

Associations of Semaglutide With Alzheimer’s disease-related
Dementias in Patients With Type 2 Diabetes:
A Real-world Target Trial Emulation StudyThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Alzheimers Dis 2025 (Jun 24): 13872877251351329 ~ FULL TEXT
William Wang • Pamela B Davis • Xin Qi • Mark Gurney • George Perry
Nora D Volkow • David C Kaelber • Rong Xu
Center for Science, Health, and Society,
Case Western Reserve University School of Medicine,
Cleveland, OH, USA
Background: Almost half of the dementia cases are preventable. Semaglutide treats several medical conditions that are risk factors for dementia.
Objective: We aim to investigate if semaglutide is associated with a decreased risk of dementia.
Methods: We conducted emulation target trials based on a nationwide population-based database of patient electronic health records (EHRs) in the US among 1,710,995 eligible patients with type 2 diabetes (T2D) comparing semaglutide with other antidiabetic medications. First-time diagnosis of Alzheimer's disease-related dementia (ADRD) including vascular dementia, frontotemporal dementia, Lewy body dementia and other dementias were examined using Cox proportional hazards and Kaplan-Meier survival analyses during a 3-year follow-up. Models were adjusted by propensity-score matching.
Results: We show that semaglutide was associated with a significantly reduced risk of overall ADRD incidence with a hazard ratio ranging from 0.54 (0.49-0.59) compared with insulin, 0.67 (0.61-0.74) compared with metformin, to 0.80 (0.72-0.89) compared with older generation glucagon-like peptide-1 agonists (GLP-1RAs). The association varied for specific dementia types, with significantly reduced risk of vascular dementia and no evidence of associations with frontotemporal and Lewy body dementias.
Conclusions: These findings provide evidence supporting protective effects of semaglutide on dementias in patients with T2D. Future works are needed to establish the causal relationships through randomized clinical trials and to characterize the underlying mechanisms.
Keywords: Alzheimer's disease related dementias; Alzheimer’s disease; Lewy body dementia; dementia; frontotemporal dementia; semaglutide; target trial emulation; type 2 diabetes; vascular dementia.
I was just reading Dr. Gabrielle Lyon's seminal text “Forever Strong: A New, Science-based Strategy for Aging Well.” Her text recommends red meat ("the original superfood“) as the ideal source for our amino acids, BUT that was because she found that most of her patients who stopped eating steak (millions at least) ended up sourcing the plant kingdom, via processed foods, for their amino acids, from such weak choices as “cereals, breads, pastries and pizzsas” (see page 100)
The good news is that Plant Proteins ARE available that come from pure, organically-grown, non-GMO sources like Soy isolate formulas and Whey protein, that are ALSO enhanced with those same naturally-sourced KEY vitamins and minerals that make red meat “the-original-superfood“.
I guess that makes (certain) plant-based proteins “the-future-superfood”. YAY!!!
You just need to be super-careful who you get your plant protein from, because huxters still abound in the new Wild West of “health-food” purveyors.
From the FULL TEXT Article:
Introduction
Dementia affects more than 6 million Americans, [1] accounting for more than 100,000 deaths and costing $600 billion each year in the US. [2, 3] Currently, dementias have no cures or effective treatments, therefore, prevention strategies are critical. About 45% of dementia cases have been linked to 14 modifiable risk factors, including diabetes, obesity, hypertension, and cardiovascular diseases, and modifying these risk factors could theoretically help prevent or delay nearly half of dementia cases. [4] Proximal mechanisms driving increased dementia risk from these factors include inflammation [5–7] and associated vascular disease. [4] Therefore, a multicomponent prevention strategy simultaneously targeting these risk factors and reducing inflammatory and vascular dysfunction could help prevent dementia.
Semaglutide, a new generation of glucagon-like peptide-1 receptor agonist (GLP-1RA), has shown a broad range of benefits, including reductions in obesity, type 2 diabetes, hypertension, and cardiovascular diseases. [8–11] Emerging research suggests that semaglutide has anti-inflammatory and immunological properties [12, 13] and improves vascular function. [8, 11] Given its ability to simultaneously target multiple risk factors and proximal mechanisms of dementia, we hypothesize that semaglutide may reduce the risk of developing dementia in high-risk patients.
Preclinical studies demonstrated that semaglutide had neuroprotective and anti-inflammatory effects in animal models of Alzheimer’s disease (AD). [14–16] Randomized clinical trials showed that liraglutide, an older generation of GLP-1RAs, reduced whole cortical gray matter, frontal, temporal, and parietal lobe volume compared to placebo in patients with early to moderate AD. [17–19] Currently, two large phase 3 placebo-controlled trials are underway to evaluate the neuroprotective effects of semaglutide in early AD. [20–22]
Another proof-of-concept trial is investigating semaglutide in patients with mild cognitive impairment. [23] In a recent real-world emulation trial in T2D patients, we showed that semaglutide was associated with a significant 40%-70% reduction in AD risk with other antidiabetic medications, including the first generation of GLP-1RAs. [24] However, it remains unknown whether semaglutide has similar effects on other AD-related dementias. In this study, we conducted target trial emulation in patients with T2D to investigate whether semaglutide was associated with a reduced risk of Alzheimer’s disease-related dementia (ADRD), including vascular, frontotemporal (FTD), and Lewy body (LBD), and to identify which patients might benefit most.
Methods
Specification of the target trialsStudy overview.
We compared the effects of semaglutide versus other antidiabetic medications on first-time diagnoses of ADRD using a target trial emulation framework. [25, 26] We assessed seven T2D patient populations who had no prior AD/ADRD diagnosis: all patients, older (≥65), younger (<65), women, men, patients with and without obesity. Supplemental Table 1 lists key protocol components. For each population, we specified seven target trials separately comparing semaglutide with insulin, metformin, dipeptidyl-peptidase-4 inhibitors (DPP-4i), sodium-glucose cotransporter-2 inhibitors (SGLT2i), sulfonylureas (SUs), thiazolidinediones (TZDs), and the first-generation GLP-1RAs (albiglutide, dulaglutide, exenatide, liraglutide, and lixisenatide). The target trials are specified as follows.
Eligibility criteria.
Eligibility criteria for all target trials included patients with T2D who had recent medical encounters for their T2D diagnosis in the past year (“active and recent T2D”), were prescribed antidiabetic medications between December 2017 and December 2021, and were diagnosed with at least one condition based on semaglutide’s prescription guidelines (e.g., obesity, hypertension, hypercholesterolemia, heart diseases, chronic kidney diseases, stroke, or A1C ≥ 8.5%). [27]
Exclusions included a history of ADRD (vascular dementias, FTD, LBD, or other unspecified dementias) or AD, co-prescription of semaglutide and comparison medications at baseline, and certain medical conditions (pancreatitis, type 1 diabetes, thyroid cancer, gastroparesis) based on contraindications, warnings, and limited use information for semaglutide. [27]
Additional criteria for subpopulations were:
Older patients: Age ≥65 (age based on the time of medication prescription)
Younger patients: Age <65 (age based on the time of medication prescription)
Women/Men: Gender-based inclusion
Obesity/no obesity: with or without a prior diagnosis of obesity
Details are in Supplemental Table 2.
Treatment strategies.
In each of the 7 target trials, the treatment strategies were the initiation of semaglutide use at baseline (time zero, index event) or the initiation of comparison antidiabetic medication use at baseline (time zero or index event), but not both. For all treatment strategies, initiation of use is defined as the first prescription for the drug, consistent with an intention-to-treat design. The treatment strategy is assigned at baseline.
Study outcomes.
The main outcomes are the first-time diagnosis of(1) overall ADRD (International Classification of Diseases, Tenth Revision (ICD-10) code F01 “Vascular dementia”, G31.0 “Frontotemporal dementia”, G31.83 “Neurocognitive disorder with Lewy bodies”, F03 “Unspecified dementia”, F02 “Dementia in other diseases classified elsewhere”),
(2) vascular dementia (F01 “Vascular dementia”),
(3) FTD (G31.0 “Frontotemporal dementia”),
(4) LBD (G31.83 “Neurocognitive disorder with Lewy bodies”), and
(5) other dementias (F03 “Unspecified dementia”, F02 “Dementia in other diseases classified elsewhere”).Dementia-related medication prescriptions (Donepezil, Rivastigmine, Galantamine, Memantine) were used as a secondary outcome. As a sensitivity analysis, we used outpatient medical encounters to measure overall healthcare utilization. Each outcome was analyzed separately (no multiple comparisons nor competing outcomes). Each eligible patient was followed starting 30 days after the index event (to mitigate reverse causation) until the occurrence of the outcome, death, loss to follow-up, or 3 years after the index event, whichever occurred first. Details of diagnosis codes for study outcomes are in Supplemental Table 3.
Analysis approach.
The causal estimates of interest represent the intention-to-treat effect of being assigned to the treatment strategies. Cumulative incidences were estimated using the Kaplan–Meier survival analysis. Cox proportional hazard analyses were used to compare rates of time-to-event daily during the follow-up time after the index event. Hazard ratios (HRs) and 95% CIs were calculated.Emulation of the target trials
We explicitly emulated the target trials described above using data and built-in analytic functions on the TriNetX Analytics platform. TriNetX is a global, federated, health research network providing access to deidentified and aggregated EHRs from approximately 118 million patients in 68 large healthcare organizations covering diverse geographic regions, age, race and ethnicity, income and insurance groups, and clinical settings. [28] This study analyzed de-identified and population-based EHR data within the TriNetX Analytics platform. The built-in analytics within the TriNetX Analytics platform analyzed patient-level data, however, only population-level results are reported to users. TriNetX data are HIPAA de-identified, and access to protected health information is not allowed. Therefore, there is no risk for protected health information disclosure, and institutional review board review was not needed. We previously performed emulation target trials and cohort studies using the TriNetX platform to examine the association of semaglutide with AD incidence, [24] substance use disorders. [29–32] and suicidal ideation. [33]
Available data elements of EHRs include extensive information on demographics, diagnoses, medications, procedures, laboratory tests, visits, and socioeconomic and lifestyle information. All covariates are either binary, categorical, or continuous, but essentially guaranteed to exist (more details of TriNetX are in the Supplemental Material).
Each component of the target trial was emulated using EHRs from the TriNetX Analytics platform (more details of target trial emulation components are in Supplemental Tables 1 to 4 and Supplemental Figure 1). Patients were classified into drug treatment groups — semaglutide versus other antidiabetic medications (insulin, metformin, DPP-4i, SGLT2i, SU, TZD, and other GLP-1RAs) — based on the first prescription in the study period (December 2017 to December 2021), which was the baseline or index event. The study period of December 2017 to December 2021 was chosen because semaglutide was approved as Ozempic to treat Type 2 Diabetes Mellitus (T2DM in December 2017, and the ending date of December 2021 allowed for a 3-year follow-up for all patients at the time of data collection and analysis on January 6, 2025.
Eligibility criteria and more than 50 baseline covariates were evaluated at baseline. The semaglutide group and each of the 7 comparison treatment groups were separately propensity-score matched (1:1 using nearest neighbor greedy matching with a caliper of 0.25 times the Standard Deviation) for covariates at the baseline to emulate randomization. Cumulative incidences were estimated using the Kaplan–Meier survival analysis in patients who were propensity-score matched. Cox proportional hazard analyses were used to compare rates of time-to-event daily during the follow-up time after the index event. Hazard ratios (HRs) and 95% CIs were calculated. All models are adjusted for confounders at baseline by propensity-score matching baseline covariates.
Statistical analysis
The data were collected and analyzed on January 6, 2025, within the TriNetX Analytics platform. All statistical analyses in this study including propensity-score matching, Kaplan–Meier survival analysis, Cox proportional hazards analyses, were done using built-in functions within the TriNetX Analytics platform that are implemented using the Survival package version 3.2–3 in R 4.0.2 and libraries and utilities for data science and statistics in Python 3.7 and Java 11.0.16. Details of clinical codes for eligibility criteria, treatment strategies, outcomes, and baseline covariates are in Supplemental Table 4.
Results
Study populations
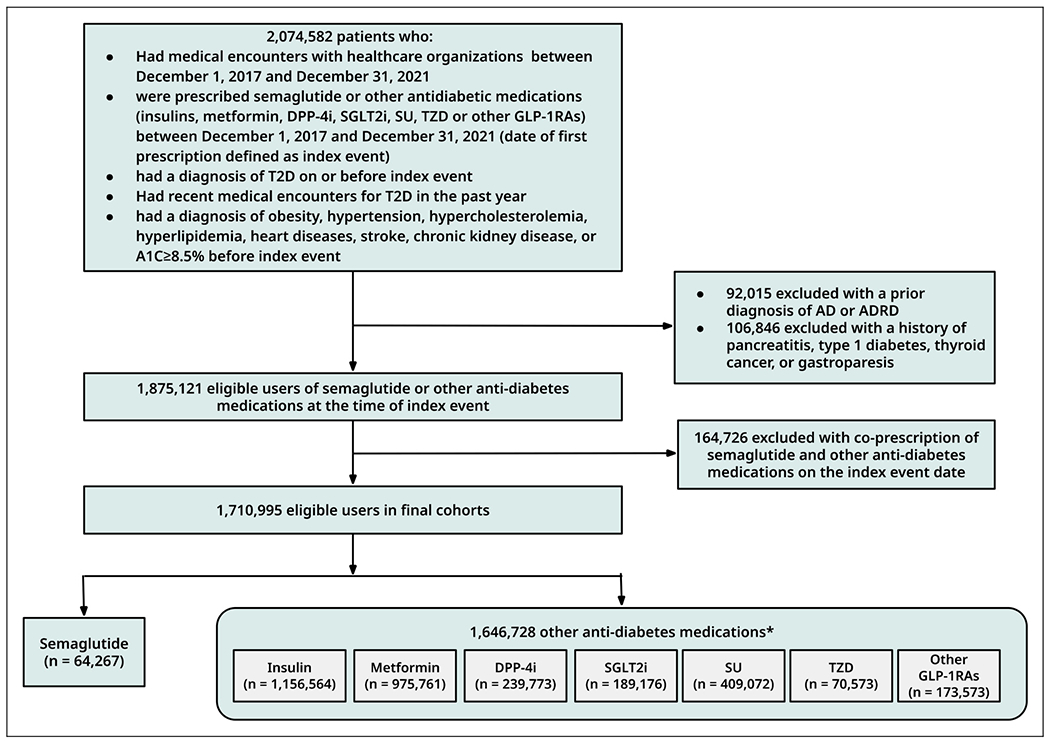
Figure 1

Table 1
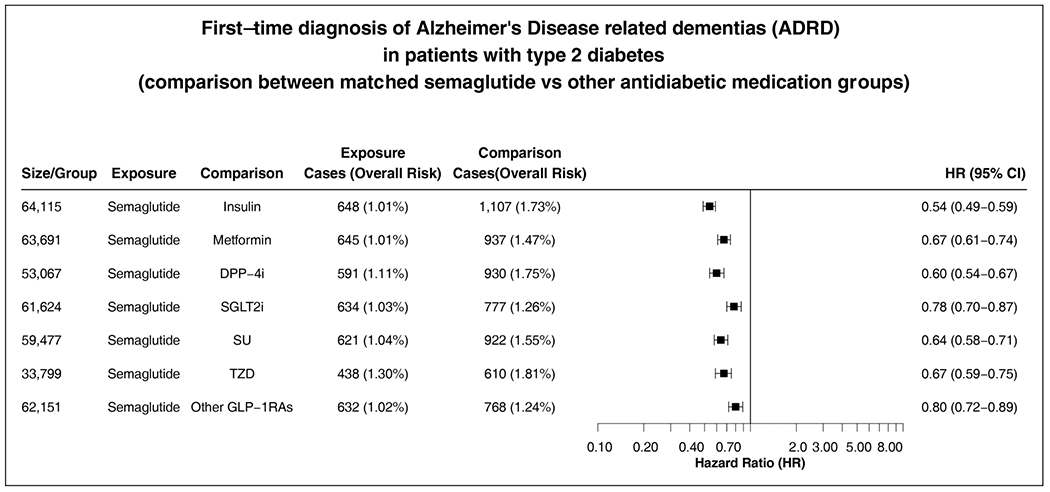
Figure 2
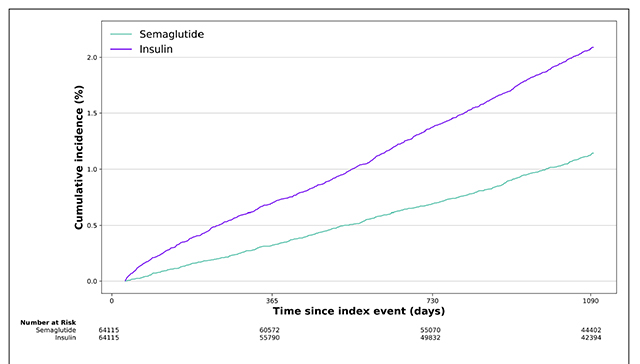
Figure 3 Figure 1 is the flow chart of cohort construction. The study included 1,710,995 T2D patients without prior AD or ADRD who were prescribed antidiabetic medications, including 64,267 prescribed semaglutide and 1,646,728 prescribed other antidiabetic medications. Semaglutide was separately compared with each of the seven other antidiabetic medication classes. Before propensity matching, the semaglutide and comparison groups differed by age, sex, and comorbidities, including dementia risk factors such as obesity, hypertension, stroke, metabolic disorders, and depression. After propensity-score matching, comparison groups were balanced (Table 1, Supplemental Tables 5–10, Supplemental Figures 2–8).
Associations of semaglutide and overall ADRD incidence
Semaglutide was associated with a significantly decreased risk of ADRD incidence compared with other antidiabetic medications in patients with T2D during a 3-year follow-up, with a hazard ratio ranging from 0.54 [0.49–0.59] compared with insulin to 0.80 [0.72–0.89] compared with other GLP-1RAs (Figure 2).
The 3-year cumulative incidence curves comparing semaglutide with each of the seven antidiabetic medications are shown in Figure 3 and Supplemental Figure 9–12. The average follow-up times for semaglutide versus each comparison group are as follows: insulin (975.4 ± 98.8 versus 900.2 ± 158.2 days), metformin (975.4 ± 99.1 versus 948.8 ± 125.1 days), DPP-4i (970.0 ± 102. versus 919.7 ± 143.9 days), SGLT2i (973.9 ± 100.1 versus 932.0 ± 132.6 days), SU (973.3 ± 100.5 versus 929.1 ± 137.7 days), TZD (962.9 ± 108.2 versus 894.6 ± 159.6 days), and other GLP-1RAs (974.1 ± 100.5 versus 946.9 ± 124.0 days). The slope for the semaglutide group was consistently lower than that for the comparison groups, indicating the potential sustained benefits of semaglutide in slowing down the development of ADRD.
Consistent reductions were observed in subgroup analyses. The overall 3-year risk of developing ADRD in younger adults age <65 (average age 51.7 ± 9.44) was 0.27% to 0.57%, lower than in the 2.32% to 3.88% in older adults age ≥65 (average age 70.9 ± 4.76). While semaglutide was associated with a significantly reduced risk of ADRD incidence in both patient groups, the association was stronger in younger adults than in older adults with largely non-overlapping confidence intervals (Supplemental Figures 13 and 14). Significant reductions were observed in both women and men, stronger in women than in men but with overlapping confidence intervals (Supplemental Figures 15 and 16). Significant reductions were observed in T2D patients with and without obesity, stronger in those with obesity than in those without obesity with overlapping confidence intervals (Supplemental Figures 15 and 16).
Associations of semaglutide and incidence of vascular dementia, FTD, LBD, and other dementias
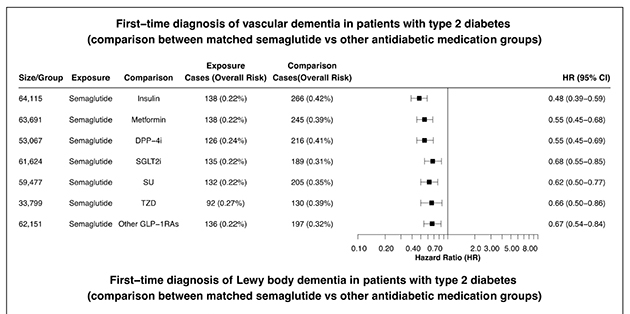
Figure 4 The association of semaglutide with ADRD varied across dementia types. Semaglutide was associated with a significantly lower risk of vascular dementia, with an HR of 0.48 (0.39–0.59) compared with insulin, 0.55 (0.45–0.68) compared with metformin, and 0.67 (0.54–0.84) compared with other GLP-1RAs. Semaglutide was not significantly associated with the risk of FTD or LBD, though the number of cases with FTD or LBD was very low, which limited the power of our analyses. Semaglutide was associated with significantly lower risks of other dementias (Figure 4).
Associations of semaglutide and dementia-related medication prescriptions
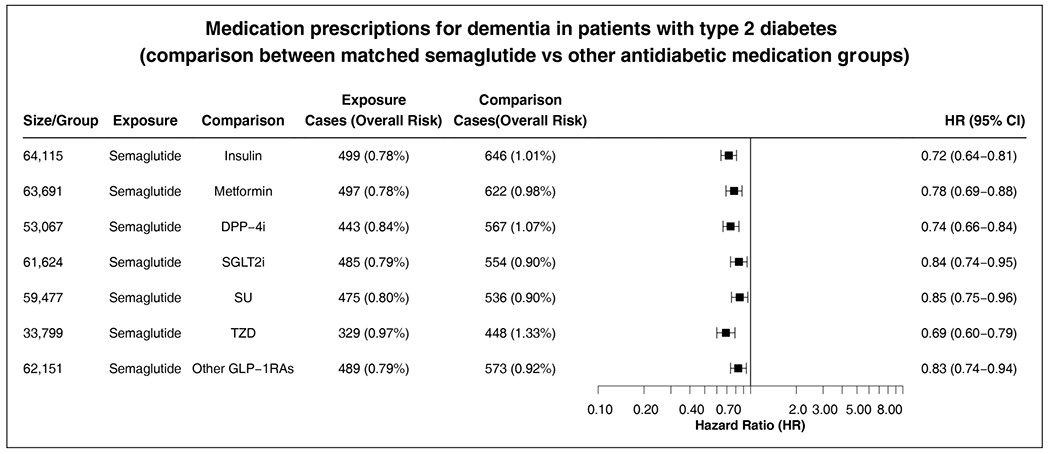
Figure 5 We examined dementia-related medication prescriptions (donepezil, rivastigmine, galantamine, memantine) as an alternative outcome measure. Semaglutide was associated with significantly lower prescriptions of these medications compared with other antidiabetic medications (Figure 5), consistent with the main finding for ADRD incidence.
Sensitivity analysis
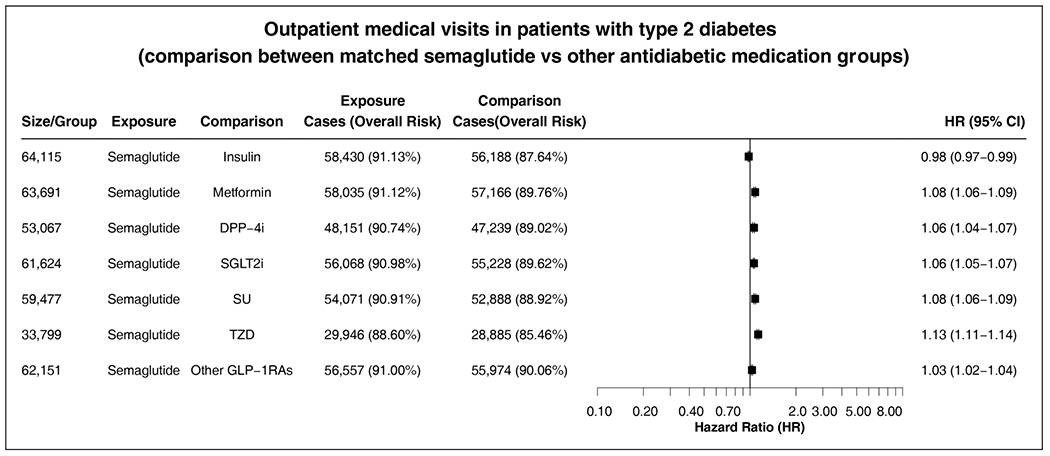
Figure 6 As a sensitivity analysis for potential differences in overall healthcare utilization, we compared the outpatient medical encounters between the matched groups during the 3-year follow-up. The semaglutide group did not differ substantially from the comparison groups in outpatient medical encounters (Figure 6).
Discussion
In a real-world population with T2D who had no prior diagnosis of AD/ADRD, our study shows that semaglutide was associated with a significantly lower risk of overall Alzheimer's disease-related dementia (ADRD) incidence compared with other antidiabetic medications, including insulin, metformin, and other GLP-1RAs. Significant reductions were observed in older and younger patients, women and men, and patients with and without obesity. The slope of the cumulative incidence curve for the semaglutide group was consistently lower than that for the comparison groups, indicating the potential sustained benefit of semaglutide in slowing down the development of ADRD. The association of semaglutide with ADRD varied across dementia types. Specifically, semaglutide was associated with reduced risks for vascular dementia but not FTD or LBD.
Nearly half of dementia cases are theoretically preventable by targeting 14 modifiable risk factors, including obesity, diabetes, hypertension, smoking, depression, physical inactivity, excessive alcohol consumption, traumatic brain injury, less education, air pollution, social isolation, and high LDL cholesterol. [4] Since the population attributable fraction for each risk factor ranges from 1%-7%, [4] targeting several risk factors simultaneously is needed for effective dementia prevention. Findings from the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large RCT of 1,260 adults at risk for cardiovascular disease, showed that a 2-year multidomain intervention consisting of diet, exercise, cognitive training, and vascular risk management could improve or maintain cognitive functioning. However, dementia incidence was not measured. [34]
The Systematic Multi-Domain Alzheimer Risk Reduction Trial (SMART), a RCT with a 2-year personalized, risk-reduction intervention with health coaching and nurse visits in 172 older adults at risk for dementia, led to modest improvements in cognition. [35]
In contrast, the 6-year Prevention of Dementia by Intensive Vascular Care (PreDIVA) did not result in a reduced incidence of all-cause dementia in an unselected population of older people. [36] Several multidomain trials of risk reduction are in progress worldwide. [37–40] While these studies show that multidomain interventions are feasible, their effects are modest and inconclusive, [41] highlighting the challenges of multidomain prevention trials for dementia.
On the other hand, semaglutide might represent a multiple-domain intervention with a single component. Semaglutide is highly effective in simultaneously targeting several dementia risk factors, including obesity, diabetes, and cardiovascular diseases, and appears to also be beneficial for smoking and alcohol drinking. [8, 29, 31, 42–45] In addition, semaglutide has anti-inflammatory effects, [12, 13] and inflammation plays a significant role in the development and progression of all-cause dementias. [5–7, 46, 47]
We previously showed that semaglutide was associated with reduced AD incidence in patients with T2D who had no prior AD. [24] A recent study showed GLP-1RAs were associated with a modestly decreased risk of dementia (HR 0.92 (0.88–0.97) in US veterans with T2D (95% men) compared with other antidiabetic medications. [48] The latter study examined GLP-1RAs and did not examine dementia incidence since patients with prior diagnoses of dementia were not excluded. In this study, we show that semaglutide was associated with a significant 20%-46% reduction in overall ADRD incidence. These results are consistent with the recognition that dementia can be prevented, and support trials to test semaglutide as a multifunctional pharmaceutical strategy for dementia prevention.
The slope of the survival curve for the semaglutide group remained flatter than that of the comparison groups throughout the 3-year follow-up, suggesting that semaglutide slowed down the development of dementia. The slope was largely constant over time, suggesting the effect of semaglutide in slowing the development of ADRD was sustained, which could allow patients to have more time with intact cognitive function. As seen in Table 1, the matched semaglutide and comparison groups are heterogeneous, even though they were well matched. Patients with different characteristics (e.g., age, comorbidities) often have varying risks for progressing to dementia. For those with a very high risk of progression, semaglutide likely slowed down dementia progression from inflammation and improved dementia risk factors, which could explain the early divergence in outcomes between the two groups.
Diabetes contributes to 2% of overall dementia cases and thus its treatment may have a role in dementia prevention. However, a systematic review of intervention trials found no evidence that intensive diabetes treatment prevented cognitive decline or dementia. [49] Given that diabetes and other dementia risk factors account for 1%-7% of the overall dementia risk, detecting modest effects of any single-component intervention would require long follow-up and large sample sizes that are difficult to achieve with RCTs. In our study, semaglutide was associated with a reduced risk of dementia compared with each of the seven different antidiabetic medications, including other GLP-1RAs. While semaglutide has superior glycemic control compared to other antidiabetic agents, [50] the significant 20%-46% reduction compared with other anti-diabetic medications suggests that semaglutide targets multiple dementia risk factors in addition to diabetes.
In our study, semaglutide was associated with reduced incidence of vascular dementia, but not LBD and FTD. Since major modifiable risk factors of vascular dementia include cardiovascular diseases, diabetes, and obesity, [51] one could speculate that semaglutide’s anti-inflammatory effects and recognized benefits in metabolic and vascular diseases could help prevent vascular dementias. [8, 11] FTD is highly heritable with early onset, and head trauma and thyroid diseases are recognized risk factors. [52] Since GLP-1RAs are associated with increased risks of thyroid diseases, [53, 54] this might underlie the trend for an increased risk of FTD we observed with semaglutide. We also failed to see an association of semaglutide with LBD, for which genetic factors account for 60 percent of disease susceptibility, [55] and traumatic brain injury is a recognized risk factor. [56] While these data also suggest a fundamental distinction between FTD, LBD, and AD and other ADRD, the anti-inflammatory effects of semaglutide might still be beneficial in FTD and LBD. [57, 58] Given the low number of FTD and LBD patients in this study, further monitoring of a possible effect of semaglutide on the risk of FTD and LBD is warranted with longer follow-up and specific populations, for example, younger populations for FTD.
In the subgroup analyses, semaglutide was consistently associated with significant reductions in overall ADRD incidence in older or younger patients, women or men, and patients with and without obesity. The association was stronger in younger adults (mean age 51.7 years) with a 32%-56% reduction in ADRD incidence than in older adults (mean age 70.9 years) with a 20%–40% reduction, with non-overlapping confidence intervals. These data suggest that semaglutide could slow down the development of dementia in the general population, but with greater protective effects in younger than older people. Women are twice as likely to develop dementia as men. [59] Our data show that the association of semaglutide with reduced ADRD incidence was stronger in women than in men, but with overlapping confidence intervals.
Future work needs to confirm the findings and understand the underlying mechanisms. We show that semaglutide was associated with a greater reduction of ADRD in T2D patients with obesity than in those without obesity, though with overlapping confidence intervals. These data suggest that semaglutide’s impact on dementia may be partly mediated by its effects on weight loss or the underlying factors of obesity, in addition to mitigating other risk factors. Future work is necessary to examine patients with other medical risk factors to identify which patients may benefit most and provide insights into potential mechanisms of semaglutide action.
The strengths of this study include its large sample size with nationwide scope, rigorous target trial emulation framework, head-to-head comparisons with seven different types of anti-diabetic medications, including the first-generation of GLP-1RAs, clinically meaningful primary outcomes (first diagnosis of ADRD) complemented by secondary outcomes of dementia medication prescriptions.
Our study has several limitations. First, retrospective observational studies using patient EHRs can suffer from inherent limitations including over-/under-/mis-diagnosis, unmeasured or uncontrolled confounders and biases; therefore, causal conclusions cannot be drawn. Second, we hypothesized that semaglutide may slow down dementia development by simultaneously targeting multiple dementia risk factors, lowering peripheral blood sugar, reducing peripheral and central inflammation, and improving overall vascular and metabolic health. However, this cohort study could not characterize the underlying mechanisms, and future mechanistic studies are necessary.
Third, our patient cohort was sourced from the TriNetX Network, necessitating validation of results in other EHR databases and analytics platforms. Fourth, due to semaglutide’s recent approval for treating T2D, our follow-up period was limited to 3 years.
Though we observed a significant reduction in Alzheimer's disease-related dementia (ADRD) risk in a high-risk population with type 2 diabetes (T2D) who had high comorbidity (63% obesity, 82% hypertension, 85% metabolic disorders), future studies should explore longer follow-ups and different populations. In addition, future works are warranted to explore newer GLP-1RAs including Tirzepatide. Tirzepatide is a dual agonist targeting GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors, making it a more powerful treatment for Type 2 Diabetes Mellitus (T2DM and obesity. [43, 60] GIP receptors are expressed in many regions of the brain. [61] Early evidence suggests that GIP may have neuroprotective and anti-inflammatory effects. [62] The dual action of GLP-1 and GIP on the brain, mediated by tirzepatide, could work synergistically to reduce neuroinflammation, protect neurons from damage, and enhance cognitive function in AD and related dementias. Fifth, we used the ICD-10 diagnosis code for ADRD as the primary outcome and corroborated the findings using dementia-related medication prescriptions. However, the ICD-10-based diagnoses are for billing purpose, which have limitations in overdiagnosis, misdiagnosis and under-diagnosis of dementia cases.
However, both the semaglutide and comparison groups in our study were drawn from the same healthcare organizations, so relative rates as measured by hazard ratio are likely valid. Finally, EHRs in TriNetX lack data on medication adherence and explicit tracking of cognitive impairment. Limited genomics data prevented further analysis by genotype status. Practice pattern variations among healthcare organizations and patient healthcare utilization could not explicitly be controlled, though sensitivity analyses indicated similar healthcare utilization between semaglutide and comparison groups, minimizing potential surveillance bias.
Our findings show evidence that semaglutide treatment in patients with diabetes appears to protect from vascular dementia and other dementia, but not FTD or LBD. As we and others have previously shown benefit for semaglutide and other GLP-1RA medications in AD, our results support the GLP-1 receptor as a target for dementia prevention.
Preclinical and clinical studies are necessary to understand the mechanisms and establish causal effects through randomized trials. Economic and policy analyses are needed to examine the cost-effectiveness of integrating semaglutide and other pharmacotherapy-based prevention strategies with existing behavior-based approaches, such as exercise and diet in in achieving substantial benefits for preserving cognitive function and preventing AD and related dementias.
Supplementary Material
Supplemental Tables 1-10, Supplemental Figures 2–18
and TriNetX Analytics Platform statement. (1.9MB, pdf)Acknowledgements
The authors have no acknowledgments to report.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We acknowledge support from National Institute on Aging (grant numbers AG057557, AG061388, AG062272, AG076649). National Center for Advancing Translational Sciences (NCATS) (grant number TR004528).
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: George Perry is an Editor-in-Chief of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer-review. The remaining authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data availability statement
This study used population-level aggregate and HIPAA de-identified data collected by the TriNetX platform (“US Collaborative Network”) and available from TriNetX, LLC (https://trinetx.com/), but third-party restrictions apply to the availability of these data. The data were used under license for this study with restrictions that do not allow for the data to be redistributed or made publicly available.
To gain access to the data, a request can be made to TriNetX (join@trinetx.com), but costs may be incurred, and a data-sharing agreement may be necessary. Data specific to this study including diagnosis codes and cohort characteristics in aggregated format are included in the manuscript as tables, figures, and supplemental files.
Data through the TriNetX platform is queried in real-time with results being returned typically in seconds to minutes. Data from the underlying electronic health records of participating healthcare organizations is refreshed in the TriNetX platform from daily to every couple of months depending on the healthcare organization.
Code availability
All the statistical analyses in this study including propensity-score matching, and Cox proportional hazards used web-based built-in functions within the TriNetX Analytics Platform that are implemented using the Survival package version 3.2-3 in R 4.0.2 and libraries/utilities for data science and statistics in Python 3.7 and Java 11.0.16.
References:
Rajan KB, Weuve J, Barnes LL, et al.
Population estimate of people with clinical Alzheimer’s disease and
mild cognitive impairment in the United States (2020-2060).
Alzheimers Dement 2021; 17: 1966–1975.Murphy S, Kochanek K, Xu J, et al.
Mortality in the United States, 2016.
NCHS Data Brief 2017; 293: 1–8.Nandi A, Counts N, Chen S, et al.
Global and regional projections of the economic burden of
Alzheimer’s disease and related dementias from 2019 to 2050:
a value of statistical life approach.
EClinicalMedicine 2022; 51: 101580.Livingston G, Huntley J, Liu KY, et al.
Dementia prevention, intervention, and care:
2024 report of the Lancet standing Commission.
Lancet 2024; 404: 572–628.Engelhart MJ, Geerlings MI, Meijer J, et al.
Inflammatory proteins in plasma and the risk of dementia:
the Rotterdam Study.
Arch Neurol 2004; 61: 668–672.Koyama A, O’Brien J, Weuve J, et al.
The role of peripheral inflammatory markers in dementia
and Alzheimer’s disease: a meta-analysis.
J Gerontol A Biol Sci Med Sci 2013; 68: 433–440.Schmidt R, Schmidt H, Curb JD, et al.
Early inflammation and dementia: a 25-year follow-up
of the Honolulu-Asia Aging Study.
Ann Neurol 2002; 52: 168–174.Marso SP, Bain SC, Consoli A, et al.
Semaglutide and cardiovascular outcomes in patients with type 2 diabetes.
N Engl J Med 2016; 375: 1834–1844.Davies M, Pieber TR, Hartoft-Nielsen M-L, et al.
Effect of oral semaglutide compared with placebo and subcutaneous
semaglutide on glycemic control in patients with type 2 diabetes:
a randomized clinical trial.
JAMA 2017; 318: 1460–1470.Davies M, Færch L, Jeppesen OK, et al.
Semaglutide 2·4 mg once a week in adults with overweight or obesity,
and type 2 diabetes (STEP 2): a randomised, double-blind,
double-dummy, placebo-controlled, phase 3 trial.
Lancet 2021; 397: 971–984.Lincoff AM, Brown-Frandsen K, Colhoun HM, et al.
Semaglutide and cardiovascular outcomes in obesity without diabetes.
N Engl J Med 2023; 389: 2221–2232.Bendotti G, Montefusco L, Lunati ME, et al.
The anti-inflammatory and immunological properties
of GLP-1 receptor agonists.
Pharmacol Res 2022; 182: 106320.Drucker DJ.
The benefits of GLP-1 drugs beyond obesity.
Science 2024; 385: 258–260.Wang Z-J, Li X-R, Chai S-F, et al.
Semaglutide ameliorates cognition and glucose metabolism dysfunction
in the 3xTg mouse model of Alzheimer’s disease via
the GLP-1R/SIRT1/GLUT4 pathway.
Neuropharmacology 2023; 240: 109716.Wang Z-J, Han W-N, Chai S-F, et al.
Semaglutide promotes the transition of microglia from M1 to
M2 type to reduce brain inflammation in APP/PS1/tau mice.
Neuroscience 2024; 563: 222–234.Zhang Y, Tang C, He Y, et al.
Semaglutide ameliorates Alzheimer’s disease and restores
oxytocin in APP/PS1 mice and human brain organoid models.
Biomed Pharmacother 2024; 180: 117540.Edison P, Femminella GD, Ritchie CW, et al.
Evaluation of liraglutide in the treatment of Alzheimer’s disease.
Alzheimers Dement 2021; 17: e057848.Edison P, Femminella GD, Ritchie C, et al.
MRI Changes following treatment of glp-1 analogue,
liraglutide in Alzheimer’s disease.
Alzheimers Dement 2023; 19: e080538.Edison P.
Evaluation of Novel GLP-1 analogue in the
treatment of Alzheimer’s disease.
Alzheimers Dement 2024; 20: e089799.Cummings JL, Atri A, Feldman HH, et al.
Evoke and evoke +: design of two large-scale, double-blind, placebo-
controlled, phase 3 studies evaluating efficacy, safety, and tolerability
of semaglutide in early-stage symptomatic Alzheimer’s disease.
Alzheimers Res Ther 2025; 17: 14.Clinicaltrials.gov.
A research study investigating Semaglutide in people
with early Alzheimer’s disease (EVOKE),
https://clinicaltrials.gov/study/NCT04777396
(2024, accessed December 1, 2024).Clinicaltrials.gov.
A research study investigating semaglutide in people
with early Alzheimer’s disease (EVOKE plus),
https://clinicaltrials.gov/study/NCT04777409
(2024, accessed December 1, 2024).Clinicaltrials.gov.
COMMETS-combination MCI metabolic syndrome,
https://clinicaltrials.gov/study/NCT06072963
(2024, accessed December 1, 2024).Wang W, Wang Q, Qi X, et al.
Associations of semaglutide with first-time diagnosis of Alzheimer’s
disease in patients with type 2 diabetes: target trial emulation
using nationwide real-world data in the US.
Alzheimers Dement 2024; 20: 8661–8672.Hernán MA and Robins JM.
Using big data to emulate a target trial when
a randomized trial is not available.
Am J Epidemiol 2016; 183: 758–764.Hernán MA, Wang W and Leaf DE.
Target trial emulation: a framework for
causal inference from observational data.
JAMA 2022; 328: 2446–2447.Nordisk Novo.
Ozempic prescribing information,
https://www.ozempic.com/prescribing-information.html
(accessed June 27, 2024).TriNetX.
The world’s largest, living ecosystem of real-world data
and evidence. TriNetX, https://trinetx.com/real-world-data/
(2021, accessed January 16, 2024).Wang W, Volkow ND, Berger NA, et al.
Association of semaglutide with tobacco use disorder in patients with
type 2 diabetes. Target trial emulation using real-world data.
Ann Intern Med 2024; 177: 1016–1027.Wang W, Volkow ND, Ms QQW, et al.
Semaglutide and opioid overdose risk in patients with
type 2 diabetes and opioid use disorder.
JAMA Netw Open 2024; 7: e2435247.Wang W, Volkow ND, Berger NA, et al.
Associations of semaglutide with incidence and recurrence
of alcohol use disorder in real-world population.
Nat Commun 2024; 15: 4548.Wang W, Volkow ND, Berger NA, et al.
Association of semaglutide with reduced incidence and relapse of cannabis
use disorder in real-world populations: a retrospective cohort study.
Mol Psychiatry 2024; 29: 2587–2598.Wang W, Volkow ND, Berger NA, et al.
Association of semaglutide with risk of suicidal ideation
in a real-world cohort.
Nat Med 2024; 30: 168–176.Ngandu T, Lehtisalo J, Solomon A, et al.
A 2 year multidomain intervention of diet, exercise, cognitive training,
and vascular risk monitoring versus control to prevent cognitive decline
in at-risk elderly people (FINGER): a randomised controlled trial.
Lancet 2015; 385: 2255–2263.Yaffe K, Vittinghoff E, Dublin S, et al.
Effect of personalized risk-reduction strategies on cognition and
dementia risk profile among older adults:
the SMARRT randomized clinical trial.
JAMA Intern Med 2024; 184: 54–62.van Charante EPM, Richard E, Eurelings LS, et al.
Effectiveness of a 6-year multidomain vascular care intervention to prevent
dementia (preDIVA): a cluster-randomised controlled trial.
Lancet 2016; 388: 797–805.Cho SH, Kang HJ, Park YK, et al.
South Korean study to PrEvent cognitive impaiRment and protect BRAIN health
through Multidomain interventions via facE-to-facE and video communication
plaTforms in mild cognitive impairment (SUPERBRAIN-MEET):
protocol for a Multicenter Randomized Controlled Trial.
Dement Neurocognitive Disord 2024; 23: 30–43.Sakurai T, Sugimoto T and Akatsu H.
Japan-multimodal intervention trial for the prevention
of dementia: a randomized controlled trial.
Alzheimers Dement 2024; 20: 3918–3930.Kivipelto M, Mangialasche F, Ngandu T, et al.
World Wide Fingers will advance dementia prevention.
Lancet Neurol 2018; 17: 27.Barbera M, Lehtisalo J, Perera D, et al.
A multimodal precision-prevention approach combining lifestyle intervention
with metformin repurposing to prevent cognitive impairment and disability:
the MET-FINGER randomised controlled trial protocol.
Alzheimers Res Ther 2024; 16: 23.The Lancet Neurology.
Pointing the way to primary prevention of dementia.
Lancet Neurol 2017; 16: 677.Rubino DM, Greenway FL, Khalid U, et al.
Effect of weekly subcutaneous semaglutide vs daily liraglutide on
body weight in adults with overweight or obesity without
diabetes: the STEP 8 randomized clinical trial.
JAMA 2022; 327: 138–150.Frías JP, Davies MJ, Rosenstock J, et al.
Tirzepatide versus semaglutide once weekly in patients
with type 2 diabetes.
N Engl J Med 2021; 385: 503–515.Perkovic V, Tuttle KR, Rossing P, et al.
Effects of semaglutide on chronic kidney disease in
patients with type 2 diabetes.
N Engl J Med 2024; 391: 109–121.Newsome PN, Buchholtz K, Cusi K, et al.
A placebo-controlled trial of subcutaneous semaglutide
in nonalcoholic steatohepatitis.
N Engl J Med 2021; 384: 1113–1124.Zhou M, Xu R, Kaelber DC, et al.
Tumor necrosis factor (TNF) blocking agents are associated with lower
risk for Alzheimer’s disease in patients with
rheumatoid arthritis and psoriasis.
PLoS One 2020; 15: e0229819.Zheng C, Fillmore NR, Ramos-Cejudo J, et al.
Potential long-term effect of tumor necrosis factor inhibitors on
dementia risk: a propensity score matched
retrospective cohort study in US Veterans.
Alzheimers Dement 2022; 18: 1248–1259.Xie Y, Choi T and Al-Aly Z.
Mapping the effectiveness and risks of GLP-1 receptor agonists.
Nat Med 2025; 31: 951–962.National Academies of Sciences Engineering, Medicine.
Preventing cognitive decline and dementia: A way forward.
Washington, DC: The National Academies Press, 2017.Andreadis P, Karagiannis T, Malandris K, et al.
Semaglutide for type 2 diabetes mellitus:
a systematic review and meta-analysis.
Diabetes Obes Metab 2018; 20: 2255–2263.Smith EE.
Clinical presentations and epidemiology of vascular dementia.
Clin Sci (Lond) 2017; 131: 1059–1068.Onyike CU and Diehl-Schmid J.
The epidemiology of frontotemporal dementia.
Int Rev Psychiatry 2013; 25: 130–137.Hu W, Song R, Cheng R, et al.
Use of GLP-1 receptor agonists and occurrence of thyroid disorders:
a meta-analysis of randomized Controlled Trials.
Front Endocrinol (Lausanne) 2022; 13: 927859.Brito JP, Herrin J, Swarna KS, et al.
GLP-1RA use and thyroid cancer risk.
JAMA Otolaryngol Head Neck Surg 2025; 151: 243–252.Guerreiro R, Ross OA, Kun-Rodrigues C, et al.
Investigating the genetic architecture of dementia with
Lewy bodies: a two-stage genome-wide association study.
Lancet Neurol 2018; 17: 64–74.Crane PK, Gibbons LE, Dams-O’Connor K, et al.
Association of traumatic brain injury with late-life
neurodegenerative conditions and neuropathologic findings.
JAMA Neurol 2016; 73: 1062–1069.Bright F, Werry EL, Dobson-Stone C, et al.
Neuroinflammation in frontotemporal dementia.
Nat Rev Neurol 2019; 15: 540–555.Surendranathan A, Su L, Mak E, et al.
Early microglial activation and peripheral inflammation
in dementia with Lewy bodies.
Brain 2018; 141: 3415–3427.Thies W and Bleiler L.
Alzheimer’s disease facts and figures.
Alzheimers Dement 2013; 9: 208–245.Rodriguez PJ, Cartwright BM, Gratzl S, et al.
Semaglutide vs tirzepatide for weight loss in adults
with overweight or obesity.
JAMA Intern Med 2024; 184: 1056–1064.Fukuda M.
The role of GIP receptor in the CNS for the pathogenesis of obesity.
Diabetes 2021; 70: 1929–1937.Zhang ZQ and Hölscher C.
GIP Has neuroprotective effects in Alzheimer and Parkinson’s disease models.
Peptides 2020; 125: 170184.
Return to ALZHEIMER's
Since 7-19-2025


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |