

The Detoxification Enzyme Systems This section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Alternative Medicine Review 1998 (Jun); 3 (3): 187–198 ~ FULL TEXT
DeAnn J. Liska, Ph.D.
HealthComm International
P.O. Box 1729, Gig Harbor, WA.
deann@healthcomm.comThe human body is exposed to a wide array of xenobiotics in one s lifetime, from food components to environmental toxins to pharmaceuticals, and has developed complex enzymatic mechanisms to detoxify these substances. These mechanisms exhibit significant individual variability, and are affected by environment, lifestyle, and genetic influences. The scientific literature suggests an association between impaired detoxification and certain diseases, including cancer, Parkinson's disease, fibromyalgia, and chronic fatigue/immune dysfunction syndrome. Data regarding these hepatic detoxification enzyme systems and the body s mechanisms of regulating them suggests the ability to efficiently detoxify and remove xenobiotics can affect these and other chronic disease processes. This article reviews the myriad detoxification enzyme systems, their regulatory mechanisms, and the dietary, lifestyle, and genetic factors influencing their activities, as well as laboratory tests available to assess their functioning.
Introduction
We are exposed to a great number of xenobiotics during the course of our lifetime, including a variety of pharmaceuticals and food components. Many of these compounds show little relationship to previously encountered compounds or metabolites, and yet our bodies are capable of managing environmental exposure by detoxifying them. To accomplish this task, our bodies have evolved complex systems of detoxification enzymes. These enzyme systems generally function adequately to minimize the potential of damage from xenobiotics. However, much literature suggests an association between impaired detoxification and disease, such as cancer, Parkinson's disease, fibromyalgia, and chronic fatigue/immune dysfunction syndrome. Therefore, accumulated data suggests an individual's ability to remove toxins from the body may play a role in etiology or exacerbation of a range of chronic conditions and diseases.
The detoxification systems are highly complex, show a great amount of individual variability, and are extremely responsive to an individual's environment, lifestyle, and genetic uniqueness. This review of the detoxification systems is meant to whet the appetite for a more in-depth look at detoxification and, as such, it may raise more questions than it answers.
Discovery of Detoxification Reactions
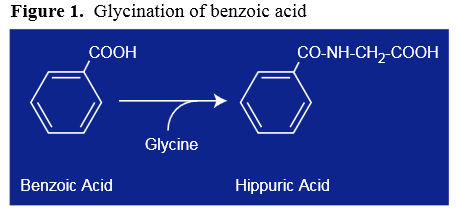
Figure 1 The hypothesis that xenobiotics consumed by animals are transformed to water-soluble substances and excreted through the urine was first put forth in the late 18th century. For years, scientists collected urine from various animals, purifying and then chemically characterizing the compounds present in an attempt to understand how the body managed to remove various xenobiotics. Hippuric acid, discovered in 1773, was one of the first metabolites identified in these early studies and, from chemical characterization, was proposed to result from the conjugation of glycine with benzoic acid (Figure 1). However, it was not until 1842 that this hypothesis was officially tested. Keller is attributed with performing the first challenge test, in which he took a dose of benzoic acid, collected his urine, and showed a direct relationship between ingestion of benzoic acid and the hippuric acid subsequently excreted. [1]

Table 1 For more than 100 years after this observation, research continued in the identification of various metabolites, and a variety of conjugation reactions were identified. During this time, glucuronic acid, sulfate, glycine, glutamine, taurine, ornithine, and glutathione were identified as conjugating substances (Table 1). Although the conjugation reactions solved the puzzle of how a non-water-soluble compound can be converted to a substance that could be excreted in urine, it raised another question. In all these cases of conjugation, the xenobiotic is required to have the ability to react with the conjugating moiety, i.e., to have an active center or "functional" group to react with, and bind, the conjugating moiety. What happens with compounds that do not have a reactive site?

Figure 2 In his landmark 1947 monograph, Detoxification Mechanisms, R.T. Williams defined the field of detoxification. Williams proposed that these non-reactive compounds could be biotransformed in two phases: functionalization, which uses oxygen to form a reactive site, and conjugation, which results in addition of a water-soluble group to the reactive site. [2] These two steps, functionalization and conjugation, are termed Phase I and Phase II detoxification, respectively. The result is the biotransformation of a lipophilic compound, not able to be excreted in urine, to a water-soluble compound able to be removed in urine (Figure 2). Therefore, detoxification is not one reaction, but rather a process that involves multiple reactions and multiple players.
Today, the challenge to understand detoxification continues. The question of how the body can handle such a wide range of compounds it has never before seen has led to considerable study in an attempt to understand the protein structure and regulation of various enzymes involved in detoxification. It is now known one mechanism the body uses is a battery of enzymes, each with broad specificity, to manage this challenge. Currently, over 10 families of Phase I enzymes have been described, which include at least 35 different genes. Phase II reactions are equally complex, and involve multiple gene families as well.
Enzyme Systems Involved in DetoxificationThe Phase I detoxification system, composed mainly of the cytochrome P450 supergene family of enzymes, is generally the first enzymatic defense against foreign compounds. Most pharmaceuticals are metabolized through Phase I biotransformation. In a typical Phase I reaction, a cytochrome P450 enzyme (CypP450) uses oxygen and, as a cofactor, NADH, to add a reactive group, such as a hydroxyl radical. As a consequence of this step in detoxification, reactive molecules, which may be more toxic than the parent molecule, are produced. If these reactive molecules are not further metabolized by Phase II conjugation, they may cause damage to proteins, RNA, and DNA within the cell. [4] Several studies have shown evidence of associations between induced Phase I and/or decreased Phase II activities and an increased risk of disease, such as cancer, systemic lupus erythematosus, and Parkinson's disease. [5–10] Compromised Phase I and/or Phase II activity has also been implicated in adverse drug responses. [5, 11, 12]

Table 2

Figure 3 The Phase I System:
As stated, at least 10 families of Phase I activities have been described in humans (Table 2). The major P450 enzymes involved in metabolism of drugs or exogenous toxins are the Cyp3A4, Cyp1A1, Cyp1A2, Cyp2D6, and the Cyp2C enzymes (Figure 3). The amount of each of these enzymes present in the liver reflects their importance in drug metabolism. 11, 13] Most information on the Phase I activities has been derived from studies with drug metabolism; however, Phase I activities are also involved in detoxifying endogenous molecules, such as steroids.

Table 3 The Phase II System: Phase II conjugation reactions generally follow Phase I activation, resulting in a xenobiotic that has been transformed into a water-soluble compound that can be excreted through urine or bile. Several types of conjugation reactions are present in the body, including glucuronidation, sulfation, and glutathione and amino acid conjugation (Table 3). These reactions require cofactors which must be replenished through dietary sources.
Much is known about the role of Phase I enzyme systems in metabolism of pharmaceuticals as well as their activation by environmental toxins and specific food components. However, the role of Phase I detoxification in clinical practice has received less consideration. The contribution of the Phase II system has received lesser attention both in academic research circles and in clinical practice. And, little is currently known about the role of the detoxification systems in metabolism of endogenous compounds.Is There a Phase III?: Recently, antiporter activity (p-glycoprotein or multi-drug resistance) has been defined as the Phase III detoxification system. [14] Antiporter activity is an important factor in the first pass metabolism of pharmaceuticals and other xenobiotics. The antiporter is an energy-dependent efflux pump, which pumps xenobiotics out of a cell, thereby decreasing the intracellular concentration of xenobiotics. [15]
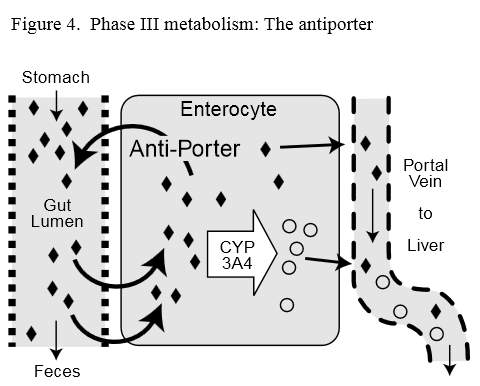
Figure 4 Antiporter activity in the intestine appears to be co-regulated with intestinal Phase I Cyp3A4 enzyme. [16] This observation suggests the antiporter may support and promote detoxification. Possibly, its function of pumping non-metabolized xenobiotics out of the cell and back into the intestinal lumen may allow more opportunities for Phase I activity to metabolize the xenobiotic before it is taken into circulation (Figure 4).
Two genes encoding antiporter activity have been described: the multi-drug resistance gene 1 (MDR1) and multi-drug resistance gene 2 (MDR2). [15] The MDR1 gene product is responsible for drug resistance of many cancer cells, and is normally found in epithelial cells in the liver, kidney, pancreas, small and large intestine, brain, and testes. MDR2 activity is expressed primarily in the liver, and may play a role similar to that of intestinal MDR1 for liver detoxification enzymes; however, its function is currently undefined.
Regulation of Detoxification Activities

Table 4 Since the detoxification systems function to help in the management of exposure to exogenous compounds, the body has developed several mechanisms to regulate detoxification activity (Table 4). Specific detoxification pathways may be induced or inhibited depending on the presence of various dietary or xenobiotic compounds, the age and sex of the individual, genetics, and lifestyle habits, such as smoking. [5, 13, 17–20] Furthermore, disease can also influence activity of the enzymes. In some disease states, detoxification activities appear to be induced or up-regulated, whereas, in other conditions these activities may be inhibited from acting or not produced at high levels. [12, 13]
Induction
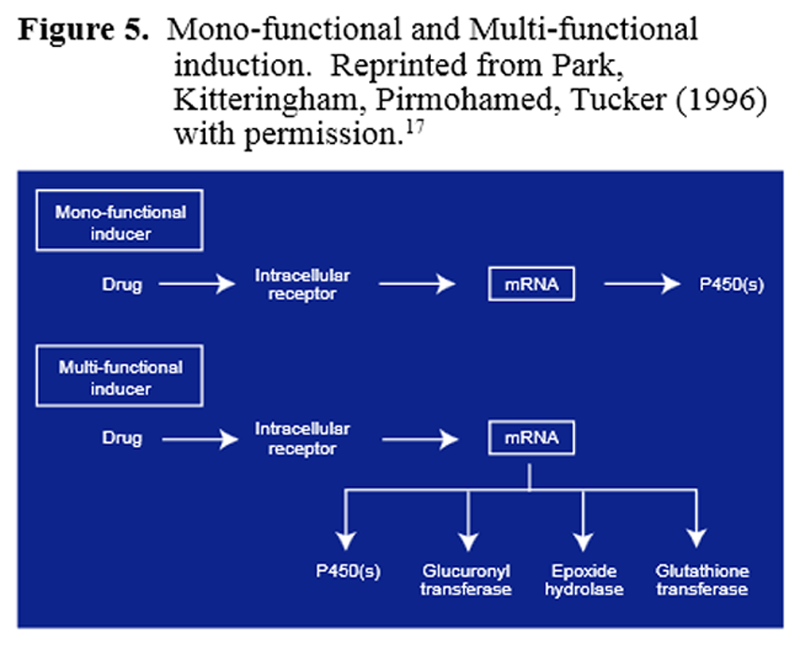
Figure 5 When the body is confronted with a high xenobiotic load, the Phase I and/or Phase II enzymes involved in detoxifying this compound can be induced, leading to more enzyme being present and a faster rate of xenobiotic detoxification. Inducers can be mono-functional or multi-functional (Figure 5). A mono-functional inducer affects only one enzyme or one phase of detoxification, whereas, a multi-functional inducer affects multiple activities. [17]
Mono-functional inducers, such as polycyclic hydrocarbons from cigarette smoke and aryl amines from charbroiled meats, result in dramatic induction of the Cyp1A1 and Cyp1A2 enzymes, leading to a substantial increase in Phase I activity, with little or no induction of Phase II enzymes. [21–25] Similarly, glucocorticoids and anti-convulsants induce Cyp3A4 activity, and ethanol, acetone, and isoniazid induce Cyp2E1. [13, 16, 17, 26, 27] Induction of these activities without co-induction of Phase II activities may lead to an uncoupling of the Phase I and Phase II balance of activity and, therefore, a higher level of reactive intermediates, which can cause damage to DNA, RNA, and proteins. [17, 24, 28]
The multi-functional inducers include many of the flavonoid molecules found in fruits and vegetables. For example, ellagic acid found in red grape skin has been shown to induce several Phase II enzymes while decreasing Phase I activity. [29–31] Garlic oil, rosemary, soy, cabbage, and brussels sprouts all contain compounds that can induce several Phase II enzyme activities. [24, 29, 32–35] Commonly, the glutathione S-transferase and glucuronyl transferases are induced by multi-functional inducers. In general, this increase in Phase II supports better detoxification in an individual and helps to promote and maintain a healthy balance between Phase I and Phase II activities. The enhancement of Phase II activity has been proposed to explain, at least in part, the ability of fruits and vegetables to protect against many cancers. [17, 24, 25, 28, 29]
Inhibition
Phase I and Phase II enzyme activities can also be inhibited. Inhibition can occur by competition between two or more compounds for the same detoxifying enzyme. Increased toxic load may lead to inhibition of detoxification of a number of compounds by simply overwhelming the systems and competing for detoxification enzyme activities. Moreover, some compounds selectively inhibit only one detoxifying activity; for example, quinidine, which competitively inhibits Cyp2D6 activity. [13] Cimetidine is an example of a compound that can bind directly to the heme iron of the cytochrome P450 reactive site to inhibit all cytochrome-dependent Phase I enzyme activities. [13] Much has been written recently about grapefruit juice, which contains high amounts of the flavonoid naringenin and its ability to inhibit first pass metabolism of many drugs that are detoxified through the Cyp3A4 enzyme-antiporter system in the intestine. [36–39]
A common mechanism of inhibition for some Phase II enzymes is the depletion of necessary cofactors. In humans, sulfation is particularly susceptible to inhibition due to compromised cofactor status. Serum sulfate concentration is a balance between absorption of inorganic sulfate and its production from cysteine, and sulfate elimination by urinary excretion and incorporation into low molecular weight substrates of sulfation. In humans, serum sulfate levels vary dramatically throughout 24 hours, and are decreased in individuals who are fasting or ingesting high levels of substances that are metabolized by sulfation, such as acetaminophen. [40–42] Humans excrete approximately 20–25 millimoles of sulfate in 24 hours; therefore, sulfate reserves must be maintained through dietary intake of sulfur-containing amino acids or inorganic sulfate, both of which have been shown to support increased serum sulfate levels. [40]
Polymorphisms
Genetic differences in the ability of an individual to metabolize xenobiotics are related to the presence of different versions of the gene encoding that activity, or genetic polymorphism. Cyp2D6 is the classic example of the influence of genetic polymorphism on phenotype. Several varieties of the Cyp2D6 gene exist in the population; some encode an enzyme with a lower activity than others. Individuals who receive two versions of the gene encoding slower Cyp2D6 activity do not detoxify substances through the Cyp2D6 pathway as fast as those who receive genes encoding faster acting enzymes. [5, 7, 11, 19, 43] These individuals have been termed "slow metabolizers." The Cyp2D6 enzyme is an important detoxifier of many narrow spectrum drugs, including antiarrhythmics, antidepressants, and antipsychotic drugs. Adverse side-effects occurring from these drugs may be reduced by decreasing dosages in those individuals who are Cyp2D6 slow metabolizers. [11, 43]
Polymorphisms in the Phase II activity of N-acetyltransferase can also lead to slow metabolizers. Associations have been found between slow metabolism through the N-acetyltransferase pathway and high risk of some types of cancer and Parkinson's disease. [5, 7, 10] Polymorphisms in Cyp2D6 have also been associated with a high risk of early onset Parkinson's disease. [5]
Other Factors
Several other factors also influence the expression and resultant activity of many of the detoxification enzymes. Like many other proteins, detoxification activities are under strict developmental control. Phase I Cyp3A enzymes and enzymes catalyzing glucuronidation, sulfation, and glutathione conjugation are present in the human fetus. By the time of birth, these enzymes are capable of catalyzing most Phase I biotransformation reactions; however, the rate of these reactions is generally slower than in adults. After two weeks of life, Phase I and Phase II detoxification systems become produced more fully. [13]
Sex and age also affect the type, amount, and activity of the various detoxification enzymes. The Cyp3A family of detoxification enzymes is particularly sensitive to hormones. For example, premenopausal women generally show 30–40 percent more Cyp3A4 activity than men or postmenopausal women. [16, 44, 45] The Cyp3A4 enzyme appears to be regulated, at least in part, by progesterone. [4] Since Cyp3A4 is the major Phase I detoxification pathway for the anti-epileptic agents phenobarbital and phenytoin, pregnant women, in whom this activity is increased, more readily eliminate these drugs and, therefore, may require a higher dose during pregnancy. [14]
Disease and health status of the individual also influence detoxification activities. Since the majority of detoxification occurs in the liver, it is not surprising that impairment of normal liver function due to alcoholic disease, fatty liver disease, biliary cirrhosis, and hepatocarcinomas can lead to lower detoxification activity in general. [12, 13] However, different enzyme systems occur in different regions of the liver; Phase I activities are membrane associated, whereas the majority of Phase II activity occurs in the cytosol. [4] Therefore, the amount of decrease in detoxification activity may vary from one isozyme to another.
Moreover, some conditions can lead to an increase or induction of activity. For example, Cyp2E1 catalyzes the oxidation of ethyl alcohol to acetaldehyde, and in addition detoxifies many of the small carbon-chain molecules, including ketone bodies, that result from gluconeogenesis and the breakdown of fatty acids. Cyp2E1 is increased in insulin-dependent diabetes, in morbidly obese individuals and, conversely, during starvation. [46, 47] The reason Cyp2E1 is induced in these conditions is not fully understood; however, it may have to do with the role Cyp2E1 plays in metabolism of products from gluconeogenesis and energy metabolism, and may influence how these pathways become imbalanced during altered metabolism. The full range of changes in other detoxification systems in response to health status is not fully understood.
Role of the Intestine in Detoxification
Most literature on detoxification refers to liver enzymes, as the liver is the site of the majority of detoxification activity for both endogenous and exogenous compounds. However, the first contact the body makes with the majority of xenobiotics is the gastrointestinal tract. Over the course of a lifetime, the gastrointestinal tract processes more than 25 tons of food, which represents the largest load of antigens and xenobiotics confronting the human body. [48] Furthermore, since most drugs are consumed orally, the gastrointestinal tract is also the first contact with many drugs. It is not surprising, then, that the gastrointestinal tract has developed a complex set of physical and biochemical systems to manage this load of exogenous compounds.
Several factors influence how much of a chemical ends up in the system, requiring detoxification by the liver. The gastrointestinal tract initially provides a physical barrier to exogenous components. As previously discussed, the gastrointestinal tract is the second major site in the body for detoxification. Detoxification enzymes such as Cyp3A4 and the antiporter activities have been found in high concentrations at the tip of villi in the intestine. [16, 49, 50] Adequate first pass metabolism of xenobiotics by the gastrointestinal tract requires integrity of the gut mucosa. Compromised barrier function of the mucosa will easily allow xenobiotics to transit into the circulation without opportunity for detoxification. Therefore, support for healthy gut mucosa is instrumental in decreasing toxic load.
The gastrointestinal tract influences detoxification in several other ways. Gut microflora can produce compounds that either induce or inhibit detoxification activities. Pathogenic bacteria can produce toxins that can enter circulation and increase toxic load. [51–54] Moreover, in a process called enterohepatic recirculation, gut microflora also have the ability to remove some conjugation moieties, such as glucuronosyl side chains, converting the xenobiotic to its original form, and allowing it to reenter circulation, leading to an increased toxic load. [54–56]
Summary
The detoxification system in humans is extensive, highly complex, and influenced by myriad regulatory mechanisms. This complexity of the detoxification systems and the individual uniqueness shown in ability to detoxify substances suggests one test or type of assay to fully assess detoxification status will not be possible. For some enzyme activities, such as the Cyp2D6, which are primarily influenced by genetics, determination of slow or fast metabolizers is possible with one gene analysis. However, to understand the detoxification profile of an individual, other approaches are necessary.
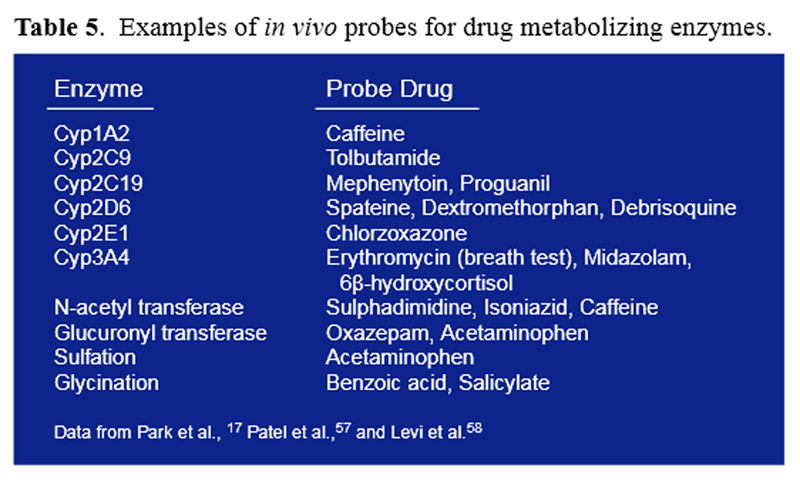
Table 5 The first analyses to identify detoxification pathways were challenge tests, in which a known amount of a substance was ingested and the amount of metabolite found in urine over a specified time period was collected and quantified (Table 5). This type of test takes into account myriad factors influencing detoxification activities and remains the standard today. The search for appropriate challenge substances and better methods to interpret the influence of the variety of detoxification activities on overall health and well-being is the challenge we face.

Return to IMMUNE FUNCTION
Return to NUTRITION ARCHIVES
Since 8-01-1998


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |