

Nociplastic Pain: An Introduction This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Can Chiropr Assoc 2025 (Aug); 69 (2): 131–144 ~ FULL TEXT
OPEN ACCESS Christopher B. Roecker, DC, MS • Samuel M. Schut, DC
VA Puget Sound Health Care System,
Care Rehabilitation Care Services,
Everett, Washington.
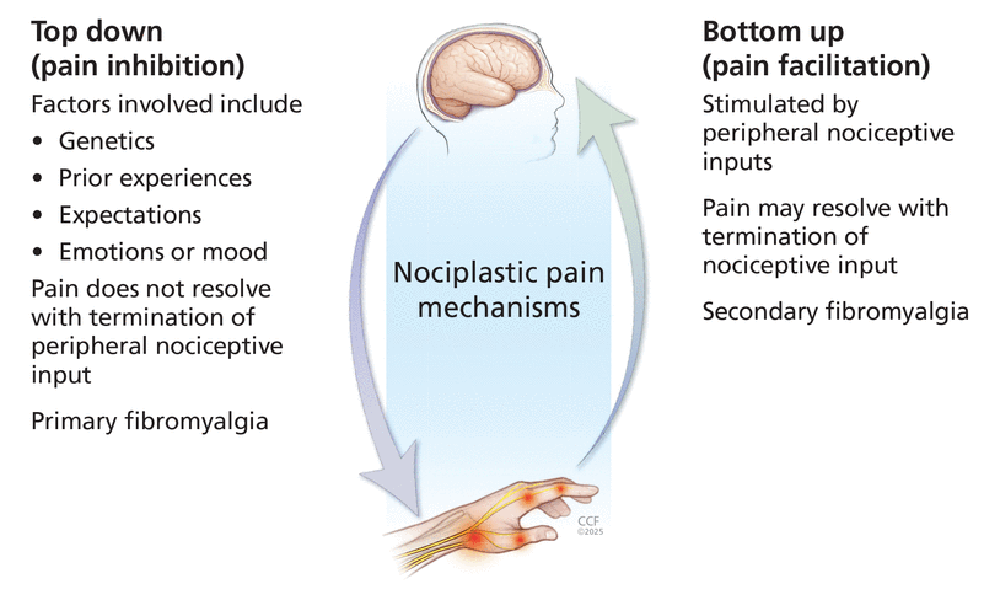
Chronic pain is common in chiropractic practice and often presents without clear evidence of tissue injury. Nociplastic pain is a recently defined concept that highlights altered nociceptive processing within the nervous system. This newer understanding of pain provides insight into chronic conditions such as chronic back or neck pain, chronic headaches, and fibromyalgia. These conditions are commonly encountered in chiropractic practice but may be challenging to address using traditional models. This commentary introduces nociplastic pain, outlining potential mechanisms and relevance to chiropractic care. We advocate a collaborative, multimodal management approach that includes patient education, exercise promotion, and functional goal-setting within a biopsychosocial framework. Understanding nociplastic pain equips chiropractors to support patients with complex chronic pain through compassionate, evidence-based care that addresses the whole person.
Keywords: back; biopsychosocial; central sensitization; chiropractic; chronic; fibromyalgia; headache; interdisciplinary health teams; management; neck; neuropathic; nociception; nociplastic; pain; widespread chronic pain.
From the FULL TEXT Article:
Introduction
Advances in pain science continue to transform our understanding of pain mechanisms. Traditionally, pain has been mechanistically classified as either nociceptive or neuropathic in nature, and cases that did not fall easily into one of these categories were often labeled as idiopathic or pejoratively suggestive of malingering. [1] This framework, however, was incomplete and left many patients without a clear explanation for their symptoms. By 2017, sufficient evidence had accumulated to describe a third pain mechanistic descriptor (i.e., type of pain), characterized by alterations in nociceptive processing. [2–4] This new understanding of pain is now recognized as nociplastic pain. [5–8]

Table 1 Nociplastic pain is defined as “pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain” (Table 1). [5, 8] The purpose of this commentary is to introduce nociplastic pain, its purported pathophysiologic mechanisms, management strategies, and its implications for clinical decision-making within the chiropractic profession.
Nociplastic pain
Nociplastic pain represents a distinct mechanistic pain category characterized by aberrant pain processing within the nervous system. [10] Unlike nociceptive or neuropathic pain, nociplastic pain is not directly attributable to tissue damage, inflammation, or nerve injury. Instead, nociplastic pain develops from abnormal neuronal signaling, causing increased sensitivity to various sensory stimuli and perpetuating the cycle of persistent pain. [6, 10]
Nociplastic pain can provide an explanation for how chronic and recurrent pain conditions, such as chronic non-traumatic low back or neck pain, become established and maintained for prolonged periods. [11] Understanding chronic pain from a nociplastic pain perspective offers a rationale for persistent pain that cannot be fully explained by traditional pathoanatomical models, while avoiding tendencies to attribute such conditions to solely psychopathologic causes. [12] The concept of nociplastic pain may provide validation and reduce stigma for patients who may have been led to believe that pain isn’t real or that it is all in their head. [13] This new mechanistic descriptor of pain promotes a more nuanced approach to evaluating and treating chronic pain, which aligns with the latest advances in pain science. [13]
Mechanisms underlying nociplastic pain
Nociplastic pain develops from altered nociceptive processing in the central and peripheral nervous systems and can occur in the absence of nociceptor activation or somatosensory pathology. [10, 14–17] Current understanding of the neurophysiologic mechanisms of nociplastic pain is complex and remains incomplete, but emerging evidence suggests that the key pathophysiologic changes associated with nociplastic pain include central sensitization, alterations in brain network connectivity, and peripheral nervous system changes. [10, 15]
Central sensitization is the hallmark of nociplastic pain and refers to heightened excitability within the central nervous system and amplification of sensory input, resulting in increased pain perception. [5, 11, 18] Clinically, this may present as an exaggerated pain response to high-threshold stimuli (i.e., hyperalgesia) or a pain response to typically non-painful, low-threshold stimuli such as light touch (i.e., allodynia), reflecting a lowered pain threshold. [10, 14, 18] Central sensitization occurs due to a variety of mechanisms that remain incompletely understood, but changes within the spinal cord and brain network connectivity are known to play a role. [10, 15, 18] Spinal mechanisms include regional clustering and convergence of signals from different pain locations, spinal cord reorganization, hyper-responsiveness of spinal dorsal horn neurons, amplified spinal reflex transmission, decreased spinal inhibition, and temporal summation. [6, 15, 18–20]
Evidence also suggests that neuroimmune activation occurs via spinal microglia along with increased concentration of substance P and glutamine levels within cerebrospinal fluid. [14–16, 21] Altered connectivity within various brain regions is also implicated in the development of central sensitization, particularly increased connectivity between the default mode network, salience network, and sensorimotor network. [10, 14, 15] These large-scale brain networks are involved in self-referential thought, attention and sensory integration, and sensory processing and motor initiation. [10, 22] Although these networks appear to become enmeshed, the mechanisms by which this contributes to sensory, emotional, or cognitive aspects of the pain remain unclear. Changes in the size and shape of the gray and white matter, in areas of the brain related to pain perception, have also been observed on magnetic resonance imaging (MRI) as a consequence of chronic pain. [23–26] The function of normal descending inhibitory spinal pathways may also be altered in cases of nociplastic pain. [10, 15, 27, 28] Disturbance of this normal inhibitory nociceptive signaling is referred to as disinhibition, but the mechanisms facilitating it are not yet well understood.
Neuronal changes may also occur in the peripheral nervous system of those with nociplastic pain, though they are generally considered to play a lesser role than central mechanisms. [10, 14, 15, 18] Peripheral sensitization refers to increased sensitivity to sensory stimuli, resulting in a heightened pain response. [29, 30] While this process serves a protective role following acute tissue injury or inflammation, by promoting healing,30 it becomes pathological when it persists beyond the acute phase of tissue repair, contributing to maladaptive nociception in cases of nociplastic pain. [8, 14, 15, 18]
Less is known about peripheral sensitization than central sensitization, but peripheral sensitization is believed to involve an expansion of the receptive field, elevated concentrations of pro-inflammatory cytokines and chemokines, proliferation of sodium channels, and abnormal coupling of primary afferent neurons by sympathetic neurons, known as sympatho-afferent coupling. [14, 15, 18, 31–33] Peripheral sensitization is believed to initiate or maintain central sensitization via perpetual bombardment of the central nervous system with nociceptive stimuli. [10, 34] Persistent nociceptive stimulus is characteristic among individuals with chronic inflammatory autoimmune conditions, such as rheumatoid arthritis, [34] and may explain why fibromyalgia is more common among individuals with co-occurring autoimmune inflammatory conditions. [34–38]
Top-down versus bottom-up nociplastic pain subtypes
Emerging research suggests that nociplastic pain may involve potential subtypes, termed bottom-up and topdown, based on their predominant mechanistic pathway. [10, 34, 39] Top-down nociplasticity or nociplastic pain arises primarily from impaired descending pain modulation, [28] and is reportedly more common in individuals with substantial psychological comorbidities, often developing at a younger age. [10, 34] In contrast, bottom-up nociplastic pain results from persistent peripheral nociceptive input, as seen in conditions like rheumatoid arthritis or advanced osteoarthritis, ultimately leading to central sensitization. [10, 34]
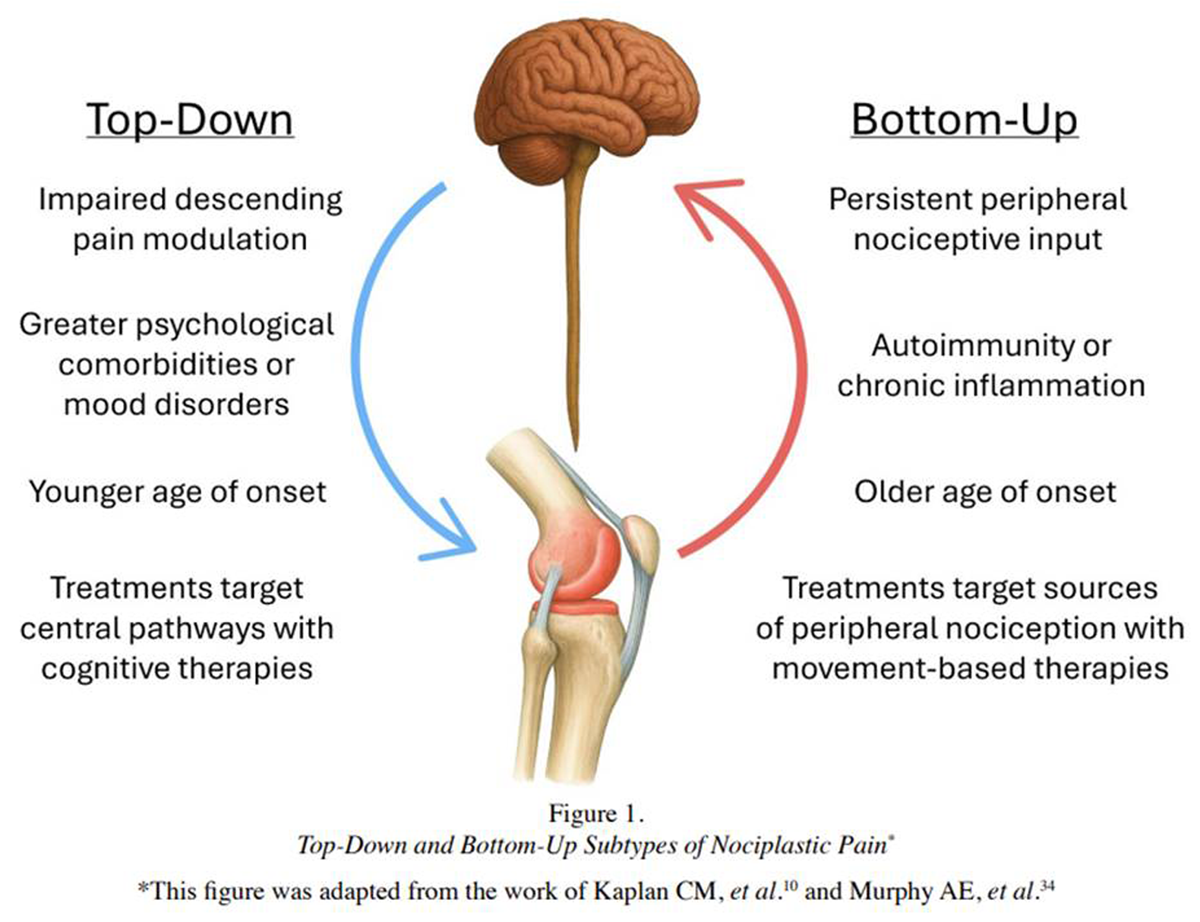
Figure 1 Understanding these subtypes may help to inform treatment. [10, 34] Treatment of bottom-up nociplastic pain may respond more favorably to treatments targeting peripheral sources of nociception, for example manual therapies, while top-down nociplastic pain is believed to respond more favorably to treatments targeting central pathways, such as cognitive-behavioral therapy (CBT), mindfulness-based strategies, or biofeedback (Figure 1). [10, 34, 40]
Nociplastic pain conditions
Nociplastic pain may be a component of any chronic pain condition, [10] but conditions characterized by nociplastic pain are those where nociplastic pain is believed to be the predominant pain mechanism. [41] These conditions are now beginning to be referred to as chronic primary pain syndromes by the International Classification of Diseases (ICD) coding system and have been described as painful conditions in their own right, [42] rather than conditions where pain emerges as a secondary feature of another disease process. [43] The most recognized nociplastic pain syndromes include a wide range of chronic pain conditions such as fibromyalgia, chronic nonspecific low back pain, migraines, chronic tension-type headaches, irritable bowel syndrome (IBS), and temporomandibular joint disorder (TMD). [10, 14, 41, 44] Other conditions involving predominant nociplastic pain mechanisms include complex regional pain syndrome type I (CRPS-I), or chronic pelvic pain syndromes (e.g., chronic prostatitis, vulvodynia) (Table 1). [5, 10]
Chronic pain conditions characterized by nociplastic pain often coexist with other chronic pain conditions (i.e., chronic overlapping pain conditions) and are more prevalent in individuals with a higher burden of comorbidities. [10, 18, 45] The relationship between nociplastic pain and the changes in neurophysiology helps to explain non-painful features that are known to accompany these chronic pain conditions. Comorbid conditions that are associated with nociplastic pain include depression, anxiety, post-traumatic stress disorder (PTSD), sleep disturbance, poor mental clarity (i.e., brain fog), chronic abdominal or pelvic pain, or other multisensory sensitivities to light, sound, or odors. [10, 14, 18] The clustering of these chronic conditions supports the concept of shared overlapping neurophysiologic mechanisms within the nervous system.
Diagnosing nociplastic pain
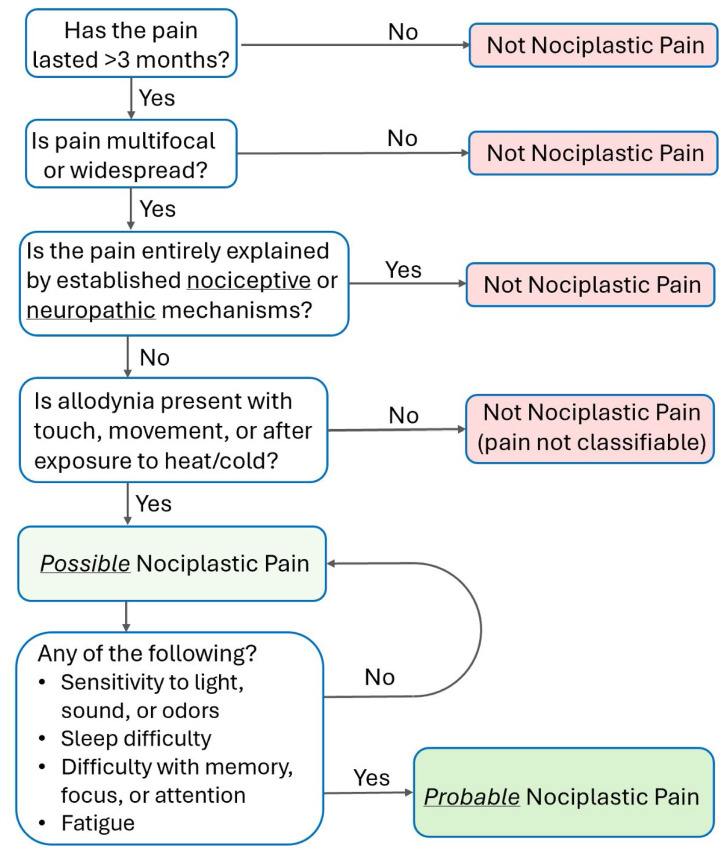
Figure 2 At present, no validated diagnostic tools or biomarkers exist to formally identify nociplastic pain and this mechanistic classification is based on a comprehensive history, physical exam, and clinical judgement. [10] In 2021 the IASP released a grading system to assist clinicians with identification, allowing for nociplastic pain to be qualified as possible or probable (Figure 2). [6, 20] Efforts to further refine this grading criteria continue, with this criteria having recently been refined to include a non-classifiable pain designation. [46–48]
Clinical tools, such as the Central Sensitization Inventory (CSI), [49] screening for yellow flags, [50] or movement-evoked pain (MEP), [51] have shown potential for measuring aspects of nociplastic pain in clinical settings, but capture only limited aspects of nociplastic pain. Abnormalities associated with nociplastic pain may be quantified via the use of quantitative sensory testing (QST), sensory evoked potentials, or functional MRI, [6, 10, 11, 15, 39] but these methods are largely reserved for research settings and are not yet recommended for use in clinical practice. [10]
The current lack of validated diagnostic criteria likely contributes to difficulties in quantifying the prevalence of nociplastic pain conditions. [10, 40] Nociplastic pain conditions are likely to be common, but are often underrecognized or attributed to other causes. [40] For context, conditions primarily involving nociplastic pain are known to be widespread. Chronic low back pain affects approximately 13% of adults, [52] fibromyalgia impacts around 5% of the population, [53] and up to 4% of the population experiences chronic tension-type headaches. [54] Increased awareness and recognition of nociplastic pain stands to help guide more targeted treatments aimed at addressing the underlying mechanisms driving these painful conditions. Many conditions now understood to be predominantly nociplastic pain in nature are among the most common conditions managed by chiropractors, [55–57] highlighting the critical importance of recognizing nociplastic pain in clinical practice.
Mixed pain mechanisms
Nociplastic pain may be present in isolation or as part of a mixed pain state. Mixed pain refers to the simultaneous involvement of nociceptive, neuropathic, or nociplastic mechanisms, with one or more mechanisms potentially predominating. [59, 60] Accordingly, nociplastic pain may occur in combination with nociceptive and/or neuropathic pain. [14, 60]
To illustrate this concept, we present a hypothetical clinical scenario. A 58-year-old male experiences persistent back pain and stiffness localized to the thoracolumbar spine and paraspinal regions. His pain is moderate in intensity and began insidiously at age 40 and is aggravated by movements in all planes of thoracolumbar motion and by prolonged sitting. Sitting for longer than 30 minutes significantly increases his pain, while movements involving lumbar lateral bending and extension provoke pain flares lasting approximately five minutes. These activities occasionally lead to pain radiating into the lateral gluteal and posterolateral thigh regions, accompanied by intermittent subjective numbness and tingling into the proximal posterolateral aspects of his calves.
Previous radiographs reveal moderate lumbar spondylosis with zygapophyseal (i.e., facet) joint arthropathy and moderate bilateral L3-L5 lumbar neuroforaminal narrowing. His health history includes class II obesity, chronic bilateral knee pain, intermittent neck and right shoulder pain, migraines, irritable bowel syndrome, depression, anxiety, sensitivity to loud sounds, and non-restorative sleep with moderate daily fatigue. He also reports hesitancy towards exercise due to worries about damaging what he describes as his “crumbling discs” (a phrase reflecting the patient’s fear-driven beliefs, rather than a formal diagnosis).
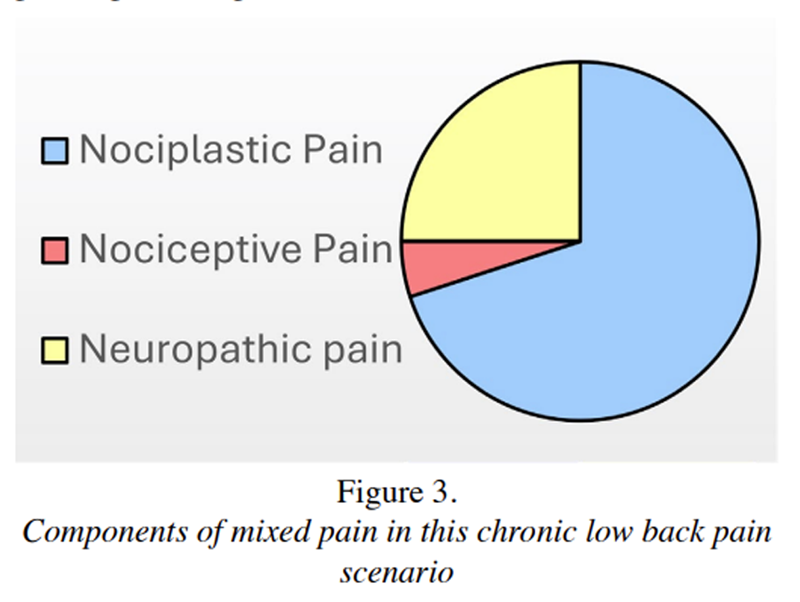
Figure 3 Figure 3 illustrates how varying degrees of overlapping pain mechanisms are present in this case. [61] Increased pain with extension and the presence of facet arthropathy are suggestive of a nociceptive component. Multilevel neuroforaminal narrowing, with pain radiating into the thighs and intermittent paresthesia in the legs, indicates a neuropathic component. Finally, his 18-year history of chronic widespread pain, multi-sensory sensitivities, sleep difficulty, and daily fatigue are suggestive of a probable nociplastic pain component.
Implications for chiropractors and other health care providers Chiropractors diagnose and manage a variety of painful neuromusculoskeletal conditions, which likely involve nociplastic pain. [55 ,62] These include both widespread pain conditions (e.g., fibromyalgia) and more localized pain conditions (e.g., chronic back pain, chronic migraine, chronic tension-type headaches, and temporomandibular joint disorders). Since some of these do not have an identifiable, peripheral lesion with which to target therapeutic interventions, patients and providers alike may experience distress and confusion regarding the best management approach. Moreover, an overemphasis towards nociceptive and/or neuropathic pain mechanisms may lead to the improper use of imaging or prioritization of structural causes of pain, which have been shown to promote concerns or confusion and limit a patient’s recovery. [63, 64] It is therefore critical for clinicians and researchers to be aware of and acknowledge nociplastic pain as a legitimate pain mechanism in order to facilitate understanding and to provide reassurance and effective management. [58]
Diagnosis of nociplastic pain can prove clinically challenging with the clinical assessment forming the evaluative backbone. [15] Patients commonly report pain that is longstanding and poorly localized. [65] Nociplastic pain is often disproportionately and unpredictably impacted by aggravating and alleviating factors. [65] Additionally, it is associated with concomitant symptoms such as fatigue, sleep disturbances, cognitive issues, mood disorders, and heightened sensitivity to environmental stimuli. [6] Psychological factors such as stress, anxiety, depression, a history of trauma (e.g., PTSD, adverse childhood experiences), catastrophizing, or fear-avoidance behaviors or kinesiophobia are thought to play a role in the development and/or maintenance of nociplastic pain. [10] Patients may also report a protracted history of pharmacologic, non-pharmacologic, interventional, and surgical treatment, with minimal or transient benefit. [34] The clinical assessment should be used to help rule out other drivers of pain and to assess for features characteristic of nociplastic pain. Patients may display signs of hyperalgesia and allodynia, hesitancy and guarded movements during range of motion testing, and difficulty maintaining prolonged positioning.
It is understandable that many health care providers may be entirely unaware of the nociplastic pain classification as a recognized pain construct, although experienced practitioners may be intuitively familiar with its clinical presentation in their patients. The degree to which current chiropractic students and recent graduates are, or have been, exposed to nociplastic pain concepts in their respective training, is unknown. Research examining whether chiropractic programs are incorporating nociplastic pain education into their curricula would help clarify this gap, and efforts to integrate this content into chiropractic training are encouraged.
Clinicians are encouraged to provide reassurance and validation to patients, emphasizing that their pain will be acknowledged and respected. [66, 67] Functional goal-setting and establishing realistic treatment expectations should be discussed from a management-focused perspective, rather than a curative one. [68, 69] Clinicians are also encouraged to explain pain constructs, such as neuroplasticity and sensitization, in a patient-centered manner that avoids the use of technical jargon and is solutions-oriented. [40, 70] The primary goal of this education is to validate the patient’s experience, provide an explanation for their chronic pain, help them understand the drivers of their chronic pain, and discuss potential methods for modifying these factors.
Stress management approaches including relaxation techniques, mindfulness, and meditation practices can be introduced to patients by chiropractors to help manage stress-related drivers of sensitivity. Moreover, considering sleep disturbances are a hallmark sign of nociplastic pain, chiropractors should discuss beneficial sleep strategies including adopting a regular sleep schedule, creating a relaxing bedtime routine, and optimizing the sleep environment. [40, 71] Prompt referrals should be coordinated to sleep specialists if more intensive approaches are required.
Chiropractors can also help formulate and supervise graded exercise programs that are tailored to individual abilities and focused on functional goals. This may include a mixture of both aerobic exercise and resistance training. Recommendations for pacing activities should be provided and may be accompanied by strategies to track latent soreness (e.g. activity diaries) given that exercising in the presence of pain poses unique barriers to engagement compared to exercising pain-free. [72, 73] More recently, a multidimensional rehabilitative approach known as cognitive functional therapy (CFT) has shown promise in reducing disabling chronic low back pain, likely driven by nociplastic pain mechanisms. [] 74–76 Through addressing negative cognitions (e.g., kinesiophobia), behaviors (e.g., guarded, non-varied movements), as well as healthy lifestyle changes, CFT aims to help patients make better sense of their pain and promote the extinction of safety behaviors through graded exposure to fearful movements. Chiropractors may consider integrating the principles of CFT, in combination with approaches to healthy lifestyle, [77] to help manage chronic nociplastic pain conditions.
The presence of nociplastic pain should not deter providers from evaluating and addressing peripheral dysfunctions as part of an overall treatment plan. Manual therapies, including joint mobilization or manipulation, soft tissue techniques, and heat or cold applications may also play a role in treating patients with nociplastic pain by reducing peripheral nociception. [11] This “bottom-up” approach is directed to peripheral tissues, rather than central ones, and may help to attenuate peripheral drivers of central sensitization. [] 10,78–80 In 2010, Srbely proposed that spinal manipulative therapy may serve as a method for modulating neurophysiological sensitization. [81] Recent research has shown that a 4-week trial of 12 sessions of spinal manipulative therapy has the capacity to alter nociplastic pain factors and reduce segmental mechanical hyperalgesia among adults with chronic low back pain. [78] Clinicians are encouraged to avoid prolonged reliance on passive treatments alone, as these approaches are unlikely to provide more than temporary relief and may contribute to learned helplessness, potentially undermining the central role of self-care and lifestyle modification for the management of chronic nociplastic pain conditions. [82, 83]
Pharmacologic treatments may play a role in nociplastic pain management. Pharmacologic agents are recommended in a stepwise approach when non-pharmacologic and self-management strategies fail to provide sufficient relief. [14, 84] Importantly, national guidelines recommend that the use of pharmacologic treatment should only occur in tandem, rather than in lieu of, non-pharmacologic approaches. [39, 84] Various central-acting medication classes have been found to be helpful for managing nociplastic pain including tricyclic antidepressants (e.g., amitriptyline, nortriptyline), selective norepinephrine reuptake inhibitors (e.g., duloxetine, venlafaxine), gabapentinoids (e.g., gabapentin, pregabalin), and low-dose naltrexone. [39, 85] Traditional analgesics, such as nonsteroidal anti-inflammatories (NSAIDs) and acetaminophen, are often ineffective for nociplastic pain, while opioids are strongly discouraged. [14, 39, 86] Considering the significant sleep disturbances often experienced by patients with nociplastic pain, pharmacologic or supplemental sleep aids (e.g., melatonin) as well as advice on general sleep hygiene may play a role in certain cases.

Table 2 Health care providers are encouraged to prioritize patients’ needs and work collaboratively with other healthcare professionals to help provide comprehensive care for nociplastic pain. By integrating evidence-based non-pharmacologic treatments within a broader interdisciplinary pain management plan (Table 2), chiropractors can play a pivotal role in addressing the multifaceted nature of chronic pain conditions involving a significant nociplastic pain component. A patient-centered, multidisciplinary approach ensures that care is tailored to individual patients and stands to improve outcomes and empower patients to actively participate in their pain management.
Conclusion
The concept of nociplastic pain represents a fundamental shift in how chronic pain is understood and managed.40 By recognizing the role of altered nociceptive processing within the nervous system, chiropractors can move beyond traditional structural and nociceptive models of diagnosis and treatment to providing more comprehensive, evidence-based, and patient-centered care. Many conditions, now considered to be primarily nociplastic in nature, are commonly encountered in chiropractic practice, emphasizing the need for a deeper understanding of this pain mechanism among clinicians.
Chiropractors are uniquely positioned to provide patient education, implement non-pharmacologic care, and collaborate within interdisciplinary teams to improve chronic pain management. While research on the direct effects of manual therapies for nociplastic pain conditions continues to evolve, evidence supports a multimodal approach that includes movement-based treatments, cognitive strategies, and lifestyle modifications within a biopsychosocial framework. [87] Integrating these strategies into chiropractic practice can enhance patient care by addressing both the physiological and psychosocial aspects of chronic pain.
Further research is needed to clarify nociplastic pain mechanisms, develop reliable diagnostic tools, and refine optimal treatment strategies. By staying informed and adaptable, chiropractors can play a pivotal role in advancing pain management strategies that validate patients’ experiences and empower them toward meaningful functional improvements and improved quality of life.
Acknowledgements
The authors would like to thank Dr. John Crouse, DC, Assistant Professor at Palmer College of Chiropractic, for his valuable feedback and expertise in reviewing this manuscript. We also acknowledge the use of OpenAI’s ChatGPT (model GPT-4o) for the creation of anatomic images shown in Figure 1.
Conflicts of Interest:
The authors have no disclaimers, sources of support, or funding to report in the preparation of this manuscript. The views and conclusions presented in this manuscript are solely those of the authors and do not necessarily reflect the official positions or policies of the U.S. Government, the Veterans Health Administration, or the Cleveland Clinic. CBR serves as a consultant on a “HEAL Initiative: Pain Research Enhancement Program (PREP)” NIH grant application. If funded, compensation for this consulting will total $6,000 over a period of 3 years, which breaks down to $2,000 per year. Additionally, CBR is the sole proprietor of Clinical Solutions, LLC. This LLC is a business structure, under which he is currently writing a book with plans to publish. SMS has no conflicts of interest to disclose
References:
Trouvin AP, Perrot S.
New concepts of pain.
Best Pract Res Clin Rheumatol. 2019;33(3):101415.
doi: 10.1016/j.berh.2019.04.007.Giesecke T, Gracely RH, Grant MAB, et al.
Evidence of augmented central pain processing
in idiopathic chronic low back pain.
Arthritis Rheum. 2004;50(2):613–623.
doi: 10.1002/art.20063.Burgmer M, Pfleiderer B, Maihöfner C, et al.
Cerebral mechanisms of experimental hyperalgesia in fibromyalgia.
Eur J Pain Lond Engl. 2012;16(5):636–647.
doi: 10.1002/j.1532-2149.2011.00058.x.Baliki MN, Mansour AR, Baria AT, Apkarian AV.
Functional reorganization of the default mode network
across chronic pain conditions.
PloS One. 2014;9(9):e106133.
doi: 10.1371/journal.pone.0106133.Kosek E, Cohen M, Baron R, et al.
Do we need a third mechanistic descriptor for chronic pain states?
Pain. 2016;157(7):1382–1386.
doi: 10.1097/j.pain.0000000000000507.Nijs J, Lahousse A, Kapreli E, et al.
Nociplastic Pain Criteria or Recognition of Central Sensitization?
Pain Phenotyping in the Past, Present and Future.
J Clin Med. 2021;10(15):3203.
doi: 10.3390/jcm10153203.Fitzcharles MA, Ste-Marie PA, Goldenberg DL, et al.
2012 Canadian Guidelines for the diagnosis and management
of fibromyalgia syndrome: executive summary.
Pain Res Manag. 2013;18(3):119–126.
doi: 10.1155/2013/918216.Terminology | International Association for the Study of Pain
International Association for the Study of Pain (IASP)
[Accessed December 29, 2024].
https://www.iasp-pain.org/resources/terminology/Nijs J, De Baets L, Hodges P.
Phenotyping nociceptive, neuropathic, and nociplastic pain:
who, how, & why?
Braz J Phys Ther. 2023;27(4):100537.
doi: 10.1016/j.bjpt.2023.100537.Kaplan CM, Kelleher E, Irani A, Schrepf A, Clauw DJ, Harte SE.
Deciphering nociplastic pain: clinical features,
risk factors and potential mechanisms.
Nat Rev Neurol. 2024;20(6):347–363.
doi: 10.1038/s41582-024-00966-8.Nijs J, George SZ, Clauw DJ, et al.
Central sensitisation in chronic pain conditions: latest
discoveries and their potential for precision medicine.
Lancet Rheumatol. 2021;3(5):e383–e392.
doi: 10.1016/S2665-9913(21)00032-1.Melzack R, Katz J.
Pain.
Wiley Interdiscip Rev Cogn Sci. 2013;4(1):1–15.
doi: 10.1002/wcs.1201.What we know about nociplastic pain.
University of Michigan Medical School; Jun 20, 2024.
[Accessed December 29, 2024].
https://medresearch.umich.edu/department-news/
what-we-know-about-nociplastic-painFitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W.
Nociplastic pain: towards an understanding of prevalent pain conditions.
Lancet Lond Engl. 2021;397(10289):2098–2110.
doi: 10.1016/S0140-6736(21)00392-5.Bu?dy? K, Górnicki T, Ka?ka D, et al.
What Do We Know about Nociplastic Pain?
Healthc Basel Switz. 2023;11(12):1794.
doi: 10.3390/healthcare11121794.McDonough KE, Hammond R, Wang J, et al.
Spinal GABAergic disinhibition allows microglial activation
mediating the development of nociplastic pain in male mice.
Brain Behav Immun. 2023;107:215–224.
doi: 10.1016/j.bbi.2022.10.013.Hankerd K, McDonough KE, Wang J, Tang SJ, Chung JM, La JH.
Post-injury stimulation triggers a transition to nociplastic pain in mice.
Pain. 2022;163(3):461–473.
doi: 10.1097/j.pain.0000000000002366.Yoo YM, Kim KH.
Current understanding of nociplastic pain.
Korean J Pain. 2024;37(2):107–118.
doi: 10.3344/kjp.23326.Alshelh Z, Brusaferri L, Saha A, et al.
Neuroimmune signatures in chronic low back pain subtypes.
Brain J Neurol. 2022;145(3):1098–1110.
doi: 10.1093/brain/awab336.Kosek E, Clauw D, Nijs J, et al.
Chronic nociplastic pain affecting the musculoskeletal system:
clinical criteria and grading system.
Pain. 2021;162(11):2629–2634.
doi: 10.1097/j.pain.0000000000002324.Ji RR, Nackley A, Huh Y, Terrando N, Maixner W.
Neuroinflammation and Central Sensitization in
Chronic and Widespread Pain.
Anesthesiology. 2018;129(2):343–366.
doi: 10.1097/ALN.0000000000002130.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC.
The human brain is intrinsically organized into
dynamic, anticorrelated functional networks.
Proc Natl Acad Sci U S A. 2005;102(27):9673–9678.
doi: 10.1073/pnas.0504136102.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A.
Brain Gray Matter Decrease in Chronic Pain Is the
Consequence and Not the Cause of Pain.
J Neurosci. 2009;29(44):13746–13750.
doi: 10.1523/JNEUROSCI.3687-09.2009.Apkarian AV, Sosa Y, Sonty S, et al.
Chronic back pain is associated with decreased prefrontal
and thalamic gray matter density.
J Neurosci Off J Soc Neurosci. 2004;24(46):10410–10415.
doi: 10.1523/JNEUROSCI.2541-04.2004.Coulombe MA, Lawrence KS, Moulin DE, et al.
Lower Functional Connectivity of the Periaqueductal Gray Is Related
to Negative Affect and Clinical Manifestations of Fibromyalgia.
Front Neuroanat. 2017;11:47.
doi: 10.3389/fnana.2017.00047.Smallwood RF, Laird AR, Ramage AE, et al.
Structural brain anomalies and chronic pain:
a quantitative meta-analysis of gray matter volume.
J Pain. 2013;14(7):663–675.
doi: 10.1016/j.jpain.2013.03.001.Kwon M, Altin M, Duenas H, Alev L.
The role of descending inhibitory pathways on chronic
pain modulation and clinical implications.
Pain Pract Off J World Inst Pain. 2014;14(7):656–667.
doi: 10.1111/papr.12145.Ossipov MH, Morimura K, Porreca F.
Descending pain modulation and chronification of pain.
Curr Opin Support Palliat Care. 2014;8(2):143–151.
doi: 10.1097/SPC.0000000000000055.Gangadharan V, Kuner R.
Pain hypersensitivity mechanisms at a glance.
Dis Model Mech. 2013;6(4):889–895.
doi: 10.1242/dmm.011502.Hoegh M.
Pain Science in Practice (Part 3): Peripheral Sensitization.
J Orthop Sports Phys Ther. 2022;52(6):303–306.
doi: 10.2519/jospt.2022.11202.Jänig W, Levine JD, Michaelis M.
Interactions of sympathetic and primary afferent
neurons following nerve injury and tissue trauma.
Prog Brain Res. 1996;113:161–184.
doi: 10.1016/s0079-6123(08)61087-0.Song Q, ES, Zhang Z, Liang Y.
Neuroplasticity in the transition from acute to chronic pain.
Neurother J Am Soc Exp Neurother. 2024;21(6):e00464.
doi: 10.1016/j.neurot.2024.e00464.Matsuda M, Huh Y, Ji RR.
Roles of inflammation, neurogenic inflammation,
and neuroinflammation in pain.
J Anesth. 2019;33(1):131–139.
doi: 10.1007/s00540-018-2579-4.Murphy AE, Minhas D, Clauw DJ, Lee YC.
Identifying and Managing Nociplastic Pain in Individuals
With Rheumatic Diseases: A Narrative Review.
Arthritis Care Res. 2023;75(10):2215–2222.
doi: 10.1002/acr.25104.Paroli M, Gioia C, Accapezzato D, Caccavale R.
Inflammation, Autoimmunity, and Infection in Fibromyalgia:
A Narrative Review.
Int J Mol Sci. 2024;25(11):5922.
doi: 10.3390/ijms25115922.Aleksi V, Elise K, Koskela TH.
Excess use of thyroid hormone treatment among patients with
fibromyalgia: a cross-sectional study in primary health care.
BMC Res Notes. 2022;15(1):83.
doi: 10.1186/s13104-022-05971-y.Greenbaum H, Weil C, Chodick G, Shalev V, Eisenberg VH.
Evidence for an association between endometriosis,
fibromyalgia, and autoimmune diseases.
Am J Reprod Immunol. 2019;81(4):e13095.
doi: 10.1111/aji.13095.Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F.
The Prevalence and Characteristics of Fibromyalgia in the
2012 National Health Interview Survey.
PloS One. 2015;10(9):e0138024.
doi: 10.1371/journal.pone.0138024.Kosek E.
The concept of nociplastic pain-where to from here?
Pain. 2024;165(11S):S50–S57.
doi: 10.1097/j.pain.0000000000003305.Ablin JN.
Nociplastic Pain: A Critical Paradigm for
Multidisciplinary Recognition and Management.
J Clin Med. 2024;13(19):5741.
doi: 10.3390/jcm13195741.Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD.
Overlapping Chronic Pain Conditions: Implications
for Diagnosis and Classification.
J Pain. 2016;17(9 Suppl):T93–T107.
doi: 10.1016/j.jpain.2016.06.002.Raffaeli W, Arnaudo E.
Pain as a disease: an overview.
J Pain Res. 2017;10:2003–2008.
doi: 10.2147/JPR.S138864.Treede RD, Rief W, Barke A, et al.
Chronic pain as a symptom or a disease: the IASP Classification of
Chronic Pain for the International Classification of Diseases (ICD-11)
Pain. 2019;160(1):19–27.
doi: 10.1097/j.pain.0000000000001384.Wirth B, Schweinhardt P.
Personalized assessment and management of non-specific low back pain.
Eur J Pain Lond Engl. 2024;28(2):181–198.
doi: 10.1002/ejp.2190.Ocay DD, Ross BD, Moscaritolo L, et al.
The Psychosocial Characteristics and Somatosensory Function of Children
and Adolescents Who Meet the Criteria for Chronic Nociplastic Pain.
J Pain Res. 2023;16:487–500.
doi: 10.2147/JPR.S397829.Russo MA, Visser E, North RB, et al.
Taxonomy and pain clinic patients.
Pain. 2022;163(8):e964.
doi: 10.1097/j.pain.0000000000002671.Kosek E, Baron R, Clauw D, et al.
Reply to Russo et al.
Pain. 2022;163(8):e964–e965.
doi: 10.1097/j.pain.0000000000002672.Schmidt H, Drusko A, Renz MP, et al.
Application of the grading system for “nociplastic pain” in chronic
primary and chronic secondary pain conditions: a field study.
Pain. 2025;166(1):196–211.
doi: 10.1097/j.pain.0000000000003355.Neblett R, Sanabria-Mazo JP, Luciano JV, et al.
Is the Central Sensitization Inventory (CSI) associated with quantitative
sensory testing (QST)? A systematic review and meta-analysis.
Neurosci Biobehav Rev. 2024;161:105612.
doi: 10.1016/j.neubiorev.2024.105612.Lentz TA, Beneciuk JM, Bialosky JE, et al.
Development of a Yellow Flag Assessment Tool for Orthopaedic
Physical Therapists: Results From the Optimal Screening
for Prediction of Referral and Outcome (OSPRO) Cohort.
J Orthop Sports Phys Ther. 2016;46(5):327–343.
doi: 10.2519/jospt.2016.6487.Leemans L, Nijs J, Wideman TH, et al.
Do measures of central sensitization relate to movement-evoked pain
in people with chronic low back pain? A longitudinal prospective study.
Braz J Phys Ther. 2024;28(6):101138.
doi: 10.1016/j.bjpt.2024.101138.Shmagel A, Foley R, Ibrahim H.
Epidemiology of Chronic Low Back Pain in US Adults:
Data From the 2009-2010 National Health
and Nutrition Examination Survey
Arthritis Care Res (Hoboken) 2016 (Nov); 68 (11): 1688–1694Ruschak I, Montesó-Curto P, Rosselló L, Aguilar Martín C.
Fibromyalgia Syndrome Pain in Men and Women:
A Scoping Review.
Healthc Basel Switz. 2023;11(2):223.
doi: 10.3390/healthcare11020223.Krishnan A, Silver N.
Headache (chronic tension-type)
BMJ Clin Evid. 2009;2009:1205.Beliveau PJH, Wong JJ, Sutton DA, et al.
The Chiropractic Profession: A Scoping Review of Utilization Rates,
Reasons for Seeking Care, Patient Profiles, and Care Provided
Chiropractic & Manual Therapies 2017 (Nov 22); 25: 35Green BN, Johnson CD, Daniels CJ, Napuli JG, Gliedt JA, Paris DJ.
Integration of Chiropractic Services in Military and Veteran
Health Care Facilities: Systematic Review of the Literature
J Evid Based Complementary Altern Med. 2016 (Apr); 21 (2): 115–130Mior S, Wong J, Sutton D, et al.
Understanding patient profiles and characteristics of current
chiropractic practice: a cross-sectional Ontario Chiropractic
Observation and Analysis STudy (O-COAST)
BMJ Open. 2019;9(8):e029851.
doi: 10.1136/bmjopen-2019-029851.Nijs J, Malfliet A, Nishigami T.
Nociplastic pain and central sensitization in patients with
chronic pain conditions: a terminology update for clinicians.
Braz J Phys Ther. 2023;27(3):100518.
doi: 10.1016/j.bjpt.2023.100518.Freynhagen R, Parada HA, Calderon-Ospina CA, et al.
Current understanding of the mixed pain concept:
a brief narrative review.
Curr Med Res Opin. 2019;35(6):1011–1018.
doi: 10.1080/03007995.2018.1552042.Freynhagen R, Rey R, Argoff C.
When to consider “mixed pain”? The right questions
can make a difference!
Curr Med Res Opin. 2020;36(12):2037–2046.
doi: 10.1080/03007995.2020.1832058.Hassan S, Nesovic K, Babineau J, Furlan AD, Kumbhare D, Carlesso LC.
Identifying chronic low back pain phenotypic domains and characteristics
accounting for individual variation: a systematic review.
Pain. 2023;164(10):2148.
doi: 10.1097/j.pain.0000000000002911.Lefebvre R, Peterson D, Haas M.
Evidence-Based Practice and Chiropractic Care
J Evid Based Comp Altern Med. 2012 (Dec 28); 18 (1): 75–79Farmer C, O’Connor DA, Lee H, et al.
Consumer understanding of terms used in imaging reports
requested for low back pain: a cross-sectional survey.
BMJ Open. 2021;11(9):e049938.
doi: 10.1136/bmjopen-2021-049938.O’Keeffe M, Ferreira GE, Harris IA, et al.
Effect of diagnostic labelling on management intentions for non-specific
low back pain: A randomized scenario-based experiment.
Eur J Pain Lond Engl. 2022;26(7):1532–1545.
doi: 10.1002/ejp.1981.Shraim MA, Massé-Alarie H, Hodges PW.
Methods to discriminate between mechanism-based categories of pain
experienced in the musculoskeletal system: a systematic review.
Pain. 2021;162(4):1007–1037.
doi: 10.1097/j.pain.0000000000002113.The Lancet.
Rethinking chronic pain.
Lancet. 2021;397(10289):2023.
doi: 10.1016/S0140-6736(21)01194-6.Young A, French SD, Traeger AC, Ayre J, Hancock M, Jenkins HJ.
Clinician experiences in providing reassurance for patients
with low back pain in primary care: a qualitative study.
J Physiother. doi: 10.1016/j.jphys.2024.11.003.
Published online December 12, 2024. S1836-9553(24)00108-5.Nijs J, Wijma AJ, Willaert W, et al.
Integrating Motivational Interviewing in Pain Neuroscience
Education for People With Chronic Pain:
A Practical Guide for Clinicians.
Phys Ther. 2020;100(5):846–859.
doi: 10.1093/ptj/pzaa021.Gardner T, Refshauge K, McAuley J, Hübscher M, Goodall S, Smith L.
Combined education and patient-led goal setting intervention reduced
chronic low back pain disability and intensity at 12 months:
a randomised controlled trial.
Br J Sports Med. 2019;53(22):1424–1431.
doi: 10.1136/bjsports-2018-100080.Haverfield MC, Giannitrapani K, Timko C, Lorenz K.
Patient-Centered Pain Management Communication from the Patient Perspective.
J Gen Intern Med. 2018;33(8):1374–1380.
doi: 10.1007/s11606-018-4490-y.Nijs J, Mairesse O, Neu D, et al.
Sleep Disturbances in Chronic Pain: Neurobiology,
Assessment, and Treatment in Physical Therapist Practice.
Phys Ther. 2018;98(5):325–335.
doi: 10.1093/ptj/pzy020.Smith BE, Hendrick P, Smith TO, et al.
Should exercises be painful in the management of chronic musculoskeletal
pain? A systematic review and meta-analysis.
Br J Sports Med. 2017;51(23):1679–1687.
doi: 10.1136/bjsports-2016-097383.Booth J, Moseley GL, Schiltenwolf M, Cashin A, Davies M, Hübscher M.
Exercise for chronic musculoskeletal pain:
A biopsychosocial approach.
Musculoskeletal Care. 2017;15(4):413–421.
doi: 10.1002/msc.1191.Ploutarchou G, Savvas C, Karagiannis C, et al.
The effectiveness of cognitive functional therapy on patients
with chronic neck pain: A systematic literature review.
J Bodyw Mov Ther. 2024;40:1394–1408.
doi: 10.1016/j.jbmt.2024.07.059.Kandeel M, Morsy MA, Khodair KMA, Alhojaily S.
Cognitive functional therapy for lower back pain: A meta-analytical assessment
of pain and disability outcomes in randomized controlled trials.
J Back Musculoskelet Rehabil.
doi: 10.3233/BMR-240230.
Published online September 3, 2024.O’Keeffe M, O’Sullivan P, Purtill H, Bargary N, O’Sullivan K.
Cognitive functional therapy compared with a group-based exercise
and education intervention for chronic low back pain:
a multicentre randomised controlled trial (RCT)
Br J Sports Med. 2020;54(13):782–789.
doi: 10.1136/bjsports-2019-100780.Ciolfi M.
Lifestyle Medicine Applications for the Chiropractic Profession.
J Contemp Chiropr. 2024;7(1):214–224.Gevers-Montoro C, Romero-Santiago B, Medina-García I, et al.
Reduction of Chronic Primary Low Back Pain by Spinal Manipulative
Therapy is Accompanied by Decreases in Segmental Mechanical
Hyperalgesia and Pain Catastrophizing: A Randomized
Placebo-controlled Dual-blind Mixed Experimental Trial.
J Pain. 2024;25(8):104500.
doi: 10.1016/j.jpain.2024.02.014.Randoll C, Gagnon-Normandin V, Tessier J, et al.
The mechanism of back pain relief by spinal manipulation
relies on decreased temporal summation of pain.
Neuroscience. 2017;349:220–228.
doi: 10.1016/j.neuroscience.2017.03.006.Haavik H, Kumari N, Holt K, et al.
The Contemporary Model of Vertebral Column Joint Dysfunction
and Impact of High-velocity, Low-amplitude Controlled
Vertebral Thrusts on Neuromuscular Function
European J Applied Physiology 2021 (Oct); 121 (10): 2675–2720Srbely J.
Chiropractic Science: A Contemporary Neurophysiologic Paradigm.
J Can Chiropr Assoc. 2010;54(3):144–146.Voogt L, de Vries J, Meeus M, Struyf F, Meuffels D, Nijs J.
Analgesic effects of manual therapy in patients
with musculoskeletal pain: a systematic review.
Man Ther. 2015;20(2):250–256.
doi: 10.1016/j.math.2014.09.001.Hohenschurz-Schmidt D, Thomson OP, Rossettini G, et al.
Avoiding nocebo and other undesirable effects in chiropractic,
osteopathy and physiotherapy: An invitation to reflect.
Musculoskelet Sci Pract. 2022;62:102677.
doi: 10.1016/j.msksp.2022.102677.Macfarlane GJ, Kronisch C, Dean LE, et al.
EULAR revised recommendations for the management of fibromyalgia.
Ann Rheum Dis. 2017;76(2):318–328.
doi: 10.1136/annrheumdis-2016-209724.Marcus NJ, Robbins L, Araki A, Gracely EJ, Theoharides TC.
Effective Doses of Low-Dose Naltrexone for Chronic Pain
- An Observational Study.
J Pain Res. 2024;17:1273–1284.
doi: 10.2147/JPR.S451183.Derry S, Wiffen PJ, Häuser W, et al.
Oral nonsteroidal anti-inflammatory drugs for fibromyalgia in adults.
Cochrane Database Syst Rev. 2017;3(3):CD012332.
doi: 10.1002/14651858.CD012332.pub2.Gliedt JA, Schneider MJ, Evans MW, King J, Eubanks JE.
The Biopsychosocial Model and Chiropractic:
A Commentary with Recommendations
for the Chiropractic Profession
Chiropractic & Manual Therapies 2017 (Jun 7); 25: 16
Return to LOW BACK PAIN
Return to CHRONIC NECK PAIN
Return to SPINAL PAIN MANAGEMENT
Since 10-20-2025


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |