

Role of Resveratrol in Prevention and Therapy
of Cancer: Preclinical and Clinical StudiesThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Anticancer Res. 2004 (Sep); 24 (5A): 2783–2840 ~ FULL TEXT
Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y.
Cytokine Research Laboratory,
Department of Bioimmunotherapy,
The University of Texas M. D. Anderson Cancer Center,
Box 143, 1515 Holcombe Boulevard,
Houston, Texas 77030, USA.
aggarwal@mdanderson.orgResveratrol, trans-3,5,4'-trihydroxystilbene, was first isolated in 1940 as a constituent of the roots of white hellebore (Veratrum grandiflorum O. Loes), but has since been found in various plants, including grapes, berries and peanuts. Besides cardioprotective effects, resveratrol exhibits anticancer properties, as suggested by its ability to suppress proliferation of a wide variety of tumor cells, including lymphoid and myeloid cancers; multiple myeloma; cancers of the breast, prostate, stomach, colon, pancreas, and thyroid; melanoma; head and neck squamous cell carcinoma; ovarian carcinoma; and cervical carcinoma. The growth-inhibitory effects of resveratrol are mediated through cell-cycle arrest; upregulation of p21Cip1/WAF1, p53 and Bax; down-regulation of survivin, cyclin D1, cyclin E, Bcl-2, Bcl-xL and clAPs; and activation of caspases. Resveratrol has been shown to suppress the activation of several transcription factors, including NF-kappaB, AP-1 and Egr-1; to inhibit protein kinases including IkappaBalpha kinase, JNK, MAPK, Akt, PKC, PKD and casein kinase II; and to down-regulate products of genes such as COX-2, 5-LOX, VEGF, IL-1, IL-6, IL-8, AR and PSA. These activities account for the suppression of angiogenesis by this stilbene. Resveratrol also has been shown to potentiate the apoptotic effects of cytokines (e.g., TRAIL), chemotherapeutic agents and gamma-radiation. Phamacokinetic studies revealed that the target organs of resveratrol are liver and kidney, where it is concentrated after absorption and is mainly converted to a sulfated form and a glucuronide conjugate. In vivo, resveratrol blocks the multistep process of carcinogenesis at various stages: it blocks carcinogen activation by inhibiting aryl hydrocarbon-induced CYP1A1 expression and activity, and suppresses tumor initiation, promotion and progression. Besides chemopreventive effects, resveratrol appears to exhibit therapeutic effects against cancer. Limited data in humans have revealed that resveratrol is pharmacologically quite safe. Currently, structural analogues of resveratrol with improved bioavailability are being pursued as potential therapeutic agents for cancer.
Key Words: Resveratrol, cell signaling, chemoprevention, metastasis, transformation, invasion, tumorigenesis, apoptosis, review
From the FULL TEXT Article:
Introduction
The history of resveratrol can be traced back thousands of years. Perhaps the first known use of grape extracts for human health can be dated over 2000 years ago, to "darakchasava", a well-known Indian herbal preparation of which the main ingredient is Vitis vinifera L. This "Ayurvedic" medicine is prescribed as a cardiotonic and also given for other disorders [1]. The use of dried grapes (also called manakka) as a cardiotonic is well documented. High- performance liquid choromatography (HPLC) analysis of darakchasava revealed polyphenols such as resveratrol and pterostilbene. This age-old formulation became interesting in the light of recently acquired knowledge on resveratrol.
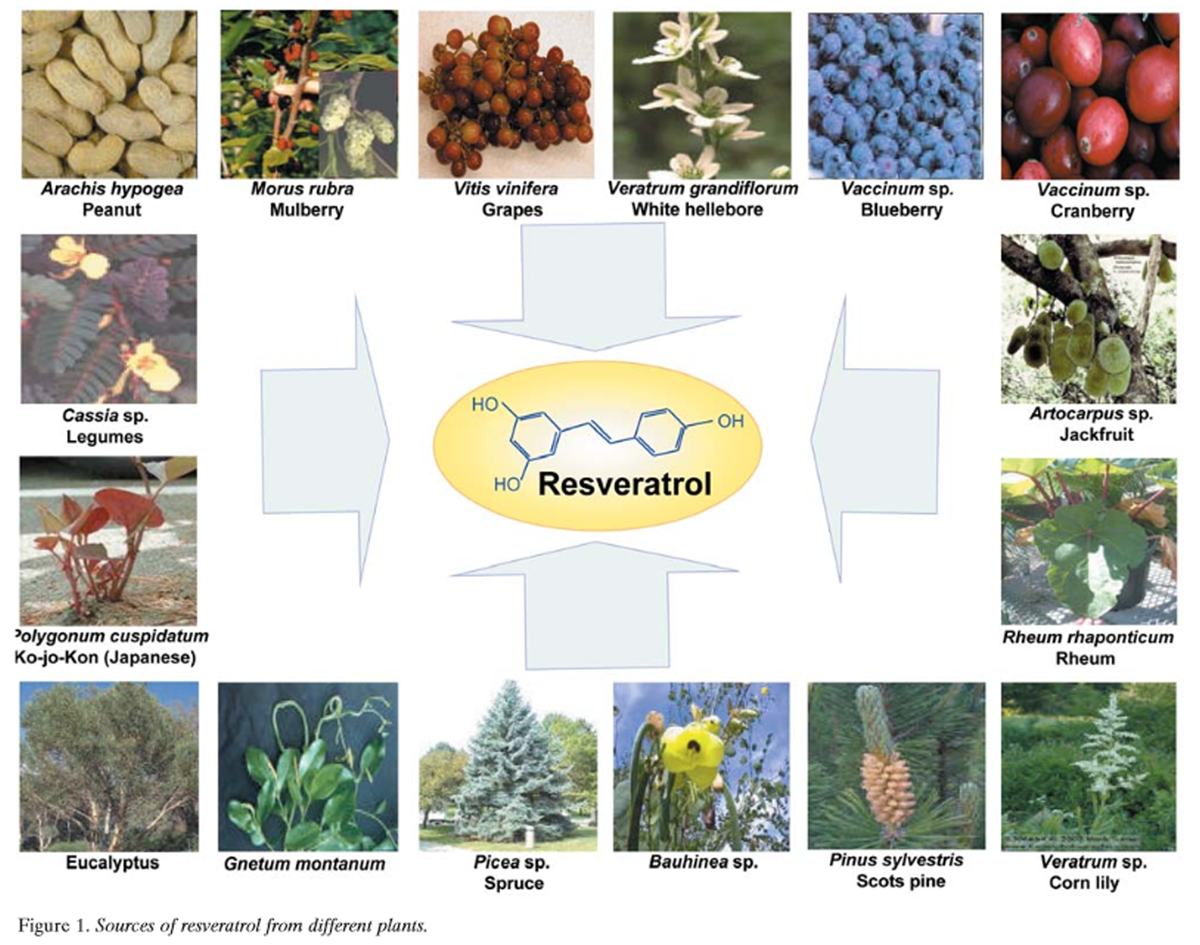
Figure 1 Resveratrol (3,5,4’-trihydroxystilbene) is a naturally occurring phytoalexin produced by a wide variety of plants, such as grapes (Vitis vinifera), peanuts (Arachis hypogea), and mulberries in response to stress, injury, ultraviolet (UV) irradiation, and fungal (e.g., Botrytis cinerea) infection. Although phytoalexins have long been inferred to be important in the defense of plants against fungal infection, few reports show that they provide resistance to infection. Several plants, including grapevine, synthesize the stilbene- type phytoalexin resveratrol when attacked by pathogens. Stilbenes with fungicidal potential are formed in several unrelated plant species, such as peanut, grapevine, and pine (Pinus sylvestris) (Figure 1). Stilbene biosynthesis specifically requires the presence of stilbene synthase. Furthermore, the precursor molecules for the formation of hydroxy-stilbenes are malonyl-coenzyme A (CoA) and p-coumaroyl-CoA, both present in plants. Hain et al. isolated the stilbene synthase gene from grapevine, transferred it into tobacco, and found that regenerated tobacco plants containing this gene are more resistant to infection by Botrytis cinerea [2].
Resveratrol was first identified in 1940 as a constituent of the roots of white hellebore (Veratrum grandiflorum O. Loes), and later in the dried roots of Polygonum cuspidatum, called Ko-jo-kon in Japanese, which is used in traditional Chinese and Japanese medicine to treat suppurative dermatitis, gonorrhea favus, athlete’s foot (tinea pedis), and hyperlipemia [3–6]. In 1976, resveratrol was detected in the leaf epidermis and the skin of grape berries but not in the flesh [7–9]. Fresh grape skins contain 50–100 mg resveratrol per gram, and the concentration in wine ranges from 0.2 mg/l to 7.7 mg/l. The epidemiological finding of an inverse relationship between consumption of red wine and incidence of cardiovascular disease has been called the "French paradox" [10, 11]. For a variety of reasons, the cardioprotective effects of red wine have been attributed to resveratrol (12). These effects include suppression of lipid peroxidation and eicosanoid synthesis, inhibition of platelet aggregation, and antioxidant, anti-inflammatory and vasorelaxant activities (13). Numerous reports indicate that resveratrol has antiviral effects against HIV-1 [14] and the herpes simplex virus [15, 16]. Heredia et al. reported that resveratrol synergistically enhances the anti- HIV-1 activity of the nucleoside analogues zidovudine (AZT), zalcitabine (ddC) and didanosine (ddI) [14].
Resveratrol also exhibits antibacterial effects [17], including inhibition of growth of different strains of Helicobacter pylori [18–20].
Extensive research during the last two decades has suggested that, besides cardioprotective effects, resveratrol also exhibits anticancer activities. How resveratrol manifests its anticancer properties, the cell signaling pathways affected, the transcription factors modulated, the genes induced, the enzyme activities regulated, the protein interactions, and the types of in vitro and in vivo model systems in which resveratrol has been examined are the focus of this review. Although several reviews have been written on resveratrol [21–28], none covers the aspects of this polyphenol discussed here.
A. Sources of Resveratrol
That red grapes or red wine are sources of resveratrol is well known [29]. However, resveratrol has been identified in a wide variety of plants, including Japanese knotweed (Polygonum cuspidatum) [4]; the peanut [30, 31]; Vaccinum spp. (including blueberry, bilberry, and cranberry) [32, 33]; Reynoutria japonica; and Scots pine (Figure 1). Other plant sources of resveratrol include Vitis spp. (including grapevines, leaves, and berryskins); Morus spp. (including mulberry); lilies (Veratrum spp.); legumes (Cassia spp., Pterolobium hexapetallum); Rheum spp. (including rhubarb); eucalyptus; spruce (Picea spp); pine (Pinus spp.); grasses (Poaceae including Festuca, Hordeum, Poa, Stipa and Lolium spp.); Trifolium spp.; Nothofagus spp.; Artocarpus spp; Gnetum spp.; Pleuropterus ciliinervis; Bauhinia racemosa; Paeonia lactiflora; Scilla nervosa; and Tetrastigma hypoglaucum. Isorhapontigenin, isolated from Belamcanda chinensis, is a derivative of stilbene. Its chemical structure is very similar to that of resveratrol and it has a potent anti-oxidative effect. Compounds that are closely related to resveratrol structurally, and thus may have similar biological effects, have been identified in a wide variety of plants (Table I).
Table 1. Sources of Resveratrol and its analogues.
Refer to pages 4–7 of 58
B. Chemistry of Resveratrol
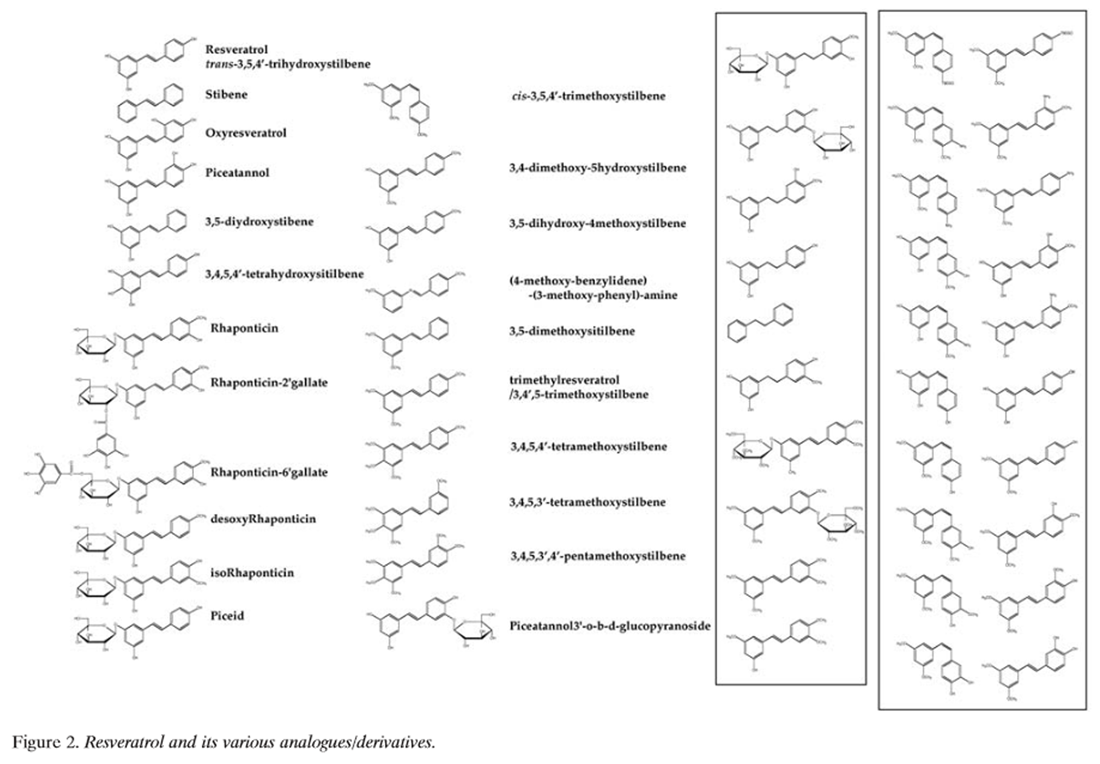
Figure 2 Resveratrol (Figure 2) is found widely in nature, and a number of its natural and synthetic analogues and their isomers, adducts, derivatives and conjugates are known [6, 26–28, 33–104] (Table I). It is an off-white powder (extracted by methanol) with a melting point of 253-255ÆC and molecular weight of 228.25. Reveratrol is insoluble in water but dissolves in ethanol and dimethylsulphoxide. The stilbene-based structure of resveratrol consists of two phenolic rings linked by a styrene double bond to generate 3,4’,5, -trihydroxystilbene. Although the presence of the double bond facilitates trans- and cis-isomeric forms of resveratrol [(E)- and (Z)-diasteromers, respectively], the trans-isomer is sterically the more stable form [105]. On spectrophotometric analysis in ethanol, trans-resveratrol absorbs maximally at 308 nm and cis-resveratrol at 288 nm, which allows for their separation by HPLC with UV detection [105, 106]. Absorptivity is greater in an ethanol: water solution (1:9 v/v), but with a small shift in Ïmax (trans-resveratrol Ïmax, 306 nm; cis-resveratrol Ïmax, 286 nm). Besides their differences in spectrophotometric UV absorptions, trans- and cis-resveratrol are also clearly distinguished by their chemical shifts in nuclear magnetic resonance spectroscopy [106].
Trans-resveratrol is commercially available and converts to the cis-form on exposure to UV irradiation [23, 24, 26–28]. Trela and Waterhouse conducted trials under various conditions and showed that trans-resveratrol is stable for months when protected from light, except in high pH buffers [105]. These workers also showed that the cis-isomer is extremely light-sensitive but can remain stable in the dark at ambient temperature in 50% ethanol for at least 35 days over the range of 5.3–52.8 µM. Low pH also causes cis-resveratrol to isomerize to trans-resveratrol. Recently, Deak and Falk studied the reactions of commercially obtained trans-resveratrol and photochemically prepared cis-resveratrol [106]. The free enthalpy difference between the two isomers was estimated to be of the order of that of common stilbenes, with the trans-isomer being more stable by about 11–14 KJ/mol. These workers also reported that the pK a values of trans-resveratrol, corresponding to the mono, di- and tri-protonation of the system, were 9.3, 10.0, and 10.6, respectively. Resveratrol occurs predominantly as the trans-isomer, and reports of the presence of the cis-isomer, for example in certain wines, are attributed to photoisomeric conversion, enzyme action during fermentation, or release from resveratrol oligomers (viniferins) [23, 24, 26–28]. Since reports about the cis-isomer are limited, when the structure of resveratrol is not specified, we refer here to trans-resveratrol.
Over the past decade, several HPLC and gas chromatographic methods have been developed to detect the presence and measure levels of resveratrol and its analogues [23, 24, 26–28]. Much attention has been focused on method development, since studying the biological properties of resveratrol requires analyses of complex mixtures containing very low amounts of stilbenes, and complete and quick extractions are required to minimize losses from isomerization or denaturation. Generally, HPLC methods using reverse phase C18 columns coupled with UV detection (photodiode array or diode array detectors) can adequately distinguish resveratrol isomers and their analogues on the basis of their different absorbance maxima. However, the use of mass spectrometry (MS) fluorimetric and electrochemical detectors, which are more specific than UV detection, has considerably improved sensitivity and decreased sample size [23, 24]. Gas chromatographic methods, with or without MS detection, although not as popular as HPLC, have been frequently employed but require trimethylsilyl derivatization of resveratrol and its analogues.
Since the first reported detection of trans-resveratrol in grapevines in 1976, and later in wine in 1992, and its implications in relation to the "French paradox" [7, 10, 107], there has been an explosion of interest in the various biological activities of this natural phytoalexin. Given the substantial number of reports on natural and synthetic analogues of resveratrol (Table I), considerable attention has been focused on structure-activity relationship studies of these compounds. Natural and synthetic resveratrol analogues include a myriad of compounds differing in the type, number and position of substituents (hydroxyl, methoxyl, halogenated, glycosylated, esterified, etc.), presence or absence of stilbenic double bonds, modified steroisomery, and oxidative dimerizations (to form oligomers). Calculations based on density functional theory studies have been used to study the structure-activity relationships of resveratrol in the chain reaction of auto- oxidation [108]. The 4’-hydroxyl group of resveratrol was reported to be more reactive than the 3’- and 5’-hydroxyl groups becase of resonance effects and, in conjunction with the trans-olefin structure of the parent stilbene skeleton, were the most important determinants of bioactivity [61–63, 108–110]. Ashikawa et al. reported that piceatannol (a tetrahydroxyl resveratrol analogue) was considerably different in biological activity to the stilbene and rhaponticin (a methoxylated and glucosylated analogue of resveratrol) [111]. Similarly, structure-activity relationship studies have shown distinct biological properties of resveratrol oligomers and resveratrol glycosides (called polydatins and piceids) [6, 26–28]. Much attention has been focused on the chemistry of resveratrol and its natural and synthetic analogues because of their biological properties and their potential in the prevention and therapy of cancer.
C. Preclinical Studies
C1. In vitro effects
C 1a. Antiproliferative effects of resveratrol
Resveratrol has been shown to suppress proliferation of a wide variety of tumor cells, including lymphoid and myeloid cancers; breast, colon, pancreas, stomach, prostate, head and neck, ovary, liver, lung and cervical cancers; melanoma; and muscles [112–188] (Table II). Besides inhibiting proliferation, resveratrol also induces apoptosis either through the caspase-8-dependent pathway (receptor- mediated; type I) or the caspase-9-dependent pathway (mitochondrial; type II), or both. The mechanisms of suppression of cell growth and induction of apoptosis for these cell types are described here.
Table 2. Antiproliferative and pro-apoptotic effects of resveratrol against tumor cells and their mechanism
Refer to pages 12 and 13 of 58
B-cell lymphoma: Several studies have shown the antiproliferative effects of resveratrol on B cells [112–115]. Billard et al. investigated the effects of resveratrol on leukemic cells from patients with chronic B-cell malignancies and found that resveratrol had antiproliferative effects and induced apoptosis in leukemic B-cells that correlated with activation of caspase-3, a drop in the mitochondrial transmembrane potential, reduction in the expression of the anti-apoptotic protein Bcl-2, and reduction in expression of inducible nitric oxide synthase (iNOS) [112]. In contrast, resveratrol had little effect on the survival of normal peripheral blood mononuclear cells (PBMC). Roman et al. reported apoptotic and growth- inhibitory effects of resveratrol in human B-cell lines derived from chronic B-cell malignancies [113]. Resveratrol inhibited the expression of the antiapoptotic proteins Bcl-2 and iNOS in WSU-CLL and ESKOL cells and cells derived from patient with B-cell choronic lymphocytic leukemia (B-CLL). Dorrie et al. showed that resveratrol induced extensive apoptotic cell death not only in Fas/CD95- sensitive leukemia lines, but also in B-lineage leukemic cells that are resistant to Fas signaling [114]. They also found that resveratrol had no cytotoxicity against normal PBMC. In each acute lymphocytic leukemia (ALL) cell line, resveratrol induced progressive loss of mitochondrial membrane potential and increase in caspase-9 activity. No evidence of caspase-8 activation or Fas signaling was observed. In BJAB Burkitt-like lymphoma cells, Wieder et al. demonstrated that resveratrol-induced cell death accompanied an increase in mitochondrial permeability transition and caspase-3 activation and was independent of the Fas signaling pathway [115]. Resveratrol was also found to induce apoptosis in leukemic lymphoblasts isolated from patients suffering from childhood ALL.
T-cell lymphoma: Several reports indicate that resveratrol modulates the growth of T cells [116, 117]. Hayashibara et al. showed that resveratrol inhibited growth in five HTLV-1- infected cell lines (adult T-cell leukemia) and induced apoptosis, which correlated with a gradual decrease in the expression of survivin, an anti-apoptotic protein [116]. Tinhofer et al. showed that resveratrol induced apoptosis in the CEM-C7H2 T-ALL cell line. They also found that resveratrol induced apoptosis via a novel mitochondrial pathway controlled by Bcl-2 [117] and that resveratrol- induced apoptosis was inhibited by Bcl-2. Resveratrol stimulation of C7H2 cells produced reactive oxygen species (ROS), and this production was significantly reduced by Bcl-2. As expected, pretreatment of cells with N-acetylcysteine protected cells from DNA fragmentation induced by resveratrol. Interestingly, resveratrol-induced apoptosis did not involve cytochrome c release, nor trigger activation of death receptor type II pathways, as no early processing of Bid could be detected. Resveratrol, however, caused activation of caspase-9, -2, -3 and -6 in the control cells, but not in the subclones overexpressing Bcl-2. These authors also found that DNA cleavage by resveratrol occurred downstream of mitochondrial signaling and was significantly blocked in the Bcl-2-overexpressing subclones. After various proapoptotic stimuli, the loss of mitochondrial transmembrane potential led to the release of apoptosis- inducing factor (AIF) from the mitochondrial intermembrane space, thus representing the link between mitochondria and nucleus in resveratrol-induced apoptosis. Resveratrol, however, did not induce translocation of AIF, suggesting that this pathway of caspase-independent activation of nucleases is not involved in resveratrol-induced apoptosis.
Myeloid leukemia: Resveratrol can induce apoptosis in myeloid cells [118–127]. Clement et al. showed that resveratrol triggered Fas signaling-dependent apoptosis in HL-60 human leukemia cells [118]. Resveratrol-treated cells exhibited increases in externalization of inner membrane phosphatidylserine and in cellular content of subdiploid DNA, indicating loss of membrane phospholipid asymmetry and DNA fragmentation. Resveratrol-induced cell death was mediated by intracellular caspases, as indicated by the increase in proteolytic cleavage of caspase substrate poly (ADP-ribose) polymerase (PARP) and the ability of caspase inhibitors to block resveratrol cytotoxicity. Furthermore, resveratrol treatment enhanced Fas ligand (FasLCD95L) expression on HL-60 cells, and resveratrol-mediated cell death was specifically Fas signaling-dependent. The expression of FasL was not unique to HL-60 cells but also was induced on T47D breast carcinoma cells. Resveratrol treatment of normal human PBMC did not affect cell survival for as long as 72 h, which correlated with the absence of a significant change in either Fas or FasL expression on treated PBMC. These data showed specific involvement of the Fas-FasL system in the anticancer activity of resveratrol (Table III).
Table 3. Effects of resveratrol on different cell signaling pathways.
Refer to pages 14 of 58
Tsan found that, in human monocytic leukemia THP-1 cells, resveratrol induced apoptosis independently of Fas signaling [119]. The effect of resveratrol on THP-1 cells was reversible after its removal from the culture medium. Surh et al. found that resveratrol inhibited proliferation and DNA synthesis in human promyelocytic leukemia HL-60 cells [120]. Resveratrol-induced cell death was characterized by internucleosomal DNA fragmentation, an increased proportion of the subdiploid cell population, and a gradual decrease in the expression of anti-apoptotic Bcl-2. In histiocytic lymphoma U-937 cells, Park et al. revealed that resveratrol treatment caused apoptosis and DNA fragmentation, which are associated with caspase-3 activation and phospholipase C-Á1 degradation. Bcl-2 was found to inhibit resveratrol-induced apoptosis by a mechanism that interfered with cytochrome c release and caspase-3 activity [121].
We examined the effect of resveratrol on fresh acute myeloid leukemia (AML) cells [122]. Interleukin (IL)-1‚ plays a key role in proliferation of AML cells, and we found that resveratrol inhibited proliferation of AML by arresting the cells at S-phase. Resveratrol significantly reduced production of IL-1‚ suppressed IL-1‚-induced activation of NF-ÎB, and suppressed colony-forming cell proliferation of fresh AML marrow cells.
Breast cancer: Several groups have investigated the effects of resveratrol on breast cancer cells [128–138]. Mgbonyebi et al. showed that resveratrol had antiproliferative effects against the breast cancer cell lines MCF-7, MCF-10F and MDA- MB-231, and these effects were independent of the estrogen receptor (ER) status of the cells [128]. Serrero et al. found that, in ER-positive MCF-7 breast cancer cells, resveratrol inhibited estradiol-induced cell proliferation by antagonizing the stimulation by estradiol of an ER element reporter gene construct and of progesterone receptor (PR) gene expression [129]. Resveratrol also inhibited proliferation of the ER- negative human breast carcinoma cell line MDA-MB-468 by a mechanism other than ER antagonism, involving alteration in autocrine growth modulators such as transforming growth factor (TGF)-α, TGF-β, PC cell-derived growth factor and insulin-like growth factor I receptor mRNA. Nakagawa et al. found that resveratrol at low concentrations caused cell proliferation in ER-positive human breast cancer cell lines (KPL-1, ≤ 22 µM; MCF-7, ≤ 4 µM), whereas it suppressed cell growth at high concentrations (≥ 44 µM). Growth suppression was due to apoptosis, as indicated by the appearance of a sub-G1-phase fraction, up-regulation of Bax and Bak proteins, down-regulation of Bcl-xL protein and activation of caspase-3. Pozo-Guisado et al. examined the effects of resveratrol in human breast cancer cell lines MCF-7 and MDA-MB-231 [131]. They showed that, although resveratrol inhibited cell proliferation and viability in both cell lines, apoptosis was induced in a concentration- and cell- specific manner. In MDA-MB-231, resveratrol (at concentrations up to 200 µM) lowered the expression and kinase activities of positive G1/S and G2/M cell-cycle regulators and inhibited ribonucleotide reductase activity in a concentration-dependent manner, without a significant effect on the low expression of tumor suppressors p21 Cip1/WAF1, p27 Kip1 and p53. These cells died by a nonapoptotic process in the absence of a significant change in cell-cycle distribution. In MCF-7, resveratrol produced a significant (< 50 µM) and transient increase in the expression and kinase activities of positive G1/S and G2/M regulators. Simultaneously, p21 Cip1/WAF1 expression was markedly induced in the presence of high levels of p27 Kip1 and p53. These opposing effects resulted in cell-cycle blockade at the S phase and induction of apoptosis in MCF-7 cells. Thus, the antiproliferative activity of resveratrol could take place through the differential regulation of the cell- cycle, leading to apoptosis or necrosis.
Colon cancer: Several reports suggest that resveratrol suppresses proliferation of colon cancer cells [143–151]. In the human wild-type p53-expressing HCT116 colon carcinoma cell line and HCT116 cells with both p53 alleles inactivated by homologous recombination, Mahyar-Roemer et al. showed that resveratrol induced apoptosis independently of p53 and that the apoptosis was mediated primarily by mitochondria and not by a receptor pathway [143]. Wolter and Stein determined that, in the colon adenocarcinoma cell line Caco-2, resveratrol enhanced the differentiation-inducing effect of butyrate, inhibited butyrate-induced TGF-β1 secretion, and did not elevate alkaline phosphatase (ALP) activity or E-cadherin protein expression (markers of epithelial differentiation) when applied alone [144]. Wolter et al. reported that resveratrol inhibited growth and proliferation of Caco-2 cells through apoptosis, which was accompanied by an increase in caspase- 3 activity and in the expression of cyclin E and cyclin A, decrease in levels of cyclin D1 and cyclin-dependent kinase (Cdk) 4, cell-cycle arrest in S- to G2-phases at lower concentrations, and reversal of S-phase arrest at higher concentrations [145]. They observed similar results for the colon carcinoma cell line HCT116 and found that cell-cycle inhibition by resveratrol was independent of COX inhibition. Delmas et al. analyzed the molecular mechanisms of resveratrol-induced apoptosis in colon cancer cells, with special attention to the role of the death receptor Fas in this pathway [146]. They showed that, at concentrations of 10–100 µM, resveratrol activated various caspases and triggered apoptosis in SW480 human colon cancer cells. Caspase activation was associated with accumulation of the pro- apoptotic proteins Bax and Bak, which underwent conformational changes and relocalization to the mitochondria. Resveratrol did not modulate the expression of Fas and Fas-ligand (FasL) at the surface of cancer cells, and inhibition of the Fas/FasL interaction did not influence the apoptotic response to the molecule. Resveratrol induced the clustering of Fas and its redistribution in cholesterol and sphingolipid-rich fractions of SW480 cells, together with Fas-associated death domain protein (FADD) and procaspase-8. This redistribution was associated with the formation of a death-inducing signaling complex (DISC). Transient transfection of a dominant-negative mutant of FADD, E8, or viral protein MC159, that interfered with DISC function, decreased the apoptotic response of SW480 cells to resveratrol and partially prevented resveratrol- induced Bax and Bak conformational changes. Altogether, these results indicated that the ability of resveratrol to induce the redistribution of Fas in membrane rafts may contribute to the molecule's ability to trigger apoptosis in colon cancer cells.
Liang et al. found that resveratrol inhibited proliferation of HT-29 colon cancer cells and resulted in their accumulation in the G2-phase of the cell-cycle, and that this was accompanied by inactivation of Cdc2/p34 protein kinase and an increase in the tyrosine phosphorylated (inactive) form of Cdc2 [147]. Kinase assays revealed that the reduction of Cdc2 activity by resveratrol was mediated through inhibition of Cdk7 kinase activity, while Cdc25A phosphatase activity was not affected. In addition, resveratrol-treated cells were shown to have a low level of Cdk7 kinase-Thr(161)-phosphorylated Cdc2. These results demonstrated that resveratrol induced cell-cycle arrest at the G2 phase through inhibition of Cdk7 kinase activity, suggesting that its antitumor activity might occur through disruption of cell division at the G2/M-phase.
Pancreatic cancer: Ding and Adrian demonstrated that, in human pancreatic cancer cell lines PANC-1 and AsPC-1, resveratrol inhibited proliferation through apoptosis and dramatically increased the fraction of sub-G0/G1-phase cells [152].
Gastric cancer: Resveratrol has been shown to suppress proliferation of gastric cancer cells [153–155]. Atten et al. reported that resveratrol inhibited proliferation of nitrosamine-stimulated human gastric adenocarcinoma KATO-III and RF-1 cells [153]. It arrested KATO-III cells in the G0/G1-phase of the cell-cycle and eventually induced apoptotic cell death by utilizing a proteinase kinase C (PKC)- mediated mechanism to deactivate these gastric adenocarcinoma cells. Holian et al. demonstrated that, in gastric adenocarcinoma cell line SNU-1, which was stimulated by hydrogen peroxide (H2O2), resveratrol suppressed synthesis of DNA and generation of endogenous O2- but stimulated NOS activity, which may have been responsible for inhibition of SNU-1 proliferation [154]. Resveratrol also inhibited the growth of esophageal cancer cell line EC-9706 [155]. Resveratrol-induced apoptosis of EC-9706 was mediated by down-regulation of Bcl-2 and up-regulation of the expression of the apoptosis-regulated gene Bax.
Prostate cancer: Proliferation of both androgen-dependent and -independent prostate cancer cells is suppressed by resveratrol [156–163]. Using cultured prostate cancer cells that mimic the initial (hormone-sensitive; LNCaP) and advanced (hormone-refractory; DU-145, PC-3, and JCA-1) stages of prostate carcinoma, Hsieh and Wu showed that resveratrol caused substantial decreases in growth of LNCaP, PC-3 and DU145 cells, but had only a modest inhibitory effect on proliferation of JCA-1 cells, and that it partially disrupted the G1/S transition in all three androgen-non- responsive cell lines [157]. It caused a significant percentage of LNCaP cells to undergo apoptosis and significantly lowered both intracellular and secreted prostate-specific antigen (PSA) levels without affecting expression of the androgen receptor (AR). Lin et al. also showed, in DU145 cells, that resveratrol induced apoptosis through activation of mitogen-activated protein kinase (MAPK,) increases in cellular p53 content, serine-15 phosphorylation of p53, p53 binding to DNA and p53-stimulated increase in p21Cip1/WAF1 mRNA [158]. Mitchell et al. found that, in a hormone- sensitive prostate cancer cell line, resveratrol repressesed different classes of androgen up-regulated genes at the protein or mRNA level, including PSA, human glandular kallikrein-2, AR-specific coactivator ARA70, and the Cdk inhibitor p21Cip1/WAF1 [159].
This inhibition is probably attributable to a reduction in AR at the transcription level, inhibiting androgen-stimulated cell growth and gene expression. Kampa et al. reported that the antiproliferative effects of resveratrol on DU145 cells could have been mediated through a decrease in NO, although resveratrol did not affect growth of PC3 and LNCaP cells [160]. Kuwajerwala et al. showed that, in androgen-sensitive LNCaP cells, the effect of resveratrol on DNA synthesis varied dramatically depending on the concentration and the duration of treatment [161]. In cells treated for 1 h, resveratrol had only an inhibitory effect on DNA synthesis, which increased with increasing concentration (IC50, 20 µM). However, when treatment duration was extended to 24 h, resveratrol had a dual effect on DNA synthesis. At 5–10 µM it caused a two- to three-fold increase in DNA synthesis, while at ≥15 µM it inhibited DNA synthesis. The increase in DNA synthesis was seen only in LNCaP cells, not in androgen-independent DU145 prostate cancer cells or in NIH/3T3 fibroblast cells. The resveratrol-induced increase in DNA synthesis was associated with enrichment of LNCaP cells in S-phase and concurrent decreases in nuclear p21Cip1/WAF1 and p27Kip1 levels. Furthermore, consistent with the entry of LNCaP cells into S-phase, there was a dramatic increase in nuclear Cdk2 activity associated with both cyclin A and cyclin E. Taken together, their observations indicate that LNCaP cells treated with resveratrol are induced to enter into S-phase, but subsequent progression through S-phase is limited by the inhibitory effect of resveratrol on DNA synthesis, particularly at concentrations greater than 15 µM. This unique ability of resveratrol to exert opposing effects on two important processes in cell-cycle progression, induction of S-phase and inhibition of DNA synthesis, may be responsible for its dual apoptotic and antiproliferative effects.
Prostate cancer prevention by key elements present in human nutrients derived from plants and fruits has been confirmed in various cell cultures and tumor models. Resveratrol has been shown to induce remarkable inhibitory effects in prostate carcinogenesis via diverse cellular mechanisms associated with tumor initiation, promotion and progression. Narayanan et al. examined whether resveratrol activates a cascade of p53-directed genes that are involved in apoptosis mechanism(s) or modifies cell growth by modifying AR and its co-activators directly or indirectly [162]. They demonstrated by DNA microarray, reverse tanscriptase-polymerase chain reaction (RT-PCR), Western blot and immunofluorescence analyses that treatment of androgen-sensitive prostate cancer cells (LNCaP) with 10 µM resveratrol for 48 h down-regulated PSA, AR co- activator ARA 24, and NF-Î B p65. Altered expression of these genes is associated with activation of p53-responsive genes such as p53, PIG 7, p21 Cip1/WAF1, p300/CBP and apoptosis protease activating factor-1 (Apaf-1). The effect of resveratrol on p300/CBP plays a central role in its cancer- preventive mechanisms in LNCaP cells. These results implicate activation of more than one set of functionally related molecular targets. At this point we have identified some of the key molecular targets associated with the AR and p53 target genes.
Melanoma: Several studies suggest that resveratrol is effective against melanoma [164–167]. Resveratrol inhibited growth and induced apoptosis in human melanoma cell lines A375 and SK-mel28 [164]. It did not alter the phosphorylation of p38 MAPK or c-Jun N-terminal kinase (JNK) in either cell line. Resveratrol induced phosphorylation of extracellular receptor kinase (ERK)1/2 in A375 but not in SK-mel28 cells. Ahmad et al. demonstrated that resveratrol, via modulations in Cdk inhibitor-cyclin-Cdk machinery, resulted in a G1-phase arrest followed by apoptosis of human epidermoid carcinoma (A431) cells [165]. It caused an induction of p21Cip1/WAF1 that inhibited cyclin D1/D2-Cdk6, cyclin D1/D2-Cdk4, and cyclin E-Cdk2 complexes, thereby imposing an artificial checkpoint at the G1/S-phase transition of the cell-cycle. These authors also showed, in the same cell line, the involvement of the retinoblastoma (Rb)-E2F/DP pathway in resveratrol-mediated cell-cycle arrest and apoptosis [166]. They suggested that resveratrol caused a down-regulation of hyperphosphorylated Rb protein with a relative increase in hypophosphorylated Rb that, in turn, compromised the availability of free E2F, which may have resulted in stoppage of cell-cycle progression at the G1/S-phase transition, thereby leading to a G0/G1 phase arrest and subsequent apoptotic cell death. Larrosa et al. showed that resveratrol and the related molecule 4-hydroxystilbene induced growth inhibition, apoptosis, S-phase arrest and up-regulation of cyclins A, E and B1 in human SK-Mel-28 melanoma cells [167].
Lung cancer: Several studies suggest that resveratrol is effective against lung carcinoma [168–170]. Kim et al. showed that resveratrol inhibited the growth of human lung carcinoma A549 cells and resulted in a concentration- dependent induction of S-phase arrest in cell-cycle progression, marked inhibition of phosphorylation of Rb and concomitant induction of Cdk inhibitor p21Cip1/WAF1, which is transcriptionally up-regulated and is p53-dependent [168]. In addition, fluorescence microscopy and flow cytometric analysis showed that treatment with resveratrol resulted in induction of apoptosis. These effects were found to correlate with activation of caspase-3 and a shift in the Bax/Bcl-xL ratio toward apoptosis. Resveratrol treatment also inhibited the transcriptional activity of NF-ÎB. These findings suggest that resveratrol has firm potential for development as an agent for prevention of human lung cancer.
Liver cancer: Several studies suggest that resveratrol is effective against liver cancer [171–174]. Delmas et al. examined the ability of resveratrol to inhibit cell proliferation in the rat hepatoma Fao cell line and the human hepatoblastoma HepG2 cell line [171]. The results showed that resveratrol strongly inhibited cell proliferation and that Fao cells were more sensitive than HepG2 cells. Interestingly, the presence of ethanol lowered the threshold of the resveratrol effect. Resveratrol appeared to prevent or delay the entry to mitosis, since no inhibition of 3 H-thymidine incorporation was observed, while the number of the cells in S- and G2/M-phases increased. Kozuki et al. revealed that 100 or 200 µM of resveratrol inhibited proliferation of AH109A hepatoma cells and suppressed invasion of the hepatoma cells even at a concentration of 25 µM [172]. This anti-invasive activity of resveratrol is independent of its antiproliferative activity and may be related to its anti-oxidative action. De Ledinghen et al. found that resveratrol decreased hepatocyte growth factor-induced scattering of HepG2 hepatoma cells and invasion by an unidentified postreceptor mechanism [173]. It decreased cell proliferation without evidence of cytotoxicity or apoptosis, with no decrease in the level of the hepatocyte growth factor receptor c-met, c-met precursor synthesis, c-met autophosphorylation, or activation of Akt-1 or ERK1/2. Moreover, resveratrol did not decrease urokinase expression and did not block the catalytic activity of urokinase.
Thyroid and head and neck cancers: Several reports suggest that resveratrol may suppress proliferation of thyroid and other head and neck cancers [174–181]. Shih et al. showed that treatment of papillary thyroid carcinoma and follicular thyroid carcinoma cell lines with resveratrol led to apoptosis, which accompanied activation and nuclear translocation of ERK1/2 [175]. Resveratrol increased the cellular abundance of p53, serine phosphorylation of p53, and abundance of c-fos, c-Jun, and p21Cip1/WAF1 mRNAs. Elattar et al. reported that resveratrol led to inhibition of human oral squamous carcinoma SCC-25 cell growth and DNA synthesis [176, 177]. Moreover, combining 50 µM resveratrol with 10, 25, or 50 µM quercetin resulted in gradual and significant increases in the inhibitory effects of the two compounds. Babich et al. demonstrated that resveratrol irreversibly caused arrest of human gingival epithelial cell growth by inhibition of DNA synthesis [178].
Ovarian and endometrial tumors: Several studies suggest that resveratrol is effective against ovarian and endometrial tumors [174, 182–186]. Yang et al. showed that resveratrol inhibited cell growth and induced apoptosis in PA-1 human ovarian cancer cells and up-regulated the NAD(P)H quinone oxidoreductase 1 (NQO-1) gene, which is involved in p53 regulation [182]. Bhat and Pezzuto reported that treatment of human endometrial adenocarcinoma (Ishikawa) cells with resveratrol did not significantly increase the levels of the estrogen-inducible marker enzyme ALP [174]. On the contrary, it decreased 17‚β-estradiol- induced ALP and PR expression and thus its effects may be mediated by both estrogen-dependent and -independent mechanisms. It inhibited Ishikawa cell proliferation by arresting cells at S-phase and increased expression of cyclins A and E but decreased Cdk2. Kaneuchi et al. showed that resveratrol suppressed the growth of Ishikawa cells through down-regulation of epidermal growth factor (EGF) [183].
Opipari et al. showed that resveratrol inhibited growth and induced death in a panel of five human ovarian carcinoma cell lines and that this response was associated with mitochondrial release of cytochrome c, formation of the apoptosome complex, and caspase activation [184]. Surprisingly, even with these molecular features of apoptosis, analysis of the resveratrol-treated cells by light and electron microscopy revealed morphological and ultrastructural changes indicative of autophagocytic, rather than apoptotic, death. This suggested that resveratrol can induce cell death through two distinct pathways. Consistent with resveratrol's ability to kill cells via nonapoptotic processes, cells transfected to express high levels of the antiapoptotic proteins Bcl-xL and Bcl-2 were equally as sensitive as control cells to resveratrol. Together, these findings show that resveratrol induces death in ovarian cancer cells through a mechanism distinct from apoptosis, suggesting that it may provide leverage to treat ovarian cancer that is chemoresistant on the basis of ineffective apoptosis.
C 1b. Resveratrol induces apoptosis
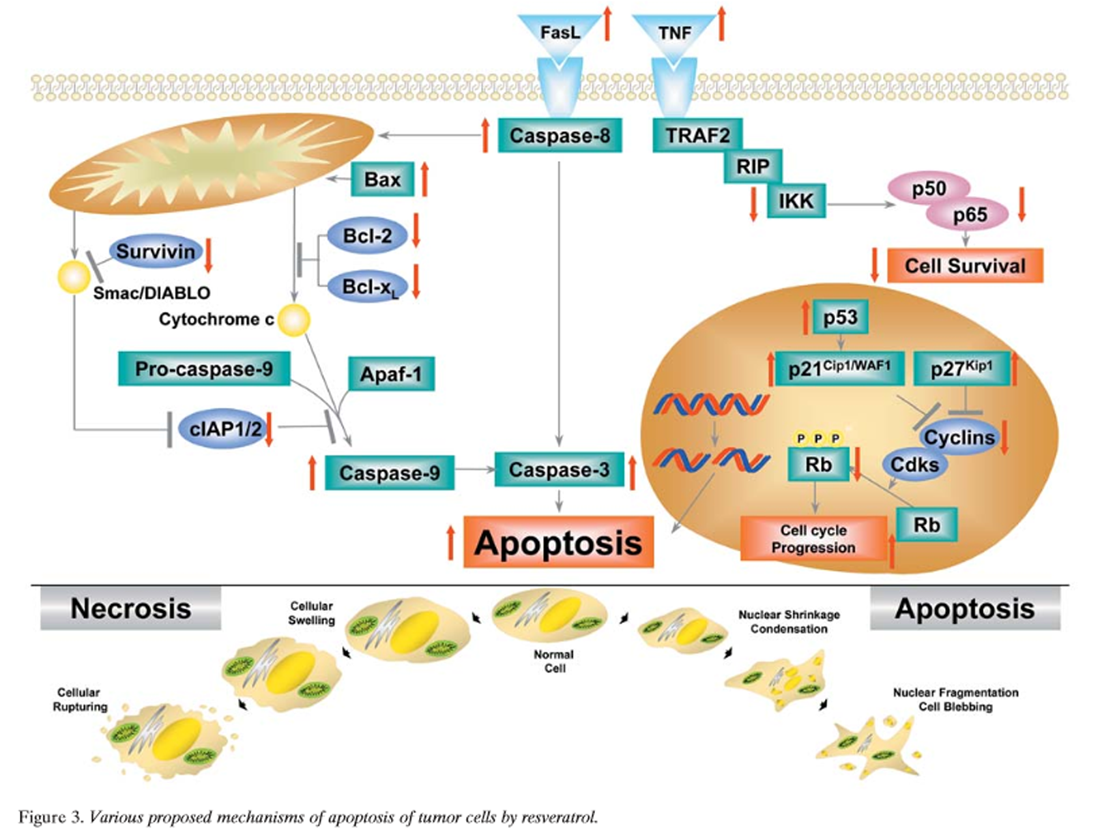
Figure 3 Apoptosis is a mode of cell death that differs from necrosis. While the former is characterized by initiation of cell death from the outside of the cell, the latter is a death mechanism initiated from inside the cell, primarily from the mitochondria [189]. Apoptosis is usually mediated through the activation of caspases. Mechanistically, two different type of apoptosis have been described; one that is caspase-8- dependent and receptor-mediated (type I), and the other that is caspase-9-dependent and usually mediated through the mitochondria (type II). Resveratrol has been shown to mediate apoptosis through a variety of different pathways (Figure 3) [51, 114, 117, 118, 131, 137, 138, 146, 148, 162, 166, 168, 175, 187, 190–199], as described below.
Fas pathway: Resveratrol has been shown to induce death receptors, that in turn activate apoptosis, through the type I pathway. Fas is one of the death receptors of the tumor necrosis factor (TNF) superfamily [200]. Clement et al. showed that resveratrol triggered FasL signaling-dependent apoptosis in human tumor cells [118]. They showed that resveratrol treatment enhanced FasL expression on HL-60 cells and T47D breast carcinoma cells, and that resveratrol- mediated cell death was specifically dependent on Fas signaling. Resveratrol treatment had no effect on normal PBMC, which correlated with the absence of a significant change in either Fas or FasL expression on treated PBMC. These data showed specific involvement of the Fas-FasL system in the anticancer activity of resveratrol. In contrast to these results, those of Bernhard et al. found that resveratrol caused arrest in the S-phase prior to Fas- independent apoptosis in CEM-C7H2 ALL cells [191]. These findings indicate that the effect of resveratrol on Fas signaling may depend on cell type. Delmas et al. showed that resveratrol-induced apoptosis was associated with Fas redistribution in the rafts and the formation of a DISC in colon cancer cells [146]. Resveratrol did not modulate the expression of Fas and FasL at the surface of cancer cells, and inhibition of the Fas-FasL interaction did not influence the apoptotic response to the molecule. Resveratrol, however, induced the clustering of Fas and its redistribution in cholesterol- and sphingolipid-rich fractions of SW480 cells, together with FADD and procaspase-8. This redistribution was associated with formation of a DISC. Transient transfection of a dominant-negative mutant of FADD, E8, or viral protein MC159 that interferes with DISC function decreased the apoptotic response of SW480 cells to resveratrol and partially prevented resveratrol- induced Bax and Bak conformational changes. Altogether, these results indicate that the ability of resveratrol to induce redistribution of the Fas receptor in membrane rafts may contribute to the molecule's ability to trigger apoptosis in colon cancer cells.
Mitochondrial pathway: Resveratrol has also been shown to activate the type II pathway. This pathway for apoptosis is mediated through the activation of the mitochondrial pathway. Dorrie et al. showed that resveratrol induced extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in ALL cells and that these effects were independent of Fas signaling [114]. Tinhofer et al. showed that resveratrol induced apoptosis via a novel mitochondrial pathway controlled by Bcl-2 [117].
Mitochondrial proton F0F1-ATPase/ATP synthase synthesizes ATP during oxidative phosphorylation. Zheng et al. found that resveratrol inhibited the enzymatic activity of both rat brain and liver F0F1-ATPase/ATP synthase (IC50, 12–28 µM) [192]. The inhibition of F0F1-ATPase by resveratrol was non-competitive in nature. Thus the mitochondrial ATP synthase is a target for this dietary phytochemical and may contribute to its potential cytotoxicity. Zheng et al. also found that piceatannol, an analogue of resveratrol, inhibited mitochondrial F0F1- ATPase activity by targeting the F1 complex [192]. Piceatannol potently inhibited rat brain mitochondrial F0F1-ATPase activity in both solubilized and submitochondrial preparations (IC50, 8–9 µM) while having a relatively small effect on Na+, K+-ATPase activity. Piceatannol inhibited the ATPase activity of purified rat liver F1 (IC50, 4 µM), while resveratrol was slightly less active (IC50, 14 µM). These results indicated that piceatannol and resveratrol inhibit the F-type ATPase by targeting the F1 sector, which is located in the inner membrane of mitochondria and the plasma membrane of normal endothelial cells and several cancer cell lines.
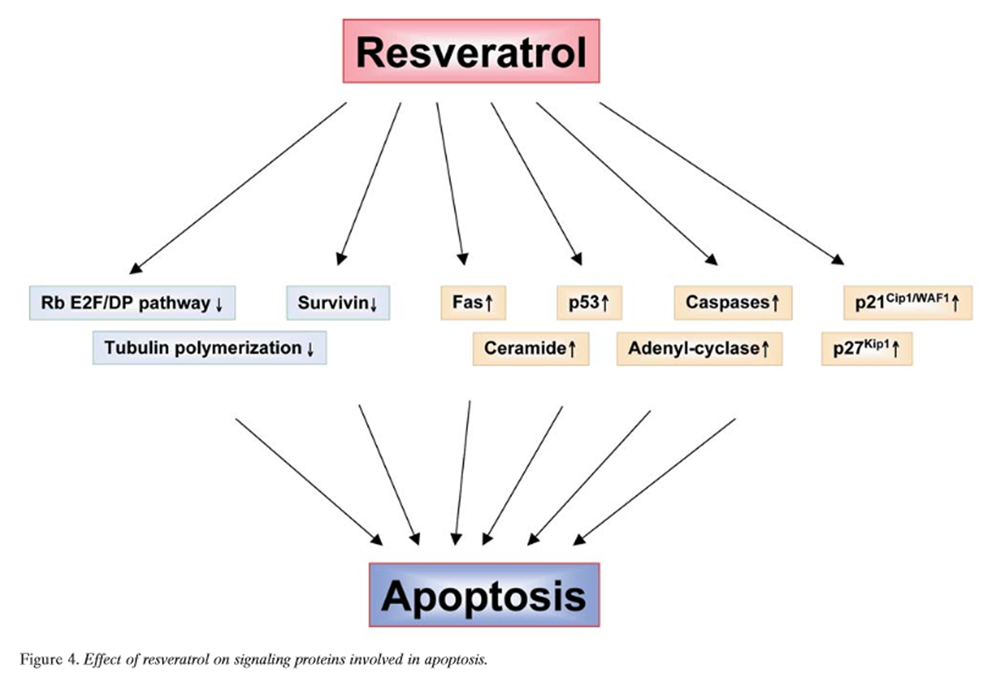
Figure 4 Rb-E2F/DP pathway: Rb and the E2F family of transcription factors are important proteins that regulate the progression of the cell-cycle at and near the G1/S-phase transition (Figure 4). Adhami et al. provided evidence for the involvement of the Rb-E2F/DP pathway as an important contributor to resveratrol-mediated cell-cycle arrest and apoptosis [166]. Immunoblot analysis demonstrated that resveratrol treatment of A431 melanoma cells resulted in a decrease in the hyperphosphorylated form of Rb and a relative increase in hypophosphorylated Rb. This response was accompanied by down-regulation of expression of all five E2F family transcription factors studied and their heterodimeric partners DP1 and DP2. This suggested that resveratrol causes down-regulation of hyperphosphorylated Rb protein with a relative increase in hypophosphorylated Rb that, in turn, compromises the availability of free E2F. These events may result in a stoppage of cell-cycle progression at the G1/S-phase transition, thereby leading to a G0/G1-phase arrest and subsequent apoptotic cell death. Kim et al. showed that resveratrol treatment of A549 cells resulted in a concentration-dependent induction of S-phase arrest in cell-cycle progression [168]. This antiproliferative effect of resveratrol was associated with a marked inhibition of phosphorylation of Rb and concomitant induction of the Cdk inhibitor p21Cip1/WAF1, which appears to be transcriptionally up-regulated and p53-dependent. Fluorescence microscopy and flow-cytometric analysis also revealed that treatment with resveratrol resulted in induction of apoptosis. These effects were found to correlate with activation of caspase-3 and a shift in the Bax/Bcl-xL ratio toward apoptosis.
p53 activation pathway: p53 is a tumor suppressor gene. There are numerous reports about the role of p53 in resveratrol-induced apoptosis [51, 162, 175, 193–198]. Huang et al. found that resveratrol-induced apoptosis occurred only in cells expressing wild-type p53 (p53+/+), but not in p53-deficient (p53-/-) cells, while there was no difference in apoptosis induction between normal lymphoblasts and sphingomyelinase-deficient cell lines [193]. These results demonstrated for the first time that resveratrol induces apoptosis through activation of p53 activity, suggesting that resveratrol’s antitumor activity may occur through induction of apoptosis. Hsieh et al. showed that resveratrol inhibited proliferation of pulmonary artery endothelial cells, which correlated with suppression of cell progression through the S- and G2-phases of the cell-cycle and was accompanied by increased expression of p53 and elevation of the level of Cdk inhibitor p21Cip1/WAF1 [194]. Lu et al. showed that resveratrol analogues significantly induced expression of p53, GADD45 and Bax genes and concomitantly suppressed expression of the Bcl-2 gene in human fibroblasts transformed with SV40 virus (WI38VA), but not in nontransfected WI38 cells [51]. A large increase in p53 DNA-binding activity and the presence of p53 in the Bax promoter binding complex suggested that p53 was responsible for the Bax gene expression induced by resveratrol in transformed cells.
She et al. elucidated the potential signaling components underlying resveratrol-induced p53 activation and induction of apoptosis [195, 196]. They found that, in the JB6 mouse epidermal cell line, resveratrol activated ERK1/2, JNK, and p38 MAPK and induced serine-15 phosphorylation of p53. Stable expression of a dominant-negative mutant of ERK2 or p38 MAPK or their respective inhibitors, PD98059 or SB202190, repressed phosphorylation of p53 at serine-15. In contrast, overexpression of a dominant-negative mutant of JNK1 had no effect on the phosphorylation. Most importantly, ERK1/2 and p38 MAPK formed a complex with p53 after treatment with resveratrol. Strikingly, resveratrol- activated ERK1/2 and p38 MAPK, but not JNKs, phosphorylated p53 at serine-15 in vitro. Furthermore, pretreatment of the cells with PD98059 or SB202190 or stable expression of a dominant-negative mutant of ERK2 or p38 MAPK impaired resveratrol-induced p53-dependent transcriptional activity and apoptosis, whereas constitutively active MEK1 increased the transcriptional activity of p53. These data strongly suggest that both ERK1/2 and p38 MAPK mediate resveratrol-induced activation of p53 and apoptosis through phosphorylation of p53 at serine-15. Shih et al. also showed that resveratrol acted via a Ras-MAPK kinase-MAPK signal transduction pathway to increase p53 expression, serine phosphorylation of p53, and p53-dependent apoptosis in thyroid carcinoma cell lines. Haider et al. showed that resveratrol led to a reversible arrest in early S phase of the vascular smooth muscle cell (VSMC), accompanied by accumulation of hyperphosphorylated Rb [197]. Resveratrol decreased cellular levels of the p21Cip1/WAF1 and p27Kip1 and increased the level of phosphorylated p53 protein (serine-15). The authors found that resveratrol only slightly inhibited phosphorylation of ERK1/2, protein kinase B/Akt, and p70(S6) kinase upon serum stimulation. Thus, inhibition of these kinases is not likely to contribute to the effects of the polyphenol on the cell-cycle. Importantly, the observed S-phase arrest was not linked to an increase in apoptotic cell death: there were no detectable increases in apoptotic nuclei or in levels of the proapoptotic protein Bax. This was the first study to elucidate the molecular pathways mediating the antiproliferative properties of resveratrol in VSMCs.
The expression of the nonsteroidal anti-inflammatory drug -activated gene-1 (NAG-1), a member of the TGF-β superfamily, has been associated with pro-apoptotic and antitumorigenic activities. Baek et al. demonstrated that resveratrol induced NAG-1 expression and apoptosis through an increase in the expression of p53 [198]. They showed that p53-binding sites within the promoter region of NAG-1 played a pivotal role in controlling NAG-1 expression by resveratrol. Derivatives of resveratrol were examined for NAG-1 induction, and the data suggest that induction of NAG-1 and p53 by resveratrol is not dependent on its anti-oxidant activity. The data may provide a linkage between p53, NAG-1 and resveratrol and, in part, a new clue to the molecular mechanism of the antitumorigenic activity of natural polyphenolic compounds.
Earlier studies showed that resveratrol alters the expression of genes involved in cell-cycle regulation and apoptosis, including cyclins, Cdks, p53, and Cdk inhibitors. However, most of the p53-controlled effects related to the role of resveratrol in transcription, either by activation or repression of a sizable number of primary and secondary target genes, have not been investigated. Narayanan et al. examined whether resveratrol activates a cascade of p53-directed genes that are involved in apoptosis mechanism(s) [162]. They demonstrated by DNA microarray, RT-PCR, Western blot and immunofluorescence analyses that treatment of androgen- sensitive prostate cancer cells (LNCaP) with resveratrol down- regulated PSA, AR co-activator ARA 24, and NF-ÎB p65. Altered expression of these genes is associated with activation of p53-responsive genes such as p53, PIG 7, p21Cip1/WAF1, p300/CBP and Apaf-1.
Ceramide activation pathway: Apoptosis induction by various cytokines has been shown to be mediated through generation of ceramide. Whether resveratrol-induced apoptosis also involves ceramide production has been investigated. Scarlatti et al. showed that resveratrol can inhibit growth and induce apoptosis in MDA-MB-231, a highly invasive and metastatic breast cancer cell line, in concomitance with a dramatic endogenous increase of growth inhibitory/pro-apoptotic ceramide [137]. They found that accumulation of ceramide derives from both de novo ceramide synthesis and sphingomyelin hydrolysis. More specifically, they demonstrated that ceramide accumulation induced by resveratrol can be traced to the activation of serine palmitoyltransferase (SPT), the key enzyme of a de novo ceramide biosynthetic pathway, and neutral sphingomyelinase (nSMase), a main enzyme of the sphingomyelin/ceramide pathway. By using specific inhibitors of SPT (myriocin and L-cycloserine) and nSMase (gluthatione and manumycin), however, they found that only the SPT inhibitors could counteract the biological effects induced by resveratrol. Thus, resveratrol seems to exert its growth-inhibitory/apoptotic effect on the metastatic breast cancer cell line MDA-MB-231 by activating the de novo ceramide synthesis pathway.
Tubulin polymerization pathway: Certain chemotherapeutic agents such as taxol induce apoptosis by interfering with tubulin polymerization. Whether resveratrol could also mediate apoptosis through this pathway has been investigated. Schneider et al. found that a methylated derivative of resveratrol (Z-3,5,4'- trimethoxystilbene; R3) at a concentration of 0.3 µM, exerted an 80% growth- inhibitory effect on human colon cancer Caco-2 cells and arrested growth completely at a concentration of 0.4 µM (R3 was 100–fold more active than resveratrol) [199]. The cis conformation of R3 was also 100–fold more potent than the trans isomer. R3 (0.3 µM) caused cell-cycle arrest at the G2/M-phase transition. The drug inhibited tubulin polymerization in a dose-dependent manner (IC50, 4 µM), and it reduced by half the activities of ornithine decarboxylase and s-adenosylmethionine decarboxylase. This caused depletion of the polyamines putrescine and spermidine, which are growth factors for cancer cells. R3 partially inhibited colchicine binding to its binding site on tubulin, indicating that R3 either partially overlaps with colchicine binding or binds to a specific site of tubulin that is not identical with the colchicine binding site, modifying colchicine binding by allosteric influences. R3 is an interesting antimitotic drug that exerts cytotoxic effects by depleting the intracellular pool of polyamines and by altering microtubule polymerization. Such a drug may be useful for the treatment of neoplastic diseases.
Adenylyl-cyclase pathway: Both cyclic GMP and cyclic AMP (cAMP) are known to regulate proliferation of cells. Whether resveratrol could modulate cell growth by modulating the levels of these nucleotides has been investigated [138]. El-Mowafy et al. examined the effects of resveratrol on the activity of the enzymes adenylate cyclase and guanylate cyclase, two known cytostatic cascades in MCF-7 breast cancer cells [138]. Resveratrol increased cAMP levels (t1/2, 6.2 min; EC50, 0.8 µM), but had no effect on cGMP levels. The stimulatory effects of resveratrol on adenylate cyclase were not altered either by the protein synthesis inhibitor actinomycin-D (5 µM) or the ER blockers tamoxifen and ICI182,780 (1 µM each). Likewise, cAMP formation by resveratrol was insensitive to both the broad-spectrum phosphodiesterase (PDE) inhibitor IBMX (0.5 µM) and the cAMP-specific PDE inhibitor rolipram (10 µM). Instead, these PDE inhibitors significantly augmented maximal cAMP formation by resveratrol. Parallel experiments showed that the antiproliferative effects of resveratrol in these cells were appreciably reversed by the protein kinase A inhibitors Rp-cAMPS (100–300 µM) and KT-5720 (10 µM). Pretreatment with the cPLA2 inhibitor arachidonyl trifluoromethyl ketone (10 µM) markedly antagonized the cytotoxic effects of resveratrol. With these findings, we demonstrated that resveratrol is an agonist for the cAMP/protein kinase A system.
C 1c: Resveratrol suppresses NF-ÎB activation
Because resveratrol exhibits anti-inflammatory, cell growth- modulatory and anticarcinogenic effects, that it mediates these effects by suppressing NF-ÎB, a nuclear transcription factor that regulates the expression of various genes involved in inflammation, cytoprotection and carcinogenesis, has been proposed [200, 201]. We investigated the effect of resveratrol on NF-ÎB activation induced by various inflammatory agents. Resveratrol blocked TNF-induced activation of NF-ÎB and suppressed TNF-induced phosphorylation and nuclear translocation of the p65 subunit of NF-ÎB and NF-ÎB-dependent reporter gene transcription [22, 71, 73, 92, 120, 122, 125–127, 129, 132, 135, 139–142, 145, 147, 151, 153, 154, 156, 159, 161, 165, 167, 168, 173–175, 179, 182, 183, 185, 187, 191, 193–196, 198, 201–284]. Suppression of TNF-induced NF-ÎB activation by resveratrol was not restricted to myeloid cells (U-937); it was also observed in lymphoid (Jurkat) and epithelial (HeLa and H4) cells. Resveratrol also blocked NF-ÎB activation induced by phorbol myristate acetate (PMA), LPS, H2O2, okadaic acid and ceramide. Holmes-McNary and Baldwin found resveratrol to be a potent inhibitor of both NF-ÎB activation and NF-ÎB-dependent gene expression through its ability to inhibit IÎB kinase activity, the key regulator in NF-ÎB activation, probably by inhibiting an upstream signaling component [202]. In addition, resveratrol blocked the expression of mRNA-encoding monocyte chemoattractant protein-1, a NF-ÎB-regulated gene. Heredia et al. found that resveratrol synergistically enhanced the anti-HIV-1 activity of the nucleoside analogues AZT, ddC, and ddI [14]. Resveratrol at a concentration of 10 µM was not toxic to cells, and by itself reduced viral replication by 20–30%. In phytohemagglutinin (PHA)-activated PBMCs infected with HTLV-IIIB, 10 µM resveratrol reduced the 90% inhibitory concentrations (IC90) of AZT, ddC and ddI by 3.5-, 5.5- and 17.8-fold, respectively. Similar antiviral activity was demonstrated when ddI was combined with 5 or 10 µM resveratrol in PBMCs infected with clinical isolates of HIV-1. The addition of resveratrol resulted in a >10–fold augmentation of ddI antiviral activity in infected monocyte- derived macrophages. In a resting cell model of T lymphocytes infected with HTLV-IIIB, resveratrol plus ddI in combination, but not individually, suppressed the establishment of a productive viral infection. In addition, resveratrol plus ddI markedly inhibited the replication of four ddI-resistant viral isolates, three of which presented mutations in the reverse transcriptase gene conferring reverse transcriptase-multidrug resistance. Finally, 10 µM resveratrol showed enhancement of ddI antiviral suppressive activity similar to that of 100 µM of hydroxyurea. However, resveratrol had less of a cellular antiproliferative effect than hydroxyurea.
Pellegatta et al. reported different short- and long-term effects of resveratrol on NF-ÎB phosphorylation and nuclear appearance in human endothelial cells [203]. They found that the nuclear appearance of p50 and p65 acutely induced by TNFα was not modified by resveratrol, but was increased after overnight incubation with resveratrol alone or in combination with TNFα. Acute treatment with resveratrol did not modify TNFα-induced cytoplasmic IÎBα serine phosphorylation but did increase IÎBα tyrosine phosphorylation. Resveratrol increased tyrosine phosphorylation (but not nitrosylation) of immunoprecipitated NF-ÎB, did not decrease cellular p21Cip1/WAF1, and did not increase peroxisome proliferator-activated receptor-α activity. They concluded that acute resveratrol treatment does not inhibit the nuclear appearance of NF-ÎB in human umbilical vein endothelial cells (HUVEC), but overnight treatment does.
We showed that resveratrol blocks IL-1‚-induced activation of NF-ÎB that leads to inhibition of proliferation, causes S-phase arrest, and induces apoptosis of AML cells [122]. Adhami et al. showed the suppression of UV B exposure- mediated activation of NF-ÎB in normal human keratinocytes by resveratrol [204]. Kim et al. showed the involvement of NF-ÎB suppression in induction of growth arrest and apoptosis by resveratrol in human lung carcinoma A549 cells [168]. These results indicate that NF-ÎB suppression by resveratrol may be essential for its antitumor activities.
C 1d. Resveratrol suppresses AP-1 activation
Activator protein-1 (AP-1) is a transcription factor transactivated by many tumor-promoting agents, such as phorbol ester, UV radiation, asbestos and crystalline silica [209, 210]. AP-1 complexes are formed by dimers of Jun proto- oncogene family members (c-Jun, JunB, and JunD) or heterodimers of Jun family members with the Fos proto- oncogene family members (c-Fos, FosB, Fra-1, and Fra-2). AP-1 binds to a specific target DNA site (also known as TRE) in the promoters of several cellular genes and mediates immediate early gene expression involved in a diverse set of transcriptional regulation processes [209, 210]. Agents that activate NF-ÎB also activate AP-1. Both of these factors are regulated by the redox status of the cell. AP-1 activation has been implicated in cell proliferation and chemical carcinogenesis. It has been shown to play a critical role in proliferation of cells. Whether resveratrol affects activation of AP-1 has been investigated by several groups. We showed that suppression of NF-ÎB by resveratrol coincided with suppression of AP-1 [201]. Resveratrol has been shown to suppress activation of AP-1 by PMA, TNF and UV. It inhibited PMA-induced IL-8 production in human monocytic U-937 cells at protein and mRNA levels which was, at least partly, due to inhibition of AP-1 activation [211]. It also suppressed PMA-mediated signaling events such as induction of COX-2 and prostaglandin synthesis in human mammary and oral epithelial cells [212]. Moreover, it inhibited PMA- mediated activation of PKC and induction of COX-2 promoter activity by c-Jun. PMA-mediated induction of AP-1 activity was blocked by resveratrol. Resveratrol also inhibited PMA- or UV-induced AP-1-mediated activity through inhibition of c-Src non-receptor tyrosine kinase and MAPK pathways and may also regulate gene expression of cellular defensive enzymes such as phase II detoxifying enzymes [213]. It also suppressed TNF-induced AP-1 activity in various cancer cell lines [201].
Resveratrol inhibited the TNF-induced activation of MAPK and JNK, which are needed for AP-1 activation. Yu et al. found that resveratrol inhibited phorbol ester and UV-induced AP-1 activation by interfering with MAPK pathways [213]. They showed that pretreatment with resveratrol also inhibited the activation of ERK2, JNK1 and p38 MAPK. Selectively blocking MAPK pathways by overexpression of dominant-negative mutants of kinases attenuated the activation of AP-1 by PMA and UVC. Interestingly, resveratrol had little effect on induction of the AP-1 reporter gene by active Raf-1, MAPK/ERK kinase kinase (MEKK)1, or MAPK kinase (MKK)6, suggesting that it inhibited MAPK pathways by targeting the signaling molecules upstream of Raf-1 or MEKK1. Indeed, incubation of resveratrol with the isolated c-Src protein tyrosine kinase and PKC diminished their kinase activities. Moreover, modulation of ER activity by 17-β-estradiol had no effect on the inhibition of AP-1 by resveratrol. In contrast to these studies, those of Wolter et al. showed that the AP-1 constituents c-Fos and c-Jun increased on resveratrol treatment of cells [214]. While the DNA-binding activity of c-Jun remained unchanged, the DNA-binding activity of c-Fos was significantly enhanced by resveratrol and piceatannol.
C 1e. Resveratrol suppresses Egr-1 activation
Early growth response–1 gene product (Egr-1) is another transcription factor that plays an important role in proliferation of cells. It is a member of a family of immediate early response genes and regulates a number of pathophysiologically relevant genes that are involved in growth, differentiation, immune response, wound healing and blood clotting. Resveratrol selectively up-regulates Egr-1 by an ERK1/2-dependent mechanism in human erythro- leukemic K562 cells, induces Á-globin synthesis, and causes erythroid differentiation due to impairment of cell proliferation, increase in p21Cip1/WAF1 expression and inhibition of Cdk2 activity [215]. Ragione et al. found that resveratrol increases Egr-1 and causes differentiation of HL-60 cells [216] and examined its effects on this transcription factor [215]. Up-regulation of p21Cip1/WAF1 transcription is prevented by cycloheximide, indicating that an intermediate protein(s) is required that, in turn, regulates gene expression. Quantitative analysis of some transcription factors involved in the erythroid lineage, namely GATA-1, GATA-2 and Egr-1, indicated that resveratrol selectively up-regulates Egr-1 by an ERK1/2-dependent mechanism. The presence of an Egr-1 consensus sequence in the p21ip1/WAF1 promoter suggests that this transcription factor directly regulates the expression of the Cdk inhibitor. Transfection studies with deleted gene promoter constructs, as well as electrophoretic mobility shift assay, pull-down and chromatin immunoprecipitation experiments, substantiated this view, demonstrating that Egr-1 binds in vitro and in vivo to the identified consensus sequence of the p21Cip1/WAF1 promoter. Moreover, an Egr-1 phosphorothioate antisense construct hinders p21Cip1/WAF1 accumulation and the antiproliferative effects of resveratrol.
C 1f. Suppression of MAPK by resveratrol
Three different MAPK have been identified: ERK1/2, JNK and p38 MAPK. While ERK1/2 have been implicated in the proliferation of cells, JNK and p38 MAPK are activated in response to different types of stress stimuli. JNK activation is needed for activation of AP-1; it also mediates apoptosis in some situations. Numerous studies suggest that resveratrol modulates all three of these protein kinases [163, 175, 179, 195, 196, 217, 218]. Miloso et al. showed that resveratrol induced activation of ERK1/2 in human neuroblastoma SH-SY5Y cells [179]. In undifferentiated cells, resveratrol 1 µM induced phosphorylation of ERK1/2, which was already evident at 2 min, peaked at 10 min and still persisted at 30 min. A wide range of resveratrol concentrations (from 1 pM to 10 µM) were able to induce phosphorylation of ERK1/2, while higher concentrations (50-100 µM) inhibited phosphorylation of MAPK. In retinoic acid-differentiated cells, resveratrol (1 µM) induced an evident increase in ERK1/2 phosphorylation. El-Mowafy et al. found short-term treatment of porcine coronary arteries with resveratrol substantially inhibited MAPK activity (IC50, 37 µM) and reduced phosphorylation of ERK1/2, JNK1 and p38 MAPK at active sites. Endothelin-1 enhanced, MAPK activity, phosphorylation and nuclear translocation in a concentration-dependent manner, but resveratrol reversed it [217]. She et al. showed that resveratrol activated ERK1/2, JNKs and p38 MAPK in the JB6 mouse epidermal cell line and induced serine-15 phosphorylation of p53 [196]. Stable expression of a dominant-negative mutant of ERK2 or p38 MAPK repressed phosphorylation of p53 at serine-15. In contrast, overexpression of a dominant-negative mutant of JNK1 had no effect on this phosphorylation. Most importantly, ERK1/2 and p38 MAPK formed a complex with p53 after treatment with resveratrol. Strikingly, resveratrol-activated ERK1/2 and p38 MAPK, but not JNKs, phosphorylated p53 at serine-15 in vitro. Shih et al. examined the effect of resveratrol on papillary and follicular thyroid carcinoma cell lines [175]. They found that treatment with resveratrol (1-10 µM) induced activation and nuclear translocation of ERK1/2. Cellular abundance of the oncogene suppressor protein p53, serine phosphorylation of p53, and abundance of c-fos, c-Jun, and p21Cip1/WAF1 mRNAs were also increased by resveratrol. Inhibition of the MAPK pathway by either H-Rasantisense transfection or PD 98059, MAPK kinase inhibitor, blocked these effects. Thus, resveratrol appears to act via a Ras-MAPK kinase-MAPK signal- transduction pathway to increase p53 expression, serine phosphorylation of p53 and p53-dependent apoptosis in thyroid carcinoma cell lines.
She et al. showed the interesting involvement of JNK in resveratrol-induced activation of p53 [195]. They found that resveratrol activated JNKs at the same dosage that inhibited tumor promoter-induced cell transformation. Stable expression of a dominant-negative mutant of JNK1 or disruption of the Jnk1 or Jnk2 gene markedly inhibited resveratrol-induced p53-dependent transcription activity and induction of apoptosis. Furthermore, resveratrol-activated JNKs were shown to phosphorylate p53 in vitro, but this activity was repressed in the cells expressing a dominant- negative mutant of JNK1 or in Jnk1 or Jnk2 knockout (Jnk1-/- or Jnk2-/-) cells. These data suggest that JNKs act as mediators of resveratrol-induced activation of p53 and apoptosis, which may occur partially through p53 phosphorylation. Woo et al. showed that resveratrol inhibited PMA-induced matrix metalloproteinase (MMP)-9 expression by inhibiting JNK [218]. From these results, it is clear that resveratrol can modulate all three MAPKs, which leads to modulation of gene expression. Resveratrol appears to cause activation of MAPK in some cells and inhibition in others. This variability may depend on the cell type and the dose of resveratrol used.
Stewart and O'Brian showed that resveratrol antagonized EGFR-dependent ERK1/2 activation in human androgen- independent prostate cancer cells with associated isozyme- selective PKC-α inhibition [163]. They found that resveratrol suppressed EGFR-dependent ERK1/2 activation pathways stimulated by EGF and PMA in human AI PrCa PC-3 cells in vitro. Resveratrol abrogation of a PKC- mediated ERK1/2 activation response in PC-3 cells correlated with isozyme-selective PKC-α inhibition.
C 1g. Suppression of protein kinases by resveratrol
PKC has been shown to play a major role in tumorigenesis. The PKC isozyme subfamily consists of cPKC-α, -β‚ and -γ, nPKC-D and - ε, and αPKC-ζ. Numerous reports indicate that resveratrol can inhibit PKC [127, 139, 153, 218-221]. Garcia-Garcia et al. showed that resveratrol was incorporated into model membranes and inhibited PKC-α activity [219]. Resveratrol activated by phosphatidylcholine/ phosphatidylserine vesicles inhibited PKC-α with an IC50 of 30 µM, whereas that activated by Triton X-100 micelles inhibited PKC-α with an IC50 of 300 µM. These results indicate that the inhibition of PKC-α by resveratrol can be mediated, at least partially, by membrane effects exerted near the lipid-water interface. Stewart et al. showed that resveratrol preferentially inhibited PKC-catalyzed phosphorylation of a cofactor-independent, arginine-rich protein substrate by a novel mechanism [139]. While resveratrol has been shown to antagonize both isolated and cellular forms of PKC, the weak inhibitory potency observed against isolated PKC cannot account for the reported efficacy of the polyphenol against PKC in cells. Stewart et al. analyzed the mechanism of PKC inhibition by resveratrol and found that resveratrol has a broad range of inhibitory potencies against purified PKC that depend on the nature of the substrate and the cofactor dependence of the phosphotransferase reaction. Resveratrol weakly inhibited the Ca2+/phosphatidylserine-stimulated activity of a purified rat brain PKC isozyme mixture (IC50, 90 µM) by competition with ATP (Ki, 55 µM). Consistent with the kinetic evidence for a catalytic domain-directed mechanism was resveratrol’s inhibition of the lipid-dependent activity of PKC isozymes with divergent the regulatory domains, and it was even more effective in inhibiting a cofactor- independent catalytic domain fragment of PKC generated by limited proteolysis. This suggested that regulatory features of PKC might impede resveratrol inhibition of the enzyme. To explore this, the authors examined the effects of resveratrol on PKC-catalyzed phosphorylation of the cofactor-independent substrate protamine sulfate, which is a polybasic protein that activates PKC by a novel mechanism. Resveratrol potently inhibited protamine sulfate phosphorylation (IC50, 10 µM) by a mechanism that entailed antagonism of the activation of PKC by protamine sulfate and did not involve competition with either substrate.
Protein kinase D (PKD) is a member of the PKC superfamily with distinctive structural, enzymic and regulatory properties. Identification of the cellular function(s) of PKD has been hampered by the absence of a selective inhibitor. Stewart et al. compared the effects of resveratrol against the autophosphorylation reactions of PKC isozymes to those against the autophosphorylation reactions of the novel phorbol ester-responsive kinase PKD [127]. They found that resveratrol inhibited PKD autophosphorylation, but had only negligible effects against the autophosphorylation reactions of representative members of each PKC isozyme subfamily (cPKC-α, -β1 and -γ, nPKC-D and -ε, and αPKC-ζ). Resveratrol was comparably effective against PKD autophosphorylation (IC50, 52 µM) and PKD phosphorylation of the exogenous substrate syntide-2 (IC50, 36 µM). The inhibitory potency of resveratrol against PKD is in line with those observed in cellular systems and against other purified enzymes and binding proteins that are implicated in the cancer chemopreventive activity of the polyphenol. Thus, PKD inhibition may contribute to the cancer chemopreventive action of resveratrol. Haworth et al. showed inhibition of PKD by resveratrol, not only in vitro but also in intact cells [220]. Atten et al. demonstrated that resveratrol treatment significantly inhibited PKC activity of KATO-III human gastric adenocarcinoma cells and of human recombinant PKC-α [153]. Woo et al. showed that resveratrol inhibited PMA-mediated PKC-Δ activation, which led to suppression of MMP-9 [218].
The COP9 signalosome (CSN), purified from human erythrocytes, possesses kinase activity that phosphorylates proteins such as c-Jun and p53, with consequences for their ubiquitin-dependent degradation. Uhle et al. showed that resveratrol could block the CSN-associated kinases protein kinase CK2 and PKD and induce degradation of c-Jun in HeLa cells [221].
C 1h. Modulation of NO/NOS expression by resveratrol
Synthesis of NO is dependent on expression of an inducible enzyme, iNOS. The expression of this enzyme is regulated by the transcription factor NF-ÎB. Production of NO has been shown to mediate antiproliferative effects in various cell types. NO also been linked with pro-inflammatory effects. Resveratrol has been reported to both enhance and suppress production of NO [92, 154, 194, 222]. Kageura et al. reported that resveratrol analogues had inhibitory activity against NO production in LPS-activated macrophages (IC50, 11-69 µM) [92]. Furthermore, the active stilbenes (rhapontigenin, piceatannol and resveratrol) did not inhibit iNOS activity, but they inhibited NF-ÎB activation following expression of iNOS. Chung et al. examined the effect of α-viniferin, a trimer of resveratrol, in a mouse model of carrageenin-induced paw edema [222]. They found that α-viniferin at doses >30 mg/kg (p.o.) or >3 mg/kg (i.v.) showed significant anti-inflammatory activity on this edema. α-Viniferin at doses of 3-10 µM inhibited NO production in LPS-activated Raw 264.7 cells when α-viniferin and LPS were applied simultaneously, but not when α-viniferin was applied 12 h after LPS stimulation. α-Viniferin inhibited synthesis of the iNOS transcript with an IC50 value of 4.7 µM.
Hsieh et al. found that resveratrol induced NOS in cultured pulmonary artery endothelial cells, which led to inhibition of their proliferation [194]. Holian et al. found that resveratrol stimulated NOS activity in human gastric adenocarcinoma SNU-1 cells [154]. They suggested that the antioxidant action of resveratrol toward gastric adenocarcinoma cells may reside in its ability to stimulate NOS to produce low levels of NO, which, in turn, exerts antioxidant action. Thus, whether resveratrol induces or inhibits NO production depends on the cell system, inducer and other conditions.
C 1i. Suppression of growth factor and associated protein tyrosine kinases by resveratrol
Because resveratrol exhibits antiproliferative effects against a wide variety of tumor cells and the effects of various growth factors are mediated through protein tyrosine kinases, it is possible that resveratrol either down-regulates the expression of growth factors and growth factor receptors or suppresses the activity of protein tyrosine kinases required for their activity. Kaneuchi et al. found that resveratrol treatment significantly decreased EGF expression in Ishikawa endometrial cancer cells [183]. Palmieri et al. found that tyrosine kinase activities from particulate and cytosolic fractions of placenta were inhibited by resveratrol and piceatannol [223]. Oliver et al. showed that piceatannol (3,4,3',5'-tetrahydroxy-trans-stilbene) preferentially inhibited the activity of Syk protein tyrosine kinase as compared with Lyn when added to in vitro assays with isolated enzymes [224]. Selective inhibition of Syk in this manner blocked receptor-mediated downstream cellular responses (inositol 1,4,5-trisphosphate production, secretion, ruffling and spreading). We showed that piceatannol inhibited H2O2- induced NF-ÎB activation through inhibition of Syk kinase [225]. These reports suggest that resveratrol and its analogues can potentially suppress growth factors, growth factor receptors and their associated protein tyrosine kinases.
Resveratrol exerts an inhibitory effect in EGF-induced cell transformation [226]. It also inhibits proliferation of the breast cancer cell line MDA-MB-468 through alteration in autocrine growth modulators such as TGF-α, TGF-β, PC cell-derived growth factor, and insulin-like growth factor I receptor mRNA [129]. Moreover, it decreases hepatocyte growth factor-induced cell scattering and invasion by an unidentified postreceptor mechanism in HepG2 cells [173].
C 1j. Suppression of COX-2 and LOX by resveratrol
The enzymes COX-2 and lipooxygenase (LOX) play important roles in inflammation. Both of these enzymes are regulated by the transcription factors NF-ÎB and AP-1. The products of these enzymes also regulate proliferation of cells. Whether resveratrol modulates expression of these enzymes has been investigated by numerous groups [141, 142, 212, 222, 227, 228]. Subbaramaiah et al. showed that resveratrol inhibits COX-2 transcription and activity in phorbol ester-treated human mammary epithelial cells [141]. Transient transfections utilizing COX-2 promoter deletion constructs and COX-2 promoter constructs, in which specific enhancer elements were mutagenized, indicated that the effects of PMA and resveratrol were mediated via a cAMP response element. Resveratrol inhibited the PMA-mediated activation of PKC. Overexpressing PKC-α, ERK1 and c-Jun led to 4.7-, 5.1- and 4-fold increases in COX-2 promoter activity, respectively. These effects were inhibited by resveratrol. Resveratrol blocked PMA-dependent activation of AP-1- mediated gene expression. In addition to these effects on gene expression, we found that resveratrol also directly inhibited the activity of COX-2. These data are likely to be important for understanding the anticancer and anti- inflammatory properties of resveratrol. Chung et al. showed that α-viniferin inhibited COX-2 activity with an IC50 value of 4.9 µM, and at doses of 3-10 µM, inhibited synthesis of COX-2 transcript in LPS-activated murine macrophages Raw 264.7 [222]. MacCarrone et al. demonstrated that resveratrol acted as a competitive inhibitor of purified 5-LOX and 15- LOX and prostaglandin H synthase, with inhibition constants of 4.5 µM (5-LOX), 40 µM (15-LOX), 35 µM (COX activity of prostaglandin H synthase), and 30 µM (peroxidase activity of prostaglandin H synthase) [227].
C 1k. Suppression of cell-cycle proteins by resveratrol
Numerous reports indicate that resveratrol inhibits proliferation of cells by inhibiting cell-cycle progression [122, 135, 145, 147, 151, 161, 165, 167, 187, 191, 194, 229]. Various reports indicate that resveratrol inhibits different cells at different stages of the cell-cycle. The arrest of cells in G1- phase [165], S-phase [122, 151, 161, 187, 191], S/G2-phase [194] and G2-phase [147] of the cell-cycle has been reported. Why the effects of resveratrol on different cell types vary so widely is not clear. Which cell-cycle proteins are modulated by resveratrol has been investigated in detail. Wolter et al. showed the down-regulation of the cyclin D1/Cdk4 complex by resveratrol in colon cancer cell lines [145]. Yu et al. showed that, following treatment of H22 tumor-bearing mice with resveratrol at 10 or 15 mg/kg bodyweight for 10 days, the growth of transplantable liver cancers was inhibited by 36.3% or 49.3%, respectively [229]. The levels of expression of cyclin B1 and Cdc2 protein were decreased in treated tumors, whereas the expression of cyclin D1 protein did not change. Liang et al. showed that resveratrol induced G2 arrest through the inhibition of Cdk7 and Cdc2 kinases in colon carcinoma HT-29 cells [147]. Larrosa et al. showed that resveratrol and the related molecule 4-hydroxystilbene induced S- phase arrest and up-regulation of cyclins A, E and B1 in human SK-Mel-28 melanoma cells [167]. Thus, it is clear that the effects of resveratrol on the cell-cycle are highly variable. Kuwajerwala et al. showed that resveratrol had a dual effect on DNA synthesis [161]. At concentrations of 5- 10 µM, it caused a 2- to 3-fold increase in DNA synthesis, and at doses ≥15 µM, it inhibited DNA synthesis. The increase in DNA synthesis was seen only in LNCaP cells, not in the androgen-independent DU145 prostate cancer cells or in NIH/3T3 fibroblast cells. The resveratrol-induced increase in DNA synthesis was associated with enrichment of LNCaP cells in S-phase and concurrent decreases in nuclear p21Cip1/WAF1 and p27Kip1 levels. Furthermore, consistent with the entry of LNCaP cells into the S-phase, there was a dramatic increase in nuclear Cdk2 activity associated with both cyclin A and cyclin E.
C 1l. Suppression of adhesion molecules by resveratrol
Various cell-surface adhesion molecules, including intracellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1 and endothelial-leukocyte adhesion melecule (ELAM)-1, are regulated by NF-ÎB. These molecules play an essential role in adhesion of tumor cells to endothelial cells and thus mediate tumor cell metastasis. Several groups have examined the effect of resveratrol on the adhesion of cells to the endothelial cells. Ferrero et al. examined the activity of resveratrol on granulocyte and monocyte adhesion to endothelium in vitro [230, 231]. They showed that resveratrol, at concentrations as low as 1 µM and 100 nM, significantly inhibited ICAM-1 and VCAM-1 expression by TNFα-stimulated HUVEC and LPS-stimulated human saphenous vein endothelial cells (HSVEC), respectively. They also showed that resveratrol induced significant inhibition of the adhesion of U-937 monocytoid cells to LPS-stimulated HSVEC. Such inhibition was comparable with that obtained when anti- VCAM-1 monoclonal antibody was used instead of resveratrol. Resveratrol also significantly inhibited the adhesion of neutrophils to TNFα-stimulated NIH/3T3 ICAM-1-transfected cells, whereas neutrophils activated by formyl-methionyl-leucyl- phenylalanine did not significantly modify adhesion to NIH/3T3 ICAM-1-transfected cells. Pendurthi et al. also showed that resveratrol suppressed agonist-induced monocyte adhesion to cultured human endothelial cells [125]. Thus, it is clear that resveratrol affects the expression of adhesion molecules, most likely through down-regulation of NF-ÎB.
C 1m. Suppression of androgen receptors by resveratrol
Via their receptor AR, androgens play a role in prostate cancer etiology [159, 285]. Mitchell et al. demonstrated that resveratrol had inhibitory effects on androgen action in the LNCaP prostate cancer cell line [159]. They found that resveratrol repressed different classes of androgen up- regulated genes at the protein or mRNA level, including PSA, human glandular kallikrein-2, AR-specific coactivator ARA70, and the Cdk inhibitor p21Cip1/WAF1. This inhibition is probably attributable to a reduction in AR level at the transcription level, inhibiting androgen-stimulated cell growth and gene expression. These results suggest that resveratrol may be a useful chemopreventive / chemotherapeutic agent for prostate cancer.
C 1n. Suppression of PSA by resveratrol
Hsieh et al. demonstrated that resveratrol inhibited the proliferation of LNCaP cells and expression of the prostate- specific gene PSA. A 4-day treatment with resveratrol reduced the levels of intracellular and secreted PSA by approximately 80%, as compared to controls [156]. They found that this change in PSA was not due to a change in AR expression. Thus, it would appear that the prostate tumor marker PSA is down-regulated by resveratrol, by a mechanism independent of changes in AR.
C 1o. Suppression of inflammatory cytokine expression by resveratrol
Because resveratrol down-regulates NF-ÎB, which is known to mediate inflammation, it is possible that resveratrol also down-regulates the expression of inflammatory cytokines. Wang et al. showed that resveratrol inhibited IL-6 production in cortical mixed glial cells under hypoxic/hypoglycemic conditions followed by reoxygenation [232]. Zhong et al. demonstrated the inhibitory effect of resveratrol on IL-6 release by stimulated peritoneal macrophages of mice [233]. Shen et al. found that resveratrol suppressed IL-8 gene transcription in phorbol ester-treated human monocytic cells [211]. Wadsworthe et al. showed that resveratrol had no effect on LPS-induced TNFα mRNA in the macrophage cell line RAW 264.7, but decreased LPS-stimulated TNFα release, as measured by ELISA [234]. Culpitt et al. determined whether resveratrol would inhibit cytokine release in vitro by alveolar macrophages from patients with chronic obstructive pulmonary disease (COPD) [235]. They showed that resveratrol inhibited basal release of IL-8 in smokers and patients with COPD by 94% and 88%, respectively, and inhibited granulocyte-macrophage colony- stimulating factor (GM-CSF) release by 79% and 76%, respectively. Resveratrol also inhibited stimulated cytokine release. Resveratrol reduced IL-1β-stimulated IL-8 and GM- CSF release in both smokers and COPD patients to below basal levels. Moreover, resveratrol inhibited cigarette smoke media (CSM)-stimulated IL-8 release by 61% and 51%, respectively, in smokers and COPD patients, and inhibited GM-CSF release by 49% in both subject groups.
Boscolo et al. elucidated the "in vitro" effects of resveratrol on human PBMC proliferation and cytokine release [236]. Spontaneous PBMC proliferation was unaffected by resveratrol, while resveratrol at a concentation of 100 µM inhibited PHA-stimulated PBMC proliferation by 69%. The proliferation stimulation index (i.e., the ratio of PHA- stimulated PBMC proliferation/spontaneous PBMC proliferation) of cultures containing 100 µM resveratrol was very low in relation to the control, while the proliferation stimulation index values at resveratrol concentrations of 10 µM and 100 nM were similar and slightly higher (without statistical significance), respectively. Resveratrol strongly inhibited PHA-stimulated interferon (IFN)-γ and TNFα release from PBMC at a concentration of 100 µM, but not concentrations of 10 µM or 100 nM. The concomitant immune effects of resveratrol on PBMC proliferation and release of IFN-γ and TNFα may be explained by an inhibitory effect on transcription factor NF-ÎB.
C 1p. Suppression of angiogenesis, invasion and metastasis by resveratrol
Angiogenesis is a process of blood vessel formation that is mediated through modulation of proliferation and gene expression by endothelial cells. This process plays an essential role in tumor growth, other diseases and wound healing. Several studies have examined the effects of resveratrol on endothelial cells and on angiogenesis [194, 218, 237-241, 243-246, 286]. Szende et al. examined the effect of resveratrol on endothelial cells and showed that low doses (0.1-1 µg/ml) of resveratrol enhanced HUVEC proliferation, while higher doses (10-100 µg/ml) induced apoptosis and decreased mitotic activity, which is reflected in changes of cell number [237]. Igura et al. found that resveratrol inhibited the growth of bovine aorta endothelial (BAE) cells in a concentration-dependent manner (6-100 µM) [238]. The migration of BAE was obviously inhibited by resveratrol. When the lengths of all tubes constructed in the 3-dimensional culture system with or without resveratrol were measured, resveratrol was found to inhibit tube formation by BAE cells. Hsieh et al. found that resveratrol induced NOS in cultured pulmonary artery endothelial cells, which inhibited the proliferation of cells, correlated with suppression of cell progression through S- and G2-phases of the cell-cycle, and was accompanied by an increase in the expression of protein p53 and elevation of the level of Vdk inhibitor p21Cip1/WAF1 [194]. Using bovine pulmonary artery endothelial cells, Bruder et al. found an increase in NOS expression that led to morphological and structural changes [239]. Lin et al. investigated the mechanism by which resveratrol inhibited vascular endothelial growth factor (VEGF)-induced angiogenic effects in HUVECs [240] and showed that resveratrol, at the dose of 1 or 2.5 µM, effectively abrogated VEGF-mediated tyrosine phosphorylation of vascular endothelial (VE)-cadherin and its complex partner, β-catenin. This inhibitory effect of resveratrol reflected on the retention of VE-cadherin at cell-cell contacts as demonstrated by immunofluorescence. They showed that VEGF stimulated an evident increase of peroxide, which was strongly attenuated by resveratrol. Their data suggested that resveratrol inhibition of VEGF- induced angiogenesis was mediated by disruption of ROS- dependent Src kinase activation and the subsequent VE- cadherin tyrosine phosphorylation.
Abou-Agag et al. showed that resveratrol increased tissue- type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) gene transcription in cultured human endothelial cells [241]. Resveratrol yielded increases in tPA and uPA antigen levels (two- to three-fold) and mRNA levels (3- to 4-fold) and correlated increases (2- to 3-fold) in sustained (24 h), surface-localized fibrinolytic activity. Used at concentrations present in human plasma following moderate wine consumption, resveratrol inhibited adhesion molecule expression by TNF-stimulated endothelial cells [286]. Resveratrol also significantly prevented cytokine-induced vascular leakage. Others have shown that resveratrol can stimulate K-Ca channels in endothelial cells, which may be the mechanism for its effect on the functional activities of endothelial cells [243]. Fulgenzi et al. showed that TNF- induced vascular permeability changes were inhibited by resveratrol, not only in vitro but also in vivo [244].
Proteolytic degradation of the extracellular matrix and tumor metastasis correlate with expression of endopeptidases known as MMPs. The Expression of MMPs is regulated by cytokines and signal transduction pathways, including those activated by PMA. Woo et al. found that resveratrol significantly inhibited PMA-induced increases in MMP-9 expression and activity [218]. These effects of resveratrol were dose-dependent and correlated with suppression of MMP-9 mRNA expression. PMA caused about a 23-fold increase in MMP-9 promoter activity, which was suppressed by resveratrol. Transient transfection utilizing MMP-9 constructs, in which specific transcriptional factors were mutated, indicated that the effects of PMA and resveratrol were mediated via an AP-1 and NF-ÎB response element. Resveratrol inhibited PMA- mediated activation of JNK and PKC-¢. Brakenhielm et al. found that resveratrol suppressed angiogenesis, tumor growth and wound healing [245].
C 1q. Effect of resveratrol on bone cells
Bone formation is regulated by the balance between osteoclasts (bone-resorbing cells) and osteoblasts (bone- forming cells). Resveratrol has been reported to promote differentiation of murine MC3T3-E1 osteoblasts. Ulsperger et al. examined the effects of resveratrol on the increased proliferation of the human AHTO-7 osteoblastic cell line, induced by conditioned medium from a panel of carcinoma cell lines [247]. This compound was found to modulate AHTO-7 proliferation in a tamoxifen-sensitive mechanism at lower concentrations but, unlike vitamin D3, it failed to induce the osteoblast differentiation marker ALP. The proliferative response of AHTO-7 cells to conditioned medium from carcinoma cell lines were diminished (30- 71.4% inhibition) upon pretreatment with 0.5 µM resveratrol. The highest degree of inhibition was demonstrated for pancreas (BxPC3 and Panc-1), breast (ZR75-1) and renal (ACHN) carcinoma cell line supernatants, whereas the effects on colon carcinoma (SW620 and Colo320DM) cell-conditioned medium and prostate cancer (PC3, DU145 and LNCaP)-conditioned medium were less pronounced. Direct addition of resveratrol affected only the supernatants of cell lines (<25% inhibition) exhibiting growth-stimulatory activity for normal WI38 lung fibroblasts. Resveratrol inhibited proliferation of DU145 and LNCaP cells at concentrations exceeding 5 µM, altered cell-cycle distribution of all prostate cancer cell lines at concentrations as low as 0.5 µM, but did not inhibit the production of osteoblastic factors by these lines. Thus, resveratrol failed to induce ALP activity as a marker of osteoblast differentiation in human osteoblastic AHTO-7 cells, although it inhibited their response to osteoblastic carcinoma-derived growth factors at concentrations significantly lower than those needed to reduce the growth of cancer cells, thus effectively modulating tumor-osteoblast interaction.
Mizutani et al. found that resveratrol directly stimulated the proliferation and differentiation of osteoblastic MC3T3- E1 cells [278]. It also increased the ALP activity and prolyl hydroxylase activity of MC3T3-E1 cells. Moreover, the antiestrogen tamoxifen reversed these effects. On the other hand, resveratrol inhibited prostaglandin E2 production in MC3T3-E1 cells.
C 1r. Effects of resveratrol on expression of cytochrome P450 and metabolism of carcinogens
Many environmental compounds are carcinogenic only after metabolic activation. Exposure to carcinogens, such as polycyclic aromatic hydrocarbons (PAH), increases expression of the enzymes responsible for this activation. These enzymes consist of members of the cytochrome p450 (CYP) 1A and 1B subfamilies. They generate genotoxic epoxide metabolites of the parent aryl hydrocarbon, which can bind to DNA, forming adducts. These adducts, if not repaired, can cause specific mutations leading to cellular transformation. Therefore, the activity and expression of carcinogen-activating enzymes in chemically-induced carcinogenesis, and inhibition of their activity, either by direct enzyme inhibition or through modulation of their expression, is thought to be an important mechanism in the prevention of carcinogenesis.
The carcinogen activation pathway is regulated by the aryl hydrocarbon receptor (AhR), which further activates the enzymes CYP1A1 and CYP1A2 in microsomes. Different carcinogens are activated by different CYP. The carcinogen 7,12-dimethylbenz[a]anthracene (DMBA) is a classic hydrocarbon that is activated through the CYP enzymes CYP1B1, CYP1A1 and CYP1A2.
Resveratrol inhibits the phase I drug-activating enzymes such as CYP and increases the activity/level of phase II drug- detoxifying enzymes [73, 140, 248-258, 287, 288]. In human hepatic microsomes, resveratrol inhibits CYP isoenzymes, such as CYP1A1, CYP1B1 and CYP2B6, which are involved in the bioactivation of numerous carcinogens [248]. Chun et al. found that rhapontigenin (3,3',5-trihydroxy-4'-methoxystilbene) exhibited a potent and selective inhibition of human CYP1A1 with an IC50 of 0.4 µM. The values for Ki and K inactivation were 0.09 µM and 0.06 min -1, respectively, suggesting that rhapontigenin is a potent mechanism-based inactivator of human CYP1A1 [73]. Others showed that resveratrol inhibits CYP1A1 through an AhR-independent posttranscriptional pathway [140]. Ciolini et al. showed that resveratrol competitively inhibited, in a concentration-dependent manner, the activity of the carcinogen-activating enzymes CYP1A1 and CYP1A2 in microsomes [249]. Resveratrol inhibits aryl hydrocarbon-induced CYP1A activity in vitro, by directly inhibiting CYP1A1 and CYP1A2 enzymes activity and by inhibiting the signal transduction pathway that up-regulates the expression of carcinogen-activating enzymes. Chang et al. found that resveratrol differentially-inhibited human CYP1 enzymes and that this occurred through two distinct mechanisms: direct inhibition (mainly CYP1B1 and CYP1A1) and mechanism-based inactivation (CYP1A2) [250].
Chan et al. demonstrated that resveratrol inactivated CYP3A4 in a time- and NADPH-dependent manner [251]. Chang et al. found that resveratrol inhibited a substrate oxidation reaction catalyzed by human recombinant CYP3A4 and CYP3A5 in vitro [252]. That resveratrol is an irreversible (probably mechanism-based) inhibitor of CYP3A4 and a non- competitive reversible inhibitor of CYP2E1 has been demonstrated [248]. Yu et al. found that resveratrol inhibited CYP with IC50 values of 11.6 µM for CYP2C19 and 1.1 µM for CYP3A4, but the IC50 values exceeded 50 µM for all the other CYP isozymes, indicating no inhibition [288].
CYP1B1 is expressed in a number of human tissues in which cancers occur (e.g., prostate, ovary, uterus, mammary gland). CYP1B1 activates many environmental mutagens and also catalyzes the 4-hydroxylation of estrogens, considered to be an important step in hormonal carcinogenesis. The enzyme CYP1B1 is overexpressed in a wide variety of human tumors and catalyzes aromatic hydroxylation reactions. Chang et al. studied whether trans-resveratrol modulates the catalytic activity and gene expression of CYP1B1 and found that resveratrol decreased human recombinant CYP1B1-catalyzed 7-ethoxyresorufin O-dealkylation activity with an value of 1.4 µM [253]. Treatment of MCF-7 cells with 10 µM resveratrol decreased relative CYP1B1 mRNA levels after 5 h, indicating that resveratrol both inhibited the catalytic activity and suppressed the constitutive expression of the CYP1B1 gene. This may explain the protection against toxicity and carcinogenicity induced by compounds that undergo CYP1B1-catalyzed bioactivation. We report here that resveratrol undergoes metabolism by CYP1B1 to give a metabolite that has been identified as the known antileukemic agent piceatannol. This demonstrates that a natural dietary cancer preventive agent can be converted to a compound with known anticancer activity by an enzyme that is found in human tumors. This also provides evidence for the concept that CYP1B1 in tumors may be functioning as a growth suppressor enzyme.
Guengerich et al. examined the activities of several of the major allelic variants of human CYP1B1 and found that resveratrol is also an inhibitor of this enzyme [255]. Further studies with rhapontigenin and synthetic stilbenes led to the discovery of 2,4,3',5'-tetramethoxystilbene, a selective inhibitor of CYP1B1 relative to other CYP enzymes. Inhibition is competitive, with a Ki value of 3 nM, and the inhibitor is resistant to metabolism. In addition to blocking 17-‚-estradiol 4-hydroxylation, this stilbene also inhibited the activation of heterocyclic amines to mutagens. 2,4,3',5'- tetramethoxystilbene also suppressed expression of CYP1B1 and growth of human mammary tumor cells. 3,3',4',5,5'- pentamethoxystilbene was a selective inhibitor of CYP1A1, showing mixed inhibition, and also suppressed CYP1A1 expression in HepG2 cells.
Dubuisson et al. investigated the effects of resveratrol on DNA binding via esterification reactions with 2- hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine (N- OH-PhIP) - a metabolite of a mammary gland carcinogen present in cooked meats [256]. Treatment of primary cultures of human mammary epithelial cells with 50 µM resveratrol led to decreases in PhIP-DNA adducts ranging from 31% to 69%. Resveratrol inhibited PhIP-DNA adduct formation by O-acetyltransferase and sulfotransferase catalysis and suppressed O-acetyltransferase and sulfotransferase activities from the breast cancer cell lines MCF-7 and ZR-75-1. It also stimulated ATP-dependent cytosolic activation of N-OH- PhIP in all human samples, but not in mouse liver samples.
Moreover, resveratrol increased the activity of NQO, a detoxifying enzyme for quinone-containing substances [182].
C 1s. Suppression of inflammation by resveratrol
Numerous lines of evidence suggest that resveratrol is a potent anti-inflammatory agent. As already described, resveratrol can suppress the activation of transcription factor NF-ÎB, which is closely linked with inflammation. It can also suppress the expression of proinflammatory cytokines such as TNF, IL-1, IL-6 and IL-8 [211, 232-236]. Resveratrol can abrogate the expression of proteins such as iNOS, COX-2 and 5-LOX, that mediate inflammation. Kimura et al. showed that resveratrol inhibits the 5-LOX products 5-hydroxy-6,8,11,14- eicosatetraenoic acid (5-HETE) 5,12-dihydroxy-6,8,10,14- eicosatetraenoic acid (5,12-diHETE) and leukotriene C4 (LTC4) at IC50 of 8.9 µM, 6.7 µM, and 1.37 µM, respectively [259]. The IC50 of 5-HETE, 5,12-diHETE and LTC4 formations of synthetic 3,3',4-trihydroxystilbene were 5.9 µM, 6.3 µM and 8.8 µM, respectively. Moreover, they inhibited the release of lysosomal enzymes such as lysozyme and ‚-glucuronidase induced by calcium ionophore A 23187 from human polymorphonuclear leukocytes (PMN). In another study, these workers examined the effects of various stilbenes (i.e., 3,4',5-trihydroxystilbene, 3,4',5-trihydroxystilbene 3-O-D- glucoside, and 2,3,4',5-tetrahydroxystilbene 2-O-D-glucoside) on COX and LOX activities in rat PMN [260]. Resveratrol inhibited the 5-LOX product, 5-HETE, and the COX products, HHT and thromboxane B2, at IC50 of 2.72 µM for 5-HETE, 0.7 µM for HHT and 0.8 mM for thromboxane B2. Piceid (3,4',5-trihydroxystilbene 3-O-D-glucoside) and 2,3,4',5- tetrahydroxystilbene 2-O-D-glucoside also inhibited the formation of 5-HETE, HHT and thromboxane B2, although less strongly. Their IC50 values were, respectively, 55.3± 15.3 µM and >1000 µM for 5-HETE, 196 µM and 300 µM for HHT, and 251 µM and 366 µM for thromboxane B2.
The expression NAG-1, a member of the TGF-β superfamily, has been shown to be associated with pro-apoptotic and antitumorigenic activities. Baek et al. demonstrated that resveratrol induced NAG-1 expression and apoptosis in a concentration-dependent manner [198]. Resveratrol increases the expression of the tumor suppressor protein p53 prior to NAG-1 induction, indicating that induction of NAG-1 expression by resveratrol is mediated by p53 expression. These authors also showed that the p53-binding sites within the promoter region of NAG-1 play a pivotal role in controlling induction of NAG-1 expression by resveratrol.
Resveratrol exerted a strong inhibitory effect on the superoxide radical (O2*) and H2O2 produced by macrophages stimulated by LPS or PMA. Resveratrol also significantly decreased 3H-arachidonic acid release induced by LPS and PMA or by exposure to O2* or H2O2. Resveratrol treatment caused a significant impairment of COX-2 induction stimulated by LPS and PMA or by O2* or H2O2 exposure. These resveratrol effects were correlated with a marked reduction of prostanglandin synthesis. These results indicate that the anti-inflammatory action of resveratrol affects arachidonic acid mobilization and COX-2 induction.
Huang et al. examined the anti-inflammatory activity of resveratrol tetramers amurensins I-L, (+)-hopeaphenol, isohopeaphenol, vitisin A, (+)-vitisifuran A and heyneanol A [261]. Among them, (+)-hopeaphenol, isohopeaphenol, vitisin A, (+)-vitisifuran A, and heyneanol A potently inhibited biosynthesis of leukotriene B4, and amurensins I and L strongly antagonized the histamine acceptor. Chung et al. examined the anti-inflammatory activity of α-viniferin, a trimer of resveratrol, in an animal model of carrageenin- induced paw edema, and its inhibitory effects on COX and iNOS [222]. α-viniferin, at doses >30 mg/kg (p.o.) or >3 mg/kg (i.v.), had significant anti-inflammatory activity on this edema in mice and an inhibitory effect on COX-2 activity (IC50, 4.9 µM), but a very weak inhibitory effect on COX-1 (55.2±2.1% of the control [100%] at 100 µM). At doses of 3- 10 µM, α-viniferin inhibited synthesis of the COX-2 transcript in LPS-activated Raw 264.7 murine macrophages. α-Viniferin inhibited NO production in LPS-activated Raw 264.7 cells at in IC50 of 2.7 µM when α-viniferin and LPS were administered simultaneously, but did not inhibit NO production when α-viniferin was administered 12 h after LPS. α-viniferin inhibited synthesis of the iNOS transcript with an IC50 of 4.7 µM. The inhibitory effect of α-viniferin on the release of prostanoids and NO may provide important evidence of its anti-inflammatory action.
C 1t. Anti-oxidant effects of resveratrol
Numerous lines of evidence suggest that resveratrol exterts anti-oxidant activity [71, 262-276]. Jang et al. found that resveratrol was a potent inhibitor of ROS production in both unopsonized zymosan-stimulated RAW 264.7 cells (IC50, 17 µM) and in human monocytes (IC50, 18 µM) and neutrophils (IC50, 23 µM) [262]. 3,5-Dihydroxy-4'-methoxystilbene and 3,4'-dimethoxy-5-hydroxystilbene exhibited IC50 values of 63 and 73 µM in RAW 264.7 cells, 51 and >100 µM in human monocytes, and 10 and 37 µM in human neutrophils. Trimethylresveratrol, piceid and 3,5- dihydroxy-4'-methoxystilbene-3-O-β-D-glucoside were weak inhibitors of ROS production. Resveratrol’s potent inhibitory action on ROS production might be one biochemical mechanism related to its anti-inflammatory and anticarcinogenic activities. The number and position of hydroxy substituents in resveratrol analogues seems to play an important role in the potency of their inhibition of ROS production. Burkitt et al. provided evidence for hydroxyl- radical scavenging and a novel, glutathione-sparing mechanism of action for resveratrol [263]. Resveratrol strongly inhibited NADPH- and ADP-Fe3+-dependent lipid peroxidation at the initial and propagation stages [264]. Moreover, phenolic stilbenes inhibited UV-induced lipid peroxidation and efficiently scavenged 2,2'-azobis-(2- amidinopropane)-dihydrochloride peroxyl radicals [264]. Tadolini et al. found that resveratrol inhibited more efficiently than either the hydrophilic analogue of vitamin E, Trolox, or vitamin C ascorbate the Fe2+-catalyzed lipid hydroperoxide-dependent peroxidation of sonicated phosphatidylcholine liposomes [265]. They also showed that resveratrol inhibited lipid peroxidation mainly by scavenging lipid peroxyl radicals within the membrane, like vitamin E. Although resveratrol is less effective, its capacity to spontaneously enter the lipid environment confers on it great anti-oxidant potential.
By using the Rancimat method and the 2, 2-diphenyl-1- picryhydrazyl (DPPH) free radical scavenging model, Wang et al. found that 3, 3',4,5'-tetrahydroxystilbene, 3,3',4,5,5'- pentahydroxystilbene and 3,4,4',5-tetrahydroxystilbene were more active than resveratrol [266]. A dimer of resveratrol was identified as the major radical reaction product when resveratrol was reacted with DPPH radicals. Murcia et al. compared the anti-oxidant activities of resveratrol and several other agents and found that the abilities to scavenge hypochlorous acid (HOCl) were, in decreasing order, propyl gallate > resveratrol > vitamin E > phenol [267]. Resveratrol (6.25-100 µg/ml) also has been shown to inhibit chemiluminescence and the generation of O2- in blood platelets [268]. It has an inhibitory effect on the production of ROS and thiobarbituric acid-reactive substances (TBARS) in platelets induced by LPS or thrombin. Isorhapontigenin, isolated from Belamcanda chinensis, is a derivative of stilbene whose chemical structure is very similar to that of resveratrol and has a potent anti-oxidant effect. Stojanovic et al. examined the efficiency and mechanism of the anti-oxidant activity of trans-resveratrol and its analogues in radical liposome oxidation [269]. They showed that the para-hydroxyl group of trans-resveratrol had greater radical-scavenging activity than its meta-hydroxyl groups. This was apparently confirmed by pulse radiolysis studies of the reactions of trans-resveratrol and its analogues with trichloromethylperoxyl radicals, CCl3OO*, which showed that the spectral and kinetic properties of the observed transients were very similar in trans-resveratrol and trans-4-hydroxystilbene reactions.
Belguendouz et al. found that trans-resveratrol, which is by far the most potent chelator of copper, does not chelate iron [270]. They also found that resveratrol protected low- density lipoprotein (LDL) against peroxidative degradation, by both chelating and free radical scavenging mechanisms. Some reports, however, suggest that resveratrol can also act as a pro-oxidant [264]. Martinez et al. showed that resveratrol exerts a strong inhibitory effect on O2- and H2O2 produced by macrophages stimulated by LPS or PMA [271]. Resveratrol also significantly decreased 3H-arachidonic acid release induced by LPS and PMA or by exposure to O2- or H2O2 and significantly impaired the COX-2 induction stimulated by LPS and PMA or by O2- or H2O2 exposure. These effects were correlated with a marked reduction in prostaglandin synthesis. These results indicate that the anti- oxidant action of resveratrol affects arachidonic acid mobilization and COX-2 induction.
C 1u. Suppression of transformation by resveratrol
Some reports suggest that resveratrol can suppress the transformation of cells. Huang et al. found that resveratrol suppressed cell transformation and induced apoptosis through a p53-dependent pathway [193]. Resveratrol suppressed tumor promoter-induced cell transformation and markedly induced apoptosis, the transactivation of p53 activity, and expression of p53 protein in the same cell line and at the same dosage. Also, resveratrol-induced apoptosis occurs only in cells expressing wild-type p53 (p53+/+), not in p53-deficient (p53-/-) cells, while apoptosis induction is no different in normal lymphoblasts and sphingomyelinase- deficient cell lines.
She et al. investigated the effect of resveratrol and its structurally related derivatives on EGF-induced cell transformation [226]. Their results provided evidence that one of the resveratrol derivatives exerted a more potent inhibitory effect than resveratrol on EGF-induced cell transformation but had less cytotoxic effects on normal nontransformed cells. The resveratrol derivative caused cell-cycle arrest in the G1-phase but, unlike resveratrol, did not induce p53 activation and apoptosis. Furthermore, this compound, unlike resveratrol, markedly inhibited EGF-induced phosphoinositide 3-kinase (PI3K) and Akt activation. Collectively, these data suggest that resveratrol derivative’s antitumor effect may be mediated through a different mechanism, by mainly targeting PI3K/Akt signaling pathways.
C 1v. Induction of cellular differentiation by resveratrol
Evidence that resveratrol is a differentiation–inducing agent has been reported in certain cell types [277-279]. Using the human erythroleukemic K562 cell line as an in vitro model, Rodrigue et al. showed that 50 µM of resveratrol induced greater hemoglobin production (7-fold) than 500 µM of hydroxyurea (3.5-fold) [277]. This erythroid differentiation was linked to the inhibition of cell proliferation associated with an equivalent increased expression of p21Cip1/WAF1 mRNA, but with the level of p21Cip1/WAF1 protein increased to a greater extent (6-fold) for cells treated with resveratrol than for those treated with hydroxyurea (1.5-fold). They also showed that 50 µM of resveratrol and 25 µM of hydroxyurea induced variable,but similar, enhancements of fetal hemoglobin synthesis in cultured erythroid progenitors for the majority of the sickle cell patients studied. These inductions were linked to, but not correlated with, variable decreases in erythroid burst-forming unit clone number. Mizutani et al. examined the effect of resveratrol on the proliferation and differentiation of osteoblastic MC3T3-E1 cells and found that it increased DNA synthesis [278]. In addition, resveratrol increased the ALP activity and prolyl hydroxylase activity of MC3T3-E1 cells. Moreover, the antiestrogen tamoxifen reversed resveratrol’s stimulation of proliferation and ALP activity in these cells. On the other hand, resveratrol inhibited prostaglandin E2 production in MC3T3-E1 cells. These results indicate that resveratrol directly stimulates the cell proliferation and differentiation of osteoblasts.
Wang et al. examined the effect of resveratrol on cell growth, differentiation and death in human medulloblastoma Med-3, UW228-1, -2 and -3 cell lines [279]. The results demonstrated that resveratrol could suppress growth, promote differentiation and commit its target cells to apoptosis in time- and dose-related fashions. Fas was constitutively expressed, but FasL was undetectable in the four lines in spite of resveratrol treatment. Anti-Fas antibody neither inhibited growth nor induced apoptosis of the cell lines. Up-regulated caspase-3 was found in resveratrol-treated populations and the appearance of its cleaved form was closely associated with the apoptotic event.
C 1w. Estrogenic/anti-estrogenic effects of resveratrol
Resveratrol has a structural similarity to diethylstilbestrol, a synthetic estrogen. Whether it is an estrogen agonist or antagonist is highly controversial. Some reports suggest that resveratrol has estrogenic activity, while others show no such effects [132, 174, 185, 280-284, 289]. Gehm et al. found that, at concentrations comparable to those required for its other biological effects (~3-10 µM), resveratrol inhibited the binding of labelled estradiol to the ER and activated the transcription of estrogen-responsive reporter genes transfected into human breast cancer cells [280]. This transcriptional activation was ER-dependent, required an estrogen response element in the reporter gene, and was inhibited by specific estrogen antagonists. In some cell types (e.g., MCF-7 cells), resveratrol functioned as a superagonist (i.e., produced a greater maximal transcriptional response than estradiol), whereas in others it produced an activation equal to or less than that of estradiol. Resveratrol also increased the expression of native estrogen-regulated genes, and it stimulated the proliferation of estrogen-dependent T47D breast cancer cells. The authors concluded that resveratrol is a phytoestrogen and that it exhibits variable degrees of ER agonism in different test systems.
Turner et al. examined the estrogenic activity of resveratrol in vivo and found that resveratrol treatment had no significant effect on body weight, serum cholesterol level, radial bone growth, epithelial cell height, or mRNA levels for insulin-like growth factor I [281]. These results, in contrast to those of prior in vitro studies, suggest that resveratrol has little or no estrogen agonism on reproductive and non- reproductive estrogen target tissues and may be an estrogen antagonist. Lu et al. showed that resveratrol inhibited the growth of ER-positive MCF-7 cells in a dose-dependent fashion [132]. Detailed studies with MCF-7 cells demonstrated that resveratrol antagonized the growth- promoting effect of 17-β-estradiol at both the cellular (cell growth) and the molecular (gene activation) levels. At a concentration of 5 µM, resveratrol abolished the growth- stimulatory effect mediated by concentrations of 17-β- estradiol as high as 1 nM. The anti-estrogenic effect of resveratrol could be observed at concentrations of 1 µM and higher. This effect was also demonstrated at the molecular level. Resveratrol antagonized, in a dose-dependent fashion, the stimulation by 17-β-estradiol of PR gene expression in MCF-7 cells. Moreover, expression of TGF-α and insulin-like growth factor-I receptor mRNAs were inhibited, while expression of TGF-β2mRNA was significantly elevated in MCF-7 cells cultivated in the presence of resveratrol (10 µM). These results show that resveratrol, a partial ER agonist itself, acts as an ER antagonist in the presence of estrogen, leading to inhibition of human breast cancer cells.
Bhat et al. characterized the estrogen-modulatory effects of resveratrol in a variety of in vitro and in vivo mammary models [185]. The effects of resveratrol alone, and in combination with 17-β-estradiol, were assessed in MCF-7, T47D, LY2 and S30 mammary cancer cell lines. In transient transfection studies in MCF-7 cells, resveratrol showed a weak estrogenic response, but when resveratrol was combined with 17-β-estradiol (1 nM), a clear dose- dependent antagonism was observed. Similar mixed estrogenic/anti-estrogenic effects were noted in S30 cells, whereas resveratrol functioned as a pure estrogen antagonist in T47D and LY2 cells. In MCF-7 cells, furthermore, resveratrol induced PR protein expression but, when resveratrol was combined with 17-β-estradiol, expression of PR was suppressed. With T47D cells, resveratrol significantly down-regulated the steady-state and 17-β-estradiol-induced levels of PR. In LY2 and S30 cells, resveratrol down-regulated presnelin 2 protein expression. In the mouse mammary organ culture model, resveratrol induced PR when administered alone, but suppressed the expression of PR in the presence of 17-β-estradiol (1 nM). Furthermore, resveratrol inhibited the formation of estrogen- dependent preneoplastic ductal lesions induced by DMBA in these mammary glands (IC50, 3.2 µM) and reduced N-methyl- N-nitrosourea-induced mammary tumorigenesis when administered to female Sprague-Dawley rats by gavage. In the absence of 17-β-estradiol, therefore, resveratrol exerts mixed estrogen agonist/antagonist activities in some mammary cancer cell lines, but in the presence of E2, resveratrol functions as an anti-estrogen.
In rodent models, carcinogen-induced preneoplastic lesions and mammary tumors are inhibited by resveratrol. Bhat et al. showed that treatment of cultured human endometrial adenocarcinoma (Ishikawa) cells with resveratrol (concentrations as high as 10 µM) did not significantly increase the levels of the estrogen-inducible marker enzyme ALP [174]. On the contrary, when ALP was induced by treatment with 1 nM of 17-β-estradiol, resveratrol exhibited a decrease in activity (IC50, 2.3 µM). Furthermore, when Ishikawa cells were treated with resveratrol alone, estrogen-inducible PR was not enhanced, and PR expression induced by treatment with 17-β-estradiol was inhibited by resveratrol in a dose-dependent fashion at both the mRNA and protein levels. Moreover, resveratrol mediated the suppression of a functional activity of PR as demonstrated by down-regulation of α1-integrin expression induced by 17-β-estradiol plus progesterone. In transient transfection experiments conducted with Ishikawa cells, anti-estrogenic effects were confirmed by dose-dependent inhibition of the 17-β-estradiol-induced estrogen response element-luciferase transcriptional activity. Resveratrol showed no discernable activity with ER-α, but with ER-‚ 17-β-estradiol was displaced with an IC50of 125 µM. However, ER-α but not ER-‚mRNA and protein expression were suppressed in Ishikawa cells by resveratrol in the concentration range of 5-15 µM. In the presence or absence of 17-β-estradiol, resveratrol inhibited Ishikawa cell proliferation in a time-dependent manner with cells accumulating in the S-phase of the cell-cycle in ≤48 h. This effect was reversible. Analysis of some critical cell-cycle proteins revealed a specific increase in expression of cyclins A and E, but a decrease in Cdk2. These data suggest that resveratrol exerts an antiproliferative effect in Ishikawa cells, and that the effect may be mediated by both estrogen- dependent and -independent mechanisms.
Basly et al. examined the estrogenic/anti-estrogenic and scavenging properties of (E)- and (Z)-resveratrol [282]. They found that both isomers increased the in vitro growth of MCF-7 cell lines at concentrations of 10-25 µM, whereas 0.1-1 µM had no effect and 50 µM decreased cell growth and was cytotoxic. The 25 µM (E)-isomer alone was able to reduce the proliferation induced by the estradiol. Low concentrations of (E)- and (Z)-resveratrol (0.1-1 µM) and moderate concentrations of (Z)-resveratrol (10 µM) did not interfere with the ER, whereas moderate concentrations of (E)-resveratrol (10 and 25 µM) and a somewhat higher concentration of (Z)-resveratrol (25 µM) both functioned as superagonists of estradiol. Bowers et al. showed that resveratrol acts as a mixed agonist/antagonist for ER-α and ERβ‚ [283].
Recent data have indicated that the ER-α, through interaction with p85, regulates PI3K activity, revealing a physiological, non-nuclear function potentially relevant in cell proliferation and apoptosis. Pozo-Guisado et al. recently showed that resveratrol modulates the PI3K pathway through an ER-α-dependent mechanism [289]. They found that resveratrol increased ER-α-associated PI3K activity with a maximum stimulatory effect at concentrations close to 10 µM; concentrations >50 µM decreased PI3K activity. The stimulation of PI3K activity by resveratrol was ER-α- dependent, since it could be blocked by the antiestrogen ICI 182,780. Resveratrol did not affect p85 protein expression but induced the proteasome-dependent degradation of ER-α.
C 1x. Effect of resveratrol on normal cells
Resveratrol appears to affect the proliferation not only of tumor cells but also of normal cells. The proliferation of keratinocytes [290], smooth muscle cells (SMC) [188, 197, 291], and endothelial cells [194, 237, 238, 245] is suppressed by resveratrol. The proliferation of normal human PBMC, however, was unaffected by resveratrol [292]. Holian et al. evaluated the viability and proliferation of cultured normal human keratinocytes exposed to resveratrol [290]. They found that resveratrol, even at submicromolar concentrations, inhibits the proliferation of these keratinocytes in vitro and, at higher concentrations, is cytotoxic to these cells.
Zou et al. investigated the effects of resveratrol on the proliferation and cell-cycle control of cultured SMC [188]. Resveratrol reduces SMC proliferation in a dose-dependent manner, with concentrations of 50-100 µM resveratrol resulting in 70-90% reduction of SMC proliferation induced by such diverse mitogens as serum, endothelin and platelet- derived growth factor (PDGF). The antimitogenic effects of resveratrol are not mediated by the induction of apoptosis, but appear to relate to a G1/S-phase block in the cell-cycle. Mnjoyan et al. found that resveratrol inhibited the growth of human aortic VSMC at concentrations as low as 1 µM, as indicated by inhibition of DNA synthesis and increased intracellular p53 and p21Cip1/WAF1 levels, and effectively blocked the cell-cycle progression of serum-stimulated VSMC [291]. Intriguingly, however, high concentrations of resveratrol could not induce apoptosis in quiescent VSMC. These differential biological effects of resveratrol on quiescent and proliferating VSMC suggest that resveratrol may be capable of selectively eliminating abnormally proliferating VSMC of the arterial walls in vivo. Haider et al. showed that resveratrol led to reversible arrest in early S-phase of VSMC, accompanied by the accumulation of hyperphosphorylated Rb [197]. In contrast to findings in other cell systems, resveratrol decreases the cellular levels of the Cdk inhibitors p21Cip1/WAF1 and p27Kip1. This is of particular interest because phosphorylated p53 protein (serine-15) is strongly enhanced by this substance. Importantly, the observed S-phase arrest was not linked to an increase in apoptotic cell death: there were no detectable increases in apoptotic nuclei or in levels of the proapoptotic protein Bax.
Lu et al. synthesized a number of polyhydroxy- and polymethoxy-stilbenes and tested their antiproliferative effects in normal and transformed human cells [51]. They showed that one of the resveratrol analogues, 3,4,5,4'- tetrahydroxystilbene (R-4), specifically inhibited the growth of SV40 virally-transformed WI38 cells (WI38VA) at a concentration of 10 µM, but had no effect on normal WI38 cells at even higher concentrations. R-4 also prominently induced apoptosis in WI38VA cells, but not in WI38 cells. An RNase protection assay showed that R-4 significantly induced the expression of p53, GADD45 and Bax genes and concomitantly suppressed expression of the Bcl-2 gene in WI38VA, but not in WI38 cells. A large increase in p53 DNA-binding activity and the presence of p53 in the Bax promoter binding complex suggested that p53 was responsible for the Bax gene expression induced by R-4 in transformed cells. Within 4 h of treatment with R-4, the Bax to Bcl-2 protein ratios in WI38 and WI38VA cells were, respectively, 0.1 and 105, a difference of three orders of magnitude. While R-4 prominently induced the p53/Bax pro-apoptotic genes, it also concomitantly suppressed the expression of COX-2 in WI38VA cells. Taken together, these findings suggest that induction of the p53 gene by R-4 in transformed cells may play a key role in the differential growth inhibition and apoptosis of transformed cells.
Cavallaro et al. investigated the effect of resveratrol on some activities of PBMC, particularly generation of the superoxide anion O2- in whole blood, HOCl and NO production by isolated cells, and chemotaxis [292]. Resveratrol had significant effects on all these activities. In particular, it inhibited O2- generation in stimulated, but not in resting, neutrophils and decreased HOCl much more than O2- production, indicating an effect on myeloperoxidase secretion, since HOCl production is directly and proportionally dependent on O2- generation and reduced cell motility. The small dose of resveratrol (4.38 nM) used is attainable by consuming a diet that includes red wine and vegetables, confirming its protective role against some pathological processes such as inflammation, coronary heart disease and cancer.
Losa et al. examined the effect of resveratrol on apoptosis and the oxidative metabolic status of normal human PBMC isolated ex vivo from healthy donors [293]. Neither apoptotic nor oxidative parameters were affected by culturing PBMC in medium containing resveratrol at concentrations as high as 20 µM for 5 days, while the frequency of cells with intermediate permeability to propidium iodide (17%) increased at a concentration of 50 µM. Thus resveratrol was slightly toxic, but there was little apoptosis in these cells. PBMC were also grown, first in medium plus resveratrol for 24 h, and then for 96 h in medium containing resveratrol plus 10 mM of oxidant 2-deoxy-D-ribose, an oxidant sugar that is apoptogenic in human lymphocytes. The apoptotic changes triggered by 2-deoxy-D-ribose were counteracted by the phytoalexin in a dose-dependent manner, but resveratrol activity was absent at the lowest concentration (5 µM) and significantly reduced at the highest concentration used (50 µM). In PBMCs co-incubated with 20 µM of resveratrol and 10 mM of 2-deoxy-D-ribose, the anti-oxidant effect of resveratrol manifested with significant reductions of caspase-3, -8, γ-glutamyltransferase, and glutathione-S-transferase activities and intracellular lipid peroxidation content.
C 1y. Suppression of mutagenesis by resveratrol
Numerous reports suggest that resveratrol exerts chemopreventive activities. The suppression of mutagenesis is one line of evidence in this direction. Sgambato et al. evaluated the antiproliferative activity of resveratrol on a panel of cell lines of various histogenetic origins, including normal rat fibroblasts, mouse mammary epithelial cells and human breast, colon and prostate cancer cells [294]. They found that resveratrol induced significant increases in the apoptotic index, reductions in the percentage of cells in the G2/M-phase, inhibition of increases in ROS following exposure to oxidative agents (e.g., tobacco-smoke condensate and H2O2), and reduced nuclear DNA fragmentation, as assessed by single cell gel electrophoresis (comet test), suggesting that resveratrol can act as an antimutagenic/anticarcinogenic agent by preventing oxidative DNA damage, which plays a pivotal role in the carcinogenic activity of many genotoxic agents.
Uenobe et al. showed that resveratrol had a suppressive effect on umu gene expression of the SOS response induced by 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1) in Salmonella typhimurium [295]. Revel et al. showed that B[a]P damaged sperm through AhR activation, phase I enzyme induction, DNA adduct formation and increased germ cell apoptosis in the testis, and that resveratrol could prevent these adverse effects. [296]. B[a]P significantly increased apoptosis, and this effect was abrogated by resveratrol. Thus B[a]P caused increased sperm cell B[a]P diol epoxidite (BPDE) DNA adduct formation and apoptosis in the mouse. The natural AhR antagonist resveratrol diminished B[a]P-induced DNA adducts and apoptosis in seminiferous tubules. Matsuoka et al. tested the genotoxicity of resveratrol in a bacterial reverse mutation assay, an in vitro chromosome aberration test, an in vitro micronucleus test and sister chromatid exchange test [169]. They found that resveratrol may preferentially induce sister chromatid exchange but not chromosome aberration, that is, it may cause S-phase arrest only when sister chromatid exchanges are induced.
Resveratrol was recently shown to induce strand breakage in DNA in the presence of copper ions. Ahmad et al. showed that resveratrol catalyzed the reduction of Cu(II) to Cu(I), which is accompanied by formation of "oxidized product(s)" of resveratrol, which in turn also appear to catalyze the reduction of Cu(II) [297]. Strand scission by the resveratrol-Cu(II) system was found to be biologically active, as assayed by bacteriophage inactivation. Fukuhara et al. demonstrated DNA cleavage by resveratrol, as indicated by relaxation of pBR322 in the presence of Cu2+ [298]. They provided evidence that resveratrol is capable of binding to DNA, and that the Cu2+-dependent DNA damage is more likely to be caused by a copper-peroxide complex than by a freely diffusible oxygen species.
C 1z. Radioprotective and radiosensitive effects of resveratrol
Various reports during the last few years have suggested that radioresistance is induced by the activation of NF-ÎBand NF-ÎB-regulated gene products such as COX-2 and 5-LOX [299, 300]. Inhibitors of NF-ÎB, COX-2, and 5-LOX have been shown to induce radiosensitivity [301-303]. Because resveratrol has also been shown to down-regulate NF-ÎB [201], COX-2 [141] and 5-LOX [227], it is possible that resveratrol will induce radiosensitization. Prostaglandins, products of COX-2, have been implicated in the cytotoxic and/or cytoprotective response of tumor cells to ionizing radiation. Using clonogenic cell survival assays, Zoberi et al. showed that HeLa and SiHa cell killing was enhaced by pretreatment with resveratrol prior to ionizing radiation exposure, and that this pretreatment induced an early S-phase cell-cycle checkpoint arrest [186]. These results suggest that resveratrol alters both cell-cycle progression and the cytotoxic response to ionizing radiation.
C 1aa. Chemosensitization by resveratrol
Several mechanisms of chemoresistance have been described. Some reports during the last few years have suggested that chemoresistance is induced by the activation of NF-ÎB and NF-ÎB-regulated gene products such as COX-2 and 5-LOX [299, 300]. Inhibitors of NF-ÎB, COX-2 and 5-LOX have been shown to induce radiosensitivity [301- 303]. Because resveratrol has also been shown to down-regulate NF-ÎB [201], COX-2 [141] and 5-LOX [227], it is possible that resveratrol will induce chemosensitization. Kubota et al. studied the in vitro biological activity of resveratrol by examining its effect on the apoptosis induced by taxol in lung cancer cell lines A549, EBC-1 and Lu65 [304]. Although simultaneous exposure to resveratrol plus taxol did not result in significant synergy, treatment with resveratrol (10 µM, 3 days) significantly enhanced the subsequent antiproliferative effect of taxol. The same resveratrol treatment similarly enhanced the subsequent apoptotic effects of taxol: when given prior to taxol, it induced p21Cip1/WAF1 expression approximately 4-fold. These results suggest that lung cancer cells exposed to resveratrol have a lowered threshold for killing by taxol.
Survivin is an inhibitor of apoptotic proteins, that is expressed at high levels in most human cancers and may facilitate evasion from apoptosis and aberrant mitotic progression. Fulda et al. discovered that resveratrol is a potent sensitizer of tumor cells to TRAIL-induced apoptosis through p53-independent induction of p21Cip1/WAF1 and p21Cip1/WAF1-mediated cell-cycle arrest associated with survivin depletion [305]. Concomitant analysis of cell-cycle, survivin expression and apoptosis revealed that resveratrol- induced G1-phase arrest was associated with down- regulation of survivin expression and sensitization for TRAIL-induced apoptosis. Importantly, resveratrol sensitized various tumor cell lines, but not normal human fibroblasts, for apoptosis induced by death receptor ligands or anticancer drugs. This combined sensitization with resveratrol as an induction (e.g., TRAIL) strategy may be a novel approach to enhancing the efficacy of TRAIL-based therapies in a variety of human cancers.
Nicolini et al. found that taxol induced apoptosis in the human neuroblastoma cell line SH-SY5Y [180]. Addition of trans-resveratrol to SH-SY5Y cultures exposed to taxol significantly reduced cellular death. Resveratrol is able to inhibit the activation of caspase-7 and degradation of PARP that occur in SH-SY5Y exposed to taxol.
Jazirehi and Bonavida found that resveratrol modified the expression of apoptotic regulatory proteins and sensitized non-Hodgkin's lymphoma and multiple myeloma cell lines to taxol-induced apoptosis [306]. Both resveratrol and taxol negatively-modulated tumor cell growth by arresting the cells at the G2/M-phase of the cell-cycle. Low concentrations of resveratrol exerted a sensitizing effect on drug-refractory non-Hodgkin's lymphoma and multiple myeloma cells to apoptosis induced by taxol. Resveratrol selectively down-regulated the expression of anti-apoptotic proteins Bcl-xL and myeloid cell differentiation factor-1 and up-regulated the expression of proapoptotic proteins Bax and Apaf-1. Combination of resveratrol with taxol had minimal cytotoxicity against quiescent and mitogenically stimulated human PBMC. Inhibition of Bcl-xL expression by resveratrol was critical for chemosensitization, and its functional impairment mimicked resveratrol-mediated sensitization to taxol-induced apoptosis. Inhibition of Bcl-xL expression by resveratrol was due to inhibition of the ERK1/2 pathway and diminished AP-1-dependent Bcl-xL expression.
Depending on the concentration, resveratrol may exhibit dual effects; potentiating the effect of cytokines and chemotherapeutic agents at higher concentrations and inhibiting them at lower concentrations. The protective/inhibitory effects at lower concentrations appear to be mediated through an anti-oxidant mechanism. Manna et al. showed that resveratrol abrogated TNF-induced cytotoxicity and caspase activation [201]. Similarily, Jang and Surh showed that resveratrol pretreatment attenuated H2O2-induced cytotoxicity, DNA fragmentation and intracellular accumulation of ROS, suggesting that resveratrol has the potential to prevent oxidative stress-induced cell death, owing to its anti-oxidant property [181]. Recently, Ahmad et al. provided evidence that exposure of human leukemia cells to low concentrations of resveratrol (4-8 µM) inhibited caspase activation, DNA fragmentation and translocation of cytochrome c induced by H2O2 or the anticancer drug C2, which is a purified photoproduct of MC540, vincristine, and daunorubicin [307]. They found that, at these concentrations, resveratrol induces an increase in intracellular superoxide and inhibits drug-induced acidification. Blocking the activation of the NADPH oxidase complex neutralized resveratrol-induced inhibition of apoptosis. Interestingly, decreasing intracellular superoxide with the NADPH oxidase inhibitor diphenyliodonium reversed the inhibitory effect of resveratrol on drug-induced H2O2 production.
C 1ab. Direct targets of resveratrol
From the preceding description, it is clear that resveratrol exhibits numerous biological activities. How resveratrol exhibits all these activities is not fully understood. Numerous molecules with which resveratrol physically interacts have been identified. These include PKC [139], PKD [127], SYK [151], 5-LOX [227], COX-2 [141], ER [132], AR [159], AhR [249], and CYP [308]. The in vitro efficiency of resveratrol was found to be due mainly to its capacity to chelate copper, although it also scavenges free radicals. Belguendouz et al. found resveratrol to associate with LDL in the order of their lipid content: high- density lipoprotein < LDL < very LDL [309]. Miura et al. found that resveratrol associated with and inactivated creatine kinase, alcohol dehydrogenase and cholinesterase [310]. Kitson et al. found that resveratrol inhibited alcohol dehydrogenase by binding to the aldehyde site on the enzyme [311]. Zhou et al. found that resveratrol bound and inhibited xanthine oxidase in vitro, and the binding was shown to be competitive with their Ki values of 9.7 µM [312]. Resveratrol competitively inhibits monoamine oxidase A with an IC50 of 26.6 µM and a Ki of 47.3 µM. Fontecave et al. showed that resveratrol bound and inhibited ribonucleotide reductase, which might have further applications as an antiproliferative or cancer chemopreventive agent in humans [313].
C 1ac. Immunomodulatory effects of resveratrol
Numerous reports suggest that resveratrol can modulate the immune system [126, 236, 259, 314-316]. Falchetti et al. evaluated the in vitro effects of resveratrol in three immune response models:i) development of cytokine-producing CD4+ and CD8+ T-cells induced by stimulation of PBMC with anti-CD3/anti-CD28;
ii) specific antigen-induced generation of CTL; and
iii) natural killer (NK) activity of PBMC [314].The results showed that in vitro exposure to resveratrol produces a biphasic effect on the anti-CD3/anti-CD28-induced development of IFN-γ-, IL2- and IL4-producing CD8+ and CD4+ T-cells, with stimulation at low resveratrol concentrations and suppression at high concentrations. Similarly, the compound was found to induce significant enhancement (at low concentrations) and suppression (at high concentrations) of both CTL and NK cell cytotoxic activities. On the whole, the results of the study indicate that resveratrol modulates several human immune cell functions and suggest that this activity may be related to its effects on cytokine production by both CD4+ and CD8+ T-cells.
Gao et al. investigated the effect of resveratrol on mitogen/antigen-induced proliferation of splenic lymphocytes, induction of CTL and lymphokine-activated killer (LAK) cells, and production of the cytokines IFN-γ, IL-2, TNFα and IL-12 [126]. They found that mitogen-, IL-2-, or alloantigen-induced proliferation of splenic lymphocytes and development of antigen-specific CTL were suppressed significantly at resveratrol concentrations of 25- 50 µM. LAK cells generated at similar concentrations were less sensitive to the suppressive effect of resveratrol. The suppression of cell proliferation and CTL generation by resveratrol was not only reversible, but in some cases the response (mitogen/IL-2-induced proliferation and CTL generation) was actually enhanced following pretreatment of cells with resveratrol. Resveratrol also inhibited the production of IFN-γ and IL-2 by splenic lymphocytes and production of TNFα and IL-12 by peritoneal macrophages. The inhibition of cytokine production by resveratrol was irreversible. Further, resveratrol blocked activation of NF-ÎB without affecting basal NF-ÎB activity. The latter result suggested that resveratrol inhibits cell proliferation, cell- mediated cytotoxicity and cytokine production, at least in part through inhibition of NF-ÎB activation. Gao et al. also compared the in vitro and in vivo effects of resveratrol on the development of various cell-mediated immune responses, including mitogen/antigen-induced T-cell proliferation, induction of CTLs, IL-2 induced LAK cells and cytokine production [315]. They found significant suppression (>90%) of mitogen/antigen-induced T-cell proliferation and development of allo-antigen specific CTLs in vitro with resveratrol at a concentration of 25 µM. Intragastric administration of resveratrol (2 mg daily) to mice for 4 weeks showed no effect on age-related gain in body weight, peripheral blood cell counts (WBC, RBC, or platelets), or the cellularity of bone marrow or spleen. The CD4+ and CD8+ T-cells in spleen or total colony-forming units in the marrow also remained unaffected by treatment with resveratrol. Spleen cells, which were stimulated in vitro after being removed from mice that had been administered resveratrol for 2 or 4 weeks, showed no significant change in IL-2- or concanavalin A-induced proliferation of T-cells or production of IL-2-induced LAK cells. Further, production of IFN-γ and IL-12 was not affected by the administration of resveratrol, but production of TNFα was reduced. Even when conducted entirely in vivo, treatment with resveratrol was found to only marginally reduce the allo-antigen-induced T-cell proliferation and generation of CTL in the draining lymph nodes. Thus, even though resveratrol strongly inhibits T-cell proliferation and production of cytolytic cells in vitro, oral administration of resveratrol for 4 weeks does not induce hematological or hematopoietic toxicity and only marginally reduces T-cell-mediated immune responses.
Rotondo et al. showed that resveratrol has a strong inhibitory effect on ROS produced by PMN stimulated with formyl methionyl leucyl phenylalamine (fMLP). Resveratrol prevented the release of elastase and ‚-glucuronidase by PMN stimulated with fMLP and C5a and also inhibited elastase and β-glucuronidase secretion and production of 5-LOX metabolites LTB4, 6-trans-LTB4 and 12-trans-epi- LTB4 by PMN stimulated with the calcium ionophore A23187 [316]. Resveratrol significantly reduced the expression and activation of the β2-integrin MAC-1 on the PBMC surface following stimulation. PMN homotypic aggregation and formation of mixed cell conjugates between PMN and thrombin-stimulated fixed platelets in a dynamic system were also prevented consistently by resveratrol. These results indicate that resveratrol interferes with the release of inflammatory mediators by activated PMN and down-regulates adhesion-dependent thrombogenic PMN functions. Kimura et al. found that resveratrol inhibited the 5-LOX products 5-HETE, 5,12-diHETE and LTC4 with IC50 of 8.9 µM, 6.70 µM and 1.37 µM, respectively [259]. The IC50 of 5-HETE, 5,12-diHETE and LTC4 formations of synthetic 3,3',4-trihydroxystilbene were 5.9 µM, 0.63 µM and 0.88 µM, respectively. Moreover, these compounds inhibited the release of lysosomal enzymes such as lysozyme and β-glucuronidase induced by calcium ionophore A 23187 from human PMN-L at concentrations of 0.1-1 mM. Boscolo et al. elucidated the in vitro effects of resveratrol on human PBMC proliferation and cytokine release [236]. Spontaneous PBMC proliferation was unaffected by resveratrol, while the compound inhibited PHA-stimulated PBMC proliferation by 69%. Resveratrol strongly inhibited PHA-stimulated IFN-γ and TNFα release from PBMC, which may be explained by its inhibitory effect on NF-ÎB.
C 1ad. Modulation of gene expression by resveratrol
The expression of numerous genes that are regulated by different transcription factors has been shown to be down- regulated by resveratrol. These include COX-2 [141], 5-LOX [227], iNOS [234], ICAM-1 [231], TNF [234], IL-1 [65], IL-6 [233] and IL-8 [211]. Fustier et al. found that resveratrol is also a phytoestrogen and binds to and activates ERs that regulate the transcription of estrogen-responsive target genes such as the breast cancer susceptibility genes BRCA1 and BRCA2 [317]. Treatment of human breast cancer cell lines (MCF-7, HBL100 and MDA-MB 231) with 30 µM resveratrol increased expression of BRCA1 and BRCA2 mRNAs without any change in the expression of the proteins. Yang et al. examined whether resveratrol has any effect on growth and gene expression in human ovarian cancer PA-1 cells [182]. They investigated the effect of resveratrol on changes of global gene expression during resveratrol-induced growth inhibition and apoptosis in PA-1 cells by using a human cDNA microarray with 7,448 sequence-verified clones. Out of the genes screened, 118 were affected in their expression levels by more than 2-fold after treatment with 50 µM resveratrol for 24 h. Following treatment of PA-1 cells at a concentration of 50 µM for 6, 12, 24 and 48 h, gene expression patterns was analyzed by microarray. Clustering of the genes modulated more than 2-fold at three of these points divided the genes into two groups. Within these groups, there were specific subgroups of genes whose expressions were substantially changed at the specified time points. One of the most strongly up-regulated genes was NQO-1, which has recently been shown to be involved in p53 regulation.
Earlier studies have shown that resveratrol alters the expression of genes involved in cell-cycle regulation and apoptosis, including cyclins, Cdks, p53 and Cdk inhibitors. Narayanan et al. examined whether resveratrol activates a cascade of p53-directed genes that are involved in apoptosis mechanism(s), or modifies the AR and its co-activators directly or indirectly and inhibits cell growth [162]. They demonstrated by DNA microarray, RT-PCR, Western blot and immunofluorescence analyses that treatment of androgen-sensitive prostate cancer cells (LNCaP) with 100 µM resveratrol for 48 h down-regulated PSA, AR co-activator ARA 24, and NF-ÎB p65. Altered expression of these genes is associated with activation of p53-responsive genes such as p53, PIG 7, p21Cip1/WAF1, p300/CBP and Apaf-1. The effect of resveratrol on p300/CBP plays a central role in its cancer-preventive mechanisms in LNCaP cells. These results implicated its targeting of more than one set of functionally-related molecules. Pendurthi et al. examined the effect of resveratrol on the induction of tissue factor expression in vascular cells that had been exposed to pathophysiological stimuli [125]. The data presented herein show that resveratrol inhibited the expression of tissue factor in endothelial cells stimulated with a variety of agonists, including IL-1β, TNF and LPS. A similar inhibition of tissue factor induction was seen in monocytes that had been pretreated with resveratrol before their stimulation with LPS. In addition, resveratrol was shown to inhibit the LPS-induced expression of TNFα mRNA in endothelial cells and of TNFα and IL-1β mRNA in monocytes.
C2 In vivo animal studies of resveratrol
Besides its effects in vitro , resveratrol has been found to be quite active in vivo. Its in vivo cancer-related effects are elaborated here.
C2 a. Metabolism, pharmacokinetics, tissue distribution and clearance of resveratrol
Numerous studies have examined the metabolism, pharmacokinetics, tissue distribution and clearance of resveratrol (see Table IV). Bertilli et al. studied the plasma kinetics and tissue bioavailability of this compound after oral administration in rats [318]. Its plasma pharmacokinetics after oral administration could be described by an open one- or two-compartment model. Tissue concentrations show a significant cardiac bioavailability and a strong affinity for the liver and kidneys. Andlauer et al. investigated the absorption and metabolism of resveratrol by using an isolated preparation of luminally and vascularly perfused rat small intestine [319]. A synthetic perfusate free from blood components was used as a vascular medium, with a perfluorocarbon as oxygen carrier. Vascular uptake of luminally administered resveratrol was 20.5%. The majority of the absorbed resveratrol was conjugated to yield resveratrol glucuronide (16.8%), which was also the main luminal metabolite (11.2%). Lesser amounts of resveratrol sulfate, 3.0% and 0.3%, were found on the luminal and vascular sides, respectively, while only minute amounts of resveratrol and resveratrol conjugates (1.9%) were found in the intestinal tissue. These results demonstrate an ample uptake and metabolic conversion of resveratrol. Kuhnle et al. studied the absorption and metabolism of resveratrol in the jejunum in an isolated rat small intestine model [320]. Only small amounts of resveratrol were absorbed unmetabolized across the enterocytes of the jejunum and ileum. The principal compound detected on the serosal side was the glucuronide conjugate of resveratrol (96.5%±4.6 of the amount absorbed), indicating the susceptibility of resveratrol to glucuronidation during transfer across the rat jejunum. These findings suggest that resveratrol is most likely to be in the form of a glucuronide conjugate after crossing the small intestine and entering the blood circulation. This will have important implications for the study of the biological functions of resveratrol in vivo.
Table 4. Pharmacokinetics, biotransformation, tissue distribution and metabolic clearance rates of resveratrol.
Refer to pages 39 of 58
De Santi et al. examined the glucuronidation of resveratrol in human liver microsomes and whether flavonoids inhibited resveratrol glucuronidation [321]. The assay employed uridine-5'-diphosphoglucuronic acid-14C and unlabelled resveratrol. They found that resveratrol underwent glucuronidation and that the flavonoid, quercetin, inhibited resveratrol glucuronidation. These results show that resveratrol is glucuronated in the human liver, which may reduce the bioavailability of this compound; however, flavonoids inhibit resveratrol glucuronidation and this inhibition might improve the bioavailability of resveratrol. Aumont et al. found that glucuronidation was regioselective and stereoselective [322]. It occurred at a faster rate with the cis-isomer and preferred the 3-position on both isomers. In addition, the glucuronidation of resveratrol was tested by using several recombinant UDP-glucuronosyltransferase (UGT) isoforms. The reaction was catalyzed by UGT of the family 1A (UGT1A1, 1A6, 1A7, 1A9, 1A10). The bilirubin-conjugating UGT1A1 was involved mainly in the 3-O-glucuronidation of trans-resveratrol, whereas the phenol-conjugating UGT1A6 activity was restricted to cis-resveratrol. The UGT1A9 and 1A10 were active toward both isomers. The activity supported by UGT2B7 and UGT2B15 was very low and restricted to cis-resveratrol. UGT1A3, 1A4, 2B4 and 2B11 did not form resveratrol glucuronides. Li et al. found that resveratrol is not a substrate for P-glycoprotein or the multidrug resistance associated proteins [243]. No phase I metabolites were observed, but the phase II conjugates resveratrol-3-glucuronide and resveratrol-3-sulfate were identified by liquid chromatography mass spectrometry (LC- MS) and liquid chromatographic-tandem mass spectrometry (LC-MS-MS) analysis and comparison with synthetic standards. Although these data indicate that resveratrol diffuses rapidly across the intestinal epithelium, extensive phase II metabolism during absorption might reduce the resveratrol bioavailability.
De Santi et al. examined the sulfation of resveratrol in the human liver and duodenum [323]. They found that resveratrol undergoes sulfation and that this sulfation is blocked by quercetin, a flavonoid present in wine, fruits and vegetables, suggesting that compounds present in the diet may inhibit the sulfation of resveratrol, thus improving its bioavailability. Bertilli et al. examined the kinetics of trans- and cis-resveratrol in rats after oral administration [324]. Resveratrol concentrations were measured in the plasma, heart, liver and kidneys. Tissue concentrations showed a significant cardiac bioavailability and strong affinity for the liver and kidneys. They found that even modest dosages of resveratrol produced an observable pharmacological effect, and that these dosages were compatible with the resveratrol concentrations obtained after oral administration [325].
Piceatannol is a closely related stilbene, that has antileukemic activity and is also a tyrosine kinase inhibitor. Piceatannol differs from resveratrol by having an additional aromatic hydroxy group. Potter et al. showed that the enzyme CYP1B1 is overexpressed in a wide variety of human tumors and catalyzes aromatic hydroxylation reactions [254]. They found that resveratrol undergoes metabolism by CYP1B1 to produce a metabolite that has been identified as piceatannol. This observation provides a novel explanation for the cancer-preventive properties of resveratrol. It demonstrates that a natural dietary agent can be converted to a compound with known anticancer activity by an enzyme that is found in human tumors. This result gives important insight into the functional role of CYP1B1 and provides evidence for the concept that CYP1B1 in tumors may function as a growth suppressor.
Corsi et al. evaluated resveratrol in a human monocytic leukemia cell line at concentrations (100 nM to 1 µM) found in the bloodstream after moderate wine intake [326]. As early as 4 h after intake, resveratrol exhibited antiproliferative and cytotoxic activity. At the same time, some apoptosis-like phenomena were detected, such as cell membrane perturbation (phosphatidylserine-annexin V binding), Fas expression and mitochondrial ΔΨ depolarization. The anticancer drug camptothecin, used as a positive control, did not significantly increase Fas levels and increased FasL only modestly. At 12 h after intake, however, resveratrol at concentrations of 100 nM and 1 µM did not exhibit the same antiproliferative activity, and increased cell proliferation was correlated with a significant increase in FasL expression. The authors concluded that treatment with low doses of resveratrol, such as those found after moderate wine intake, is not sufficient to stop human leukemia cell line proliferation and that cell resistance, marked by high FasL expression, could be mediated by low ΔΨ mitochondria-released anti- apoptotic factors such as Bcl-2.
Whether resveratrol could be absorbed in human and enter the systemic circulation was examined by Kaldas et al. [327]. This was examined by measuring the transport and metabolism of resveratrol (5-40 µM) by the human intestinal epithelial cell line Caco-2 cultured in Transwells. Transport across the Caco-2 monolayer occurred in a direction- independent manner with Papp values of approximately 70 nm/s, much higher than for the paracellular transport marker mannitol (approximately 4 nm/s), suggesting efficient absorption in vivo. At the highest resveratrol concentration, the absorption increased, possibly owing to saturation of metabolism. In sharp contrast to previous findings in the rat, the metabolism of resveratrol in Caco-2 cells involved mainly sulfation and, to a minor extent, glucuronidation. At low resveratrol concentrations, most of the sulfate conjugate was exported to the apical side, presumably by multidrug resistance protein 2, which is strongly expressed in these cells. At high concentrations, there was a shift toward the basolateral side, possibly involving multidrug resistance protein 3. These results indicate that the absorption of resveratrol in vivo may be high but with limited bioavailability owing to efficient sulfate conjugation.
Yu et al. examined in vitro and ikn vivo the metabolism of trans-resveratrol [328]. The in vitro experiments included incubation with human liver microsomes, human hepatocytes and rat hepatocytes, and the in vivo studies included oral or intraperitoneal administration of resveratrol to rats and mice. No resveratrol metabolites were detected in the microsomal incubations, and no phase I metabolites, such as oxidation, reduction, or hydrolysis products, were observed in any samples. However, abundant trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-sulfate were identified in rat urine, mouse serum and incubations with rat and human hepatocytes. Incubation with β-glucuronidase and sulfatase to release free resveratrol was used to confirm the structures of these conjugates. Only trace amounts of cis-resveratrol were detected, indicating that isomerization is not an important factor in the metabolism and elimination of resveratrol. These results indicated that trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-sulfate are the most abundant metabolites of resveratrol. Virtually no unconjugated resveratrol was detected in urine or serum samples, which might have implications regarding the significance of in vitro studies that used only unconjugated resveratrol.
Sale et al. examined the pharmacokinetics in mice and growth-inhibitory properties of resveratrol and the synthetic analogue trans-3,4,5,4'-tetramethoxystilbene (DMU 212) [329]. The latter showed greater growth-inhibitory and pro- apoptotic properties in transformed cells than in untransformed cells. The authors compared the pharmacokinetic properties of DMU 212 with those of resveratrol in the plasma, liver, kidney, lung, heart, brain and small intestinal and colonic mucosa of mice. DMU 212 or resveratrol (240 mg/kg) was administered intragastrically, and drug concentrations were measured by HPLC. Metabolites were characterised by cochromatography with authentic reference compounds and were identified by MS. The ratios of area of plasma or tissue concentration vs time curves of resveratrol over DMU 212 (AUC(res)/AUC(DMU212)) for the plasma, liver and small intestinal and colonic mucosa were 3.5, 5, 0.1 and 0.15, respectively. Thus, resveratrol afforded significantly higher levels in the plasma and liver, while DMU 212 exhibited superior availability in the small intestine and colon. Resveratrol was metabolized to its sulfate or glucuronate conjugate, while DMU 212 underwent metabolic hydroxylation or single and double O-demethylation. DMU 212 and resveratrol inhibited the growth of human- derived colon cancer cells HCA-7 and HT-29 in vitro with IC50 values of between 6 and 26 µM.
Vitrac et al. investigated the distribution of 14C-trans-resveratrol in mouse tissues after oral administration [330]. Male Balb/c mice were given a single oral dose of 14C-trans-resveratrol and were sacrificed at 1.5, 3, or 6 h later. The distribution of radioactivity in the tissues was evaluated by using whole-body autoradiography, quantitative organ-level determination and microautoradiography. Radioactive compounds in the kidney and liver were identified by HPLC. An autoradiographic survey of mice sections, as well as radioactivity quantification in various organs, revealed a preferential fixation of 14C-trans-resveratrol in the organs and biological liquids of absorption and elimination (stomach, liver, kidney, intestine, bile, urine). Moreover, they showed that 14C-trans-resveratrol-derived radioactivity is able to penetrate the tissues of liver and kidney, a finding supported by microautoradiography. These tissue contained intact 14C-trans-resveratrol together with glucuronoconjugates and/or sulfoconjugates. This study demonstrated that trans-resveratrol is bioavailable following oral administration and remains mostly in the intact form. The results also suggest a wide range of target organs for cancer chemoprevention by wine polyphenols in humans.
Meng et al. examined the urinary and plasma levels of resveratrol in humans, mice and rats after ingestion of pure compounds [331]. Oral administration of resveratrol in humans yielded detectable levels of resveratrol and their derivatives in the plasma and urine. After intragastric administration of resveratrol to rats (2 mg/kg), levels of resveratrol as high as 1.2 µM were observed in the plasma.
C2 b. Chemopreventive effects of resveratrol in animals
Chemoprevention can be defined as the use of substances to interfere with the process of cancer development. Chemoprevention prevents or delays the process of carcinogenesis by administration of natural or synthetic compounds. That resveratrol may have chemopreventive effects has been tested in several cancer model systems (Table V). Resveratrol has been shown to have a chemopreventive role in a wide variety of tumors including skin [332-336], liver [229, 337], colon [149], breast [185, 332, 338, 339], lung [340, 341] and esophageal [342] cancers. Resveratrol suppresses tumor initiation and tumor progression by a wide variety of inducers (Table IV). It can inhibit the tumor initiation process induced by B[a]P,DMBA, azoxymethane and nitrosamines and tumor promotion induced by PMA [149, 185, 229, 332-343].
Table 5. Chemopreventive and thrapeutic effects of resveratrol.
Refer to pages 42 of 58
The first report of the chemopreventive effects of resveratrol appeared in 1997, when Jang et al. demonstrated its cancer chemopreventive activity in assays representing three major stages of carcinogenesis [332]. Resveratrol was found to act as an antioxidant and antimutagen and to induce phase II drug- metabolizing enzymes (anti-initiation activity); it mediated anti-inflammatory effects and inhibited COX and hydroperoxidase functions (antipromotion activity); and it induced human promyelocytic leukemia cell differentiation (antiprogression activity). In addition, it inhibited the development of preneoplastic lesions in carcinogen-treated mouse mammary glands in culture and inhibited tumorigenesis in a mouse skin cancer model. In another study by the same group, resveratrol was shown to inhibit carcinogen-induced preneoplastic lesions in mouse mammary organ culture and PMA-promoted mouse skin tumors. The authors also found that resveratrol inhibited tumorigenesis in mouse skin through interference with pathways of reactive oxidants and possibly by modulating the expression of c-fos and TGF-β1 [333]. Pretreatment of mouse skin with resveratrol negated several PMA-induced effects such as elevation in the expression of COX-1, COX-2, c-myc, c-fos, c-Jun, TGF-β1 and TNFα, which could be responsible for inhibition in mouse skin tumorigenesis. Kapadia et al. demonstrated that at a 50–fold molar ratio to PMA, resveratrol reduced by 60% the papillomas in DMBA-initiated and PMA-promoted mouse skin two-stage carcinogenesis protocols at 20 weeks [334]. In another study in a two-stage skin cancer model, in CD-1 mice using DMBA as initiator and PMA as promoter, resveratrol moderately inhibited the rate of tumor formation in individual mice and the number of mice developing one or more tumors [335]. Afaq et al. reported that resveratrol possesses the potential to ameliorate the damage caused by short-term UVB exposure to SKH-1 hairless mouse skin through inhibition of the UVB-mediated induction of COX, ornithine decarboxylase, and lipid peroxidation [336].
Soleas et al. used a two-stage CD-1 mouse skin cancer model, with DMBA as initiator and PMA as promoter, to compare the antitumorigenic activities of resveratrol [335]. Animals were treated with specific polyphenols, at doses ranging from 0 to 25 µMoles (dissolved in 200 µL acetone), twice a week for 18 weeks. The solution was applied topically to the shaved dorsal region of each animal. The relative potencies of the polyphenol were compared by evaluating the percentage inhibition of tumor formation in individual mice and the number of mice developing one or more tumors with the different dose schedules. They found that resveratrol was absorbed much more efficiently and was effective in suppressing the tumors. Ignatowicz et al. investigated resveratrol for its inhibitory effects on the covalent binding of DMBA to DNA in vitro and its suppression of the oxidative burst in PMA-stimulated human PMN [344]. 32P-postlabelling analysis of DNA incubated with DMBA in the presence of 3-methylcholanthrene-induced microsomes produced three major adducts derived from anti-, syn- and anti-dihydrodiol epoxides, respectively, through reactions with 2'-deoxyguanosine and 2'-deoxyadenosine. Phenolic compounds at the concentration of 150 µM reduced the levels of all DMBA-DNA adducts by 55-98%. Human neutrophils showed a significant dose-related decrease of PMA-induced chemiluminescence after pretreatment with phenolic compounds. These results suggest that suppression of ROS and carcinogen-DNA adduct formation may be important for the anticarcinogenic activity of these phenolics.
Hecht et al. examined resveratrol and some other stilbene derivatives as a chemopreventive agent against lung tumor induction in A/J mice by the tobacco smoke carcinogens B[a]P and 4-(methyl-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK) [340]. Groups of 20 A/J mice were treated weekly by gavage with a mixture of B[a]P and NNK (3 mmol each) for 8 weeks, then sacrificed 26 weeks after the first carcinogen treatment. In mice treated with butylated hydroxyanisole (BHA) (20 or 40 µMol) by gavage 2 h before each dose of B[a]P and NNK, lung tumor multiplicity was significantly reduced. Treatment with resveratrol (500 ppm) from 1 week after carcinogen treatment until termination had no effect on lung tumor multiplicity. Cadenas et al. found that resveratrol prevented the oxidative DNA damage induced in the kidney by the carcinogen KBrO3 [345]. We investigated the chemopreventive potential of resveratrol by testing it against mammary carcinogenesis induced by DMBA in female Sprague-Dawley rats [338]. Dietary administration of resveratrol (10 ppm) had no effect on the body weight or tumor volume, but strikingly reduced the incidence (45%; p<0.05), multiplicity (55%; p<0.001) and extended latency period of tumor development relative to DMBA-treated animals. Histopathological analysis of the tumors revealed that DMBA induced ductal carcinomas and focal microinvasion in situ (7/7), whereas treatment with resveratrol suppressed DMBA-induced ductal carcinoma. Immunohistochemical and Western blot analysis revealed that resveratrol suppressed DMBA-induced COX-2 and MMP-9 expression in the breast tumors. Gel-shift analysis showed that resveratrol suppressed DMBA-induced NF-ÎB activation. Treatment of human breast cancer MCF-7 cells with resveratrol suppressed NF-ÎB activation and inhibited proliferation at S-, G2-, and M-phases. Overall, our results suggest that resveratrol suppresses DMBA-induced mammary carcinogenesis, and that this suppression correlates with the down-regulation of NF-ÎB, COX-2 and MMP-9 expression.
Ziegler et al. demonstrated that resveratrol consumed ad libitum in the diet does not modify tumorigenesis in Apc(Min/+) mice [346]. B[a]P is an agonistic ligand for the AhR and a major environmental carcinogen implicated in the etiology of lung cancer through induction of BPDE and BPDE-DNA adducts. Because B[a]P metabolization requires CYP1A1 induction through activation of AhR, Revel et al. hypothesized that resveratrol, a natural competitive inhibitor of AhR, could prevent B[a]P’s adverse effects on the lung [341]. Balb/c mice were injected for 5 weeks with corn oil, B[a]P (5 mg/kg/week), resveratrol (50 mg kg/ week) or B[a]P with resveratrol. Immuno-histochemical analysis was then performed on sections of their lungs for determination of CYP1A1 protein, BPDE- DNA adducts and apoptosis. Mice exposed to B[a]P had a significantly greater induction of lung BPDE-DNA adducts than controls (H scores: control, 26, interquartile range 18- 33; B[a]P, 276, interquartile range 269-288; p<0.01). The induction of BPDE-DNA adduct by B[a]P was significantly abrogated by resveratrol (H score: B[a]P + resveratrol, 103, interquartile range 96-113). A similar pattern was found in the analysis for apoptosis (H scores: control, 121, interquartile range 102-137; BaP, 288, interquartile range 282-292, p<0.05; B[a]P with resveratrol, 132, interquartile range 121-141, p=NS) and CYP1A1 (H scores: control, 170.3, interquartile range 164-175; B[a]P, 302.3, interquartile range 291-315, p<0.05; B[a]P with resveratrol, 200.7, interquartile range 174-215, p=NS). Western blot analysis confirmed that resveratrol prevented B[a]P-induced CYP1A1 expression. This increase in CYP1A1 expression in response to B[a]P administration most probably causes B[a]P metabolism, BPDE-DNA adduct formation and subsequent apoptosis. All B[a]P-induced effects could be prevented by resveratrol, suggesting a possible chemopreventive role for this natural phytoalexin against the development of lung cancer.
Resveratrol also inhibits colon cancers in mice and rats. Resveratrol pretreatment (200 µg/kg/day in drinking water) and treatment in the initiation phase of azoxymethane-induced colon cancer in F344 rats inhibited the number of aberrant crypt foci (ACF)/colon and their multiplicity and completely abolished the large ACF [149]. In resveratrol-treated rats, bax expression was enhanced in ACF but not in the surrounding mucosa. In both controls and resveratrol- treated rats, proliferation was higher in ACF than in normal mucosa. Resveratrol prevents colon carcinogenesis with a mechanism involving changes in Bax and p21Cip1/WAF1 expression. Resveratrol also prevents colon cancer in mice. Breinholt et al. showed that moderate to high doses of resveratrol (1-100 mg/kg diet) induced DNA oxidation products in plasma in male F344 rats, increased the area of GST-placental form-positive foci in the liver and increased the number of ACF to a number comparable to that induced by dietary carcinogen 2-amino-3-methylimidazo[4,5-f]quinoline [337]. This study suggests the possibility that long-term exposure to moderate to high doses of anti-oxidants via pro-oxidative mechanisms and non-oxidative mechanisms can modulate carcinogenesis.
In the transplanted hepatoma H22 murine model, the antitumor activity of resveratrol was studied by treating the tumor-bearing mice with the agent at 10 or 15 mg/kg bodyweight for 10 days. Resveratrol inhibited the growth of this murine transplantable liver cancer [229]. The underlying antitumor mechanism of resveratrol might involve inhibition of cell-cycle progression by decreasing the expression of cyclinB1 and Cdc2 proteins.
In addition to several in vitro studies on MCF-7 human breast cancer cells showing that resveratrol has superestrogenic effects and studies in ER-transfected cell lines showing that resveratrol acts as a mixed agonist/antagonist, there are some in vivo studies that characterize the estrogen- modulatory effects of resveratrol. Bhat et al. demonstrated that resveratrol alone induced PR and, in combination with estradiol, suppressed the expression of PR in mammary glands of Balb/c mice placed in organ culture [185]. Moreover, resveratrol inhibited the formation of the estrogen-dependent preneoplastic ductal lesions induced by DMBA in these mammary glands (IC50, 3.2 µM). Furthermore, resveratrol reduced MNU-induced mammary tumorigenesis in female Sprague-Dawley rats. On the other hand, prepubertal treatment with resveratrol for 5 days accelerated MNU- induced mammary carcinogenesis in female Sprague-Dawley rats [339]. Resveratrol (100 mg/kg) significantly increased the incidence of mammary carcinomas ≥1 cm and multiplicity, but did not affect latency. It did not increase body weight, but did cause slightly earlier vaginal opening. Resveratrol-treated animals exhibited significantly increased irregularity of the estrous cycle, spending more time in the estrus phase. Thus, short-term resveratrol treatment of prepubertal female rats affected the endocrine function and accelerated development of MNU-induced mammary carcinomas.
Li et al. investigated whether resveratrol inhibits N-nitrosomethylbenzylamine (NMBA)-induced rat esophageal tumorigenesis in F344 male rats and found that the number of NMBA-induced esophageal tumors per rat was significantly reduced. The maximum size of tumors in each group treated with resveratrol was significantly smaller than that in the group treated with NMBA alone, which correlated with decreases in COX and prostanglandin E [342].
C2 c. Antitumor effects of resveratrol in animals
Numerous reports suggest that resveratrol exerts therapeutic effects against cancer (Table V). Carbo et al. found that administration of resveratrol to rats inoculated with a fast- growing tumour (the Yoshida AH-130 ascites hepatoma) caused a very significant decrease (25%) in the tumor cell content [347]. The effects of the diphenol were associated with an increase in the number of cells in the G2/M cell-cycle phase. Interestingly, flow cytometric analysis of the tumor cell population revealed the existence of an aneuploid peak (representing 28% of total), which suggests that resveratrol decreases tumor cell numbers by inducing apoptosis. Caltagirone et al. investigated the effects of resveratrol on the growth and metastatic potential of B16-BL6 melanoma cells in vivo [348]. Intraperitoneal administration of resveratrol, at the time of intramuscular injection of B16- BL6 cells into syngeneic mice, resulted in a significant, dose- dependent delay of tumor growth without toxicity. Furthermore, the polyphenol significantly potentiated the inhibitory effect of a non-toxic dose of cisplatin. Kimura et al. found that resveratrol significantly reduced tumor volume (42%), tumor weight (44%) and metastasis to the lung (56%) in mice bearing highly metastatic Lewis lung carcinoma (LLC) tumors at doses of 2.5 and 10 mg/kg but not at 0.6 mg/kg [349]. Resveratrol did not affect the number of CD4+, CD8+ and NK1.1+ T-cells in the spleen. Therefore, the inhibitory effects of resveratrol on tumor growth and lung metastasis could not be explained by NK cell or CTL activation. Resveratrol inhibited DNA synthesis most strongly in LLC cells (IC50, 6.8 µM). Resveratrol at 100 µM increased apoptosis to 20.6±1.35% from 12.1±0.36% (p<0.05) in LLC cells, and decreased the S-phase population to 22.1±1.03% and 29.2±0.27% from 35.2±1.72% (p<0.05) at concentrations of 50 and 100 µM, respectively. Resveratrol inhibited tumor-induced neovascularization at doses of 2.5 and 10 mg/kg in an in vivo model. Moreover, it significantly inhibited the formation of capillary-like tubes from HUVEC at concentrations of 10-100 µM; the degree of inhibition of capillary-like tube formation by resveratrol was 45.5% at 10 µM, 50.2% at 50 µM and 52.6% at 100 µM. Resveratrol inhibited the binding of VEGF to HUVEC at concentrations of 10-100 µM, but not at concentrations of 1 or 5 µM. The degree of inhibition of VEGF-binding to HUVEC by resveratrol was 16.9% at 10 µM, 53.2% at 50 µM and 47.8% at 100 µM. The authors suggested that the antitumor and antimetastatic activities of resveratrol might be due to inhibition of DNA synthesis in LLC cells and of LLC- induced neovascularization and tube formation (angiogensis) in HUVEC.
Min mice are congenic mice genetically predisposed to develop intestinal tumors as a result of a mutation of the Apc gene. Scheider et al. studied the effect of oral administration of resveratrol on tumorigenesis in these mice [350]. Resveratrol (0.01% in the drinking water containing 0.4% ethanol) was administered for 7 weeks to Min mice, starting at 5 weeks of age. The control group was fed the same diet and received water containing 0.4% ethanol. Resveratrol prevented the formation of colon tumors and reduced the formation of small intestinal tumors by 70%. Comparison of the expression of 588 genes in the small intestinal mucosa showed that resveratrol down-regulated genes that are directly involved in cell-cycle progression or cell proliferation (cyclins D1 and D2, DP-1 transcription factor and Y-box binding protein) and up-regulated several genes that are involved in recruitment and activation of immune cells (CTL Ag-4, leukemia inhibitory factor receptor and monocyte chemotactic protein 3) or in inhibition of the carcinogenic process and tumor expansion (tumor susceptibility protein TSG101, TGF-β, inhibin-β A subunit and desmocollin 2). Thus, the high potency and efficacy of resveratrol supported its use as a therapeutic and chemopreventive agent in the management of intestinal carcinogenesis.
Bove et al. found that resveratrol inhibited the in vitro growth of 4T1 breast cancer cells in a dose- and time- dependent manner [133]. In vivo , however, resveratrol had no effect on the time to tumor take, tumor growth, or metastasis when administered intraperitoneally (1, 3, or 5 mg/kg) daily for 23 days starting at the time of tumor inoculation. Resveratrol had no effect on body weight, organ histology, or estrous cycling of the tumor-bearing mice. Resveratrol, therefore, is a potent inhibitor of 4T1 breast cancer cells in vitro , is nontoxic to mice at 1-5 mg/kg, and has no growth- inhibitory effect on 4T1 breast cancer in vivo.
Kimura et al. studied the effects of stilbene glucosides, isolated from medicinal plants and grapes, on tumor growth and lung metastasis in mice bearing highly metastatic LLC tumors [351]. They also studied the inhibitory effects of stilbene glucosides on the differentiation of HUVEC to form a capillary network. Tumor growth in the right hind paw and lung metastasis were inhibited by oral administration of the stilbene glucoside piceid or 2,3,5,4'- tetrahydroxystilbene-2-O-D-glucoside for 33 consecutive days in LLC-bearing mice. As the numbers of CD8+ and NK1.1+ T-cells in the spleen were not affected, the inhibitory effects of these stilbene glucosides on tumor growth and lung metastasis could not be explained by NK or CTL activation. Piceid inhibited DNA synthesis in LLC cells at a concentration of 1000 µM, but not at lower concentrations (10-100 µM). 2,3,5,4'-tetra-hydroxystilbene-2-O-D-glucoside also inhibited DNA synthesis in LLC cells (IC50, 81 µM). Both stilbene glucosides inhibited formation of capillary-like tube networks (angiogenesis) in HUVEC at concentrations of 100 to 1000 µM. The authors suggested that the antitumour and antimetastatic activity of these stilbene glucosides might be due to the inhibition of DNA synthesis in LLC cells and angiogenesis of HUVEC.
Kozuki et al. found that resveratrol inhibited the proliferation of hepatoma cells and suppressed their invasion even at a concentration of 25 µM [172]. Sera from rats given resveratrol by mouth restrained only the invasion of AH109A cells; resveratrol and resveratrol-loaded rat serum suppressed ROS-potentiated invasive capacity. These results suggest that the anti-invasive activity of resveratrol is independent of its antiproliferative activity, and that its anti-oxidant property may be linked to its anti- invasive action. Mishima et al. found that vaticanol C, a resveratrol tetramer, exhibited strong cytotoxicity against various tumor cell lines [352]. They examined the antitumor activity of the ethanol extract from the stem bark of Vateria indica, which is used for health and healing diseases in the Indian Ayurvedic tradition. HPLC analysis showed that the extract contains bergenin, hopeaphenol, vaticanol B, vaticanol C and epsilon- viniferin. An in vitro assay displayed the extract's anticancer activity against mouse sarcoma 180 cells (IC50, 29.5 µM). Growth of sarcoma 180 cells subcutaneously allografted in DDY mice was significantly retarded by oral administration of the extract at the dose of 30 or 100 mg/kg body weight (p<0.001). The extract did not show significant toxicity to mice even at a dosage of 1000 mg/kg body weight administered daily for 28 days. De Ledinghen et al. showed that liver myofibroblasts stimulated the in vitro invasion of hepatocellular carcinoma cell lines through a hepatocyte growth factor/urokinase-dependent mechanism [173]. They further evaluated the effects of trans-resveratrol on invasion of the human hepatoma cell line HepG2 and demonstrated that trans-resveratrol decreased the hepatocyte growth factor-induced HepG2 cell invasion by an, as yet, unidentified postreceptor mechanism. Juan et al. evaluated whether high doses of trans-resveratrol have harmful effects on Sprague-Dawley rats [353]. trans-Resveratrol was administered orally to male rats for 28 day at a daily dose of 20 mg/kg, 1000 times the amount consumed by a 70- kg person taking 1.4 g of trans-resveratrol/day. Neither body weight nor food and water consumption differed between rats treated with trans-resveratrol and the control group. Hematological and biochemical variables were not affected by the treatment. Histopathological examination of the organs obtained at autopsy revealed no alterations. These results support the view that repeated consumption of trans-resveratrol at 20 mg/kg/day does not adversely affect the variables tested in rats.
Mollerup et al. studied the effect of resveratrol on the expression of genes involved in the metabolism of PAH in the human bronchial epithelial cell line BEP2D [170]. Expression of the CYP1A1 and CYP1B1, microsomal epoxide hydrolase (mEH), and GSTP1 genes were measured by RT-PCR. The cells were treated with either B[a]P or 2,3,7,8-tetrachlorodibenzo-p-dioxin in the presence or absence of resveratrol. Resveratrol inhibited both the constitutive and induced expression of CYP1A1 and CYP1B1. In contrast, resveratrol increased the expression of the mEH gene and elicited no change in the expression of GSTP1. The altered gene expression in response to resveratrol was reflected in a reduced overall level of B[a]P metabolism. These data indicate that resveratrol may exert lung cancer chemopreventive activity through altering the expression of genes involved in the metabolism of PAH, resulting in altered formation of carcinogenic B[a]P metabolites in human bronchial epithelial cells.
Liu et al. examined the antitumor and immunomodulatory activity of resveratrol on experimentally-implanted H22 tumors in Balb/c mice [343]. Intraperitoneal resveratrol, at a dose of 500 mg/kg, 1000 mg/kg, or 1500 mg/kg, could curb the growth of implanted H22 tumors in mice. The inhibitory rates were 31.5%, 45.6% and 48.7%, respectively (p<0.05), which could raise the level of serum immunoglobulin G and plaque- forming cell response to sheep red blood cells. Intraperitoneal resveratrol at doses of 1000 mg/kg or 1500 mg/kg or bacillus Calmette-Guerin 200 mg/kg could increase the production of serum TNFα in H22 tumors in mice. The effect of resveratrol, however, was insignificant (p>0.05). Thus resveratrol could inhibit the growth of H22 tumors in Balb/c mice. This antitumor effect might be related directly to the inhibition of H22 cell growth and indirectly to inhibition of the agent’s potential effect on nonspecific host immunomodulatory activity.
Morales et al. showed that trans-resveratrol has a protective effect on gentamycin-induced nephrotoxicity [354]. This is related to resveratrol’s strong affinity for the kidneys [324, 345]
D. Clinical studies with resveratrol
Despite the fact that an enormous amount of data is available on resveratrol’s anticancer effects in vitro and in animals, few clinical studies have been performed in humans. The data available from these studies are limited. Gautam et al. found that ex vivo purging of contaminating tumor cells may reduce the incidence of relapse in patients undergoing bone marrow transplantation [355]. In this study, they demonstrated that resveratrol exhibits antileukemic activity against mouse (32Dp210 and L1210) and human (U-937 and HL-60) leukemic cell lines by inhibiting cell proliferation. Long-term exposure to resveratrol also inhibits the clonal growth of normal hematopoietic progenitor cells, but at a higher IC50 than for most of the leukemia cell lines tested. The inhibitory effect of resveratrol on hematopoietic progenitors is partially reversible, whereas the effect on leukemia cells is largely irreversible. The inhibition of leukemia cells by resveratrol involves nucleosomal DNA fragmentation (apoptosis). On the other hand, resveratrol does not induce or enhance spontaneously occurring apoptotic death in normal hematopoietic progenitor cells. In vivo experiments, performed with untreated and resveratrol-treated bone marrow, showed comparable hematopoietic reconstitution in lethally irradiated mice (10 Gy) as determined by survival, hematological recovery and the number of hematopoietic progenitor cells present in the marrow of reconstituted animals. Taken together, these results indicate the potential for the use of resveratrol for ex vivo pharmacological purging of leukemia cells from bone marrow autografts without significant loss in the hematopoietic activity of progenitor cells. We showed that resveratrol suppressed the colony- forming cell proliferation of fresh AML marrow cells from five patients with newly diagnosed AML in a dose-dependent fashion, showing that resveratrol is an effective in vitro inhibitor of AML cells and suggesting that this compound may have a role in future therapies for AML [122].
Goldberg et al. reported that, after an oral dose of resveratrol (25 mg/70 kg) to healthy human subjects, the compound appears in serum and urine predominantly as glucuronide and sulfate conjugates and reaches peak concentrations (10-40 nM) in serum around 30 min after consumption [356]. Free polyphenols account for 1.7-1.9% of the peak serum concentrations and more than 80% is absorbed. Pace-Asciak et al. reported that trans-resveratrol can be absorbed from grape juice in biologically active quantities and in amounts that are likely to cause reduction in the risk of atherosclerosis [357]. That red wines (which have 20 times more polyphenols than white wines) show no advantages over other forms of ethanol suggests that, in vivo, ethanol is the dominant anti-aggregatory component in these beverages, which are more potent than grape juices in preventing platelet aggregation in humans. A study by Wang et al. , suggested that resveratrol (at doses of 10-1000 µM) significantly inhibits the in vitro platelet aggregation induced by collagen [358]. Thrombin, at a concentration of 4 mg/kg/day, inhibits ADP-induced platelet aggregation in humans and rabbits, despite not changing serum lipid levels. Resveratrol also causes an increase in plasma adenosine levels and blood nucleosides in human subjects [359].
Conclusions
From the studies described in this review, it is clear that resveratrol holds great potential in the prevention and therapy of a wide variety of tumors. Resveratrol has antiproliferative effects through the induction of apoptosis in cell lines of various origin such as leukemias and breast, prostate, colon, pancreas, and head and neck carcinomas. It induces Fas-dependent apoptosis in some cell lines and Fas- independent apoptosis in others. Most, but not all, studies indicate that resveratrol does not induce apoptosis in normal cells. Some in vitro studies showing that resveratrol has antiproliferative effects at certain dose ranges but not at other doses could explain the small number of in vivo animal studies in which resveratrol was ineffective in inhibiting certain cancer conditions. Some studies have reported that resveratrol has a biphasic behavior with respect to its antiproliferative effects. Thus, systematic studies are required to test a range of resveratrol concentrations in vitro and then apply those doses in vivo to a wide variety of tumors. In vivo studies clearly demonstrate that resveratrol is pharmacologically safe and can be used for the prevention and therapy of cancer. Resveratrol’s ability to radiosensitize and chemosensitize opens up additional opportunities. That the structure of resveratrol is simple, and the presence of hydroxyl groups is strongly linked with its biological activity, provides additional opportunities for structure-activity relationship studies to improve its biopotency and bio- availability. Lastly, resveratrol has potential for treating diseases other than cancer and cardiovascular ailments. Howitz et al. found evidence in yeast that resveratrol mimics calorie restriction and thus extends the lifespan by 70% [360].
Acknowledgements
We would like to thank Katy Hale for the critical review of the manuscript. We would also thank various summer students for their assistance with the references. This work was supported by the Clayton Foundation for Research (to BBA), Department of Defense US Army Breast Cancer Research Program grant (BC010610, to BBA), a PO1 grant (CA91844) from the National Institutes of Health on lung chemoprevention (to BBA), and a P50 Head and Neck SPORE grant from the National Institutes of Health, USA (to BBA).
REFERENCES
Refer to pages 46 through 58

Return to RESVERATROL
Since 8-31-2008


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |