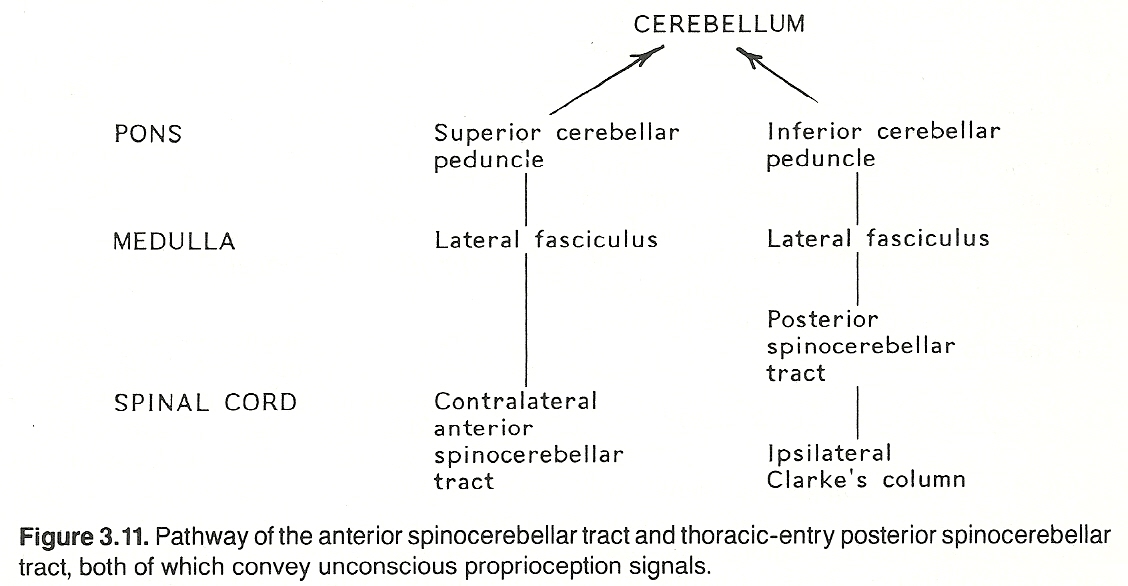
The anterior spinocerebellar pathway. First-order neurons entering the spinal cord at the lumbar and sacral levels convey signals from the pelvis and lower extremities. They synapse at the level of entry in the posterior gray horn, second-order neurons remain uncrossed or cross to the opposite side of the cord, ascend through the spinal cord, medulla, and pons in the lateral fasciculus as the anterior spinocerebellar tract, and most fibers project to the cerebellum via a circular route as the superior cerebellar peduncle (brachium conjunctivum). To offer a means for bilateral input, some fibers at the posterior fossa level re-cross to the ipsilateral side before they are projected to the cerebellum.
The posterior spinocerebellar pathway. First-order neurons entering the spinal cord at the thoracic level convey signals from the thorax and abdomen. They synapse at the level of entry, if they enter the CNS between T1 and L1, in the nucleus dorsalis of Clarke (Clarke's column) of the posterior gray horn. Second-order neurons enter the ipsilateral lateral funiculus and ascend the spinal cord as the posterior spinocerebellar tract, and then project to the cerebellum as the inferior cerebellar peduncle (restiform body). See Figure 3.11 This course changes somewhat above T1 and below L1 segments: Fibers carrying unconscious proprioception signals that enter the CNS above T1 ascend in the posterior columns to the lower medulla's representation of Clarke's column (the lateral cuneate nucleus). Here they synapse with second-order neurons (the cuneocerebellar tract) that run with posterior spinocerebellar fibers of the ipsilateral inferior cerebellar peduncle to the cerebellum.
Similar fibers entering the CNS below L1 ascend in the posterior columns until they reach a point where they can enter Clarke's column. They then synapse there with second-order neurons that ascend as the posterior spinocerebellar tract.
Lesions within pathways for unconscious proprioception messages manifest primarily as motor incoordination in the extremities (eg, past pointing, ataxia). However, rarely are such pathways involved singularly. Spinocerebellar tract deficits usually play a part in a larger clinical picture of cord injury or pathology.
(1) simple touch, which is the crude perception of localized cutaneous pressure; and
It has been previously described that fibers conveying tactile discrimination signals for stereognostic perception ascend in the posterior column as the fasciculus gracilis with those conveying conscious proprioception data. Some fibers carrying messages of simple touch may also take this route. Other fibers conveying touch signals extend cephalad as the anterior spinothalamic tract. Peripheral neurons conveying touch signals have their cell bodies in the posterior root ganglia. They project myelinated processes distally to receptors in the skin, around hair follicles, and in subcutaneous tissues. The proximal axons pass through the posterior root, enter the posterior root entry zone of the spinal cord, ascend and descend for several segments, and then synapse with second-order neurons near the core of the posterior gray horn.
These secondary neurons cross to the opposite side in the anterior white commissure, turn upward and ascend the cord as the anterior spinothalamic tract, and terminate in the anterior posterolateral nucleus of the thalamus, where they synapse with third-order neurons.
At the level of the pons and above, the course is the same as that taken by those of the fasciculus gracilis. Third-order thalamocortical axons arise from the posterolateral nucleus, extend through the posterior limb of the internal capsule, and then end in the somesthetic area of the postcentral gyrus of the parietal lobe in an homunculus-like manner.
(1) the sensory receptors are intact,
(2) the sense-conveying fibers are normal,
(3) the sense-interpreting centers are active, and
(4) the associative centers of consciousness are intact.
Common clinical terms used to describe sensory perception are listed in Table 3.6.
Localization: General Principles
Note: Authorities differ somewhat as to exact levels, and variances of a segment above or below are commonly stated by different authorities. The above data are a composite of the findings from several sources (Courtesy of Associated Chiropractic Academic Press).
The sensation of light touch is commonly measured clinically with a wisp of cotton, pressure by using thumb pressure, pain with a blunted pin, and two-point discrimination by using calipers. These and other sensory tests will be described in Chapter 8.
Thalamic Syndrome. If a lesion involves the sensory fibers of the thalamus, all somatic afferent sensations will be lost in the contralateral half of the body. The pain is usually persistent with associated outbursts of poorly localized pain. The character of the pain may be described as highly distressing burning, drawing, pulling, swelling, or tension sensations. Homonymous hemianopsia will be part of the clinical picture if that portion of the thalamus related to the optic nerve is involved. Ataxia, hyperkinesia, inability to discriminate tactile and joint signals, paresthesia, and weakness are also typical components of the syndrome. A slight stimulus may provoke a seizure of severe abnormal sensations.
Spinothalamic Tract Syndrome. If a lateral spinothalamic tract is interrupted, the patient will exhibit a contralateral loss of pain and temperature sensation from 23 segments below the level of the lesion through the lower extremity. Because fibers of the spinothalamic tract run in courses determined by their entry to the CNS, small midspine anterior lesions may spare fibers entering at the sacral area.
Skully lists the more important areas as follows: The radial sensory area, located on the back of the hand and the lateral aspect of the thumb.
The median area, occupying more than half of the palm and the posterior tips of the index finger, middle finger, and half of the ring finger.
The ulna area, limited to the anterior and posterior surfaces occupied by half of the ring finger and the small finger, from fingertip to wrist.
The musculocutaneous area, located on the medial aspect of the forearm.
The axillary area, found over the point of the shoulder.
The femoral area, situated on the anterior medial aspect of the thigh.
The femoral lateral cutaneous area, located over the distal part of the lateral surface of the thigh.
The common peroneal area, exhibited as a vertical strip on the front of the ankle.
The sciatic sensory area, occupying the entire foot and ankle except the medial aspect.
Neuralgia. Neuralgia is a general term that refers to any sharp, severe, stabbing, paroxysmal, remittent pain with temporary abatement in severity that travels along the course of one or more nerves. It is usually limited to tissues supplied by the branches of a specific nerve. The sharp pain, often described in terms of "lightning-like" or "like an electric shock," is usually associated with tenderness along the course of the nerve and violent episodic spasms in the muscles innervated. Although the term neuralgia is nondiagnostic, it is often used in situations where the exact etiology and pathology involved are idiopathic. Neuralgia rarely subsides spontaneously, and it is often so severe that the victim becomes totally incapacitated and frequently addicted to narcotics. Depression is a common associated factor, and suicidal tendencies are not infrequently seen. Morphologic changes cannot usually be detected early in a pure neuralgia or neurodynia. The term neurodynia is often used to describe a similar pain that is less severe; ie, a deep ache.
Nerve Tracing. There may be a condition that will cause tenderness if pressure is exerted, but no abnormal sensation is felt if there is no pressure. It is upon this fact that nerve tracing is based. Nerve tracing is the palpable act of following the course of tenderness over spinal nerves that are irritated or impinged that will usually assist in locating the focus of pain, tenderness, or headache. It is a diagnostic art that is used more in chiropractic than any other healing art. The technique of nerve tracing will be described in a subsequent chapter. Peripheral lesions give a segmental loss picture, and the loss will be on the same side as the lesion (eg, nerve root compression).
Central lesions cause a sensory loss from the level of the lesion caudally in the following manner: (a) the loss will be on the side opposite the lesion for exteroceptive sensations; (b) the loss will be on the same side as the lesion for proprioceptive sensations. The reason for this is that exteroceptive fibers transverse the cord while proprioceptive fibers do not.
The reception of homeostatic-threatening stimuli (mechanical, chemical, thermal, psychic) by algesic substances acting on transducer-like pain receptors. The most common algesic substances appear to be accumulations of bradykinins, certain prostaglandins, hydrogen ions, and potassium chloride following cellular damage by pathologic inflammation or trauma. Cailliet and Travell/Rinzler point out that certain functional disorders may also enhance the excitability of pain receptors. For example, the pain receptors located within muscles and tendons are activated or primed by excessive tone such as seen in hypertonic syndromes or the effects of poor biomechanics.
The transduction of energy changes from its origin at pain receptors into sequential nerve impulses.
The conduction of the pain impulses by intact peripheral, spinal, and higher center nerves.
The unconscious modulation and integration of impulses within the central nervous system.
The conscious perception (experience) of pain.
The voluntary and automatic reactions to pain such as are contained within various physical and psychologic responses.
Thus, pain is a complex "mind-body" experience involving the total person rather than only the mind or the body; ie, the mental and physical experiences of pain are inseparable.
Fast vs Slow Pain. A painful stimulus may cause two sensations:
(1) an initial sharp, localized sensation is perceived;
Guyton, Daube/Sandok, Chusid, and others frequently refer to these different sensations as fast or first pain and slowor second pain, respectively. Various combinations of fast and slow pain determine the character of the pain experienced; eg, local, diffuse, sharp, dull, burning, prickling, gnawing, knife-like, etc. These descriptors and whether the pain is constant or intermittent frequently help to localize the underlying site and character of the distress.
Central Objective Pain. Central pains are mainly due to lesions in the optic thalamus (eg, thrombosis of a branch of the posterior cerebral artery perfusing the thalamus), which causes transient paralysis, marked loss of position, abnormal vibratory and touch sensations on half of the body, and moderate loss of pin-prick perceptions that are followed by severe painful dysesthesias. A central lesion that can cause extremity pain is secondary to paralysis from either a loss of spinal cord or corticospinal tract function that is always related to immobilization of a joint or spasticity. It is differentiated from thalamic pain in that there is no dysesthesia, and movement of an extremity, especially of the proximal joints, increases the pain. Peripheral pain of intrinsic origin. In a sprain, for example, the ligaments are excessively stretched. There are sensory impressions constantly arising from the injured tissues that reach the brain, are interpreted, and then efferent impulses are directed to the point from which the impression originated. The experience of pain, however, occurs in the brain. Other examples of intrinsic pain include the parenchymatous types that arise from inflammations, masses, colic contractions, or displacements.
Peripheral pain of extrinsic origin. Pain from a bruise, for example, has a protective function to prevent further use of the injured part until it can be properly and naturally repaired. After a scratch, signals are sent to the brain where they are interpreted as pain. Motor impulses are then sent back to the muscles, which causes them to contract to withdraw the part from further injury. Other examples of extrinsic pain are those that register from functional pressure upon nerves or their terminals such as new growths, swollen organs, tensed tendons, stretched ligaments, and spastic muscles.
The mechanisms of pain, its perception, and the various characteristics of reported pain that aid the diagnosis of its cause will be described from additional viewpoints in Chapter 8.
In a manner similar to that of the afferent sensory system, the efferent motor system consists of a complex network of pathways and centers at all levels of the neuraxis, which interact with multiple integrating sensory centers that modify and initiate appropriate motor responses.
(1) a single cell body of a lower motor neuron (located in the anterior gray horn of the spinal cord or brain stem),
A motor unit is also called a lower motor neuron or an alpha motor neuron. In addition to alpha neurons, there are also smaller neurons in the anterior horn, the gamma motor neurons, which enervate muscle spindles, and the interneurons, which provide connections with other neurons. A neuron conveying impulses from the cells of the motor area of the cerebrum, through the internal capsule, to an anterior horn cell in the brain stem or spinal cord is called an upper motor neuron.
(1) the sensory system (eg, simple reflexes),
The direct activation pathway conveys unsynapting impulses directly from the cerebral cortex to contralateral anterior horn cells to initiate voluntary movements (especially fine, skilled motions). The major pyramidal tract is the corticospinal tract. The shorter, more complex, multisynapsing tracts of the indirect activation pathway also convey impulses from the cerebral cortex to the anterior horn cells but they essentially mediate conscious and unconscious gross automatic activities (eg, posture) where the coordination of many muscles is required. The major coordinating tracts are the reticulospinal, vestibulospinal, and rubrospinal tracts. The activity of the descending (a) corticospinal, (b) rubrospinal (from the contralateral red nucleus), (c) vestibulospinal (from the ipsilateral dorsal vestibular nuclei and contralateral vestibular nuclei), (d) olivospinal (from the ipsilateral olivary nucleus), (e) reticulospinal (from the ipsilateral reticular nuclei), and (f) tectospinal (from the contralateral tectum) tracts.
Various intersegmental and intrasegmental reflex neurons such as (a) ipsilateral reflexes of the same segment of the cord, (b) contralateral reflexes of the same segment of the cord, (c) ipsilateral reflexes from other segments of the cord, and (d) contralateral reflexes from other segments of the cord.
The convergence from many afferent sources. That is, sensory impulses from several segments (involving many types of receptors) may influence the function of the anterior horn cells. If input from several sources compete for the final common pathway at one time, allied impulses may reinforce each other. If incompatible, those impulses (usually nociceptive) that are protective have priority.
Muscle Fibers
(1) the receiver mechanism or afferent (sensory) limb of the arc, The sensory limb originates in the receptors of the skin, muscles, joints, ligaments, and viscera. Impulses travel the afferent fibers to parts of the CNS such as the spinal cord, brain stem, cerebral cortex, and/or cerebellum. General sensory fibers have their cells of origin located in the dorsal root ganglia. Somatic afferent fibers carry the exteroceptive and the proprioceptive impulses from the sensory endings in the body wall, tendons, and joints. Visceral afferent fibers carry sensory impulses from the internal organs.
The internuncial limb carries impulses to an interpretative center and thus connects the stimulating point with a center that will create a responsive impulse.
After interpretation of the stimulus, a center of the CNS creates an impulse that is sent to an effector organ such as a muscle or gland via the efferent or motor limb of the arc. Somatic motor fibers enervate the striated musculature derived from the myotomes of somites. Visceral (autonomic) motor fibers enervate smooth and cardiac muscle and regulate glandular secretions.
Conduction Blocks. Conduction blocks of the pyramidal tracts are especially characterized by impairment of fine voluntary digital movements, hypotonia, a lack of atrophy, and minor, if any, hyporeflexia. There are, however, some exceptions to the hyporeflexia being minor. For example, although tendon reflexes may appear near normal, a unilateral loss of the segmental abdominal and cremasteric reflexes is a cardinal feature because these reflexes depend upon an intact corticospinal tract. Babinski's plantar reflex and the grasp reflex are also prominent because the withdrawal effect expressed in these pathologic reflexes is normally inhibited by corticospinal action potentials. Limited cortical lesions manifest on one side of the face or in one limb. Pyramidal medulla lesions exhibit contralaterally below the level of the lesion on one side of the body. The unusual occurrence of a positive Babinski response and flaccid paralysis distally signifies a lesion in the primary motor area of the cerebral cortex.
Lesions of the anterior aspect of the frontal lobe often spare the primary motor centers. If this occurs, there will be no somatic weakness but delicate volitional control will be severely impaired (viz, motor apraxia).
Irritative Lesions. Stimulating lesions of the direct activation pathway are rarely found below the corona radiata level. One common exception to this is seen in the early stages of amyotrophic lateral sclerosis in which the anterior horn cells are highly irritable and produce widespread fasciculations and spasticity. Once the lower motor neurons degenerate, atrophy and flaccid paralysis occur.
(1) the red nucleus and the rubrospinal tract,
Influences from the Red Nucleus
(1) two areas of the system produce contrasting (excitatory and inhibitory) yet consistent motor effects, and Posterolateral reticular fiber influences. Action potentials traveling this course activate the gamma motor neurons in the anterior horn of the spinal cord that serve to activate extensor motor neurons and inhibit flexormotor neurons in the maintenance of postural tone.
Anteromedial reticular fiber influences. Action potentials traveling this course have mixed effects depending upon various synaptic influences, but their primary function (like rubrospinal impulses) is to activate flexor motor neurons and inhibit extensor motor neurons in the maintenance of postural tone. Thus, they tend to counterbalance posterolateral reticular influences.
(1) the cerebellum,
Influences of the Tectospinal Tract Conduction block lesions in the rubrospinal system and anteromedial reticulospinal system manifest as flexor weakness.
Conduction block lesions in the posterolateral reticulospinal system feature extensor weakness.
Conduction block lesions of the vestibulospinal system are characterized by a tendency to fall after rapid turning movements.
Conduction block lesions of the tectospinal tract result in delayed postural responses of the neck and head to potentially harmful visual and auditory stimuli.
The level of a lesion in the indirect activation pathway will determine its effects. Examples are shown in Table 3.9.
If the descending tracts to alpha and gamma motor neurons of the spinal cord are severed, all motor and reflex activity is initially lost, and muscles become flaccid. In time, however, the lower motor neurons usually recover their excitability so that reflexes become overactive but postural and voluntary motor activity remain lost. Abnormal activity in this circuit leads to slowed skeletal muscle activity (hypokinesia), hypertonicity, stiffness, and increased resistance to passive motion (rigidity). Abnormal postures are assumed, and motions are difficult to stop precisely (eg, past-pointing phenomena). The substantia nigra is the most common site for a lesion in the basal ganglia control circuit.
Subnormal activity in this circuit leads to somatic hyperactivity (hyperkinesia), resting tremor, athetosis, chorea, hemiballismus, and dystonia. Parkinsonism and Huntington's chorea are classic examples of subnormal basal ganglia control circuit function.
(1) In upper motor neuron or pyramidal fiber lesions, the deep tendon reflexes are exaggerated but the superficial skin reflexes are decreased or absent.
The classical clinical picture of an upper motor neuron lesion is described by Tran and others as follows: Spastic type of paralysis or paresis on the opposite side of the body and below the level of the lesion if the lesion is at or above the medullary pyramidal decussation; eg, in the motor cortex, internal capsule, pes pedunculi, or pons. Weakness is especially pronounced in the musculature of the limbs, and there is great difficulty with movements of the hands. Increased muscle tone will be witnessed as firmness and stiffness, especially in the arm flexors and the leg extensors.
Minimal or no atrophy of the muscles involved. However, there will be a later disuse atrophy. After a few days to a few weeks, stretch reflexes return in the involved muscles and usually become more active than usual. Muscle resistance to passive movements is exaggerated. It is often strong at the beginning of movement and then collapses in a peculiar "clasp-knife" method as more force is applied.
An increase response of deep reflexes since the normal operation of gravity against the weight of the body may initiate stretch reflexes. The reflex contractions are exaggerated because of a loss of the inhibitory mechanism from the higher central level. The combination of this effect with contractions resulting from continuous discharge of brain stem excitatory mechanisms leads to a hypertonic state. Increased tone is characterized by firmness and stiffness, especially in the flexor muscles of the arm and the extensors of the leg.
The superficial reflexes (eg, abdominal, cremasteric) are abolished.
The nociceptive flexor reflex and a positive Babinski sign can be observed. The nociceptive reflex evokes withdrawal of a body part by action of flexor muscles at one or several joints, depending on the severity of the stimulus, in response to actual or potential injurious stimuli such as pricking, pinching, or burning. A variation of the flexor reflex is the famous Babinski reflex. In a patient with a pyramidal tract lesion or an infant in whom the tract is not yet functional, the Babinski reflex will be positive. The anatomical basis of the change of response is not totally understood. Hoffmann's sign is usually present, but it is not a reliable sign since it is seen occasionally in normal individuals.
No reaction of degeneration will be present in an upper motor neuron lesion. If the peripheral nerve responds to galvanic or faradic stimulation, it can be assumed that the lesion is in an upper motor neuron.
In a lower motor neuron lesion, neuronal impulses from the upper central level or from the anterior gray horn of the spinal cord cannot reach the appropriate muscle fibers; thus, the characteristic clinical picture will be as follows: A flaccid paralysis is seen if there is no regeneration; ie, the muscles involved eventually atrophy and bulk is possibly replaced by connective and adipose cells. Muscle tone no longer exists because the peripheral nerve is unable to maintain muscle tone. The involved muscles become soft and limp.
Severe muscle atrophy is observed within a few weeks due to a lack of efferent impulses that results in degenerated muscle fibers.
Fibrillations and fasciculations are present only in the early stage during which the muscles are undergoing atrophic changes.
There is a loss of both superficial and deep reflexes. Stretch and tendon reflexes are abolished, thus producing a hypotonic state, and the classic pathologic reflexes are not exhibited.
Reaction of degeneration is seen 1014 days after the injury. The nerve is unable to conduct a galvanic or faradic current because of structural alterations. A site on the body wall, cranium, or limb to another site on the body wall, cranium, or a limb (somatosomatic reflexes); ie, a somatosomatic reflex develops when a sensory receptor in the skin, subcutaneous tissues, fascia, striated muscle, a tendon, a ligament, or a joint is stimulated to trigger a volley of action potentials to another anatomical location of this type via efferent somatic or autonomic fibers.
A site (cutaneous, subcutaneous, musculoskeletal) in the body wall, cranium, or a limb to an internal organ or gland (somatovisceral reflexes); ie, a somatovisceral reflex is initiated when a sensory receptor in the skin, subcutaneous tissue, fascia, striated muscle, a tendon, a ligament, or a joint is stimulated to trigger a volley of reflex efferent impulses to viscera or glands.
An internal organ or gland to a site on the body wall, cranium, or a limb (viscerosomatic reflexes); ie, a viscerosomatic reflex develops when a sensory receptor in an internal organ, a gland, or a vessel is stimulated to trigger a volley of reflex efferent impulses to the skin, subcutaneous tissues, fascia, a striated muscle, a tendon, a ligament, or a joint.
An internal organ or gland to another internal organ or gland (viscerovisceral reflexes); ie, a viscerovisceral reflex develops when a sensory receptor in an internal organ, a gland, or a vessel is stimulated to trigger a volley of reflex impulses to another anatomical location of this type via efferents of the autonomic nervous system.
It must also be considered that these reflexes usually have segmental, propriospinal, and/or suprasegmental implications.
The reticular activating system (RAS) plays dual roles: it is both an afferent and an efferent system. It receives direct or indirect signals from the spinoreticular, spinotectal, spinothalamic, auditory, and visual tracts, as well as others. It also transmits signals superiorly to the cerebrum and inferiorly to the spinal cord (as previously described). In fact, many RAS neurons divide so that one branch is sent upward while another is sent downward.
The visceral nervous system (the magnificent servant) attends to the voluminous neural activities that must be conducted each second to maintain homeostasis. Because of this, and unlike the somatic system, the vegetative system is continually active.
(1) along the spinal column as the gangliated cord or more peripherally at convenient sites for sending out nerve fibers to supply the various viscera, or
(2) they may lie in the walls of the viscera themselves (eg, plexuses of Auerbach and Meisner).
The ganglia are connected to the CNS by connector nerves arising from cells within the gray matter of certain segments of the spinal cord and similarly in the medulla and midbrain. Also, certain ganglia are located in the brain and cord that serve as control stations for receiving and distributing impulses which result in the correlation and integration of complicated acts involving several widely separated structures. These stations distribute impulses to visceral, glandular, and skeletal structures, thus bringing the total body habitus into a unity of and for action.
Preganglionic Fibers. Preganglionic sympathetic neurons are found with thoracic nerves T1T12 and lumbar nerves L1L2. Their cell bodies lie in the intermediolateral horn of the spinal cord. Their fibers pass through the anterior root, enter and course with spinal nerves until they exit their IVFs, then separate and pass through white rami into the ganglia of the paravertebral sympathetic chain. From here, these fibers can take one of three courses: Synapse with postganglionic neurons in the ganglion entered at that level.
Ascend or descend longitudinally in the chain to synapse with postganglionic neurons at higher or lower horizontal levels.
Ascend or descend longitudinally in the chain and then project horizontally to synapse in a peripheral ganglion.
Preganglionic parasympathetic neurons are found with cranial nerves I, III, VII, IX, and X, which have their cell bodies in the gray matter of the brain stem, and with sacral nerves S2S4, which have their cell bodies in the middle three segments of the sacral cord. Except for a few cranial parasympathetic fibers and those sacral axons that supply the plexuses of Meissner and Auerbach in the intestinal tract, parasympathetic neurons are preganglionic; ie, they pass without synapsing directly to their peripheral synapses with short postganglionic fibers in the wall of the organ or gland innervated.
(1) activating or inhibiting to a given smooth muscle or secreting cell or
Thus, vegetative nerves often consist of two systems that are reciprocally antagonistic for the most part. In addition, each system contains neurons that often produce effects opposite to those which are common to that system, and sometimes the function of one system in a dual innervated organ has not be determined. This challenges the common idea that the two systems are always antagonistic.
Visceral nerve action varies according to
(1) the state of activity of the organ when it receives the stimulus and
Peripheral Visceral Receptors. There are three types of peripheral visceral receptors, and they are widely distributed throughout the body:
(1) slowly adapting mechanoreceptors that respond to tension (stretch);
Keep in mind that there are also somatic nociceptors present in visceral surfaces and blood vessels that respond to noxious stimuli. Impulses from any receptor, however, if stimulated intensely and long enough, can be interpreted in the CNS as pain if they reach consciousness. This is true for both the somatic and the visceral systems.
Threshold Factors. Full activation of a somatic nerve is in the range of 75200 impulses per second (ips). The threshold for an autonomic nerve is much lower and an important consideration in electrotherapy. An autonomic nerve, sympathetic or parasympathetic, will generally produce a response sufficient to maintain minimal effect with 1 ips and reach full activation with a 1020 ips discharge.
(1) follow along with splanchnic, hypogastric, or pelvic nerves to join the paravertebral sympathetic chain of ganglia or
(1) follow the blood vessels centrally to join the sympathetic chain and then pass to the spinal nerve and posterior root via the rami communicates or
(1) with somatomotor axons to produce related somatic activity (eg, parietal spasm) or
For longitudinal correspondence upward, two routes are also taken: (1) Synapses are made with ascending fibers in the lateral spinothalamic tract and posterior columns to the hypothalamus, thalamus, and reticular formation. (2) Synapses are made with sensory neurons in the posterior horns that also receive peripheral somatosensory input. This convergence of viscerosensory and somatosensory impulses is the basis for the overlying segmental hyperesthesia (superficial referral) so often seen with irritating visceral disorders. Adaptation. Smooth muscle contains an inherent ability to adapt (alter) the strength and rhythm of contractions that is unrelated to mechanical influences (eg, distention stretch). Smooth muscle also readily adapts to humoral and chemical influences.
Automatism. The term automatism refers to the ability of smooth muscle that has been isolated from any innervation to continue to spontaneously contract in a rhythmic manner in response to local stimuli. The stimuli for this effect arise from functional pacemakers whose impulses spread from one area of tissue to another. These are functional areas, not structural entities, as they may shift from one area to another as the result of changing needs. In this manner, smooth muscle and many glands may continue to function in the absence of an outside source of action potentials. It is for this reason that autonomic neural disorders express clinically in a far less overt manner in their early stages than do somatic neural disorders.
Intramural conduction. If a local area of a vegetative organ (eg, intestine) is stimulated, local contraction occurs that slowly travels along the length of the organ. This spread is made possible by intramural connections.
Preganglionic Autonomic Axons. All preganglionic autonomic neurons, sympathetic and parasympathetic, stimulate the release of acetylcholine. From this, it can be deduced that a preganglionic sympathetic fiber will excite both postganglionic parasympathetic and sympathetic fibers, and vice versa. Some preganglionic sympathetic axons extend to the medullae of the adrenal glands and terminate on special cells where they stimulate the release of epinephrine and norepinephrine directly into the blood stream to produce widespread sympathetic effects.
There are two types of adrenergic receptors: alpha and beta (1 and 2). Norepinephrine activates alpha-adrenergic receptors, with a minimal effect on beta-adrenergic receptors. Epinephrine activates both alpha-adrenergic and beta-adrenergic receptors to an equal degree.
It is well to keep in mind when treating patients who are on drugs that affect the autonomic nervous system that synthetic drugs may or may not act upon receptors in the same manner that these natural substances do. For example, synthetic isopropyl norepinephrine activates a strong reaction in beta receptors but has no effect on alpha receptors.
Trophic Reactions. Trophic lesions of the joints, muscles (atrophy), skin, and nails are common. They blend and are somewhat explained as the result of vascular changes (vasomotor). Herpes labialis (cold sores) and herpes zoster (shingles) appear to give evidence of being due to nutritive disorders in the ganglia, not to vascular changes, predisposing to virus infection. In brain lesions, trophic and vasomotor changes are much less common than in diseases of the cord and peripheral nerves. The acute bedsores that form in myelitis, the angioneurotic local swellings that appear here and there in certain people, and the local syncope or asphyxia that sometimes leads to Raynaud's form of gangrene seem to require both nerve and vascular changes to explain them.
(1) an uneasy dull discomfort in the viscera itself and
Head's law states:
Psychic Influences. Stimuli to the sympathetic-accelerator side of the autonomic nervous system are initiated frequently by emotions as well as by related exercise. Within a reasonable time, the symptoms created by the stimuli should return to normal. However, if these stimuli are too prolonged or pronounced, then the balancing parasympathetics (eg, the vagus nerve) should be investigated to see why it is not exerting its proper opposition force or why it is underactive.
In the longitudinal systems described previously, the signs of dysfunction were the result of direct neural damage. This is not true for the vascular system. Being a supporting system, defects in the vascular system supplying the nervous system generally manifest as secondary neurologic deficits.
Arterial Supply. Each cervical vertebral artery offers a branch at the brain stem to form a single anterior spinal artery that runs the length of the spinal cord, lying over the median fissure. It tapers as it courses downward in the thoracic cord and gives off central branches at intervals of about 2 mm that, in turn, branch both peripherally and centrally. The anterior spinal artery supplies all the cord with the exception of the posterior columns and horns.
(1) internal plexuses, whose pia branches drain the contents of the vertebral canal, vertebral arch, and posterior vertebral body and leave via the IVF and
The Arterial System. An anterior artery, descending in the ventral median sulcus, and two posterior arteries, descending along the posterolateral sulcus, supply the spine. The latter arise from either the vertebral arteries or the posteroinferior cerebellar arteries. These anterior and posterior axial conduits are often small, irregular, and must be reinforced at intervals by radicular arteries that branch from nearby spinal arteries arising outside the vertebral column.
(1) an anterior branch, which
(a) supplies the posterior aspect of the vertebral body to anastomose with the lateral and anterior twigs of that part of the artery that did not enter the IVF, and
(2) an intermediate branch, which supplies the nerve root and spinal dura; and
The Venous System. The venous system of the spine (Batson's plexus) is a valveless plexus separate from that of the thoracoabdominal cavities. It is derived from the veins of the extremities, body wall, neck, and head. Any increase in intrathoracic or intra-abdominal pressure (eg, Valsalva maneuver) shunts venous blood to the vertebral system. The resulting engorgement contributes to the spinal pain of many patients with spinal faults. The close tie between spinous venous pressure and neurologic integrity is clearly exhibited when the jugular compression test elicits paresthesia in the lower extremities.
Extradural Hematoma
Subdural Hematoma
Acute Subdural Hematoma. This is the most frequent cause of death in falls and sports injuries. It is much more common than extradural hematoma and shows no lucid interval. Lateralizing signs, however, are similar. Inequality of pupils (anisocoria) is of considerable localizing value. Hutchinson's pupil from compression of the 3rd cranial nerve against the free edge of the tentorium is often seen. Widely dilated and fixed pupils bilaterally indicate that death is approaching. Severe acute subdural hemorrhage is usually associated with cerebral laceration. Bleeding from the brain itself seeps into the subdural space, adding to probable dura, pia, and arachnoid bleeding. In the late stage, signs of herniation and oculomotor paralysis appear. The incidence is high in the elderly because cortical atrophy increases the space in which the veins must traverse. The incidence is also high in boxers, other contact sport athletes, and alcoholics because of increased head trauma.
Apoplexy
The onset may be slow or spontaneous (stroke), and symptoms will vary depending upon the severity and site of the hemorrhage; eg, abrupt headache (usually suboccipital), torticollis, vomiting, mental confusion, weakness, possible sensory loss, visual field defects, possible skull bruit, possible aphasia, unconsciousness, xanthochromia, late upper motor lesion signs, hemiparesis, and coma. Blurred vision
Headache
Decreased alertness
Hiccuping
Diplopia
Nausea
Dizziness
Papilledema (usually late)
Fever (possibly)
Projectile vomiting
Frequent yawning
Tinnitus
Specific disorders causing increased intracranial pressure are listed in Table 3.21. Some other cerebrovascular disorders not previously mentioned are briefly described below.
Cerebral Aneurysm. The most common cause of a cerebral aneurysm is an atheroma of the internal carotid. The primary symptoms vary, depending upon the exact site of the lesion; eg:
(1) frontal headache and isolated oculomotor paralysis (origin of posterior communicating artery);
Medial temporal lobes (bilateral): global amnesia
Medial temporal lobe (unilateral): specific memory defect(s)
Occipital lobe: visual field defect
Subthalamus: hemiballismus
Thalamus: loss of tactile sensation and pain contralaterally.
Sagittal Sinus Thrombosis. Possible skull fracture should be ruled out. In addition to the general characteristics of intracranial pressure, seizures are common that slowly progress in severity. There are neurologic focal signs, a negative brain scan, and EEG findings are normal or show diffuse slowing.
The term consciousness refers to the mental state of perception and responsiveness to environmental stimuli. Perception is the process of being aware; the receiving, elaboration, and usually the localization of a sensory impressions; the mental linking of cause and effect. Apperception means full comprehension such as the recognition or identification of the source of a stimulus, and this depends greatly on association with experiences. These faculties of awareness are primarily integrated, modulated, and coordinated by the cerebral cortex.
(1) the brain stem from the reptilian period,
The structurally ideal biped would be ambidextrous, but most people present with a hemispheric dominance as expressed, for example, in right- or left-handedness, as well as in a dominant eye, ear, and foot. About 90% of the population has a genetically dominant right side that becomes firmly established about the age of 8 years. Attempts in maturity to acquire balanced bilateral motor activity interferes with one's normal speed, rhythm, and endurance.
Coma. Coma is an abnormally deep and prolonged state of unconsciousness from which the patient cannot be aroused by even painful stimulation. It is the most severe form of unconsciousness. The patient exhibits absent or depressed pupillary, stretch, and plantar reflexes; a periodic respiratory rhythm and slowed pulse, especially if the lesion is in the brain stem; and little or no spontaneous movement. Coma vigil is a severe and grave form of coma in which the patient lies with the eyes open but is entirely unconscious of surroundings. It may be accompanied by a low muttering delirium that is similar to that of a person talking during deep sleep. Semicoma is a light state of coma in which the patient is restless (often exhibiting tremors and twitches) and reacts somewhat to painful stimuli.
Functional Anatomy: An Overview
(1) the inherent motility of the brain and spinal cord,
Involuntary Cranial Motion. Upledger and other osteopathic researchers have conducted numerous studies of craniosacral motion, its causes, and the results of impairment in the system. They explain that every organ in the body exhibits a pulsation or inherent rhythmic action that features a slow, worm-like movement. The brain and spinal cord are no exception to this: manifesting a slow, rhythmic coiling and uncoiling of the hemispheres and a longitudinal movement of the spinal cord within the spinal dura.
This combined motility of the central nervous system and movement of CSF manifest as a hydrodynamic "pump" and a bioelectric interchange. Upon light palpation of the cranium, this pulsation can be felt to have a rate of about 1014 cycles/min. It is the result of the pull of the dural membranes, the fluctuating CSF, and the inherent motility of the CNS. The sphenobasilar symphysis appears to be the key cranial articulation. Prior to 25 years of age, it has a cartilaginous union; after 25, it has the resiliency of cancellous bone.
Adrian ED:
THE SENSORY SYSTEM
Daube/Sandok classify afferent (sensory) impulses according to their origin and specificity. See Table 3.2.
Table 3.2. Classification of Sensory Impulses
Type Origin
General somatic afferent (GSA) Joints, skin, striated muscle.
Special somatic afferent (SSA) Equilibrium, hearing, vision.
General visceral afferent (GVA) Smooth muscle and viscera (essentially
unconscious perception).
Special visceral afferent (SVA) Smell, taste.
Basic Types of Sensory Perception
(b) topognosis, the ability to localize and perceive cutaneous stimuli.
Classic literature often uses Head's two groups in classifying cutaneous sensibility.
Sensory Neuron Levels
The function of the transducer-like receptor organs is to convert chemical, mechanical, photic, and thermal stimuli into neuronal potentials that can be transmitted to and within the CNS by specific pathways so that they can be perceived, interpreted, and integrated. This process is usually (but not always) conducted, processed, and used over three orders of neurons: primary, secondary, and tertiary.
Primary Neurons
The dendrites of peripheral sensory neurons receive impulses from sensory receptors, and their unipolar cell bodies lie outside the CNS in a spinal root ganglion. There are no synapses in a posterior (dorsal) spinal ganglion.
Each ganglion cell has a single nerve process (dendraxon) that divides so that the distal branch enervates the receptor and the proximal branch enters the CNS by a posterior root of the spinal cord or brain stem and then through the posterolateral sulcus at the posterior root entry zone. (See Fig 3.1) The proximal axons of a primary neuron may synapse immediately with secondary neurons in the CNS or ascend within a tract (usually a white matter fasciculus) for a considerable distance before they synapse. (See Figure 3.2 and Table 3.3).
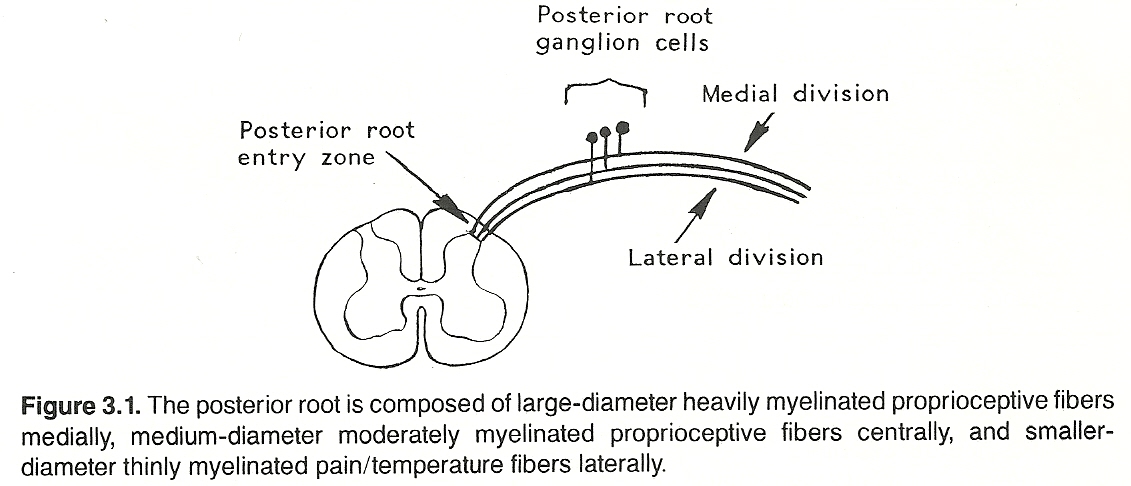
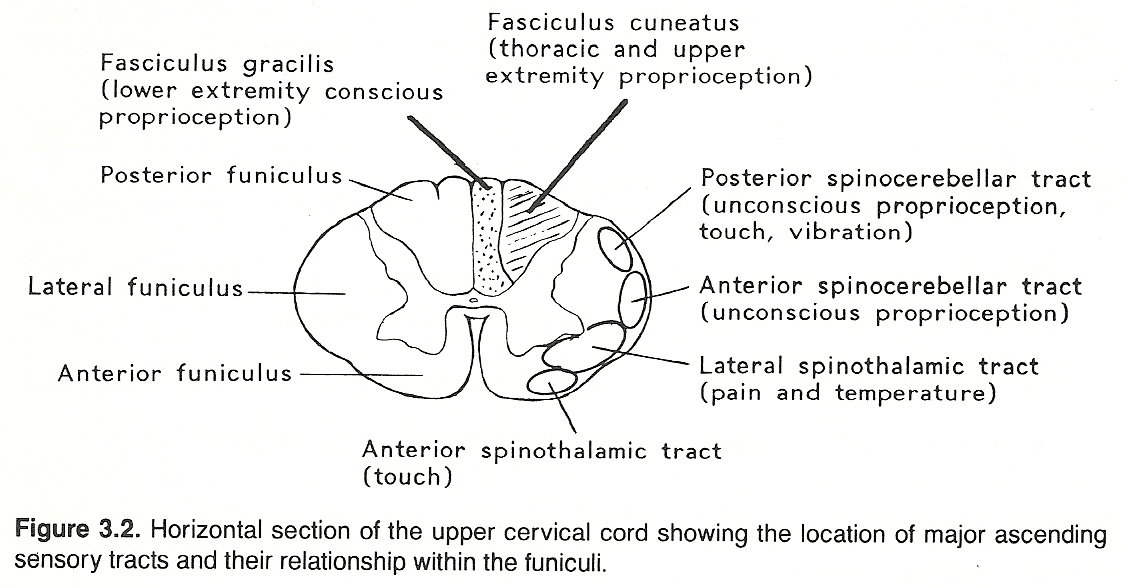
Table 3.3. Primary Sensory Pathways
Pathway Primary Function
Posterior (dorsal) columns:
Fasciculus gracilis Conscious proprioception, tactile
discrimination, stereognosis,
barognosis, vibration
Fasciculus cuneatus Conscious proprioception
Posterior (dorsal) Unconscious proprioception
spinocerebellar tract
Anterior (ventral) Unconscious proprioception
spinocerebellar tract
Lateral spinothalamic tract Pain, temperature
Anterior (ventral) Simple touch
spinothalamic tract
The axons of primary sensory neurons synapse close to the cell bodies of secondary neurons that lie within the posterior gray matter of the spinal cord or an analogous area of the brain stem (derivatives of the embryonic alar plate.
The axons of secondary sensory neurons cross the midline (decussate) in their cephalad course and then ascend to the brain stem to end in specific sensory nuclei of the thalamus, where they synapse with the cell bodies of tertiary sensory neurons. The crude recognition of general somatic information enters consciousness at this level.
Tertiary Neurons
The axons projecting from the cell bodies of the tertiary sensory neurons of the thalamus extend to the somesthetic area of the sensory cortex through the thalamocortical radiation. Auditory and visual fibers extend to the temporal and occipital lobes, respectively. However, the primary area of the cortex involved for somatic sensory impulses is in the postcentral gyrus of the parietal lobe and distributed in a homunculus-like manner. (See Fig 3.3) It is at this level where the finer, more discriminative, aspects of somatic sensory perception occur.

Receptors: General Considerations
It was described in the previous chapter that whenever a stimulus of adequate threshold acts upon a receptor, the receptor organ depolarizes and a graded generator potential initiates. If this is strong enough, an electrotonic potential is produced that induces a train of centralward action potentials of uniform amplitude.
Receptor Adaptation
Because all action potentials are of the same amplitude, the intensity of stimulation is coded by the quantity of neurons activated and the frequency (modulation) of the discharges. The axonal discharge frequency is high initially but gradually decreases (adapts) when receptor stimulation is sustained. If a sensory receptor is repetitively stimulated, successively smaller generator potentials are produced (receptor fatigue).
Recovery depends on the type of receptor stimulated. Some receptors (eg, tonic) adapt slowly and respond variably according to the strength of the stimuli applied; other receptors (eg, phasic) adapt quickly and initiate impulses almost continuously.
Receptor Specificity
Sensory receptors are specialized cells that detect particular environmental changes. There are various types of receptor organs and each is more sensitive to one or more types of stimuli but not sensitive to other types. This specificity is determined by the location of the receptor organ and the organ's general structural and chemical composition (surface membrane, organelles).
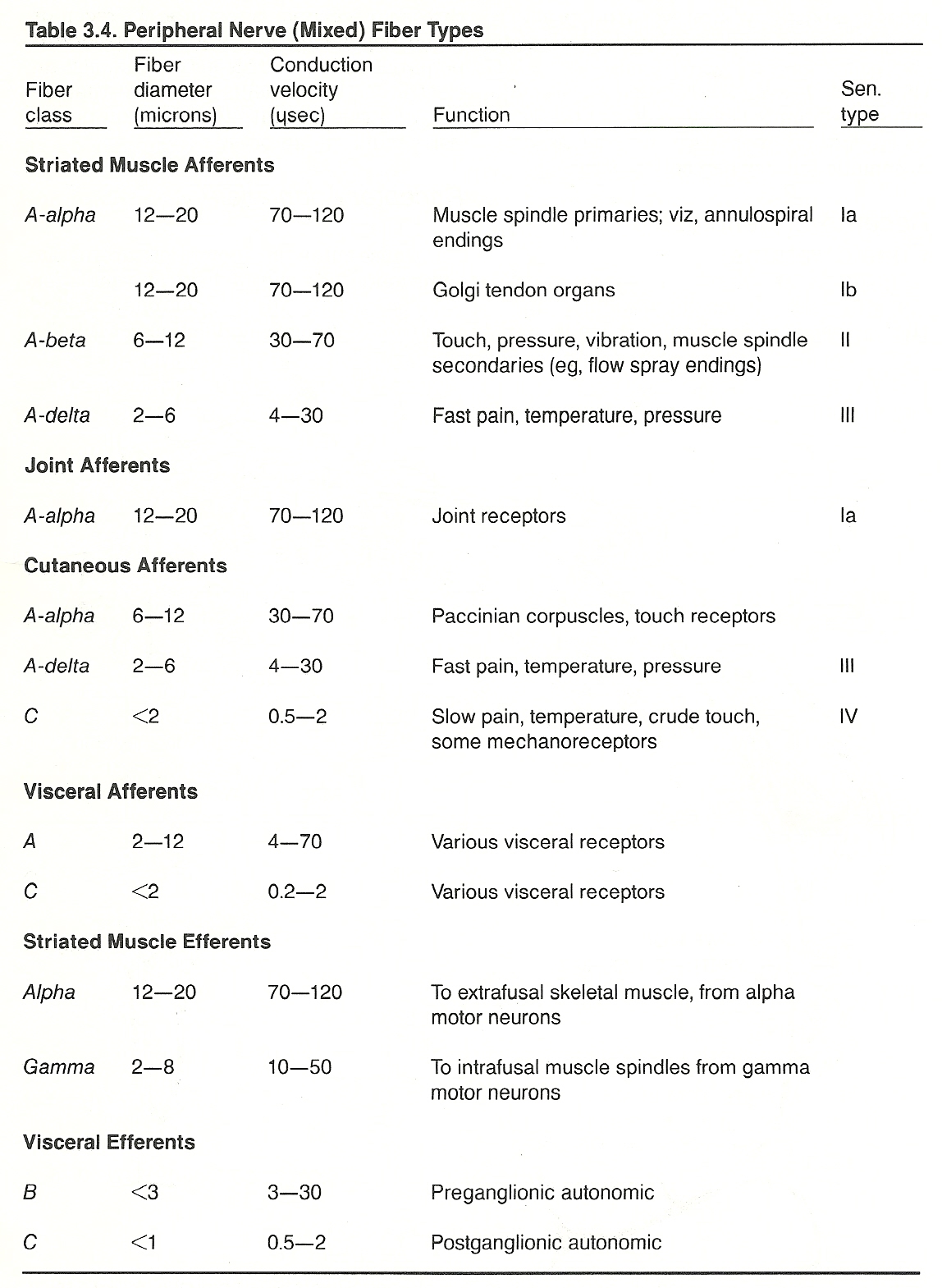
Hirschy describes five general types of sensory receptors:
All sensory receptors initiate impulses from their sites to the spinal cord through Type IIV sensory afferent fibers. See Table 3.4.
Sensory receptors can also be described from other viewpoints. For example, a teleceptor is any sensory receptor that is sensitive to distant stimuli such as those for sight, hearing, smell, and, to some extent, temperature. Exteroceptors include the teleceptors and those receptive to mechanical, electromagnetic, and chemical contact (eg, retinal receptors, Merkel's and Meissner's corpuscles, Krause's end bulbs). They are primarily affected by changes in the external environment.
Proprioceptors relay input from muscle spindles, Golgi tendon organs, pacinian corpuscles, labyrinthine receptors, and the soft tissues of joints to offer data about posture, position, stretch, and compression. Interoceptors are sensitive to changes within blood vessels, respiration, and the viscera. These latter types also convey signals of hunger, thirst, nausea, and sexual sensations. Special interoceptors convey smell and taste sensations.
Skin receptors concerned with touch, pressure, or pain function as both somesthetic exteroceptors and as proprioceptors. They initiate many of the basic inborn protective reflexes such as grasp reflexes, withdrawal reflexes, and placement reactions.
Golgi tendon organs and muscle spindles transmit changes in muscle length, contraction, and tension, and the specialized receptors in the eye and ear are quite specific for type of stimuli to which they will respond. Such specialization, however, is not true of most receptors found in or near the skin. The stimuli from touch or pressure, for example, may excite bare nerve endings, hair follicle endings, Krause's end-bulbs, Meissner's corpuscles, Merkel's discs, Pacinian corpuscles, or Ruffini's corpuscles. Pacinian corpuscles are also activated by vibration, Krause's end-bulbs by cold, and Ruffini's corpuscles by heat.
While bare nerve endings are commonly thought of as the primary receptors of noxious stimuli (pain), they have also been shown to react to temperature, light touch, and deep pressure stimuli. Thus, while it was once thought that receptors were quite specific, it is now known that they have considerable overlap in function.
A few joint-related receptors are insensitive to movement but discharge in proportion to the joint angle. There is no position in which all joint receptors are silent.
Balance Recovery
Besides those receptors involved in receiving impressions from the surface and in maintaining the erect posture against gravity, there are other postural mechanisms concerned with sustaining body equilibrium. These are necessary to keep the body in the correct position during complex motor activities. For example, when the body is thrown off balance (eg, from a stumble or blow), compensatory movements beyond conscious control are made to restore normal posture.
These reflex movements receive their sensory input from inner-ear proprioceptors, stretch receptors of the cervical musculature, other joint and muscle proprioceptors, the sensory organs of touch (especially of the plantar surfaces of the feet), and visual stimulation. The orientation of visual impressions is important in maintaining erect posture by establishing relationships to objects about the individual.
Labyrinthine Receptors
Inner ear sensory organs contribute stimuli to postural tone through impulses arising when the neck is moved. Small particles of calcium (otoliths) are suspended within the fluid of the semicircular canals. When the head is tilted, the otoliths drift from one part of the canal's wall to another. As these floating otoliths touch the sides of the canal's walls, they press against a few of the many hair-like sensory nerve receptors that line the canal's walls. This slight pressure stimulates impulses that ultimately produce a sensation of position and a reflex righting of the head relative to gravitational pull.
Only slight stimulation is necessary to evoke the reflexes necessary to maintain the body erect. Equilibrium reflexes evoked by stimulation of the semicircular canals dominate all other righting reflexes. Once these reflexes are lost (eg, pathology), one must rely solely on muscle and tendon proprioceptors, visual stimuli, and cutaneous touch and pressure to maintain an erect position.
Cervical Receptors
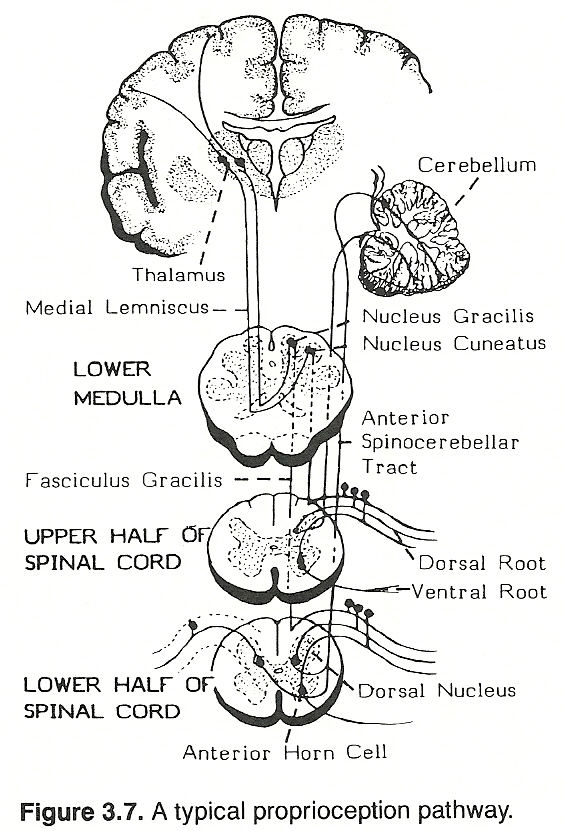
With the head erect, the labyrinths are placed in an optimum position to act synergistically with the neck reflexes, and these in turn react with other existing proprioceptive and exeroceptive impulses to supply a symmetric distribution of tone in proper quantities to postural muscles. Specifically, neck righting reflexes are evoked from impulses arising in joint receptors of the neck to produce contraction to align the body with the head. A typical spinal proprioception pathway is shown in Figure 3.7.
The cervical muscles are richly supplied with proprioceptors, and the atlanto-occipital and atlantoaxial joints are especially endowed with receptors. These facts, besides the specialized proprioception receptors of the inner ear, make head position important in maintaining structural balance.
Almost all movements are started with a head movement in the direction desired, and only a slight poorly directed head movement during a complicated movement is necessary to throw the entire body out of alignment. In contrast, cervical reflexes appear to be dominant on the upper limbs, while labyrinthine reflexes appear to be dominant on the lower limbs.
It is not unlikely that proprioceptive impulses combined with the interacting postural reflexes of good body mechanics play a role in the maintenance of good health and optimal performance. Conversely, the maladjustment of nervous impulses within the CNS as a result of pressure, irritation, or poor posture may be a causative factor in the production of poor health and hindered performance by contributing to dysfunction from the subtle yet persistent stress involved.
Joint Receptors for Pain
Wyke, Newton, and others point out that articular capsules are richly supplied with nociceptors. See Table 3.5.
Table 3.5. Joint Receptors*
Type I II III IV
Resembles Ruffini corpuscles Pacinian corpuscles Golgi end-organs
Location Outer layers of Sparsely found in Joint ligaments, Joint
joint capsule, deep layers of grape-like clusters (except
grape-like clus- joint capsule and (2-3) or found synovial
ters (3-8) and in fat pads, individually ligaments
grape-like clusters fat pads
(2-4)
Endings Encapsulated Thickly encapsulated Thinly encapsulated Bare
nerve
ending
plexus
Fiber type Myelinated, small Myelinated, medium Myelinated, large Myelin-
and size (69 microns) (912 microns) (1317 microns) and
unmyelinated
Threshold Very low Low High High
Conduction Slow Medium Rapid Slow
Adaptation Slow Rapid Very slow Not
adapting
Action Mechanoreceptor Dynamic mecha- Dynamic mecha- Noci-
noreceptor noreceptor ceptor
Function Signals static Signals only rapid Signals direction Signals
position of joint, changes in move- of movement; noxic
speed and direc- ment, acceleration, guards against change
tion of joint deceleration; helps excessive joint
movement; con- initiate momentum, movement by
stantly firing; does not signal regulating muscle
aids in regulat- joint velocity, tone (braking
ing posture and amplitude, or mechanism)
muscle tone dur- direction
ing joint motion
_________________________________________
*Adapted from Kessler/Hertling and Wyke.
(2) a fine network with numerous terminations in the histiocytic cell layer; and
(3) an extremely delicate network intermeshed within the fine network that is closely attached to the capillaries, connective tissue, and histiocytes.
If a blindfolded person maintains a limb in an asymmetrical posture for some time, it is typically misplaced in the direction of previous asymmetry when an attempt is made to restore it to a symmetrical position. This effect, called apostural persistence, is thought to be caused by adaptation in joint receptors or muscular forces such as are involved, for example, after contraction.
In studies conducted by Howard/Anstis, the after-effects of holding the head to one side were compared with the after-effects of holding it straight while straining against a torque. After-effects in the self-perceived position of the head were evident in only the former condition and were found to be reduced when the head was in the opposite direction to that in which it was adapted. It was thus concluded that postural persistence is a function of adaptation in joint receptors, at least in moderate loading.
Major Pathways
The major structures involved in the detection and transmission of painful stimuli are the first-order neurons and posterior root, the lateral spinothalamic tract of the spinal cord, the thalamus, parietal cortex, and the prefrontal cortex. It should be noted, however, that lesions in these structures greatly modify pain (+/), but they rarely abolish it. From a clinical standpoint, the major pathways are those that convey sensations of pain/temperature, touch, proprioception, and discrimination.
Receptors. Pain receptors are the most primitive of all neuroreceptors, and reflex responses to painful stimuli appear early during embryogeny. The primary locations for pain receptors are the
(2) periosteum,
(3) vascular walls,
(4) joint structures (eg, capsules, para-articular soft tissues),
(5) falx and tentorium of the cranial vault, and
(6) deep tissues diffusely distributed throughout the body.
It should be noted that the overstimulation of almost any receptor can give rise to pain. Whether this is do to a normal response in nonnociceptors or an effect on neighboring bare nerve ending is unclear. Prichard states that excessive receptor stimulation causes cells in any tissue to release, by cell rupture or increased permeability, substances (eg, bradykinin) that are not ordinarily present in extracellular fluid. Such substances are thought to act on bare nerve ending in the area in a manner similar to transmitter substances on postsynaptic membranes so that action potentials are initiated in nociceptor fibers.
Primary Tracts. Spinal cord impulses conveying pain and temperature signals appear to travel the same major course:
The points shown in Figure 3.9 describe the course of the major pathway for pain/temperature impulses, bit it should also be recognized that collateral fibers are given off at various levels of the neuraxis during this ascending course. One important level is in the brain stem where synapses are made with neurons of the centrally located reticular formation that provide data to the ascending activating system. These neuronal connections, whose signals are widely dispersed to the cortex, are important in maintaining an alert state of consciousness. This process will be explained later in this chapter.
Cranial vs Spinal Pain-Conducting Neurons. The pathways for pain described above are essentially those of structures located below the foramen magnum. Painful stimuli from the face, for example, are conducted primarily by the trigeminal (cranial V) nerve, which has its primary sensory cell bodies in the semilunar (gasserian) ganglion. Proximal axons enter the brain stem in the pontine region, descend ipsilaterally to the upper cervical cord, synapse with second-order neurons, cross to the contralateral side, and then ascend to the anterior posteromedial nucleus of the thalamus. It is for this slightly divergent course taken by nerves entering the CNS above and below the foramen magnum that lesions in the lower part of the brain stem may alter pain perception in the face ipsilaterally and in the lower body contralaterally.
Localization of Pain. The manifestations of pain/temperature-pathway lesions vary according to which level the neuraxis is involved:
Conscious Proprioception and Tactile Discrimination
Proprioception refers to the inborn kinesthetic awareness of body posture, position, movement, weight, pressure, tension, changes in equilibrium, resistance of external objects, and the associated stereotyped response patterns. This awareness is the result of impulses generated in afferent receptors located within muscles, tendons, joints, skin, viscera, middle ears, and eyes. The responsive reflex patterns consist of highly coordinated movements of numerous muscle groups and joints that must be precisely regulated and timed as to sequence, speed, intensity, and duration.
Sensory data from the musculoskeletal system is necessary to direct and control motor function. This is accomplished through conscious and unconscious input.
The primary course of conscious proprioception is as follows:
Unconscious Proprioception
From 40%60% of nerve fibers supplying a muscle is sensory in function. Some of these afferent fibers are concerned with pain, but most are concerned with proprioception to register muscle fiber contraction or stretch. This is invariably an unconscious process, yet it plays a vital part in controlling muscular force and timing.
The complete process is a combined effect from afferent fibers from muscles, tendons, and joints. These sensory muscle fibers originate from sites on perimysium, endomysium, capillaries, or in interstitial fat. Other sensory fibers originate from receptors concerned purely with pressure. These are abundant in joint tissues and offer conscious information as to limb position.
The primary course of unconscious proprioception impulses is as follows:
The proximal axons enter the medial division of the posterior root entry zone of the spinal cord. From here, two primary courses are taken:
(b) Other fibers ascend as either the anterior or posterior spinocerebellar tracts so that the cerebellum can modify the unconscious afferent impulses to assure smooth, well-coordinated motion by cerebellar efferent directives.
Touch
The perception of touch can be divided into two general types:
(2) tactile discrimination, which perceives the depth and quality of the pressure as well as the approximate shape and size of the stimulus as modified by the recall of associated past experiences.
The primary course of touch signals by way of the anterior spinothalamic tract is as follows:
Pathways of Other Sensations
The tracts conveying visceral signals will be described later in this chapter. The pathways for vision, hearing, equilibrium, taste, and smell will be described in Chapter 4.
Clinical Considerations
The diagnosis of neurologic diseases can often be frustrating because of the transient nature of many symptoms and signs. Besides frequent symptomatic peaks and valleys, neurologic symptoms may mimic a large variety of organic and functional disturbances. In general, the objective of the neurologic examination is to evaluate disturbances of motion, sensation, reflexes, skills, neuronutrition, and the general psyche.
The basic equipment usually required for evaluating the sensory system are a wisp of cotton or camel's hair brush, a pin or pinwheel, tubes of hot and cold water, a tuning fork (C-128), olfactory solutions, taste test solutions, an ophthalmoscope, a penlight, a skin pencil, eye and color charts, and objects to test stereognosis objects. Common accessory equipment includes devices to detect localized areas of skin resistance and skin temperature variations.
The most common features associated with diseases of the sensory system are headache, vomiting, spinal and extremity pain, paresthesia, anesthesia, tinnitus, vertigo, deafness, and visual disturbances. Less common afferent nerve lesions take the form of sudden behavioral changes, disorders of memory and thinking, disturbances of consciousness, and hallucinations.
To experience any sensation, it is necessary that:
Table 3.6. Common Terms Used to Describe Abnormal Sensory Perceptions
Term Description
Acroesthesia Abnormal sensitivity, especially pain, of the extremities.
Acroparesthesia Numbness, prickling, or tingling sensations of the distal
extremities (eg, fingers and toes).
Allesthesia A single stimulation that is perceived as being displaced
across the midline to a homologous region; ie, perceived
contralaterally. This is sometimes referred to as
allochiria or allochesthesia.
Alliesthesia The perception of an external stimulus as being pleasant
at one time and unpleasant at another.
Analgesia Complete loss of normal pain sensibility.
Anesthesia Complete loss of sensation. Dissociated anesthesia is the
loss of some forms of cutaneous sensibility (usually pain/
temperature) with the preservation of others (eg, tactile).
Astereognosis Inability to recognize familiar objects by touch even if
anesthesia is not present; usually indicates a lesion in
the parietal cortex of the cerebrum.
Atopognosis Inability to localize tactile stimuli without visual aid.
Baragnosis Inability to distinguish objects of different weight
without the aid of vision; usually indicates a lesion
in the parietal cortex of the cerebrum.
Causalgia Autonomic nerve fiber injury. The median or posterior
tibial nerve is usually involved.
Colic A nondiagnostic term that refers to any symptom complex
whose major feature is acute paroxysmal pain.
Dysesthesia Impaired sensitivity; a painful sensation from a stimulus
that is normally not painful; formication.
Formication False perception of insects crawling upon the skin; a
common effect of cocaine withdrawal.
Hyperalgesia Increased sensibility to pain-provoking stimuli; also
called hyperalgia.
Hyperaphia Excessive sensitivity to touch stimuli.
Hyperesthesia Excessive sensitivity to any sensory stimulus; often
restricted to increased tactile sensibility.
Hypoesthesia Diminished sensation, especially tactile. This is often
referred to as hypesthesia.
Neuralgia Severe sharp pain along the course of a nerve; typically
a functional disorder from nerve trunk pressure, faulty
nerve nutrition, toxicosis, neuritis, or hysteria. This
is sometimes referred to as neurodynia.
Paresthesia Any disagreeable sensation such as numbness, tingling,
prickling, or formication; often restricted to abnormal
nonpainful sensory aberrations.
Phantom pain A projection of the sensation of pain to a part of the
body that hasbeen lost (eg, amputated). It is due to the
stimulation of pain fibers that previously innervated the
lost part.
Sensory When two stimuli are applied simultaneously, one is well
displacement localized while the other is displaced toward it.
Sensory When two stimuli are applied simultaneously, one is well
extinction localized while the other is perceived poorly or not at all.
Synesthesia Two sensations are perceived from a single
stimulus, one of which is well localized while the other
is perceived contralaterally or in an area in which there
is dysesthesia or burning pain. This is sometimes referred
to as synchiria.
Correlating the patient's signs and symptoms of sensory dysfunction with the specific anatomical pathway level involved will be a distinct aid in determining the site of the lesion. Supratentorial lesions, for example, exhibit contralateral sensory deficits. In posterior fossa lesions, the primary signs will be contralateral sensory features in the trunk and extremities and, possibly, ipsilateral sensory loss in the face.
In spinal cord lesions, signs and symptoms manifest as sensory loss at the lesion level, with a varying degree of loss at all levels below the level of the lesion. In peripheral nerve or nerve root lesions, manifestations occur in a segmental manner and involve all peripheral sensory functions (often including pain). See Table 3.7.
Table 3.7. Classic Locations of Segmental Pain
Priority Priority
Suspect Suspect
Nerve(s) Area of Localized Pain Nerve(s) Area of Localized Pain
Trigeminal Anterior head and face T58 Esophagus (caudal)
C12, T712 Occiput T512 Peritoneum
C23 Forehead T610 Pancreas, spleen
C3, T15 Neck T79 Ascending colon
C34, T13 Aortic arch T89 Gallbladder
C3 4, T15 Heart T910 Small intestines
C34, T18 Head and face T911 Transverse colon
C34, T35 Lungs T1011 Umbilical area, ovary,
testicle
C34, T67 Stomach, cardiac aspect T1012 Crown of head, scrotum,
lower limbs
C34, T810 Stomach, pyloric aspect T1012, S13 Prostate
C34, T79 Liver T10L1 Kidney, uterine body
C4 Shoulder girdle, temple T11L1 Urethra, epididymis
C5 Deltoid area T11L2 Bladder neck, descending
colon
C6 Thumb T11L1 Suprarenal area
C7 First or index finger T12L1, S14 Uterine neck
C8 Fourth finger T12L2 Ureter
T1 Fifth finger L1 Groin
T14 Thorax L13, S14 Bladder body, rectum,
genitals
T2 Nipple area L3 Knee, medial aspect
T24 Bronchi L5 Great toe
T25 Upper limbs S1 Fifth toe
T212 Pleura S2 Thigh, posterior aspect
T45 Mammae bodies S24 Cervix
T47 Thoracic aorta
Supratentorial Level Lesions
All major sensory pathways have decussated once they reach the supratentorial level; thus, lesions at this level generally manifest as extensive contralateral sensory loss. Two important syndromes that are initiated at this level are described below.
Suprathalamic Syndrome. Lesions involving thalamocortical sensory fibers also alter somatic afferent sensations contralaterally; however, the loss is not as complete as it is in a thalamic syndrome. In a suprathalamic syndrome (eg, Dejerine's cortical sensory syndrome), there is a severe loss of tactile discrimination, two-point discrimination, passively moved joint-positions, baresthesia, the ability to recognize textures, the ability to recognize numbers drawn on the skin, and stereognosis because cortical function is necessary. However, only a minor or moderate loss occurs of pain, temperature, simple touch, and vibration sensations.
Posterior Fossa Level Lesions
Lesions at the posterior fossa level feature contralateral intersegmental sensory loss below the neck because of interruption of the major ascending tracts and, usually, an ipsilateral sensory loss in the face because of involvement of the trigeminal nucleus. The most common cause of this syndrome is inferior cerebellar artery occlusion (Wallenberg's syndrome).
Spinal Level Lesions
Severe lesions of the spinal cord, proximal to root entry, that interrupt major ascending and descending pathways will produce sensory signs and symptoms at and below the lesion level. Typical examples are paralysis, loss of superficial and deep reflexes, and autonomic disturbances. If sensory tracts are interrupted but reflex motor arcs are not, deep tendon reflexes at and below the level of the lesion will be hyperactive.
Commissural Syndrome. This syndrome is seen when the central region of the spinal cord is impaired for several segments. It is characterized by a bilateral segmental loss of pain and temperature sensations, because both ipsilateral and decussating fibers are involved. Syringomyelia (cavitation), cord neoplasms, and trauma are the common causes.
Syringomyelia, which produces gliosis around the central canal of the spinal cord, features a severe loss of pain and temperature sensations but retains touch and pressure sensations in the affected parts; ie, dissociated anesthesia.
Brown-Sιquard's Syndrome. This syndrome is rarely witnessed, but it offers a classic picture of the effects of a unilateral lesion of the spinal cord. The classic picture is described as follows: (1) ipsilateral lower motor neuron paralysis and a zone of anesthesia in the segment of the lesion; (2) ipsilateral hyperesthesia below the zone of anesthesia; (3) ipsilateral upper motor neuron paralysis, loss of proprioception, loss of vibration sense, and loss of two-point discrimination below the level of the lesion; (4) a contralateral zone of hyperesthesia in the segment of the lesion; and (5) a contralateral loss of pain and temperature sensation below the level of the lesion. Common causes include a bullet wound, laceration, hematomyelia, cord tumor, and syringomyelia.
Tabes Dorsalis. Because of the interruption of proprioception pathways in the dorsal roots and posterior columns, this syndrome features marked sensory ataxia and a characteristic gait. At times, subjective sensory disturbances (tabetic crises) manifest as severe painful cramps in the larynx, stomach, and other viscera.
Posterolateral Sclerosis. In advanced cases of pernicious anemia, for example, combined degeneration of the posterior and lateral columns of the spinal cord occurs. The syndrome features marked sensory and motor disturbances. An early sign is a diminished vibration sense, with diminished discrimination faculties following.
Multiple Sclerosis. This disorder is caused by disseminated patches of gliosis in the spinal cord and brain. It features early patches of numbness and paresthesias, followed by marked sensory and motor disturbances.
Root Lesions. Nerve root syndromes are frequently met with in chiropractic practice. They may involve neuronal compression, stretching, or inflammation and induce sensory disturbances such as pain, hyperesthesia or hypesthesia, and paresthesias along the anatomical distribution of the affected root. This typically manifests in a dermatomal pattern. The associated pain (usually lancinating) is commonly aggravated by any act that increases intrathecal pressure such as in coughing, sneezing, abdominal straining, or any maneuver that stretches the involved root. Typical causes include acute subluxations, IVD protrusions, spondylitis, cord tumors, vertebral fractures, and meningitis.
In singular lesions, the perception of touch in dorsal root lesions is retained to a large degree because of the segmental overlap to adjacent segments; eg, the segmental distribution of T5 overlaps half of T4 and T6, T6 overlaps half of T5 and T7, etc. As most of the skin is innervated by an overlapping nerve supply from adjacent nerve branches, it is difficult in testing cutaneous sensations to isolate a specific spinal nerve that may be affected. In certain parts of the body, however, there are isolated areas of cutaneous innervation by a specific nerve. These areas are known as diagnostic sensory areas.
Peripheral Lesions
Nerve course varies little with body type. Some nerves that normally pass over or under a muscle, may pass through it thus subjecting them to abnormal stress during motion. The sciatic nerve, for example, may pass over, under, of through the piriformis muscle, or it may be divided. Peripheral nerve courses sometimes do not follow the specific descriptions shown in textbooks, which exhibit average paths.
Because a peripheral nerve contains mingled sensory, motor, and autonomic fibers, it is almost impossible that a peripheral nerve injury would result in only a sensory loss. Thus, numbness, absent stretch reflexes, flaccid paralysis, atrophy, and trophic disturbances can be expected if a peripheral nerve is severed. If a peripheral nerve is affected by an irritating lesion that results in hypersensitivity, pain, hyperesthesia, tenderness, paresthesias, possible hyperreflexia, and hypertonicity can be expected. In either situation, the sensory signs will manifest in a segmental manner.
A noxious peripheral stimulus is usually perceived in a well localized area. It is commonly described as a burning or prickling type of pain that is often worse at night and unrelated to position.
Neurogenic pain manifests within the involved nerve's distribution, on the surface or deep, and it usually radiates. There is an excessive response to stimulation. It is difficult for the patient to describe its character as it is unlike any other type of pain and usually is a combination of painful sensations. It is provoked by any peripheral stimulation in the involved zone, and stimulated trigger points cause spontaneous paroxysms. The patient vigilantly guards the involved part and shows great apprehension.
Pain that is accentuated by heat suggests neuritis. In contrast, pain that is relieved by heat usually suggests something producing abnormal myotonia. Pain of intrinsic neurologic origin is generally accompanied by paresthesias and root signs. When throbbing pain is present, vascular congestion, crush syndrome, a vasomotor disturbance, or possibly Paget's disease should be the first suspicions.
Neuritis. The term neuritis technically refers to inflammation of any nerve or nerves, but it is commonly restricted to inflammatory disorders of peripheral nerve disorders. The syndrome may consist of neuralgia in the affected part, hyperesthesia, anesthesia, paresthesia, dysesthesia, muscular atrophy, possible paralysis, and diminished reflexes depending upon the cause and the scope of degenerative processes involved. During the early stages, pain and tenderness is prominent along the course of the nerve involved. Later, motor and sensory deficits occur and involved normal reflexes are lost or greatly diminished. Causes include (1) mechanical factors (eg, trauma, compression, contusion); (2) infections (eg, leprosy, malaria, measles, tetanus, tuberculosis); (3) vascular factors (eg, peripheral vascular disease); (4) toxicosis (eg, heavy metal poisoning, alcohol, carbon tetrachloride); or (5) metabolic factors (eg, diabetes, gastrointestinal malfunction, thiamin deficiency, toxemia of pregnancy).
Radiculalgia. Radiculalgia refers to the pain of spinal root disease, which arises primarily from the posterior roots. Inflammation or a grating compression of posterior nerve roots irritates nociceptive fibers and commonly produces pain that is felt along the anatomical distribution of the roots affected. The pain radiates to the periphery, is usually deep seated, and is directly related to paravertebral muscle tension enhanced by any movement that would cause stretch or contraction of the involved muscle(s). The unmyelinated fibers in the posterior roots are responsible for dull pain, while the myelinated fibers are responsible for sharp pain. In either case, the pain is aggravated by any maneuver (eg, Valsalva's) that increases intraspinal pressure. Thus, coughing, laughing, sneezing, or straining at the stool will aggravate the pain of spinal root disease. Sensory changes such as hyperesthesia and abnormal reflexes may also accompany inflammatory spinal root disease.
Causalgia. Causalgia is an agonizing burning pain, usually associated with a history of severe trauma, that is essentially a reflex vasomotor dystrophy. It consists chiefly of sympathetic phenomena involving one or more limbs. It is often followed by organic changes such as bone atrophy and mottling, resulting from persistently recurring nutrient artery spasms as well as skin and muscle dystrophy and atrophy. The vasospasm produces an excruciating, diffuse burning pain that may involve either or both the lower or upper extremities. Joint immobility with or without pain, scleroderma, and contractures may occur. Emotional disturbances are often associated. Any slight tactile, thermal, or even psychic stimulus may produce an explosive attack.
Hyperalgesia. Hyperalgesia literally means an excessive sensitivity to pain. A painful tenderness produced by external pressure frequently results from trigger points, traumatic lesions of sensitive subdermal tissue, the development of a toxic accumulation, or a deep-seated inflammatory irritation. An extremely "ticklish" person is one whose superficial reflexes (skin and tendon) are very lively, thus low pain and temperature thresholds can be anticipated.
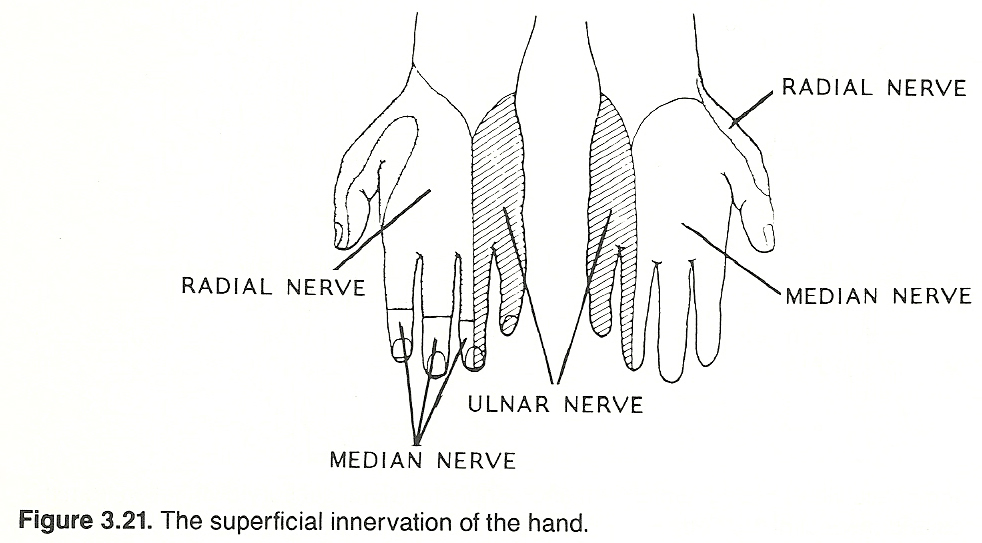
Peripheral vs Central Lesions
By evaluating the sensory bed, it is often possible to determine if a lesion is in the peripheral nerves or whether it is in the central (upper) system such as within the cord or brain.
The Components of Pain
Pain can be defined as any sensation of severe discomfort or distress because of sensory receptor provocation that is difficult to ignore. It is the result of central interpretation arising from an abnormal condition within the body or from an external stimulus that has a detrimental effect upon the body. It is a feature of physical as well as psychologic illness, and its perception may vary from mild discomfort to excruciating, intolerable agony.
In regard to pain, Chatton defines the clinician's concern: "The reaction to pain, a function of the higher centers, is extremely variable and influenced by many factors depending upon the individual patient (the affective component) and the situation (the sensory component). When pain becomes chronic, the multifactorial influences (eg, anxiety, depression, social, cultural, and economic factors; and secondary gain) play an even larger role." Thus, whenever possible, the primary cause, its pathophysiology, and the contributing factors involved must be determined.
The experience of pain contains six sequential components:
Pain gives information about a patient's biologic status; but, unlike other reported sensations, it does not indicate the nature of the stimuli. With healthy afferent pathways, pain results from tissue damage; but with hypersensitive receptors, normally nonnoxious stimuli may also evoke pain.
Basic Types of Pain
(2) a following dull, aching, more diffuse and longer lasting response.
The primary explanation for these two sensations has been that fast pain is mediated centrally by small myelinated A-delta (type III) fibers and transmitted cephally by direct lemniscal pathways, while slow pain is conducted centrally by extremely small and slower conducting C (type IV) unmyelinated fibers and transmitted cephally by multisynaptic nonlemniscal tracts. Guyton states that C fibers constitute over two-thirds of all fibers within the peripheral nervous system.
It has also been noted that the farther the initial stimulus is from the brain, the greater the temporal separation of these two painful sensations. Lewis/Pochin feel that much of this temporal separation between the perception of fast pain and slow pain is due to the fact that nerve fibers conveying pain impulses from muscles and those conveying sense of position, or of local tension, are separate. While the difference in conduction rates between type III and IV sensory fibers can explain the timing difference, it does not explain the difference in the quality of pain perceived.
It is generally taught that afferent fibers enter the spinal cord through the posterior roots and efferent fibers leave the cord through the anterior roots. In 1977, however, Coggeshall and Ito showed that some C fiber afferents enter the cord through the anterior roots. Whether these are nociceptive fibers or not and how they connect with ascending sensory tracts has not been firmly determined, but this finding has certainly added to the complexity of neuroanatomy and associated clinical concepts.
Superficial vs Deep Pain. Unlike superficial pain, deep pain is poorly localized and often associated with autonomic symptoms such as nausea, sweating, and blood pressure alterations. Reflex contractions of skeletal muscles near the origin of the noxious stimuli are often found, and such contractions will lead to myoischemia, stimulating muscle nociceptors, which will contribute to the painful syndrome. Deep somatic pain and visceral pain are frequently not perceived at the site of the lesion but referred to distant somatic tissues. Such pain is usually referred to a structure that developed from the same embryonic dermatome, myotome, or sclerotome.
Pain can also be generally classified into the two general divisions of objective pain and subjective pain. See Table 3.8. Objective pains are those arising from some foreign agent or condition that is abnormal to the area in which it is excited. Objective pain can be further divided into central objective pain and peripheral objective pain. Central pain is that for which no peripheral cause exists at the time the pain is perceived by the patient. Subjective pains are those that have no organic cause; rather, they arise primarily through a mental process.
Table 3.8. General Categories of Pain
1. Objective pain 2. Subjective (psychic) pain
a. Central objective pain a. Emotional pain
b. Peripheral objective pain b. Habitual pain
(1) Intrinsic c. Hysterical pain
(2) Extrinsic d. Occupational pain
Causalgias, phantom limb pains, and central pains are sometimes referred to collectively as central pain syndromes even though their etiologies differ. Causalgia from injury to a peripheral nerve is sometimes listed separately from central pain and from lesions within the CNS that affect pain pathways, but the fact remains that causalgia, phantom limb pain, and central pain are all related.
Peripheral Objective Pain. Peripheral objective pain may be either intrinsic or extrinsic:
THE MOTOR SYSTEM
All body movements (somatic, visceral, or glandular) result from muscle contraction. The integration of sensory and motor impulses is carried out essentially by stereotyped responses to specific sensory stimuli (reflexes) in which action potentials from sensory neurons activate action potentials of motor neurons, which, in turn, initiate a striated muscle or autonomic motor response.
During the neurologic examination, testing procedures are directed to the functioning of the entire reflex arc, all of its parts, as well as the interrelation between various structures of the nervous system. The most simple reflex is that exhibited by a deep tendon reflex (eg, knee jerk), which occurs strictly on the horizontal level of a spinal cord segment. It consists of a first-order sensory neuron, an internuncial neuron that connects the anterior and posterior horns of the spinal cord, and the final common efferent pathway.
Basic Sensory and Motor Segmental Mechanisms
Sensory impulses initiated from receptors in muscles, joints, skin, or viscera travel to the posterior root ganglia by way of unipolar neurons and then to the spinal cord. Some of these impulses are transmitted to higher control centers where they may or may not reach conscious awareness. The majority of these sensory impulses are instantly relayed via cord interneurons (flexor, extensor, excitatory, inhibitory) to motor neurons located at the same segmental level (ipsilaterally or contralaterally) as the entering posterior root to determine whether muscles will act as prime movers, accessories, antagonists, or synergists. A minority of sensory impulses synapse directly with motor neurons without assistance from interneurons; these allow the stretch reflex.
Because most afferent impulses travel a multisynaptic course, each motor neuron at the segmental level is subject to numerous contralateral and ipsilateral influences, communications from superior and inferior adjacent segments, and influences from higher centers transmitted via the descending pathways. The integrating net effect of these influences determines motor neuron potential and the resulting state of effector organ excitability.
Functional Anatomy: An Overview
The primary efferent component is the motor unit. A motor unit consists of
(2) its axon and many branches (extending from the CNS through an anterior root via a peripheral nerve),
(3) the motor end plates and neuromuscular junctions, and
(4) the particular muscle fibers innervated.
Gamma motor neurons have a lower firing threshold than do alpha motor neurons. Thus, stimuli sufficient to fire a gamma neuron may be insufficient to fire an alpha neuron and stimuli just strong enough to fire an alpha neuron may exert a pronounced effect on gamma neurons.
Each lower motor neuron is directly influenced by input from
(2) the direct activation pathway (pyramidal tract), and
(3) the indirect activation pathway (extrapyramidal tract). It is also indirectly influenced by the control circuits.
The motor pathways originating in the cerebral cortex include five basic systems: the corticospinal, corticobulbar, corticotectal, corticorubrospinal, and corticoreticulospinal tracts. In the brain stem, the descending corticospinal tracts are joined by tracts from the midbrain, pons, medulla, and vestibular apparatus. In general, the pathways that pass through the spinal cord to the anterior portion of the gray matter generally tend to facilitate extensor activity and inhibit flexor activity. Conversely, the pathways that synapse in the posterior portion of the gray matter tend to facilitate flexor and inhibit extensor neurons. This point is helpful during the analysis of paralysis (spastic or flaccid).
The two major components of the control circuits are the cerebellum, the basal ganglia, and their connections to components of the motor system. The cerebellum, which coordinates and refines muscle motion, and the basal ganglia, which provides background support of static motor activity and some autonomic activity, receive input from the direct and indirect activation pathways, and, in turn, the cerebellar and basal ganglia systems modulate the activities of the direct and indirect activation pathways.
Some authorities view motor control in a slightly different manner. Skeletal activity is described as the net result of the influences upon alpha and gamma motor neurons of the spinal cord and upon the motor components of the cranial nerve nuclei. Such activity is primarily controlled by several descending motor pathways which directly or indirectly are under the management of the cerebral cortex, certain brain stem nuclei, the basal ganglia, and the cerebellum. These structures are, as a whole, called the suprasegmental motor system.
The Final Common Pathway
It has been previously explained that each lower motor neuron is directly influenced by input from sensory neurons, the direct activation pathway, and the indirect activation pathway, and is indirectly influenced by the control circuits. The neurologic unit through which the motor system finally acts via the lower motor neuron is called the final common pathway.
It is well to think of a skeletal muscle as one part of a three-part nerve-muscle-skeleton unit. That is, an intact spinal level and motor nerve are necessary to stimulate muscle contraction, the muscle itself must be able to contract and to relax, and the power of the contraction must be transmitted to a skeletal attachment to produce the desired movement. When any one part of this three-part unit cannot function normally, total function suffers.
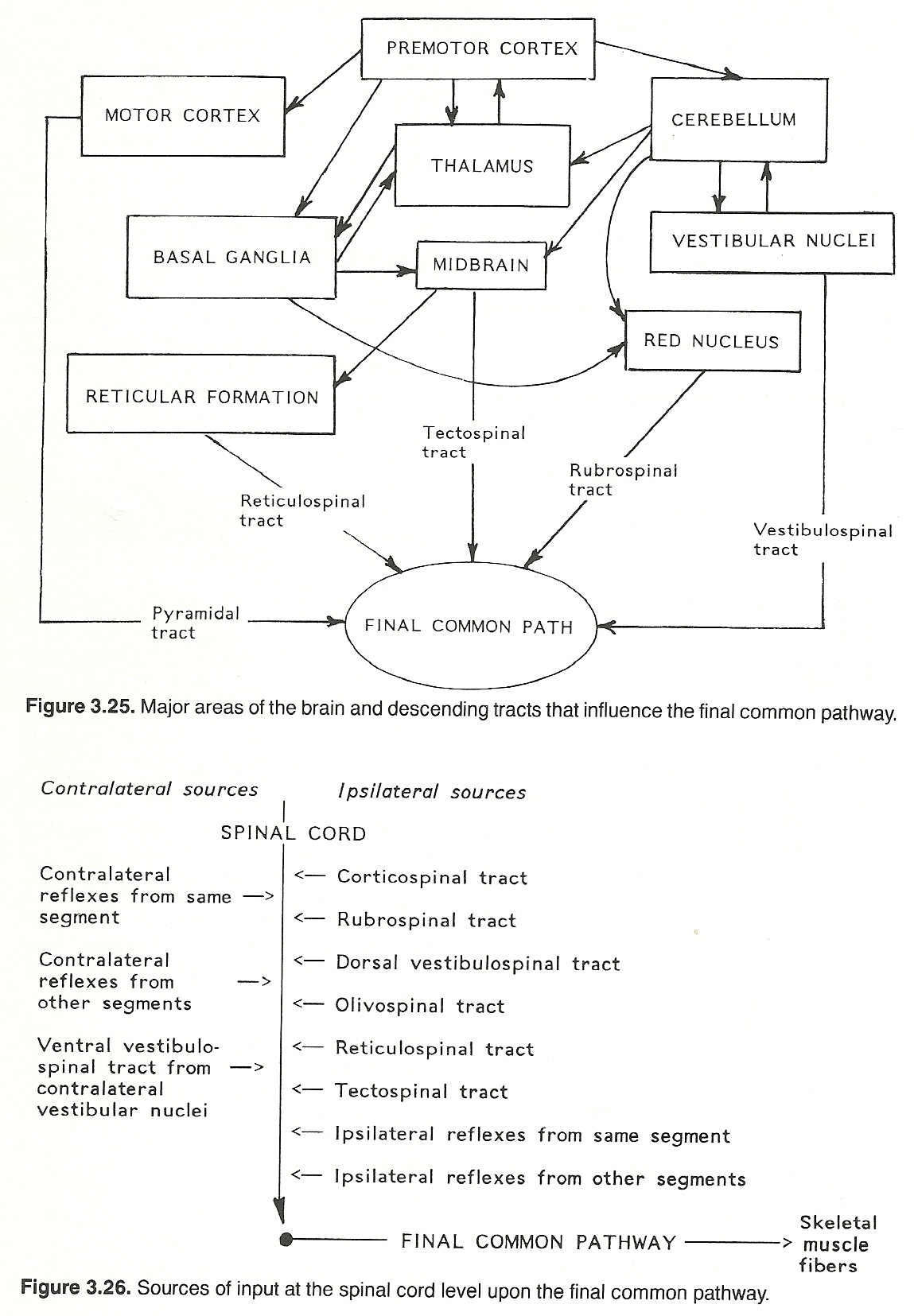
The Spinal Cord and Peripheral Motor Cells
The final common pathway consists of lower motor (alpha) neurons. The bodies of the alpha motor cells in the anterior horn of the spinal cord are comparatively large. About half the myelinated peripheral nerves extending from the anterior root consist of large alpha motor neurons that enervate muscle fibers (extrafusal muscle fibers) and about half consist of smaller diameter gamma motor neurons that extend to muscle spindles (intrafusal muscle fibers). Alpha fibers bifurcate several times to richly supply numerous muscle fibers. The smaller gamma fibers that supply muscle spindles provide indirect central control via a neural loop.
The relatively large motor cells arranged in columns within the anterior gray horn of the spinal cord and brain stem are influenced by:
Basic muscle tissue consists of (1) active contractive elements within the muscle fibers and (2) inactive spring-like yielding connective tissue that allows muscle tension to be transmitted smoothly and for elongated fibers to recover. The quantity of noncontractile connective tissue decreases and the length of muscle fibers increases as muscles are located more proximally in a limb. Much of the connective tissue element of muscle tissue is quite elastic. During work, there is no load on or shortening of the active contractile fibers until the load first stretches the inactive elastic connective-tissue components.
Muscle Fiber Blood Supply
Muscle tissue is rich in blood vessels. Arteries and veins course through the epimysium, arterioles and venules travel along the perimysia, and capillaries run longitudinally between muscle fibers in the endomysia. There are many collateral links and tiny reservoirs between many of these vessels to form a rich pool-like network that supplies oxygen and other necessary nutrients during prolonged effort where severe contraction may restrict capillary flow. During rest, many of these pools are closed, but they open when contraction makes it necessary. In this manner, the blood supply to a muscle is normally in accord with the degree of physical activity.
Typical Motor Reflexes
The body's ability to respond to internal and external environments depends on the functional integrity of all parts of simple and complex reflex arcs. If any part of the arc is disturbed, the function of the body with regard to responsiveness to a particular stimulus is disturbed and can be evaluated.
Although motor reflexes will be described in the next and several subsequent chapters, brief mention of their function will be made here so that an overview of their action and association with the longitudinal neurologic systems can be recognized.
The Reflex Arc and Its Components
A reflex arc can be described as that mechanism whereby an internal or external originating stimulus is received, where the nerve impulse so created is sent to central interpretive centers, and where various centers within the CNS are stimulated to cause an impulse to be created to accomplish a specific purposeful function such as to contract a muscle. Thus, the basic parts of the reflex arc are
(2) the interpretation center or internuncial limb of the arc, and
(3) the activator mechanism or efferent (motor) limb of the arc.
Stretch Reflexes
Some of the proximal fibers conveying unconscious proprioception signals from muscle spindles pass directly through the posterior gray matter to the anterior gray matter. These fibers constitute the afferent limb of stretch reflexes such as are induced clinically by testing tendon reflexes with a percussion hammer. The normal result is contraction. This reaction is the effect of a typical local segmental reflex.
Lengthening Reflexes
The Golgi organs offer the most important sensory feedback from tendons. As contrasted with stretch receptors, Golgi organ impulses reflexly inhibit contraction of the muscle of origin and its synergists. For example, if a spastic muscle is stretched to the threshold of the Golgi tendon organs, the muscle will suddenly give way (clasp-knife reflex). This reflex restraint, a protective mechanism, is called autogenic inhibition and has an important therapeutic application in relaxing functionally spastic (splinting) muscles.
Nocifensive Reflexes
A skin receptor that is stimulated by injury (nociceptor) or the anticipation of traumatic stimuli will induce a protective reflex response. For example, a limb will automatically withdraw from a stimulus that is irritating or perceived to be irritating. This usually unilateral and usually contraction response will also involve an opposite reaction in the ipsilateral antagonist and contralateral limb's flexors and extensors.
Reciprocal Innervation
The term reciprocal innervation is used to describe the cord level reflex activity that coordinates functionally opposite groups of muscles such as agonist-antagonist coordination of the hip and lower extremity muscles during gait. This mechanism helps to make possible self-regulation of rhythmical movements. Reciprocal innervation is thought to be due to division within the spinal cord of connector neurons for each afferent nerve so that one branch excites extensor motor neurons and the other branch inhibits flexor motor neurons.
The Direct Activation Pathway
The direct activation pathway derives its name from the fact that fibers from the cerebral cortex (especially the motor areas of the frontal lobe) course to the anterior horns of the spinal cord without synapsing (ie, primarily corticospinal). The major function of this pathway is involved in the planning (based on past experience) and directing of skilled voluntary motions. In right-handed people, for example, a large part of the facial area of the lower left hemisphere provides motor centers for speech. The same is true of the lower right hemisphere in left-handed individuals.
Course
Because corticospinal fibers mainly arise from the precentral cortex (only a few arise from the postcentral cortex and several other lobes) and pass through the pyramid of the medulla during their course, they are often referred to as the pyramidal tract. About 80% of corticospinal fibers decussate in the pyramidal decussation of the brain stem and descend contralaterally in the lateral column via the lateral corticospinal tract. From the lateral corticospinal tract, action potentials from the cerebrum (essentially phasic) synapse directly with lower motor neurons or connect via interneurons. The influence is on all cells of the anterior horn; ie, alpha motor neurons especially but also gamma neurons.
The 20% of uncrossed fibers descend in the much smaller anterior column via the anterior corticospinal tract. Whether or not some of these decussate in their descent is undetermined.
Corticobulbar fibers are also considered part of this pathway because they are grouped with corticospinal fibers in the cerebrum; however, they do not pass through the pyramids. Corticobulbar fibers separate from corticospinal fibers at several levels in the brain stem, with most decussating, to connect with the motor nuclei of eight of the twelve cranial nerves.
Because 80% of pyramidal fibers decussate, lesions will primarily exhibit themselves on the contralateral side relative to the hemispheric origin of the neurons involved. Thus, understanding this course is important during the diagnosis of many common motor disturbances.
Clinical Significance
Disturbances of the corticospinal tract may be either stimulative (irritative) such as in epileptic seizures or depressive (inhibiting) such as in flaccid paralysis, depending upon the type of lesion present and how it is affecting the transmission of action potentials.
A general rule holds that muscle groups which commonly act in unison in an agonist-antagonist manner (eg, the paraspinal muscles) are usually innervated bilaterally; ie, they receive input from both hemispheres. In contrast, delicate muscles that habitually act in a learned, isolated manner (eg, lower facial muscles and the muscles of the fingers) are primarily activated by impulses originating in the contralateral hemisphere. For this reason, a unilateral cerebral lesion (eg, hemorrhage, tumor) involving motor areas typically manifests as slurred speech and severe contralateral body weakness that spares to a considerable extent the paraspinal muscles and the muscles of the upper face.
The extent and location of the somatic peripheral weakness depend upon the scope and site of the lesion. For example:
The Indirect Activation Pathway
The indirect activation pathway is more complicated than the direct pathway. This extrapyramidal system consists of neurons originating in the cerebral hemispheres that extend to
(2) the reticular formation and the reticulospinal tract,
(3) the vestibular nuclei and the vestibulospinal tract, and (4) the tectospinal tract. The major function of this pathway is involved in the maintenance of muscle tone and gross posture, thus providing a framework on which action potentials for skilled volitional skills expressed through the direct activation pathway may manifest.
The red nucleus receives input from the cerebral cortex, basal ganglia, and cerebellum. The comparatively scarce descending rubrospinal fibers are closely intertwined with those of the corticospinal tract in the spinal cord and their terminations are essentially at the same areas. The effect of rubrospinal impulses is to activate flexor muscles and inhibit extensor muscles, thus providing muscle coordination. Because few rubrospinal fibers extend past midthoracic levels, their influence is mainly on the muscles of the upper extremity.
Postural Control: Neuronal Reciprocity
The motor neurons controlling posture are directed supraspinally from neurons originating in the pons and medulla. Their basic function is to activate extensor activity and inhibit flexor activity, and these pontobulbar functions are modulated by impulses from forebrain structures activating flexor and inhibiting extensor activity. If the counterbalancing impulse conduction from the forebrain structures is suppressed, pontobulbar function is left unchecked and extensor spasticity (or at least hyperexcitability) results. Just which specific extensors will be affected depends upon the level of the lesion.
Influences from the Reticular Formation
The reticular formation is a collection of small nuclei scattered between the large nuclei and tracts of the medulla, pontine tegmentum, and mesencephalic tegmentum. Its purpose is to control the overall degree of CNS activity.
The ascending reticular activating system has its origin in the lower brain stem, extends upward through the mesencephalon and thalamus, and is distributed throughout the cerebral cortex via two paths. One pathway extends through the brain stem portion of the reticular formation and the other smaller pathway extends through subthalamic, hypothalamic, and adjacent structures.
The reticular activating system has two general functions:
(2) it mediates ascending sensory activity and consciousness (eg, attention, wakefulness, and sleep states). This second general function will be described later in this chapter. The emphasis here concerns the postural motor effects:
Influences from the Vestibular Nuclei
The vestibular nuclei of the brain stem receive input from the vestibular apparatus and cerebellum, and, in turn, project efferents to
(2) other cells within the brain stem via ascending and descending fibers (medial longitudinal fasciculus) to modulate eye and neck muscle activity, and
(3) the spinal cord via descending fibers (lateral vestibular fasciculus) to modulate spinal motor neurons (essentially the activation of motor neurons supplying flexor muscles).
Few facts about this tract are known. It arises from the superior colliculus, decussates in the brain stem, descends near the medial longitudinal fasciculus, and then travels inferiorly via the anterior funiculus to terminate in the anteromedial region of the cervical cord. Fibers are not found below the cervical level. It is generally believed that this tract plays a role in mediating reflex movements of the neck in response to visual and auditory stimuli.
Clinical Significance
Severe brain stem lesions produce effects that are not conducive to sustaining life. Thus, from a clinical standpoint, the common diagnostic concern involves isolated lesions. When vital functions are not impaired, specific muscle weakness and poor reflex reactions will often point to a lesion of the indirect activation pathway. For example:
Table 3.9. Lesions of the Indirect Activation Pathway
Level Effects
Above midbrain and red nucleus Increased flexor tone in arms
and increased extensor tone
in legs (decorticate posture)
because of loss of descending
pathway inhibition.
Midbrain below red nucleus Weak arm flexors, spastic
and hypertonic arm extensors
(decerebrate posture). A bilateral
lesion will produce extensor spasm
in all extremities.
Below the medulla Generalized flaccidity because of
loss of descending input.
Major points in differentiating an indirect activation pathway lesion fom a direct activation pathway lesion are shown in Table 3.10.
Table 3.10. Signs of Activation Pathway Lesions
Indirect Activation Direct Activation
Pathway Lesions Pathway Lesions
Clasp-knife response Absent abdominal reflexes
to passive stretch
Clonus Babinski plantar reflex positive
Crude exaggerated motions Distal weakness
Decerebrate or Grasp reflex positive
decorticate posture
Hypertonic arm flexors Tendon hyporeflexia
and leg extensors
Tendon hyperreflexia Muscle hypotonia
Loss of fine, skilled,
voluntary motions

The Control Circuits
For motor activity to be smooth and well coordinated, the integration of a large amount of sensory input is necessary. This input is derived from skin receptors, muscle receptors, joint receptors, the coordination of the activity in the direct and indirect activation pathways just described, and the highly important control circuits of the basal ganglia and cerebellum. To sustain smooth and efficient skeletal muscle activity, these circuits have some influence on the descending tracts but their major function is to integrate and coordinate motor action potentials at the cerebral cortex level.
Input from the cerebellum integrates, coordinates, and directs especially carefully synchronized agonist-antagonist motor activity. Input from the basal ganglia integrates and coordinates motor activity concerned with posture and background static muscle contractions. In addition, circuits exist for intercommunication between the cerebellum and basal ganglia.
Basal Ganglia Influences
The cerebrum exerts its control over lower motor neuron activity either directly through the corticospinal and corticobulbar (pyramidal) tracts or indirectly through the polysynaptic extrapyramidal pathways such as the corticoreticulospinal and corticorubrospinal tracts. The many circuits and feedback loops within and between the structures related to the basal ganglia of the hemispheric subsubstance and rostral part of the brain stem interact with the cerebral cortex and cerebellum to affect indirectly the activity of the lower motor neurons reflex centers via thalamic circuits and midbrain nuclei.
The basal ganglia control circuits provide a braking system, thus:
Cerebellar Influences
The cerebellum is the director of somatic synergy throughout the body. It coordinates the activity of muscle groups spatially and times contractions so that motion will be smooth and accurate. In spite of the fact that the cerebellum receives an abundance of afferent fibers, its efferent fibers do not give rise to consciousness of sensation in the cerebellum, nor are these sensations perceived elsewhere in the brain. Cerebellar input is derived primarily from the cerebral cortex, the vestibular apparatus, the vestibular nuclei of the brain stem, muscle spindle stretch receptors, Golgi tendon organs, and joint and periarticular proprioceptive afferents.
Cerebellar afferents transmit impulses from the vestibular system, the cerebral cortex (pontine nuclei), the basal ganglia (rubroreticulo-olivocerebellar tract), and/or the spinal cord (posterior spinocerebellar tract). Cerebellar efferents transmit impulses from the flocculonodular lobe to vestibular nuclei, anterior lobe and vermis to the red nucleus and reticular formation, and posterior lobe to the red nucleus, thalamus, and cerebral cortex.
In contrast to the cerebrum, the cerebellar influence on movement is ipsilateral. Voluntary movement and its power, intellectual faculties, and sensory perception can be achieved without cerebellar assistance, but the motions will be awkward and disorganized such as are typified in the inaccuracy and intention tremor of dyssynergia, cerebellar ataxia and hypotonia, dysmetria, and certain speech impairments (ataxic dysarthria) where deficits in rapidity, pitch, rhythm, and volume are heard.
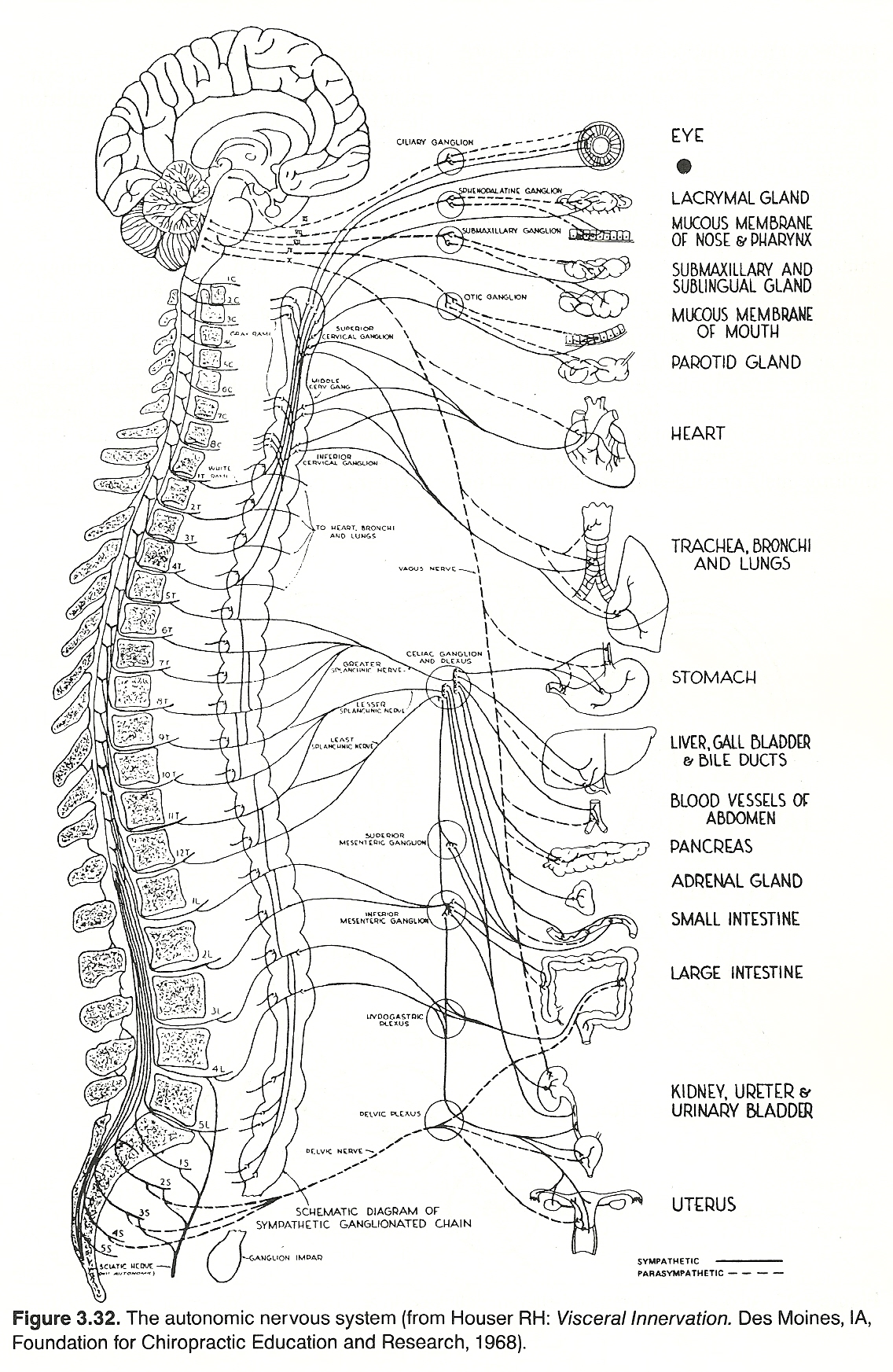
Clinical Considerations
The most common features associated with diseases of the motor system are muscle weakness, spasm, stiffness, paralysis, atrophy and wasting, incoordination, and smooth muscle dysfunction. Less common efferent nerve lesions take the form of tremors, tics, gait disturbances, loss of interest or drive or energy, major or minor convulsions, dysuria, and difficulties in talking, swallowing, or writing.
In evaluating the motor system, the equipment usually required includes a percussion hammer, tongue blades, sterile pads, penlight, ophthalmoscope, laryngoscope, skin pencil, flexible tape, wristwatch, drinking water, and a solid food. Common accessory equipment includes an electric muscle stimulator and/or an electromyograph.
Any lesion producing an interruption of connections between the motor cortex or subcortical levels and the motor axons of the anterior horn of the spinal cord is considered to be an upper motor neuron lesion. Such a lesion may interrupt direct or indirect activation pathway fibers, or both, because the pyramidal and extrapyramidal systems are so closely associated that lesions involving one will invariably affect both. On the other hand, any lesion damaging motor neurons whose axons reach skeletal muscle fibers produces a lower motor neuron lesion, whether the destruction is in the anterior horn, ventral roots, peripheral nerves, or neuromuscular junction.
Diagnostic Reflexes
While a diagnosis of a neurologic disorder cannot be determined by reflexes alone, they are important aids in establishing the type and location of a lesion. Diagnostic reflexes are divided into deep reflexes (eg, tendon, periosteal), superficial reflexes (skin, pharyngeal), visceral reflexes (eg, pupillary, carotid sinus, bladder), and pathologic reflexes (eg, Babinski's). Deep reflexes vary in direct proportion to muscle tone. Superficial reflexes involve any reflex phenomenon that may be induced by a light tactile stimulus (eg, stroking the skin with a wisp of cotton, resulting in horripilation [gooseflesh] or muscle contraction).
It is important to realize that:
(2) In lesions of a lower motor neuron or the motor fibers from the anterior horn cells of the cord, both the deep and the superficial reflexes are decreased or absent.
Upper Motor Neuron Lesions: Summary Review
Upper motor neurons convey impulses from the motor area of the cerebrum via fibers that pass through the internal capsule, the brain stem, and the spinal cord to synapse in the anterior gray horn cells of the spinal cord. At the synapse, connections are made with lower motor neurons.
Lesions of the upper motor neurons may occur anywhere along their course and may be the result of hemorrhage, thrombosis, inflammation, birth injuries, neoplasms, trauma, or a degenerative process. From a clinical standpoint, any lesion producing an interruption of connections between the motor cortex, especially the cortical area 4 of Broadman, or subcortical levels and the motor axons found in the anterior horn of the spinal cord is considered to be an upper motor neuron lesion. Such a lesion may interrupt corticospinal fibers or extrapyramidal fibers, or both, since the pyramidal and extrapyramidal systems are so closely associated that lesions involving one will invariably encompass both.
On the other hand, any lesion damaging motor neurons whose axons reach skeletal muscle fibers produces a lower motor neuron lesion, whether the destruction is in the anterior horn cells of the spinal cord, ventral nerve roots, or the spinal or peripheral nerves. See Table 3.11.
Table 3.11. Differentiation of Upper and Lower Motor Neuron Lesions
Consideration Upper Motor Neuron Lesion Lower Motor Neuron Lesion
Site Cerebral cortex or Anterior horn or peripheral
pyramidal tract motor neuron.
Distribution Diffuse or patchy Segmental (number)
Reflexes
Superficial Absent Absent
Deep Exaggerated Absent or hypotonic
Atrophy Disuse, not prominent Rapid extension, trophic
Trophic lesions Minimal Intense and extensive
Pathologic signs Present Absent
and reflexes
Fasciculations Absent Present
Paralysis
Type Spastic and rigid Flaccid
Location Contralateral or Paresis limited to
ipsilateral specific muscles.
hemiparesis,
depending on
lesion site.
Lower Motor Neuron Lesions: Summary Review
The lower motor neuron has its cell body in the anterior gray column of the cord and its axon passes via the peripheral nerves to the motor end plates of the muscles associated. This course is known as the final common pathway because the corticospinal, rubrospinal, olivospinal, vestibulospinal, reticulospinal, and tectospinal tracts as well as the intersegmental and intrasegmental reflex neurons travel this route as a final pathway through which efferent impulses reach the muscles.
Lesions of lower motor neurons result from infection, toxins, trauma, fractures, vascular disorders, tumors, congenital malformations, and degenerative processes. Lesions involving spinal nerves or peripheral nerves induce both motor and sensory losses and often reflex changes. Major neurologic signs for cervical and lumbosacral radiculopathies are shown in Table 3.12.
Table 3.12. Neurologic Signs in the Cervical and Lumbosacral Radiculopathies
Nerve Root Major Sensory Disorder Major Motor Disorder Reflex Changes
Affected (Hypalgesia) (Weakness) (Reflexes )
C5C6 Lateral arm Biceps, supraspinatus, Biceps
infraspinatus, deltoid
C6C7 Middle finger Triceps Triceps
C7T1 Lateral forearm and thumb Brachioradialis Brachioradial
C8 Little finger Wrist and finger flexors None
T1 Medial forearm Intrinsic hand muscles Finger flexors
L2L4 Medial calf Quadriceps, iliopsoas, Knee jerk
sometimes thigh
adductors
L5 Lateral calf, dorsomedial Toe/ankle extensors Usually none, foot and great toe possibly
ankle jerk
L5S2 Posterior calf; sometimes Possible weakness of Ankle jerk
lateral aspect of the foot toe extensors
Control of Locomotion Patterns
Large motor units are required for powerful gross movements where the muscle fasciculi are large and coarse. Fine precise movements require smaller motor units where the fasciculi are small in proportion to adjacent connective tissue. Different motor units respond differently to identical stimulus intensities and manifest different frequency peaks and discharge rhythms.
In repetitive activities such as during gait and exercising, muscle flexor and extensor groups must be fired and depressed at the proper time to afford smooth reciprocal actions, body part coordination, and adjustments to speed and external forces and conditions. During moderate gait, conscious control is only primary when gait is started, stopped, or during fatigue. This requires various sustaining excitatory and inhibitory spinal cord mechanisms acting on ipsilateral and contralateral muscle groups.
A pair of genetically determined pattern generators in the cervical spinal cord for the upper extremities and a pair in the lumbosacral cord for the lower extremities are believed to control action by exciting or inhibiting alpha and gamma motor neurons in proper sequence. The activity levels of the patterns are thought to be set by the midbrain. The generators receive specific and nonspecific input from the periphery, especially specific prompters from hip and shoulder capsules, and they are also modified by the descending tracts and spinal reflexes to adapt to load and balance changes.
The Development of Motor Skills
Motor skill development is a complex process of adjustment where constant attention must be concentrated on the component spatial movements, the power and extent of movements, and the correlation of visual and proprioceptive input. At this early stage, performance is under the control of the motor area of the cerebral cortex. With practice, the proper sequence of movements is "learned" and control is transferred to the premotor cerebral area where constant attention is no longer necessary. Concurrently, visual and proprioceptive data have been so correlated that the action can be carried out blindfolded and guided solely by proprioceptive input.
Clinical Reflexes
The human body exhibits an astonishingly complex array of neural circuitry. While the study of reflex communication between tissues under "voluntary" control and tissues under "autonomic" control (and their excitatory and inhibitory effect on one another) is still in its infancy, the answers to why so many visceral disorders mimic musculoskeletal disorders and why so many musculoskeletal disorders mimic visceral disorders appear to be on the horizon.
Hypotheses are also being presented that help to explain the progressive reaction spread of some disorders that fail to respond to conventional therapies. These basic types of clinical reflexes have numerous diagnostic and therapeutic significance that will become obvious in subsequent chapters.
These reflexes can be classified into four broad categories; ie, those communicating from:
Evaluation of the Motor System
Although the basic points involved in the clinical evaluation of the motor system have been described in this chapter, this subject will be described in greater depth in Chapter 9.
THE RETICULAR ACTIVATING SYSTEM
Functional Anatomy: An Overview
It has been previously described that the ascending reticular activating system extends from the lower brain stem upward through the mesencephalon and thalamus and is distributed throughout the cerebral cortex via one pathway that passes through the brain stem portion of the reticular formation and another smaller pathway that passes through the thalamic area.
The mesencephalic portion of the RAS is basically responsible for maintaining the degree of wakefulness of consciousness. The thalamic portion has two basic functions: (1) it transmits general facilitatory signals from the mesencephalon to all parts of the cerebral cortex to activate consciousness. (2) it transmits specific facilitatory signals to selected areas of the cerebral cortex. This latter role is thought to be involved in the direction of attention to specific mental activities (eg, imagination, conceptual development).
The reticular activating system merges into that part of the brain called the limbic system. The main structure of the limbic system is the U-shaped hippocampus, which processes messages from short- and long-term memory so vital for learning. Lateral to the hippocampus is the amygdala and above is the hypothalamus, which together generate sex drives, thirst, hunger, heightened emotions, and many altered states of consciousness. The hypothalamus is responsible for both the sympathetic "fight-or-flight" response and the parasympathetic relaxation response.
Arousal and Sleep States
Our ability to think, to concentrate, to perceive, and to respond to a stimulus with anything beyond a simple reflex is due to the cerebral cortex complex. But the cortex cannot function adequately unless it is aroused or awake, and the cortex cannot awaken itself from the sleep state. The RAS, at the superior level of the brain stem, is extremely important in arousing the awareness level of the cerebral cortex. Without it, consciousness is impossible. Without it, we cannot be aware.
During the sleep state, the RAS is depressed but almost any sensory input (proprioceptive, nociceptive, visual, auditory, visceral) can reactivate the system. Guyton states that pain and somatic proprioception impulses appear to be the most potent arousal stimuli. Motor activity also has the effect of arousing and maintaining wakefulness.
In addition to arousing the cortex from a sleep state, the RAS monitors and regulates all muscular activity and sensory perceptions, switches on the cortex during sleep to produce vivid dreams, and inhibits the whole spinal cord to prevent sleepwalking. Stimulating some areas of the RAS with an electric current causes all muscular efforts and sensory perceptions to become exaggerated, as if the impulses themselves were stronger. Stimulating other areas of the RAS causes muscles and sensory perceptions to become weaker.
Attention
During normal wakefulness, a normal individual has the ability to focus attention on certain mental activities or aspects of the external environment to the exclusion of others. How this occurs from an anatomical/physiological standpoint is not known although the faculty has been explored from diverse applied viewpoints in neurology, psychology, religion, and metaphysics.
Recent studies, however, have revealed three clues. First, the degree of overall attentiveness seems to be controlled by the pons and mesencephalon, probably in the same manner that wakefulness is controlled. Second, mild stimuli in the thalamic portion of the RAS activate only a small area in the cerebral cortex. Third, the pathways from all sensory areas of the brain extend centrifugally towards the lower centers so that the intensity of input at the cortex can be either facilitated or inhibited at the cortical level. For example, the auditory, visual, and somesthetic areas of the cortex can either enhance or dampen signals from the cochlea, retina, and musculoskeletal tissues.
Clinical Observations
The cortex and reticular system operate in a feedback mode in an attempt to maintain an optimum level of stimulation. Impulses reaching the cerebral cortex are fed back to the RAS. If the activity level becomes too high, the RAS sends inhibitory signals to the cortex to reduce the level of excitation, or vice versa if need be. It can thus be postulated that many spastic/anxiety disorders or subtonic states can be attributed to a failure of the inhibitory or excitatory function of the RAS to maintain control within optimum levels.
When faced with stress (functional, physical, or emotional), it appears that the dominant hemisphere delegates, via the RAS, automatic chores to the less dominant and less competent side for temporary control. When the stress level is reduced, control is "switched back" to the finely organized dominant side. For some reason, this "switch back" may fail, and the result may be abnormal neuromuscular effects. Frequently reversing requested directions, mirror writing, and other "switched" reactions are said to be associated overt signs.
According to the empiric studies of applied kinesiologists, unilateral neuromuscular disorders (eg, torticollis or low back pain) are frequently related to contralateral cerebral hemisphere dominance. Examination will typically reveal specific muscle hypotonicity with contralateral hypertonicity.
In such disorders, Walther and Stoner (in reference to Goodheart's studies in applied kinesiology) note that if the head of a supine patient is turned contralaterally toward the side of hypotonicity and the patient goes through the active motions of cross-crawling 1012 times, a reduction in the tone of the spastic muscles will result. This effect can apparently be reversed by having the patient move the arm and leg in unison on the side of hypertonicity, with the head again turned towards the weak muscle.
THE VISCERAL SYSTEM
The regulation of heart and pulmonary rate, body temperature, perspiration, endocrine activity, and digestive and elimination processes are just a few examples of the complex functions that must be coordinated and regulated. The vast majority of these functions is conducted subconsciously. Conscious awareness of visceral function is minimal except in disease states, and, even then, conscious control is normally meager.
Almost all organs are innervated through the generally antagonistic sympathetic and parasympathetic divisions of the autonomic nervous system. The sympathetics are widespread in their distribution. Through their innervation of blood vessels, sympathetic fibers reach every tissue of the body. They control blood vessel constriction, subdermal structures, heart muscle, sphincter system of the gut and urinary apparatus, parts of the bladder and genitalia and reproductive organs, inhibit many structures in the head and chest, and reach the enteral system's muscles and glands.
The sympathetics are concerned with the expenditure of energy, protection of the organism, and influencing environmental relationships. Thus, the sympathetics are referred to as the catabolic system.
The parasympathetics activate the intrinsic eye muscles, glands of the peripheral head, bronchi muscles and glands, the entire enteral system, body of the bladder, and inhibit the heart and provide vasodilation in many structures (especially of the head and penis). The major functions of the parasympathetics are the digestion of food, storing of energy, and maintaining of function holistically. Thus, the parasympathetics are referred to as the anabolic system.
Disturbed activity balance of these two divisions invariably results in some degree of visceral dysfunction. In health, the divisions are tonically stimulated to maintain a reciprocal physiologic balance, even though most organs appear dominantly controlled by one system or the other. For various reasons, this harmony may be shifted in favor of one or the other division and create visceral dysfunction and maladjustments.
Functional Anatomy: An Overview
There are both intrinsic and extrinsic factors responsible for physiologic function. The three major intrinsic systems are the electrolytes, the endocrine glands, and the visceral organs together forming the activating, correlating, and integrating systems that preside over vegetative structures.
The vegetative effector system comprises the muscle of the heart, the smooth muscle system, and all glandular structures of the body. All life-essential functions such as respiration, circulation, digestion, reproduction, and all other parts essential to maintain a person as an individual and to perpetuate the species are parts of the vegetative system. Embryologically, the somatic structures appear late in development as compared to the vegetative nervous system, which serves as the chief integrating and correlating system of all visceral and glandular structures.
General Organization
The vegetative nervous system is a peripheral system whose ganglia contain cells from which fibers arise and are located without the CNS. These ganglia are arranged either
Postganglionic Fibers. Postganglionic sympathetic neurons originate in either a ganglion of the paravertebral sympathetic chain or in one of the prevertebral ganglia. From either source, the fibers then extend to their destination (ie, visceral tissue or other ganglia). Many postganglionic sympathetic fibers pass back from the paravertebral chain at all levels via the gray rami and travel along with spinal nerves. Guyton states that about 8% of a typical spinal nerve consists of these type C sympathetic fibers.
Postganglionic fibers outnumber preganglionic fibers by a ratio of 32:1. Because of this design, a single preganglionic axon serves to discharge a large number of ganglionic cells. It is in this manner that a few CNS connections can transmit impulses to a large number of effectors.
As mentioned above, most postganglionic parasympathetic neurons are extremely short because their cell bodies are located in or near the tissues innervated. Thus, their length is quite different from that of the majority of postganglionic sympathetic fibers, which have their cell bodies in the paravertebral or prevertebral ganglia.
Activation and Inhibition Mechanisms
Stimuli that act upon muscle or secreting cells exhibit either an activating or inhibiting effect. As noted in the study of skeletal muscle, a nerve activating a flexor is associated with a stimulus that also inhibits the opposing extensor, and vice versa. This action and inhibition produced by opposing musculature is the result of innervation by different nerves of the voluntary system. However, in the vegetative system, the divisions themselves either are
(2) nerves of the same system produce opposing action by different chemical substances through which they transmit their effects to motor cells; ie, cholinergic or adrenergic functions.
The sympathetics arising from the thoracic and lumbar cord are reciprocal opponents of the parasympathetics arising from the midbrain, medulla, and sacral cord segments when they supply the same viscera, but this is true only when the transmitting substance (which results from stimulating the nerves) is that which is usual to that system. A few structures are supplied by one system only, but most are supplied by both systems. For example, a situation that causes one system to activate an organ simultaneously produces an inhibition of the opposing system.
In addition to the specific effects of sympathetic and parasympathetic stimulation shown in Table 3.13, there are also various systemic effects. Effects thought to be under the sole control of the sympathetic division are shown in Table 3.14.
Table 3.13. Effects of Organic Sympathetic and Parasympathetic Activation
Sympathetic Division Parasympathetic Division
Structure Supply Effect of Stimulation Supply Effect of Stimulation
Thyroid T1 Increases secretion X Decreases secretion
Mucous membranes T12 Vasoconstriction VII Vasodilation
of head
Salivary glands T12 Increases organic IX Increases watery
substances
Pupils T12 Dilation III Constriction
Lacrimal glands T13 Vasoconstriction VII Secretion
Heart T15 Increases rate and X Decreases rate and
force of contraction, force of contraction,
dilates coronary constricts coronary
arteries arteries
Upper limbs T16 Vasoconstriction,
sweating, piloerection
Bronchi and lungs T17 Dilation, X Constriction,
vasoconstriction vasodilation
Sphincter of Oddi T48 Constricts X Relaxes
Gallbladder T48 Relaxes muscle, X Constricts muscle,
constricts sphincter relaxes sphincter
Stomach T59 Decreases secretion X Increases secretion
and motility and motility
Spleen T68 Contracts smooth muscle X Relaxes smooth muscle
Pancreas T69 Decreases secretion X Increases secretion
Liver T810 Increases glycogen X Opposite
to glucose, increases
protein metabolism,
vasoconstriction
Pyloric sphincter T9 Increased tone, X Relaxation
contraction
Adrenals T910 Increases secretion X ? (unknown)
Small intestine T9L1 Slightly decreases X Increases peristalsis
peristalsis and and secretions,
secretions, relaxes sphincters
vasoconstriction
Kidneys T10-L1 Vasoconstriction X ? (unknown)
Prostate T10-L1 Contracts muscle and S24 Increases secretion
spermatic vein
Fallopian tubes T10-L1 Contracts muscle ? (unknown)
Urinary bladder T12-L2 Constricts sphincter, S24 Relaxes sphincter,
relaxes wall constricts wall
Lower limbs T12L2 Vasoconstriction,
sweating, piloerection
Uterus L1 Contracts body S24 Relaxes body,
contracts cervix relaxes cervix
Ileocecal valve L1 Contracts S24 Relaxes
Penis, clitoris L12 Duct contraction, S24 Erection
ejaculation
Colon and rectum L13 Decreased peristalsis S35 Increased peristalsis
Anal sphincter L3 Contract S35 Relaxes
Table 3.14. Effects of Systemic Autonomic Stimulation
Effect of Effect of
Sympathetic Parasympahetic
Structure/ Process Stimulation Stimulation
Adrenals Secretion increased Unknown
Apocrine glands Thick, odoriferous secretion Unknown
Basal metabolism Increased (>100%) Unknown
Blood coagulation Increased Unknown
Blood glucose Increased Unknown
Blood vessels Vasoconstriction Unknown
Kidney function Decreased output Unknown
Mental activity Increased Unknown
Piloerector muscles Excited Unknown
Skeletal muscle Increased glycogenolysis Unknown
& strength
Sweat glands Hyperhidrosis Unknown
(2) the transmitter substances that are liberated at the site of action. For the most part, parasympathetic effects in the visceral tissues are cholinergic and the sympathetic effects are adrenergic. But this is not exclusively true because there are some cholinergic sympathetic fibers and adrenergic parasympathetic fibers.
Viscerosensory Pathways
In the vegetative system, the afferent sensory component is much larger than the efferent motor component. For example, the vagus nerve is one of the largest nerves of the body, second only to the sciatic nerve, and consists of 80% viscerosensory fibers. In spite of this large number of sensory fibers, the vagus does not transmit pain impulses.
Viscerosensory Receptors
(2) rapidly adapting mechanoreceptors that react to pressure and motion stimuli; and
(3) chemoreceptors that respond to chemical changes (eg, gastric pH, intestinal osmotic pressure, plasma oxygen level, etc).
The specialized receptors for taste and smell closely resemble visceral chemoreceptors. Mechanoreceptors are also found in the somatic system, but chemoreceptors are not (according to current belief).
Impulses from visceroreceptors are conveyed via plexuses to autonomic ganglia located in or near their associated organ, and from there they may either synapse to initiate a local peripheral reflex or be transmitted to the CNS. Visceral afferents from thoracic, abdominal, and pelvic organs enter the cord via the pelvic, hypogastric, and splanchnic nerves. The response is usually a diffuse reaction (including pain). Visceral afferents in the cranial nerves go directly to the brain stem, but the response is far more discrete and localized.
Central Visceral Receptors. In addition to the peripheral visceral receptors described above, there are also mechanoreceptors and chemoreceptors in the CNS that respond to circulating hormones, toxins, and changes in blood pressure, pH, oxygen and carbon dioxide levels.
Distal Viscerosensory Fibers
Peripheral visceroafferents are small, slow conducting fibers, some of which are unmyelinated. Axons involved in a local peripheral reflex (ie, synapse with a visceroefferent within the organ) or that connect with a peripheral ganglion have their cell bodies in or near the organ innervated. Some authorities believe that these circuits provide a degree of control that is independent of the CNS system. Axons entering the CNS have their cell bodies in posterior root ganglia or cranial nerve nuclei.
Proximal Viscerosensory Fibers
Visceral afferents entering the spinal cord have their cell bodies in the posterior root ganglia, as do those of the somatic sensory system. Fibers from viscera either
(2) join with mesentery nerves that travel directly to a posterior root.
Fibers from the extremities either
(2) join with somatic nerves that travel directly to a posterior root.
Spinal Cord Level Entry. Viscerosensory fibers entering the spinal cord make many connections. The two major connections for horizontal activity are
(2) with visceromotor axons for simple viscerovisceral reflex activity.
Posterior Fossa Level Entry. At this level, viscerosensory impulses through various cranial nerves reach the CNS via the midbrain (III), pons (VII), and medulla (IX and X). Impulse connections are made in the brain stem that are transmitted to the hypothalamus, thalamus, and reticular formation, with a few extending to the cerebral cortex.
Supratentorial Level Input. Some fibers within the olfactory nerve transmit impulses to the rhinencephalon. These fibers terminate in the amygdala, temporal lobe, and cerebral cortex (medial and frontal lobes). A few fibers from other viscerosensory nerves communicate with the cerebral cortex and most of these arise from the hypothalamus.
Central Visceral Control
Central control of the autonomic nervous system is conducted in the reticular formation in the brain stem and in the hypothalamus and cerebral cortex at the supratentorial level. Impulses from the rhinocephalic cortex participate in bladder and bowel control.
Because we know that the vegetative nervous system is abundant in afferents and only a few reach the conscious level, it can be deduced that much afferent activity is involved in reflex activity mediated in the spinal cord and brain stem. The spinal cord's cervical segments and the lower lumbar and upper sacral segments are not connected with the vegetative ganglia by efferent connective fibers; rather, they are left to enervate the arms and legs, as far as their nervous outflow is concerned.
Cortical Control
Healthy individuals are rarely aware of visceral sensations except for those of sweating and a distended stomach, bladder, or rectum. During illness, however, visceral-related pain or nausea may be severe. Unfortunately or fortunately, depending upon the circumstances involved, the conscious awareness of visceral dysfunction is minimal.
Human Will, at least for normal consciousness, has limited power to direct visceral effects as one would direct a skeletal muscle because vegetative functions must be conducted whether one is awake or asleep. In certain acts, however, voluntary and vegetative nerves supplement one another such as in swallowing, breathing, defecation, and urination. Thus, the voluntary and vegetative nervous systems are intimately connected and brought into reflex connection so that visceral stimulation has somatic expression and skeletal muscle messages are expressed in visceral tissues. The body is a whole in neural integration.
Although the number of viscerosensory fibers in the body far exceeds that of somatosensory fibers, the number of visceral sensations that can be interpreted consciously is quite limited and these are frequently misinterpreted. For example, the perception of a burning sensation arising from visceral tension, compression, or ischemia resembles the feeling perceived when superficial thermal receptors are stimulated.
To further complicate clinical discrimination, a sensation arising from one site may be perceived by the patient to be located in an entirely different area. Nowhere else within the study of clinical neurology is the differentiation of referred symptoms more difficult or more frustrating.
Role of the Limbic System and Hypothalamus
Autonomic and somatic afferent and efferent activity is integrated and coordinated at the supratentorial level by the limbic system. The limbic system consists of an array of cortical and subcortical structures (especially the hipocampus, amygdala, and gyrus formation), which are located at or near the edge (limbus) of the medial wall of the cerebral hemispheres. Connections are made with the septal area, hypothalamus, and medial zone of mesencephalic tegmentum. By the latters connection fibers, the limbic system exerts an important influence upon the autonomic nervous system especially those that affect mood and motivation and direct other affective and emotional states.
The hypothalamus, the center of homeostatic activity, forms the floor and a part of the anterolateral walls of the third ventricle. It contains the optic chiasm, the stalk of the pituitary, the mammillary bodies, and an abundance of hypothalamic nuclei. Fiber connections are numerous and complex, with connections with the cerebrum, brain stem, spinal cord, and intrahypothalamic fibers.
The hypothalamus mediates the major reflex activity for the vegetative system (via fast, specific, short duration stimuli over neural pathways to the brain stem and spinal cord) and endocrine system (via slower, more generalized, longer lasting stimuli over hormonal pathways).
A lesion in the rostral (supraoptic) region or caudal portion results in sympathetic dominance. Other effects of a hypothalamic lesion are diabetes insipidus, appetite abnormalities, Frohlich's syndrome, neurogenic fever, and antisocial behavior.
Reticular Formation Control Centers
The reticular formation is a group of nonspecific neural centers that surround the central core of the brain stem from the medulla to the pons. It receives viscerosensory impulses from the hypothalamus and the periphery.
At the medulla level, centers controlling vomiting, respiration, and cardiovascular activity are located. It also has specialized receptors at the medulla level that are sensitive to various alterations in the blood (eg, hormones, metabolic debris, pH, carbon dioxide levels) and blood pressure (pressor and depressor centers). There is also a respiratory center located at the pons level.
Visceromotor Pathways
Unlike the somatic motor system, in which the spinal pathway from the cord to a skeletal muscle consists of a single fiber, there is no final common pathway in the autonomic system. This is because of the dual paths in the sympathetic system, consisting of two fibers (preganglionic and postganglionic). In addition, some activities are controlled entirely in the periphery.
Tracts: Summary Review
Sympathetic impulses from the hypothalamus and reticular formation are conveyed through the spinal cord via the lateral funiculus to thoracic and upper lumbar neurons that mediate the visceral activities of the thorax, abdomen, and pelvis. Impulses are then transmitted over short preganglionic fibers, which primarily terminate in the nearby paravertebral ganglia and act upon long postgangionic axons that extend to visceral receptors.
Parasympathetic impulses are transmitted to the nuclei and axons of several cranial nerves (III, VII, IX, and X) and down the lateral funiculus of the spinal cord to connect with sacral autonomic efferents. Impulses are then conveyed over long preganglionic axons, which ultimately synapse in peripheral ganglia with the shorter postganglionic fibers that enervate the visceral effectors.
Visceral Ganglia and Plexuses
A ganglion is a group of nerve cell bodies and associated fibers, lying outside the CNS, that is contained within a connective tissue capsule. Some visceral neurons are found in the paravertebral ganglia or in peripheral ganglia near the organs they enervate, and others are found in the posterior root ganglia.
A nerve plexus is a complex network of axons that extend from one area to another. There are 41 differentiated nerve plexuses associated with the peripheral autonomic nervous system. The large plexuses contain mingled direct viscerosensory axons, preganglionic parasympathetic axons, and postganglionic sympathetic axons.
Hormonal Mechanisms
The hormonal pathway is a nonneural pathway. It consists of the hypophyseal portal system and various releasing factors secreted by the hypothalamus that modulate secretions of the anterior pituitary gland such as somatostatin, the thyrotropin-releasing factor, and the luteotropin-releasing factor.
Visceral Effector Organs
Most end organs of autonomic efferents act on smooth muscle fibers of the viscera, glands, blood vessels, skin, eyes, and genitals. Although smooth muscle has the same characteristics of excitability and contractility as striated muscle, it also has three unique properties:
Types of Autonomic Neurotransmitters
An autonomic nerve that stimulates the release of epinephrine or an epinephrine-like substance is said to be functionally adrenergic (a term derived from adrenaline, the British word for epinephrine). An autonomic nerve that stimulates the release of acetylcholine is called cholinergic.
Postganglionic Autonomic Axons. All parasympathetic postganglionic axons are cholinergic. Most postganglionic sympathetic axons are adrenergic, but postganglionic sympathetic fibers to the sweat glands and a few blood vessels are cholinergic.
Autonomic Neurotransmitter Actions
Adrenergic and cholinergic neurotransmitter substances have a different effect on different areas of cell membrane according to the type of receptor present. For example, acetylcholine activates nicotinic and muscarinic receptors. These receptors are so named because these poisons, nicotine and muscarine, activate only these receptors.
Muscarinic receptors are activated by postganglionic parasympathetic neurons and postganglionic sympathetic cholinergic neurons. Nicotinic receptors are found at the synapses of both preganglionic sympathetic and parasympathetic neurons and at the terminal connections of both sympathetic and parasympathetic neurons. See Table 3.15.
Table 3.15. Transmitter-Receptor Effect in the Vegetative System
Usual Transmitter Typical Receptor Action Location Effect
Acetylcholine Muscarinic Smooth and cardiac Temporary
muscle depolarization
Acetylcholine Nicotinic Smooth muscle, Prolonged
ganglia, and depolarization
striated muscle
Norepinephrine Alpha-adrenergic Smooth muscle Depolarization, contraction
Epinephrine Alpha-adrenergic Smooth muscle Depolarization,
contraction
Beta-adrenergic Smooth muscle Hyperpolarization,
relaxation
Either type of adrenergic receptor (alpha or beta) can be either excitatory or inhibitory, depending upon the affinity of the hormone for the receptors in a particular effector organ. Functions of adrenergic receptors are shown in Table 3.16.
Table 3.16. Functions of Adrenergic Receptors
Alpha Receptors 1 Receptors 2 Receptors
Intestinal relaxation Cardiac acceleration Bronchodilation
Intestinal sphincter Increased heart strength Calorigenesis
contraction Lipolysis Glycogenesis
Pilomotor contraction Intestinal relaxation
Pupil dilation Urinary bladder relaxation
Urinary bladder sphincter Uterine relaxation
contraction Vasodilation
Vasoconstriction
The Adrenal Medullae
Activation of the entire sympathetic system is the typical effect when a significant portion of it is stimulated. Thus, epinephrine and norepinephrine are invariably released by the adrenal medullae into systemic circulation simultaneously whenever local sympathetic stimulation occurs. In this manner, vegetative tissues are acted upon by two supporting influences: neural and hormonal. Because hormones are removed from the circulation slowly, the effects of adrenal medulla secretion last about 10 times longer than direct sympathetic stimulation.
Because of the dual action of sympathetic innervation and the adrenal medulla hormones, complete loss of local innervation will be supported by the adrenals or bilateral loss of the adrenals will be supported by local innervation. In most cases, these compensating events will completely hide deficits from the clinical picture.
Epinephrine and norepinephrine react on tissues in a similar manner, but there are some differences. In comparison to norepinephrine, epinephrine has a greater effect on heart muscle and output; has a greater effect on basal metabolic rate, liver and muscle glycogenolysis, and glucose release into the blood; but causes weaker vasoconstriction in skeletal muscle.
Adrenal medullae hormones also stimulate processes that are not directly innervated by sympathetic fibers. For example, although a small percentage of body cells receives direct sympathetic innervation, epinephrine and norepinephrine increase the metabolic rate of every cell in the body.
Clinical Considerations
For most of the history of chiropractic, most chiropractic practices were family oriented; ie, almost any type of case was accepted that was within the scope of a particular state's practice act except those that required surgical, pharmaceutical, or psychiatric attention. About 75%80% of entering complaints reported could be classified under the broad category of visceral dysfunction and minor infections (eg, colds, influenza, childhood diseases), with the remaining 20%25% classified under musculoskeletal disorders.
During the 1970s, these percentages became almost reversed parallel with the inclusion of chiropractic within Medicare, Medicaid, and the passing of insurance equality laws. While it is fortunate that this turn in events has increased our knowledge and improved our efficiency in the treatment of biomechanical disorders, it is unfortunate that recent years have seen a neglect of the role of chiropractic in treating visceral disorders.
Wiles/Diakow reported in 1982 that bronchial asthma, idiopathic dysmenorrhea, and essential hypertension are three of many visceral disorders frequently treated in a chiropractic office. Structural adjustments, nutritional therapy, and a variety of therapeutic modalities are commonly used.
Imbalance Manifestations
The contributions of Pottenger cannot be overlooked in proving that imbalance in the vegetative nerves is capable of producing a wide variety of symptoms. Clinically, a recognizable imbalance is produced in all visceral diseases that reach a sufficient magnitude to be recognized by the patient.
Autonomic imbalance implies visceral instability wherein the normal physiologic control of visceral activity throughout the body, or in some organ or system, is so unstable that a comparatively mild stimulus, when applied to the system of unstable cells, is sufficient to produce a marked effect. This effect often overcomes all antagonistic forces and produces a pathologic state. Patients having such an imbalance show reactions beyond what is natural for the stimulus applied. For instance, such factors as subluxations, emotions, fatigue, weather changes, and toxemia produce changes in such individuals that are greater than would normally be expected.
Skin Reactions
The piloerector muscles, sweat glands, and blood vessels of the skin are innervated by cholinergic sympathetic fibers arising from T1T6 for the upper limbs and T12L2 for the lower limbs. These sympathetics also supply the apocrine glands, but the fibers are adrenergic. It is on this rationale of sympathetic dominance of the skin that instruments which measure skin resistance or surface temperature are often used in chiropractic to gather data as to the efficiency of local sympathetic innervation. The function of parasympathetic fibers in the extremities has not been determined.
A point of interest made by Guyton is that the sympathetic fibers supplying most of the structures of the skin are activated primarily by hypothalamic centers that are usually considered to be parasympathetic centers. Fibers supplying the apocrine glands are controlled by CNS sympathetic centers rather than parasympathetic centers.
During general sympathetic overactivity (eg, anxiety, fear), the blood vessels constrict, the sweat glands secrete, "goose pimples" arise, and hairs sometimes erect. The result is a cool, clammy, rough skin. During sympathetic underactivity (eg, Horner's syndrome), the blood vessels dilate, the sweat glands are inhibited, and the piloerectors relax. The result is a warm, dry, smooth skin.
Circulatory Reactions
Besides the influence upon smooth muscle, much of visceral function is regulated by blood vessel size and heart output. Vessel lumen diameter is under the control of the sympathetic system, heart rate and contraction strength are the product of both sympathetic and parasympathetic influences, and both vascular size and heart efficiency receive specific and nonspecific sensory input from receptors within the blood vessels, heart, and CNS. If lesions affect these receptors, functional blood pressure abnormalities and cardiac disorders arise, which are often the result of poor adaptation to normal stress.
During undemanding activity, sympathetic stimulation to blood vessels keeps the vessels of the body constantly constricted to about 50% of their maximum. This is the basis of peripheral vasculature tone. If it were not for this fact, inhibited sympathetic control could not produce vasodilation.
Inasmuch as activated sympathetics increase heart strength and constrict blood flow within peripheral circulation, the role of sympathetic overactivity is an important factor to be considered in managing cases of vasotonic high blood pressure.
Studies conducted so far indicate that stimulation of the parasympathetics does not appear to have any effect on arterial diameter; ie, it does not induce vascular relaxation. One exception to this is facial blushing. As sympathetic stimulation induces vascular contraction and sympathetic inhibition induces vascular relaxation, the peripheral vascular system is currently considered under the essential control of the sympathetics. The same appears to be true for the sweat glands, pilomotor muscles, apocrine glands, kidneys, skeletal muscle, adrenal cortex, basal metabolism, and mental activity. However, it would be a narrow belief that the parasympathetic system has no role on these structures or processes even if that purpose has not yet been determined.
Pupil Reactions
The size of the pupils is under the control of the autonomic nervous system, and size changes are responses to both external (light) and emotional stimuli. Pupil constrictors and the degree of lens convexity are controlled by parasympathetic fibers traveling in the oculomotor nerve. Pupil dilators are regulated by sympathetic fibers originating from spinal segments T1 and T2 via the sympathetic chain. Thus, a pupil that does not respond to light during examination likely indicates either sympathetic underactivity or parasympathetic overactivity.
Duct Glands and Gut Reactions
The lacrimal, nasal, salivary, upper gastrointestinal glands (from mouth to stomach), and pancreas are under the dominance of the parasympathetics. The glands of the enteric system are controlled for the most part by local factors rather than autonomic innervation from the spinal cord.
Although the autonomics play a minimal role in intestinal glandular activity, they serve a primary role in intestinal tone and motility. Sympathetic stimulation (T9L1) decreases peristalsis. Parasympathetic stimulation (Cranial X) increases peristalsis and plays an essential role in maintaining the sustained tone of intestinal smooth muscles.
Endocrine Reactions
Sympathetic activation increases the secretion of the adrenal medulla, pancreatic islets, pineal gland, and thyroid. The parasympathetics have a reverse or unknown effect.
Micturition
The urinary bladder is innervated by sympathetic, parasympathetic, and some somatic fibers (external sphincter), and they all arise from the lower segments of the spinal cord. Sympathetic afferents are believed to originate in detrusor muscles of the body of the bladder, and efferents are provided by the T12L2 segments of the spinal cord. Parasympathetic afferents pass via the pudendal nerve to the S2S4 segments of the cord, and efferents extend from these segments to the bladder via the hypogastric plexus.
The voluntary starting and stopping of micturition arise primarily from impulses in the paracentral lobule of the cerebral cortex. Sympathetic stimulation relaxes the muscular wall and constricts the sphincter; parasympathetic stimulation has the opposite effect. Dysfunction in micturition may be the result of inadequate sensory input, poor motor output, a lesion involving reflex interneurons in the spinal cord, or a lesion disrupting longitudinal ascending or descending impulses.
Defecation
The rectum, as the urinary bladder, is innervated by sympathetic, parasympathetic, and somatic fibers (abdomen, external anal sphincter). Sympathetic innervation is provided from L1L3 segments, and the parasympathetic supply is from the S3S5 segments of the spinal cord. Sympathetic stimulation contracts the internal anal sphincter and decreases peristalsis. Parasympathetic activation has the reverse effect. Voluntary assistance by the abdominal muscles is provided by somatic motor nerves T6T12.
Sexual Function
Sexual function is a product of both sympathetic and parasympathetic influences. In the female, sympathetic stimulation contracts the fallopian tubes (T10L1), contracts the body of the uterus (L1), inhibits vaginal secretion, and relaxes the clitoris. Generally, parasympathetic stimulation (S2S4) has the opposite effect.
In the male, sympathetic stimulation (T10L1) contracts the prostate muscle, spermatic vein, and genital ducts, and initiates ejaculation. Parasympathetic stimulation (S2S4), produces vasodilation for penile erection, relaxes the genital ducts, and increases prostate secretion.
Factors Involved in Vegetative Imbalance
Vegetative functional activity and control are complex affairs conducted by the cells, the electrolytes, chemicals of the various hormones, and the sympathetic and parasympathetic nerves. The action of these systems is interdependent and can be understood only when considered together because imbalance in one may mean imbalance in all. Imbalance may have as its chief cause a disturbance in any one or more of these factors.
The cells may be at fault, the electrolytes that have an important part to play in cellular activity may be abnormal in their proportions, or the chemical or nervous regulators may create too little or too much activity. Usually, the cells will do their job if the environment is right. This puts the onus on nerve supply, electrolytes, lymph circulation, and nutrition. Regardless, deviations from the norm result in abnormal or subnormal function.
Normal vegetative function can be maintained if proper nutrition is supplied the cells and their colloidal phases are usual for the structure under consideration provided (1) the necessary electrolytes are supplied in the right proportions, (2) the hormones from the various endocrine glands and vitamins are supplied in normal amounts, and (3) the activating and inhibiting impulses that are carried to the cells by nerves balance each other or at least fail to overbalance each other. If these factors are not provided, deviations from the normal will lead to vegetative imbalance.
The Character of Visceral Pain. The character of visceral pain reaching consciousness that is associated with a visceral dysfunction is sometimes helpful during diagnosis. Visceral pain entering directly with peripheral nerves is sharp and quite localized, while pain entering directly from pelvic nerves is perceived as various degrees of fullness. Visceral pain impulse entering through the sympathetic trunk is usually perceived as being diffuse, dull, and aching, regardless of cause (tension, compression, or ischemia).
Associated Factors in Visceral-Related Pain. In the context of pain-related visceral dysfunction, Pottenger pointed out that hyperalgesia of soft tissues is not uncommon in the areas that have been the seat of reflex sensory pain. For example, subcutaneous soreness within the shoulder and upper arm muscles is often associated with inflammatory diseases of the lungs. He also reported that cutaneous hyperalgesia is a common finding in visceral disease. Hyperalgesic skin frequently overlies an area of pleurisy, a tubercular cavity, a peptic ulcer, or an inflamed ovary.
Zones of hyperalgesia (often associated with cutaneous vasoconstriction and hypermyotonia) are more commonly associated with acute and subacute visceral disease rather than chronic disorders. The afferent fibers occupying the pre- and post-ganglionic pathways of the autonomic system from soma and viscera have a segmental arrangement when pain is a factor.
Head's Law. During the differentiation of visceral pain with hyperalgesia, it is well to keep in mind that visceral distress is manifested in two ways:
(2) a truly painful sensation on the overlying surface of the body.
When a painful stimulus is applied to a part of low sensibility (eg, a viscus) in close central connection with a part of much greater sensibility (eg, the skin), the pain produced is felt in the part of higher sensibility to a far greater extent than is perceived in the part of lower sensibility to which the stimulus was applied.
Sympathicotonia and Vagotonia: Basic Considerations
The parasympathetic system tends to express itself in a local, specific manner (eg, anabolic activity); while the sympathetic system expresses in a diffuse, less specific manner (eg, preparatory "flight or fight" responses).
Any deviation from normal vegetative functional activity is called a vegetative imbalance. The two common deviations are called sympathicotonia and parasympathicotonia (vagotonia). Vagotonia is a condition indicating excessive action in cranial X expressing itself in the tissues of the body. The opposite is sympathicotonia. These disorders may be general or limited to certain systems or organs.
Although vegetative disorders are expressed in terms of sympathetic and parasympathetic action, this does not mean that the nervous system is primarily at fault: remembering that the fault may lie with the cells, electrolytes, hormones, vitamins, or the autonomic nerves. Regardless of cause, there are large numbers of people who have a marked tendency to respond to stimuli that act on one or the other part of the vegetative system to such a degree that it amounts to disease.
Pottenger stated that sympathicotonics react more markedly than normal to cold, toxins, and emotions in an epinephrine-like manner. They readily produce reflexes wherein the sympathetics are involved. Contrariwise, parasympathicotonics are particularly susceptible to allergic excitants and respond in an atropine-like manner.
These syndromes may be produced by stimuli that act either centrally or peripherally. Toxins, for example, act centrally and result in widespread sympathetic effects. Inflammation in an organ, on the other hand, may act upon the nerve endings therein and create reflex sympathetic or parasympathetic effects only in the specific organs or tissues whose nerves receive the reflex stimulation.
A patient need not be sympathicotonic or vagotonic throughout the vegetative structures. He may have a sympathicotonic heart while the rest of the structures are normal, or he may express symptoms of vagotonia elsewhere such as hyperchlorhydria or spastic colon.
An important thing during diagnosis is to assign these phenomena when they arise to the components of the vegetative nervous system to which they belong. Exophthalmic goiter is a good example where sympathetic imbalance can be found in one organ and parasympathetic imbalance in another.
Activation of the parasympathetic-brake side of the autonomic system is often initiated by bad news, fright, or shock. Again, within a reasonable time, the patient should recover from the effects of these stimuli and the emotions returned to normal. If these stimuli are too prolonged or pronounced, then the balancing sympathetics should be investigated to see why they are not exerting their proper opposition forces or why they are underactive.
The clinical evaluation of the autonomic nervous system and interpretation of findings are described in Chapter 10.
THE VASCULAR SYSTEM
Functional Anatomy: An Overview
Lassen and associates found that even at rest the blood flow to the brain is not uniform. They found that engaging the senses increased cortical blood flow and brought into action cerebral regions in a special pattern. The major blood supply of the brain has been described in Chapter 2.
Circulation of the Spinal Cord
The vertebral arteries also contribute a branch to unilateral posterior spinal arteries that form longitudinal plexiform channels as they progress caudad to supply the posterior columns and horns. The anterior spinal artery appears to be the main source of blood supply to the posterior arteries below the upper thoracic level.
Radicular Support. The arterial system of the cord does not have extensive collateral circulation. It relies heavily on extraspinal radicular artery sources. Any interruption of these (eg, IVF encroachment) can produce serious neurologic damage. The superior portion of the anterior spinal artery is assisted by radicular arteries that enter through the IVFs of the midcervical, lower cervical, and upper thoracic levels. A large radicular artery (great spinal) enters between the T9 and 12 levels and is believed to be responsible for the blood supply of the cord below this level. A severe drop in blood pressure for 35 minutes can so compromise radicular circulation that necrosis of the thoracic neurons occurs.
Venous Supply. The abundant veins of the spinal cord drain into the intervertebral veins that communicate with other plexuses. The immediate part of the system involves the
(2) the external plexuses, which drain the anterior and lateral aspects of the vertebral body and its associated tissues. If the prostate is cancerous, pelvic blood returning to the heart via the vertebral plexuses rather than the inferior vena cava may initiate spinal metastasis.
Circulation of the Spine
In the cervical area, the vertebral artery runs in the foramen transversarium of the transverse processes of C2C6 and offers branches to each segment. Similar arteries arise from the intercostal and lumbar arteries branching from the aorta.
The lateral sacral arteries give branches to the sacral segments. In each instance, the segmental artery offers several anterior twigs to the front and sides of the vertebral body and posterior divisions to the spinal muscles and IVFs. After a posterior division passes through the IVF, it enters the vertebral canal and divides into three branches:
(b) ascending and descending twigs;
(3) a posterior branch, which supplies the vertebral arch, extradural contents, and dura.
Clinical Considerations
Space-occupying lesions within the cranium commonly result from hemorrhage, an aneurysm, or a tumor. See Table 3.17.
Table 3.17. Primary Features of CNS Space-Occupying Lesions
Above the Foramen Magnum
Cranial nerve palsies, unilateral Papilledema
Headache, persistent Perceptive deafness, unilateral
Hemiplegia, slowly progressing Personality changes
History of seizures Progressive onset of symptoms
Increased intracranial pressure Visual field cuts
Below the Foramen Magnum
Bladder disturbances Paralysis, slowly progressing
Cutaneous electrical resistance Progressive onset of symptoms
changes Radicular pain
Muscle weakness and atrophy, Rectal sphincter disturbances
unilateral or bilateral Trophic changes
The dura mater in the adult does not adhere to the entire skull. Thus, it is possible for fluid to be forced between the cranium and the dura, and the increased pressure tends to dislodge attached dura from its points of skull connection. As branches of the middle meningeal artery nourish both the dura and the surrounding bone of the skull, bleeding from torn vessels forms between the skull and the dura and produces an extradural hematoma. It is controversial whether this condition is commonly associated with concussion: opinions vary from frequently to rarely.
Extradural hematoma can result when a fracture tears the middle meningeal artery. A characteristic feature is the appearance of paralysis of the arm, leg, and face on the contralateral side of the lesion. The semiconscious patient will not respond to supraorbital pressure on the affected side. When hemiplegia is suspected, corroborating signs must be sought such as increased deep reflexes, absent abdominal reflexes, and a positive Babinski response. In early cases, the arm is more affected than the leg. Other features include early but brief unconsciousness, followed by drowsiness, headache, and vomiting.
The probability of extradural hematoma should always be suspected when hematoma of the temporalis muscle, gradual onset of hemiplegia, deepening coma, Hutchinson's pupils, and the lucid interval are exhibited. The differential diagnosis should first exclude a depressed skull fracture, intracerebral hematoma, and subdural hematoma.
In addition to the general characteristics of increased intracranial pressure, the typical clinical picture exhibits an abrupt onset, hemispheric or brain stem compression signs, and rapidly evolving coma. X-ray films will likely show a possible fracture across the middle meningeal artery, and a pineal shift is probable. There is a positive brain scan, and EEG depression over the site.
Many characteristics of neurovascular injury following severe head injury are listed in Table 3.18.
Table 3.18. Neurovascular Features of Severe Head Injury
Abnormal auscultated vascular Hyperglycemia
sounds in skull
Abnormal auscultated vocal Impaired sensory interpretation
sounds in skull
Abnormal lip and/or mouth color Irritability
Abnormal movement of head or fac Lucid interval
Amnesia Mouth breathing
Anxiety Nausea
Aphasia Nuchal rigidity
Autonomic dysfunction Palpable abnormal
elevations/depressions
Battle sign Palsy or paralysis
Behavioral changes Papilledema (late usually)
Bloody or nonbloody otorrhea Postnasal bleeding
Bloody or nonbloody rhinorrhea Progressing fever
Bloody spinal fluid Pulsating exophthalmos
Consciousness disturbances Raccoon sign
"Cracked-pot" sound on Respiratory rhythm changes
skull percussion
Cranial nerve malfunction Restlessness (severe)
Cyanosis Roentgenographic findings
Decerebrate rigidity Scalp laceration (possibly)
Diplopia Shock
Disorientation Speech impairment
Dizziness/vertigo Stertorous breathing with
cheek puffing
Ecchymosis Strabismus
Eyelid edema and/or malfunction Tongue protrusion deviation
Facial swelling Unequal pupils
Headache Unilateral facial palsy
Hemianopsia Vomiting
Subdural hemorrhages are caused by rupture of bridging veins crossing the space between the dura and the arachnoid. They are characterized by headaches, drowsiness, poor concentration, mild confusion, progressively decreasing levels of consciousness, and motor deficits (eg, hemiparesis). These symptoms may be immediate or delayed for weeks or months after injury. Although larger than an extradural hemorrhage, a subdural hemorrhage is confined unilaterally because the dura is firmly fixed to the falx between the hemispheres.
Features include pronounced and progressive headache, drowsiness, stupor, epileptic attacks, temperature increase, reduced pulse and respiratory rates, increased pulse pressure, progressive contralateral paralysis, sphincter relaxation, severe shock, and coma. Besides the general characteristics of increased intracranial pressure, other major signs are: skull fracture (probably), possible pineal shift shown on x-ray films, positive brain scan, EEG depression over site, inhibited mental functions, lateralizing signs (if unilateral), and progressive obtundation. Localizing featues following intracranial trauma are shown in Table 3.19.
Chronic Subdural Hemorrhage. Chronic subdural hemorrhage presents with symptoms of anorexia, vomiting, blurred vision, drowsiness, personality changes, and gait disturbances. These not infrequent outcomes of head injury are often related to a mild initial cranial trauma, insufficient to cause loss of consciousness. The accident is often forgotten by the patient. Headache, mental changes, and drowsiness develop some months later. This is caused by the clot attracting nonprotein fluids that cause gradual enlargement of the mass; ie, the osmotic tendency of any fluid of lighter density to pass through a semipermeable membrane to join fluid of greater density.
Symptoms are often marked at times, disappear, and return later with greater severity. Albuminuria is a striking concomitant finding, and xanthochromic spinal fluid is a common finding. Motor involvements, emotional disturbances, and greatly altered deep reflexes are usually present. Less frequently, cranial nerve involvement, Jacksonian convulsions, aphasia, vomiting, slow pulse, and choked disc are exhibited. The differential diagnosis should exclude bromide intoxication, general paresis, and subdural hygroma and other space-occupying cerebral lesions.
Table 3.19. Localizing Features Following Intracranial Trauma
Feature Location
Impaired recognition of Postcentral gyrus
ordinary sensations
Irregular explosive speech Cerebellar involvement
Movement difficulties with Precentral gyrus and adjacent
spastic paralysis and/or frontal gyri
hypertonicity and rigidity
of contralateral side
Lucid interval Middle meningeal (extradural)
artery hemorrhage, usually
Nasal speech Palatal paralysis
Olfactory hallucinations Temporal lobe
Personality changes Prefrontal area of cerebrum
Recent-event amnesia, poor Temporal lobe
recognition of sounds and
their significance
Severe, intractable, poorly Thalamic lesion
localized pain
Slurred speech Upper or lower motor lesion
Vasomotor and autonomic Hypothalamic lesion
disturbances
Visual disturbances Occipital lobe
of recognition
The most dangerous effect of high blood pressure is hemorrhage. This commonly occurs into the cerebrum from a terminal branch of the middle cerebral artery. Hemorrhages into the ventricles of the brain or meninges may also be found. Intracranial hemorrhages may be small and localized or massive and diffuse. They may be suddenly fatal or cause a paralysis that corresponds to the portion of the brain damaged. Nosebleed is a common warning sign. Intracerebral hemorrhage can be associated with either extradural or subdural hematoma, or it may be the sole lesion. Lateralizing signs are usually absent. Hemorrhages less frequently occur into the spinal cord. See Table 3.20.
Table 3.20. Common Sites and Causes of Cerebral Hemorrhage
Site Cause(s)
Intracerebral Aneurysm Hypertension
hemorrhage Arteriovenous malformation Trauma
Bleeding disorders
Subarachnoid Aneurysm Bleeding disorders
hemorrhage Arteriovenous malformation Trauma
In cases of intracranial hemorrhage, Babinski's sign is most significant and the one most frequently present. It usually denotes a hematoma on the opposite side of the brain. Aphasia may be the first lateralizing sign in a left side lesion in a right-handed person. Broca's area is usually on the left side.
Infarction from either embolism or thrombosis often mimics hemorrhage. Hemorrhage gives little warning, infarction usually does. Cortical defects are rare with hemorrhage but common to infarction. Stiff neck, papilledema, and a shifted pineal gland are typical signs of hemorrhage but rare with thrombosis. However, inasmuch as both are medical emergencies, the need for differentiation in a chiropractic office is rare. When suspicions arise, immediate neurologic consultation is recommended.
Vertebrobasilar Insufficiency
The posterior vertebral arteries pass through the transverse foramina of the cervical vertebrae and the foramen magnum before forming the posterior portion of the circle of Willis. As Zeoli points out, cervical subluxation syndromes, muscular spasms, scolioses, and the size and position of the foramen magnum may adversely affect cranial blood supply. These syndromes will be described in subsequent chapters. Major features such as headache, nausea, vertigo, lip and tongue tingling, dysarthria, diplopia or blindness, ataxia, increased cranial pressure, and hemiparesis or tetraparesis may result.
Other Common Considerations
A large number of intracranial lesions produce increased intracranial pressure. The general features of pressure build-up, which are common to most all etiologies, include:
Table 3.21. Disorders That Produce Increased Intracranial Pressure
Cardiopulmonary disease Hydrocephalus Paget's disease
Cerebral edema Hypertensive encephalo- Parasitic infestation of brain
Cerebral vasculitis pathy leading to apoplexy Polyneuropathy
Cerebral venous thrombosis Intracranial abscess Poststatus epilepticus
Demyelinating diseases Leptomeningeal cyst Primary neoplasm
Diabetes mellitus Mediastinal disease Reye's syndrome
Empyema, epidural/subdural Meningeal neoplasm Sagittal sinus thrombus
Epidural hematoma Meningismus Spinal cord tumor
Fluid/electrolyte imbalances Meningitis Subarachnoid bleeding
Granulomatous diseases Metastatic neoplasm Subdural hematoma
Heavy metal poisoning Mycotic aneurysm Viral encephalitis
(2) ophthalmoplegia, possible ocular bruit, and trigeminal sensory loss (carvernous sinus); or
(3) optic atrophy and visual loss (suprasellar region).
Cerebral Angioma. This is a congenital arteriovenous defect that usually exhibits in early to middle adulthood. Major symptoms include focal epilepsy, a cranial bruit, and constant migraine-like headaches.
Cranial Arteritis (Cerebral Vasculitis). This inflammatory disorder leads to a clinical picture of peripheral symmetric or mononeuropathy multiplex. In addition to the general characteristics of intracranial pressure, the onset is sudden, and the signs are similar to those of hypertensive encephalopathy. Skull x-ray films are negative, brain scan is positive (usually), and an EEG shows focal-to-diffuse abnormalities.
Diffuse Cerebral Atherosclerosis. As a result of a series of minor strokes, progressively deepening symptoms of intellectual impairment, personality changes, dysarthria, dysphagia, bladder disturbances, shuffling gait, parkinsonism, and signs of progressive pseudobulbar palsy are typical.
Internal Carotid Disease. Brief but frequent episodes of confusion, transient ipsilateral monocular blindness, possible contralateral hemiplegia and sensory loss, dysphagia (dominant hemisphere), and Horner's syndrome are characteristic.
Migraine. The cardinal symptom is paroxysmal headache that is usually unilateral and of long duration (from several hours to days). An early prodromal aura and facial or arm paresthesia are often associated. Depending on severity, photosensitivity, hyperlacrimation, nasal congestion, unilateral ptosis and contracted pupil, anorexia, and vomiting may be associated.
Posterior Cerebral Artery Syndrome. The major feature of this disorder specifically depends on the site of the lesion. For example:
Venous Sinus Thrombosis. Whether or not the cause is trauma or an infection, this disorder has three major forms; ie, (1) lateral sinus thrombosis from mastoid disease exhibits headache, papilledema, fever, and vomiting; (2) cavernous sinus thrombosis from facial or nasal sinus disease is associated with ocular proptosis, severe eye pain, chemosis, and possible papilledema and ophthalmoplegia; and (3) superior sagittal sinus thrombosis from facial or nasal sinus disease manifests as focal neurologic and epileptic signs.
In addition to the general characteristics of any cause of increased intracranial pressure, the syndrome features include a sudden onset, focal neurologic signs, and seizures. Skull x-ray films are negative, brain scan is possibly positive, and EEG shows focal-to-diffuse abnormalities.
THE CONSCIOUSNESS SYSTEM
Central Neural Control Mechanisms
Whether a person is awake or asleep, the brain is constantly bombarded by input from all skin and internal receptors. This barrage of incoming messages is examined, valued, and translated relative to a framework composed of instincts, experiences, and mental conditioning.
In some as yet undiscovered manner, an appropriate decision is arrived at that is transmitted to all muscles necessary for the response desired. By means of varying synaptic facilitation and restraints within the appropriate circuits, an almost limitless variety of neural integration and signal transmission is possible.
In terms of motor function, the cerebral cortex can be discussed as three distinct regions that control specific muscle groups associated with specific joints. The primary motor region of the cerebral cortex is primarily concerned with delicate voluntary movements, particularly of the facial and distal flexor muscles of the extremities. Its left aspect controls the right side of the body, and its right aspect controls the left side.
The supplementary motor area is concerned primarily with bilateral synergistic movements. The premotor area, the cortical extrapyramidal center, is involved in the development of motor skills, and its fibers are linked to the cerebellum via thalamic nuclei.
Memory
The vast majority of activity of the nervous system is initiated by stimuli acting on one or more types of receptor end organs that are transmitted into the CNS via spinal and cranial nerves. This sensory experience may or may not initiate a motor response (eg, muscular or glandular activity).
Because of the vast number of sensory receptors of the body and because of the multitude of stimuli to which it is exposed to each second, Guyton states that more than 99% of all sensory information entering the CNS is discarded by the brain as being irrelevant and unimportant. Much of this selective filtering process is conducted at the synapses.
Whether a motor response to sensory impulses is initiated or not, the memory of the experience may be stored (from seconds to years) within the nervous system (primarily by facilitation at the supratentorial level) and be used to modify similar experiences that might occur in the future. Without such sensory input and its memory, the conscious being would have no communication with or knowledge of its environment, have no capacity to learn and adapt, or be unable to draw attention to or away from specific impressions.
Just as there are certain tensions, mechanical vibrations (sounds, pressure waves), electromagnetic wavelengths, smells, and tastes that are above and below conscious perception, it can be postulated that many events occur within the external and internal environment to which the brain at either conscious or unconscious levels is unaware because there are no receptors that are sensitive to the type or level of stimuli produced.
Cortical Hemispheric Dominance
It can be said that the brain has three levels corresponding to its evolutionary ancestry:
(2) the limbic system from the mammalian level plus the cerebellum added to the brain stem, and
(3) the higher cortex. The later levels are further divided into right and left hemispheres, and the whole brain has certain preferred communication paths. The brain stem is linked through the limbic levels to the cortex, and there are massive communication links between the two halves of the cortex.
Primary consciousness resides in the dominant hemisphere, and this side is responsible for such operations as logical thought, time-sequential analysis, categorizing, and speech. The nondominant side is responsible for such abilities as recognizing familiar people, places, and things; understanding maps and other abstractions; appreciating art and music; and perceiving holistic concepts.
Cerebral Asymmetry
While accepting the fact of hemispheric dominance, most people think of the hemispheres of the brain as being symmetrical. They are not. Asymmetries in the occipital lobes and lateral ventricles are correlated with hand preference. Asymmetries in the auditory region and in the Sylvian fissure are present even in the fetus, and it is possible that these differences relate to right-left differences in function and to language lateralization.
The studies of Galaburda and associates have led them to believe that anatomical asymmetries help to explain the range of human talents, the recovery from acquired disorders of language function, certain childhood learning disabilities, and some dementing illnesses of middle life.
Brain Waves
The brain constantly emits varying waves of electrical activity. These patterns can be recorded from the outer surface of the head or from the surface of the brain during surgery. The recording process is called electroencephalography(EEG).
The undulating patterns of brain activity vary in intensity and frequency during various levels of excitation during consciousness and sleep, and certain brain disorders offer characteristic patterns (eg, epilepsy, hypertensive encephalopathy, cerebral embolism or thrombosis, brain tumors or abscesses, etc).
The higher the degree of cerebral activity, the higher the frequency of the wave pattern. For example, during general anesthesia the frequency may be 1 ips, while during a seizure it may be 100 ips or more.
For teaching purposes, certain distinct brain wave patterns have been classified into four transient levels of consciousness: alpha, beta, theta, and delta.
Beta Waves
This is the normal level of consciousness in which a person conducts everyday affairs. Typical beta waves have a fast frequency of 1430 Hz and their greatest intensity is found over the frontal and parietal lobes.
Beta waves are divided into two types: beta I and beta II. Beta I waves disappear during mental activity, thus they are inhibited by cortical activity. Beta II waves appear during typical mental activity (eg, tension), thus are excited by cortical activity. During normal daily activities, the patterns constantly shift from beta I to beta II waves as attention to sensory stimuli rises and falls.
Alpha Waves
Alpha waves have a typical frequency of 813 Hz. They usually have their greatest intensity in the occipital area, but sometimes they are located over the parietal or frontal lobes. These waves are found in healthy conscious individuals during deep relaxation with the eyes closed but are quickly replaced with low-voltage patterns when the eyes are opened. They are not found during sleep or during conscious sensory impression from the environment (eg, visual, auditory, etc). They are prominent just before entering sleep and before full awakening occurs after sleep.
The alpha level has a typical voltage of 50 v, but this voltage drops immediately when mental activity is aroused. Alpha waves do not occur in the cortex unless thalamic connections are intact, thus it is thought that they originate from a generalized activity of the thalamocortical system.
The alpha level of consciousness has also been associated with yoga and other forms of prolonged meditation, in which thought processes are stilled and the autonomic nervous system is quieted. It is thought by many that this level, when prolonged, affords the optimal level for creativity, inspiration, intuitive perceptions, and unusual psychic phenomena.
Theta Waves
Theta waves have a frequency of 47 Hz and usually have their greatest intensity in the parietal and temporal lobes in children. These levels are recorded in adults during periods of emotional stress (eg, frustration, disappointment) and during certain brain disorders. Theta waves are sometimes found in the parietal and temporal lobes of healthy children and drowsy adults.
Delta Waves
Delta waves, the slowest of brain waves. They appear to originate from the thalamus and occur during normal deep sleep states, stupor, and general anesthesia; ie, when the cerebral cortex is not being influenced by the reticular activity system. They are also frequently recorded from healthy infants and in severe organic brain disease.
Disorders of Consciousness
Consciousness is the ability of the mind to cognize sensory impulses that are capable of producing physical or mental sensations. It is a function of the combined activity of the cerebral cortex and the ascending reticular activating system (ARAS), thus a product of both supratentorial and posterior fossa activity. If the function of either the cortex or the ARAS becomes impaired, temporary or permanent consciousness alterations can be expected. Signs may vary from mild sleepiness to deep coma depending upon the site and scope of the lesion.
The conscious patient is awake, alert, and oriented in time and space. A semiconscious patient responds to painful stimuli but makes no spontaneous movements. An unconscious patient is in a condition in which there is no cerebral appreciation of environmental stimuli.
Loss of consciousness may occur gradually or suddenly and may have varying degrees of completeness such as in somnolence, stupor, coma, delirium, and delusion. A patient may also appear dazed, stunned, irritable, irrational, etc. Several disorders of consciousness are briefly described below.
Concussion. A sudden blow to the head often results in a brief loss of consciousness that is followed by a variable duration of partial amnesia. This loss of consciousness is usually attributed to mechanically altered neuronal function (from stretching or pressure) of neurons critical for consciousness, cerebral ischemia, and/or neuronal hyperpolization or depolarization.
Confusion. A mentally confused individual is often irritable, impatient, angry, and may refuse to talk or cooperate. The ability to think clearly and respond quickly is impaired. The patient is alert but disoriented and excited, presenting some coherent conversation. The disorientation to environment and resulting excitement, which are not in keeping with the total situation, may be temporary and have a psychologic basis in addition to or rather than organic injury.
Convulsions. A convulsion is a series of transient involuntary muscle contractions and relaxations involving the voluntary muscles of a major portion of the body. Convulsive seizures are sudden and violent, and they may vary in intensity from a momentary lapse in consciousness (petit mal) to prolonged violent convulsions (grand mal). Generalized seizures are commonly associated with a loss of consciousness; focal seizures are usually not. Several authorities place some abnormal sensations (eg, paresthesias, hallucinations, and overt behavioral disturbances) under the category of "seizures."
Delirium. Delirium is a state of mental unrest, usually of short duration, which often follows unconsciousness. It often features incoherent speech, delusions, illusions, hallucinations, restlessness, excitement, and sensory perversions a profound agitated state of confusion.
Delusions, Illusions, and Hallucinations. These states should be differentiated. A delusion is an absurd and unfounded belief. An illusion is a false interpretation of sensory impressions received from objects that really exist. A hallucination is a sense perception without a physical basis. Any or all of these disturbances of consciousness may be found in the insanities, profound neuroses, or typhoid states.
Dizziness. Dizziness, a disturbed subjective relationship with space, is a sensation of whirling, unsteadiness, or being subjected to a tendency to fall. Sometimes a feeling of movement within the head is experienced. It may be a mild form of vertigo or a state of impending syncope, from which it should be differentiated. Dizziness is sometimes caused by simple anxious overbreathing that reduces blood carbon dioxide, which in turn inhibits the metabolism of the balancing center.
Faintness. Faintness, a mild form of syncope, is a sensation of weakness and impending loss of consciousness.
Irrationality. This state, which usually precedes delirium, is characterized by loquaciousness and belligerence. Blunt forehead trauma may be sufficient to injure the frontal lobes of the brain and cause marked behavioral changes, irritability, and seizures (rare).
Stupor. Stupor is a decided loss of consciousness into which the patient may pass and from which he can be aroused only by extraordinary means. It is a borderline state from which a patient may sink into coma or enter when recovering from coma. In stupor, few sensible answers are obtained but the patient might respond to simple forceful commands.
Syncope. Syncope is a brief faint, a transient loss of consciousness. It is usually the result of a generalized cerebral ischemia, for whatever reason. Typical causes include factors producing a reduced pulse, a reduced cardiac output, or pooling of the blood in the periphery. Syncope is usually preceded by faintness, which is characterized by a sensation of weakness, giddiness, light-headedness, blurred vision, and impending loss of consciousness. Pallor, a weak pulse, hypotension, and cool perspiration are generally associated.
Transient Ischemia Attacks (TIAs). Transient disorientation, weakness, dysarthria, and numbness result from insufficiency of the carotid artery or one of its branches. These attacks usually precede a major cerebrovascular accident.
Vertigo. Vertigo is not a synonym of dizziness (spatial disorientation), but it is closely related. Vertigo is a hallucination sensation of turning or rotating either of the self (subjective) or the surroundings (objective). The cause of vertigo is usually a disturbance in the equilibratory apparatus anywhere from the middle ear (semicircular canals, labyrinthitis) to the brain stem through cranial nerve VIII.
THE CEREBROSPINAL FLUID SYSTEM
The brain and spinal cord are suspended in cerebrospinal fluid (CSF) that fills the surrounding subarachnoid space. The four ventricles are also filled with this clear watery liquid. The system is constantly being renewed by production and reabsorption equaling a total replacement every 8 hours. Most of the fluid originates from the choroid plexuses of the ventricle walls.
In its superior aspect, there is movement of fluid from the ventricles into the subarachnoid spaces where it is absorbed by cerebral veins. In its inferior aspect, spinal fluid fills the sleeve-like tubular extension of the subarachnoid space around the spinal cord to about S2, far below the end of the spinal cord.
Although this sleeve ends as a blind pocket, the exchange of spinal fluid is enhanced by a slow mixing process induced by postural changes. Some authorities believe that this is important from a clinical viewpoint because biomechanical spinal and cranial disorders may interfere with normal spinal dynamics that can result in CSF stagnancy.
Clinical Considerations
Effects of Flow Impairment. Impairment to normal cerebrospinal fluid (CSF) circulation results in ventricular backup, leading to an increase in intracranial pressure. Severe facial or skull trauma, edema, meningitis, brain mass, or anything that will cause blockage along the passageways will produce fluid accumulation in the system resulting in a degree of hydrocephalus. In a few days or weeks, increased pressure inside the sleeve of the dura surrounding the optic nerve may cause the retinal veins to dilate and the pale pink optic nerve head to exhibit papilledema and a choked disc.
Brain tumor compressing part of the ventricular system is the most common cause of papilledema, associated commonly with headaches (dura mater stretching) and vomiting (a parasympathetic center reflex). Meningitis, trauma, or anything that will cause blockage along the passageways will produce fluid accumulation in the system resulting in some degree of hydrocephalus. The features of increased intracranial pressure have been previously described in this chapter relative to vascular system disorders.
The Dynamic Craniosacral System
Although cranial sutures are immovable in cadavers and dried skulls, they are minutely movable in the living body, although it is often taught that they are not. The uniting and intervening layers between the edges of adjacent bones in the skull offer a strong bond of union yet one that permits definite but limited movement.
It is thought by many that this movement is necessary for tissue respiration of the brain and spinal cord. It is controlled by the following structures and motions:
(2) the fluctuations of the cerebrospinal fluid,
(3) the mobility of the intracranial and intraspinal membranes,
(4) the articular mobility of the cranial bones, and
(5) the involuntary mobility of the sacrum between the ilia.
Flexion of both the sphenoid and occiput increases the dorsal convexity of the skull and results in elevation of the sphenobasilar symphysis towards the vertex. Extension does the reverse. In other words, flexion of the midline bones appears as a slight increase in convexity; extension, a slight decrease in convexity; ie, the occiput, sphenoid, ethmoid, and vomer. The paired temporals, parietals, and maxillae move in synchronized internal and external rotation with the midline bones. Fixation resulting in impaired reciprocal motion of these bones is the basic hypothesis of cranial therapy in osteopathy and chiropractic.
Associated Involuntary Extracranial Motion. In addition to normal voluntary and postural motions of the sacral between the ilia, the sacrum responds to the inherent motility of the central nervous system, fluctuation of the CSF, and pull of the intracranial and intraspinal membranes in a similar fashion as do the cranial bones. With light palpation, it is felt as a slight rocking synchronized with cranial motion.
Since all voluntary and involuntary systems are encased in fascial envelopes connected directly or indirectly to the base of the skull through the cervical, thoracic, abdominal, pelvic, and appendicular fascial connections, all organs are subjected to this rhythm in addition to the rhythms of their particular voluntary or involuntary activity throughout life.
Several authorities state that dysfunction of inherent craniospinal motion is noted in almost every case of temporomandibular joint (TMJ) pain and dysfunction, thus the wide range of effects from this disturbance from cranial nerve impairments to lower extremity disorders. Likewise, the spine, pelvis, and extremities also affect the head and TMJ via the temporals because of the dural strap connecting the sacrum to the cervical spine and occiput.
BIBLIOGRAPHY
Sensory mechanisms: Introduction.
In Field J, Magoun HW (eds): Handbook of Physiology.
Washington, DC, American Physiological Society, Vol 1, 1959
Anderson KV:
Endogenous opioid peptides and the control of pain.
In Brena SF, Chapman SL (eds): Management of Patients with Chronic Pain.
New York, Spectrum, 1983, pp 33-46
Arbuckle BE:
Reflexes.
Journal of the American Osteopathic Association, 46(7), July 12, 1946
Balduc HA:
Overview of contemporary chiropractic science for the Chiropractic Association of Oklahoma.
Northwestern College of Chiropractic, Convention notes, April 24, 1983.
Beck JW (ed):
The Management of Pain.
Amsterdam, Elsevier, 1979, pp 306-316.
Berthold CH:
Morphology of normal peripheral axons.
In Waxman SG (ed): Physiology and Pathobiology of Axons.
New York, Raven Press, 1978.
Bishop B:
Pain: Its physiology and rationale for management. Part I, neuroanatomical substrate of pain.
Physical Therapy, 60:13-23, 1980; Part II, analgesia systems of the CNS, 60:21-23, 1980.
Black RG:
The chronic pain syndrome.
Surgical Clinics of North America, 55:999-1011, 1975.
Blacker HM:
Evaluating sensory and motor systems.
Patient Care, 17(16):121-136, 1983.
Bonica JJ:
Neurophysiologic and pathologic aspects of acute and chronic pain.
Archives of Surgery, 112:750-761, 1977.
Brazier MAB:
Electrical Activity of the Nervous System, ed 4.
Baltimore, Williams & Wilkins, 1977, pp 1-12.
Brena SF, Chapman SL:
Chronic pain: Physiology, diagnosis, management.
In Leek JC, Gershwin ME, Fowler WM Jr: Principles of Physical Medicine and Rehabilitation in the Musculoskeletal Diseases.
Orlando, FL, Grune & Stratton, 1986, pp 189-216.
Bullock TH:
The neuron doctrine and electrophysiology.
Science, 129:17, 1959.
Burke DC, Murray DD:
Handbook of Spinal Cord Medicine. Toronto, Macmillan of Canada, 1975, pp 65-70.
Cailliet R:
Disuse syndrome: fibrositic and degenerative changes.
In Brena SF, Chapman SL (eds): Management of Patients with Chronic Pain.
New York, Spectrum Publications, 1983, pp 63-71.
Calbe DB, Eisler T:
The pathogenesis and medical treatment of extrapyramidal disease. Medical Clinics of North America, 63:715-727, 1979.
Cervero F:
Deep and visceral pain. In Kosterlitz HW, Terenius LY (eds):
Pain and Society. Weinheim, Verlag Chemic, 1980, pp 263-282.
Chusid JG:
Correlative Neuroanatomy & Functional Neurology, ed 19.
Los Altos, CA, Lange Medical, 1985, pp 19-37, 66-80, 162-243, 254-257, 269-278, 338-352, 448-470.
Clark WC, Hunt HF:
Pain. In Downey J, Darling R (eds):
Physiological Basis of Rehabilitation Medicine.
Philadelphia, W.B. Saunders, 1971, pp 373-401.
Coggeshall RE, Ito H:
Sensory fibers in ventral roots L7 and S1 in the cat.
Journal of Physiology, 267:215, 1977.
Conforti MF:
The mind as in a computer.
Occupational Nursing, 18(12), December 1970.
Coote JH, et al:
Reflex discharges into thoracic white rami elicited by somatic and visceral afferent excitation.
Journal of Physiology, 202:141-159, 1969.
Crue BB, et al:
Neurophysiology of pain peripheral aspects.
Bulletin of the Los Angeles Neurological Society, 41(1):13-42, 1976.
Curtis DR, Eccles JC:
Synaptic action during and after repetitive stimulation.
Journal of Physiology (British), 150:374, 1960.
Daube JR, Sandok BA:
Medical Neurosciences.
Boston, Little, Brown, 1978, pp 92-111, 114-133, 138-153, 156-191, 194-227, 230-257.
Davis NJ, Kovac MP:
The command neuron and the organization of movement.
TINS, 4(3):73-76, 1981.
DeBacher G:
Biofeedback in spasticity. In Basmajian JV (ed):
Biofeedback: Principles and Practice for Clinicians.
Baltimore, Williams & Wilkins, 1979, p 61.
Ditmar E:
Cutaneo-visceral neural pathways.
Journal of Physical Medicine (British), 15:208, 1952.
Eisen A, Hoirch M:
The electrodiagnosis evaluation of spinal root lesions.
Spine, 8(1):98-106, 1983.
Fitz-Ritson D:
The anatomy and physiology of the muscle spindle, and its role in posture and movement.
Journal of the Canadian Chiropractic Association, 26(4): 144-150, 1982.
Fowler WM Jr, Taylor RG:
Differential diagnosis of muscle disease.
In D'Ambrosia RD: Musculoskeletal Disorders: Regional Examination and Differential Diagnosis.
Philadelphia, J.B. Lippincott, 1977, pp 93-95.
Freedman BG:
The biodynamical aspect of cerebrospinal fluid in disease and health.
Journal of Manipulative and Physiological Therapeutics, 2:79-84, 1979.
Galaburda AM, LeMay M, Kemper TL, Geschwind N:
Rightleft asymmetries in the brain.
Science, 199:852-856, 1978.
Gandhavadi B, et al:
Autonomic pain: Features and methods of assessment.
Postgraduate Medicine, 7(1):85-90, 1982.
Goodman CE:
Pathophysiology of pain.
Archives of Internal Medicine, 143:527, 1983.
Guyton AC:
Medical Physiology, ed 4.
Philadelphia, W.B. Saunders, 1971, p 557.
Guyton AC:
Textbook of Medical Physiology, ed 6.
Philadelphia, W.B. Saunders, 1981, pp 560-564, 671-677, 679-682, 684-698, 710-718.
Haldeman S:
The electrodiagnostic evaluation of nerve root function.
Spine, 9(1):42-48, 1984.
Haldeman S:
Interactions between the somatic and visceral nervous systems.
ACA Journal of Chiropractic, V:57-64, August 1971.
Haldeman S:
Neurophysiological concept of importance to chiropractors.
Journal of the Canadian Chiropractic Association, 18(3):6, 1974.
Hart FD (ed):
The Treatment of Chronic Pain.
Philadelphia, F.A. Davis, 1974, pp 1, 4, 8, 15, 64, 113.
Hibbard D:
Effects of manipulation on gait muscle activity: Preliminary EMG research.
ACA Journal of Chiropractic, 20(10):49-52, 1983.
Hirschy LD:
Utilization of TENS in conjunction with chiropractic methodology in the treatment of acute and chronic pain.
Ortho-Briefs, ACA Council on Orthopedics, pp 23-29, April 1984.
Howard IP, Anstis T:
Muscular and joint-receptor components in postural persistence.
Journal of Experimental Psychology, 103(1):167-170, 1974.
Huskisson EC:
Measurement of pain.
]Journal of Rheumatology, 9(5):768-769, 1982.
Igneizi RJ, et al:
Pain and its modulation.
Neurosurgery, 6(5):577-590, 1980.
Inman VT, Saunders JB:
Referred pain from skeletal structures.
Journal of Nervous and Mental Disease, 99:660-667, 1944.
Janda V:
Muscle and joint correlations. In Lewis K, Gutmann G (ed): Rehabilitcia,
Proceedings of the IVth Congress Prague International Federation of Manual Medicine,
Supplement 10-11:154-157, 1975.
Janse J:
A concept: The neurological element as it relates to clinical chiropractic.
The Chirogram, December 1975.
Janse J:
The integrative purpose and function of the nervous system: A review of classical literature.
Journal of Manipulative and Physiological Therapeutics, 1(3), September 1978.
Janse J:
Principles and Practice of Chiropractic. Lombard, IL,
National College of Chiropractic, 1976, pp 243-250.
Jaskoviak PA, Schafer RC:
Applied Physiotherapy: Practical Applications Within Clinical Chiropractic.
Arlington, VA, American Chiropractic Association, 1986, pp 15-44.
Jewett DL, Rayner MD:
Basic Concepts of Neuronal Function.
Boston, Little, Brown, 1983.
Kandel ER:
Small systems of neurons.
Scientific American, 24(3):66, 1979.
Karvinen E, Komi PV:
Neuromuscular performance. In Larson LA (ed):
Fitness, Health, and Work Capacity. New York, Macmillan, 1974, pp 73-78, 81-86.
Keegan JJ, Garrett FD:
The segmental distribution of the cutaneous nerves in the limbs of man. Anatomical Record, 102:409-439, 1948.
Kellgren JH:
Observations on referred pain arising from muscle.
Clinical Science, 3:175, 1938.
Kellgren JH:
On the distribution of pain arising from deep somatic structures with charts of segmental pain areas.
Clinical Science, 4:35-46, 1939.
Kellgren JH, Samuel EP:
The sensitivity and innervation of the articular capsule.
Journal of Bone & Joint Surgery, 32(B):84-92, 1950.
Kessler RM, Hertling D (eds):
Management of Common Musculoskeletal Disorders.
Philadelphia, Harper & Row, 1983, pp 27-30, 91-98.
Kimber DC, Gray CE, Stackpole CE:
Textbook of Anatomy and Physiology, ed 11.
New York, Macmillan, pp 194-197, 250-260.
Korr IM:
The spinal cord as organizer of disease processes: Some preliminary perspectives.
Journal of the American Osteopathic Association, September 1976.
Krupp MA, Tierney LM Jr, Jawetz E, Roe RL, Camargo CA:
Physician's Handbook, ed 21.
Los Altos, CA, Lange Medical, 1985, pp 34-53.
Kuntz A:
Anatomic and physiologic properties of cutaneo-visceral vasomotor reflex arcs.
Journal of Neurophysiology, 8:421-429, 1943.
Lamb DW:
The neurology of spinal pain.
Physical Therapy, 59:971-973, 1979.
Laporte Y, Emonet-Denand F, Jami L:
The skeletal-fusimotor or beta-innervation of mammalian muscle spindles.
TINS, 4(4):97-99, 1981.
Lassen NA, Ingvar DH, Skinhoj E:
Brain function and blood flow.
Scientific American, 239(4):62-71, 1978.
Laufenberg PC:
New dimensions in neurologic evaluation.
The Digest of Chiropractic Economics, pp 10-12, 105-106, May/June 1979.
Leavitt F, Garron DC, Whisler WW, Sheinkop MB:
Affective and sensory dimensions of back pain.
Pain, 4:273-281, 1978.
Lewis T, Pochin EE:
Effects of asphyxia and pressure on sensory nerves of man.
Clinical Science, 3:141-155, 1938.
Lewis T:
Suggestions relating to the study of somatic pain.
British Medical Journal, 1:321-327, 1938.
Lynch MK, Kessler RM: Pain.
In Kessler RM, Hertling D (eds): Management of Common Musculoskeletal Disorders.
Philadelphia, Harper & Row, 1983, pp 51-72.
MacDonald ML, Stanish WD:
Neuromuscular system. In Scott WN, Nisonson B, Nicholas JA: Principles of Sports Medicine. Baltimore, Williams & Wilkins, 1984, pp 15-24.
McKenzie RA:
The Lumbar Spine. Upper Hutt, New Zealand, Spinal Publications Ltd, 1981, pp 9-14.
McNaught AB, Callander R:
Illustrated Physiology, ed 4.
New York, Churchill Livingstone, 1983, pp 220, 246-256, 260, 264-265, 268-270.
Melzack R, et al:
Neurophysiological foundations of pain. In Sternbach RA (ed): The Psychology of Pain.
New York, Raven Press, 1978, pp 1-26.
Melzack R, Wall PD:
On the nature of cutaneous sensory mechanisms.
Brain, 85:331-356, 1962.
Merskey H, et al:
The lateralization of pain.
Pain, 7:271-280, 1979.
Newton RA:
Joint receptor contributions to reflexive and kinesthetic responses.
Physical Therapy, 62(1):22-29, 1982.
Nouwen A, Bush C:
The relationship between paraspinal EMG and chronic low back pain.
Pain, 20(2):109-123.
Olson WH, Brumback RA, Gascon G, Christoferson LA:
Practical Neurology for the Primary Care Physician.
Springfield, IL, Charles C Thomas, 1981, pp 1-32.
Pastan RS:
Diffuse musculoskeletal pain.
Diagnosis, 5(3):42-46, 1983.
Penfield W, Rasmussen T:
The Cerebral Cortex of Man: A Clinical Study of Localization of Function.
New York, Macmillan, 1950.
Perl ER:
Mode of action of nociceptors. In Hirsch C, et al (eds):
Cervical Pain. Oxford, England, Pergamon, 1972, pp 157-163.
Phillips RB:
The irritable reflex mechanism.
ACA Journal of Chiropractic, January 1974.
Pinkenburg C:
A basis for the theory of manipulative medicine.
Journal of Manipulative and Physiological Therapeutics, 3(2):81-85, June 1980.
Pomeranz B, Wall PD, Weber WV:
Cord cells responding to fine myelinated afferents from viscera, muscle, and skin.
Journal of Physiology (British), 199:511-532, 1968
Poole PB:
Considerations of neurogenic pain.
Ortho-Briefs, Council on Chiropractic Orthopedics of the American Chiropractic Association, Fall 1982.
Pottenger FM:
Symptoms of Visceral Disease, ed 6. St. Louis, C.V. Mosby, 1944, pp 183, 187, 189, 266.
Prichard JW:
Nerve. In Albright JA, Brand RA (eds):
The Scientific Basis of Orthopaedics. New York, Appleton-Century-Crofts, 1979, pp 399-405, 408-412.
Rosse C:
Muscle action and its control. In Rosse C, Clawson DK (eds):
The Musculoskeletal System in Health & Disease. Hagerstown, PA, Harper & Row, 1980, pp 64-65, 70-75.
Rossi F:
The fine innervation of the articular capsule.
Acta Anatomy, 10:161-232, 1950.
Rozeiu AM:
Clinical decisions: A normative approach.
Journal of the Canadian Chiropractic Association, 26:102-106, 1982.
Sato A, Kaufman A, Koizumi K, Brooks C:
Afferent nerve groups and sympathetic reflex pathways.
Brain Research, 14:575-587, 1969.
Schafer RC:
Clinical Biomechanics: Musculoskeletal Actions and Reactions.
Baltimore, Williams & Wilkins, 1983, pp 137, 154-156, 221-223, 233, 240-243, 247-249, 397-401, 405-408, 427-428.
Schafer RC:
Physical Diagnosis: Procedures and Methodology in Chiropractic Practice.
Prepublication manuscript, chapters 4 (Clinical interpretation of pain) and 7 (Basic neurologic considerations).
Arlington, VA, American Chiropractic Association, 1987.
Schafer RC:
Symptomatology and Differential Diagnosis. Arlington, VA,
American Chiropractic Association, 1986, pp 8-9, 203-219.
Searles RP, Maldinich EK, Messner RP:
Isolated trigeminal sensory neuropathy: early manipulation of mixed connective tissue disease.
Neurology, 28:1286-1289, 1978.
Shepherd GM:
Microcircuits in the nervous system.
Scientific American, 238(2): 92, 1978.
Sinclair DC, Weddell G, Feindel WH:
Referred pain and associated phenomena.
Brain, 7:184-211, 1948.
Skully RL:
The nervous system and its disorders.
The Texas Chiropractor, March 1975.
Stoner F:
The Eclectic Approach in Chiropractic. Las Vegas, FLS Publishing, 1975, pp 341-342.
Sutton DC, Lueth HC:
Pain.
AMA Archives of Internal Medicine, 45:827, 1930.
Terrett ACJ, Vernon H:
Manipulation and pain tolerance: A controlled study of the effect of spinal manipulation on paraspinal cutaneous pain tolerance levels.
American Journal of Physical Medicine, 63(5):217-224.
Thomas AH:
The anatomy, physiology, and psychology of pain.
Journal of Occupational Medicine, 7:526-534, 1965.
Tran TA:
Clinical correlations of the pyramidal system.
The Chirogram, November 1976.
Tran TA:
Essentials of the neurological examination: Tests for cerebral function.
The Chirogram, July 1975.
Tran TA:
The motor system: A general look at its components and their clinical correlations.
The Chirogram, October 1976.
Travell JG, Simons DG:
Myofascial Pain and Dysfunction: The Trigger Point Manual.
Baltimore, Williams & Wilkins, 1983, p 663.
Ufberg SH:
Muscular nociception.
ACA Journal of Chiropractic, 14:93-96, September 1980.
Upledger JE:
The relationship of craniosacral examination findings in grade school children with developmental problems.
Journal of the American Osteopathic Association, 77(9):760-776, 1978.
Upledger JE, Vredevoogd JD:
Craniosacral Therapy, Seattle, Eastland Press, 1983.
Wall PD:
Gate control theory of pain mechanisms: a re-examination and re-statement.
Brain, 101:1-18, 1978.
Walther DS:
Applied Kinesiology. Pueblo, CO, Systems DC, 1981, vol 1, pp 121-129.
Wayne KM:
Disturbances of the nervous system as related to chiropractic.
Journal of the Canadian Chiropractic Association, 13(2):20-23, 1969.
Werner JK:
Trophic influence of nerves on the development and maintenance of sensory receptors.
American Journal of Physical Medicine, 53(3):127-142, 1974.
Weiss S, Davis D:
The significance of the afferent impulses from the skin in the mechanisms of visceral pain: skin infiltration as a useful therapeutic measure.
American Journal of Medical Science, 176:517-536, 1928.
Wiles MR, Diakow P:
Chiropractic and visceral disease: A brief survey.
Journal of the Canadian Chiropractic Association, 26:65-68, 1982.
Williams HG:
An anatomical-functional review of selected CNS motor control structures.
The Texas Chiropractor, April 1977.
Wood KW:
Myospasm, myosprain and myalgia.
The Digest of Chiropractic Economics, pp 126-127, May/June 1985.
Wyke B:
Articular neurology and manipulative therapy. In Aspects of Manipulative Therapy.
Proceedings of Multidisciplinary International Conference on Manipulative Therapy.
Melbourne, Lincoln Institute of Health Sciences, Carlton, Victoria, Australia, August 1979, pp 67-72.
Wyke B:
Articular neurology: A review.
Physiotherapy, 38(3):94-99, 1972.
Wyke B:
Neurological mechanisms in the experience of pain. Acupuncture and Electro-Therapeutical Research International Journal, 4:27-35, 1979.
Zeoli NJ:
Anatomical and pathological consideration of the circle of Willis.
Digest of Chiropractic Economics, 14(3):44-45, 1971.