

Guideline for Reporting Interventions on Spinal
Manipulative Therapy: Consensus on Interventions
Reporting Criteria List for Spinal Manipulative
Therapy (CIRCLe SMT)This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Manipulative Physiol Ther 2017 (Feb); 40 (2): 61–70 ~ FULL TEXT
OPEN ACCESS Ruud Groeneweg, MSc • Sidney M. Rubinstein, PhD • Rob A.B. Oostendorp, PhD
Raymond W.J.G. Ostelo, PhD • Maurits W. van Tulder, PhD
Department of Health Sciences & EMGO Institute
for Health and Care Research,
Faculty of Earth & Life Sciences,
VU University Amsterdam,
Amsterdam, The Netherlands
Objective: The aim of the Consensus on Interventions Reporting Criteria List for Spinal Manipulative Therapy (CIRCLe SMT) study was to develop a criteria list for reporting spinal manipulative therapy (SMT).
Methods: Methods: A Delphi procedure was conducted from September 2011 to April 2013 and consisted of international experts in the field of SMT. The authors formed a steering committee and invited participants, selected initial items, structured the comments of the participants after each Delphi round, and formulated the feedback. To ensure content validity, a large number of international experts from different SMT-related disciplines were invited to participate. A workshop was organized following the consensus phase, and it was used to discuss and refine the wording of the items.
Results: In total, 123 experts from 18 countries participated. These experts included clinicians (70%), researchers (93%), and academics working in the area of SMT (27%), as well as journal editors (14%). (Note: The total is more than 100% because most participants reported 2 jobs.) Three Delphi rounds were necessary to reach a consensus.
The criteria list comprised 24 items under 5 domains, including(1) rationale of the therapy,
(2) description of the intervention,
(3) SMT techniques,
(4) additional intervention/techniques, and
(5) quantitative data.Conclusions: A valid criteria list was constructed with the aim of promoting consistency in reporting SMT intervention in scientific publications.
Keywords: Manipulation, Spinal; Publications; Randomized Controlled Trials.
From the FULL TEXT Article:
Introduction
A randomized controlled trial (RCT) is considered the most robust design to study the effectiveness of treatments. Accurate reporting of RCTs is important for critical appraisal of study validity, adequate interpretation of the results, and for optimal implementation of the findings in clinical practice. In that regard, the CONsolidated Standard Of Reporting Trials Statement (CONSORT) was designed to improve the reporting of trials and has been modified for nonpharmacologic trials. [1]
Descriptions of the study design and method often constitute a substantial part of reports of RCTs, whereas the intervention is often described in a few sentences. [2, 3] Glasziou [4] indicated that many RCT reports often lack crucial details about the intervention. To best interpret the results of individual studies, it is important to have more detailed information about the “who, what, when, and where” of the intervention, especially given that interventions are typically tailored to meet individual needs. [4] Development of a criteria list for the reporting of interventions has been undertaken for a variety of conservative interventions, including acupuncture and homeopathy for musculoskeletal complaints. [5–7] Descriptions and classifications of mobilizations and/or manipulations have been published, [8, 9] but no item list for reporting spinal manipulative therapy (SMT) has yet been developed. Creating such a list seems essential given the fact that many different techniques are used in SMT. [10]
The potential benefits of good reporting are significant, [11] and this also applies to a detailed description of the intervention. For example, such reporting improves the completeness and transparency of the research reports, which enables a more accurate interpretation of the RCT. In addition, it allows clinicians and researchers to replicate the intervention. The specific characteristics of the application of SMT techniques are critical to adequate interpretation of the outcomes of RCTs and make them applicable to clinical practice. Therefore, CIRCLe SMT (Consensus on Interventions Reporting Criteria List for Spinal Manipulative Therapy) aims to develop a minimum set of items for the description of SMT in RCTs by obtaining consensus via a Delphi procedure among experts in the field of SMT.
Methods
The article “Guidance for Developers of Health Research Reporting Guidelines” was used for this project. [12] A Delphi process was used as the facilitation technique for reaching consensus. [13] This project was exempted from ethics review under Dutch law.
Steering Committee
In September 2011, the project team formed a steering committee that was responsible for the construction of the list of items, selection of participants, construction of the Delphi questionnaires, analysis of the responses of the participants, and handling the feedback from the participants after each round.
Phase ISelection of Items
Items to be included in the questionnaires were selected on the basis of articles on mobilization and manipulation techniques, [14–18] systematic reviews [10] and textbooks on SMT, [19–22 ] and other guidelines for description of interventions. [6–9, 23–25]
A scheme consisting of relevant domains that were thought to influence treatment outcome was established.
In a pilot study, participants with various clinical backgrounds were invited to evaluate these items and to formulate additional items to ensure that all potentially relevant items would be included in the initial draft of the criteria list to be used in the first Delphi round.
Selection of Participants
To ensure content validity, a large number of international experts from different disciplines were invited to participate, including authors of RCTs or systematic reviews in the field of SMT from the previous 5 years; participants of the International Forum XI on Low-Back Pain Research in Primary Care in Melbourne, Australia (2011); and clinical experts identified by the steering committee.
Phase II: Procedure Delphi Rounds
During the Delphi procedure, the project team used structured questions. Additionally, participants were invited to give comments on the suggested items and suggestions for additional items. Consensus was defined as 70% of the participants or more answering “yes” on an item.Round 1
First, demographics of the participants were ascertained (eg, type of profession), and questions about participation in (planning) RCTs or systematic reviews concerning SMT in the last 5 years were posed. For each item, the project team asked the participants if that item should be included in the final criteria list. In addition, the participants were asked whether manipulation and mobilization techniques should be described in the same terms or separately.
Round 2
On the basis of the results from Round 1, questions were rephrased and presented for the second round. To compile a minimum criteria list, the project team asked the participants to state whether they thought inclusion of an item was “absolutely required” or “desirable.” In addition, participants were asked to indicate whether a global description would suffice for an item or if a detailed description was necessary.
Workshop Meeting
Items that were identified from the second round were discussed during a workshop at the International Low-Back Pain Forum XII in Odense, Denmark, in 2012. Moreover, the wording was refined where necessary. Also, an example of good reporting of SMT was formulated by the participants.
Round 3
Based on the outcomes of Rounds 1 and 2, the steering committee formulated 3 possible choices to determine which items should be included in the final list:(1) because of the small number of items chosen (by consensus) as “absolutely required,” the first option was to use all of the original items from Round 1 and disregard the results of Round 2;
(2) include items that were considered important in Round 2 by at least 50% of the participants; or
(3) include items that were considered important in Round 2 by at least 70% of participants.A list with the 3 options was presented, and participants were asked to rank these options. The first choice (highest preference) was assigned 3 points, the second choice 2, and the third 1 point. The option with the highest score was used to compose the final criteria list.
Final Criteria List
On the basis of the Delphi procedure and workshop, the final criteria list was composed. Items were modified, if necessary, by the steering committee according to the input of the participants in the Delphi rounds and the comments received during the workshop.
Results
Participants

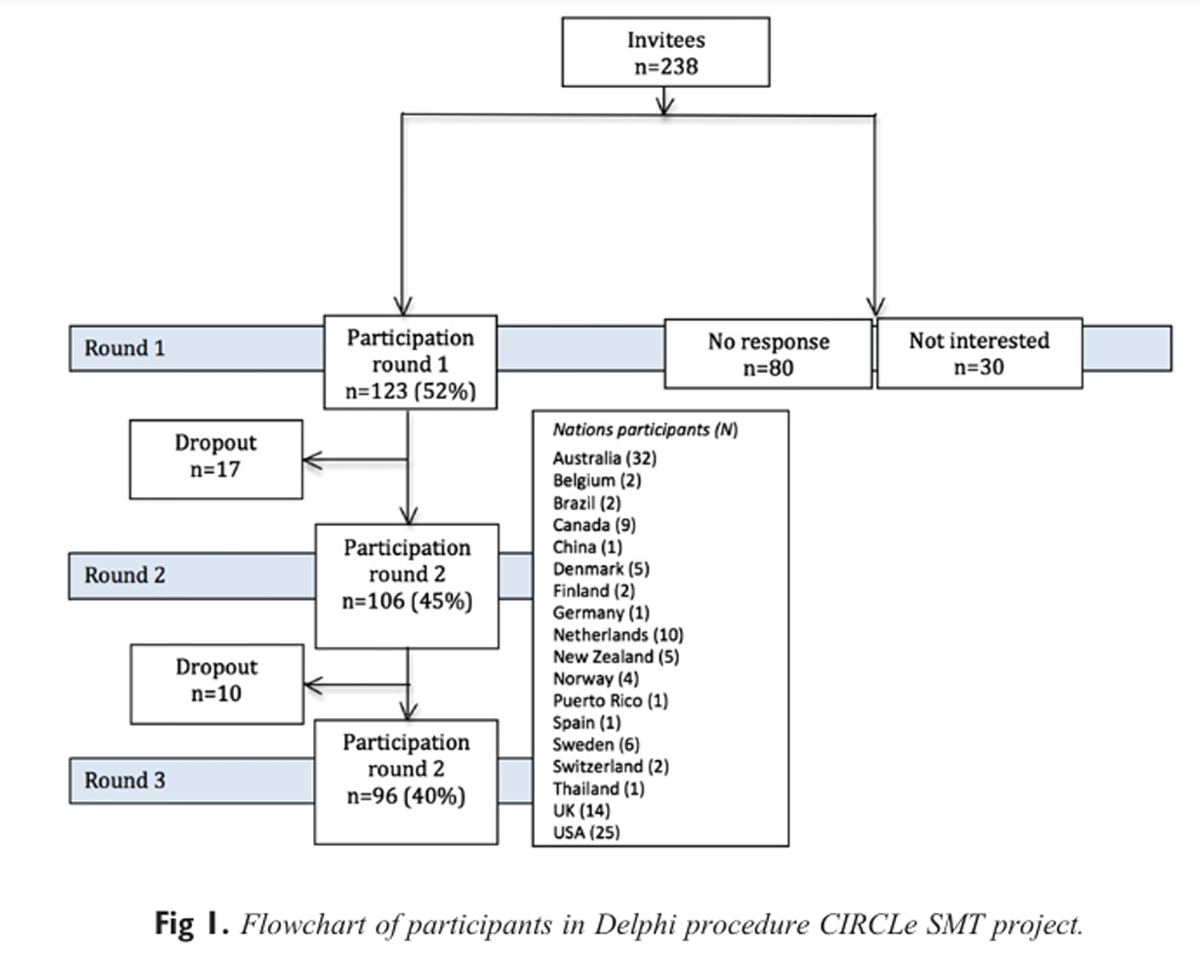
Figure 1 In total, 238 experts were invited via e-mail, and 123 (52%) completed the first round. Figure 1 depicts the flow of the study. The experts were from 18 countries (Figure 1) and included clinicians (70%), researchers in the field of musculoskeletal pain (93%), and academics working in the area of SMT (27%), as well as journal editors (14%). (Note: The total is more than 100% because most reported more than 1 job.) Among the clinicians, there were 42 manual therapists, 19 chiropractors, 6 osteopaths, and 18 with another profession. In total, 55% had participated in an RCT, with 41% of those in 3 or more trials, and 50% had participated in a systematic review, with 44% of those in 4 or more reviews.
Round 1
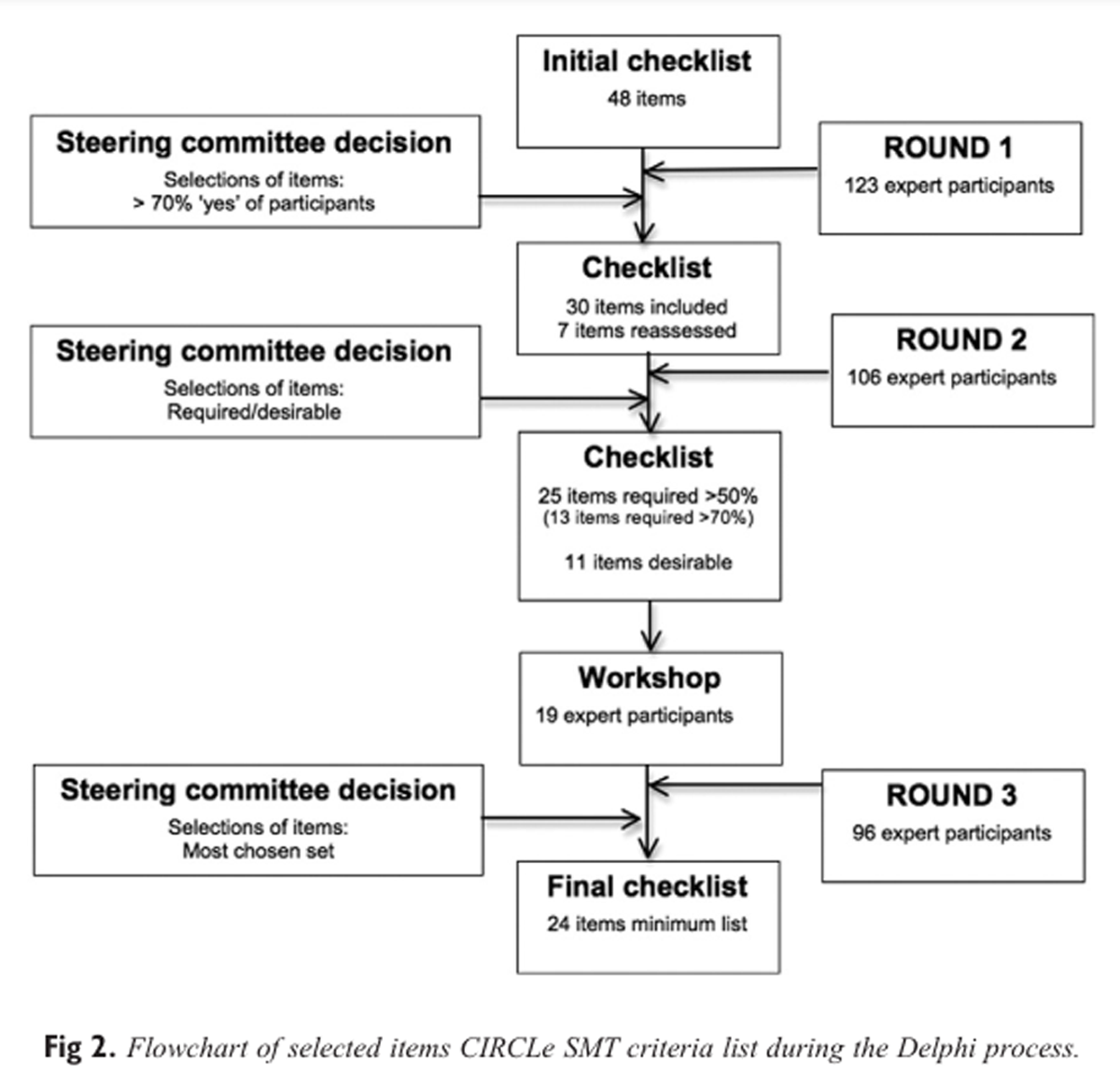
Figure 2 In total, 48 items in 6 domains were included in the first round (Figure 2). The majority of the participants (66%) stated that manipulation and mobilization techniques should be described separately. Eleven items were excluded from this round (Figure 2).
Round 2
Of those invited, 86% completed the second round. In the first round, 4 items were considered to reach consensus, namely, active movement tests, passive movement tests, pain sensitivity tests, and additional techniques/interventions. However, no consensus was reached as to whether their description should be global or detailed. Hence, these items were presented in the second round again. Round 3
Workshop Meeting
In total, 19 participants joined the workshop in Odense, Denmark. During the workshop, items from the second round were discussed and rephrased, if suggested by the participants. The included items were then processed in a sample text of reporting SMT.
Round 3
Of the participants, 78% completed Round 3. The results of this round were as follows: Option 1 (ie, all items from Round 1) received 198 points, option 2 (ie, 50% cutoff) had 215 points, and option 3 (70% cutoff) had 163 points. The items that were excluded by choosing option 2 were as follows:
Rationale of the therapy: (1) Qualification of practitioners; (2) Supposed theoretical and/or underlying mechanism of the therapy;
Description of the intervention: (3) Method for increasing adherence to the protocol;
Patient assessment: (4) Active movement tests; (5) Passive movement tests; (6) Pain sensitivity tests;
Description of SMT techniques: (7) Type of movement component of the technique (eg, traction, translation, angulation, spin); (8) Amplitude of the technique; (9) Target of force (the location of the intended force) of the technique; (10) Manually assisted procedures allowed (eg, table adjustments, instrument adjustments); and
Quantitative data: (11) Treatment duration per session (in minutes).
The procedure was completed in April 2013.
Final Version CIRCLe SMT Criteria List
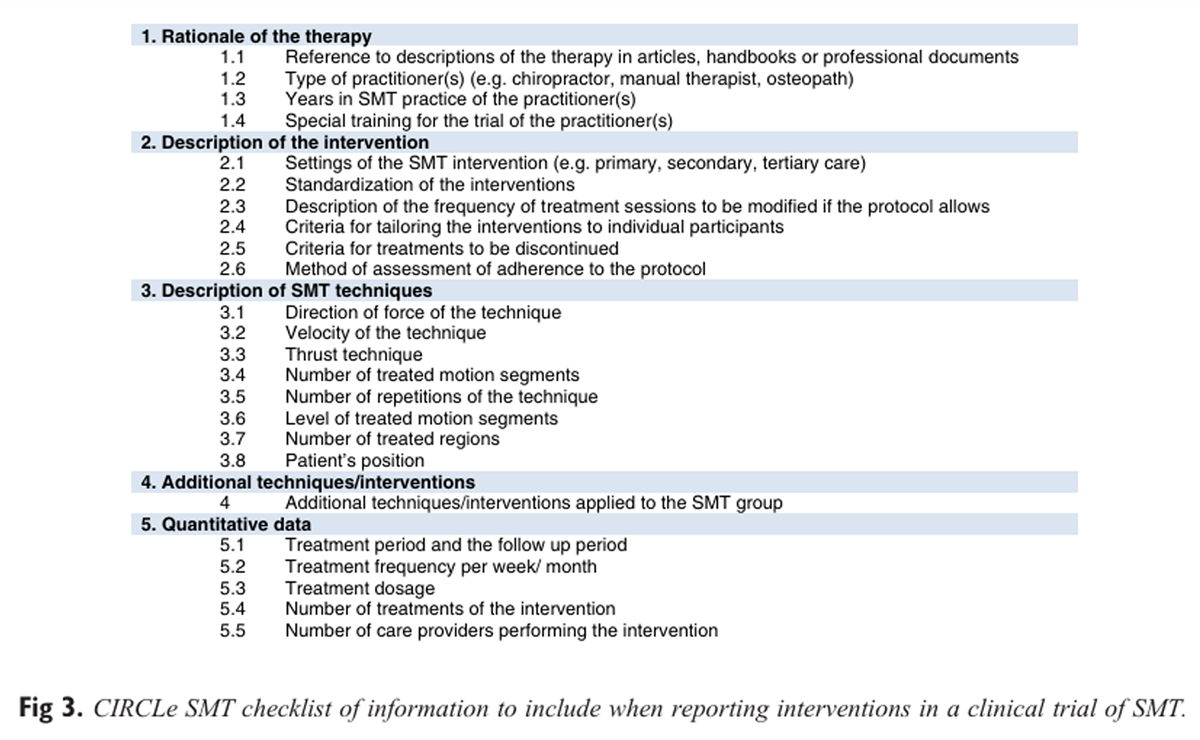
Figure 3 The 5 domains of the CIRCLe SMT list and the 24 items with the explanations on the need for adequate reporting and the operationalization of the items are presented. In Figure 3, the domains and the items are summarized.
Rationale of the TherapyReference to Descriptions of the Therapy in Articles, Handbooks, or Professional Documents
To be able to replicate the trial or implement the performed treatment in clinical practice, the fullest possible description of the underlying concepts that are the basis for the therapeutic constructs should be stated, including references to literature and to documents pertaining to the trial.
Type of Practitioner(s)
Spinal manipulative therapy is characterized by different conceptions and styles/types. The overall styles, types, or approach on which the treatment is based should be described to contextualize the trial within the range of current clinical practices.
Years in Practice
To clarify potential variations and expertise bias and to help improve the applicability of the results, it is informative to report the years of practice of the care providers involved in the trial.
Special Training of the Practitioner(s) for the Trial
It is necessary to describe whether participating care providers received any specific training for the trial.
Description of the InterventionSettings of the SMT Intervention (eg, Primary, Secondary, Tertiary Care)
To be clinically useful, the result should be relevant to a definable group of patients in a particular clinical setting (ie, they must be externally valid). There needs to be a description of where the treatment was delivered, even if in general terms (ie, in private practices or in a hospital setting).
Standardization of the Interventions
Spinal manipulative therapy could be defined as a complex intervention, as there are several interacting components both independently and interdependently. A highly specific protocol would provide a word-for-word, session-by-session script for the therapist to follow with every patient. A flexible treatment protocol would provide a general framework within which to operate but would not constrain the selection of specific activities or topics. [26] Both treatment protocols are possible; therefore, the treatment protocol should be adequately described.
Description of the Frequency of Treatment Sessions to Be Modified if the Protocol Allows
The planned number and frequency of treatments should be clearly documented. Deviations from the protocol during the study should be described in the Results section, including, where relevant, the reasons for this deviation.
Criteria for Tailoring the Interventions to Individual Patients
Describing the details of the process of tailoring the treatment for individual patients is necessary for enabling replication, whereby the algorithm might be done as a figure, flowchart, or a table rather than as text. It is important to use relevant standardized terminology (eg, items of our list) in the study protocol to facilitate documentation of the interventions and to determine the amount of individualization in the intervention.
Criteria for Treatments to Be Discontinued
Criteria for discontinuation of therapy should be preset. These criteria may depend on the symptoms of the patient or the complaint’s course.
Method of Assessment of Adherence to the Protocol
Assessing treatment adherence is essential to appraising the feasibility and reproducibility of the intervention in clinical practice. Therefore, the method of assessment of adherence to the protocol should be described.
Description of SMT TechniquesDirection of Force of the Technique
The direction in which the therapist applies the force should be described using standard anatomical and biomechanical terminology.
Velocity of the Technique
The velocity should be recorded in general terms (eg, slow or fast).
Thrust Technique (Name/System)
A description of whether or not a thrust technique is used should be given (including name or system), as well as the position in which the restricted barrier is provided.
Number of Treated Motion Segments/Joints
The number of treated motion segments of the spine should be described. If the number varies among patients, the mean and range should be reported.
Number of Repetitions of the Technique
The number of repetitions of each applied technique should be mentioned by mean and range.
Level of Treated Motion Segments/Joints
When the study is on a specific level (eg, C0–C1 or L5–S1), the level should be specified with the description of how the segments were localized.
Number of Treated Regions
It is suggested that the number of treated regions, at least the subregions (eg, upper, middle, and lower cervical regions), be described.
Patient's Position
A description of the position of the patient should be included (eg, supine, prone, side-lying). This includes any prepositioning of a region of the body, such as positioning the patient in rotation or side bending.
Additional Techniques/Interventions
A clear and detailed description of all additional components, whether carried out by the practitioner or the patient, should be reported so that the factors that might be responsible for any change observed are made known. Additional techniques and interventions refer to, for example, exercises, acupuncture, auxiliary techniques, prescribed self-treatment (including medication usage), and (lifestyle) advice provided by the practitioner. These additions should be described in detail so that readers are well informed about the package of treatment. If appropriate, guidelines or recommendations on reporting of interventions or techniques should be followed, such as for exercises [27] or acupuncture. [5]
Quantitative DataTreatment Period and the Follow-up Period
Both the treatment period and the follow-up period should be clearly described.
Treatment Frequency per Week or Month
The frequency of treatment should be accurately described, for example, if participants have been treated twice a week for the first 2 weeks and then once a week for an additional 4 weeks.
Treatment Dosage
Therapy dose can be described in terms of time spent in therapy or in terms of effort expended, or both. Description of time includes minutes per session, sessions per day or week, and number of days or weeks.
Number of Treatments of the Intervention
The mean and range should be reported if there is variation among the participants. The frequency and duration of sessions should be described, with mean and range if there are differences among the participants.
Number of Care Providers Performing the Intervention

Table 1
page 6To describe the potential clustering of practitioners and centers, which is essential for accurate statistical analyses, the number of care providers for each trial arm should be documented in detail.
An example of reporting SMT using all items of the criteria list is presented in Table 1.
Discussion
This is the first study to develop a criteria list for the reporting of SMT, which may to serve as an extension of the CONSORT Statement. A similar extension exists for nonpharmacologic interventions; however, a list specific to SMT is lacking. This is deemed important for a number of reasons, namely, SMT is not one entity but, rather, represents a broad description of an intervention. A better description of SMT will make it possible for clinicians to better interpret results and determine to what extent this may influence their clinical practice. Such a guideline also makes it possible to better interpret and compare results across trials. In that regard, our study represents an important step forward; that is, this work, which is based on a Delphi procedure including a large group of representatives throughout the field of SMT, defines a (minimal) criteria list for reporting trials of SMT. In the end, this encompasses 24 items clustered in 5 domains. This effort represents the first guideline for reporting items to be included in trials of SMT. Although designed for RCTs, this guideline can also be used for study designs.
Items of the Criteria List
Most of the participants were in favor of describing manipulation and mobilization separately. However, at the end of the Delphi procedure, they selected the same items for manipulation as for mobilization.
There was a preference for keeping track of subjective descriptions of items that can have a positive impact on the clinical practice. For example, the intentions of the practitioner should be recorded as well as the actual treatment applied. The description of SMT techniques is supposed to be in a language that is theoretically neutral. The description should only provide information about where and how the force is applied. [8]
Interestingly, no consensus was reached on diagnosis-related items, even though diagnosis is considered the keystone of therapeutic treatment. A potential explanation for this could be the low-to-moderate reliability of current manual diagnostic tests, [28–31] although clinicians feel confident in their conclusions drawn from them. [32, 33]
Recommendations
Authors of clinical trials in the field of manipulation-based therapy and mobilization-based therapy should use the recommendations presented in this report, together with the items from the CONSORT Statement, with the extension for nonpharmaceutical trials. Spinal manipulative therapy is, in general, a complex intervention with a large number of interacting components. For example, the biomechanical parameters in patients may change with the patients’ physical characteristics, clinical condition, and clinical progress. Thus, each related item on the list should be described in as detailed a manner as possible.
Journal endorsement of our criteria list is encouraged, as it has been shown that such lists help improve the quality and completeness of reporting in medical journals. [34, 35] The authors also encourage “umbrella” organizations (eg, the International Federation of Orthopaedic Manipulative Therapists, the European Chiropractic Union) and national associations related to SMT to endorse the criteria list to facilitate its implementation in clinical practice and educational programs.
The control intervention should be described in as much detail as the SMT intervention, if possible, following the Enhancing the QUAlity and Transparency Of health Research (EQUATOR) guidelines. [36]
Finally, the specified length of articles in scientific journals often do not allow for detailed descriptions of interventions. Therefore, it is recommended that additional information be made available by publishing a separate article, preferably a design article describing the complete protocol, including a detailed description of the intervention. Other opportunities would be using an online appendix or link to a website or a publicly available video.
Limitations and Strengths
As with any Delphi study, a limitation of our study is that the findings are based only on expert opinion. Although a large number of people participated, the idea that a consensus among a group of experts results in the correct and valid criteria list could be debated. The steering committee also may not have included all relevant experts. The invited participants have a strong focus on SMT research, although the majority of participants are also active in clinical practice. However, the target population (clinicians) may have been underrepresented.
It is important to emphasize that the aim of the CIRCLe SMT project was to reach consensus on a criteria list for the description of SMT, so not all participants involved to the Delphi process (fully) agree about the items in the final criteria list, but the criteria list is the result of a “communis opinio.”
The strength of the study is the participation of a large group of experts from various backgrounds, and thus the results are likely to be largely transferable. Importantly, we followed a standard procedure in accordance with the guidance for developers of health research reporting guidelines, so the methodologic quality should be viewed to be good. [12]
Conclusions
This is the first effort to develop a criteria list designed to improve the reporting of trials of SMT, and this list should be used in conjunction with the CONSORT Statement and its extension for nonpharmaceutical trials. This initiative represents an important step toward improving the quality of reporting SMT and will help clinicians to interpret the results of trials and effectively apply them to clinical practice.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research):
R.G., S.M.R., R.A.B.O., R.W.J.G.O., M.W.v.T.
Design (planned the methods to generate the results):
R.G., S.M.R., R.A.B.O., R.W.J.G.O., M.W.v.T.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript):
R.G., S.M.R., R.A.B.O., R.W.J.G.O., M.W.v.T.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data):
R.G., S.M.R., R.A.B.O., R.W.J.G.O., M.W.v.T.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results):
RG R.G., S.M.R., R.A.B.O., R.W.J.G.O., M.W.v.T.
Literature search (performed the literature search):
R.G., S.M.R., R.A.B.O., R.W.J.G.O., M.W.v.T.
Writing (responsible for writing a substantive part of the manuscript):
R.G., S.M.R., R.A.B.O., R.W.J.G.O., M.W.v.T.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking):
R.G., S.M.R., R.A.B.O., R.W.J.G.O., M.W.v.T.
References:
Boutron I Moher D Tugwell P et al. A checklist to evaluate a report of a nonpharmacological trial (CLEAR NPT) was developed using consensus. J Clin Epidemiol. 2005; 58: 1233-1240
Manual physical therapy in the Netherlands: reflecting on the past and planning for the future in an international perspective. J Man Manip Ther. 2007; 15: 133-141
Vincent K Maigne J-Y Fischhoff C Lanlo O Dagenais S Systematic review of manual therapies for nonspecific neck pain. Joint Bone Spine. 2013; 80: 508-515
Glasziou P Meats E Heneghan C Shepperd S What is missing from descriptions of treatment in trials and reviews?. BMJ. 2008; 336: 1472-1474
MacPherson H Altman DG Hammerschlag R et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. 2010; 7: e1000261
Dean ME Coulter MK Fisher P Jobst KA Walach H Reporting Data on Homeopathic Treatments (RedHot): a supplement to CONSORT. J Alternat Complement Med. 2007; 13: 19-24
Stock-SchrOer B Albrecht H Betti L et al. Reporting experiments in homeopathic basic research (REHBaR)—a detailed guideline for authors. Homeopathy. 2009; 98: 287-298
Barbaix E Hoe doseer ik mijn therapie?: het cockpitmodel. Stimulus. 2003; 22: 273-289
Mintken PE Derosa C Little T Smith B American Academy of Orthopaedic Manual Physical Therapists A model for standardizing manipulation terminology in physical therapy practice. J Man Manip Ther. 2008; 16: 50-56
Rubinstein SM van Middelkoop M Assendelft WJ de Boer MR van Tulder MW Spinal manipulative therapy for chronic low-back pain. Cochrane Database Syst Rev. 2011; 2: CD008112
Moher D Weeks L Ocampo M et al. Describing reporting guidelines for health research: a systematic review. J Clin Epidemiol. 2011; 64: 718-742
Moher D Schulz KF Simera I Altman DG Guidance for developers of health research reporting guidelines. PLoS Med. 2010; 7: e1000217
Hasson F Keeney S McKenna H Research guidelines for the Delphi survey technique. J Adv Nurs. 2000; 32: 1008-1015
Downie AS Vemulpad S Bull PW Quantifying the high-velocity, low-amplitude spinal manipulative thrust: a systematic review. J Manipulative Physiol Ther. 2010; 33: 542-553
Evans DW Lucas N What is manipulation? A reappraisal. Man Ther. 2010; 15: 286-291
Evans DW Why do spinal manipulation techniques take the form they do? Towards a general model of spinal manipulation. Man Ther. 2010; 15: 212-219
Herzog W
The Biomechanics of Spinal Manipulation
J Bodyw Mov Ther. 2010 (Jul); 14 (3): 280–286van de Veen EA de Vet HCW Pool JJM Schuller W de Zoete A Bouter LM Variance in manual treatment of nonspecific low back pain between orthomanual physicians, manual therapists, and chiropractors. J Manipulative Physiol Ther. 2005; 28: 108-116
Maitland GD English K Banks K Hengeveld E English K Maitland’s Vertebral Manipulation. 6th ed. Elsevier Health Sciences, St. Louis, MO2001
McKenzie R May S Cervical and Thoracic Spine: Mechanical Diagnosis and Therapy. Orthopedic Physical Therapy Products, Windham, NH2006
McKenzie RA May S The Lumbar Spine Mechanical Diagnosis & Therapy. Spinal Publications New Zealand, Waikanae, New Zealand2013
Kaltenborn FM Evjenth O Kaltenborn TB Manual Mobilization of the Joints, Volume II: The Spine. Orthopedic Physical Therapy Products, Windham, NH2009
MacPherson H Altman DG Hammerschlag R et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. J Alternat Complement Med. 2010; 16: ST1-ST14
Bossuyt PM Reitsma JB Bruns DE et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Fam Pract. 2004; 21: 4-10
Boutron I Moher D Altman DG Schulz KF Ravaud P; CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008; 148: 295-309
Hart T Treatment definition in complex rehabilitation interventions. Neuropsychol Rehabil. 2009; 19: 824-840
Slade SC Keating JL Exercise prescription: a case for standardised reporting. Br J Br J Sports Med. 2012; 46: 1110-1113
van Trijffel E Anderegg Q Bossuyt PMM Lucas C Inter-examiner reliability of passive assessment of intervertebral motion in the cervical and lumbar spine: a systematic review. Man Ther. 2005; 10: 256-269
Stochkendahl MJ Christensen HW Hartvigsen J et al. Manual examination of the spine: a systematic critical literature review of reproducibility. J Manipulative Physiol Ther. 2006; 29: 475-485
Haneline MT Young M A review of intraexaminer and interexaminer reliability of static spinal palpation: a literature synthesis. J Manipulative Physiol Ther. 2009; 32: 379-386
Smith J Bolton PS What are the clinical criteria justifying spinal manipulative therapy for neck pain?—a systematic review of randomized controlled trials. Pain Med. 2013; 14: 460-468
Trijffel EV Oostendorp RAB Lindeboom R Bossuyt PMM Lucas C Perceptions and use of passive intervertebral motion assessment of the spine. Man Ther. 2009; 14: 243-251
Abbott JH Flynn TW Fritz JM Hing WA Reid D Whitman JM Manual physical assessment of spinal segmental motion: intent and validity. Man Ther. 2009; 14: 36-44
Hopewell S Dutton S Yu L-M Chan A-W Altman DG The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in PubMed. BMJ. 2010; 340: c723
Turner L Shamseer L Altman DG et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst Rev. 2012; 11: MR000030
EQUATOR. (Available at:) http://www.equator-network.org

Return to GUIDELINES
Since 6-21-2024


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |