

Effect of Vitamin D2 Supplementation on 25-Hydroxyvitamin
D3 Status: A Systematic Review and Meta-Analysis
of Randomized Controlled TrialsThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Nutrition Reviews 2025 (Sep 18): nuaf166 ~ FULL TEXT
Emily I G Brown • Andrea L Darling • Tracey M Robertson • Kathryn H Hart
Jie Li • Cathie Martin • Martin J Warren • Colin P Smith
Susan A Lanham-New • Ruan M Elliott
Discipline of Nutrition, Exercise, Chronobiology and Sleep,
School of Biosciences, Faculty of Health and Medical Sciences,
University of Surrey,
Guildford, GU2 7XH, United Kingdom.
Context: Researchers have identified differences in metabolic activity between vitamins D2 and D3. Moreover, it is suspected from randomized controlled trial data that vitamin D2 supplementation increases the metabolic clearance of 25-hydroxyvitamin D3 [25(OH)D3], but this effect has yet to be quantified.
Objective: This study sought to undertake a systematic review and meta-analysis of the effect of vitamin D2 supplementation on serum 25(OH)D3 concentrations.
Data sources: PUBMED was searched for articles published from January 1, 1975, to February 1, 2023. Of the 202 articles retrieved, 20 were included in this review, and of those, 11 were suitable for meta-analysis.
Data extraction: Randomized controlled trials reporting either baseline and postintervention serum 25(OH)D3 concentrations (nmol/L) or absolute changes in concentrations were included. Random-effects meta-analyses were calculated using Review Manager (version 5.3; The Cochrane Collaboration). Mean differences were reported with 95% CIs.
Data analysis: In meta-analyses there was a reduction in serum 25(OH)D3 after vitamin D2 supplementation compared with control for end-of-trial between-groups data (random weighted mean difference [WMD] = -17.99 nmol/L; 95% CI, -25.86 to -10.12; P < .00001) and absolute change over the trial (random WMD = -9.25 nmol/L; 95% CI, -14.40 to -4.10; P = .0004).
Conclusions: Study participants who received vitamin D2 supplementation showed statistically significant reductions in serum 25(OH)D3 concentrations, compared to controls without supplementation. An inverse relationship between vitamin D2 and D3 concentrations has been proposed in the literature. A regulatory mechanism that increases the disposal rate of 25(OH)D after an increase in vitamin D concentrations could explain these results. However, further research is needed to establish whether vitamins D2 and D3 elicit different changes in overall vitamin D metabolism that might influence clinical advice to recommend vitamin D3 supplements over vitamin D2 supplements, where appropriate.
Keywords: 25(OH)D3; meta-analysis; systematic review; vitamin D2; vitamin D3.
From the FULL TEXT Article:
INTRODUCTION
Vitamin D is a fat-soluble prohormone that helps to maintain calcium homeostasis and influences immune function and cell turnover. [1, 2] Functions of vitamin D continue to be explored as research further elucidates the relationships between serum 25-hydroxyvitamin D [25(OH)D] levels and both musculoskeletal and non-musculoskeletal health outcomes. [3] Positive associations between vitamin D sufficiency and health benefits have been observed for osteomalacia and rickets, cancer, cardiovascular disease, oral health, and some autoimmune and allergic conditions. [3–5] However, larger-scale randomized controlled trials (RCTs) may be required to further explore the promising results of epidemiological and molecular studies.
There are 2 forms of vitamin D: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D3 is the form of vitamin D synthesized in human and animal skin upon exposure to ultraviolet B (UVB) radiation from sunlight. UVB radiation causes photolysis of 7-dehydrocholesterol (7-DHC) in the skin epidermis, which leads to production of cutaneous previtamin D and is subsequently thermally isomerized into vitamin D3. [6, 7] This vitamin D3 produced from UVB radiation, in addition to vitamins D2 and D3 consumed from dietary sources, then undergo serial hydroxylations in the liver and kidneys to form 1,25-dihydroxyvitamin D, the hormonally active form of vitamin D. [8] This hydroxylation process is mediated by cytochrome P450 (CYP) enzymes, starting with cytochrome P450 25-hydroxylase (CYP2R1), which is the primary enzyme responsible for the 25-hydroxylation of vitamins D2 and D3 into calcidiol [25-hydroxyvitamin D or 25(OH)D] in the liver. Subsequently, in the kidneys, cytochrome P450 1α-hydroxylase (CYP27B1) synthesizes the active metabolite calcitriol [1,25-dihydroxyvitamin D or 1,25(OH)2D]. The UVB wavelengths need to be in the range of 290-315 nm for production of dermal vitamin D. [9] This means that for latitudes of 40 degrees North or South and higher, for at least 1 month per year (and at more extreme latitudes for 6 months per year) no meaningful amounts of vitamin D can be produced. Therefore, adequate serum concentrations need to be achieved through consumption of vitamin D–rich foods, and/or supplementation.
Endogenous vitamin D synthesis is dependent on the percentage of skin exposed to the sun, as well as exposure time. [10] Many individuals do not synthesize sufficient vitamin D due to limited skin exposure, lifestyle, seasonality, conservative clothing, or use of sunscreens with high sun protection factors. Recent research also suggests that individuals of older age and those belonging to ethnic groups with darker skin pigmentation may have reduced dermal vitamin D3 synthesis. For older individuals, this reduction is attributable to decreasing quantities of 7-DHC in the skin epidermis as age increases. [11, 12] For individuals with darker skin, the pigment melanin can absorb UVB radiations which reduces production of pre-vitamin D. [13]
Vitamin D3 is also present in some animal products including oily fish (such as salmon, sardines, and mackerel), egg yolks, and meat. [14] Mushrooms are the main source of vitamin D2 in foods, including wild and UVB-treated mushrooms. [15] Quantities of both D2 and D3 vary depending on the climate and environment of the animal or fungus and their resulting sun exposure.
Vitamin D2 and D3 are structurally similar, but vitamin D2 is differentiated by an additional methyl group linked to carbon 24 and a double bond between carbons 22 and 23. [8] The D2 and D3 forms of vitamin D have been studied for possible variability when influencing total serum 25(OH)D levels and were previously considered to be of equal potency in their ability to increase serum 25(OH)D. [16, 17] However, recent research has shown vitamin D3 to be more effective, particularly when pre-intervention serum 25(OH)D concentrations are <50 nmol/L or in a state of deficiency. [18, 19] These findings suggest that research should continue into the comparative metabolism and functions of vitamins D2 and D3.
The article search for this publication did not reveal any systematic reviews or meta-analyses performed to investigate the impact of vitamin D2 supplementation on vitamin D3 concentrations; however, several RCTs have examined this relationship. Indeed, an inverse relationship between D2 and D3 has been observed on several occasions, but this relationship has often been an incidental finding within studies performed with a different research aim. [20, 21] Stephenson and coworkers [22] reported a proportional decrease in serum 25(OH)D3 in a study performed to investigate the effects of vitamin D2 supplementation on serum 25(OH)D and 25(OH)D2 concentrations. Following this finding, Hammami and co-workers suggested it to be a reciprocal phenomenon in which serum 25(OH)D2 decreases upon vitamin D3 supplementation, and 25(OH)D3 decreases with vitamin D2 supplementation. [23] In many RCTs, 25(OH)D3 concentrations for the vitamin D2 supplemented cohort appear to be diminished below concentrations of the placebo supplemented groups. [18, 23–25] However, the biological mechanism for this finding is currently unclear.
The aim of the present study was to review current RCT evidence to quantify the amount by which serum vitamin 25(OH)D3 concentrations decrease when study participants receive vitamin D2 supplementation compared to those who received a placebo or were unsupplemented controls.
METHODOLOGY
Study Identification and Search Procedures
PUBMED was searched using the Boolean operator terms “Vitamin D2” OR “25OHD2” OR “ergocalciferol” AND “Vitamin D3” OR “25OHD3” OR “cholecalciferol” and the search results were filtered by date (from January 1, 1975, up to February 1, 2023) and type of study (RCT). The reference list of a relevant, recent publication by Balachandar and co-workers [26] was also searched for additional articles.
Initial screening involved selecting studies with relevant titles and abstracts for further analysis. Subsequent inspection was performed to assess whether baseline and postintervention serum 25(OH)D3 concentrations, or the absolute change, were reported in the results of each article. Screening was conducted by E.I.G.B., according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, [27] with inclusions inspected by A.L.D prior to analysis and any disagreements resolved by a third party (S.A.L-N).
Eligibility Criteria for Inclusion

Table 1 Table 1 details the eligibility criteria according to the PICOS (Population, Intervention, Comparison, Outcomes and Study) framework. [28] Study characteristics were tabulated and compared against PICOS criteria to assess eligibility. Included studies were randomized, controlled studies in humans of any sex. They needed to report the baseline and post-intervention serum concentration of 25(OH)D3. Supplementation types in the study interventions could be either dietary food fortification, or standardized supplements. The strength and frequency of dose was not a basis for exclusion. Studies involving pregnant or breastfeeding women were excluded. Studies involving children were included for systematic review but were excluded from meta-analysis as they were dissimilar to adult studies.
Data Extraction
For each study, lead author surnames, year of publication, country of origin, study design, number of participants (in both D2 supplemented and control groups), type of control, duration of study, supplementation frequency and dose strength were extracted. For studies in which the supplement or food fortification dose was tested, the revised and confirmed actual dose was extracted. The mean baseline and post-intervention serum concentrations and SD of vitamin D metabolites (25(OH)D, 25(OH)D2, and 25(OH)D3) were also extracted for all participants collectively. If the post-intervention concentrations were not recorded, the measures from the latest time point were used as an alternative.
The SD values were calculated from the SEM values or CIs where required. Vitamin D supplement doses from the RCTs were, if not already, translated into international units (IU) for data synthesis (µg × 40.0). For consistency in quantifying units, serum concentrations of 25(OH)D, 25(OH)D2 and 25(OH)D3 were expressed in nanomoles per litre (equivalent to nanograms per millilitre × 2.5).
For consistency, for articles reporting studies with multiple vitamin D2 supplemented study arms, the highest supplement dose was chosen. When studies provided both tablet and food supplements, the tablet form was chosen to be more consistent with the other included studies. Food fortification studies most often used fortified mushrooms; however, the study by Tripkovic et al. [18] involved juice or biscuit supplementation. The juice was selected as a closer comparison to the fortified mushrooms reported in other articles, because the plant fibers of the juice were more similar to the food matrix of the fungi than that of the biscuit, which contains animal fats and protein.
Statistical Analysis
The included studies were grouped and meta-analyses were performed according to the type of data published in the articles: either the end of trial mean and SD or the absolute change and SD for the course of the intervention. Review Manager (version 5.3; The Cochrane Collaboration) was used to conduct the meta-analyses and produce forest plots. Mean differences were reported with 95% CIs. For the meta-analyses, vitamin D2 supplementation was compared to control. Random effects models were used due to potential heterogeneity, with interpretation of I2 statistics. Sensitivity analyses were conducted to evaluate the robustness of the meta-analyses. Risk of publication bias was not assessed due to the numbers of studies in the meta-analysis being too small for inclusion in funnel plots. Included studies were assessed for methodological quality using the Jadad scale. [29]
RESULTS
Study Selection
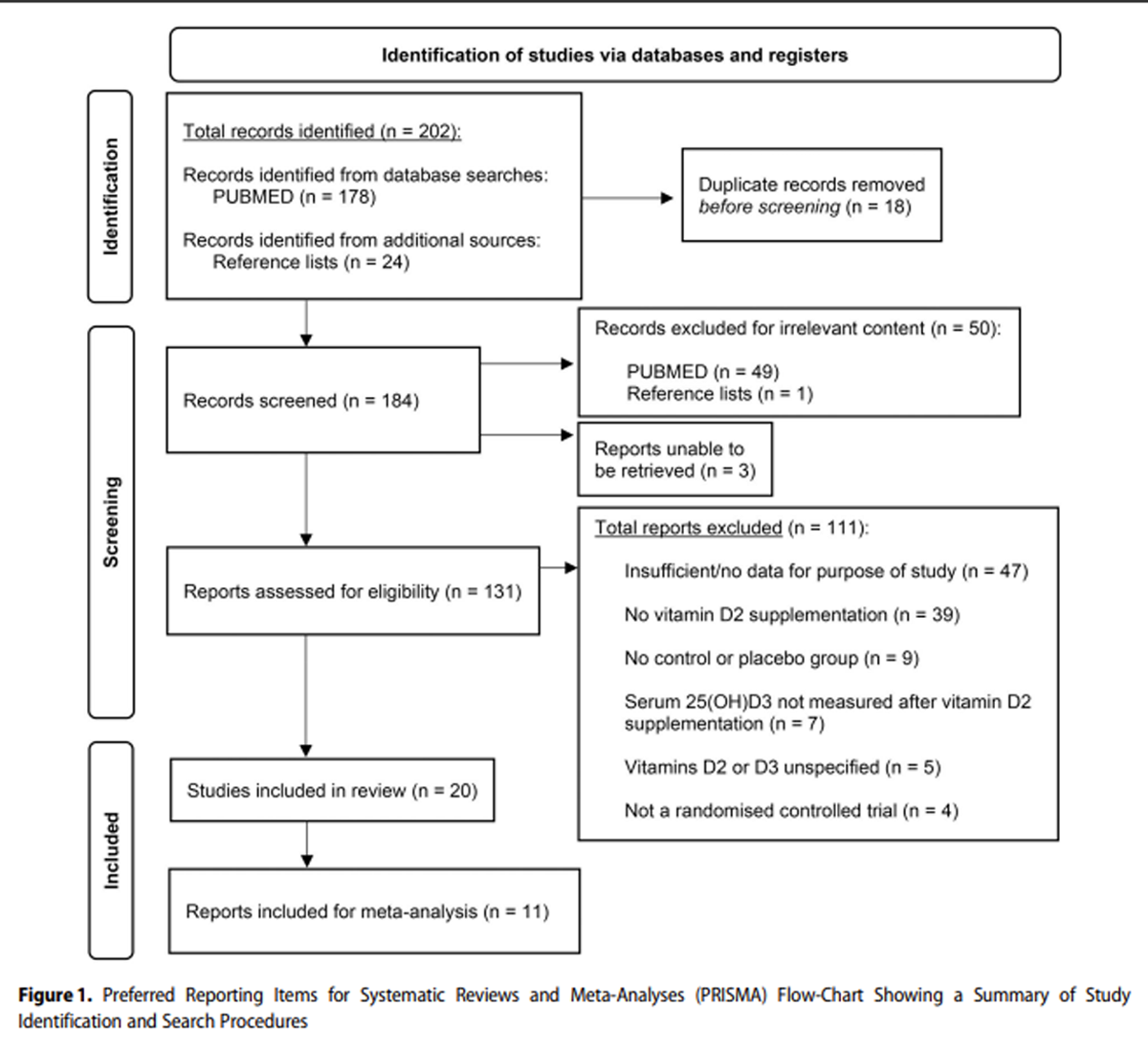
Figure 1

Table 2
see page 5The PRISMA flow chart in Figure 1 details the process and outcomes of study selection for the systematic review and meta-analysis. Of the 184 records screened, 50 had irrelevant content. Seven publications were successfully retrieved by inter-library loan for screening; however, 3 articles were irretrievable and were therefore excluded from review. From the 131 reports assessed for eligibility, 111 studies were excluded. A total of 20 articles were included in the systematic review, with 11 also included in meta-analysis. The final set of studies meeting the inclusion/exclusion criteria of the systematic literature search are displayed in Table 2. [16, 18, 20, 22–25, 30–42]
Characteristics of Included Studies
Table 2 shows the 20 studies included in the review. Study durations ranged from 3 to 25 weeks. Supplementation frequency varied between a single bolus dose to daily supplements with 300-100 000 IU in a single dose. In total, there were 1080 participants in the control and vitamin D2 supplementation groups, of whom 60 participants were aged <18 years across an infant study and a pediatric study. [17, 31, 32] Of the 20 included studies, 18 identified reductions in serum 25(OH)D3 after vitamin D2 supplementation. Of those 18 studies, 16 studies reported that compared with the placebo group the participants given vitamin D2 supplementation demonstrated greater reduced change or lower mean serum 25(OH)D3 concentrations. [18, 22–25, 31, 33–42] Reductions in serum 25(OH)D3 were reported in 2 studies, [16, 30] but the reduction levels did not differ significantly between the vitamin D2-supplemented groups and the placebo groups. Two articles reported increases in serum 25(OH)D3 after D2 supplementation. [20, 32] However, in 1 article the increase remained lower in the participants in the D2-supplemented group than those in the placebo group. [32]
Meta-Analysis
From the 20 articles included in the systematic review, 11 contained sufficient data for inclusion in meta-analysis. Two articles that reported on infants were excluded [31, 32] and a further 7 articles were excluded due to missing or unconvertible data.
There were 655 participants (342 vitamin D2 supplemented vs. 313 control) across the 11 studies in the meta-analyses. All except 1 of the studies involved healthy participants. [39] One article reported a study in which participants had a health condition that included exercise-induced muscle damage; however, the study was included as the condition was deemed unlikely to have a negative impact upon the study results of vitamin D absorption and concentrations. [39] Eight of the 11 studies had mixed male and female participants. One study observed a male-only population that was split into young (n = 9) and old (n = 9) participant groups, so the finding for the younger population was included in the present review as a closer comparison to the other included studies. [33] One other study focused on a female-only population but compared South Asian (n = 63) and White European (n = 228) participants. [18] The final study did not specify the sexes of the study participants (n = 28). [39]
Nine of the studies used a placebo supplement, whereas Harris et al. [33] and Nimitphong et al. [40] had instead a control group with no supplementation. The frequency of supplement consumption and dose of vitamin D were both highly variable between the included studies. Supplementation frequency ranged from a single bolus dose to daily supplementation, with 7 of the studies following a frequency of once daily. Studies varied considerably in duration, with the study by Fisk et al. [16] having the shortest duration of 4-weeks, while the study by Zajac et al., had the longest duration of a 6-month supplementation period. [42] The dose ranged from 300 IU up to 50 000 IU as a result of the varied frequency of supplementation.
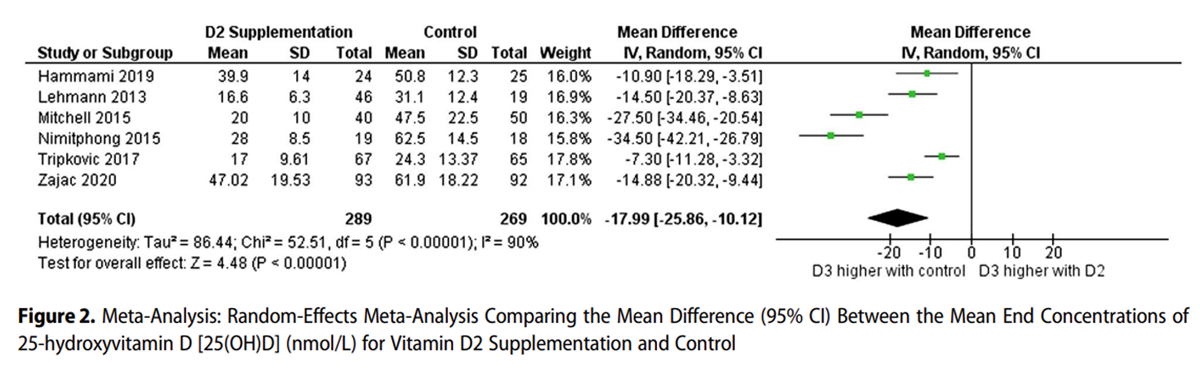
Figure 2
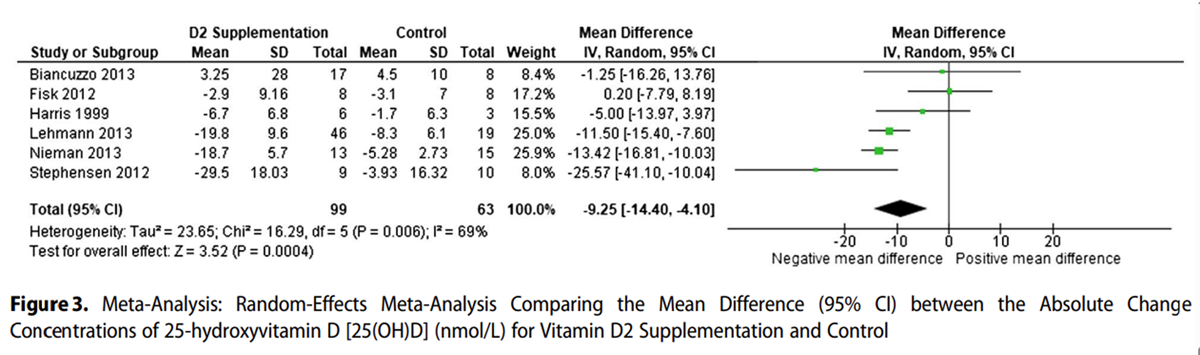
Figure 3 Figure 2 shows that the mean end concentrations of 25(OH)D3 were significantly lower in the D2 supplemented group, with a weighted mean difference of –17.99 nmol/L (95% CI, –25.86 to –10.12; z = 4.48; P < .00001). Serum 25(OH)D3 concentrations were higher in the non-supplemented and placebo groups than in the vitamin D2 supplemented groups. Five of 6 studies showed a negative absolute change in serum 25(OH)D3 with both D2 and control supplementation, with a larger negative change in the D2 group (Figure 3).
One study showed a positive mean change in both groups, with the control group having a slightly higher positive change. The mean difference in the meta-analysis was statistically significant, with the D2 supplemented group having a larger negative change in serum 25(OH)D3 than the control group, with a weighted mean difference of –9.25 nmol/L (95% CI, –14.40 to –4.10; z = 3.52; P = .0004).
Risk of Bias

Table 3 Table 3 [16, 18, 20, 22, 23, 33, 36, 38–40, 42] displays the risk of bias analysis for the 11 RCTs included in the meta-analyses. Eight of the studies involved double blinding in their methodology; 7 of these studies included detailed use of an appropriate placebo. For 10 of the 11 studies the articles stated their randomized nature, and 8 of these articles included descriptions of the exact method of randomization utilized. Only 4 studies detailed the number of participants who withdrew and the reason for withdrawal. Overall, risk of bias analysis suggests that there is some risk of bias in the studies included in the meta-analyses.
Heterogeneity and Sensitivity Analysis
The heterogeneity data presented in Figures 2 and 3 show that I2 values in the meta-analysis were high (83%) and moderate (69%), respectively. For the analysis presented in Figure 2 (D2 vs control, end of trial data), the I2 level varied from 59% to 85%, depending on which study was removed. A sensitivity analysis was conducted for this meta-analysis (data not shown), and statistical significance was not altered when any study was removed from the analysis; effect-size changes were small, with no effect size change larger than 2.5 nmol/L. Similarly, for the analysis presented in Figure 3 (D2 vs control, absolute change data), the I2 level varied from 50% to 75%, depending on which study was removed. For the sensitivity analysis (data not shown), statistical significance was not altered when any individual study was removed from the analysis and effect size changes were small, with no effect size change larger than 2.2 nmol/L.
DISCUSSION
The aim of this study was to review the current RCT evidence to quantify the amount by which serum vitamin 25(OH)D3 concentrations decrease in study participants who receive vitamin D2 supplementation, compared participants who received a placebo or were unsupplemented controls. Of the 20 articles in the systematic review, 18 found that vitamin D2 supplementation was associated with a decrease in serum 25(OH)D3 concentrations. Although a rise in serum 25(OH)D3 after vitamin D2 supplementation was reported in 1 article, [20] the increase was less than that of the placebo group. The rise in 25(OH)D3 could be the result of confounding UV exposure from sunlight, as the study ran from February into May when UVB wavelengths in Boston can produce dermal vitamin D. [43]
Meta-analysis revealed a statistically significant decrease of approximately 18 nmol/L in serum 25(OH)D3 concentration in study participants after vitamin D2 supplementation compared to non-supplemented participants who received a control or placebo. When analyzing absolute change data, compared with the control group the D2 supplemented group had a significant reduction in serum 25(OH)D3, of approximately 9 nmol/L. Therefore, these meta-analyses confirm that D2 supplementation reduces serum 25(OH)D3 concentrations.
There are no previous reviews to compare the findings reported here, and the physiological mechanisms behind these results have not yet been fully elucidated. Hammami et al. [23] attempted to explain the physiological response by drawing a direct comparison between the functioning of vitamins D2 and D3, and these investigators proposed that the 2 vitamins have inverse mechanisms and decrease each other to achieve control over total serum 25(OH)D concentrations. All of the 11 studies included in these meta-analyses reported concentrations of 25(OH)D2 as well as 25(OH)D3. Interestingly, 10 of these studies found a significant difference in the concentration of 25(OH)D2, either a significant change from baseline concentrations, a significant difference between the vitamin D2 supplemented group compared to the control, or a significant increase in the number of participants with a detectable 25(OH)D2 concentration >5 nmol/L. The remaining study did also find a rise in 25(OH)D2 concentrations in the vitamin D2 supplemented group from undetectable levels to a mean of 39.8 nmol/L at day 14 and 19.4 nmol/L at day 56; contrastingly, the mean 25(OH)D2 concentrations remained undetectable for the duration of the study in the placebo group. [23]
However, the theory by Hammami et al. [23] does not explain why/how supplementation of 1 vitamin D metabolite leads to decreased concentrations of the other vitamin D metabolite. Hammami and Yusuf [25] suggested that rather than the decrease in 25(OH)D3 concentrations being a direct result of vitamin D2 functioning, that it may be the result of a regulatory mechanism that disposes of 25(OH)D after an increase in vitamin D concentration. There has been no quantification to specify at what serum 25(OH)D concentrations this effect would be enacted. This theory may also explain why vitamin D3 supplementation can also cause a decrease in serum 25(OH)D2 concentrations. [25]
Durrant et al. [44] conducted a blood transcriptome analysis and identified differentially expressed genes after vitamin D2 or D3 supplementation. Their findings suggested that both vitamin D2 and D3 can potentially cause the immune system to be more tolerogenic. However, only vitamin D3 had a stimulatory effect on type 1 and II interferon activities, which play essential roles in the innate immune response to infections. [44] Although the study by Durrant et al. [44] focused on the respective functioning of vitamins D2 and D3 in the context of the blood transcriptome and gene expression, this study highlights the possibility that vitamin D2 and D3 are not of equivalent benefit when supplemented. Further evidence is needed, but vitamin D3 might be the preferential type of vitamin D supplementation, subject to individual preference, due to its potential additional benefits to human health over vitamin D2.
The more rapid decline in 25(OH)D3 levels observed following dietary supplementation with vitamin D2 is likely a result of homeostatic mechanisms activated by the increase in total 25(OH)D due to the supplementation. The primary enzyme responsible for the conversion of vitamin D to 25(OH)D is CYP2R1, which has similar activity on vitamins D2 and D3. [45] However, it is not the sole enzyme responsible for 25-hydroxylation of vitamin D, and at least one of the other candidate enzymes, cytochrome P450 25-hydroxylase (CYP27A1), acts preferentially to 25-hydroxylate the D3 form. [46] Thus, there is scope for differential metabolism of the D2 and D3 forms at this stage in the pathway. However, based on current knowledge, it is not clear whether CYP2R1 and CYP27A1 are regulated in response to changing vitamin D status. In contrast, the 1-α hydroxylase CYP27B1, which converts 25(OH)D into 1,25(OH)2D, and cytochrome P450 24-hydroxylase (CYP24A1), which is responsible for the first step in the catabolism of both 25(OH)D and 1,25(OH)2D, are both well known to be regulated by vitamin D status. [45] CYP27B1 activity is upregulated by parathyroid hormone (PTH), which is increased when vitamin D status is low, and downregulated by fibroblast growth factor 23 (FGF-23), which is released from bone in response to 1,25(OH)2D. Conversely, CYP24A1 is inhibited by PTH and activated by FGF-23. Thus, activation of CYP24A1 in response to vitamin D2 supplementation is a plausible explanation for the observed enhanced clearance of 25(OH)D3. [47]
In terms of the limitations of this analysis, meta-analysis heterogeneity was moderate to high, and in the future when more studies have been published it will be important to undertake meta-regression to assess the possible factors contributing to this heterogeneity. Risk of quality bias was not negligible and the Jadad scale [29] suggested some weaknesses in quality of included studies in the meta-analysis. This was primarily a result of 7 of the 11 included studies failing to detail the number of participants who withdrew from the RCT. In addition, only 8 of the 11 studies utilized double blinding, with 1 of these studies not detailing the method used. Publication bias could not be assessed due to insufficient available studies in either meta-analysis to construct a valid funnel plot. Publication bias is unlikely to have unduly affected this analysis due to much of the data being drawn from secondary research hypotheses or from incidental findings.
In the full systematic review of 20 studies, several RCTs contained participant populations with diagnosed disease. However, the meta-analysis only included 1 study out of the 11 that recruited participants who had a health condition, which minimizes the impact that nonhealthy participants could have had on the meta-analysis. When the study with nonhealthy participants was removed in the sensitivity analysis, the meta-analysis results remained statistically significant, which demonstrates that the health outcome was not affecting the statistical significance of the result.
The mean body mass index (BMI) at baseline, specifically within the participant groups included for this meta-analysis, ranged between 22.1 and 28.1 kg/m2 across 9 of the 11 included studies in meta-analysis. [16, 18, 22, 23, 33, 36, 38, 40, 42] Of these 9 studies, 2 used BMI as a criterion on which to base participant eligibility, [16, 22] while the remaining 2 studies out of the included 11 did not detail any information on participant BMI. [20, 39] As all mean BMI data, as detailed above, fell within the healthy to overweight range, it is therefore unlikely that participant BMI had a significant impact on the supplementation outcomes of the included studies.
Of the 11 studies included in meta-analysis, 5 of them had used liquid chromatography–tandem mass spectrometry (LC-MS/MS) assays to measure 25(OH)D3 concentrations, [20, 36, 38, 40, 42] while a further 2 used high-performance liquid chromatography (HPLC)-MS/MS18,39 and 2 used ultraperformance liquid chromatography (UPLC)-MS/MS. [16, 22] To date, LC-MS/MS assays are considered to be the “gold-standard” assay when quantifying 25(OH)D because of its high level of sensitivity. This left 2 RCTs having measured 25(OH)D3 using an HPLC [33] or reversed-phase (RP)-HPLC [23] assay which, although not as well reputed or utilized compared to LC-MS/MS assays, is still thought to be more accurate compared to immunologically based methods, which have variable analytical performance. The variations in the assays used in the analytical methodologies of the included RCTs are likely to have had an impact upon the absolute mean difference values calculated. However, the statistical comparisons drawn between groups from each individual study should be valid, and so the variation in methods is unlikely to have had a major impact upon the findings of this review. In 2 of the 11 included studies, [18, 42] the laboratories which performed the assays adhered to the Vitamin D External Quality Assessment Scheme (DEQAS), which ensures the quality and standardization of assays by assessing the accuracy of laboratories in measuring serum total 25(OH)D compared to specifically distributed human serum samples.
Overall, the meta-analysis reported here is, to the knowledge of the authors, the first to quantify the effect of vitamin D2 supplementation on vitamin D3 concentrations. Previous research has examined the relative function of vitamin D2 and D3 to increase total 25(OH)D levels after supplementation and determined that vitamin D3 appears to be more effective. However, current recommendations surrounding vitamin D deficiency and sufficiency all focus on total serum 25(OH)D concentrations. To develop current knowledge of optimal concentrations of 25(OH)D2 and 25(OH)D3, further research is required to first understand vitamin D2 and D3 functioning after supplementation. The majority of RCTs utilized for this meta-analysis have only incidentally published data for vitamin D3 levels, while studying other outcomes of vitamin D2 supplementation. There is, therefore, limited research in existence that has deliberately investigated this inverse relationship. This systematic review and meta-analysis will hopefully bring attention to the continued research required to better understand vitamin D and to initiate continued investigation into the consequences of vitamin D2 supplementation.
This study extends present understanding of the physiological responses that adult humans have after vitamin D2 supplementation and affirmed the directional influence this response has upon serum vitamin D3 levels. The physiological impact of decreased vitamin D3 concentrations in persons with serum 25(OH)D sufficiency appears to generally be unquestioned and is unknown. Future research is needed to discern whether there is a negative physiological impact on vitamin D functioning when vitamin D3 levels are lowered but total 25(OH)D levels are replete, as this would have further influence upon the limiting of vitamin D2 supplementation. A similar meta-analysis is now required to assess the inverse relationship between vitamin D3 supplementation and serum 25(OH)D2 concentrations.
The implications of this research will hopefully inform future policy surrounding the use of vitamin D3 over vitamin D2 for supplementation purposes. Regardless of the changes reported in this meta-analysis with respect to vitamin D3 levels when vitamin D2 is supplemented, it has now been reported in the literature that vitamin D3 raises total 25(OH)D status more effectively than vitamin D2. [48] Vitamin D2 supplement production would of course remain necessary to facilitate availability for personal requirements, as nearly all vitamin D3 supplements are derived from animal sources and are therefore not applicable to vegans. However, the production and prescription of vitamin D3 as a first-line recommendation may be a useful policy to implement due to the potential detrimental influence vitamin D2 supplements may have upon serum 25(OH)D3 status.
CONCLUSIONS
A statistically significant reduction of approximately 18 nmol/L in serum 25(OH)D3 concentration was found after vitamin D2 supplementation compared to control, using end of trial data, and a reduction of approximately 9 nmol/L using absolute change data. The demonstration of this result in RCTs suggests there is a causal relationship between vitamin D2 supplementation and a subsequent decline in 25(OH)D3 levels. Further research into the mechanistic and physiological function of vitamin D2 and D3 supplementation should be a priority, in order to assess whether vitamin D3 should be the first line choice for supplementation, subject to personal considerations.
Acknowledgments
We gratefully acknowledge the support of the Biotechnology and Biological Sciences Research Council (BBSRC).
Author Contributions
Conceptualization, E.I.G.B., A.L.D., T.M.R., K.H.H., C.M., M.J.W., S.A.L-N., and R.M.E.;
methodological design, E.I.G.B., A.L.D., and S.A.L-N.;
data collection and formal analysis, E.I.G.B. and A.L.D.;
data curation and interpretation, E.I.G.B., A.L.D., T.M.R., K.H.H., S.A.L-N., and R.M.E.;
manuscript writing, E.I.G.B., A.L.D., T.M.R., K.H.H., J.L., C.M., M.J.W., C.P.S., S.A.L-N., and R.M.E.;
manuscript revision, E.I.G.B., A.L.D., T.M.R., K.H.H., J.L., C.M., M.J.W., C.P.S., S.A.L-N., and R.M.E.;
project administration, E.I.G.B., A.L.D., and S.A.L-N.;
funding acquisition, C.M., M.J.W., and S.A.L-N.
All authors have read and approved the published version of the manuscript.
Susan A. Lanham-New and Ruan M. Elliott contributed equally and are considered co–senior authors of this work. In addition, they had full access to all the data in the study and take responsibility for the integrity of data and accuracy of the data analysis.
Funding
This research was funded by the BBSRC Institute Strategic Programme Food Microbiome and Health, grant number BB/X011054/1, and its partner project BB/X020029/1. The APC was funded by the partner project BB/X020029/1 to the University of Surrey. E.I.G.B. is the recipient of a UKRI BBSRC FoodBioSystems Doctoral Training Partnership (DTP), grant number BB/T008776/1.
Conflicts of Interest
E.I.G.B., A.L.D., T.M.R., K.H.H., J.L., C.M., M.J.W., C.P.S., and R.M.E. declare no conflicts of interest. S.A.L-N reports being a member of the main SACN Committee (2010 to present) and the SACN Vitamin D Working Group (2010–2016; 2022-present) and also the European Food Safety Authority (EFSA) Committee on the Tolerable Upper Limit for Vitamin D (2021-2023). S.A.L-N. is Research Director of D3Tex Ltd, which holds the United Kingdom and Gulf Corporation Council Patents for the use of UVB material for combatting vitamin D deficiency in women who dress for cultural style.
References:
Holick MF.
Vitamin D: evolutionary, physiological and health perspectives.
Curr Drug Targets. 2011;12:4-18.Martens PJ, Gysemans C, Verstuyf A, Mathieu AC.
Vitamin D's effect on immune function.
Nutrients. 2020;12:1248.Holick MF.
Vitamin D deficiency.
N Engl J Med. 2007;357:266-281.Botelho J, Machado V, Proenca L, Delgado AS, Mendes JJ.
Vitamin D deficiency and oral health: a comprehensive review.
Nutrients. 2020;12:1471.Umar M, Sastry KS, Chouchane AI.
Role of vitamin D beyond the skeletal function:
a review of the molecular and clinical studies.
Int J Mol Sci. 2018;19:1618.DeLuca HF.
Overview of general physiologic features and functions of vitamin D.
Am J Clin Nutr. 2004;80:1689S-1696S.Tian XQ, Chen TC, Matsuoka LY, Wortsman J, Holick MF.
Kinetic and thermodynamic studies of the conversion
of previtamin D3 to vitamin D3 in human skin.
J Biol Chem. 1993;268:14888-14892.Norman AW.
From vitamin D to hormone D: fundamentals of the
vitamin D endocrine system essential for good health.
Am J Clin Nutr. 2008;88:491S-499S.Chandra P, Wolfenden LL, Ziegler TR, et al.
Treatment of vitamin D deficiency with UV light in patients
with malabsorption syndromes: a case series.
Photodermatol Photoimmunol Photomed. 2007;23:179-185.Webb AR.
Who, what, where and when-influences on cutaneous vitamin D synthesis.
Prog Biophys Mol Biol. 2006;92:17-25.Baker MR, Peacock M, Nordin BE.
The decline in vitamin D status with age.
Age Ageing. 1980;9:249-252.MacLaughlin J, Holick MF.
Aging decreases the capacity of human skin to produce vitamin D3.
J Clin Invest. 1985;76:1536-1538.Armas LA, Dowell S, Akhter M, et al.
Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels:
the effect of UVB dose and skin color.
J Am Acad Dermatol. 2007;57:588-593.England PH.
McCance and Widdowson’s The Composition of Foods
Integrated Dataset 2021 User guide.
Accessed April 6, 2023.
https://assets.publishing.service.gov.uk/government/uploads/system/
uploads/attachment_data/file/971021/McCance_and_Widdowsons_
Composition_of_Foods_integrated_dataset_2021.pdfMattila PH, Piironen VI, Uusi-Rauva EJ, Koivistoinen PE.
Vitamin D contents in edible mushrooms.
J Agric Food Chem. 1994;42:2449-2453.Fisk CM, Theobald HE, Sanders TA.
Fortified malted milk drinks containing low-dose ergocalciferol
and cholecalciferol do not differ in their capacity to raise
serum 25-hydroxyvitamin D concentrations in
healthy men and women not exposed to UV-B.
J Nutr. 2012;142:1286-1290.Gallo S, Phan A, Vanstone CA, Rodd C, Weiler HA.
The change in plasma 25-hydroxyvitamin D did not differ between
breast-fed infants that received a daily supplement of
ergocalciferol or cholecalciferol for 3 months.
J Nutr. 2013;143:148-153.Tripkovic L, Wilson LR, Hart K, et al.
Daily supplementation with 15 µg vitamin D(2) compared with
vitamin D(3) to increase wintertime 25-hydroxyvitamin D status
in healthy South Asian and white European women:
a 12-wk randomized, placebo-controlled food-fortification trial.
Am J Clin Nutr. 2017;106:481-490.Wetmore JB, Kimber C, Mahnken JD, Stubbs JR.
Cholecalciferol v. ergocalciferol for 25-hydroxyvitamin D (25(OH)D)
repletion in chronic kidney disease: a randomised clinical trial.
Br J Nutr. 2016;116:2074-2081.Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF.
Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-
dihydroxyvitamin D3 in response to vitamin D2 and
vitamin D3 supplementation.
J Clin Endocrinol Metab. 2013;98:973-979.Tjellesen L, Hummer L, Christiansen C, Rodbro P.
Serum concentration of vitamin D metabolites during treatment
with vitamin D2 and D3 in normal premenopausal women.
Bone Miner. 1986;1:407-413.Stephensen CB, Zerofsky M, Burnett DJ, et al.
Ergocalciferol from mushrooms or supplements consumed with a standard
meal increases 25-hydroxyergocalciferol but decreases
25-hydroxycholecalciferol in the serum of healthy adults.
J Nutr. 2012;142:1246-1252.Hammami MM, Abuhdeeb K, Hammami S, Yusuf A.
Vitamin-D2 treatment-associated decrease in 25(OH)D3 level is
a reciprocal phenomenon: a randomized controlled trial.
BMC Endocr Disord. 2019;19:8.Armas LA, Hollis BW, Heaney RP.
Vitamin D2 is much less effective than vitamin D3 in humans.
J Clin Endocrinol Metab. 2004;89:5387-5391.Hammami MM, Yusuf A.
Differential effects of vitamin D2 and D3 supplements on 25-
hydroxyvitamin D level are dose, sex, and time dependent:
a randomized controlled trial.
BMC Endocr Disord. 2017;17:12.Balachandar R, Pullakhandam R, Kulkarni B, Sachdev HS.
Relative efficacy of vitamin D(2) and vitamin D(3) in improving
vitamin D status: systematic review and meta-analysis.
Nutrients. 2021;13:3328.Page MJ, McKenzie JE, Bossuyt PM, et al.
The PRISMA 2020 statement:
an updated guideline for reporting systematic reviews.
BMJ. 2021;372:n71.McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV, JT.
Cochrane Handbook for Systematic Reviews of Interventions
version 6.4 (updated August. 2023).
Cochrane, 2023. Accessed May 14, 2024.
www.training.cochrane.org/handbookJadad AR, Moore RA, Carroll D, et al.
Assessing the quality of reports of randomized clinical trials:
is blinding necessary?
Control Clin Trials. 1996;17:1-12.Forouhi NG, Menon RK, Sharp SJ, et al.
Effects of vitamin D2 or D3 supplementation on glycaemic control and
cardiometabolic risk among people at risk of type 2 diabetes:
results of a randomized double-blind placebo-controlled trial.
Diabetes Obes Metab. 2016;18:392-400.Gottschlich MM, Mayes T, Khoury J, Kagan RJ.
Clinical trial of vitamin D(2) vs D(3) supplementation
in critically Ill pediatric burn patients.
JPEN J Parenter Enteral Nutr. 2017;41:412-421.Greer FR, Marshall S.
Bone mineral content, serum vitamin D metabolite concentrations,
and ultraviolet B light exposure in infants fed human milk
with and without vitamin D2 supplements.
J Pediatr. 1989;114:204-212.Harris SS, Dawson-Hughes B, Perrone GA.
Plasma 25-hydroxyvitamin D responses of younger and older men
to three weeks of supplementation with 1800 IU/day of vitamin D.
J Am Coll Nutr. 1999;18:470-474.Holick MF, Biancuzzo RM, Chen TC, et al.
Vitamin D2 is as effective as vitamin D3 in maintaining
circulating concentrations of 25-hydroxyvitamin D.
J Clin Endocrinol Metab. 2008;93:677-681.Itkonen ST, Skaffari E, Saaristo P, et al.
Effects of vitamin D2-fortified bread v. supplementation with
vitamin D2 or D3 on serum 25-hydroxyvitamin D metabolites:
an 8-week randomised-controlled trial in young adult Finnish women.
Br J Nutr. 2016;115:1232-1239.Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, Dierkes J.
Bioavailability of vitamin D(2) and D(3) in healthy volunteers,
a randomized placebo-controlled trial.
J Clin Endocrinol Metab. 2013;98:4339-4345.Logan VF, Gray AR, Peddie MC, Harper MJ, Houghton LA.
Long-term vitamin D3 supplementation is more effective than
vitamin D2 in maintaining serum 25-hydroxyvitamin D status
over the winter months.
Br J Nutr. 2013;109:1082-1088.Mitchell DM, Leder BZ, Cagliero E, et al.
Insulin secretion and sensitivity in healthy adults with low
vitamin D are not affected by high-dose ergocalciferol
administration: a randomized controlled trial.
Am J Clin Nutr. 2015;102:385-392.Nieman DC, Gillitt ND, Shanely RA, Dew D, Meaney MP, Luo B.
Vitamin D2 supplementation amplifies eccentric
exercise-induced muscle damage in NASCAR pit crew athletes.
Nutrients. 2013;6:63-75.Nimitphong H, Samittarucksa R, Saetung S, Bhirommuang N, Chailurkit LO.
The effect of vitamin D supplementation on metabolic
phenotypes in Thais with prediabetes.
J Med Assoc Thai. 2015;98:1169-1178.Pinto JM, Merzbach V, Willmott AGB, Antonio J, Roberts J.
Assessing the impact of a mushroom-derived food ingredient
on vitamin D levels in healthy volunteers.
J Int Soc Sports Nutr. 2020;17:54.Zajac IT, Barnes M, Cavuoto P, Wittert G, Noakes M.
The effects of vitamin D-enriched mushrooms and vitamin D3 on
cognitive performance and mood in healthy elderly adults:
a randomised, double-blinded, placebo-controlled trial.
Nutrients. 2020;12:3847.Webb AR, Kline L, Holick MF.
Influence of season and latitude on the cutaneous synthesis of
vitamin D3: exposure to winter sunlight in Boston and
Edmonton will not promote vitamin D3 synthesis in human skin.
J Clin Endocrinol Metab. 1988;67:373-378.Durrant LR, Bucca G, Hesketh A, et al.
Vitamins D(2) and D(3) have overlapping but different effects
on the human immune system revealed through analysis
of the blood transcriptome.
Front Immunol. 2022;13:790444.Strushkevich N, Usanov SA, Plotnikov AN, Jones G, Park H-W.
Structural analysis of CYP2R1 in complex with vitamin D3.
J Mol Biol. 2008;380:95-106.Guo YD, Strugnell S, Back DW, Jones G.
Transfected human liver cytochrome P-450 hydroxylates
vitamin D analogs at different side-chain positions.
Proc Natl Acad Sci USA. 1993;90:8668-8672.Francic V, Ursem SR, Dirks NF, et al.
The effect of vitamin D supplementation on its metabolism
and the vitamin D metabolite ratio.
Nutrients. 2019;11:2539.van den Heuvel EG, Lips P, Schoonmade LJ, Lanham-New SA, van Schoor NM.
Comparison of the effect of daily vitamin D2 and vitamin D3
supplementation on serum 25-Hydroxyvitamin D
concentration (Total 25(OH)D, 25(OH)D2, and
25(OH)D3) and importance of body mass index:
a systematic review and meta-analysis.
Adv Nutr. 2024;15:100133.
Return to VITAMIN D
Since 10-09-2025


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |