

Changes in Muscle Spasticity in Patients with
Cerebral Palsy After Spinal Manipulation:
Case SeriesThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Chiropractic Medicine 2016 (Dec); 15 (4): 299—304 ~ FULL TEXT
OPEN ACCESS Oleh Kachmar, MD, PhD, Taras Voloshyn, MD, and
Mykhailo Hordiyevych, MD
Innovative Technologies Department,
International Clinic of Rehabilitation,
Truskavets, Ukraine.OBJECTIVE: The purpose of this case series was to report quantitative changes in wrist muscle spasticity in children with cerebral palsy after 1 spinal manipulation (SM) and a 2-week course of treatment.
METHODS: Twenty-nine patients, aged 7 to 18 years, with spastic forms of cerebral palsy and without fixed contracture of the wrist, were evaluated before initiation of treatment, after 1 SM, and at the end of a 2-week course of treatment. Along with daily SM, the program included physical therapy, massage, reflexotherapy, extremity joint mobilization, mechanotherapy, and rehabilitation computer games for 3 to 4 hours' duration. Spasticity of the wrist flexor was measured quantitatively using a Neuroflexor device, which calculates the neural component (NC) of muscle tone, representing true spasticity, and excluding nonneural components, caused by altered muscle properties: elasticity and viscosity.
RESULTS: Substantial decrease in spasticity was noted in all patient groups after SM. The average NC values decreased by 1.65 newtons (from 7.6 ± 6.2 to 5.9 ± 6.5) after 1 SM. Another slight decrease of 0.5 newtons was noted after a 2-week course of treatment. In the group of patients with minimal spasticity, the decrease in NC after the first SM was almost twofold-from 3.93 ± 2.9 to 2.01 ± 1.0. In cases of moderate spasticity, NC reduction was noted only after the 2-week course of intensive treatment.
CONCLUSIONS: In this sample of patients with cerebral palsy, a decrease in wrist muscle spasticity was noted after SM. Spasticity reduction was potentiated during the 2-week course of treatment.
KEYWORDS: Cerebral Palsy; Muscle Spasticity; Spinal Manipulation
From the FULL TEXT Article:
Introduction
The term cerebral palsy (CP) refers to a group of permanent disorders of the development of movement and posture, which cause activity limitations and are attributed to nonprogressive disturbances of a developing brain. [1] It is the most common motor disorder among children, affecting approximately 2 children per 1000 births. One in 5 children with CP (20%) has a severe intellectual deficit and is unable to walk. [2]
Muscle spasticity is a clinical syndrome of CP resulting from upper motor neuron lesions, and the reduction of these lesions is an important therapeutic target for optimizing motor performance. The treatment program for a child with spasticity may include different options: exercises, casting, constraint-induced therapy, oral medications, chemodenervation, intrathecal baclofen, selective dorsal rhizotomy, and orthopedic surgery. [3] Because of the limited efficiency of “traditional” treatments, a wide range of complementary and alternative therapies are used for muscle tone management in patients with CP, including spinal manipulation (SM). [4, 5]
Spinal manipulation could possibly be used as a separate intervention in CP treatment and as part of an integrated treatment program called the intensive neurophysiologic rehabilitation system, which includes different treatment modalities: physical and occupational therapy, extremity joint mobilization, reflexotherapy, body massage, and mechanotherapy. This treatment may be performed in intensive 2-week courses lasting 3 to 4 hours daily. [6]
Descriptive studies of this rehabilitation approach have reported improvements in gross motor functions [7] and a decrease in muscle spasticity in 94% of the cases. [8] However, these studies had methodologic limitations, and spasticity was measured using the Modified Ashworth Scale, [9] whose validity and reliability have been questioned by many authors. [10]
A more precise quantitative evaluation of spasticity is possible using the Neuroflexor device, developed by the Swedish company Aggero MedTech AB (Stockholm, Sweden) and validated by a research team from the Karolinska Institute (Solna, Sweden). [11] Recent studies have indicated that Neuroflexor is a reliable measurement tool with high test–retest and interrater reliability, [12] and its sensitivity is good enough to measure changes in spasticity during CP treatment. [13]
The purpose of this case series is to describe the quantitative changes in wrist muscle spasticity in children with CP after 1 SM and after a 2-week course of treatment.
Methods
Patient Selection
Patients were selected for this prospective case series according to the established inclusion criteria and evaluated 3 times. Initial evaluation was followed by SM in 10 to 15 minutes, and the second evaluation was carried out after 15 minutes. The third evaluation was performed at the end of the 2-week course of treatment. All procedures were performed in accordance with the ethical standards of the institutional committee on human experimentation and the Helsinki Declaration of 1975, as revised in 2000; written informed consent was obtained from all patients included in the study. Research work was approved by the Medical Ethics Commission of the International Clinic of Rehabilitation, located in Truskavets, Ukraine.
A total of 30 children admitted to the Rehabilitation Clinic took part in the study. Inclusion criteria were as follows: unilateral and bilateral forms of spastic CP, age 7 to 18 years, and Manual Ability Classification Scale levels I–IV. Exclusion criteria were as follows: ataxic or dyskinetic form of CP, fixed contractures of the wrist with less than 50° of passive wrist extension, and inability to understand and comply with instructions. The clinical diagnosis was confirmed by a child neurologist before the subjects were included in the study.

Table 1 One patient failed to participate in the final evaluation because of somatic disease and was excluded from the study; analysis was carried out in 29 children. The demographic characteristics of the group are presented in Table 1.
Patients were divided into 3 groups according to the spasticity level: minimal spasticity (“1” by the Modified Ashworth scale), 10 children; mild spasticity (“1+” by the Modified Ashworth scale), 10 children; moderate spasticity (“2” by the Modified Ashworth scale), 9 children.
Intervention
Spinal manipulation was performed by an orthopedic medical doctor certified in Manual Therapy. After manual evaluation, high-velocity low-amplitude SM was carried out in all regions of the spine, including thoracic adjustments in the prone position, lumbar manipulation in lateral recumbent position, and cervical manipulation in sitting position.
Spinal manipulation was repeated every day, with a total of 12 manipulations during the 2-week period. The program for children with CP also included daily sessions of physical therapy, massage, reflexotherapy, extremity joint mobilization, mechanotherapy, and rehabilitation computer games with average daily duration of 3 to 4 hours. A detailed description of the treatment is provided in the manual. [6] No side effects were detected by the researcher and doctor in charge or reported by the patients or their parents.
Evaluation Procedure
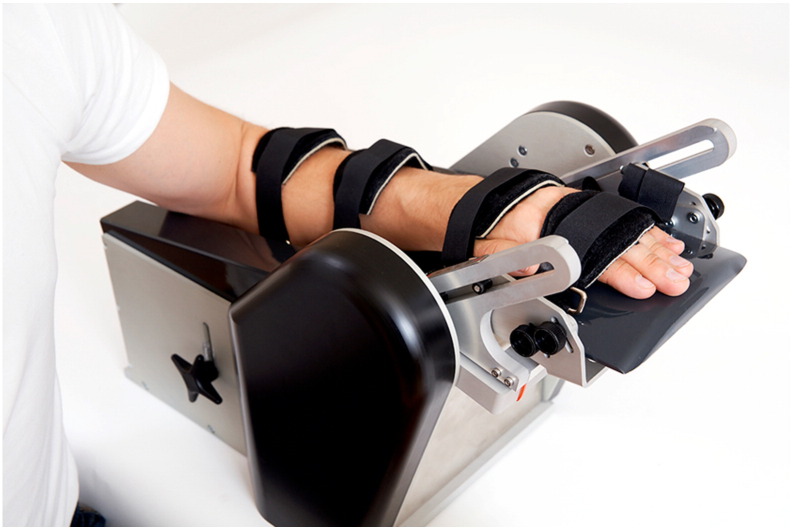
Figure 1 Muscle tone was measured using the Neuroflexor device. This instrument extends the wrist and stretches the muscles at 2 different constant velocities, while the force transducers measure resistance during movements (Figure 1).
Total movement resistance testifies to true spasticity, called the neural component (NC) of muscle tone, which is induced by the stretch reflex, and nonneural components, caused by altered muscle properties: inertia, elasticity, and viscosity. One test session consisted of 5 slow movements and 10 fast movements; dedicated software was used to separate total resistance into its elastic, viscous, and neural components. Lower NC values correspond to lower spasticity levels.
A Modified Ashworth Scale score of wrist spasticity was obtained with the child seated with the elbow flexed to 90° and the forearm pronated. [9] The children’s gross motor functions were evaluated according to the Gross Motor Function Classification System.14 Hand function was evaluated according to the Manual Ability Classification System. [15]
Results
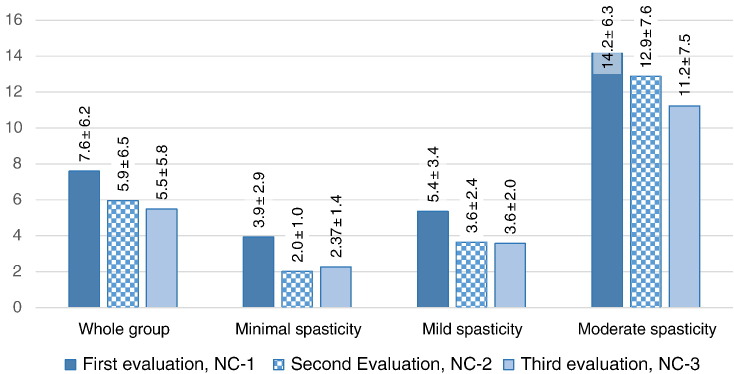
Figure 2

Table 2 Measurement results are summarized in Figure 2 and Table 2, which present NC values before intervention (NC-1), after 1 SM (NC-2), and after the 2-week course of treatment (NC-3).
Data are presented for the whole group, for patients with minimal spasticity (“1” by the Modified Ashworth scale), for patients with mild spasticity (“1+” by the Modified Ashworth scale), and for patients with moderate spasticity (“2” by the Modified Ashworth scale).
Differences between NC-1 and NC-2 indicate changes in spasticity that occurred after 1 SM; differences between NC-1 and NC-3 show changes after the 2-week course of treatment that included daily SM.
Substantial decrease in spasticity was noted both after 1 SM and after the 2-week course of treatment. The average values of spasticity decreased by 1.65 newtons (from 7.6 ± 6.2 to 5.9 ± 6.5) after 1 SM. After a 2-week course of intensive treatment with daily SM, there was another slight decrease in spasticity by 0.5 newtons.
In the group of patients with minimal spasticity, the NC decrease after the first SM was almost twofold—from 3.93 ± 2.9 to 2.01 ± 1.0. During the course of treatment, there was a small “rebound” effect, with NC values returning to 2.27 ± 1.4.
In cases of mild spasticity, changes in NC were also noted after the first SM (from 5.35 ± 3.4 to 3.64 ± 2.4) with subsequent stabilization at 3.57 ± 2.0.
In the moderate spasticity group, changes in NC after the first SM were not substantial (from 14.16 ± 3.3 to 12.88 ± 7.6), but spasticity levels continued to decrease to 11.23 ± 7.5 newtons during the course of treatment.
Discussion
Spinal manipulation is a common treatment modality for musculoskeletal problems, and in many cases, it is used for nonmusculoskeletal conditions. [16] There is growing evidence from research studies of the effectiveness of chiropractic and osteopathic manipulation for nonmusculoskeletal conditions, especially in patients with migraine and headache, [17, 18] hypertension, [19, 20] chronic obstructive pulmonary disease, [21] and different pediatric conditions, [22] including CP. [23–26]
Our study was aimed at evaluating changes in wrist muscle spasticity in children with CP after 1 SM and a 2-week intensive rehabilitation program with daily SM together with other treatment modalities: physical therapy, massage, reflexotherapy, extremity joint mobilization, mechanotherapy, and rehabilitation computer games.
In our case series, reduction in spasticity was noted after the first manipulation—the NC values of muscle tone decreased from 7.6 ± 6.2 newtons to 5.9 ± 6.5. After the 2-week course of intensive treatment with daily SM, there was another small decrease in spasticity by 0.5 newtons.
The most pronounced decrease in spasticity after 1 SM was observed in children with minimal spasticity. In cases of moderate spasticity, NC reduction after 1 SM was less pronounced but became more prominent after the 2-week course of treatment.
Because decrease in spasticity was noted after 1 SM and this effect was potentiated by a multicomponent treatment course, we can formulate the hypothesis that SM might have an impact on muscle tone regulation.
The influence of SM on muscle spasticity is not fully understood at present. However, an experimental body of evidence indicates that SM could impact primary afferent neurons from paraspinal tissues and influence muscle spindle afferents and Golgi tendon organs, [27, 28] which are directly involved in muscle tone regulation.
The literature points to the influence of SM on spinal cord neural circuits [29, 30] possibly modifying stretch reflexes. Interesting information about neural responses to SM has been included in reports of studies on animal models. [31, 32] Studies have also indicated that SM has an influence on the H-reflex, [33, 34] which is a direct electrophysiologic equivalent for spasticity measurement. This explorative study describes decrease in spasticity after SM in a group of children with CP.
Limitations
As this was a case series, there was no control group with randomized allocation or blind testing of participants or examiners, and the sample size was small. Therefore, we can only note observed phenomena and cannot calculate inferential statistics or draw conclusions on causation. The findings of this study may not necessarily be replicable for other patients with CP or spasticity. Future randomized controlled trials are required to evaluate this effect. The authors aim to conduct double-blind randomized clinical trials comparing SM and “sham” manipulation to investigate the possible influence of SM on spasticity.
Conclusions
Decrease in wrist muscle spasticity after SM in patients with CP was reported in this sample of young patients. Reduction in spasticity was further potentiated during the 2-week course of treatment.
Practical Applications
Muscle spasticity was measured quantitatively in 29 patients with CP before treatment,
after 1 SM, and after 2 weeks of treatment.This study indicates that SM may decrease spasticity of wrist muscles in patients with CP.
Further studies, including randomized control trials, are required.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): O.K., T.V., M.H.
Design (planned the methods to generate the results): O.K., T.V., M.H.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): O.K.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): T.V., M.H.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): O.K., T.V.
Literature search (performed the literature search): O.K., T.V.
Writing (responsible for writing a substantive part of the manuscript): O.K.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): O.K., T.V., M.H.
References:
Rosenbaum P, Paneth N, Leviton A.
A report: the definition and classification of cerebral palsy.
Dev Med Child Neurol. 2007;109:8–14Prevalence and characteristics of children with cerebral palsy in Europe
Dev Med Child Neurol. 2002;44(9):633–640Tilton A.
Management of spasticity in children with cerebral palsy.
Semin Pediatr Neurol. 2009;16:82–89Oppenheim WL.
Complementary and alternative methods in cerebral palsy.
Dev Med Child Neurol. 2009;51(4):122–129Liptak GS.
Complementary and alternative therapies for cerebral palsy.
Ment Retard Dev Disabil Res Rev. 2005;11(2):156–163Kozyavkin VI, Babadagly MO, Lun GP. Design studio Papuga; Lviv, Ukraine: 2012.
Intensive Neurophysiological Rehabilitation System—the Kozyavkin Method.
A Manual for Rehabilitation Specialists.Koziavkin VI, Voloshin TB, Hordievich MS, Kachmar OA.
Changes of motor function in patients with cerebral palsy during the treatment using the intensive neurophysiological rehabilitation system.
Zh Nevrol Psikhiatr Im S S Korsakova. 2012;112(7 Pt 2):14–17Kozyavkin VI, Kachmar OO.
Rehabilitation outcome assessment methods in Intensive neurophysiological rehabilitation system.
Ukrayinskyj Medychnyj Chasopys. 2003;3(35):61–66. [in Ukrainian]Bohannon RW, Smith MB.
Inter-rater reliability of a modified Ashworth scale of muscle spasticity.
Phys Ther. 1987;67(2):206–207Fleuren JF, Voerman GE, Erren-Wolters CV.
Stop using the Ashworth Scale for the assessment of spasticity.
J Neurol Neurosurg Psychiatry. 2010;81(1):46–52Lindberg PG, Gäverth J, Islam M, Fagergren A, Borg J, Forssberg H.
Validation of a new biomechanical model to measure muscle tone in spastic muscles.
Neurorehabil Neural Repair. 2011;25(7):617–625Gäverth J, Sandgren M, Lindberg PG, Forssberg H, Eliasson AC.
Test-retest and inter-rater reliability of a method to measure wrist and finger spasticity.
J Rehabil Med. 2013;45(7):630–636Gäverth J, Eliasson AC, Kullander K, Borg J, Lindberg PG, Forssberg H.
Sensitivity of the NeuroFlexor method to measure change in spasticity after treatment with botulinum toxin A in wrist and finger muscles.
J Rehabil Med. 2014;46(7):629–634Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH.
Content validity of the expanded and revised Gross Motor Function Classification System.
Dev Med Child Neurol. 2008;50(10):744–750Manual Ability Classification System for children with cerebral palsy.
http://www.macs.nu/
[cited 2016 March 30]Clar C, Tsertsvadze A, Court R, Hundt G, Clarke A, Sutcliffe P.
Clinical Effectiveness of Manual Therapy for the Management of
Musculoskeletal and Non-Musculoskeletal Conditions:
Systematic Review and Update of UK Evidence Report
Chiropractic & Manual Therapies 2014 (Mar 28); 22 (1): 12Ohlsen BA.
Combination of Acupuncture and Spinal Manipulative Therapy: Management of a
32-year-old Patient With Chronic Tension-type Headache and Migraine
Journal of Chiropractic Medicine 2012 (Sep); 11 (3): 192–201Gemma V EL, Antonia GC:
Efficacy of Manual and Manipulative Therapy in the Perception of Pain and Cervical Motion
in Patients with Tension-type Headache: A Randomized, Controlled Clinical Trial
Journal of Chiropractic Medicine 2014 (Mar); 13 (1): 4—13Win NN, Jorgensen AMS, Chen YS, Haneline MT.
Effects of Upper and Lower Cervical Spinal Manipulative Therapy on Blood Pressure and Heart Rate Variability
in Volunteers and Patients With Neck Pain: A Randomized Controlled, Cross-Over, Preliminary Study
Journal of Chiropractic Medicine 2015 (Mar); 14 (1): 1–9Yu X, Wang X, Zhang J, Wang Y.
Changes in pressure pain thresholds and basal electromyographic activity after instrument-assisted spinal manipulative therapy in asymptomatic participants: a randomized, controlled trial.
J Manipulative Physiol Ther. 2012;35(6):437–445Wearing J, Beaumont S, Forbes D, Brown B, Engel R.
The use of spinal manipulative therapy in the management of chronic obstructive pulmonary disease: a systematic review.
J Altern Complement Med. 2016;22(2):108–114Hawk C, Schneider MJ, Vallone S, Hewitt EG.
Best Practices for Chiropractic Care of Children:
A Consensus Update
J Manipulative Physiol Ther. 2016 (Mar); 39 (3): 158–168Davis MF, Worden K, Clawson D, Meaney FJ, Duncan B.
Confirmatory factor analysis in osteopathic medicine: fascial and spinal motion restrictions as correlates of muscle spasticity in children with cerebral palsy.
J Am Osteopath Assoc. 2007;107(6):226–232Duncan B, McDonough-Means S, Worden K, Schnyer R, Andrews J, Meaney FJ.
Effectiveness of osteopathy in the cranial field and myofascial release versus acupuncture as complementary treatment for children with spastic cerebral palsy: a pilot study.
J Am Osteopath Assoc. 2008;108(10):559–570Wyatt K, Edwards V, Franck L.
Cranial osteopathy for children with cerebral palsy: a randomised controlled trial.
Arch Dis Child. 2011;96(6):505–512Posadzki P, Lee MS, Ernst E.
Osteopathic manipulative treatment for pediatric conditions: a systematic review.
Pediatrics. 2013;132(1):140–152Pickar JG.
Neurophysiological Effects of Spinal Manipulation
Spine J (N American Spine Society) 2002 (Sep); 2 (5): 357–371Clark BC, Thomas JS, Walkowski SA, Howell JN.
The biology of manual therapies.
J Am Osteopath Assoc. 2012;112(9):617–629Pickar JG, Bolton PS.
Spinal Manipulative Therapy and Somatosensory Activation
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 785–794Chu J, Allen DD, Pawlowsky S, Smoot B.
Peripheral response to cervical or thoracic spinal manual therapy: an evidence-based review with meta-analysis.
J Man Manip Ther. 2014;22(4):220–229Reed WR, Long CR, Kawchuk GN, Pickar JG.
Neural Responses to the Mechanical Characteristics of High Velocity,
Low Amplitude Spinal Manipulation: Effect of Specific Contact Site
Man Ther. 2015 (Dec); 20 (6): 797–804Reed WR, Liebschner MA, Sozio RS, Pickar JG, Gudavalli MR.
Neural Response During a Mechanically Assisted Spinal Manipulation
in an Animal Model: A Pilot Study
J Nov Physiother Phys Rehabil. 2015 (Sep); 2 (2): 20–27Niazi IK, Turker KS, Flavel S, Kinget M, Duehr J, Haavik H.
Changes in H-reflex and V-waves Following Spinal Manipulation
Experimental Brain Research 2015 (Apr); 233 (4): 1165–1173Dishman JD, Bulbulian R.
Spinal reflex attenuation associated with spinal manipulation.
Spine. 2000;25(19):2519–2524
Return to CEREBRAL PALSY
Since 6–17–2017


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |