The Challenge of
Antibiotic ResistanceThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




Thanks to Scientific American (March 1998) for acces to this article!
Certain bacterial infections now defy all antibiotics. The resistance problem may be reversible, but only if society begins to consider how the drugs affect "good" bacteria as well as "bad"
by Stuart B. LevyLast year an event doctors had been fearing finally occurred. In three geographically separate patients, an often deadly bacterium, Staphylococcus aureus, responded poorly to a once reliable antidote--the antibiotic vancomycin. Fortunately, in those patients, the staph microbe remained susceptible to other drugs and was eradicated. But the appearance of S. aureus not readily cleared by vancomycin foreshadows trouble.
ANTIBIOTIC-RESISTANT BACTERIA
Worldwide, many strains of S. aureus are already resistant to all antibiotics except vancomycin. Emergence of forms lacking sensitivity to vancomycin signifies that variants untreatable by every known antibiotic are on their way. S. aureus, a major cause of hospital-acquired infections, has thus moved one step closer to becoming an unstoppable killer.
The looming threat of incurable S. aureus is just the latest twist in an international public health nightmare: increasing bacterial resistance to many antibiotics that once cured bacterial diseases readily. Ever since antibiotics became widely available in the 1940s, they have been hailed as miracle drugs--magic bullets able to eliminate bacteria without doing much harm to the cells of treated individuals. Yet with each passing decade, bacteria that defy not only single but multiple antibiotics--and therefore are extremely difficult to control--have become increasingly common.
What is more, strains of at least three bacterial species capable of causing life-threatening illnesses (Enterococcus faecalis, Mycobacterium tuberculosis and Pseudomonas aeruginosa) already evade every antibiotic in the clinician's armamentarium, a stockpile of more than 100 drugs. In part because of the rise in resistance to antibiotics, the death rates for some communicable diseases (such as tuberculosis) have started to rise again, after having declined in the industrial nations.
How did we end up in this worrisome, and worsening, situation? Several interacting processes are at fault. Analyses of them point to a number of actions that could help reverse the trend, if individuals, businesses and governments around the world can find the will to implement them.
One component of the solution is recognizing that bacteria are a natural, and needed, part of life. Bacteria, which are microscopic, single-cell entities, abound on inanimate surfaces and on parts of the body that make contact with the outer world, including the skin, the mucous membranes and the lining of the intestinal tract. Most live blamelessly. In fact, they often protect us from disease, because they compete with, and thus limit the proliferation of, pathogenic bacteria--the minority of species that can multiply aggressively (into the millions) and damage tissues or otherwise cause illness. The benign competitors can be important allies in the fight against antibiotic-resistant pathogens.
People should also realize that although antibiotics are needed to control bacterial infections, they can have broad, undesirable effects on microbial ecology. That is, they can produce long-lasting change in the kinds and proportions of bacteria--and the mix of antibiotic-resistant and antibiotic-susceptible types--not only in the treated individual but also in the environment and society at large. The compounds should thus be used only when they are truly needed, and they should not be administered for viral infections, over which they have no power.
A Bad Combination
Although many factors can influence whether bacteria in a person or in a community will become insensitive to an antibiotic, the two main forces are the prevalence of resistance genes (which give rise to proteins that shield bacteria from an antibiotic's effects) and the extent of antibiotic use. If the collective bacterial flora in a community have no genes conferring resistance to a given antibiotic, the antibiotic will successfully eliminate infection caused by any of the bacterial species in the collection. On the other hand, if the flora possess resistance genes and the community uses the drug persistently, bacteria able to defy eradication by the compound will emerge and multiply.
Antibiotic-resistant pathogens are not more virulent than susceptible ones: the same numbers of resistant and susceptible bacterial cells are required to produce disease. But the resistant forms are harder to destroy. Those that are slightly insensitive to an antibiotic can often be eliminated by using more of the drug; those that are highly resistant require other therapies.
To understand how resistance genes enable bacteria to survive an attack by an antibiotic, it helps to know exactly what antibiotics are and how they harm bacteria. Strictly speaking, the compounds are defined as natural substances (made by living organisms) that inhibit the growth, or proliferation, of bacteria or kill them directly. In practice, though, most commercial antibiotics have been chemically altered in the laboratory to improve their potency or to increase the range of species they affect. Here I will also use the term to encompass completely synthetic medicines, such as quinolones and sulfonamides, which technically fit under the broader rubric of antimicrobials.
PICKING UP RESISTANCE GENES
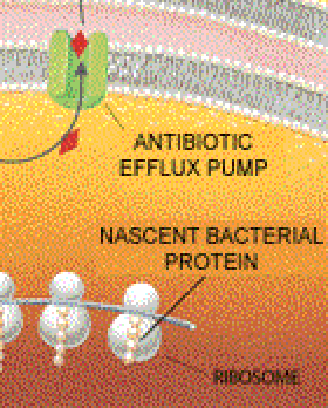
Whatever their monikers, antibiotics, by inhibiting bacterial growth, give a host's immune defenses a chance to outflank the bugs that remain. The drugs typically retard bacterial proliferation by entering the microbes and interfering with the production of components needed to form new bacterial cells. For instance, the antibiotic tetracycline binds to ribosomes (internal structures that make new proteins) and, in so doing, impairs protein manufacture; penicillin and vancomycin impede proper synthesis of the bacterial cell wall.
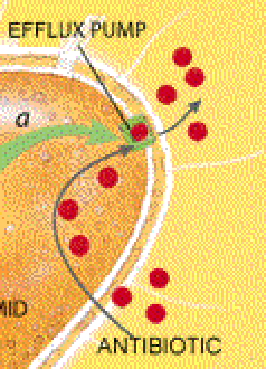
Certain resistance genes ward off destruction by giving rise to enzymes that degrade antibiotics or that chemically modify, and so inactivate, the drugs. Alternatively, some resistance genes cause bacteria to alter or replace molecules that are normally bound by an antibiotic--changes that essentially eliminate the drug's targets in bacterial cells. Bacteria might also eliminate entry ports for the drugs or, more effectively, may manufacture pumps that export antibiotics before the medicines have a chance to find their intracellular targets.
My Resistance Is Your Resistance
Bacteria can acquire resistance genes through a few routes. Many inherit the genes from their forerunners. Other times, genetic mutations, which occur readily in bacteria, will spontaneously produce a new resistance trait or will strengthen an existing one. And frequently, bacteria will gain a defense against an antibiotic by taking up resistance genes from other bacterial cells in the vicinity. Indeed, the exchange of genes is so pervasive that the entire bacterial world can be thought of as one huge multicellular organism in which the cells interchange their genes with ease.
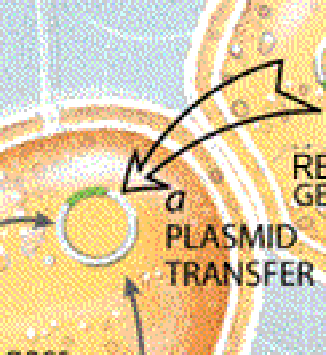
Bacteria have evolved several ways to share their resistance traits with one another [see "Bacterial Gene Swapping in Nature," by Robert V. Miller; Scientific American, January]. Resistance genes commonly are carried on plasmids, tiny loops of DNA that can help bacteria survive various hazards in the environment. But the genes may also occur on the bacterial chromosome, the larger DNA molecule that stores the genes needed for the reproduction and routine maintenance of a bacterial cell.
Often one bacterium will pass resistance traits to others by giving them a useful plasmid. Resistance genes can also be transferred by viruses that occasionally extract a gene from one bacterial cell and inject it into a different one. In addition, after a bacterium dies and releases its contents into the environment, another will occasionally take up a liberated gene for itself.
In the last two situations, the gene will survive and provide protection from an antibiotic only if integrated stably into a plasmid or chromosome. Such integration occurs frequently, though, because resistance genes are often embedded in small units of DNA, called transposons, that readily hop into other DNA molecules. In a regrettable twist of fate for human beings, many bacteria play host to specialized transposons, termed integrons, that are like flypaper in their propensity for capturing new genes. These integrons can consist of several different resistance genes, which are passed to other bacteria as whole regiments of antibiotic-defying guerrillas.
Many bacteria possessed resistance genes even before commercial antibiotics came into use. Scientists do not know exactly why these genes evolved and were maintained. A logical argument holds that natural antibiotics were initially elaborated as the result of chance genetic mutations. Then the compounds, which turned out to eliminate competitors, enabled the manufacturers to survive and proliferate--if they were also lucky enough to possess genes that protected them from their own chemical weapons. Later, these protective genes found their way into other species, some of which were pathogenic.
Regardless of how bacteria acquire resistance genes today, commercial antibiotics can select for--promote the survival and propagation of--antibiotic-resistant strains. In other words, by encouraging the growth of resistant pathogens, an antibiotic can actually contribute to its own undoing.
How Antibiotics Promote Resistance
The selection process is fairly straightforward. When an antibiotic attacks a group of bacteria, cells that are highly susceptible to the medicine will die. But cells that have some resistance from the start, or that acquire it later (through mutation or gene exchange), may survive, especially if too little drug is given to overwhelm the cells that are present. Those cells, facing reduced competition from susceptible bacteria, will then go on to proliferate. When confronted with an antibiotic, the most resistant cells in a group will inevitably outcompete all others.
RESISTANT POPULATION
Promoting resistance in known pathogens is not the only self-defeating activity of antibiotics. When the medicines attack disease-causing bacteria, they also affect benign bacteria -- innocent bystanders -- in their path. They eliminate drug-susceptible bystanders that could otherwise limit the expansion of pathogens, and they simultaneously encourage the growth of resistant bystanders. Propagation of these resistant, nonpathogenic bacteria increases the reservoir of resistance traits in the bacterial population as a whole and raises the odds that such traits will spread to pathogens. In addition, sometimes the growing populations of bystanders themselves become agents of disease.
Widespread use of cephalosporin antibiotics, for example, has promoted the proliferation of the once benign intestinal bacterium E. faecalis, which is naturally resistant to those drugs. In most people, the immune system is able to check the growth of even multidrug-resistant E. faecalis, so that it does not produce illness. But in hospitalized patients with compromised immunity, the enterococcus can spread to the heart valves and other organs and establish deadly systemic disease.
Moreover, administration of vancomycin over the years has turned E. faecalis into a dangerous reservoir of vancomycin-resistance traits. Recall that some strains of the pathogen S. aureus are multidrug-resistant and are responsive only to vancomycin. Because vancomycin-resistant E. faecalis has become quite common, public health experts fear that it will soon deliver strong vancomycin resistance to those S. aureus strains, making them incurable.
The bystander effect has also enabled multidrug-resistant strains of Acinetobacter and Xanthomonas to emerge and become agents of potentially fatal blood-borne infections in hospitalized patients. These formerly innocuous microbes were virtually unheard of just five years ago.
As I noted earlier, antibiotics affect the mix of resistant and nonresistant bacteria both in the individual being treated and in the environment. When resistant bacteria arise in treated individuals, these microbes, like other bacteria, spread readily to the surrounds and to new hosts. Investigators have shown that when one member of a household chronically takes an antibiotic to treat acne, the concentration of antibiotic-resistant bacteria on the skin of family members rises. Similarly, heavy use of antibiotics in such settings as hospitals, day care centers and farms (where the drugs are often given to livestock for nonmedicinal purposes) increases the levels of resistant bacteria in people and other organisms who are not being treated--including in individuals who live near those epicenters of high consumption or who pass through the centers.
Given that antibiotics and other antimicrobials, such as fungicides, affect the kinds of bacteria in the environment and people around the individual being treated, I often refer to these substances as societal drugs -- the only class of therapeutics that can be so designated. Anticancer drugs, in contrast, affect only the person taking the medicines.
On a larger scale, antibiotic resistance that emerges in one place can often spread far and wide. The ever increasing volume of international travel has hastened transfer to the U.S. of multidrug-resistant tuberculosis from other countries. And investigators have documented the migration of a strain of multidrug-resistant Streptococcus pneumoniae from Spain to the U.K., the U.S., South Africa and elsewhere. This bacterium, also known as the pneumococcus, is a cause of pneumonia and meningitis, among other diseases.
Antibiotic Use Is Out of Control
For those who understand that antibiotic delivery selects for resistance, it is not surprising that the international community currently faces a major public health crisis. Antibiotic use (and misuse) has soared since the first commercial versions were introduced and now includes many nonmedicinal applications. In 1954 two million pounds were produced in the U.S.; today the figure exceeds 50 million pounds.
Human treatment accounts for roughly half the antibiotics consumed every year in the U.S. Perhaps only half that use is appropriate, meant to cure bacterial infections and administered correctly--in ways that do not strongly encourage resistance.
Notably, many physicians acquiesce to misguided patients who demand antibiotics to treat colds and other viral infections that cannot be cured by the drugs. Researchers at the Centers for Disease Control and Prevention have estimated that some 50 million of the 150 million outpatient prescriptions for antibiotics every year are unneeded. At a seminar I conducted, more than 80 percent of the physicians present admitted to having written antibiotic prescriptions on demand against their better judgment.
In the industrial world, most antibiotics are available only by prescription, but this restriction does not ensure proper use. People often fail to finish the full course of treatment. Patients then stockpile the leftover doses and medicate themselves, or their family and friends, in less than therapeutic amounts. In both circumstances, the improper dosing will fail to eliminate the disease agent completely and will, furthermore, encourage growth of the most resistant strains, which may later produce hard-to-treat disorders. In the developing world, antibiotic use is even less controlled. Many of the same drugs marketed in the industrial nations are available over the counter. Unfortunately, when resistance becomes a clinical problem, those countries, which often do not have access to expensive drugs, may have no substitutes available.
A PHARMACEUTICAL STRATEGY
The same drugs prescribed for human therapy are widely exploited in animal husbandry and agriculture. More than 40 percent of the antibiotics manufactured in the U.S. are given to animals. Some of that amount goes to treating or preventing infection, but the lion's share is mixed into feed to promote growth. In this last application, amounts too small to combat infection are delivered for weeks or months at a time. No one is entirely sure how the drugs support growth. Clearly, though, this long-term exposure to low doses is the perfect formula for selecting increasing numbers of resistant bacteria in the treated animals--which may then pass the microbes to caretakers and, more broadly, to people who prepare and consume undercooked meat.
In agriculture, antibiotics are applied as aerosols to acres of fruit trees, for controlling or preventing bacterial infections. High concentrations may kill all the bacteria on the trees at the time of spraying, but lingering antibiotic residues can encourage the growth of resistant bacteria that later colonize the fruit during processing and shipping. The aerosols also hit more than the targeted trees. They can be carried considerable distances to other trees and food plants, where they are too dilute to eliminate full-blown infections but are still capable of killing off sensitive bacteria and thus giving the edge to resistant versions. Here, again, resistant bacteria can make their way into people through the food chain, finding a home in the intestinal tract after the produce is eaten.
The amount of resistant bacteria people acquire from food apparently is not trivial. Denis E. Corpet of the National Institute for Agricultural Research in Toulouse, France, showed that when human volunteers went on a diet consisting only of bacteria-free foods, the number of resistant bacteria in their feces decreased 1,000-fold. This finding suggests that we deliver a supply of resistant strains to our intestinal tract whenever we eat raw or undercooked items. These bacteria usually are not harmful, but they could be if by chance a disease-causing type contaminated the food.
The extensive worldwide exploitation of antibiotics in medicine, animal care and agriculture constantly selects for strains of bacteria that are resistant to the drugs. Must all antibiotic use be halted to stem the rise of intractable bacteria? Certainly not. But if the drugs are to retain their power over pathogens, they have to be used more responsibly. Society can accept some increase in the fraction of resistant bacteria when a disease needs to be treated; the rise is unacceptable when antibiotic use is not essential.
Reversing Resistance
A number of corrective measures can be taken right now. As a start, farmers should be helped to find inexpensive alternatives for encouraging animal growth and protecting fruit trees. Improved hygiene, for instance, could go a long way to enhancing livestock development.
The public can wash raw fruit and vegetables thoroughly to clear off both resistant bacteria and possible antibiotic residues. When they receive prescriptions for antibiotics, they should complete the full course of therapy (to ensure that all the pathogenic bacteria die) and should not "save" any pills for later use. Consumers also should refrain from demanding antibiotics for colds and other viral infections and might consider seeking nonantibiotic therapies for minor conditions, such as certain cases of acne. They can continue to put antibiotic ointments on small cuts, but they should think twice about routinely using hand lotions and a proliferation of other products now imbued with antibacterial agents. New laboratory findings indicate that certain of the bacteria-fighting chemicals being incorporated into consumer products can select for bacteria resistant both to the antibacterial preparations and to antibiotic drugs.
Physicians, for their part, can take some immediate steps to minimize any resistance ensuing from required uses of antibiotics. When possible, they should try to identify the causative pathogen before beginning therapy, so they can prescribe an antibiotic targeted specifically to that microbe instead of having to choose a broad-spectrum product. Washing hands after seeing each patient is a major and obvious, but too often overlooked, precaution.
To avoid spreading multidrug-resistant infections between hospitalized patients, hospitals place the affected patients in separate rooms, where they are seen by gloved and gowned health workers and visitors. This practice should continue.
Having new antibiotics could provide more options for treatment. In the 1980s pharmaceutical manufacturers, thinking infectious diseases were essentially conquered, cut back severely on searching for additional antibiotics. At the time, if one drug failed, another in the arsenal would usually work (at least in the industrial nations, where supplies are plentiful). Now that this happy state of affairs is coming to an end, researchers are searching for novel antibiotics again. Regrettably, though, few drugs are likely to pass soon all technical and regulatory hurdles needed to reach the market. Furthermore, those that are close to being ready are structurally similar to existing antibiotics; they could easily encounter bacteria that already have defenses against them.
With such concerns in mind, scientists are also working on strategies that will give new life to existing antibiotics. Many bacteria evade penicillin and its relatives by switching on an enzyme, penicillinase, that degrades those compounds. An antidote already on pharmacy shelves contains an inhibitor of penicillinase; it prevents the breakdown of penicillin and so frees the antibiotic to work normally. In one of the strategies under study, my laboratory at Tufts University is developing a compound to jam a microbial pump that ejects tetracycline from bacteria; with the pump inactivated, tetracycline can penetrate bacterial cells effectively.
Considering the Environmental Impact
As exciting as the pharmaceutical research is, overall reversal of the bacterial resistance problem will require public health officials, physicians, farmers and others to think about the effects of antibiotics in new ways. Each time an antibiotic is delivered, the fraction of resistant bacteria in the treated individual and, potentially, in others, increases. These resistant strains endure for some time -- often for weeks -- after the drug is removed.
The main way resistant strains disappear is by squaring off with susceptible versions that persist in--or enter--a treated person after antibiotic use has stopped. In the absence of antibiotics, susceptible strains have a slight survival advantage, because the resistant bacteria have to divert some of their valuable energy from reproduction to maintaining antibiotic-fighting traits. Ultimately, the susceptible microbes will win out, if they are available in the first place and are not hit by more of the drug before they can prevail.
Correcting a resistance problem, then, requires both improved management of antibiotic use and restoration of the environmental bacteria susceptible to these drugs. If all reservoirs of susceptible bacteria were eliminated, resistant forms would face no competition for survival and would persist indefinitely.
In the ideal world, public health officials would know the extent of antibiotic resistance in both the infectious and benign bacteria in a community. To treat a specific pathogen, physicians would favor an antibiotic most likely to encounter little resistance from any bacteria in the community. And they would deliver enough antibiotic to clear the infection completely but would not prolong therapy so much as to destroy all susceptible bystanders in the body.
Prescribers would also take into account the number of other individuals in the setting who are being treated with the same antibiotic. If many patients in a hospital ward were being given a particular antibiotic, this high density of use would strongly select for bacterial strains unsubmissive to that drug and would eliminate susceptible strains. The ecological effect on the ward would be broader than if the total amount of the antibiotic were divided among just a few people. If physicians considered the effects beyond their individual patients, they might decide to prescribe different antibiotics for different patients, or in different wards, thereby minimizing the selective force for resistance to a single medication.
Put another way, prescribers and public health officials might envision an "antibiotic threshold": a level of antibiotic usage able to correct the infections within a hospital or community but still falling below a threshold level that would strongly encourage propagation of resistant strains or would eliminate large numbers of competing, susceptible microbes. Keeping treatment levels below the threshold would ensure that the original microbial flora in a person or a community could be restored rapidly by susceptible bacteria in the vicinity after treatment ceased.
The problem, of course, is that no one yet knows how to determine where that threshold lies, and most hospitals and communities lack detailed data on the nature of their microbial populations. Yet with some dedicated work, researchers should be able to obtain both kinds of information.
Control of antibiotic resistance on a wider, international scale will require cooperation among countries around the globe and concerted efforts to educate the world's populations about drug resistance and the impact of improper antibiotic use. As a step in this direction, various groups are now attempting to track the emergence of resistant bacterial strains. For example, an international organization, the Alliance for the Prudent Use of Antibiotics (P.O. Box 1372, Boston, MA 02117), has been monitoring the worldwide emergence of such strains since 1981. The group shares information with members in more than 90 countries. It also produces educational brochures for the public and for health professionals.
The time has come for global society to accept bacteria as normal, generally beneficial components of the world and not try to eliminate them -- except when they give rise to disease. Reversal of resistance requires a new awareness of the broad consequences of antibiotic use -- a perspective that concerns itself not only with curing bacterial disease at the moment but also with preserving microbial communities in the long run, so that bacteria susceptible to antibiotics will always be there to outcompete resistant strains. Similar enlightenment should influence the use of drugs to combat parasites, fungi and viruses. Now that consumption of those medicines has begun to rise dramatically, troubling resistance to these other microorganisms has begun to climb as well.
Further Reading
THE ANTIBIOTIC PARADOX: HOW MIRACLE DRUGS ARE DESTROYING THE MIRACLE. S. B. Levy. Plenum Publishers, 1992.
DRUG RESISTANCE: THE NEW APOCALYPSE. Special issue of Trends in Microbiology, Vol. 2, No. 10, pages 341-425; October 1, 1994.
ANTIBIOTIC RESISTANCE: ORIGINS, EVOLUTION, SELECTION AND SPREAD. Edited by D. J. Chadwick and J. Goode. John Wiley & Sons, 1997.
The Author
STUART B. LEVY is professor of molecular biology and microbiology, professor of medcine and director of the Center for Adaptation Genetics and Drug Resistance at the Tufts University School of Medicine. He is also president of the Alliance for the Prudent Use of Antibiotics and president-elect of the American Society for Microbiology.
Contact : Washington State Department of Health/ Romesh Gautom, Public Health Laboratory, 206/361-2885
Matt Ashworth, Communication Office, 360/753-3237
http://epsilon.doh.wa.gov/
Invitational EU Conference
"The Microbial Threat"
Health of the Population : Strategies to prevent and control the emergence and spread of antimicrobial-resistant microorganisms.
Copenhagen, 9-10 September 1998.
(Workshops, 7-8 September 1998).

Return to ANTIBIOTIC ABUSE
Return to IATROGENIC INJURY
Return to ChiroZINE ARCHIVES


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |