Deconstructing Chronic Low Back Pain in the Older Adult -
Shifting the Paradigm from the Spine to the PersonThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Pain Medicine 2015 (May); 16 (5): 881–885 ~ FULL TEXT
OPEN ACCESS Debra K. Weiner
Geriatric Research, Education & Clinical Center,
VA Pittsburgh Healthcare System,
University of Pittsburgh,
Pittsburgh, PA, USA.
Over the past decade, the estimated prevalence of low back pain (LBP) among older adults (typically defined as those ≥age 65) has more than doubled [1], and the utilization of advanced spinal imaging (e.g., computerized tomography (CT), magnetic resonance imaging [MRI]) and procedures guided by this imaging (e.g., epidural corticosteroids, spinal surgery) have continued to skyrocket. [1-3]
Treatment outcomes, however, have not improved apace. Why? Part of the answer lies in the fact that treatment may in part be misdirected.
This issue of Pain Medicine contains the first in a series of articles on how to systematically and comprehensively rethink our approach to evaluating and designing management for older adults with chronic low back pain (CLBP). The series is entitled “Deconstructing Chronic Low Back Pain in the Older Adult: Step-by-Step Evidence and Expert-Based Recommendations for Evaluation and Treatment” and the article in this issue focuses on hip osteoarthritis (OA), an important potential contributor to CLBP in older adults.
KEYWORDS: Back Pain; Chronic Low Back Pain; Chronic Pain; Elderly; Geriatric; Homeostenosis; Low Back Pain; Lumbar; Magnetic Resonance Imaging; Older Adults; Pain Management; Treatment Outcome
From the FULL TEXT Article:
Current Practice
To understand how we might attempt to improve the care of older adults with CLBP, let us start by examining current approaches. Management of patients with CLBP often begins with a search for the cause of pain using spinal imaging.
The vast majority of people with CLBP do not require imaging because they do not have “red flag” pathology, that is, serious disorders such as cancer or infection that require urgent treatment. [4]
Spinal imaging in the older adult who does not have red flags on history or physical examination will almost certainly reveal “abnormalities.” Imaging evidence of lumbar degenerative disc and facet disease is nearly ubiquitous in older adults, even those who are pain-free. [5]
An estimated 20% of older adults without neurogenic claudication have moderate to severe lumbar spinal stenosis on magnetic resonance imaging. [6]
Thus older adults may be especially susceptible to receiving invasive treatment guided principally by imaging-identified degenerative spinal pathology and it is not surprising that such treatment is often inefficacious.
While CLBP can be a management challenge for patients of all ages, we focus on older adults because of their vulnerability to undergoing degenerative spine disease-focused procedures that may not be necessary, as well as adverse drug effects and invasive treatment-associated morbidity. [7, 8]
If we attempt to manage the older adult with CLBP solely using spinal imaging, there may be one of three results:1) The physical cause of pain is identified and appropriately targeted treatment is prescribed (e.g., severe central canal stenosis is identified and decompressive laminectomy results in reduction of pain and disability).
2) Pathology is identified that may be incidental (e.g., asymptomatic central canal stenosis, bulging discs, degenerative disc disease); the cause(s) of pain and disability lies outside of the lumbar spine (e.g., sacroiliac joint [SIJ] syndrome, iliotibial band pain, myofascial pain of the erector spinae or quadratus lumborum, hip OA), thus treatment may be inappropriately targeted.
3) Spinal pathology is identified that, when combined with biopsychosocial factors outside of the lumbar spine (e.g., anxiety, depression, fear avoidance beliefs, insomnia, fibromyalgia syndrome [FMS], hip OA), CLBP and disability results. If our treatment targets only degenerative spine disease in these patients, suboptimal outcomes are likely.
Deconstructing CLBP in Older Adults:
A Geriatric Medicine Approach
The overarching goal when treating patients with chronic pain is to optimize their capacity to function despite the persistence of some pain. This aligns well with the philosophical practice of geriatric medicine, that is, optimizing patient function despite practitioners' inability to eradicate disease. At the core of aging is a concept called homeostenosis, defined as the progressive restriction of an organism's ability to respond to stress as it ages (the antithesis of homeostasis, an organism's capacity to maintain stability in the face of change; [9]). Older adults, by virtue of being alive, have accumulated a host of changes at the cellular and tissue level, such as decrease in bulk and quality of skeletal muscle (i.e., sarcopenia), loss of skin elasticity, and vascular stiffening, to name a few. These changes in and of themselves are often not associated with disease. Instead, they serve as a source of vulnerability or homeostenosis, that is, a “weak link”. [10]
This series conceptualizes degenerative discs and facet joints of the lumbar spine as weak links instead of diseases and CLBP as a syndrome, that is, a final common pathway for the expression of multiple contributors. [11] A common syndrome familiar to those who care for older adults across multiple settings of care is delirium (acute confusional state), defined as an acute disorder of attention and cognition. [12] When a hospitalized older adult becomes acutely confused, the most likely culprit is an infection (e.g., urinary tract infections, pneumonia) or an adverse drug reaction. [12] Despite the patient's symptoms being indicative of brain dysfunction, brain imaging is not routinely recommended for those with delirium. [12] Instead, a search for causative factors outside of the central nervous system is recommended, that is, those factors that make the vulnerable aging brain overtly dysfunctional. Our goal is to move the treatment of CLBP in a similar direction by emphasizing identification and treatment of the multiple factors that when combined with degenerative disease of the lumbar spine, cause disability.
The Series
Our series of articles is written from the vantage point that the lumbar spine is a weak link or one of multiple treatment targets rather than the sole treatment target in older adults with CLBP. Each article published over the next months will contain a focused literature review and an illustrative clinical case. Our presentation of CLBP in older adults as a syndrome aligns with pain physiology. Pain is a complex physiological process contributed to by peripheral nociceptive stimuli and interpretation of those stimuli by the brain. In older adults with CLBP, factors outside of the lumbar skeleton that alter spinal biomechanics such as hip OA and leg length discrepancy (e.g., following joint replacement) may drive nociception. Factors that alter perception of nociceptive stimuli (i.e., top down inhibition) such as FMS, cognitive impairment, psychological maladaptation (e.g., fear avoidance beliefs, catastrophizing), anxiety, and depression also may contribute to pain and pain-associated disability.
Data indicate that older adults with chronic pain tend to be more psychologically robust than their younger counterparts [13], and interdisciplinary pain management programs that treat patients who fail first and second line therapy routinely use psychological interventions and other strategies to help patients improve their ability to cope and function with pain [14, 15], focusing less on identifying and treating physical pain contributors. Older adults with CLBP often have multiple physical contributors to their pain and difficulty functioning [16], thus thorough hands-on physical assessment is critical to optimizing treatment outcomes. Because of an increased risk of social isolation and dementia, they also are likely to have unique psychosocial contributors to CLBP-associated disability that traditional pain management programs do not address.
Over the ensuing months, we will discuss conditions that occur commonly in older adults with CLBP ± leg pain and that should be evaluated routinely as potential contributors to pain and disability:1) hip OA,
2) myofascial pain,
3) lumbar spinal stenosis,
4) SIJ joint syndrome,
5) lateral hip/thigh pain syndrome,
6) leg length discrepancy,
7) insomnia,
8) fibromyalgia,
9) depression,
10) anxiety,
11) psychological maladaptation, and
12) cognitive impairment.We have drawn from data within the pain, spine, orthopedics, pharmacology, physical therapy, psychology, psychiatry, rheumatology, rehabilitation, and gerontology literature, as well as expert opinion (when strong evidence was not available) to create algorithms usable in the clinical setting.
Methods
Each algorithm was created using a modified Delphi technique and takes into account resources within Veterans Health Administration (VHA) facilities to facilitate broad uptake. As VHA facilities have a more restricted formulary than non-VHA facilities, we recommend medications that are available in both VHA and civilian settings. The Delphi technique is based on the premise that pooled intelligence enhances individual judgment and captures the collective opinions of a group of experts without being physically assembled. [17, 18]
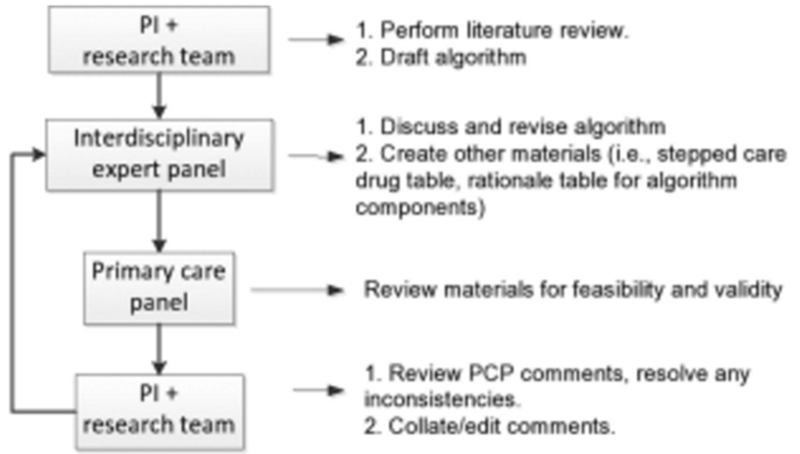
An overview of the process used in this project is shown in Figure 1 and described here.
To begin, the Principal Investigator (DW) drafted an evidence-based treatment algorithm based on a comprehensive review of the literature and knowledge of medications and services available within VHA facilities.
The draft algorithm was distributed to an interdisciplinary expert panel, chosen based on recognition in their individual fields and/or their expertise in providing clinical care to older adults. This panel discussed the algorithm via teleconference, refined it and created accompanying tables (i.e., a table providing the rationale and corresponding citations for individual algorithm components; and for the majority of algorithms, a stepped-care drug table predicated on changes in aging-associated pharmacokinetics and pharmacodynamics). When strong evidence was not available, the expert opinion and clinical experience of panelists was drawn upon for guidance.
The refined materials were distributed to a 9-member primary care provider (PCP) panel who reviewed the materials using the questionnaire shown in Figure 2, focusing on the feasibility of implementing the algorithm in the primary care setting in general and in the VA in particular, as well as the validity of the recommendations for older adults.
A research assistant collated the PCP panel comments and the PI reviewed and clarified them as needed. For example, if one of the PCP reviewer's rating of an item in Figure 2 was an outlier as compared with that of the other reviewers, she communicated with them via e-mail to resolve any inconsistencies. Revisions to the collated comments were then made and distributed to the expert panel.
The expert panel reviewed the feedback and revised the algorithm and accompanying materials. They prepared a point-by-point response to the feedback that was distributed along with the revised materials to the PCP panel for review.
The entire process was repeated until no further revisions were recommended.
Our hope is that these algorithms and accompanying materials will provide a concrete framework to guide evaluating and managing the multiple contributors to CLBP ± leg pain in older adults. The ultimate goal is to optimize function and minimize morbidity in these vulnerable patients. Consider, for example, the 68-year-old woman with LBP and bilateral leg pain as well as FMS, fear avoidance beliefs, and a lumbar MRI that shows moderate central canal stenosis. She is treated with epidural corticosteroid injections and ultimately decompressive laminectomy, without relief. In fact, her pain worsens. Her FMS was never treated, despite the fact that axial pain is a central feature of FMS, as is pain in multiple body regions, including the legs. Her fear avoidance beliefs also were not addressed. Could surgery have been avoided if her treatment began with aerobic exercise and/or medication that targeted her FMS and cognitive behavioral therapy for her fear avoidance beliefs?
We present the algorithms individually for the sake of clarity, but practitioners will likely use them together, rather than as separate guidelines. Ensuring realistic treatment expectations lie at the core of each algorithm, along with a strong emphasis on patient education, so that each person being treated understands both the factors contributing to their symptoms and how to engage in self-management. These concepts are fundamental to providing rational and high quality care to all patients, especially to those with chronic pain.
There is an art involved in caring for patients with chronic pain, a genuine therapeutic value of providers communicating positively with patients and instilling hope. Knowing how to prioritize the treatment of multiple contributors to pain and disability is also an art, and we hope to provide the artists' tools to as many providers as possible with the articles and algorithms that will appear in the following months. By deconstructing CLBP into separate components, we provide practitioners with a different framework to consider and utilize. Without a doubt, there will be subsequent projects that test the effectiveness of this kind of approach.
Epilogue: A Look to the Future
Why do nearly all of older adults have degenerative disease of the lumbar spine, but only a small fraction has CLBP, and still fewer are disabled by their pain? We know that many biopsychosocial factors influence the course and outcome of CLBP and that response to treatment of these factors is variable. It is likely that some of the discrepancy between imaging findings, symptoms and function, and the variability of treatment response has to do with as yet unidentified genetic and other biological factors, and one day, we may be able to more precisely prescribe biologic treatments based on one or more tests. That time is likely in the distant future. How in the meantime should we rationally approach the evaluation and treatment of older adults with CLBP? The United States healthcare system is in crisis. Our excessive expenditures compared with other countries are generally not matched by superior quality or quantity of life. [19] Our society is aging rapidly and there is a burgeoning of older adults with CLBP for whom we must provide rational care. Our series of articles, “Deconstructing Chronic Low Back Pain in the Older Adult—Step by Step Evidence and Expert-Based Recommendations for Evaluation and Treatment,” has been created to do exactly that.
Acknowledgments
This material is based on work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service. The contents of this report do not represent the views of the Department of Veterans Affairs or the US government. The author thanks Dave Newman, Dr. Rollin Gallagher, and Dr. Eric Rodriguez for their thoughtful review of the manuscript.
Conflicts of interest:
The author has indicated that she has no conflicts of interest regarding the content of this article.
References:
Weiner DK, Kim Y-S, Bonino P, Wang T.
Low back pain in older adults: Are we utilizing health care resources wisely?
Pain Med 2006;7(2):143–50Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, et al.
Expenditures and Health Status Among Adults With Back and Neck Problems
JAMA 2008 (Feb 13); 299 (6): 656–664Friedly J, Chan L, Deyo R.
Increases in lumbosacral injections in the Medicare population—1994 to 2001.
Spine 2007;32(16):1754–60Deyo RA, Rainville J, Kent DL.
What can the history and physical examination tell us about low back pain?
J Am Med Assoc 1992;268(6):760–5Hicks G, Morone N, Weiner D.
Degenerative lumbar disc and facet disease in older adults: Prevalence and clinical correlates.
Spine 2009;34:1301–6Jarvik J, Hollingworth W, Heagerty P, Haynor D, Deyo R.
The longitudinal assessment of imaging and disability of the back (LAIDBack) study: Baseline data.
Spine 2001;26(10):1158–66Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG.
Trends, Major Medical Complications, and Charges Associated
with Surgery for Lumbar Spinal Stenosis in Older Adults
JAMA 2010 (Apr 7); 303 (13): 1259–1265The American Geriatrics Society 2012 Beers Criteria Update Expert Panel.
American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults.
J Am Geriatr Soc 2012;60:616–31Becker P, Cohen H.
The functional approach to the care of the elderly: A conceptual framework.
J Am Geriatr Soc 1984;32:923–9Resnick NM, Marcantonio ER.
How should clinical care of the aged differ?
Lancet 1997;350:1157–8Inouye S, Studenski S, Tinetti M, Kuchel G.
Geriatric syndromes: Clinical, research and policy implications of a core geriatric concept.
J Am Geriatr Soc. 2007;55(5):780–91Inouye S.
Delirium in older persons.
N Engl J Med. 2006;354(11):1157–65Wittink H, Rogers W, Lipman A, et al.
Older and younger adults in pain management programs in the United States: Differences and similarities.
Pain Med 2006;7(2):151–63Gatchel R, McGeary D, McGeary C, Lippe B.
Interdisciplinary chronic pain management: Past, present, and future.
Am Psychol 2014;69(2):119–30Stanos S.
Focused review of interdisciplinary pain rehabilitation programs for chronic pain management.
Curr Pain Headache Rep. 2012;16:147–52Weiner D, Sakamoto S, Perera S, Breuer P.
Chronic low back pain in older adults: Prevalence, reliability, and validity of physical examination findings.
J Am Geriatr Soc 2006;54:11–20Delbecq AL, Van de Ven AH, Gustafson DH, eds.
Group Techniques for Program Planning: A Guide for Nominal Group and Delphi Processes.
Glencoe, IL: Scott, Foresman, and Company; 1975Jones J, Hunter D.
Consensus methods for medical and health services research.
Br Med J 1995;311:376–80Fuchs V.
Critiquing US health care.
JAMA 2014;312(20):2095–96
Return to MEDICARE
Return to LOW BACK PAIN
Return to PRESCRIPTION RIGHTS DEBATE
Since 1-23-2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |