

Phytoalexins in Cancer Prevention This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Frontiers in Bioscience (Landmark Ed). 2012 (Jun 1); 17: 2035–2058 ~ FULL TEXT
Donato F. Romagnolo, Cindy D. Davis, John A. Milner
Department of Nutritional Sciences and Arizona Cancer Center,
The University of Arizona,
Tucson, AZ 85721-0038, USA.
donato@u.arizona.eduPlant phytoalexins are a class of low molecular weight compounds that accumulate in response to biotic and abiotic elicitors such as pathogens, wounding, freezing, UV light, and exposure to agricultural chemicals. Phytoalexins have been identified in at least 75 plants including cruciferous vegetables, soybean, garlic, tomato, rice, beans, and potatoes suggesting plants may be a rich source of cancer-fighting compounds. Preclinical evidence suggests these compounds possess anticancer properties including an inhibition of microbial activity, cell proliferation, invasion and metastasis, hormonal stimulation, and stimulatory effects on expression of metabolizing enzymes. This review highlights the plausible molecular mechanisms through which phytoalexins regulate biological processes that can impinge cancer development. Targets of phytoalexins include signal transduction pathways, transcription factors, cell cycle checkpoints, intrinsic and extrinsic apoptotic pathways, cell invasion and matrix metalloproteinase, nuclear receptors, and the phase II detoxification pathway. Additional research should address physiological relevant dietary concentrations, combinations of phytoalexins and interactions with other dietary compounds, duration of exposure, and tissue specificity as variables that influence the effectiveness of phytoalexins on normal and cancerous processes.
Key Words: Phytoalexins, Plants, Nutrition, Cancer process, Cancer prevention, Review
From the FULL TEXT Article:
INTRODUCTION
Phytoalexins are a class of low molecular weight compounds that accumulate in plants in response to biotic and abiotic elicitors including pathogens, stress due to wounding, freezing, UV light, and exposure to agricultural chemicals. [1, 2] For example, the exposure to yeast elicitors stimulates the production of the pterocapan phytoalexin medicarpin from phenylalanine and malonyl-CoA in the legume Medicago trunculata (Barrel clover). [3] Because plants with increased levels of phytoalexins display improved resistance to various stresses, it is possible that these compounds may provide benefits in non-plant circumstances where stress might induce abnormalities including cancer. [4]

Table 1 The development of genetically engineered plants such as kiwis [5], apples [6], tomatos [7], and brussel sprouts [8] that produce higher levels of resveratrol provides an example of potential strategies to increase the dietary intake of phytoalexins. Other plants that have been genetically engineered to increase the production of phytoalexins include peanut [9] and alfalfa (medicarpin). [10] Interestingly, phytoalexin compounds have been identified in at least 75 plant species including cruciferous vegetables, peas, soybean, grapes, peanut, garlic, tomato, and beans (Table 1). In Brassica Sp. alone, close to 40 phytoalexin compounds have been isolated. [11] Therefore, plant phytoalexins represent a rich source of compounds for the development of functional foods with cancer prevention properties.
Several factors influence the content of phytoalexins in plants and include the stage of maturity at harvest. For example, the levels of tomato glycoalkaloids, which comprise the phytoalexin alpha-tomatine, decline from 20-50 mg/100 g in green tomatoes, to 1-3 mg/100 g as the fruit ripens. [12] The content of alpha-chaconine and alpha-solanine, two phytoalexins found in potatoes, varies depending on the potato cultivar and it is higher in potato sprouts and potato cortex. Moreover, the content of phytoalexin can change in response to treatment. For example, synthetic elicitors have been developed as green pesticides to increase phytoalexin protection of growing fruits from attack by harmful pathogens. [13] There is ample research evidence documenting that time of harvest, cultivar selection, growing conditions, and post-harvest processing impact the content of phytoalexins in plants, and consequently, the potential nutritional and cancer prevention properties of plant-derived foods. [14]
Several classes of phytoalexins have been identified in plants. Many cruciferous phytoalexins are produced from tryptophan and include camalexin, brassinin, 1-methoxy-brassinin, cyclobrassinin, rapalexin, and rutalexin. [15] 3-deoxyanthocyanidin phytoalexins are a rare type of flavonoid induced in Sorghum by Colletotrichum, the anthracnose fungus. [16] Sorghum is the only reported dietary source of 3-deoxyanthocyanidin available for human consumption. The major components of the 3-deoxyanthocyanidins group include apigeninidin and luteolinidin. [17] These compounds are structurally related to anthocyanidins except for the absence of C-3 hydroxylation in the C-ring.
Although adverse health consequences have been reported for glycoalkaloids, a selected group of glycoalkaloid phytoalexins have drawn attention because of their anticancer properties. Glycoalkaloids are nitrogen-containing steroidal glycosides and include alpha-solanine and alpha-chaconine from potatoes (Solanum tunerosum), and alpha-tomatine found in tomatoes (Solanum lycopersicum). Alpha-solanine and alpha-chaconine comprise more than 95% of the glycoalkaloid content in potatoes and differ only in the structure of the carbohydrate moiety attached to the 3-OH group of solanidine. [18] Postharvest conditions can dramatically increase their content in potatoes by 300-fold. [19]
Stilbenes are synthesized from coumaryl-CoA and three molecules of malonyl-CoA, and possess structural similarities to estrogen. [20] Stilbene phytoalexins are structurally characterized by the presence of a 1,2-diphenylethylene nucleus and comprise monomeric (resveratrol, pterostilbene) and dimeric (viniferin) compounds. [21] Resveratrol (3,5,4'-trihydroxy-trans-stilbene) is found predominantly in grapes, peanuts and blueberries. Pterostilbene (trans-3,5-dimethoxy-4'-hydroxy-stilbene) is found in grapes, grape leaves, some berries, and extracts of heartwood of Pterocapus marsupium used in Ayurvedic medicine. Stilbenes synthesis in grapes is influenced by grape variety with red grapes containing more stilbenes than white grapes, and growing conditions with stilbenes content increasing with elevation). [22]
Phenolic coumarins include psoralidin which is found in Psoralea coryfolia, a herbal plant used in traditional Chinese medicine for the cure of endocrine disorders and depression. Psoralidin is also one among several phytoalexins (phaseolin, kievitone, phaseollidin, coumestrol) isolated from Phaseolus vulgaris. [23] Psoralidin possesses an isopentenyl group at the C-2 position of coumestrol.
Phytoalexins have also been isolated from garlic (Allium sativum L.) and include the thiosulfinate allicin, which originates from alliin by action of the enzyme aliinase upon crushing of garlic cloves. Alliin is a sulfoxide that is synthesized from the amino acid cysteine. [24] Di-terpene phytoalexins are composed of isoprene units and include momilactones, oryzalexins, and phytocassanes found in rice (Oryza sativa) seedlings. [25]
Although much of the research dealing with the health effects of soy products has focused on the isoflavonoids genistein and daidzein, many phytoalexins with health benefiting properties are also found in soy. Their content varies greatly in response to stress factors and physical stimuli. The enrichment of the soybean phytoalexins coumestrol and glyceollin through inoculation with food-grade, non toxin producing Aspergillus Sp. (A. sojae, A oryza) has been proposed as a strategy to increase the health benefits of fermented soybean foods. [26]
Glyceollins are pterocarpan compounds induced in soybean in response to exposure to various elicitors including the fungus Diaporthe phaseolorum f. sp. meridionalis [27] and disease-inducing herbicides. [28] They share the core structure with coumestrol and consist primarily of three major isomers (I, II, and III) derived from the parent isoflavone daidzein through the intermediate glycinol that is prenylated to produce glyceollidin I and II followed by cyclization. Other pterocarpan phytoalexins include those isolated from Phaseolus vulgaris L. Analysis of extracts from red kidney bean elicited with Aspergillus sojae achieved levels of the pterocarpan kievitone at about 1200 mg/Kg and phaseollin at about 228 mg/Kg. [29]
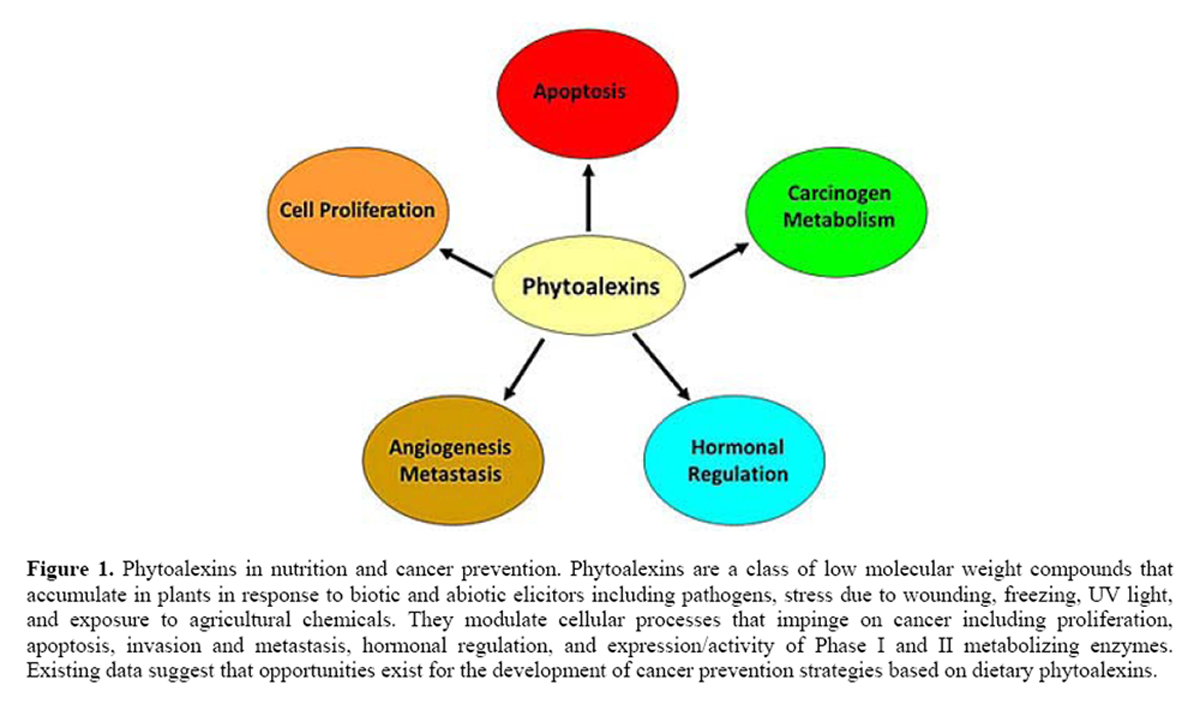
Figure 1

Table 2 Finally, 2-arylbenzofurans are phytoalexins found in rice, leguminose, Morus alba and other crops. They have been shown to protect plants against invasion by pathogens and insects, possess cytotoxic activities against various tumor cells [30], and modulate endocrine functions. [31]
The objective of this review is to summarize and discuss experimental evidence related to the anticancer effects of phytoalexins with specific interest on modulation of antimicrobial activities, proliferation, apoptosis, invasion and metastasis, hormonal regulation, and expression/activity of Phase I and II metabolizing enzymes (Figure 1). The existing data suggest that opportunities exist for the development of cancer prevention strategies based on dietary phytoalexins (Table 2). Nevertheless, before phytoalexin-based diets can be formulated for cancer prevention, future studies need to better identify molecular targets; the effects of duration, dose, and timing of exposure; tissue-specific responses and effects in normal versus cancer cells.
Phytoalexins and Cancer Prevention Mechanisms
Antimicrobial activity
The human body contains 100 times more microbes than mammalian cells. The amounts and types of microbes continue to be viewed as an important variable in many human conditions. They have been implicated in the etiology of many different types of cancer. Infections due to bacteria, viruses and parasites have been estimated to cause 20-25% and 7-10% of cancer deaths in developing and industrialized countries, respectively. [32] One mechanism whereby dietary phytoalexins may be protective against cancer is through their antimicrobial activity.
Helicobacter pylori (H. pylori), a gram-negative bacterium, causes one of the most widespread infections in humans. It afflicts up to 50% of the world's populations. [33] In 1994, H. pylori was classified by the International Agency for Research on Cancer as a group I carcinogen and a definite cause of gastric cancer in humans. [34] Epidemiologic studies have linked H. pylori infection with gastric adenocarcinoma [35] and colorectal adenomas. [36] CagA is the strain-specific H. pylori gene that has been associated with development of both premalignant and malignant lesions. [37]
Phytoalexins that have been investigated for their inhibitory effects on H. pylori include resveratrol, which has been reported to inhibit the growth of 15 different strains of H. pylori in vitro. [38] These studies suggested that antimicrobial activity may contribute to resveratrol's cancer protective effects. [39] Complex mixtures such as red wine extracts have also been shown to inhibit the growth of cagA+ strains of H .pylori in vitro. [40]
Preincubation of MKN-45 human gastric cancer cells with resveratrol (1-100 micromol/L) significantly inhibited the secretion of interleukin (IL)-8 from H. pylori infected cells and suppressed H. pylori-induced ROS generation in a concentration dependent manner. [41] IL-8 is one of the cytokines that mediate the gastric inflammatory response to H. pylori infections. [42] Further evidence of the protective effects of grape compounds against H. pylori was provided by evidence that the addition of red wine in the drinking water significantly reduced the localization of bacteria and VacA to the surface of the gastric epithelium and prevented gastritis in H. pylori-infected mice. [43]
Human studies have also suggested that wine consumption may inhibit H. pylori infection. A study of 10,537 subjects in England found an 11% lower risk of H. pylori infection in wine drinkers (3-6 glasses/wk) compared with those who drank no wine (OR=0.89, 95% CI=0.80-0.99), and higher wine consumption (more than 7 glasses/wk) was associated with a further 6% reduction in the risk of infection (OR=0.83, 95% CI=0.64-1.07). [44] Similarly, in a sample of 3,608 Danish adults there was a lower rate of H. pylori infection among wine drinkers (OR=0.6, 95% CI 0.5-0.7) compared to non-drinkers. [45] Since resveratrol is an important bioactive component in berries and peanuts, these studies suggest that resveratrol-rich diets may have preventative effects against H. pylori infection in humans, and possibly, one mechanism by which such compounds may exert anticancer properties.
Other food phyoalexins may also influence microbial populations. For centuries, garlic has been widely used as an antimicrobial agent against bacteria, viruses and fungi. [46] Allicin and allyl-methyl plus methyl-allyl thiosulfinate from acetonic garlic extracts were shown to inhibit the in vitro growth of H. pylori. [47] Allitridi is a proprietary garlic derivative and has been used to treat both systemic fungal and bacterial infections in China. [48] A proteomic analysis revealed that the bacteriostatic mechanisms of allitridi against H. pylori could be attributed to its inhibitory effects on a number of molecular targets associated with energy metabolism, and reduced biosynthesis of amino acids, proteins, mRNA, and fatty acids. Additionally, allitridi was found to suppress the production of virulence factors such as CagA, VacA and NapA leading to the pathogenic attenuation of H. pylori. [49]
Animal and human studies also suggested that garlic components were protective against the adverse effects of H. pylori infection. A study that investigated the effect of various doses (1, 2, and 4% in the diet) of garlic extracts on H. pylori-induced gastritis in Mongolian gerbils revealed that gastritis was decreased in a dose-dependent manner compared with the control group, even though the number of viable H. pylori was unchanged. [50] The Shandong Intervention Trial sought to determine whether any of three interventions with amoxicillin and omeprazole (1 g, 2 times/d for 2 wks), garlic supplement (aged garlic extract, 400 mg, 2 times/d, and steam-distilled garlic oil, 2 mg, 2 times/d for 7.3 years), or a vitamin supplement ( 100 IU vitamin E, 250 mg vitamin C, 27.5 microg selenium 2 times/d for 7.3 years) could reduce the prevalence of precancerous gastric lesions in Shandong Province in China, a region with high gastric cancer mortality rates and about 67% prevalence of H. pylori infection. [51] Although long-term administration of garlic did not appear to influence the prevalence of H. pylori infection, garlic components may be protective against the adverse effects associated with infection. Other microbes may also be influenced by allyl sulfur compounds arising from garlic. For example, ear infections appear to be sensitive to the amount of garlic consumed confirming the physiological relevance of these phytoalexins as modifiers of microbial proliferation. Many foods may influence microbial activity as reviewed several years ago by Billings and Sherman. [52]
ProliferationCruciferous phytoalexins In organ culture, mammary glands incubated with brassinin and cyclobrassinin (1-10 micromol/L) had respectively, a 70% and 90% maximal reduction in the number of lesions compared to control glands. [53] Protective effects of brassinin against 7, 12-dimethylbenz (a)anthracene (DMBA)-induced mammary lesions in organ culture have been reported at concentrations as low as 0.1 micromol/L. [54, 55] Similarly, concentrations of 1 micromol/L for brassinin, 1-methoxybrassinin, (+/-)-spirobrassinin, (+/-)-1-methoxyspirobrassinin, and (+/-)-1-methoxyspirobrassinol were reported to markedly inhibit (60-70%) in vitro proliferation of T-lymphoblastic leukemia. [56] In vivo, brassinin treatment during the promotion phase at a concentration of 1% reduced skin tumor incidence by 50%. [53]
The treatment of solid tumors and leukemia cell lines with analogues of 1-methoxyspirobrassinol exerted cytostatic/cytotoxic effects, which were similar to those induced by the drugs cisplatin, etoposide, and doxorubicin. [57] Brassinin and isobrassinin, a regioisomer of brassinin, inhibited by 25-80% the growth of Hela, A431, and MCF-7 cell lines. [58] Brassinin and its structural derivative 5-bromo-brassinin were reported to inhibit idolamine 2,3-dioxygenase (IDO), a pro-toleragenic enzyme involved in immune escape in cancer. The protective effects of brassinin and 5-bromo-brassinin on established breast tumors appeared to be dependent on coincident IDO expression since response to treatment was lost in IDO -/-mice. [59]
One of the mechanisms that contribute to the anticancer effects of cruciferous phytoalexins is induction of cell cycle arrest. For example, 1-methoxybrassinin was reported to induce cell cycle arrest with 90% of cells positioned in sub-G0/G1 after 72 h incubation in vitro. [56]
Deoxyanthocyanidins Studies that investigated the biological activities of 3-deoxyanthocyanidins reported luteolinidin and apigeninidin were more cytotoxic on human HL-60 and HepG2 cancer cells than the 3-hydroxylated anthocyanidin analogues pelargonidin and cyanidin. Because the production of 3-deoxyanthocyanidins can occur rapidly (about 3 d) in seedlings inoculated with fungi, the process of inoculation could be exploited for the large-scale enrichment of these phytoalexins in Sorghum products. [60]
Glycoalkaloids In vitro studies documented that glycoalkaloid phytoalexins effectively suppressed the growth of cervical, liver, lymphoma, stomach, and colon cancer cells. In particular, alpha-solanine, and alpha-chaconine isolated from potatoes (Solanum tuberosum), and alpha-tomatine found in tomatoes (Solanum lycopersicum) at concentrations of 1-10 mg/L inhibited by 60- 80% the growth of human colon (HT29) and liver (HepG2) cancer cells within 4 h with alpha-tomatine being the most active compound at lower concentrations (0.1 mg/L, 40% growth inhibition). These antiproliferative effects were stronger than those elicited by similar levels (1 mg/L) of the anticancer drugs doxorubicin and camptothecin. [61] Compared to treatments with alpha-chaconine and alpha-solanine alone, their combination offered synergistic advantages against proliferation of HepG2 cells. [62] These data suggested that combinations of phytoalexins may additively prevent proliferation of cancer cells.
Stilbenes Resveratrol was reported to induce cell cycle arrest in various cell lines including leukemia [63], liver HepG2 [64], B16 melanoma [65], and prostate LNCaP. [66] The antiproliferative effects of resveratrol may be influenced by interactions between dose and duration of exposure. For example, coincident arrest in G1 and downregulation of cyclin D1 were observed at concentrations of 1-5 micromol/L within 24 h, whereas repression of cyclin E and cdk2/4/6, and accumulation of hyperphosphorylated Rb, p53, and p21 were seen at higher doses (10-25 micromol/L) or after longer periods (24-48 h) of exposure. [67] At relatively higher doses (10-100 micromol/L) resveratrol was reported to induce accumulation of p27 and repression of signaling through mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase (ERK)-1 (MEK-1), ERK1/2, activator protein-1 (AP-1) [68], and nuclear factor kappa beta (NFkappaB). [69] Resveratrol-induced G1-arrest has been also observed in cancer (A431) cells lacking active p53, suggesting it may induce growth arrest through p53-independent activation of p21. [67]
Resveratrol has also been reported to induce S-phase arrest. [68-72] In human malignant-B myeloma [73] and ovarian carcinoma Ovcar-3 [74] cells, resveratrol induced S-phase arrest and the activation of the ataxia telangiectasia mutated (ATM)/ ataxia telangiectasia and Rad3-related (ATR)/Chk/cdc25c pathway. These effects were indicative of activation by resveratrol of a DNA damage response. For review of the interactions of resveratrol with ATM and ATR please refer to the excellent review by Gatz and Wiesmuller. [75] The induction of S-phase arrest has also been documented in a panel of breast cancer cells (MCF-7, MDA-MB-231) following treatment with 10-25 micromol/L resveratrol for 4 d. Biochemical changes that accompanied the growth arrest included inhibition of the phosphatidylinositol 3-kinases (PI3K)/ mammalian target of rapamycin (mTOR)/p70S6L signaling pathway. [76]
In HT29 colon cancer cells, resveratrol induced G2 phase arrest, which was accompanied by inactivation of p34/cdc2 protein kinase through inhibition of cdk7. [77] Collectively, these data suggest that resveratrol can affect multiple cell cycle targets depending on the concentration, length of exposure, and type of cell.
The resveratrol analogue pterostilbene has been investigated for its antiproliferative properties. It was shown to inhibit (ED50=4.8 micromol/L) the incidence of DMBA-induced mammary lesions in organ cultures [78], induce cytotoxicity of breast and murine lymphoid cancers [79], and inhibit proliferation of pancreatic [80] and bladder [81] cancers. The administration of pterostilbene to azoxymethane (AOM)-treated F344 rats (15/mg/kg BW pterostilbene, once weekly for 2 wks) or male ICR mice (50 or 250 ppm for 6 or 23 wks) antagonized the formation of aberrant crypt foci (ACF) and iNOS expression. [82] The mechanistic actions of pterostilbene against ACF were related to lowered proliferating cell nuclear antigen (PCNA), downregulated expression of beta-catenin and cyclin D1, reduced expression of inflammatory markers (tumor necrosis factor (TNF)alpha, IL-1beta, IL-4, phosphorylated p65) [83], inhibition of rat sarcoma (Ras), PI3K/Akt, and epidermal growth factor (EGF)-receptor (EGFR) signaling [84], and activation of nuclear factor-erythroid2-related factor2 (Nrf2)-mediated antioxidant signaling pathways. [85]
In human HT-29 colon cancer cells treated with pterostilbene, the inhibition of cell proliferation was accompanied by reduced expression of proinflammatory iNOS. [82] Downregulation of iNOS by pterostilbene (1-20 micromol/L) in macrophages was attributed to inhibition of NFkappaB, PI3K/Akt/IKK, and MAPK pathways [86]. Pterostilbene reduced the expression of c-Myc and cyclin D1 and was more effective than resveratrol in inhibiting proliferation of HT-29 colon cancer cells [87] and AOM-induced colon tumorigenesis via activation of Nrf2. [85]
Phenolic coumarins and garlic phytoalexins Psoralidin was documented to inhibit NFkappaB activation in RAW 264.7 macrophages [88] and androgen-independent prostate PC-3 and DU-145 cancer cells. [89] Other studies reported that psoralidin downregulated EGFR-regulated MAPK signaling and inhibited cell proliferation, and that the administration of psoralidin (15 and 25 mg/kg BW) for 5 d/wk for 4 wks suppressed PC-3 (prostate) xenograft tumors in nude mice. [90] Therefore, similar to other food phytoalexins, psoralidin may exert anticarcinogenic effects through inhibition of proinflammatory NFkappaB and MAPK pathways.
Whereas the garlic precursor alliin displayed no antiproliferative properties, its product allicin inhibited the growth of mammary (MCF-7), colon (HT-29), and endometrial (Ishikawa) cells in vitro. [91] The growth inhibition of MCF-7 cells was associated with cell cycle arrest in G0/G1 and G2/M. Because allicin is rapidly metabolized, derived compounds including diallyl disulfide (DADS) may contribute to the anti-tumorigenic properties of allicin. [24] v These cumulative data clearly suggest that dietary phytoalexins are of interest as anti-proliferative agents. Their cancer preventive effects are likely influenced by concentrations, association with other bioactive compounds, duration of exposure, and cell and tissue-type. The tumor growth inhibitory properties of phytoalexins appear to be related to repression of signaling through PI3K, MAPK, AP-1, and NFkappaB, and activation of G1, S, and G2 cell cycle checkpoints. The induction of cell cycle arrest by phytoalexins may predispose cells to undergo apoptosis or become more sensitive to other antiproliferative dietary compounds or drugs. For example, certain phytoalexins (i.e. resveratrol) have been shown to function as sensitizers by lowering the threshold response to other anticancer agents [92] or food compounds.
ApoptosisStilbenes Resveratrol alone or in combination with other therapeutic agents has been reported to induce apoptosis. [93, 94] The intrinsic pathway, which is triggered by DNA damage and other types of cellular stress, was induced by resveratrol (50-100 micromol/L) through release of cytochrome-c (Cyt-c) from mitochondria, caspase-3 activation, elevation of Bax, and down-regulation of Mdm2 and the anti-apoptotic B-cell lymphoma-2 (Bcl2) protein. The p53 protein is a component of the intrinsic pathway and it was shown to mediate the proapoptotic effects of resveratrol in human colon [95, 96], head and neck squamous [97], breast [98, 99], neuroblastoma [100], and melanoma [65] cancer cells. In addition, resveratrol was reported to stimulate apoptosis through a p53-independent pathway. However, in p53-null cells that had lower caspase-3 activity, resveratrol did not influence the expression of Bcl-2-associated X protein (Bax). [101]
Resveratrol may also activate the extrinsic pathway through stimulation of the cell surface receptor apoptosis stimulating fragment (Fas) (CD95) and TNF-related apoptosis-inducing ligand (TRAIL)/DR4, and the forkhead transcription factor-1 (FKHRL1). [95] The proapoptotic effects of resveratrol were attributed to inhibition of signaling through PI3K/Akt, prevention of Akt-dependent phosphorylation of forkhead box O (FOXO) transcription factors, reduced binding of 14-3-3 to phosphorylated FOXO, and the subsequent sequestration of FOXO transcription factors to the cytoplasm. Direct transcriptional targets of FOXO include the proapototic factors Bim, p27, TRAIL, DR4, and DR5, and the antiapoptotic cyclin D1. The role of FOXO in the resveratrol-induced apoptosis was demonstrated through inhibition of FOXO expression by shRNA, which abrogated resveratrol-induced caspase-3 activity. [102] Kinases that phosphorylate FOXO factors (i.e. FOXO4, Thr447 and 451) leading to its activation include reactive oxygen species (ROS)-induced c-Jun N-terminal kinase (JNK) and 5' adenosine monophosphate-activated protein kinase (AMPK) (FOXO3a, and Thr179, 399, 413, 555, 588, and 626). [103] JNK activity was required for resveratrol-induced FasL expression and the subsequent induction of apoptosis in leukemia HL-60 cells. [104]
Resveratrol is a known activator of ROS [105] and AMPK [106], which in turn, represses mTOR. [107] Because the mTOR pathway is altered in a variety of malignancies, activation of AMPK by resveratrol provides a molecular target in cancer prevention. In prostate cancer PC-3 xenografted tumors, resveratrol stimulated the expressions of TRAIL-R1/DR4, TRAIL-R2/DR5, Bax and p27, and inhibited the expression of Bcl-2 and cyclin D1. [108] Overall, resveratrol may contribute to stimulation of apoptosis through activation of both the intrinsic and extrinsic pathways. Similar proapoptotic effects have been documented for the resveratrol dimer, viniferin (50 micromol/L) in leukemia cells. [109]
The resveratrol analogue, pterostilbene, has also been investigated for its proapoptotic properties. In metastatic melanoma SK-MEL cells, it produced caspase 3/7-dependent apoptosis. Combination treatments with pterostilbene plus inositol-6-phosphate produced synergistic growth inhibition. [110] Dose-dependent (10-100 micromol/L) induction of apoptosis related to increased caspase activities were observed in lung NCI-H460, SK, MES-1 [111], breast MCF-7 and MDA-MB-231 [112], and pancreatic MIA PaCa-2 [80] cancer cells. In gastric carcinoma AGS cells, the induction of apoptosis via Fas/FasL pathway and caspases-3 was associated with enhanced expression of the growth arrest DNA-damage-inducible (GADD)-45 and GADD-153 genes, and cell cycle arrest in G1. The latter effect was marked by increased expression of the tumor suppressor genes p53, p21, p27, and p16; accumulation of unphosphorylated retinoblastoma (Rb); release of cyt-c in the cytosol; and decreased levels of cyclin A, E, cdk-2, cdk4, and cdk6. [113]
In human bladder cancer cells, pterostilbene induced autophagy through inhibition of PI3K/Akt and the mTOR/p70S6K pathways, and activation of MEK/ERK1/2). These changes preceded apoptosis characterized by cell cycle arrest in G0/G1, reduced levels of cyclins (A, B, D1, and pRb), and induction of caspase-3 activity. [81] In HT-29 colon cancer cells, the treatment with pterostilbene increased the levels of cleaved poly (ADP-ribose) polymerase (PARP). [87] Consistent with the induction of proapoptotic responses in cancer cells in vitro, pterostilbene was found to induce caspase-3/8/9 activities, PARP cleavage products, and Fas/Fas-L in colonic mucosa of male ICR mice. [84] In the model yeast Saccharomyces cerevisiae a large number of mitochondrial genes were induced by pterostilbene offering additional mechanistic evidence that apoptosis is a process targeted by this dietary phytoalexin. [114]
Phenolic coumarins Studies reported that psoralidin was cytotoxic against HT-29 (colon), MCF-7 (breast), [115], and SNU (stomach) cancer [116] cell lines. In androgen independent prostate cancer (AIPC) cells (PC-3, DU-145), psoralidin-induced apoptosis was accompanied by downregulation of antiapoptotic Bcl-2, Bcl-xL, and survivin. [89] The induction of apoptosis was also accompanied by inhibition of EGF-induced Bcl-2 and survivin, upregulation of Bax and activated caspase-3, and activation of stress-activated protein kinase (SAPK) signaling. [90] Interestingly, psoralidin did not cause any toxicity to normal prostate epithelial cells.
In prostate cancer, TNF-mediated signaling is the predominant pathway leading to cell survival and resistance to therapy. In AIPC cells, psoralidin inhibited the constitutive and TNF-induced expression of TNF-alpha and expression of the prosurvival signaling molecules NF-kB and Bcl-2. [117] Psoralidin was identified as one of the active compounds isolated from seeds of Psoralea coryfolia and believed to be responsible for the inhibition of NO production in LPS-activated microphages. [118]
Proapoptotic effects have been reported for the coumarin-related phytoalexin scopoletin (6-methoxy-7-hydroxycoumarin), which has been isolated from Erycibe obtusifolia, Artemisia Montana, and Gelsemium sempervirens. In HL-60 promyelocytic cells, scopoletin induced apoptosis accompanied by activation of caspase-3, cleavage of PARP, and DNA fragmentation. [119] In AHH-1 TK+/? human lymphoblastoid cells, exposure to coumestrol dose-dependently (10-50 micromol/L) induced a significant increase in micronuclei. The highest frequency of micronuclei occurred 2 d after exposure to coumestrol and decreased afterwards. Parallel to the decrease in the micronucleus frequency, coumestrol increased the percentage of cells undergoing apoptosis. [120]
Glycoalkaloids The potato phytoalexins alpha-chaconine and alpha-solanine were reported to induce apoptotic cell death of HT-29 human colon cancer cells. However, alpha-chaconine was more potent than alpha-solanine, and it induced apoptosis through caspase-3 activation and inhibition of ERK 1/2 phosphorylation. [18] Similar effects by alpha-chaconine were documented in LNCaP cells through induction of PARP cleavage and activation of JNK, and this response played a major role in the induction by alpha-chaconine of caspase-dependent apoptosis. [121] These results suggest that apoptosis induced by whole potato extracts in prostate cancer cell lines may be in part due to alpha-chaconine. [122]
Garlic phytoalexins Allicin is a garlic thiosulfinate known to induce apoptosis through both extrinsic and intrinsic pathways. In cancer cells, it induced cyt-c release from the mitochondria, increased caspase-3, -8, and -9 activities, and upregulated the expression of Bax and Fas. [123, 124] Similar proapoptotic effects were observed in colon cancer HCT-116 [125] and promyeolocytic-leukemia HL-60 [126] cells in which allicin decreased the Bcl-2/Bax ratio, while stimulating the mitochondrial release of cyt-c and activation of caspase 3/9. Interestingly, the proapoptotic properties of allicin have been exploited for the development of site-directed apoptotic killing. Using a rituximab monoclonal CDC20 antibody-aliinase conjugate, upon addition of alliin, allicin was formed in situ, killing CD20+ tumor B-chronic lymphocytic leukemia cells via apoptosis. Following treatment with alliin for 72 h, an 85% reduction was observed in the number of viable cells. The in situ generation of allicin in cells was also proposed as a strategy to overcome the known problem of the short-lived allicin molecules in the circulation. [127]
Di-terpenes One of the conditions that characterize solid tumor growth is hypoxia which makes tumors less responsive to therapy. Studies with the phytoalexin and di-terpene momilactone-B found in rice (Oryza sativa L.) hulls documented this food component suppressed hypoxia-induced cyclin D1, cdk4, hypophosphorylated Rb, and Bcl-2 levels, whereas it increased the cellular content of proapoptotic Bax and caspase-3 protein. [128] Anticarcinogenic effects of momilactone B were also documented in human lymphoma cells (Jurkat) through elevation of PARP, caspase-3, cytosolic cyt-c, and reduced phosphorylated Bad, [129] and in colon cancer HT-29 and SW620 cells through reduced survival. [130] Overall, these studies suggest phytoalexin compounds can impact mitochondrial functions and death receptors distribution leading to activation of cell-death complexes characteristic of apoptosis.
Invasion and metastasis
Tumor cell invasion is a key event in the metastatic process. It includes attachment of tumor cells to extracellular proteins through tumor-cell receptors and degradation of extracellular matrix (ECM) components via proteinases such as matrix metalloproteinase (MMP), of which four subclasses have been characterized: collagenase, gelatinase, stromelysin, and membrane-associated MMP. The breakdown of the extracellular matrix is followed by penetration through the basement membrane of capillary and lymphatic vessels, intravasation, and growth in new tissue (reviewed in [131]).Glycoalkaloids A phytoalexin with anti-invasion and anti-metastatic properties is alpha-solanine. At concentrations ranging from 5 to 20 micromol/L, it was reported to inhibit human melanoma A2058 cells migration and invasion by reducing MMP-2 (gelatinase-A) and MMP-9 (gelatinase-B) activities. The MMP-2 and MMP-9 are known to play a key role in the process of metastasis. The expression of MMP-9 is stimulated by TNFalpha, vascular endothelial growth factor (VEGF), EGF, transforming growth factor (TGF)beta, and Ras through various signaling pathways. Transcription factors that activate MMP-9 include AP-1 and NFkappaB, which are activated through MAPK and PI3K pathways. In A2058 cells, alpha-solanine inhibited the phosphorylation of JNK, PI3K, and Akt, and reduced the levels of nuclear NFkappaB. [132] Similar antimetastatic effects were reported for a related potato phytoalexin, alpha-chaconine, which inhibited MMP-2/9 activities and suppressed JNK and PI3K/AkT/NFkappaB signaling pathways in human lung adenocarcinoma A549 cells [133], and tube formation of bovine aortic endothelial (BAEC) cells. [134]
Garlic phytoalexins Anti-invasion and -metastatic mechanisms of action have been documented for allicin, which in human endothelial cells antagonized gamma-radiation-induced JNK/AP-1 activity and reduced the expression of the downstream target intercellular adhesion molecule-1 (ICAM-1/CD54). The ICAM-1 is an inducible surface glycoprotein that facilitates adhesion-dependent cell-to-cell interactions and invasion of cells through the ECM. [135] Therefore, by reducing ICAM-1 levels allicin may contribute to reducing invasion.
Stilbenes In rodent models, the treatment with resveratrol reduced angiogenesis, VEGF and VEGF receptor-2 (VEGFR2)-positive cells, and markers of metastasis (MMP-2 and MMP-9). [108] Similar repressive effects on MAPK, PI3K/Akt, NFkappaB, and AP-1 pathways leading to MMP-9, and reduced invasion and metastasis were reported for pterosilbene in liver HepG2 [136] and breast cancer MCF-7 cells activated with heregulin-beta1, a ligand of human EGFR-2 (HER-2) [137]. The blockage of heregulin functions is known to inhibit tumorigenesis and metastasis of breast cancer cells. [138] Repressive effects on heregulin-beta1-mediated MMP-9 expression and cell invasion have been also reported for resveratrol in human breast cancer cells. [139]
Phenolic coumarins The potential for preventing invasion has been documented by investigations with the phytoalexin coumestrol, which inhibited (2.5 to 10 micromol/L) cell invasion of MDA-MB-231 breast cancer cells in matrigel invasion assays [140], and reduced intercellular adhesion and migration in vivo. [141] In human unmbilical vein endothelial cells, scopoletin inhibited VEGF-induced tube formation, proliferation and migration. These effects were associated with inhibition of VEGF-induced autophosphorylation of VEGFR2, and downregulation of ERK1/2 and p38 MAPK activities. [142] Overall, the multiple targeting by dietary phytoalexins of MAPK, PI3K/Akt, NFkappaB, and AP-1 pathways leading to activation of factors involved in the breakdown of ECM components and invasion highlights the potential use of these compounds in the prevention of the metastatic process.
Hormonal regulationSoy phytoalexins The intake of soy food components has been linked to reduced risk of endocrine tumors. The inhibition of estrogen binding to estrogen receptor (ER) by the soy phytoalexin glyceollin occurs at low micromolar concentration and it is 3-fold higher for the ERalpha (IC50 about 6 micromol/L compared to ERbeta (IC50 of about 16 micromol/L). [143] Compared to genistein and daidzein, glyceollins are stronger inhibitors of ER signaling in breast cancer cells [144], and suppress estrogen-dependent breast and ovarian tumorigenesis in ovariectomized athymic mice. [145] In contrast to tamoxifen, glyceollins do not exhibit agonistic effects on uterine morphology and antagonize the uterotropic stimulation of estrogen. The absence of uterotropic activity is characteristic of pure antiestrogens [146] and it is of particular interest for glyceollins since antiestrogen therapies based on tamoxifen have been shown to increase the risk of endometrial cancers. [147]
The intraperitoneal treatment of nu/nu immune-compromised ovariectomized mice with 20 mg/kg/d for 15 d of a glyceollin mixture reduced estrogen-induced mammary tumor formation. In postmenopausal female monkeys, the supplementation with glyceollins (134 mg/d) prevented the estradiol-dependent stimulation of mammary proliferation. Also, glyceollin increased the expression of ERalpha while reducing the expression of the progesterone receptor (PR) and trefoil factor-1 (TFF1). [148] The dose of 134 mg/d used in the latter study is theoretically achievable in humans through diet but it is considerably higher than the typical dietary isoflavone consumption observed in Western populations (lower than 5 mg/d) and the one reported (about 60 mg/d) for some Asian diets. [149] Glyceollin-enriched soy products have been suggested as a strategy to increase the intake of glyceollins and ameliorate the estrogenic effects of soy isoflavones. For example, soy yogurt produced from black soybeans (Glycine max L. Merrill) after fungal stress with food-grade Rhizopus oliosporus was enriched (1 mg/g) in total glyceollins and had reduced levels of undesirable oligosaccharides stachyose and raffinose. [150] v In contrast to glyceollins, glycinol, a precursor of glyceollins, exhibited potent estrogenic activities including stimulation of proliferation of breast cancer cells, expression of PR, and transactivation at estrogen response elements (ERE). Because the production of glyceollins is influenced by the type and duration of stress (i.e. fermentation process), the cancer preventative effects of soy-based foods may be influenced by the relative content of glycinol and glyceollins. [151] These observations suggest caution should be exercised when recommending the use of soy-derived products for cancer prevention.
Studies with androgen-responsive human prostate (LNCaP) cancer cells documented that a mixture of glyceollins (I=68%, II=21%, III=11%) had growth inhibitory properties. The glyceollin mixture inhibited growth of LNCaP cells in a dose-dependent fashion (0.25 to 25 micromol/L). These effects were due to inhibition of G1/S progression and correlated with upregulation of cdk inhibitor 1A and 1B. In addition, glyceollin at concentrations ranging from 0.25 to 12.5 micromol/L inhibited estrogen-, but not dihydrotestosterone (DHT)-induced growth and prostate-specific antigen (PSA) expression in LNCaP cells. Furthermore, the glyceollin mixture reduced the expression of the androgen/estrogen-responsive genes NKX3.1 and insulin-like growth factor-1 receptor (IGF-1R). Intraperitoneal treatment of nu/nu immune-compromised ovariectomized mice with 20 mg/kg/d for 15 d of glyceollin mixture reduced estrogen-induced mammary tumor growth derived from xenografted MCF-7 cells injected into the mammary fat pad. [152]
The inhibition of cell proliferation by glyceollin mixtures has been attributed to antagonistic actions on the ERalpha by the (-)-glyceollin-I isoform in the cis (6aS, 11aS) configuration. In ER competition studies, this isomer had the highest affinity for the ERalpha with displacement of 50% estradiol bound to ERalpha at a concentration of about 2.0 nanomol/L. It also had antagonistic actions on proliferation of MCF-7 and ovarian (BG-1) cancer cells similar to those observed for 4-OH-tamoxifen and ICI 182,780. [153] Compared to other glyceollin isoforms, the stronger antiestrogenic effects of (-)-glyceollin-I were attributed to substitutions at the A ring. The (-)-glyceollin I isomer was documented to decrease the transcriptional activity of ER and downregulate the expression of the PR. [154]
In vitro, glyceollin I, but not glyceollin II and III showed significant inhibition of ERE activation suggesting this isomer is primarily responsible for the antiestrogenic properties of soy-derived glyceollins. [155] Overall, these studies suggest glyceolllins have unique ER-modulator properties without agonist activity.
Phenolic coumarins Compared to glyceollins, the soy phytoalexin coumestrol competes with estrogen for binding to ER at lower, nanomolar, levels and its binding affinity for ERbeta (IC50= 35 nanomol/L) is higher compared to that of the ERalpha IC50=109 nanomol/L). Coumestrol has been investigated as a potential alternative to hormone replacement therapies. [156] However, both estrogenic and antiestrogenic effects have been reported for coumestrol. At low estrogen levels (0.01 nanomol/L), coumestrol (less than 10 micromol/L) acted as an estrogen and stimulated cell growth of ER-positive breast cancer cells. Conversely, at higher estrogen levels (1 nanomol/L) coumestrol (10micromol/L) inhibited cell proliferation. [157] The administration of coumestrol (60 mg/kg/d) to immature intact rats stimulated uterine hyperplasia [158] suggesting coumestrol-rich diets may actually induce uterus growth during conditions of low estrogen exposure ( i.e. prepuberty, postmenopause). These effects were not in agreement with those of other reports documenting dietary coumestrol reduced the burden of colon tumorigenesis in the Apc Min/+ mouse model. The anticarcinogenic actions of coumestrol may be due to restoration of E-cadherin-beta-catenin interactions, which are usually observed following treatment with chemopreventive drugs such as sulindac. [141]
Although studies of coumestrol consumption reported very low dietary exposure to this phytoalexin compared to other phytoestrogens (i.e. genistein) [159, 160], coumestrol has been shown to have higher binding affinity for ERalpha (IC50=21 nanomol/L) and ERbeta (9 nanomol/L) compared to genistein (520 and 16 nanomol/L) and resveratrol (1 and 0.7 micromol/L). [161] Therefore, timing of exposure, tissue-type, and doses relative to endogenous estrogen concentrations may be important determinants of the effects of coumestrol on cancer risk. In support of this concept, case-control studies that examined the relationship between dietary intake of coumestrol and testicular cancer risk reported a multivariate U-shaped relationship with increased cancer risk at low ( less than 19 microg) and high ( more than 84 microg) levels of dietary coumestrol/1,000 kcal. [162] An inverse relationship between daily intake of coumestrol and prostate cancer risk (OR=0.48) was also observed in a case-control study in Caucasian subjects [163] with median intake levels of coumestrol of 67.5 and 45 microg/d, respectively in control and case subjects. The latter studies further highlight the impact of levels of coumestrol intake on cancer risk.
Bean phytoalexins In binding assays, kievitone had higher affinity and increased ERE transcriptional activity for ERalpha, whereas phaseollin had higher affinity and increased transcriptional activity for ERbeta. However, concentrations of 0.1 micromol/L kievitone and phaseollin had respectively only 10% and 2% the estrogenic activity for ERalpha compared to equimolar concentrations of genistein. Both kievitone and phaseollin at the concentration of 10 micromol/L reduced by about 50% the MCF-7 colony formation induced by estrogen. [29] Kievitone inhibited proliferation of MCF-7 cells stimulated with estrogen (IC50=5-18 micromol/L) and various growth factors (IGF-1, IGF-2, TGF; IC50=1-3 micromol/L). [164] Therefore, kievitone may inhibit signaling components that are common to growth factor pathways. These results suggest legume phytoalexins possess antiestrogenic activity and may hold potential for cancer prevention in estrogen-responsive tissue.
Arylbenzofurans The treatment with the arylbenzofuran compound ebenfuran-III isolated from Onobrychis ebenoides (Leguminosae) was reported to inhibit proliferation of MCF-7 cells at concentrations of 1 micromol/L while inducing G1 phase arrest. It also suppressed estradiol-induced Bcl2 expression in MCF-7 cells and induced apoptotic death. [165] Other studies revealed that ebenfuran II exhibited antiestrogenic activity in breast cancer cells via the ER, with no stimulatory effects on cervix adenocarcinoma cells. [31] These studies suggest that plant arylobenzofuran phytoalexins may be useful for the prevention of breast cancer.
Stilbenes The effects of resveratrol in breast tissue have been investigated based on early evidence it exerted agonistic effects on the ER. [166] It binds to ERalpha and ERbeta with comparable affinity, which however, is about 7,000-fold lower compared to that of estradiol. [167] Resveratrol significantly reduced the Bcl-2/Bax ratio in ER-positive MCF-7 breast cancer cells. This effect, however, was abolished by ERalpha silencing. Because estrogen is a known activator of Bcl-2, resveratrol may induce apoptosis in the absence of apoptosis-inducing stimuli, at least in part via inhibition of ERalpha functions in breast cancer cells. [168] Resveratrol is also a ligand of the aromatic hydrocarbon receptor (AhR), which physically binds the ERalpha. It was reported to reverse AhR-induced DNA adducts formation [169] and repression of BRCA-1 in breast cancer cells. [170] The binding of resveratrol to nuclear receptors has been proposed to induce changes in receptor-complexes conformation altering interactions with cofactors and DNA at promoter elements. This inhibitory mechanism of resveratrol on transcriptional activity has been suggested for the androgen receptor (AR) at androgen response elements [171], the AhR at xenobiotic response elements (XRE) [172], and the ER at ERE. [167] Consistent with these mechanisms, resveratrol was shown to inhibit estradiol and androgen-induced proliferation of AR-positive LNCaP cells in vitro. [173] Collectively, these studies suggest that phytoalexin-rich diets may be useful in the prevention of endocrine-related tumors.
Modulation of phase-I/II metabolizing enzymes activities
Whereas the ideal balance between Phase I and phase II enzymes for cancer prevention remains elusive, protective effects of phytoalexins have been related to inhibition of Phase I bioactivating and/or induction of Phase II detoxifying enzymes. One transcription factor that induces Phase II metabolizing activities is Nrf2, which binds to antioxidant response elements (ARE) harbored in the promoter region of genes coding for detoxifying enzymes. Nrf2 is activated and translocated from the cytosol to the nucleus under conditions of exposure to xenobiotics and oxidative stress. [174, 175] Phase I enzymes are induced by the AhR at XRE. Crosstalk between Nrf2 and the AhR has been proposed since the AhR activates the transcription of Nrf2 and Phase I (i.e. CYP) and Phase II enzymes such as quinone reductase (QR), glutathione-s-transferase (GST), and uridine 5'-diphospho-glucuronosyltransferase (UGT) genes at XRE [176].
The activation of Nrf2 activity was proposed to mediate the anticarcinogenic properties of brassinin and cyclobrassinin, which inhibited DMBA-induced mammary lesions in organ culture through induction of QR, also known as NAD (P)H:quinine oxidoreductase (NQO1). [54] The QR is one among many of the antioxidant response element (ARE)-dependent genes regulated by Nrf2. [177] Similarly, the phenolic coumarin psoralidin isolated from an ethyl acetate-soluble fraction of Psoralea coryfolia and coumestrol were reported to induce QR activity in Hepa 1c1c7 murine hepatoma [178] and colonic Colo205 [157] cells. Also, siRNA studies with HCT116 colon cancer cells documented that the garlic phytoalexin allicin stimulated the translocation of Nrf2 to the nucleus. [125] A role for allicin in protecting against oxidative stress has been suggested by other studies documenting activation of cellular glutathione (GSH) levels in vascular endothelial cells [179], possibly through upregulation of the phase II enzyme glutamate-cysteine-ligase modifier (GCLM), which is the rate limiting enzyme for de-novo glutathione biosynthesis. [180]
An alternative mechanism of protection by phytoalexins is related to inhibition of Phase I enzymes that participate in the bioactivation of carcinogens. For example, protective effects of increasing levels of alliin in garlic powder (5%) in the diet were reported in rat liver and colon tissue through inhibition of DNA damage induced with N-nitrosodimethylamine (NDMA), 1,2-dimethylhydrazine (DMH), methylmethane sulfonate (MMS), and aflatoxin B1 (AFB1). The alliin-dependent decrease in DNA damage was attributed at least in part to reduced activity of CYP enzymes, which participate in activation of these carcinogens. [181, 182]
The anticarcinogenic effects of the phytoalexin resveratrol have also been related at least in part to its repressive effects on expression of CYP enzymes [183-185] through inhibition of the AhR [186] and activation of Phase II enzymes (i.e. QR1). [187, 188] However, resveratrol had minimal effects on levels of Phase II enzymes in a rodent (CD1) model (20 or 50 mg/kg BW) [189] and significant effects (1 g resveratrol/d for 4 wks) only in humans subjects with low baseline activity (GST-pi, UGT1A1). [190] The lack of strong induction of Phase II enzymes is somewhat in contrast with the reported stimulatory effects of resveratrol on Nrf2 activation [191] and prevention of diethylnitrosamine-induced liver tumorigenesis. [192]
Bioavailability of phytoalexins
Although many prevention studies have focused on the anticancer effects of sulforaphane (SFN), recent investigations have reported conflicting data about its natural phytoalexin precursor 4-methylsulphinylbutyl glucosinolate also known as glucoraphanin (GRP). GRP is consumed in diets containing cruciferous vegetables. In human liver carcinoma HepG2 cells, GRP was shown to induce Nrf2 translocation and QR activity. [193] However, in a rodent model, the repeated administration of GRP led to significant induction of hepatic Phase I enzymes including CYP1A1/2, CYP3A1/2, and CYP2E1 with only modest activation of GST. [194] Activation of CYP enzymes by GRP (1 micromol/L) has been confirmed by more recent studies. [195] These investigations raise the possibility that the consumption of cruciferous varieties containing or engineered to produce higher levels of GRP may actually promote deleterious health effects through activation of Phase I enzymes. Moreover, conditions that alter bioavailability of phytoalexins (i.e. GRP, resveratrol) through higher intake or deactivation of modifying enzymes (i.e. myrosinase activity for GRP) may interfere with metabolizing activities, and consequently, influence cancer risk. For example, modulation of metabolism or competition by other dietary agents for the same Phase II enzymes may actually increase the concentration of parent resveratrol [196] or increase its cancer protective effects (85). Likewise, several studies provide evidence that allyl sulfur compounds can induce GST activity and thereby enhance removal of foreign compounds, which may otherwise induce cancer. [197]
The content of phytoalexins in plant tissues depends on the plant species, cultivar, agricultural practices, and stage of maturity. One important question related to the cancer prevention effects of phytoalexins is whether they reach and accumulate in human target tissue at concentrations that mimic those tested in vitro and animal studies. For example, most in vitro studies that have investigated the effects of resveratrol have used concentrations ranging from 10 to 50 micromol/L. However, plasma concentrations of resveratrol in humans were reported to reach maximal levels of about 2.4 micromol/L following supplementation with a single dose (5 g) of resveratrol. [198] Clinical studies suggested that target plasma concentrations of resveratrol should not surpass 1 micromol/L through the intake of 1g/d or less. [199] However, only a few preclinical studies have adopted this micromolar range (Table 2) complicating the interpretation of resveratrol data from in vitro studies. Similar concerns persist about the concentrations of other phytoalexins used in preclinical studies.
SUMMARY AND PERSPECTIVE
Phytoalexins exert pleiotropic effects against cancer via inhibition of microbial activity, cell proliferation, invasion, and metastasis. They can also influence hormonal regulation and drug detoxification mechanisms. Moreover, phytoalexins regulate the expression of many metabolizing enzymes that may alter multiple pathways in normal and cancerous cells. Although opportunities exist for the development of cancer prevention strategies based on dietary phytoalexins, future research should address whether concentrations and regimens that show efficacy in preclinical studies are attainable in humans. Also, knowledge gaps persist about tissue-specific effects and differential responses in normal vs cancer cells. Future research focusing on interactions of phytoalexins with other food components and additive effects of combinations of phytoalexins on cancer processes may help in developing cancer prevention regimens that are nutritionally relevant while alleviating potential problems associated with the supplemental use of supraphysiological doses.
ACKNOWLEDGMENTS
This work was supported by an IPA from the National Cancer Institute, National Institutes of Health, Bethesda, MD, to Donato F. Romagnolo.
References:
Return to PHYTOALEXINS
Since 8-02-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |