

Soyabean Glyceollins:
Biological Effects and Relevance to Human HealthThis section is compiled by Franκ M. Painter, D.C.
Send all comments or additions to: Franκp@chiro.org




FROM: Proc Nutr Soc. 2012 (Feb); 71 (1): 166174 ~ FULL TEXT
Hyo Jung Kim, Ji-Sun Lim, Woo-Keun Kim and Jong-Sang Kim
School of Applied Bioscience,
School of Food Science and Biotechnology,
Bκ21 Research Team for Developing Functional Health Food Materials,
κyungpooκ National University,
Deagu 702-701, Republic of κorea.Glyceollins, one family of phytoalexins, are de novo synthesised from daidzein in the soyabean upon exposure to some types of fungus. The efficiency of glyceollin production appears to be influenced by soyabean variety, fungal species, and the degree of physical damage to the soyabean. The compounds have been shown to have strong antioxidant and anti-inflammatory activities, and to inhibit the proliferation and migration of human aortic smooth muscle cells, suggesting their potential to prevent atherosclerosis. It has also been reported that glyceollins have inhibited the growth of prostate and breast cancer cells in xenograft animal models, which is probably due to their anti-oestrogenic activity. In essence, glyceollins deserve further animal and clinical studies to confirm their health benefits.
From the FULL TEXT Article:
Background
Since plants cannot move to avoid attack, they have evolved a wide array of both inducible and constitutive chemicals to defend themselves (1). Plants produce an enormously diverse array of over 100 000 secondary metabolites that have low molecular weight and are distinct from the components of primary metabolism. They are generally non-essential for the basic metabolic processes of the plant (2). Among those chemicals, phytoalexins are defined as low-molecular-weight, anti-microbial compounds that are biosynthesised de novo in response to stress, including microbial attack, heavy metal salts, or UV radiation (3,4). In contrast, phytoanticipins are constitutive plant defences whose concentrations can increase under stress conditions (4,5).
It is possible that the same chemical may serve as a phytoalexin as well as a phytoanticipin, even in the same plant, because distinction is not based on chemical structure but on how it is produced.
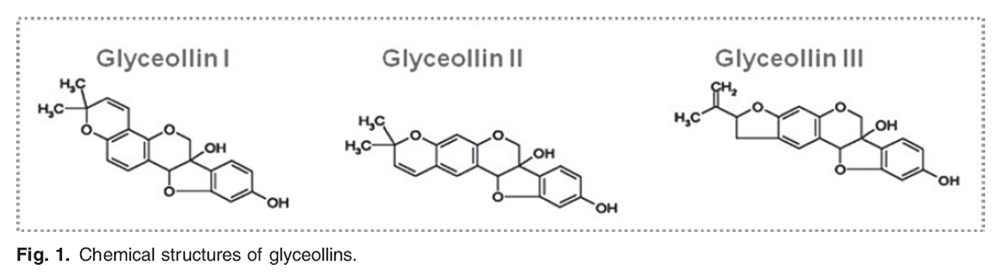
Figure 1 Although toxic to the microbes, several phytoalexins are found to have health benefits for human subjects and to have properties of chronic disease prevention (6). One of the most widely studied phytoalexins is resveratrol, which is found in fungus-infected grape skins and Rhizoma Polygoni Cuspidatisa, a type of traditional Chinese herbal medicine. Resveratrol has been shown to have several potential beneficial health effects including the prevention of cancer (7) and age-related chronic diseases (8) although its efficacy should be demonstrated by larger well-controlled clinical studies (9,10). Compared with the well-studied resveratrol, glyceollins, the fungus-induced soyabean phytoalexins, are less known for their biological activities. However, it is a quite plausible that these compounds have positive health effects because their chemical structures are similar to bioactive flavonoids (Figure 1). Our previous studies have provided evidence that glyceollins might have anti-carcinogenic and anti-atherosclerotic activities through their antioxidant potential (1012). Furthermore, glyceollins have been reported to suppress human breast and ovarian cancer formation through their anti-oestrogenic effect (1315).
This review will discuss the potential health beneficial effects of glyceollins and their mechanisms of action.
Biosynthesis of glyceollins
Soyabeans contain abundant isoflavones, a varied group of polycyclic compounds, which have been reported to have numerous biological activities including suppression of sex-hormone dependent cancers (1619) and amelioration of post-menopausal complications, including osteoporosis and hot flashes (20). Isoflavonoids could be classified as phytoanticipins because they are stored in plant cells in anticipation of pathogenic attack (21). All soyabeans and their products contain significant amounts of the isoflavones daidzein and genistein, either as the aglycone or as different types of glycoside conjugates. These include 60-O-malonylglucosides, 60-O-acetylglucosides and the b-glucosides of daidzein and genistein (22,23), all of which can be separated by reversed-phase HPLC. Smaller amounts of glycitein conjugates are often found in soyabean or soya protein, whereas conjugates of glycitein are abundantly present hypocotyledon or germ (23). The malonyl and acetyl glycosides are susceptible to heat and readily convert to the more stable b-glycoside (24). Therefore, the relative proportions of these conjugates can vary considerably among different soya foods depending on the extent of processing of the soyabean (25).
Glyceollins are de novo synthesised from daidzein in the soyabean in response to environmental stresses such as fungal infection. Although the glyceollins are detected at high concentrations in soyabeans during stress, they have also been detected at trace levels in non-elicitor-treated soyabean seeds (26).
The compounds possess pterocarpanoid skeletons with cyclic ether decoration originating from a C5 prenyl moiety. Enzymes involved in glyceollin biosynthesis have been thoroughly characterised during the early era of modern plant biochemistry, and many genes encoding enzymes of isoflavonoid biosynthesis have been cloned. However, some genes need to be identified for later biosynthetic steps (27). According to a recent study by Akashi et al., dimethylallyl diphosphate: (6aS, 11aS)-3,9, 6a-trihydroxypterocarpan ((2)-glycinol) 4-dimethylallyltransferase appears to be involved in yielding the direct precursor of glyceollin I (27).
Glyceollins are derived from the precursor molecule, daidzein through several intermediate structures including glycinol (28). Glycinol, which was previously shown to be oestrogenic, is derived from daidzein via a pterocarpan by cyclisation and 6a-hydroxylation (29). For the biosynthesis of the glyceollins, glycinol is prenylated to produce glyceollidin I and II, followed by cyclisation into the corresponding glyceollins (30).
The most well-known elicitors that effectively stimulate biosynthesis of glyceollins include some fungal strains such as Pseudomonas glycinea, Meloidogyne incognita, Heterodera glycines, Aspergillus sojae, Aspergillus awamori, Aspergillus oryzae and Rhizopus oligosporous (21,31).
Whether an elicitor is a fragment of a fungal cell or a defined chemical molecule such as a b-glucan, host plants must recognise these phytoalexin-inducing factors. Such molecules from both pathogens and mutualists are presumably recognised by receptors located on the cell wall or membrane. Typical biotic elicitors causing glyceollin production in soyabean include b-1,3-1,6-oligoglucoside, b-1,3-glucan and cyclic b-1,6-1,3-glucan, which are cell wall components of pathogenic or mutualistic symbiotic micro-organisms (32). For instance, cyclic b-1,6-1,3-glucans synthesised by both free-living cells and bacteroids of B. japonicum are active elicitors of glyceollins in soyabean (33,34).
Glyceollin synthesis is also induced by abiotic elicitors including iodoacetate, UV, Tx-100, and metal ions such as Fe, Cu, Hg and Ag (21) as well as during fungal infection. The mechanisms by which biotic and abiotic factors affect isoflavonoid phytoalexin formation in plants are unclear. Because elicitors have diverse structural features, they may act simply by injuring plant cells, which then stimulates the phytoalexin biosynthetic pathway. Or they may cause the host plant to release a constitutive elicitor that triggers phytoalexin formation (21).
Antifungal activity
According to our previous study glyceollins were shown to inhibit several lines of fungal species. More specifically, the glyceollins (200 and 600 mg/disk) revealed a remarkable antifungal effect against Phytophthora capsici and Sclerotinia sclerotiorum, and to a lesser degree Fusarium oxysporum and Botrytis cinerea, within the growth inhibition range of 10.961.0%, along with their respective minimum inhibitory concentration values ranging from 25 to 750 mg/ml. The glyceollins also had a strong suppressive effect on spore germination of all tested plant pathogens along with concentration- and time-dependent kinetic inhibition of P. capsici, which is responsible for pepper disease (35). Phytoalexins appear to exert their antifungal activity by altering membrane permeability although the presence of other mechanisms could not be excluded (36).
Antioxidant activity
In healthy aerobes, the production of reactive oxygen species (ROS) is approximately balanced with the antioxidant defence systems (37). Oxidative stress can be defined as a disturbance in the pro-oxidantantioxidant balance in favour of the former, leading to potential damage (38), or a serious imbalance between ROS production and antioxidant defences (37). Although ROS might be harmful at the high concentration, the generation of ROS, within certain boundaries, is essential to maintain homoeostasis. For instance, macrophages utilise ROS to combat infective agents. Likewise, cytosolic ROS may be involved in the regulation of some important cellular events such as proliferation, gene expression and signal transmission. However, it is generally agreed that an excessive production of ROS is associated with ageing, cancer development, atherosclerosis and neurodegenerative disorders (39,40).

Figure 2 Glyceollins isolated from soyabean that sprouted in the presence of A. sojae showed strong antioxidant activity and ROS scavenging potential when assessed by an in vitro model (13). The antioxidant activities of glyceollins were confirmed by measuring ferric reducing antioxidant power, 2,2-diphenyl-1-picrylhydrazyl radical scavenging, singlet oxygen quenching, 2,20-azinobis-(3-ethylbenzothiazoline- 6-sulfonic acid) radical scavenging, hydroxyl radical scavenging activity and lipid peroxidation inhibition. In addition, the antioxidant potential of glyceollins were measured by a fluorescent probe, 2,7-dichlorofluorescin diacetate, and dihydroethidium in mouse hepatoma hepa1c1c7 cells in which they were insulted with H2O2 to generate ROS. The compound showed a strong reducing power and inhibited lipid peroxidation and significant scavenging activities of radicals including singlet oxygen superoxide anion, 2,20-azinobis-(3-ethylbenzothiazoline-6- sulfonic acid) and 2,2-diphenyl-1-picrylhydrazyl (13). It was also found that glyceollins significantly suppressed H2O2- induced LDL oxidation production (Figure 2), suggesting their potential as natural antioxidants and nutraceuticals.
Antioxidant enzyme induction
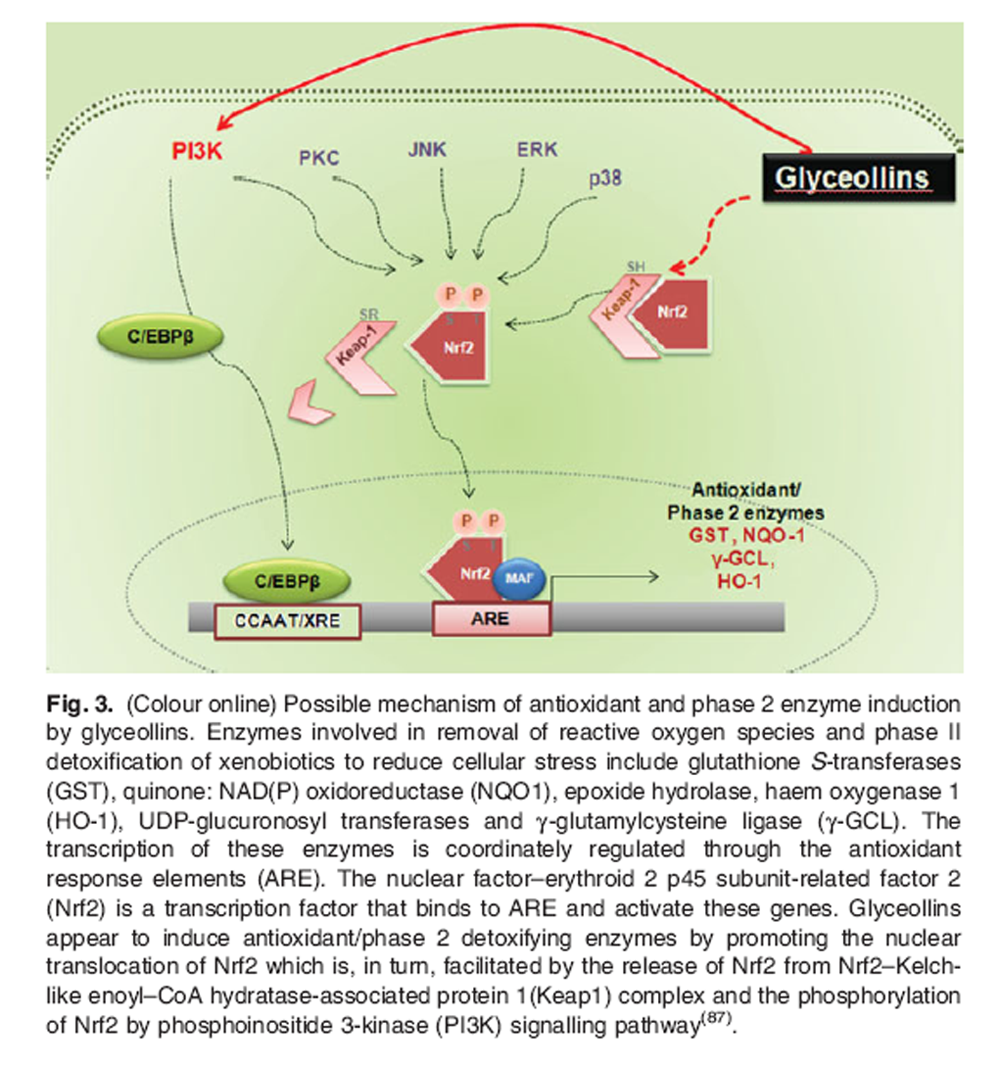
Figure 3 Many natural antioxidants have been found to induce phase 2/antioxidant enzymes such as NAD(P)H:quinone oxidoreductase 1, haem oxygenase 1, glutathione reductase, glutathione S-transferase A1 (also known as glutathione S-transferase Ya in mouse), and glutamate-cysteine ligase via the nuclear factor-erythroid 2 p45 subunit-related factor 2 (Nrf2)-mediated pathway (4144). Our recent study indicated that glyceollins have the potential to induce antioxidant enzymes and phase 2 detoxifying enzymes through the Nrf2-Kelch-like enoyl-CoA hydratase-associated protein 1 (Keap1) pathway (11), although the mechanism of action how the compounds activate the Nrf2 signalling pathway needs to be defined (Figure 3).
Under unstressed conditions, Nrf2 is present in cytosol in the form of a heterodimer with Keap1, and it is rapidly degraded by the proteosomal pathway. Once activated by oxidative stress or electrophiles, it migrates into the nucleus and binds to the antioxidant response element of specific genes, enhancing their transcription. Although the whole mechanism by which some natural compounds cause liberation of Nrf2 from Keap1 remains unclear, the conformational change of Keap1 by direct interaction with the compounds or an indirect signal generated from the binding of the compounds to cellular membrane seems to be responsible for the nuclear translocation of Nrf2 following the transcriptional activation of antioxidant enzymes.
In particular, cysteine residues abundantly present in Keap1 could be modified by exogenous oxidants or antioxidants, facilitating Keap1 separation from Nrf2 (45,46). It is also speculated that the Nrf2Keap1 complex is separated from each other by phosphorylation of either Nrf2 or Keap1 by certain signalling pathways including mitogen-activated protein kinase or phosphoinositide 3-kinase, which causes a conformational change (4749).
The importance of Nrf2 in cytoprotection has been proven from the result of when the knockout of Keap1 in mice led to juvenile lethality due to hyperkeratosis of the oesophagus (50). Hepatocyte-specific knockout of the Keap1 gene consistently elevates the accumulation of Nrf2 in the nucleus and protects hepatocytes against acute drug toxicity and inflammatory liver injury (51,52).
Halliwell pointed out that antioxidants would only significantly influence a disease process if free radicals or other reactive species caused or significantly contributed to the progression of the disease (37,53). Evidence supports the view that increased free radical formation is usually a consequence of tissue damage by a disease or toxin (54).
Nrf2-mediated phase 2 enzyme induction has been widely accepted as a promising approach for cancer chemoprevention as well as protection against oxidative stress (44,5558). Considering Nrf2-mediated antioxidant enzyme induction, glyceollins have the potential to prevent chemically induced carcinogenesis, neurodegenerative diseases such as Alzheimers disease and atherosclerosis.
Anti-inflammatory activity
Inflammation is a process that includes multiple steps, which are regulated by activating inflammatory or immune cells (59). It is widely accepted that inflammation plays a key role in coronary artery disease and in the progression of atherosclerosis (60). Similarly, inflammation has long been considered a major precursor for the development of cancer in both infectious and non-infectious conditions. Non-infectious chronic inflammatory disorders such as inflammatory bowel disease, which are typically associated with chemokine signalling, increased cell proliferation, reduced cell cycles, accumulation of mutations, and inadequate DNA repair are well-known harbingers of malignancy (61). Thus, chronic inflammation is considered to be common cause of atherosclerosis and may contribute to the incidence and/or promotion of certain types of cancer (62).
Macrophages play a central role in the regulation of the inflammatory response by releasing pro-inflammatory cytokines, chemokines and other mediators that induce migration of additional cells to inflamed tissue (63). The activated macrophages secrete inflammatory cytokines such as TNFa, IL-6, and induce ROS and PGE2 (64).
Phyto-oestrogen is known to have antioxidative, and anti-inflammatory properties by the inhibition of inducible NO synthase gene expression and NO production, as well as inhibition of the expression of inflammatory cytokines (65). The molecular mechanisms are implicated in the oestrogen receptor (ER)-dependent pathway or by the activation of the inflammatory NF-kB transcription factor through mitogen-activated protein kinases (66,67).
Our recent study showed that glyceollins markedly suppressed the inflammatory response in lipopolysaccharide (LPS)-activated murine macrophages and the skin in vivo (68). Interestingly, glyceollins suppressed LPS-dependent secretion of IL-6, NO and expression of cyclo-oxygenase 2 (COX-2) from RAW264.7 cells. NO is a molecule produced by inducible NO synthase in a reaction that converts arginine and O2 into citrulline and NO (69). It plays an important role in inflammation (69). Upregulation of COX-2 expression by the transcriptional activation of the COX-2 gene, results in the increased production of PGE2 which is a critical factor in the inflammatory condition (70).
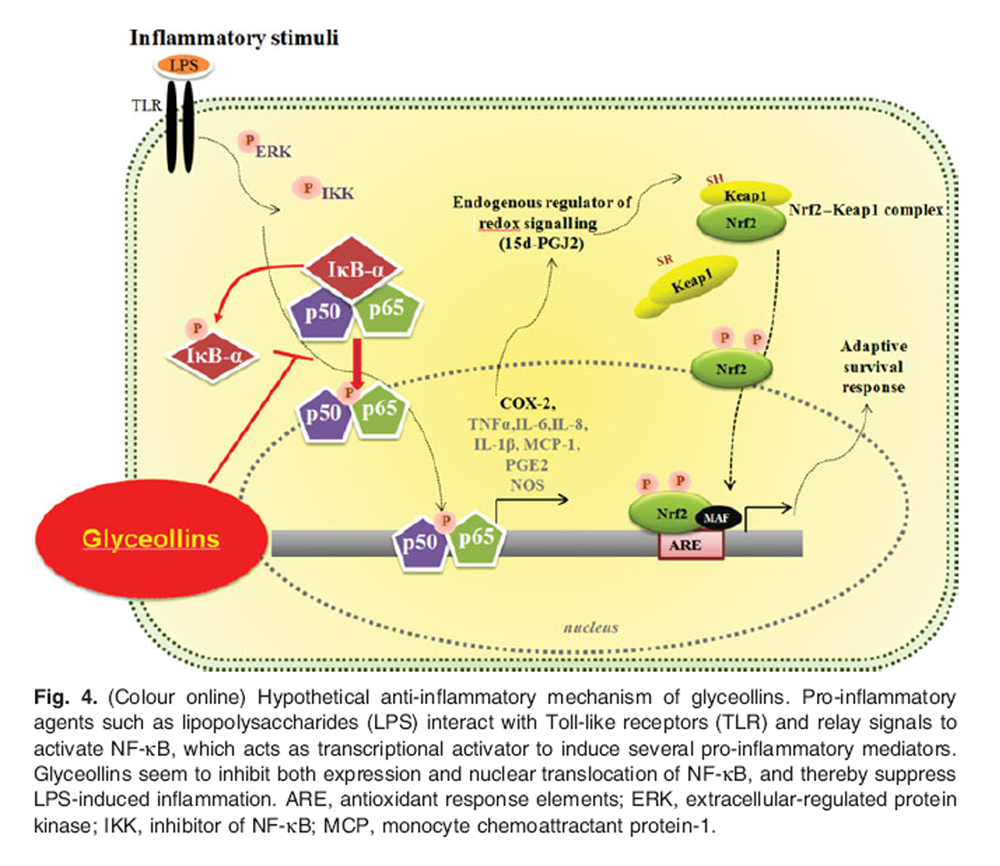
Figure 4 It seems that the anti-inflammatory activity of glyceollins is associated with the suppression of LPS-dependent phosphorylation and the induction of NF-κB (68). It is κnown that inactive NF-κB normally stays bound to inhibitory κB in the cytosol. NF-κB can be activated by LPS and pro-inflammatory cytoκines, which induce increased protein phosphorylation and proteolysis of inhibitory κB protein (71). The activated NF-κB is, in turn, translocated into the nucleus, binding to NF-κB-binding sites in the promoter regions of target genes and inducing the transcription of pro-inflammatory mediators such as inducible NO synthase, COX-2, TNFα, IL-1b, IL-6 and IL-8 (72). Activation of macrophages plays an important role in the initiation and propagation of inflammatory responses by the production of cytoκines and mediators, such as IL-1b, TNFα, NO and COX-2 (73,74).
Glyceollins also suppress LPS-dependent phosphorylation of extracellular-signal-regulated kinase 1 and 2 and p38, suggesting their ability to inhibit the essential targets responsible for the inflammation process (68). Thus, glyceollins appear to exert anti-inflammatory activity via regulating the NF-kB signalling pathway (Figure 4).
Anti-cancer and oestrogen-modulating activities
Oestrogen antagonists have been exploited to prevent or treat oestrogen-positive breast cancer. Soya isoflavones have been extensively studied for their potential to prevent and/or treat sex-hormone-dependent tumours (14,1720). This key finding led to the proposal that a diet containing soyabean may be beneficial in the prevention or treatment of hormone-dependent diseases because of the presence of these bioactive non-nutrients (1720,75).
Soyabeans and their products have long been copiously consumed by the people of East Asian countries including Korea, China and Japan (7678), countries where the incidence of breast cancer is relatively low (79,80). Therefore, it is quite plausible that isoflavones could prevent breast cancer by antagonising oestrogen action. Salvo et al. reported that treatment with glyceollin suppressed E2-stimulated tumour growth of MCF-7 cells (- 53.4%) and BG-1 cells (- 73.1%) in ovariectomized athymic mice (15). These tumour inhibitory effects corresponded with significantly lower E2-induced progesterone receptor expression in the tumours, suggesting that the glyceollin mixture may be functioning as selective ER modulators, selectively antagonising ER function in a tissue type-specific manner (14,15).
In contrast to these reports, our data consistently suggested the oestrogenic activity of glyceollins, as shown in the E-screen assay and in the enhanced proliferation of MCF-7 cells carrying ER, and the increased expression of pS2, a typical biomarker for oestrogenic activity (11). Therefore, we speculate that like isoflavones, glyceollins appear to exert both oestrogen-like and anti-oestrogenic activities depending on the physiological condition. That is, glyceollins could act as oestrogen agonists under conditions that are lacking oestrogen, as could be seen in post-menopausal women. Conversely, these compounds could act as anti-oestrogens in the presence of endogenous oestrogen, and may also compete with oestradiol for binding to ER.
Meanwhile, glyceollins were found to inhibit the proliferation and cause the apoptosis of murine hepatoma cells (W-K Kim and J-S Lim, unpublished results). This has nothing to do with the oestrogenic activity of the compounds, but it may be associated with their inhibitory activity on the cell signalling pathway related to apoptosis. In particular, apoptotic activity of the compounds is believed to be associated with their stimulation of the c-Jun N-terminal kinase signalling pathway because cytochrome c release, increased by glyceollins, was suppressed by c-Jun N-terminal kinase inhibitor (W-K Kim and J-S Lim, unpublished results). Cytochrome c release from the mitochondria is known to be essential for the activation of caspase 3, one of the terminator enzymes executing the apoptotic process.
Anti-atherosclerosis
Due to their antioxidant properties (12), glyceollins may have an effect on preventing and/or treating atherosclerosis. In addition, glyceollins inhibited LPS-induced inflammation in macrophage cells and suppressed the proliferation and migration of human aortic smooth muscle cells, suggesting that the compounds had potential to retard the atherosclerotic process (68,81). However, currently there is no direct evidence supporting anti-atherosclerotic effect in human subjects.
Other beneficial effects
Skin whitening effect
Melanin is a dark pigment produced by melanocytes and plays an important role as a natural protection agent of skin from UV light. However, the accumulation of over-produced melanin causes serious dermatological disorders such as melasma, freckles, post-inflammatory melanoderma and solar lentigo (82,83). Melanogenesis has been reported to be controlled by not only tyrosinase but also several factors including cytokines, growth factors, microphthalmia-associated transcription factor, melanocortins (α-melanocyte-stimulating hormone/MCR-1 and β-melanocyte-stimulating hormone), adrenocorticotrophic hormone β-endorphin, catecholamines, acetylcholine, corticosteroids and oestrogens with their specific receptors (84).
Glyceollin inhibited tyrosinase and tyrosine related protein-1 expression. Additionally, glyceollin effectively inhibited intracellular cAMP levels in B16 melanoma cells stimulated by α-melanocyte stimulating hormone (85). These results suggest that the whitening activity of glyceollin may be due to the inhibition of cAMP involved in the signal pathway of α-melanocyte stimulating hormone in B16 melanoma cells (85).
Insulinotropic action
Park et al. hypothesised that glyceollins play an important role in glucose homoeostasis by regulating glucose utilisation in adipocytes and improving b-cell function and survival (86). Glyceollins increased insulin-stimulated glucose uptake in 3T3-L1 adipocytes without affecting the PPARg agonist (86). The compounds also reduced TAG accumulation in adipocytes. In addition, glyceollins slightly promoted glucose-stimulated insulin secretion without palmitate treatment in Min6 cells, and they potentiated insulinotropic actions in the presence of 500 mM of palmitate used to induce b-cell dysfunction. This insulinotropic action might be associated with decreased b-cell apoptosis through the attenuation of endoplasmic reticulum stress, as assessed by mRNA levels of X-box binding protein-1, activating transcription factor-4 and -6 and C/EBP homologous protein. Glyceollins also increased glucagon-like peptide-1 secretion, resulting in insulinotropic actions in enteroendocrine cells (86). These data suggest that glyceollins help normalize glucose homoeostasis by potentiating b-cell function and survival and improving glucose utilisation in adipocytes.
Conclusion
Although some studies implicated the potential health benefits of glyceollins, there is not yet solid evidence that the compounds have significant pharmacological and/or physiological effects in human subjects. In particular, it is not understood how well they are absorbed in the digestive tract. Unlike isoflavones that are mainly present in glycoside form in the soyabean and absorbed in large intestine after being metabolised by intestinal microflora, glyceollins, which are present in aglycone form in elicited soyabean and more hydrophobic than aglycone forms of isoflavones, are more likely to be better absorbed than soya isoflavones. However, it remains to be determined whether the compounds can be absorbed in the small intestine by passive diffusion and if they require any transporter for absorption. In relation to their bioactive function, glyceollins are just starting to draw attention because they may pose potential preventive actions from some chronic diseases, such as cancer, and merit further animal and human studies to evaluate their dietary and/or medical usefulness.
Acκnowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of κorea funded by the Ministry of Education, Science and Technology (2010-0027204 and 2011-0009782). W. κ. κ. and J. S. L. conducted the experiments of unpublished results and did artworκs; H. J. κ. and J. S. κ wrote the majority part of the paper; J. S. κ. is a principal investigator. All authors read the paper and contributed to its final form. The authors declare no conflicts of interest.
References:
Jeandet P, Douillet-Breuil AC, Bessis R et al. (2002)
Phytoalexins from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants,
antifungal activity, and metabolism.
J Agric Food Chem 50, 27312741.Dixon RA (2001)
Natural products and plant disease resistance.
Nature 411, 843847.Paxton JD (1980)
A new working definition of the term phytoalexin.
Plant Dis 64, 734.Paxton JD (1981)
Phytoalexins-a working redefinition.
J Phytopathol 101, 106109.VanEtten HD, Mansfield JW, Bailey JA et al. (1984)
Two classes of plant antibiotics: phytoalexins versus phytoanticipins.
Plant Cell 6, 11911192.Feng S, Saw CL, Lee YK et al. (2008)
Novel process of fermenting black soybean [Glycine max (L.) Merrill] yogurt with dramatically reduced
flatulence-causing oligosaccharides but enriched soy phytoalexins.
J Agric Food Chem 56, 1007810084.Jang M, Cai L, Udeani GO et al. (1997)
Cancer chemopreventive activity of resveratrol, a natural product derived from grapes.
Science 275, 218220.Harikumar KB & Aggarwal BB (2008)
Resveratrol: a multitargeted agent for age-associated chronic diseases.
Cell Cycle 7, 10201035.Baur JA, Pearson KJ, Price NL et al. (2006)
Resveratrol improves health and survival of mice on a high-calorie diet.
Nature 444, 337342.Dolinsky VW & Dyck JR. (2011)
Calorie restriction and resveratrol in cardiovascular health and disease.
Biochim Biophys Acta 812, 14771489.Kim HJ, di Luccio E, Kong AN et al. (2011)
Nrf2-mediated induction of phase 2 detoxifying enzymes by glyceollins derived from soybean
exposed to Aspergillus sojae.
Biotechnol J 6, 525536.Kim HJ, Suh HJ, Kim JH et al. (2010)
Estrogenic activity of glyceollins isolated from soybean elicited with Aspergillus sojae.
J Med Food 13, 382390.Kim HJ, Suh HJ, Kim JH et al. (2010)
Antioxidant activity of glyceollins derived from soybean elicited with Aspergillus sojae.
J Agric Food Chem 58, 1163311638.Burow ME, Boue SM, Collins-Burow BM et al. (2001)
Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor a and b.
J Clin Endocrinol Metab 86, 17501758.Salvo VA, Boue SM, Fonseca JP et al. (2006)
Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis.
Clin Cancer Res 12, 159164.Wood CE, Clarkson TB, Appt SE et al. (2006)
Effects of soybean glyceollins and estradiol in postmenopausal female monkeys.
Nutr Cancer 56, 7481.Dong JY & Qin LQ. (2011)
Soy isoflavones consumption and risk of breast cancer incidence or recurrence:
a meta-analysis of prospective studies.
Breast Cancer Res Treat 125, 31523.Taylor CK, Levy RM, Elliott JC et al. (2009)
The effect of genistein aglycone on cancer and cancer risk:
a review of in vitro, preclinical, and clinical studies.
Nutr Rev 67, 398415.Hwang YW, Kim SY, Jee SH et al. (2009)
Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies.
Nutr Cancer 61, 98606.Messina M (2010)
Insights gained from 20 years of soy research.
J Nutr 140, 2289S2295S.Dakora FD & Phillips DA (1996)
Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins
Physiol Mol Plant Pathol 49, 120.Farmakalidis E & Murphy PA (1985)
Isolation of 60-Oacetyldaidzein and 60-O-acetylgenistein from toasted defatted soy flakes.
J Agric Food Chem 33, 385389.Kudou S, Fleury Y, Welt D et al. (1991)
Malonyl isoflavone glycosides in soybean seeds (Glycine max Merrill).
Agric Biol Chem 55, 22272233.Barnes S, Kirk M & Coward L (1994)
Isoflavones and their conjugates in soy foods: extraction conditions and analysis by HPLC-mass spectrometry.
J Agric Food Chem 42, 24662474.Setchell KD (1998)
Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones.
Am J Clin Nutr 68, 1333S1346S.Kraus C, Spiteller G, Mitho¨fer A et al. (1995)
Quantification of glyceollins in nonelicited seedlings of Glycine max by gas chromatography-mass spectrometry.
Phytochemistry 40, 739743.Akashi T, Sasaki K, Aoki T et al. (2009)
Molecular cloning and characterization of a cDNA for pterocarpan 4-dimethylallyltransferase catalyzing
the key prenylation step in the biosynthesis of glyceollin, a soybean phytoalexin.
Plant Physiol 149, 683693.Tilghman SL, Boue΄ SM & Burow ME (2010)
Glyceollins, a novel class of antiestrogenic phytoalexins.
Mol Cell Pharmacol 2, 155160.Boue SM, Tilghman SL, Elliott S et al. (2009)
Identification of the potent phytoestrogen glycinol in elicited soybean (Glycine max).
Endocrinology 150, 24462453.Banks SW & Dewick PM (1993)
Biosynthesis of glyceollins I, II, and III in soybean.
Phytochemistry 22, 27292733.Lee MR, Kim JY, Chun J et al. (2011)
Induction of glyceollins by fungal infection in varieties of Korean soybean.
J Microbiol Biotechnol 20, 12261229.Smith DA & Banks SW (1986)
Biosynthesis, elicitation and biological activity of isoflavonoid phytoalexins.
Phytochemistry 25, 979995.Gore RS & Miller KJ (1993)
Cyclic [b]-1,6 -1,3 glucans are synthesized by Bradyrhizobium japonicum bacteroids
within soybean (Glycine max) root nodules.
Plant Physiol 102, 191194.Miller KJ, Hadley JA & Gustine DL (1994)
Cyclic [b]-1,6-1,3-glucans of Bradyrhizobium japonicum USDA 110 elicit isoflavonoid
production in the soybean (Glycine max) host.
Plant Physiol 104, 917923.Kim HJ, Suh HJ, Lee CH et al. (2010)
Antifungal activity of glyceollins isolated from soybean elicited with Aspergillus sojae.
J Agric Food Chem 58, 94839487.Keen NT & Bruegger B (1977)
Phytoalexins and chemicals that elicit their production in plants.
ACS Symp Ser 62, 126.Halliwell B (2007)
Biochemistry of oxidative stress.
Biochem Soc Trans 35, 11471150.Sies H (1991)
Oxidative stress: from basic research to clinical application.
Am J Med 91, 31S38S.Markesbery WR (1999)
The role of oxidative stress in Alzheimer disease.
Arch Neurol 56, 14491452.Finkel T & Holbrook NJ (2000)
Oxidants, oxidative stress and the biology of ageing.
Nature 408, 239247.Kensler TW, Wakabayashi N & Biswal S (2007)
Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway.
Annu Rev Pharmacol Toxicol 47, 89116.Itoh K, Chiba T, Takahashi S, Ishii T et al. (1997)
An Nrf2 small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes
through antioxidant response elements.
Biochem Biophys Res Commun 236, 313322.Dinkova-Kostova AT, Massiah MA, Bozak RE et al. (2001)
Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis
depends on their reactivity with sulfhydryl groups.
Proc Natl Acad Sci USA 2001, 98, 34043409.Jeong WS, Jun M & Kong AN (2006)
Nrf2: a potential molecular target for cancer chemoprevention by natural compounds.
Antioxid Redox Signal 8, 99106.Kobayashi M, Li L, Iwamoto N et al. (2009)
The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding
to a wide range of chemical compounds.
Mol Cell Biol 29, 493502.Ogura T, Tong KI, Mio K et al. (2010)
Keap1 is a forkedstem dimer structure with two large spheres enclosing the intervening,
double glycine repeat, and C-terminal domains.
Proc Natl Acad Sci USA 107, 28422847.Sun Z, Huang Z & Zhang DD (2009)
Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating
the Nrf2-dependent antioxidant response.
PLoS One 4, e6588.Nakaso K, Yano H, Fukuhara Y et al. (2003)
PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human
neuroblastoma cells.
FEBS Lett 546, 181184.Lim JH, Kim KM, Kim SW et al. (2008)
Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: novel cytoprotective mechanism
against oxidative damage.
Pharmacol Res 57, 325331.Wakabayashi N, Itoh K, Wakabayashi J et al. (2003)
Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation.
Nat Genet 35, 238245.Okawa H, Motohashi H, Kobayashi A et al. (2006)
Hepatocyte-specific deletion of the Keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity.
Biochem Biophys Res Commun 339, 7988.Osburn WO, Yates MS, Dolan PD et al. (2008)
Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice.
Toxicol Sci 104, 218227.Halliwell B (1992)
Reactive oxygen species and the central nervous system.
J Neurochem 59, 16091623.Gutteridge JM & Halliwell B (2010)
Antioxidants: molecules, medicines, and myths.
Biochem Biophys Res Commun 393, 561564.Yu X & Kensler T (2005)
Nrf2 as a target for cancer chemoprevention.
Mutat Res 591, 93102.Lee JS & Surh YJ (2005)
Nrf2 as a novel molecular target for chemoprevention.
Cancer Lett 224, 171184.Kwak MK, Wakabayashi N & Kensler TW (2004)
Chemoprevention through the Keap1Nrf2 signaling pathway by phase 2 enzyme inducers.
Mutat Res 555, 133148.Seo JY, Park J, Kim HJ et al. (2009)
Isoalantolactone from Inula helenium caused Nrf2-mediated induction of detoxifying enzymes.
J Med Food 12, 10381045.Lundberg IE (2000)
The role of cytokines, chemokines, and adhesion molecules in the pathogenesis of idiopathic inflammatory myopathies.
Curr Rheumatol Rep 2, 216224.Libby P, Ridker PM & Maseri A (2002)
Inflammation and atherosclerosis.
Circulation 105, 11351143.Xavier RJ & Podolsky DK (2007)
Unravelling the pathogenesis of inflammatory bowel disease.
Nature 448, 427434.Ross JS, Stagliano NE, Donovan MJ et al. (2001)
Atherosclerosis and cancer: common molecular pathways of disease development and progression.
Ann NY Acad Sci 947, 271292.Bosca L, Zeini M, Traves PG et al. (2005)
Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate.
Toxicology 208, 249258.Kaplanski G, Marin V, Montero-Julian F et al. (2003)
IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation.
Trends Immunol 24, 2529.Labinskyy N, Csiszar A, Veress G et al. (2006)
Vascular dysfunction in aging: potential effects of resveratrol, an antiinflammatory phytoestrogen.
Curr Med Chem 13, 989996.Park MJ, Lee EK, Heo HS et al. (2009)
The antiinflammatory effect of kaempferol in aged kidney tissues:
the involvement of nuclear factor-kB via nuclear factor-inducing kinase/IkB kinase
and mitogen-activated protein kinase pathways.
J Med Food 12, 351358.Fan GW, Gao XM, Wang H et al. (2009)
The antiinflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by
estrogen receptor activation and inhibition of iNOS.
J Steroid Biochem Mol Biol 113, 275280.Kim HJ, Sung MK & Kim JS (2011)
Anti-inflammatory effects of glyceollins derived from soybean by elicitation with Aspergillus sojae.
Inflamm Res 60, 909917.Kanwar JR, Kanwar RK, Burrow H et al. (2009)
Recent advances on the roles of NO in cancer and chronic inflammatory disorders.
Curr Med Chem 16, 23732394.Lee SH, Soyoola E, Chanmugam P et al. (1992)
Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide.
J Biol Chem 267, 2593425938.Verma IM, Stevenson JK, Schwarz EM et al. (1995)
Rel/NFkB/ IkB family: intimate tales of association and dissociation.
Genes Dev 9, 27232735.Baeuerle PA & Baltimore D (1996)
NF-kB: ten years after.
Cell 87, 1320.Reddy DB & Reddanna P (2009)
Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-kB and MAPK activation
in RAW 264.7 macrophages.
Biochem Biophys Res Commun 381, 112117.Hamalainen M, Nieminen R, Vuorela P et al. (2007)
Antiinflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and
NF-kB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kB
activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages.
Mediators Inflamm 2007, article ID 45673.Setchell KD, Borriello SP, Hulme P et al. (1984)
Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease.
Am J Clin Nutr 40, 569578.Kim J & Kwon C (2001)
Estimated dietary isoflavone intake of Korean population based on National Nutrition
Survey. Nutr Res 21, 947953.Chan SG, Ho SC, Kreiger N et al. (2007)
Dietary sources and determinants of soy isoflavone intake among midlife Chinese Women in Hong Kong.
J Nutr 137, 24512455.Yamamoto S, Sobue T, Sasaki S et al. (2001)
Validity and reproducibility of a self-administered food-frequency questionnaire to assess
isoflavone intake in a Japanese population in comparison with dietary records and blood
and urine isoflavones.
J Nutr 131, 27412747.Messina M (1999)
Soy, soy phytoestrogens (isoflavones), and breast cancer.
Am J Clin Nutr 70, 574575.Wu AH, Ziegler RG, Nomura AM et al. (1998)
Soy intake and risk of breast cancer in Asians and Asian Americans.
Am J Clin Nutr 68, 1437S1443S.Kim HJ, Cha BY, Choi B et al. (2011)
Glyceollins inhibit platelet-derived growth factor-mediated human arterial smooth muscle cell
proliferation and migration.
Br J Nutr (Epublication ahead of print version)Urabe K, Nakayama J & Hori Y (1998)
Mixed epidermal and dermal hypermelanoses. In The Pigmentary System:
Physiology and Pathophysiology, pp. 909911
[JJ Norlund, RE Boissy, VJ Hearing, RA King & JP Ortonne, editors].
New York: Oxford University Press.Cullen MK (1998)
Genetic epidermal syndromes: disorders characterized by lentigines.
In The Pigmentary System: Physiology and Pathophysiology, pp. 760766
[JJ Norlund, RE Boissy, VJ Hearing, RA King & JP Ortonne, editors].
New York: Oxford University Press.Schallreuter KU, Kothari S, Chavan B et al. (2008)
Regulation of melanogenesiscontroversies and new concepts.
Exp Dermatol 17, 395404.Lee YS, Kim HK, Lee KJ et al. (2010)
Inhibitory effect of glyceollin isolated from soybean against melanogenesis in B16 melanoma cells.
BMB Rep 43, 461467.Park S, Ahn IS, Kim JH, et al. (2010)
Glyceollins, one of the phytoalexins derived from soybeans under fungal stress, enhance insulin
sensitivity and exert insulinotropic actions.
J Agric Food Chem 58, 15511557.Surh YJ (2003)
Cancer prevention with dietary phytochemicals.
Nat Rev Cancer 3, 768780.
Return to PHYTOALEXINS
Since 8-06-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |