

Coenzyme Q10 Supplementation in Reducing Inflammation:
An Umbrella ReviewThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Chiropractic Medicine 2023 (Jun); 22 (2): 131137 ~ FULL TEXT
Marc P. McRae DC
Department of Basic Sciences,
National University of Health Sciences,
Lombard, Illinois
Objective: The purpose of this study was to review meta-analyses on the effectiveness of coenzyme Q10 supplementation in reducing inflammation through changes in the inflammatory biomarkers C-reactive protein, interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α).
Methods: An umbrella review of all published meta-analyses was performed. A PubMed search from January 1, 1980, to December 31, 2021, was conducted using the following search strategy: "(coenzyme q10 OR CoQ10 OR ubiquinone OR ubiquinol) AND (meta-analysis OR systematic review)". Only English language publications that provided quantitative statistical analysis on coenzyme Q10 supplementation and markers of inflammation were retrieved.
Results: Seven meta-analyses were retrieved for inclusion in this umbrella review, and for all 3 inflammatory biomarker marker groups, the median intake of supplemental coenzyme Q10 was 200 mg/d for a median duration of 12 weeks. For C-reactive protein, only 3 of the 7 meta-analyses presented with statistically significant reductions, while statistically significant reductions in IL-6 and TNF-α for were observed in 4 of the 5 meta-analyses and 3 of the 4 meta-analyses, respectively. However, statistically significant heterogeneity was observed in the majority of these meta-analyses.
Conclusion: The majority of included meta-analyses showed that coenzyme Q10 supplementation significantly decreased the proinflammatory cytokines IL-6 and TNF-α. However, heterogeneity was observed in the majority of these meta-analyses, and therefore the results should be interpreted with caution.
Keywords: Coenzyme Q10; Inflammation; Meta-Analysis.
From the FULL TEXT Article:
Introduction
In the United States, more than 525,000 people annually will have a new myocardial infarction, and more than 15 million are estimated to be living with coronary heart disease. [1] A systematic review of systematic reviews concluded that coenzyme Q10 supplementation might be beneficial for patients with heart failure, [2] and a separate meta-analysis on patients with heart disease supplemented with coenzyme Q10 has shown a statistically significant decrease in mortality. [3] Furthermore, a statistically significant increase in cardiac ejection fraction, [4, 5] as well as improved endothelial function as measured using flow-mediated dilation. [6]
The mechanisms explaining coenzyme Q10 supplementation benefits are typically cited as being due to increased cellular energy generation and reduced oxidative stress. [2] However, heart disease is a disease of inflammation, [7] and since coenzyme Q10 has been shown to have anti-inflammatory properties via nuclear factor kappa B (NFκB) antagonism, [8] then it may be hypothesized that the benefits of coenzyme Q10 supplementation on heart disease is due to its anti-inflammatory actions.
This umbrella review gathered meta-analyses to determine if the cardiac benefits advocated in the literature with coenzyme Q10 supplementation are due in part to the anti-inflammatory properties of these supplements and the effect of coenzyme Q10 supplementation on the inflammatory biomarkers C-reactive protein (CRP), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α).
Methods
An umbrella review summarizes existing published meta-analyses and systematic reviews and determines whether authors addressing similar review questions independently observe similar results and arrive at similar conclusions. [9]
Since the first published meta-analyses started appearing in 1980, a systematic literature search of PubMed, Cochrane Library, and Cumulative Index to Nursing and Allied Health Literature from January 1, 1980, to December 31, 2021, was conducted using the following search strategy: (coenzyme q10 OR CoQ10 OR ubiquinone OR ubiquinol) AND (meta-analysis OR systematic review). Only English language full-text articles were retrieved, which provided quantitative statistical analysis of pooled treatment effects on changes in CRP, IL-6, and TNFα. Only meta-analyses conducted on clinical trials, which included randomized, double-blind, placebo-controlled trials with human participants receiving coenzyme Q10 supplementation alone, were included. Meta-analyses or systematic reviews that did not present study-specific summary data using a minimum of 3 randomized controlled trials were excluded.
For the published papers that were accepted into this review, the following information was extracted and entered into an Excel (Microsoft) spreadsheet: number of publications included in the meta-analysis, number of total participants, median coenzyme Q10 dose, and pooled treatment effects on CRP, IL-6, and TNF-α. Papers were also assessed for their disclosure of quality assessment (using either the Cochrane Collaboration Risk of Bias Tool or the Jadad scale), statistical heterogeneity (Cochrane's Q test and I2 statistic), and publication bias (visual inspection of funnel plots and Egger or Begg regression test). Because this is a descriptive summary review of meta-analyses, no statistical analyses were performed.
Results

Figure 1

Table 1

Table 2

Table 3 The initial search strategy identified 97 articles, and after review, 10 meta-analyses were retrieved for inclusion. However, 3 of the 10 meta-analyses were excluded because they fell outside the selection criteria, as they utilized only 2 trials in their analysis. [1012] A flow chart of the meta-analysis selection process is summarized in Figure 1, and Table 1, Table 2, Table 3 provide the detailed analysis of the 7 meta-analyses entered into this umbrella review. [1319]
The results of 7 meta-analyses in Table 1 show that median intakes of 200 mg/d of coenzyme Q10 for a median duration of 12 weeks provided statistically significant reductions in CRP for only 3 of the 7 meta-analyses. Statistically significant heterogeneity was only observed in 3 of the 7 meta-analyses, and no publication bias was observed in any of the 7 meta-analyses that used Egger or Begg regression tests.
The results of 5 meta-analyses in Table 2 show that median intakes of 200 mg/d of coenzyme Q10 for a median duration of 12 weeks provided statistically significant reductions in IL-6 for 4 of the 5 meta-analyses. However, statistically significant heterogeneity was observed in 3 of the 5 meta-analyses, but no publication bias was observed in any of the 5 meta-analyses that used Egger or Begg regression tests.
The results of 4 meta-analyses in Table 3 show that median intakes of 200 mg/d of coenzyme Q10 for a median duration of 12 weeks provided statistically significant reductions in TNF-α for 3 of the 4 meta-analyses. However, statistically significant heterogeneity was observed in 3 of the 4 meta-analyses, but no publication bias was observed in any of the 4 meta-analyses that used Egger or Begg regression tests.
Discussion
Heart failure and coronary heart disease are correlated with decreases in coenzyme Q10 myocardial tissue concentrations,20 and though coenzyme Q10 has been lauded as an antioxidant, it is possible that the reduction in heart injury may be due to the anti-inflammatory actions of coenzyme Q10. The modest decreases in IL-6 and TNF-α demonstrate that coenzyme Q10 supplementation has a potential anti-inflammatory effect.
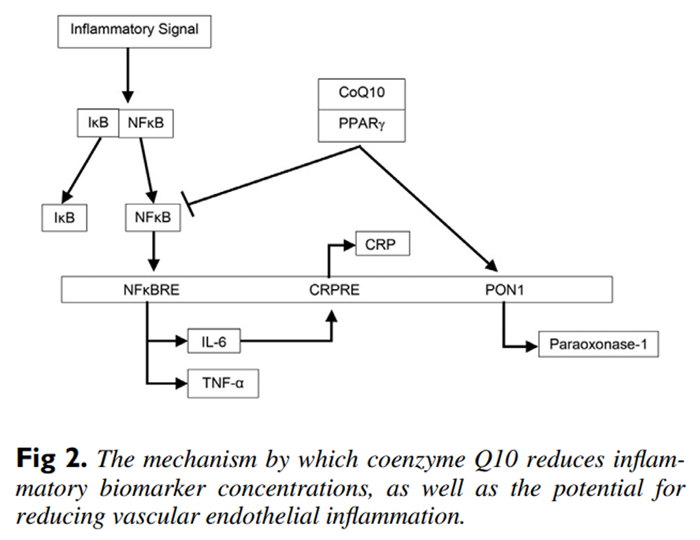
Figure 2 Nuclear factor kappa B is an inducible transcription factor that up-regulates a large array of proinflammatory cytokines that include the gene expression of IL-6 and TNF-α.21 Concerning the mechanism of action of coenzyme Q10, it has been determined that coenzyme Q10 is a ligand for peroxisome proliferator-activated receptor gamma (PPAR-γ), and when coenzyme Q10 is bound to this nuclear receptor protein, PPAR-γ inhibits NFκB activation.7,22, 23 Therefore, coenzyme Q10 antagonism of NFκB through PPAR-γ inhibits the proinflammatory cytokine expression of both IL-6 and TNF-α (see Figure 2).
However, if CRP is a marker of inflammation, then the anti-inflammatory benefits of coenzyme Q10 supplementation are questionable, as fewer than half of the meta-analyses showed statistically significant decreases in CRP concentrations. C-reactive protein is a product of hepatic stimulation by IL-6, and so it may be that the proinflammatory marker IL-6 reflects the status of inflammatory reactions with more sensitivity than CRP.14 In addition, TNF-α upregulates the production of other cytokines such as IL-6, which in turn leads to further inflammation.17 Thus, IL-6 and TNF-α reflect the status of inflammatory reactions with more sensitivity than CRP, and, moreover, both IL-6 and TNF-α are more strongly associated with cardiovascular disease when compared to CRP.24
The coenzyme Q10-PPAR-γ complex also activates the paraoxonase-1 (PON1) gene, and this increases the synthesis and release of PON1 from the liver. Paraoxonase-1 plays an important role as an antioxidant in preventing the oxidation of low-density lipoproteins and therefore reducing the inflammatory response in vascular endothelial cells.25 Coupled to coenzyme Q10s role in activating PON1, coenzyme Q10 also possesses free-radical scavenging ability, which can reduce the development of atherogenic oxidized low-density lipoproteins, which play a significant role in the multifactorial development of cardiovascular disease.26 Coenzyme Q10 acting as an antioxidant reduces the levels of reactive oxygen species that can cause mitochondrial injury and induce apoptosis of cardiovascular cells.27 Moreover, coenzyme Q10 promotes recycling of α-tocopherol, which increases the protection against cardiac cell plasma membrane peroxidation.28 And finally, limited cardiac ejection fraction plays a role in the multifactorial development of cardiovascular disease, and because coenzyme Q10 is an integral component of the inner mitochondrial membrane for electron transport, coenzyme Q10 is required for augmenting mitochondrial adenosine triphosphate energy production, which is essential for ventricular myocardial contraction.26
Although coenzyme Q10 has been shown to be beneficial for reducing the inflammatory markers observed in coronary heart disease, coenzyme Q10s anti-inflammatory potential may be helpful for many other inflammatory conditions, such as rheumatoid arthritis, osteoarthritis, nonalcoholic fatty liver disease, multiple sclerosis, dermatitis, ulcerative colitis, polycystic ovary syndrome, migraine, bipolar disorder, and even after strenuous exercise.29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41
No mention of adverse side effects associated with coenzyme Q10 supplementation was discussed in the 7 meta-analyses included in this umbrella review. This is expected since the long-term safety and tolerability of coenzyme Q10 supplementation at doses of 100 to 300 mg/d has been deemed safe.42
Limitations
The statistically significant heterogeneity observed in these meta-analyses may be due to differences in study design, population characteristics, the dosage and/or form of coenzyme Q10 used, and the duration of intervention. The diversity of the individual clinical trials combined in a meta-analysis represents a big limitation when pooling outcomes in a meta-analysis. Most of the individual clinical trials used in the meta-analyses were performed on small sample sizes of 20 to 60 participants of various population types (patients with coronary artery disease, obesity, hypertension, diabetes, and chronic kidney disease). The duration of the clinical trials ranged from 4 to 36 weeks, and the coenzyme Q10 dosages ranged from 60 to 400 mg. Finally, although data on the different forms of coenzyme Q10 supplementation (such as powder, suspension, oil solution, or solubilized forms, as well as oxidized versus reduced forms) were never extracted and presented by the 7 meta-analyses analyzed in this umbrella review, it is possible that these different forms of coenzyme Q10 can affect coenzyme Q10 bioavailability, thus increasing heterogeneity and confounding the pooled effect.17
Additional research utilizing large, well-designed randomized controlled studies with homogeneous etiologies and longer durations of time is required to confirm the efficacy of coenzyme Q10 as an anti-inflammatory in heart failure patients. And finally, as in all literature reviews, the quality of this umbrella review is directly related to the quality of the included meta-analyses, which are dependent upon the design and reporting quality of the individual meta-analysis itself, as well as on the quality of the individual studies used to conduct the meta-analysis. The majority (70%) of the meta-analyses in this umbrella review were appraised as having high methodological quality.
Conclusion
The meta-analyses included in this study show that coenzyme Q10 supplementation significantly decreases the proinflammatory cytokines IL-6 and TNF-α. Because of the heterogeneity observed in these meta-analyses, their pooled effect sizes should be interpreted with caution.
Practical Applications
Supplemental coenzyme Q10 has been used for many years as an adjunctive treatment for heart disease, and its efficacy was touted as being due to its cellular energy generation and free radical scavenging ability.
However, coenzyme Q10 may have anti-inflammatory actions.
This property has potential to address heart disease, a disease of inflammation, but also inflammatory conditions such as arthritis, inflammatory bowel disease, and multiple sclerosis.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): M.P.M.
Design (planned the methods to generate the results): M.P.M.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): M.P.M.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): M.P.M.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): M.P.M.
Literature search (performed the literature search): M.P.M.
Writing (responsible for writing a substantive part of the manuscript): M.P.M.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): M.P.M.
References:
Tsao CW, Aday AW, Almarzooq ZI, et al.
Heart disease and stroke statistics-2022 update:
a report from the American Heart Association.
Circulation. 2022;145(8):e153e639.Jafari M, Mousavi SM, Asgharzadeh A, Yazdani N.
Coenzyme Q10 in the treatment of heart failure:
a systematic review of systematic reviews.
Indian Heart J. 2018;70(1):S111S117.Lei L, Liu Y.
Efficacy of coenzyme Q10 in patients with cardiac failure:
a meta-analysis of clinical trials.
BMC Cardiovasc Disord. 2017;17(1):196.Sander S, Coleman CI, Patel AA, Kluger J, White CM.
The impact of coenzyme Q10 on systolic function in patients
with chronic heart failure.
J Card Fail. 2006;12(6):464472.Fotino AD, Thompson-Paul AM, Bazzano LA.
Effect of coenzyme Q10 supplementation on heart failure:
a meta-analysis.
Am J Clin Nutr. 2013;97(2):268275.Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L.
Effects of coenzyme Q10 on vascular endothelial function in humans:
a meta-analysis of randomized controlled trials.
Atherosclerosis. 2012;221(2):311316.Sarwar N, Thompson AJ, Di Angelantonio E.
Markers of inflammation and risk of coronary heart disease.
Dis Markers. 2009;26(5-6):217225.Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Dφring F.
Functions of coenzyme Q10 in inflammation and gene expression.
Biofactors. 2008;32(1-4):179183.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P.
Summarizing systematic reviews: methodological development, conduct
and reporting of an umbrella review approach.
Int J Evid Based Healthc. 2015;13(3):132140.Dludla PV, Orlando P, Silvestri S, et al.
Coenzyme Q10 supplementation improves adipokine levels and alleviates
inflammation and lipid peroxidation in conditions of metabolic syndrome:
a meta-analysis of randomized controlled trials.
Int J Mol Sci. 2020;21(9):3247.Alimohammadi M, Rahimi A, Faramarzi F, et al.
Effects of coenzyme Q10 supplementation on inflammation, angiogenesis,
and oxidative stress in breast cancer patients: a systematic
review and meta-analysis of randomized controlled- trials.
Inflammopharmacology. 2021;29(3):579593.Xu Y, Yang G, Zuo X, et al.
A systematic review for the efficacy of coenzyme Q10
in patients with chronic kidney disease.
Int Urol Nephrol. 2022;54(1):173184.Farsi F, Heshmati J, Keshtkar A, et al.
Can coenzyme Q10 supplementation effectively reduce human tumor necrosis
factor-α and interleukin-6 levels in chronic inflammatory diseases?
A systematic review and meta-analysis of randomized controlled trials.
Pharmacol Res. 2019;148Jorat MV, Tabrizi R, Kolahdooz F, et al.
The effects of coenzyme Q10 supplementation on biomarkers of inflammation
and oxidative stress in among coronary artery disease:
a systematic review and meta-analysis of
randomized controlled trials.
Inflammopharmacology. 2019;27(2):233248.Aslani Z, Shab-Bidar S, Fatahi S, Djafarian K.
Effect of coenzyme Q10 supplementation on serum of high sensitivity
c-reactive protein level in patients with cardiovascular diseases:
a systematic review and meta-analysis of randomized controlled trials.
Int J Prev Med. 2018;9:82.Mazidi M, Kengne AP, Banach M.
Lipid and Blood Pressure Meta-analysis Collaboration Group.
Effects of coenzyme Q10 supplementation on plasma C-reactive protein
concentrations: a systematic review and meta-analysis
of randomized controlled trials.
Pharmacol Res. 2018;128:130136.Fan L, Feng Y, Chen GC, Qin LQ, Fu CL, Chen LH.
Effects of coenzyme Q10 supplementation on inflammatory markers:
a systematic review and meta-analysis of randomized controlled trials.
Pharmacol Res. 2017;119:128136.Zhai J, Bo Y, Lu Y, Liu C, Zhang L.
Effects of coenzyme Q10 on markers of inflammation:
a systematic review and meta-analysis.
PLoS One. 2017;12(1)Bakhshayeshkaram M, Lankarani KB, Mirhosseini N, et al.
The effects of coenzyme Q10 supplementation on metabolic profiles
of patients with chronic kidney disease: a systematic review and
meta-analysis of randomized controlled trials.
Curr Pharm Des. 2018;24(31):37103723.Zozina VI, Covantev S, Goroshko OA, Krasnykh LM, Kukes VG.
Coenzyme Q10 in cardiovascular and metabolic diseases:
current state of the problem.
Curr Cardiol Rev. 2018;14(3):164174.Rahmani E, Jamilian M, Samimi M, et al.
The effects of coenzyme Q10 supplementation on gene expression related to
insulin, lipid and inflammation in patients with polycystic ovary syndrome.
Gynecol Endocrinol. 2018;34(3):217222.Liu T, Zhang L, Joo D, Sun SC.
NF-κB signaling in inflammation.
Signal Transduct Target Ther. 2017;2:17023.Lee TI, Kao YH, Chen YC, Chen YJ.
Proinflammatory cytokine and ligands modulate cardiac
peroxisome proliferator-activated receptors.
Eur J Clin Invest. 2009;39(1):2330.Heidari A, Hamidi G, Soleimani A, Aghadavod E, Asemi Z.
Effects of coenzyme Q10 supplementation on gene expressions related
to insulin, lipid, and inflammation pathways in patients with diabetic nephropathy.
Iran J Kidney Dis. 2018;12(1):1421.Khateeb J, Gantman A, Kreitenberg AJ, Aviram M, Paraoxonase Fuhrman B.
1 (PON1) expression in hepatocytes is upregulated by
pomegranate polyphenols: a role for PPAR-gamma pathway.
Atherosclerosis. 2010;208(1):119125.Garrido-Maraver J, Cordero MD, Oropesa-Avila M, et al.
Clinical applications of coenzyme Q10.
Front Biosci (Landmark Ed) 2014;19(4):619633.DiNicolantonio JJ, Bhutani J, McCarty MF, O'Keefe JH.
Coenzyme Q10 for the treatment of heart failure:
a review of the literature.
Open Heart. 2015;2(1)Kamzalov S, Sumien N, Forster MJ, Sohal RS.
Coenzyme Q intake elevates the mitochondrial and tissue levels
of Coenzyme Q and alpha-tocopherol in young mice.
J Nutr. 2003;133(10):31753180.Lee BJ, Huang YC, Chen SJ, Lin PT.
Effects of coenzyme Q10 supplementation on inflammatory markers
(high-sensitivity C-reactive protein, interleukin-6, and
homocysteine) in patients with coronary artery disease.
Nutrition. 2012;28(7-8):767772.Bauerova K, Paulovicova E, Mihalova D, et al.
Combined methotrexate and coenzyme Q10 therapy in adjuvant-induced
arthritis evaluated using parameters of inflammation and oxidative stress.
Acta Biochim Pol. 2010;57(3):347354.Lee J, Hong YS, Jeong JH, et al.
Coenzyme Q10 ameliorates pain and cartilage degradation in a rat model
of osteoarthritis by regulating nitric oxide and inflammatory cytokines.
PLoS One. 2013;8(7):e69362.Abdollahzad H, Aghdashi MA, Asghari J, Alipour B.
Effects of coenzyme Q10 supplementation on inflammatory cytokines
(TNF-α, IL-6) and oxidative stress in rheumatoid
arthritis patients: a randomized controlled trial.
Arch Med Res. 2015;46(7):527533.Farsi F, Mohammadshahi M, Alavinejad P, Rezazadeh A, Zarei M, Engali KA.
Functions of coenzyme Q10 supplementation on liver enzymes, markers
of systemic inflammation, and adipokines in patients affected by
nonalcoholic fatty liver disease: a double-blind,
placebo-controlled, randomized clinical trial.
J Am Coll Nutr. 2016;35(4):346353.Sanoobar M, Eghtesadi S, Azimi A, et al.
Coenzyme Q10 supplementation ameliorates inflammatory markers in
patients with multiple sclerosis: a double blind, placebo,
controlled randomized clinical trial.
Nutr Neurosci. 2015;18(4):169176.Moccia M, Capacchione A, Lanzillo R, et al.
Coenzyme Q10 supplementation reduces peripheral oxidative stress and
inflammation in interferon-β1a-treated multiple sclerosis.
Ther Adv Neurol Disord. 2019;12Li W, Wu X, Xu X, et al.
Coenzyme Q10 suppresses tnf-α-induced inflammatory reaction
in vitro and attenuates severity of dermatitis in mice. Inflammation.
2016;39(1):281289.Farsi F, Ebrahimi-Daryani N, Golab F, et al.
A randomized controlled trial on the coloprotective effect of coenzyme Q10
on immune-inflammatory cytokines, oxidative status, antimicrobial
peptides, and microRNA-146a expression in patients with
mild-to-moderate ulcerative colitis.
Eur J Nutr. 2021;60(6):33973410.Taghizadeh S, Izadi A, Shirazi S, Parizad M, Pourghassem B.
The effect of coenzyme Q10 supplementation on inflammatory and endothelial
dysfunction markers in overweight/obese polycystic ovary syndrome patients.
Gynecol Endocrinol. 2021;37(1):2630.Dahri M, Tarighat-Esfanjani A, Asghari-Jafarabadi M, Hashemilar M.
Oral coenzyme Q10 supplementation in patients with migraine:
effects on clinical features and inflammatory markers.
Nutr Neurosci. 2019;22(9):607615.Jahangard L, Yasrebifar F, Haghighi M, Ranjbar A, Mehrpooya M.
Influence of adjuvant Coenzyme Q10 on inflammatory and oxidative stress
biomarkers in patients with bipolar disorders during the depressive episode.
Mol Biol Rep. 2019;46(5):53335343.Dνaz-Castro J, Guisado R, Kajarabille N, et al.
Coenzyme Q(10) supplementation ameliorates inflammatory signaling
and oxidative stress associated with strenuous exercise.
Eur J Nutr. 2012;51(7):791799.Arenas-Jal M, Suρι-Negre JM, Garcνa-Montoya E.
Coenzyme Q10 supplementation: efficacy, safety, and formulation challenges.
Compr Rev Food Sci Food Saf. 2020;19(2):574594
Return to Co-Q10
Since 7-27-2024


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |