

Deconstructing Chronic Low Back Pain in the Older Adult -
Step by Step Evidence and Expert-Based Recommendations
for Evaluation and Treatment.
Part VI: Lumbar Spinal StenosisThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Pain Medicine 2016 (Mar); 17 (3): 501–510 ~ FULL TEXT
OPEN ACCESS Julie M. Fritz PT, PhD, Sean D. Rundell PT, DPT, PhD, Paul Dougherty DC,
Angela Gentili MD, Gary Kochersberger MD, Natalia E. Morone MD, MS,
Srinivasa Naga Raja MD, Eric Rodriguez MD, Michelle I. Rossi MD, MPH,
Joseph Shega MD, Gwendolyn Sowa MD, PhD, Debra K. Weiner MD
Department of Physical Therapy and College of Health,
University of Utah,
Salt Lake City, Utah.OBJECTIVE: To present the sixth in a series of articles designed to deconstruct chronic low back pain (CLBP) in older adults. This article focuses on the evaluation and management of lumbar spinal stenosis (LSS), the most common condition for which older adults undergo spinal surgery.
METHODS: The evaluation and treatment algorithm, a table articulating the rationale for the individual algorithm components, and stepped-care drug recommendations were developed using a modified Delphi approach. The Principal Investigator, a five-member content expert panel and a nine-member primary care panel were involved in the iterative development of these materials. The illustrative clinical case was taken from the clinical practice of a contributor's colleague (SR).
RESULTS: We present an algorithm and supportive materials to help guide the care of older adults with LSS, a condition that occurs not uncommonly in those with CLBP. The case illustrates the importance of function-focused management and a rational approach to conservative care.
CONCLUSIONS: Lumbar spinal stenosis exists not uncommonly in older adults with CLBP and management often can be accomplished without surgery. Treatment should address all conditions in addition to LSS contributing to pain and disability.
KEYWORDS: Aged; Assessment; Chronic Low Back Pain; Chronic Pain; Elderly; Low Back Pain; Lumbar Spinal Stenosis; Primary Care; Spinal Stenosis
From the FULL TEXT Article:
Introduction
Lumbar spinal stenosis (LSS) is a common source of pain and diminished function among older adults with chronic low back pain (CLBP). Lumbar spinal stenosis results from narrowing of the lumbar spinal canal, and/or intervertebral foramina most often resulting from degenerative changes in the spine including facet joint arthrosis, loss of intervertebral disk height, degenerative spondylolisthesis, ligament thickening, post-surgical fibrosis, etc. [1] The prevalence of LSS based on imaging criteria is estimated to be almost 50% in individuals over age 60, but many older adults with imaging evidence of anatomical stenosis are asymptomatic. [2] Lumbar spinal stenosis is the most common indication for spinal surgery among Medicare recipients, [3, 4] occurring at a rate of 135.5 surgeries per 100,000 Medicare beneficiaries in 2007. [5]
Symptomatic LSS is often characterized by neurogenic claudication which is defined as symptoms of pain, weakness and/or numbness emanating from the spine and radiating into one or both buttock, thigh, or lower leg. [6] It is theorized that since extension of the spine and weight-bearing forces cause greater narrowing of the spinal canals [7, 8] that the symptoms of LSS are exacerbated by standing, walking and bending backwards and relieved by sitting, lying or forward flexion movements. Other common clinical findings can include a wide-based gait, positive Romberg sign, and sensory or motor deficits in one or both lower extremities. [9] Because these symptoms are frequently present in other conditions common among older adults (i.e., hip osteoarthritis, vascular claudication, etc.), careful differential diagnosis is important. [10] Lumbar spinal stenosis can co-occur with these and other chronic conditions and thus may be an important contributor to a syndrome of functional compromise in older adults. [11]
Despite the prevalence of LSS, there remains a good deal of uncertainty and variability in clinical management of the condition. The natural history of LSS is not well-understood, but it appears that many symptomatic individuals remain stable or improve over time [12] and those with asymptomatic LSS often remain free from symptoms for many years. [13] Except in rare instances of progressive neurologic deficits or cauda equina involvement, a period of non-operative management is generally advocated as an initial strategy. [14, 15] Various non-surgical approaches have been recommended including watchful waiting, medications, physical therapy using a variety of interventions, and epidural steroid injections [16, 17]; however there is little evidence to inform the selection or sequencing of these options. [18] An increasing number of individuals with LSS receive surgery, particularly complex fusion procedures. [4, 5, 19, 20] More than 37,000 surgical procedures for LSS were performed in 2007 among Medicare recipients at a total cost of $1.65 billion. [5] Despite this level of utilization, a lack of consensus regarding appropriate indications for surgery is evidenced by high rates of geographic variation in LSS surgical procedures. [21]
The number of older adults living with degenerative LSS will continue to increase given the aging of the population. With the desire of older adults to remain active and independent, there will be an increasing need for effective management strategies to mitigate the pain and resulting disablement that can occur with LSS. We present a case of an older adult with chronic low back pain related to LSS. The case highlights the pragmatic application of an algorithm developed to guide the diagnostic and treatment processes for older adults with LSS.
Methods
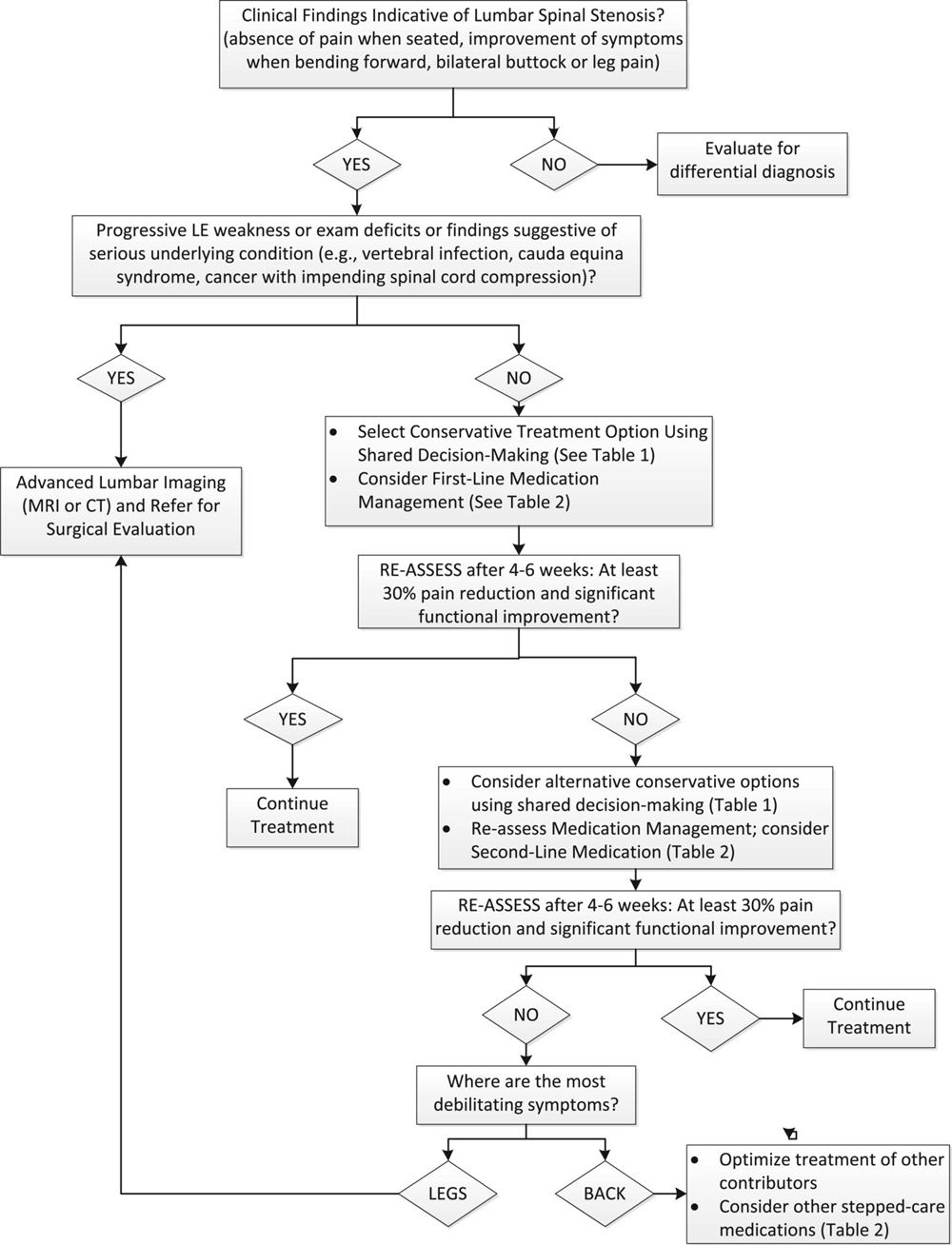
Figure 1

Table 1 A modified Delphi technique involving a content expert panel and a primary care review panel, as described in the series overview [23] was used to create the LSS algorithm (Figure 1), the table providing the rationale for the various components of the algorithm (Table 1), and the stepped-care medication table (Table 2). Expertise represented among the 5 Delphi expert panel members for the LSS algorithm included geriatric medicine, physical therapy, physiatry, pain medicine and chiropractic.
Table 2. Lumbar spinal stenosis:
Theoretical and pragmatic underpinnings of algorithm recommendations
Drug Dose/titration Important adverse events/precautions First-line medication Acetaminophen 325-1000 mg q4-6h while awake, max 3000 mg/d Adjust dosing interval for renal function: CRcl 10-50: q6 hrs; CRcl < 10: q8h Ask about all OTCs with acetaminophen; increased toxicity from chronic use if heavy EtOH use, malnourishment, pre-existing liver disease–decrease maximum daily dose to 2 gm. Salsalate choline
magnesium trisalicylate500-750 mg twice daily; maximum dose 3000 mg/day 750 mg three times daily Does not interfere with platelet function; GI bleeding and nephrotoxicity rare; salicylate concentrations can be monitored if toxicity suspected. Providers should educate patients about symptoms associated with salicylism (e.g, nausea, vomiting, tinnitus, vertigo, reversible hearing loss, etc.). Second-line medication Gabapentin 100 mg tid; consider 300 mg qhs if there is difficulty sleeping associated with pain May cause dizziness and increase fall risk. May cause sedation and worsen peripheral edema. Withdrawal seizures possible with abrupt withdrawal from high doses. Tramadol 25 mg once a day; increase by 25-50 mg daily in divided doses every 3-7 days as tolerated to max dose of 100 mg 4 times a day. Renal dosing (CRcl < 30 ml/min) 100 mg twice a day. Seizures and orthostatic hypotension. Other side effects similar to traditional opioids including constipation, sedation, confusion, respiratory depression. Potential for serotonin syndrome if patient is on other serotonergics. Hydrocodone/acetaminophen 2.5/325 or 5/325-10/325 mg q4-6h; max acetaminophen dose 3gm/d For all opioids, increased fall risk in patients with dysmobility. May worsen or precipitate urinary retention when BPH present. Increased risk of delirium in those with dementia.
Because of increased opioid sensitivity, older adults are at greater risk for sedation, nausea, vomiting, constipation, respiratory depression, urinary retention and cognitive impairment.
Start stimulant laxative to prevent/treat constipation. Many would start at opioid initiation if patient has existing complaints of constipation or other risk factors. Some providers advocate ensuring all patients initiating opioids have a stimulant laxative available to start at the first sign of constipation.
Exercise caution and follow closely if opioids are started in patients who drive. Avoid concomitant prescription of opioids and other CNS depressants.
Risk of addiction/diversion present with all opioids. Before starting assess risk with the Opioid Risk Tool. During maintenance, monitor using tool such as Current Opioid Misuse Measure. (www.painedu.org)Oxycodone or morphine Start with 2.5-5 mg oxycodone or morphine q4h and titrate no more frequently than q7d; assess total needs after 7d on stable dose, then convert to long acting.
Morphine
Renal impairment: Clcr 10-50 mL/minute: Administer at 75% of normal dose; Clcr <10 mL/minute: Administer at 50% of normal dose.
Hepatic impairment: No dosage adjustment provided in manufacturer's labeling. Pharmaco-kinetics unchanged in mild liver disease; substantial extrahepatic metabolism may occur. In cirrhosis, increases in half-life and AUC suggest dosage adjustment required.
Oxycodone
Renal impairment: Serum concentrations are increased ~50% in patients with Clcr <60 mL/minute; adjust dose based on clinical situation.
Hepatic impairment:
immediate release: Reduced initial dose may be necessary (use a conservative approach to initial dosing); adjust dose based on clinical situation.
Controlled release: Decrease initial dose to one-third to one-half the usual starting dose; titrate carefully.Side effects and risks of addiction/diversion as per hydrocodone. NEVER start long acting opioid before determining needs with short acting. Duloxetine Start 20-30 mg/d; increase to 60 mg/d in 7d. Not recommended in ESRD or CLcr<30. May precipitate serotonin syndrome when combined with triptans, tramadol, and other antidepressants. Key drug-disease interactions: HTN, uncontrolled narrow-angle glaucoma, seizure disorder. Precipitation of mania in patients with bipolar disorder. Important adverse effects include nausea, dry mouth, sedation/falls, urinary retention, constipation. Contra-indicated with hepatic disease and heavy alcohol use. Abrupt discontinuation may result in withdrawal syndrome. Contraindicated within 14 days of MAOI use.
Case Presentation
Relevant History
This patient is a 74–year-old female who is a retired cashier/business manager. She presented to a physiatry clinic with complaints of chronic, recurrent low back and lower extremity pain with episodes dating back approximately 20 years. Her chief complaint is back and left lower extremity pain extending to her dorsal foot limiting her walking to 20–30 minutes and her ability to stand upright when walking. Her symptoms have been gradually worsening over the past 2–3 years, and her current episode began suddenly two months ago when she began having pain down to her left foot and ankle that was further limiting her ability to walk. Any prolonged standing or walking exacerbates symptoms while sitting, leaning on a shopping cart, and using a walking stick relieves her symptoms. She denies any recent falls or concerns for falls. Her medical history includes osteoarthritis at the cervical spine, lumbar spine, and hands. She has a remote history of a right lower leg fracture and a resulting right knee flexion contracture. She has no history of any surgeries. Her current medications and supplements included: Lovastatin, Vitamin D-3; calcium citrate, fish oil, Coenzyme Q10, and Magnesium. She did not have any prescribed pharmaceutical pain management, but she uses non-prescription acetaminophen as needed several times a week to reduce pain with walking. She had three previous epidural steroid injections to manage past episodes. Her primary goal is to return to her usual routine of walking 45–60 minutes at least 4 days per week without pain or having to forward flex her trunk to limit symptoms.
Relevant Physical Examination
The patient is alert and oriented with no apparent distress. Her standing posture reveals increased flexion of lumbar spine and hips as well as the right knee due a flexion contracture of her knee. Her weight bearing is shifted to be greater on the left lower extremity. Her gait reveals increased forward flexion in the lumbar spine and hip flexion. Her spinal range of motion is full without pain in flexion; lumbar extension is limited to a few degrees with complaints of stiffness in her low back. There were no hip range-of-motion deficits. Her right knee range of motion reveals a 10 degree extension lag. Neurological assessment of her lower extremities reveals symmetrical strength and intact sensation with symmetrical Patellar and Achilles reflexes graded as 1+ bilaterally. Select muscle strength testing shows hip extension weakness graded as 4/5 bilaterally, and abdominal weakness graded as a 3/5 using Kendall’s leg lowering test. Examination of muscle length indicates tightness of her hip flexors (iliopsoas and rectus femoris) and her iliotibial band bilaterally.
Imaging
Spinal radiographs were performed indicating multi-level degenerative disc disease and facet joint hypertrophy. There was no indication of degenerative spondylolisthesis. MRI was also performed and the results were characterized as severe central canal stenosis at the L3/4 and L4/5 spinal levels in the radiology report.
Clinical Course
The patient initially received an epidural steroid injection which abated her left lower extremity symptoms and back pain. However, she continued to have difficulty walking without leaning on a shopping cart, her exercise program was limited to walking 1.5 miles using a walking stick and approximately two rest breaks, and she was unable to stand fully upright after walking two blocks. Due to these continued activity limitations she began physical therapy care three weeks after her injection. Over six visits of physical therapy focused on manual therapy and exercises to improve hip extension and hip flexor flexibility, she was able to stand more erect statically, but she still needed to flex forward after approximately 5 minutes of walking. Her walking distance was still limited to 1.5 miles, but she indicated she needed breaks due to “breathing hard” rather than pain. Throughout her treatment, she continued going to a group exercise class for older adults that involved sitting and standing exercises, which was very important to her.
Approach to Management
Diagnostic Considerations
The patient presented in this case was diagnosed with degenerative LSS based on clinical criteria that were consistent with imaging findings. The high false-positive rate for imaging in older adults [23] makes clinical correlation of any imaging findings a key consideration in the diagnosis of LSS as described in the expert panel algorithm (Figure 1). This patient was most debilitated by her leg symptoms, providing a rationale for the imaging that had been performed. As noted in the expert panel algorithm, imaging for patients in the absence of debilitating leg symptoms or other indications should be approached cautiously because of the risk for false positive results. Although rates of surgery of LSS are increasing [5], this patient responded favorably to a course of non-surgical management. Many patients diagnosed with LSS will have satisfactory clinical outcomes without surgery based on the natural history of the condition and/or benefits of various non-surgical treatment strategies. [20]
The patient’s complaints of leg pain that worsened with walking and was relieved by sitting (i.e., neurogenic claudication), and improvement of symptoms with forward flexion of her spine are important clinical findings that can be related to narrowing of spinal canals [6, 24] and corroborate this patient’s imaging findings. Claudication symptoms can also occur from arterial insufficiency. Vascular claudication, unlike claudication of neurogenic origin, is not affected by spinal position and can be relieved by ceasing activity and standing instead of having to sit down. [25] Further confirmation of the neurogenic versus vascular origins of this patient’s claudication symptoms may have been accomplished by recording a normal ankle-brachial index, a measurement of the blood pressure in the lower leg compared to the arm. [26] Hip osteoarthritis is another differential diagnosis whose symptoms of increased pain while walking that is relieved by sitting can mimic LSS, and the two conditions frequently co-occur. [10] This patient however lacked hip pain and did not exhibit a loss of hip internal rotation range of motion at the time of evaluation. If present, findings of loss of hip ROM or provocation of pain would suggest need for further diagnostic work-up for hip osteoarthritis, as described in an earlier algorithm in this series. [27]
Therapeutic Options
Several non-surgical treatment options have been recommended for patients diagnosed with LSS including watchful waiting, physical therapy, spinal injections as well as various complementary and alternative strategies such as chiropractic, acupuncture and massage. [15, 20] Research is currently lacking to clarify the most effective options or specify optimal sequencing of treatments, thus a process of shared decision-making on the preferred options for an individual patient is recommended (Figure 1). Re-assessing the benefits of whichever non-surgical treatment approach is selected is an important consideration for effective management since there is little information to a priori identify which treatment may be helpful for any particular patient. The impact of treatment on patients’ pain and function should be the focus of the re-assessment process. On average, an improvement of 30% in pain (2 points on a 0–10 numeric pain-rating scale) or function assessed with a validated questionnaire such as the Oswestry or Roland Morris can be considered as clinically meaningful improvement. [28, 29] If meaningful improvement is not achieved, an alternative treatment strategy may be recommended. Surgery for degenerative LSS can be effective for many patients who fail to respond to non-surgical treatments. [30] In the absence of spondylolisthesis or instability, decompression surgery without fusion is recommended. [20]
The patient in this case opted to first pursue epidural steroid injections based on receiving relief with these injections for prior episodes of LSS symptoms. Recent systematic reviews suggest epidural injections may provide short-term (2 weeks to 6 months) symptom relief in patients with neurogenic claudication due to LSS. [20, 31, 32] Existing evidence is limited and lacking in methodological rigor, and thus opinions on the efficacy of epidural injections differ. Despite this uncertainty, epidural steroids are commonly used for patients with LSS and utilization has been increasing rapidly. [34] This patient experienced a clinically meaningful reduction in pain following her epidural injection but her functional deficits persisted. Because her recovery was incomplete, the determination was next made to pursue physical therapy as a non-surgical treatment strategy.
The evidence for the benefits of physical therapy for LSS has also historically been sparse. Previous recommendations based largely on evidence from patients with chronic back pain have advocated that if physical therapy is chosen, an active approach focused on exercise with manual therapy and instruction for an ongoing self-monitored exercise program should be used, as opposed to physical therapy approaches focused on use of passive modalities (e.g, ultrasound, moist heat, etc.). [20, 34, 35] A study by Delitto and colleagues [36] published since the most recent systematic reviews supports a physical therapy approach emphasizing exercise, specifically lumbar flexion exercises, exercises directed to patient-specific strength or flexibility deficits and general conditioning, as well as patient education about LSS, the favorable natural history in many patients, and the importance of remaining active. Results from this randomized trial found this physical therapy approach equivalent to surgical decompression after 2 years, although over half (57%) of the patients randomized to physical therapy crossed over to receive surgery by 2 years. [36] The patient in this case responded favorably to physical therapy with improvement in her function. At the conclusion of her physical therapy treatment, she continued to experience deficits in endurance with physical activities, highlighting the need for an ongoing exercise and physical activity program.
The goal of pharmacologic management for patients with LSS is to manage pain so that function can be improved. The patient in this case opted to use over-the-counter nonsteroidal anti-inflammatories (NSAIDs) for this purpose. It should be highlighted that chronic use of NSAIDs is not recommended for older adults [38] and the patient presented used them only on an as needed basis, specifically to help her comply with her walking exercise program. Opioids should be considered a second-line analgesic (Table 2). Both opioids and NSAIDs have serious potential adverse effects in older adults (e.g., constipation, falls, hip fractures, depression, disrupted sleep architecture, and delirium with opioids; renal failure, painless gastrointestinal bleeding, hypertension, congestive heart failure with NSAIDs) and careful monitoring with ongoing assessment of risk versus observed benefits is essential. [36, 37]
One of the most important aspects of treating older adults with LSS is to treat patients comprehensively, targeting all contributors to their CLBP and leg symptoms. We have gathered preliminary data suggesting that as many as half of older adults with CLBP and neurogenic claudication may have other important contributors to their symptoms, including hip osteoarthritis, sacroiliac joint syndrome, myofascial pain and fibromyalgia. [38] It is critical that providers exhaust treatments that address all functionally limiting conditions as a part of treating the older patient with LSS and neurogenic claudication. [39, 40]
Resolution of Case
This case represents a fairly typical presentation of an older adult with LSS. Her clinical presentation included pathognomonic findings for the condition including a long history of episodic back pain with postural-dependent symptoms that worsen with activities or postures that emphasize spinal extension or involve compression forces and ease with flexion positions. As is typical in cases of LSS, differential diagnosis is a key consideration as the symptoms can mimic other chronic conditions common in older adults such as hip osteoarthritis or vascular claudication and imaging is prone to false positive findings. The patient in this case, like many with LSS, was able to effectively manage her symptoms with pain relieving treatments including injections and nonsteroidal anti-inflammatory medication and maintain her function through an ongoing exercise program with assistance from physical therapy to work on specific impairments of strength and flexibility. Because of the episodic nature of LSS symptoms, this patient continues to experience intermittent back pain, however she is able to self-manage these symptoms with her exercise routine and occasional use of nonsteroidal medication.
Summary
Lumbar spinal stenosis is a very common source of pain and disability and should be considered as part of the differential diagnosis of older adults with low back pain. Due to the fact that degenerative changes in the lumbar spine are present on spinal imaging in most older adults, even those without any back pain, careful clinical examination is essential to accurate diagnosis. Differential diagnosis for conditions with similar symptom presentation is also an important consideration. Common conditions such as hip osteoarthritis and vascular claudication can present with similar symptoms and may co-occur with LSS.
Lumbar spinal stenosis is the most common indication for spinal surgery in the United States [4] and rates of surgery, particularly complex procedures involving fusion continue to increase. [41] The patient presented in this case however highlights the reality that many older adults with LSS can maintain function and manage their condition without surgery. A wide variety of non-surgical treatment options are available for LSS, and choosing among them for a particular patient can be difficult. The patient in this case found benefit in maintaining a regular exercise routine and intermittently using acetaminophen for symptom exacerbations, as well as epidural steroid injections and physical therapy for more severe symptoms. All of these strategies were used in an effort to maintain her function. The maintenance of function should be an overarching goal in developing a management plan for all older adults with LSS, and all contributors to pain and disability should be targeted.
Key Points
Lumbar spinal stenosis (LSS) is a clinical diagnosis that is corroborated
by advanced imaging. Asymptomatic anatomical LSS is common in
older adults, thus imaging should not be ordered without
first conducting a thorough clinical assessment.In the absence of progressive neurological deficits or cauda equina symptoms,
management of LSS should begin conservatively (e.g., physical
therapy, epidural injection, oral analgesics).Many older adults with LSS can expect to remain symptomatically stable or
improve over time, thus practitioners should educate patients
about the importance of remaining active and attempt to
quell fears of LSS-associated disablement.LSS often co-occurs with other conditions that contribute to pain and
disability in older adults, (i.e., the other conditions covered
in this series such as hip osteoarthritis, depression,
myofascial pain and fibromyalgia). Comprehensive
assessment and treatment is needed to optimize outcomes.
Acknowledgments
The authors thank Dave Newman for his thoughtful review of the manuscript. The authors would also like to thank Kim Bennett, PT, PhD, for identifying the case presented.
Funding sources:
This material is based on work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service.
Disclosure and conflicts of interest:
The contents of this report do not represent the views of the Department of Veterans Affairs or the U.S. government. The authors have no conflicts of interest to report.
References:
Amundsen T, Weber H, Lilleas F, et al.
Lumbar spinal stenosis. Clinical and radiologic features.
Spine 1995;20:1178–86Kalichman L, Cole R, Kim DH, et al.
Spinal stenosis prevalence and association with symptoms: The Framingham Study.
Spine J 2009;9:545–50.Ciol MA, Deyo RA, Howell E, Kreif S.
An assessment of surgery for lumbar spinal stenosis: Time trends, geopgraphic variations,
complications and reoperations.
J Am Geriatr Soc 1996;44:285–90.Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES.
United States trends and regional variations in lumbar spine surgery: 1992-2003.
Spine 2006;31:2707–14.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG.
Trends, Major Medical Complications, and Charges Associated
with Surgery for Lumbar Spinal Stenosis in Older Adults
JAMA 2010 (Apr 7); 303 (13): 1259–1265Katz JN, Harris MB.
Clinical practice. Lumbar spinal stenosis.
N Engl J Med 2008;358: 818–25Hansson T, Suzuki N, Hebelka H, Gaulitz A.
The narrowing of the lumbar spinal canal during loaded MRI: The effects of the disc
and ligamentum flavum.
Eur Spine J 2009;18:679–86.Singh V, Montgomery SR, Aghdasi B, et al.
Factors affecting dynamic foraminal stenosis in the lumbar spine.
Spine J 2013;13:1080–7.Suri P, Rainville J, Kalichman L, Katz JN.
Does this older adult with lower extremity pain have the clinical syndrome of lumbar
spinal stenosis?
JAMA 2010;304:2628–36.Saito J, Ohtori S, Kishida S, et al.
Difficulty of diagnosing the origin of lower leg pain in patients with both lumbar spinal
stenosis and hip joint osteoarthritis.
Spine 2012;37:2089–93.Inouye S, Studenski S, Tinetti M, Kuchel G.
Geriatric syndromes: Clinical, research and policy implications of a core geriatric concept.
J Am Geriatr Soc 2007;55:780–91.Benoist M.
The natural history of lumbar degenerative spinal stenosis.
J Bone Spine 2002;69:450–7.Tsutsumimoto T, Shimogata M, Yui M, Ohta H, Misawa H.
The natural history of asymptomatic lumbar canal stenosis in patients undergoing
surgery for cervical myelopathy.
J Bone Joint Surg Br 2012;94:378–84.U.S. Department of Health and Human Services,
Agency for Healthcare Quality and Research.
Treatment of Degenerative Lumbar Spinal Stenosis: Summary.
Evidence Report/Technology Assessment Number 32.
Washington, DC: U.S. Government Printing Office; 2001.Kreiner DS, Shaffer WO, Summers J, et al.
Clinical Guidelines for Multidisciplinary Spine Care:
Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis.
Burr Ridge, IL: North American Spine Society (NASS); 2007.Atlas SJ, Delitto A.
Spinal stenosis: Surgery versus nonsurgical treatment.
Clin Orthop Relat Res 2006;443:198–207Haig AJ, Tomkins CC.
Diagnosis and management of lumbar spinal stenosis.
JAMA 2010;303:71–2de Tran QH, Duong S, Finlayson RJ.
Lumbar spinal stenosis: A brief review of the nonsurgical management.
Can J Anaesth 2010;57:694–703.Kovacs FM, Urrutia G, Alarcon JD.
Surgery versus conservative treatment for symptomatic lumbar spinal stenosis:
A systematic review of randomized controlled trials.
Spine 2011;36:E1335–51.Kreiner DS, Shaffer WO, Baisden JL, et al.
An evidence-based clinical guideline for the diagnosis and treatment of degenerative
lumbar spinal stenosis (update).
Spine J 2013;13:734–43Weinstein JN, Bronner KK, Morgan TS, Wennberg JE.
Trends and geographic variations in major surgery for degenerative diseases of the hip,
knee, and spine.
Health Aff 2004;Var81–9.Weiner DK.
Deconstructing Chronic Low Back Pain in the Older Adult -
Shifting the Paradigm from the Spine to the Person
Pain Medicine 2015 (May); 16 (5): 881-885Brinjikji W, Luetmer PH, Comstock B, et al.
Systematic literature review of imaging features of spinal degeneration
in asymptomatic populations.
AJNR 2015;36:811–6.Takahashi K, Takino T, Matsui T, Miyazaki T, Shima I.
Changes in epidural pressure during walking in patients with lumbar spinal stenosis.
Spine 1995;20:2746–9.Fritz JM, Erhard RE, Delitto A, Welch WC, Nowakowski P.
Preliminary results of the use of a two-stage treadmill test as a clinical diagnostic
tool in the differential diagnosis of lumbar spinal stenosis.
J Spinal Dis 1997;10:410–6.Jeon CH, Han SH, Chung NS, Hyun HS.
The validity of ankle-brachial index for the differential diagnosis of peripheral arterial
disease and lumbar spinal stenosis in patients with atypical claudication.
Eur Spine J 2012;21:1165–70.Weiner DK, Fang M, Gentili A, et al.
Deconstructing Chronic Low Back Pain in the Older Adult - Step by Step Evidence
and Expert-Based Recommendations for Evaluation and Treatment.
Part I: Hip Osteoarthritis
Pain Medicine 2015 (May); 16 (5): 886-897Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole MR.
Clinical Importance of Changes in Chronic Pain Intensity
Measured on an 11-point Numerical Pain Rating Scale
Pain 2001 (Nov); 94 (2): 149-158Fritz JM, Hebert J, Koppenhaver S, Parent EA.
Beyond minimally important change: Defining a successful outcome of physical therapy
for patients with low back pain.
Spine 2009;34:2803–9.Weinstein JN, Tosteson TD, Lurie JD, et al.
Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results
of the Spine Patient Outcomes Research Trial.
Spine 2010;35:1329–38Chou R, Hashimoto R, Friedly J, et al.
Epidural corticosteroid injections for radiculopathy and spinal stenosis:
A systematic review and meta-analysis.
Ann Intern Med 2015;163:373–81Manchikanti L, Kaye AD, Manchikanti K, et al.
Efficacy of epidural injections in the treatment of lumbar central spinal stenosis:
A systematic review.
Anesth Pain Med 2015;5:e23139.Manchikanti L, Pampati V, Boswell MV, Smith HS, Hirsch JA.
Analysis of the growth of epidural injections and costs in the Medicare population:
A comparative evaluation of 1997, 2002, and 2006 data.
Pain Physician 2010;13:199–212Whitman JM, Flynn TW, Childs JD, et al.
A comparison between two physical therapy treatment programs for patients with
lumbar spinal stenosis: A randomized clinical trial.
Spine 2006;31:2541–9Chou R, Huffman LH; American Pain Society.
Nonpharmacologic Therapies for Acute and Chronic Low Back Pain:
A Review of the Evidence for an American Pain Society/
American College of Physicians Clinical Practice Guideline
Annals of Internal Medicine 2007 (Oct 2); 147 (7): 492–504Delitto A, Piva SR, Moore CG, et al.
Surgery versus nonsurgical treatment of lumbar spinal stenosis: A randomized trial.
Ann Intern Med 2015;162:465–73American Geriatrics Society 2012 Beers Criteria Update Expert Panel.
Updated Beers Criteria for potentially inappropriate medication use in older adults.
J Am Geriatr Soc 2012;60:616–31Weiner DK, Sakamoto S, Perera S, Breuer P.
Chronic low back pain in older adults: Prevalence, reliability, and validity of physical
examination findings.
J Am Geriatr Soc 2006;54:11–20.Barkin RL, Beckerman M, Blum SL, et al.
Should nonsteroidal anti-inflammatory drugs (NSAIDs) be prescribed to the older adult?
Drugs Aging 2010;27:775–89Issack PS, Cunningham ME, Pumberger M, Hughes AP, Cammisa FP.
Degenerative lumbar spinal stenosis: Evaluation and management.
J Am Acad Orthop Surg 2012;20:527–35Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI.
United States trends in lumbar fusion surgery for degenerative conditions.
Spine 2005;30:1441–5Fritz JM, Lurie JD, Zhao W, et al.
Associations between physical therapy and long-term outcomes for individuals with lumbar
spinal stenosis in the SPORT study.
Spine J 2014;14:1611–21Liu L, Skinner M, McDonough S, Mabire L, Baxter GD.
Acupuncture for low back pain: An overview of systematic reviews.
Evid Based Complement Alternat Med 2015;15:328196.Liu K, Liu P, Liu R, Wu X, Cai M.
Steroid for epidural injection in spinal stenosis: A systematic review and meta-analysis.
Drug Des Devel Ther 2015;9:707–16.Henschke N, Ostelo RW, van Tulder MW, et al.
Behavioural treatment for chronic low-back pain.
Cochrane Database Syst Rev 2010;7:CD002014Macedo LG, Hum A, Kuleba L, et al.
Physical therapy interventions for degenerative lumbar spinal stenosis: A systematic review.
Phys Ther 2013;93:1646–60
Return to LOW BACK PAIN
Since 6-21-2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |