

Women Who Take N-3 long-chain Polyunsaturated Fatty
Acid Supplements During Pregnancy and Lactation
Meet the Recommended IntakeThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Appl Physiol Nutr Metab. 2015 (May); 40 (5): 474–481 ~ FULL TEXT
Xiaoming Jia, Mohammadreza Pakseresht, Nour Wattar, Jamie Wildgrube,
Stephanie Sontag, Murphy Andrews, Fatheema Begum Subhan, Linda McCargar,
Catherine J. Field, the APrON study team
Department of Agricultural,
Food and Nutritional Science,
University of Alberta,
Edmonton, AB T6G 2E1, Canada.
catherine.field@ualberta.ca
3/4 OF PREGNANT MOMS DON'T GET ENOUGH OMEGA-3 IN THEIR DIET
This recently published study, assessing the diet of over 600 pregnant women (Canadian Alberta Pregnancy Outcomes and Nutrition Trial) found that only 27% of them met the current European Union (EU) consensus recommendation for DHA blood levels during pregnancy. DHA (one of the key Omega-3 fatty acids) is so very important for fetal brain (nervous system) development.The aim of the current study was to estimate total intake and dietary sources of eicosapentaenoic acid (EPA), docosapentanoic (DPA), and docosahexaenoic acid (DHA) and compare DHA intakes with the recommended intakes in a cohort of pregnant and lactating women. Twenty-four-hour dietary recalls and supplement intake questionnaires were collected from 600 women in the Alberta Pregnancy Outcomes and Nutrition (APrON) cohort at each trimester of pregnancy and 3 months postpartum. Dietary intake was estimated in 2 ways: by using a commercial software program and by using a database created for APrON. Only 27% of women during pregnancy and 25% at 3 months postpartum met the current European Union (EU) consensus recommendation for DHA. Seafood, fish, and seaweed products contributed to 79% of overall n-3 long-chain polyunsaturated fatty acids intake from foods, with the majority from salmon. The estimated intake of DHA and EPA was similar between databases, but the estimated DPA intake was 20%-30% higher using the comprehensive database built for this study. Women who took a supplement containing DHA were 10.6 and 11.1 times more likely to meet the current EU consensus recommendation for pregnancy (95% confidence interval (CI): 6.952-16.07; P < 0.001) and postpartum (95% CI: 6.803-18.14; P < 0.001), respectively. Our results suggest that the majority of women in the cohort were not meeting the EU recommendation for DHA during pregnancy and lactation, but taking a supplement significantly improved the likelihood that they would meet recommendations.
KEYWORDS: Alberta Pregnancy Outcomes and Nutrition (APrON); Alberta Pregnancy Outcomes and Nutrition (« APrON »); acide docosahexaénoïque (« DHA »); acide docosapentaénoïque (« DPA »); acide eicosapentaénoïque (« EPA »); acides gras polyinsaturés à longue chaîne (AGPLC) de type n-3; docosahexaenoic acid (DHA); docosapentaenoic acid (DPA); eicosapentaenoic acid (EPA); n-3 long-chain polyunsaturated fatty acids (LCPUFA)
From the FULL TEXT Article:
Introduction
The dietary n-3 polyunsaturated fatty acids (PUFA) include α-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). EPA, DPA, and DHA are usually referred to as n-3 long-chain PUFA (n-3 LCPUFA). A source of these long-chain fatty acids is required during pregnancy for fetal and placental development (reviewed by Mennitti et al. 2015; Jones et al. 2014). Maternal intake/status of n-3 LCPUFA during pregnancy and lactation has been found to positively impact maternal, infant, and child health in many systematic reviews (Yang et al. 2013; Imhoff-Kunsch et al. 2012; Larqué et al. 2012; Horvath et al. 2007). The importance of a dietary source of n-3 LCPUFA is supported by stable isotope tracer studies that found only 1%–4% of dietary ALA is converted to DHA (Pawlosky et al. 2001; Emken et al. 1994). Although the conversion of ALA to DHA is reported to increase during pregnancy (Williams and Burdge 2006), maternal supplementation with ALA together with linoleic acid during pregnancy was not found to be effective in increasing blood DHA concentration in pregnant women or their newborn infants (de Groot et al. 2004).
Assuming that only a small amount of n-3 LCPUFA can be synthesized from the dietary precursor, at least in some women, a dietary source of n-3 LCPUFA is required during pregnancy and lactation to meet maternal and infant requirements. Although less is known about the role of DPA in fetal development, it has been suggested that there is some conversion to DHA and retro-conversion to EPA (Kaur et al. 2011). There is also recent evidence that dietary intake of DPA is associated with neuroprotective effects (Kelly et al. 2011) and heart health (Sun et al. 2008), suggesting that DPA intake may have additional benefits if consumed during pregnancy and lactation.
The American Dietetic Association (ADA) with the Dietitians of Canada (Kris-Etherton and Innis 2007) recommend at least 500 mg/day of LCPUFA for all healthy adults including pregnant and lactating women. The European Commission with the International Society for the Study of Fatty Acids and Lipids (ISSFAL) specifically recommends that pregnant and lactating women consume a minimum of 200 mg DHA per day (Koletzko et al. 2008, 2007). These recommendations could be met by consuming 1 to 2 portions per week of fish high in n-3 fatty acids, which is the recommendation by Health Canada (Health Canada 2002) and the United States Dietary Guidelines Advisory Committee (Dietary Guidelines for Americans 2005) for all women. There is currently no specific recommendation for dietary DPA.
Although maternal intake of n-3 LCPUFA is important for infant brain and retina development before and after birth (reviewed by Innis 2007), studies done in Canada, Australia, the United States, and Europe have reported that pregnant and lactating women are not meeting the suggested dietary recommendations (Cosatto et al. 2010; Sioen et al. 2010; Friesen and Innis 2009; Denomme et al. 2005; Oken et al. 2004; Innis and Elias 2003). This is likely contributed to by the low fish consumption reported by North American women (Coletto and Morrison 2011), concerns of mercury contamination (Oken et al. 2003), and low supplement use (Friesen and Innis 2009; Denomme et al. 2005; Oken et al. 2004; Innis and Elias 2003). Although n-3 supplements (Natural Health Products (NHP)) are reported to be a major source of EPA and DHA (EFSA Panel on Dietetic Products, Nutrition and Allergies 2012), the prevalence of supplement use was found to be positively associated with socioeconomic status (SES) in the Canadian Community Health survey (Vatanparast et al. 2010).
To estimate intake of dietary n-3 LCPUFA, commercial nutrient analysis programs based on the United States Department of Agriculture (USDA) and the Canadian Nutrient File (CNF) are commonly used in North America. A previous study identified problems with this data base for estimating LCPUFA intake because of missing the content of selective n-3 LCPUFA in the 2007 version of the CNF (Patterson et al. 2012). Although the nutrient file has been updated since this publication, it is not known if it still has missing values that would result in underestimating the intake of n-3 LCPUFA.
The objectives of this study were to estimate in a large maternal-infant cohort(i) dietary intake and sources of EPA, DPA, and DHA in each trimester during pregnancy and at 3 months postpartum and to compare intake with various recommendations; and
(ii) to determine the contribution of supplements to total n-3 LCPUFA intake.
Materials and methods
Participants and sample collection
Participants for this study were the first 600 women enrolled in the Alberta Pregnancy Outcomes and Nutrition (APrON) study, a prospective cohort study that recruited pregnant women in Alberta, Canada. Detailed information on recruitment criteria and data collection methodology has been published elsewhere (Kaplan et al. 2014). Briefly, the inclusion criteria were females of child-bearing age (>16 years), less than 26 weeks gestation, and able to speak and write in English. Twenty-four–hour recalls and supplement intake questionnaires (SIQs) including n-3 LCPUFA supplements were collected by face-to-face interviews with participants during each pregnant visit (2–3 times during pregnancy) and at 3 months postpartum. Ethical approval for this study was obtained from the Health Research Ethics Biomedical Panel at the University of Alberta and the Health Research Ethics Board at the University of Calgary.
Developing the n-3 LCPUFA database
Dietary intake of n-3 LCPUFA (EPA, DPA, and DHA) was estimated from the face-to-face 24-h recalls using 2 methods: (i) a commercial program (Food Processor version 10.6; ESHA Research, Salem, Ore., USA), and (ii) a database created for APrON. Similar to the study developed by Innis and Elias (2003), the APrON database for n-3 LCPUFA (EPA, DPA, and DHA) included all foods reported in the 24-hour recalls that would contribute to n-3 LCPUFA intake. Foods assumed to have negligible amounts of n-3 LCPUFA, including items such as breads, fruits, and vegetables, were not included in the database. The CNF and the USDA nutrient databases were used as the primary source of n-3 LCPUFA information. Judgments based on similar energy and compositions were used to substitute food items that were not found in either the USDA or the CNF database.
Eighty-five recipes were created and n-3 LCPUFA information was estimated from each individual ingredient. Examples of these recipes included sauces, dips, baked goods, and mixed seafood, fish, poultry, meat, egg, or dairy dishes. Utilizing the assigned EPA, DPA, and DHA values for all food items, the foods in the 24-h recalls were linked to the n-3 LCPUFA database using Microsoft Office Excel 2010. Formulas and functions were used to automatically calculate the individual and total n-3 LCPUFA intake for each participant at each time-point. To determine food sources contributing to intake, foods were grouped into food groups (n = 10), and seafood, fish, and seaweed products were further categorized into subcategories (n = 7), which is defined in the USDA database.
Estimating n-3 LCPUFA intake from supplements
A specially designed and validated SIQ, adapted from Health Canada and Statistics Canada (Canadian Community Health Survey - Nutrition 2004, Dietary Supplements and Prescription Medication Survey 2005–2006, and The Tomorrow Project questionnaires 2006), was used to collect NHP intake information during pregnancy and postpartum as previously described (Gómez et al. 2013). A NHP database (including 935 products) was developed for APrON to estimate supplement intake and more details on the development have been published elsewhere (Gómez et al. 2013). Briefly, at each visit during pregnancy and postpartum, women were asked to describe the type and quantity (i.e., frequency and dosage of intake) of NHPs consumed. A comprehensive file was created for each participant for all consumed NHPs with the detailed correction factor for days of week, weeks of trimester, and dosage at each time-point. This file was then linked to the NHP database to estimate the contribution of n-3 LCPUFA intake from supplements.
Statistical analysis
Data for all nutrients were tested for normality using the Kologrov–Smirnov test. If data were not normally distributed, it was log-transformed and retested to ensure normal distribution prior to using parametric statistics. A 1-way ANOVA, Kruskal–Wallis, Mann–Whitney U test, and Wilcoxon Signed Ranks Test were conducted, as appropriate, to compare the differences among sampling time-points. The binary logistic regression model was used to identify the likelihood of meeting the current DHA recommendation based on supplement consumption during pregnancy and at 3 months postpartum. All data were analyzed using IBM SPSS Statistics for Windows (version 21; IBM Corp., Armonk, N.Y., USA) and a 2-tailed P value of ≤0.05 was considered significant.
Results
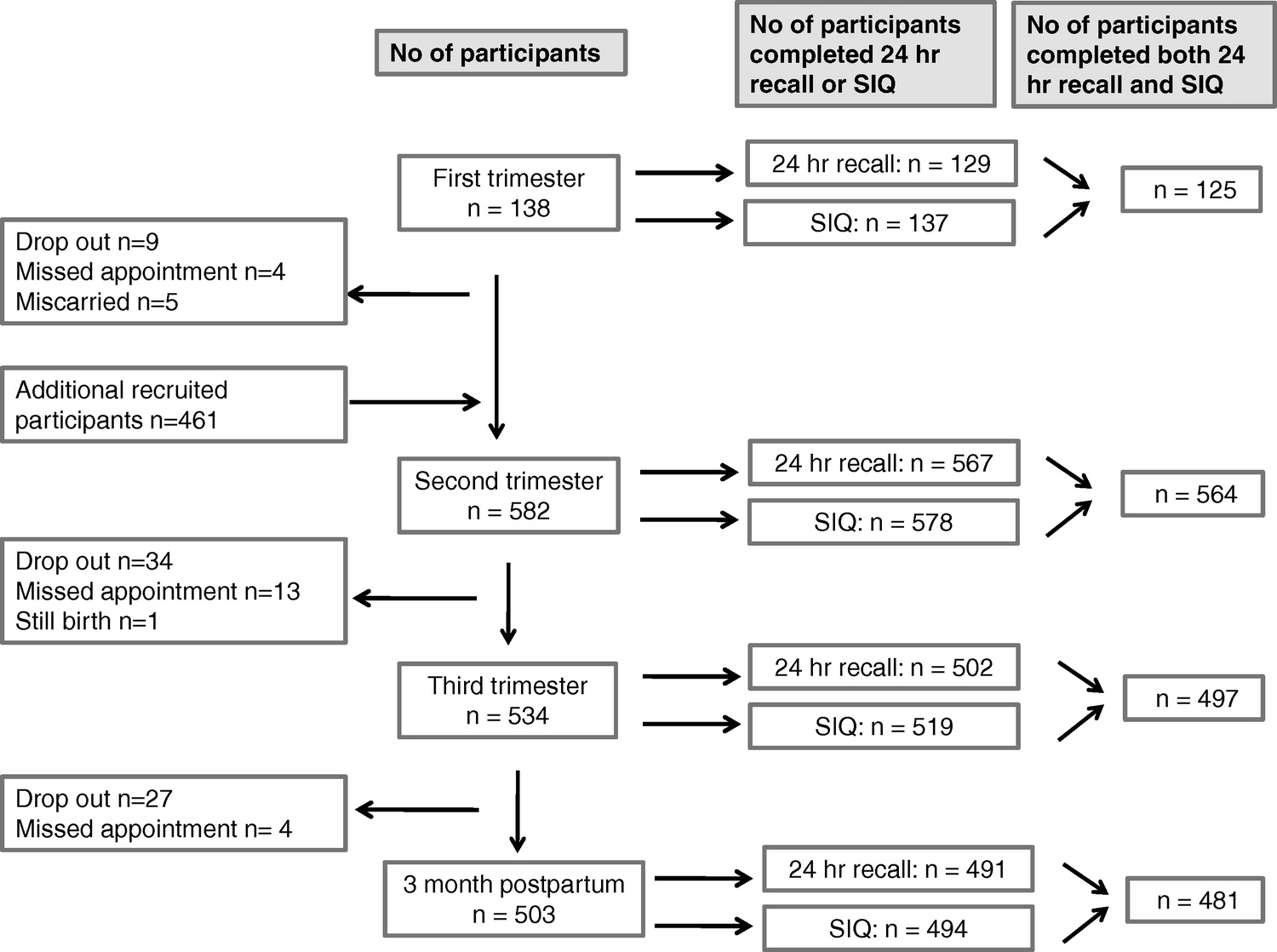
Figure 1

Table 1 Of the 600 women from the first cohort of the APrON study, 592 participants provided at least one 24-h recall and SIQ. Among those, 130, 572, 510, and 500 women provided the 24-h recalls and 137, 578, 519, and 494 women provided supplement questionnaires at the first, second, and third trimesters and 3 months postpartum visit, respectively. Among those, 1, 5, 8, and 9 subjects were excluded at each time-point, respectively, as they were defined to have nonrepresentative 24-h recalls (defined as estimated intakes of calories and/or fibre greater than 3 standard deviations from the group mean). After ensuring the complete intake (dietary and supplement) was available, 125, 564, 497, and 481 participants were included for analysis (Figure 1).
Participant characteristics are presented in Table 1. The majority of the women had completed postsecondary education, had an annual household income ≥$100,000, and were in their second trimester. Their ages ranged from 17–44 years with a mean age of 31.6 years. Approximately one-third (n = 187) of participants reported taking a supplement containing n-3 LCPUFA at 1 or more of the measured time-points. At 3 months postpartum, 92% of the women (n = 402) reported breastfeeding their infants (Jessri et al. 2013). Among these women, 54% were exclusively breastfeeding at 3 months postpartum, while the nonexclusive breastfeeding women reported providing formula as complementary to breastfeeding (Jessri et al. 2013).
Estimated daily intake of macronutrients and fatty acids

Table 2 Table 2 shows the estimated daily intake of energy, carbohydrate, protein, and fat for the first, second, and third trimesters of pregnancy and at 3 months postpartum. The mean intake of all macronutrients met the Accepted Macronutrient Distribution Range recommended by the Institute of Medicine. With the exception of energy, carbohydrate, and saturated fatty acids (SFA), there was no significant difference in the mean intake of these nutrients among the 4 time-points (Table 2). The mean daily energy, carbohydrate, and SFA intakes were significantly higher in the third trimester compared with the first trimester and 3 months postpartum.
After correcting for energy intake, carbohydrate intakes in the second and third trimesters were still significantly higher than the postpartum period (139 ± 23 and 139 ± 22 vs 132 ± 25 g, respectively, P < 0.001). The mean estimated intake of arachidonic acid (AA) and the median intake of n-3 LCPUFA (EPA+DPA+DHA) ranged from 104 to 113 mg and 58 to 86 mg per day, respectively. The ratio of total n-6 PUFA (linoleic acid+AA+gamma-linolenic acid) to n-3 PUFA (ALA+EPA+DPA+DHA) ranged from 8.3 to 9.0, but did not differ among time-points.

Table 3 Using the developed database, total dietary intake of n-3 LCPUFA, EPA, and DHA was found to be significantly higher in trimester 3 than at any of the other time-points (Table 2). When the mean intake from all trimesters was calculated for each woman (for an estimation during pregnancy) and compared with 3 months postpartum, the estimated intake of EPA, DHA, and total n-3 LCPUFA was significantly lower at 3 months postpartum (Table 3). At all time-points, DHA represented approximately 50%–53% of total n-3 LCPUFA intake. The mean intake of DHA met the current European Union (EU) consensus recommendation at the third trimester of pregnancy but not at the first and second trimester of pregnancy and 3 months postpartum (Table 3).
Only 27% of pregnant (means of 2 or 3 trimesters) and 25% of postpartum women met the current EU consensus recommendation of 200 mg DHA daily. When only dietary sources were considered, the median (inter-quartile range) value of DHA dropped to 28 (9–72) and 17 (3–60) mg/day and only 13% of participants met the EU consensus recommendation for both pregnancy and 3 months postpartum (data not shown). The mean intake of total n-3 LCPUFA at all time-points during pregnancy and 3 months postpartum was below the ISSFAL/EU and ADA recommendations of a minimum daily intake of 500 mg n-3 LCPUFA.
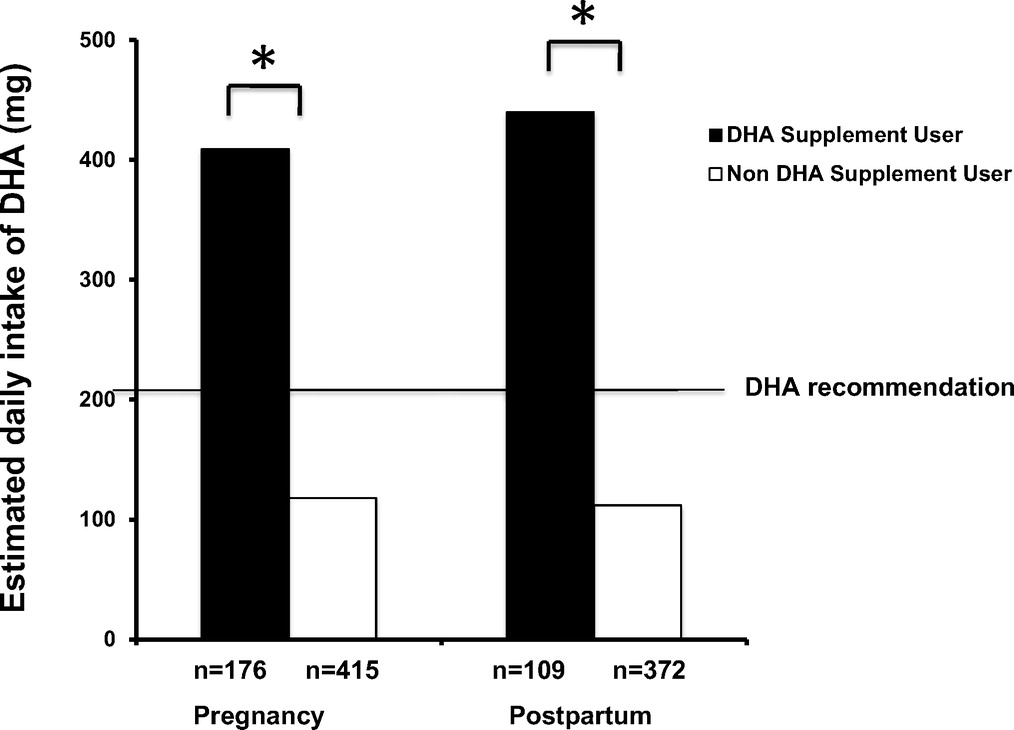
Figure 2 Thirty percent of the cohort reported taking a DHA supplement during at least 1 trimester of pregnancy and 23% reported taking a DHA supplement at 3 months postpartum. Among participants taking DHA supplements, 60% of pregnant and 63% of postpartum women met the EU consensus recommendation, whereas only 13% of both pregnant and postpartum women met the recommendation through diet alone (Figure 2). Taking a DHA supplement increased the likelihood of meeting the EU recommendation during pregnancy by 10.6 times (95% confidence interval (CI): 6.952–16.07; P < 0.001) and at postpartum by 11.1 times (95% CI: 6.803–18.14; P < 0.001).
Food sources of EPA, DPA, and DHA in the diet of pregnant and postpartum women
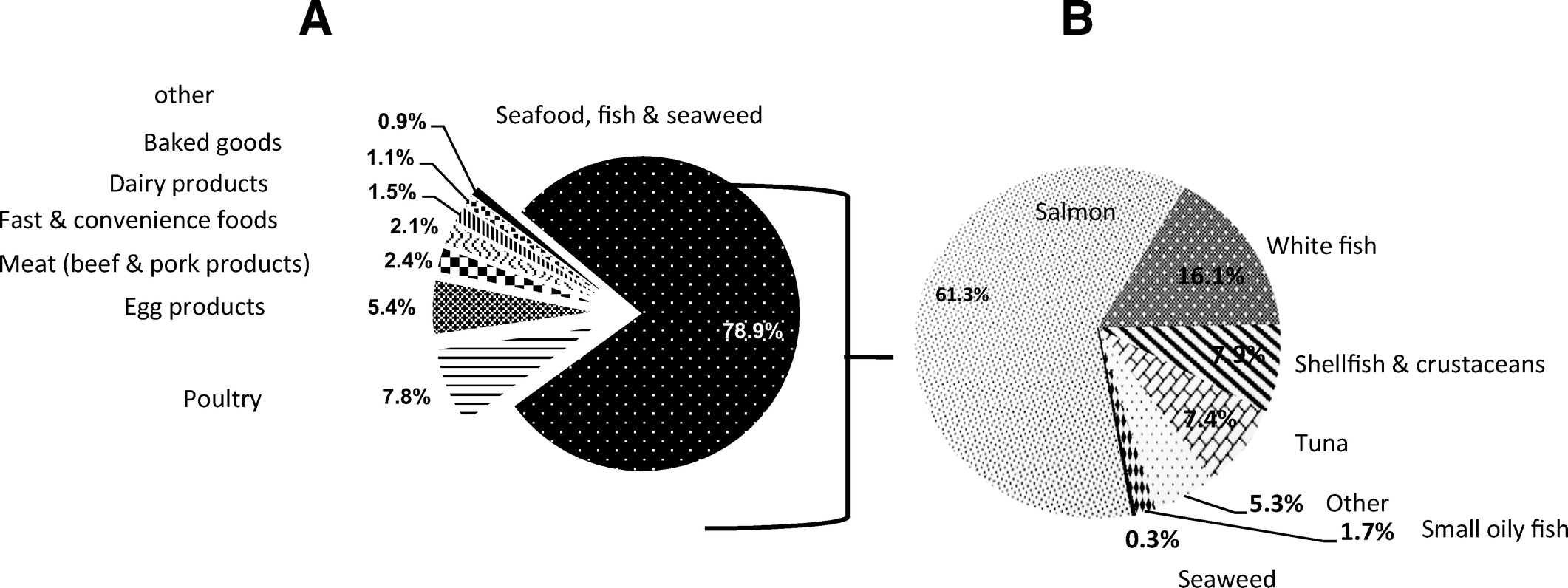
Figure 3 When all time-points were combined, seafood, fish and seaweed products were the major food contributors (79%) to total daily n-3 LCPUFA intake, 87% of EPA intake, 59% of DPA intake, and 81% of DHA intake among pregnant and postpartum women (Figure 3A; Table A1). Poultry products (14%) and meat products (11%) also made significant contributions to estimated intake of DPA intake across all time-points (Table A1). Within the seafood, fish, and seaweed category, salmon was found to be the major contributor, contributing to total n-3 LCPUFA (61%), EPA (55%), DPA (74%), and DHA (62%) intake (Figure 3B; Table A2). The other food sources within this food category included whitefish, shellfish and crustaceans, and tuna (Figure 3B).
Comparison for the estimated dietary n-3 LCPUFA intake between methods

Table 4 To determine the completeness of the commercial nutrient analysis program, the estimated n-3 LCPUFA intake presented above was compared with the output from the commercial program. The estimated dietary n-3 LCPUFA intake from the commercial program was significantly lower (5%–10%) than the data from the developed database during pregnancy (mean of the trimesters measured for each women) and postpartum (Table 4). The major contributor to the difference was DPA, which was estimated to be 20%–30% lower than the estimate using the developed database (Table 4).
Discussion
Consistent with previous studies in Canada (Friesen and Innis 2009; Sontrop et al. 2008; Denomme et al. 2005; Innis and Elias 2003), the United States (Nochera et al. 2011; Hibbeln et al. 2007; Oken et al. 2004), and Europe (Rodriquez-Bernal et al. 2013; Franke et al. 2008), the majority of pregnant and lactating women in the APrON cohort were not meeting any of the various agencies’ n-3 LCPUFA recommendations for pregnancy and lactation. However, our study is, to our knowledge, the first to report the estimated intake and sources of n-3 LCPUFA across all trimesters of pregnancy and at 3 months postpartum. The estimated intake of n-3 LCPUFA (EPA and DHA) increased significantly at the third trimester of pregnancy. As there was no difference in intake when expressed as a percentage of energy or a percentage of fat, this suggests that the increase was related to the increase in energy intake observed in this trimester.
Unlike the majority of other studies reporting n-3 LCPUFA intake during pregnancy, the majority of the participants in the APrON cohort are from high SES and 97% of the women report taking a multivitamin supplement during pregnancy (Gómez et al. 2013) Supplement use contributed to the mean intake of DHA meeting the current EU consensus recommendation during the third trimester of pregnancy in the current study. Despite this, only 27% of the women met the EU recommendation during the third trimester of pregnancy. The vast majority (73% in pregnancy and 75% in postpartum) of women in the APrON cohort were not meeting the current EU consensus recommendation. However, participants who reported taking a DHA supplement were 10.6 and 11.1 times more likely to meet the current recommendation during pregnancy and at 3 months postpartum, respectively.
Although 10 food categories (defined in the USDA and CNF databases) contributed to total n-3 LCPUFA intake in women in this study, the largest contributor to intake was seafood, fish, and seaweed products. Other foods contributing to n-3 LCPUFA intake were poultry, egg, and meat products. These sources are consistent with other North American and Australian studies, which report that seafood, fish, meat, poultry, and eggs are the primary dietary sources of total n-3 LCPUFA (Innis et al. 2013; Sioen et al. 2010; Friesen and Innis 2009; Denomme et al. 2005; Ervin et al. 2004; Meyer et al. 2003). A few studies have evaluated food source contribution to DPA intake (Rahmawaty et al. 2013; Garneau et al. 2012; Howe et al. 2006; Meyer et al. 2003), albeit not in pregnant or lactating women. We found that seafood, fish, poultry, and meat products contributed the most to overall DPA intake throughout pregnancy and lactation. These sources of DPA are consistent with that reported by Rahmawaty et al. (2013) in children and adolescents in Australia and Howe et al. (2006) and Meyer et al. (2003) in adults in Australia.
The majority (79%) of dietary n-3 LCPUFA came from the fish and seafood group (primarily salmon) and this source is almost always accessible for people living in Edmonton and Calgary (Alberta, Canada), particularly for our cohort where the participants had medium to high levels of income. The dietary sources of n-3 LCPUFA were not found to vary significantly across pregnancy and lactation. The present study also found that the estimated intake of n-3 LCPUFA was lower at 3 months postpartum. This was found to be due to a decrease in the proportion of women who reported taking an EPA/DHA supplement (30% during pregnancy and 23% at 3 months postpartum) and not their dietary intake. Our findings are similar to Sioen et al. (2010) who reported that mean intakes of EPA+DHA decreased from 328 mg/day in pregnancy to 299 mg/day during lactation. This could be a concern as maternal intake of DHA significantly affects the concentration in breast milk (Innis 2007) and 92% of the women in APrON reported breast feeding at 3 months postpartum (Jessri et al. 2013).
A strength of this study was the development of a comprehensive dietary database for n-3 LCPUFA applicable to the maternal cohort. In the present study, we found that the estimated intake of EPA and DHA were very similar between methods, confirming that the use of a commercial database provides a reasonable estimate of intake for this cohort. However, the estimated intake of DPA was 20%–30% lower using the commercial nutrient analysis program. An underestimation of DPA intake was previously reported when a food frequency questionaire was analyzed using the Australia database (that contained DPA) and the 2007b CNF (Patterson et al. 2012). Our study suggests that there are still missing estimates for DPA in foods (mixed dishes, particularly those that contain seafood, fish, poultry, and meat) in the CNF that can be partially overcome by creating a database where the DPA content is entered from the USDA Nutrient file. However, conclusions on the accuracy of our estimation requires some caution as the created database in this study was not based on fatty acid analysis of the foods and assumed that the higher intake was due to missing values for foods rather than inaccurate measurement.
Howe et al. (2006) found that, after updating the Australian database with new compositional data, the estimation of n-3 LCPUFA was 30% higher than a previously published estimate, the difference being attributable to inaccuracies in DPA content of meats. Although DPA contributes only a small amount to total n-3 LCPUFA intake, this suggests that our estimated intake, even with our developed database, may still be underestimating DPA intake. Obtaining an estimation of the intake of DPA is of interest to many as there is an emerging interest in DPA and its association with chronic diseases (Amano et al. 2011; Mozaffarian et al. 2011; Sun et al. 2008). DPA is also found in significant concentration in human breast milk (Koletzko et al. 1988), but the contribution of dietary intake of DPA to the concentration of DPA in breast milk and the role in infant development are not known.
The current study provides useful information for health practitioners and for future interventions aimed at improving the n-3 LCPUFA status of women during pregnancy and lactation. In summary, salmon and other foods in the USDA category of seafood, fish, and seaweed products contributed to 79% of overall n-3 LCPUFA intake from foods. The majority of participants in the first APrON cohort, despite a high level of education and income, were not meeting recommendations for DHA or n-3 LCPUFA that have been suggested by a number of academic and health agencies. Nutrition counselling would be one approach to improve intake as it was recently demonstrated that nutrition counselling during the first trimester of pregnancy improved n-3 LCPUFA status during pregnancy (Hautero et al. 2013). However, the current study demonstrated that taking a supplement of DHA (of approximately 275 mg during pregnancy and 299 mg at 3 months postpartum) can significantly improve a woman’s chance of meeting recommendations. However, 44% percent of the women in the cohort who reported taking a supplement during pregnancy were no longer taking these supplements when breast feeding at 3 months postpartum, supporting that education on the benefits of supplements should continue after pregnancy.
Conflict of interest statement
The authors report no conflict of interest.
Acknowledgements
The authors would like to acknowledge Anne Gilbert, Sarah Loehr, Marg Fedyna, and Erin D. Lewis who helped with data collection and database development. We are also sincerely thankful to all APrON participants and team members. This study was funded by a grant from Alberta Innovates-Health Solutions.
References:
Amano T, Matsubara T, Uetani T, Kato M, Kato B, Yoshida T, et al. 2011.
Impact of omega-3 polyunsaturated fatty acids on coronary plaque instability: an integrated backscatter intravascular ultrasound study.
Atherosclerosis 218(1): 110-116Coletto, D., and Morrison, J. 2011.
Seafood Survey: Public Opinion on Aquaculture and a National Aquaculture Act.
Available from
http://www.aquaculture.ca/files/CAIA-PUBLIC-REPORT-May-2011.pdf
[Accessed 30 July 2014.]Cosatto VF, Else PL, Meyer BJ. 2010.
Do pregnant women and those at risk of developing post-natal depression consume lower amounts of long chain omega-3 polyunsaturated fatty acids?
Nutrients 2(2): 198-213de Groot RH, Hornstra G, van Houwelingen AC, Roumen F. 2004.
Effect of ?-linolenic acid supplementation during pregnancy on maternal and neonatal polyunsaturated fatty acid status and pregnancy outcome.
Am. J. Clin. Nutr. 79(2): 251-260Denomme J, Stark KD, Holub BJ. 2005.
Directly quantitated dietary (n-3) fatty acid intakes of pregnant Canadian women are lower than current dietary recommendations.
J. Nutr. 135(2): 206-211Dietary Guidelines for Americans. 2005.
Report of the Dietary Guidelines Advisory Committee on Dietary Guidelines for Americans.
Available from
www.health.gov/dietaryguidelines/dga2005/report/
[Accessed May 2014.]EFSA Panel on Dietetic Products, Nutrition and Allergies. 2012.
Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid.
Eur. Food Safety Auth. J. 10(7): 1-48Emken EA, Adlof RO, Gulley RM. 1994.
Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males.
Biochim. Biophys. Acta 1213(3): 277-288Ervin RB, Wright JD, Wang C-Y, Kennedy-Stephenson J. 2004.
Dietary intake of fats and fatty acids for the United States population: 1999-2000.
Adv. Data 348: 1-6Franke C, Verwied-Jorky S, Campoy C, Trak-Fellermeier M,
Decsi T, Dolz V, Koletzko B. 2008.
Dietary intake of natural sources of docosahexaenoic acid and folate in pregnant women of three European cohorts.
Ann. Nutr. Metab. 53(3–4): 167-174Friesen RW, Innis SM. 2009.
Dietary arachidonic acid to EPA and DHA balance is increased among Canadian pregnant women with low fish intake.
J. Nutr. 139(12): 2344-2350Garneau V, Rudkowska I, Paradis AM, Godin G,
Julien P, Pérusse L, Vohl MC. 2012.
Omega-3 fatty acids status in human subjects estimated using a food frequency questionnaire and plasma phospholipids levels.
Nutr. J. 11(1): 46Gómez, M.F., Field, C.J., Olstad, D.L., Loehr, S.,
Ramage, S., and McCargar, L.J. 2013.
Use of micronutrient supplements among pregnant women in Alberta: Results from the Alberta Pregnancy Outcomes and Nutrition (APrON) cohort.
Matern. Child. Nutr. [Epub ahead of print.]Hautero U, Laakso P, Linderborg K, Niinivirta K,
Poussa T, Isolauri E, Laitinen K. 2013.
Proportions and concentrations of serum n-3 fatty acids can be increased by dietary counseling during pregnancy. Eur.
J. Clin. Nutr. 67(11): 1163-1168Health Canada. 2002.
Prenatal Nutrition. Health Canada, Ottawa, Ont., Canada.
Available from
www.hc-sc.gc.ca/fn-an/nutrition/prenatal/index-eng.php
[Accessed May 2014.]Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I,
Williams C, Golding J. 2007.
Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study.
Lancet 369(9561): 578-585Horvath A, Koletzko B, Szajewska H. 2007.
Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials.
Br. J. Nutr. 98(2): 253-259Howe P, Meyer B, Record S, Baghurst K. 2006.
Dietary intake of long-chain omega-3 polyunsaturated fatty acids: contribution of meat sources.
Nutrition 22(1): 47-53Imhoff-Kunsch B, Briggs V, Goldenberg T, Ramakrishnan U. 2012.
Effect of n-3 long-chain polyunsaturated fatty acid intake during pregnancy on maternal, infant, and child health outcomes: a systematic review.
Paediatr. Perinat. Epidemiol. 26: 91-107Innis SM. 2007.
Human milk: maternal dietary lipids and infant development.
Proc. Nutr. Soc. 66(3): 397-404Innis SM, Elias SL. 2003.
Intakes of essential n-6 and n-3 polyunsaturated fatty acids among pregnant Canadian women.
Am. J. Clin. Nutr. 77(2): 473-478Innis SM, Novak EM, Keller BO. 2013.
Long chain omega-3 fatty acids: micronutrients in disguise. Prostaglandins Leukotrienes Essent.
Fatty Acids 88(1): 91-95Jessri M, Farmer AP, Maximova K, Willows ND, Bell RC. 2013.
Predictors of exclusive breastfeeding: observations from the Alberta Pregnancy Outcomes and Nutrition (APrON) study.
B.M.C. Pediatr. 13(1): 77Jones ML, Mark PJ, Waddell BJ. 2014.
Maternal dietary omega-3 fatty acids and placental function.
Reproduction 147(5): R143-R152Kaplan BJ, Giesbrecht GF, Leung BM, Field CJ,
Dewey D, Bell RC, et al. 2014.
The Alberta Pregnancy Outcomes and Nutrition (APrON) cohort study: rationale and methods. Matern.
Child. Nutr. 10(1): 44-60Kaur G, Cameron-Smith D, Garg M, Sinclair AJ. 2011.
Docosapentaenoic acid (22:5n-3): a review of its biological effects.
Prog. Lipid Res. 50(1): 28-34Kelly L, Grehan B, Chiesa AD, O’Mara SM,
Downer E, Sahyoun G, et al. 2011.
The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol.
Aging 32(12): 2318.e1-2318.e15Koletzko B, Mrotzek M, Bremer HJ. 1988.
Fatty acid composition of mature human milk in Germany.
Am. J. Clin. Nutr. 47(6): 954-959Koletzko B, Cetin I, Brenna JT. 2007.
Dietary fat intakes for pregnant and lactating women.
Br. J. Nutr. 98(5): 873-877Koletzko B, Lien E, Agostoni C, Böhles H,
Campoy C, Cetin I, et al. 2008.
The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations.
J. Perinat. Med. 36(1): 5-14Kris-Etherton PM, Innis S. 2007.
American Dietetic Association, Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids.
J. Am. Diet. Assoc. 107(9): 1599-1611Larque E, Gil-Sanchez A,
Prieto-Sanchez MT, Koletzko B. 2012.
Omega 3 fatty acids, gestation and pregnancy outcomes.
Br. J. Nutr. 107: S77-S84Mennitti LV, Oliveira JL, Morais CA, Estadella D,
Oyama LM, Oller do Nascimento CM, Pisani LP. 2015.
Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring.
J. Nutr. Biochem. 26(2): 99-111Meyer BJ, Mann NJ, Lewis JL, Milligan GC,
Sinclair AJ, Howe PRC. 2003.
Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids.
Lipids 38(4): 391-398Mozaffarian D, Lemaitre RN, King IB, Song X,
Spiegelman D, Sacks FM, et al. 2011.
Circulating long-chain ?-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study.
Ann. Intern. Med. 155(3): 160-170Nochera CL, Goossen LH, Brutus AR,
Cristales M, Eastman B. 2011.
Consumption of DHA + EPA by low-income women during pregnancy and lactation.
Nutr. Clin. Pract. 26(4): 445-450Oken E, Kleinman KP, Berland WE, Simon SR,
Rich-Edwards JW, Gillman MW. 2003.
Decline in fish consumption among pregnant women after a national mercury advisory.
Obstet. Gynecol. 102(2): 346-351Oken E, Kleinman KP, Olsen SF,
Rich-Edwards JW, Gillman MW. 2004.
Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort.
Am. J. Epidemiol. 160(8): 774-783Patterson AC, Hogg RC, Kishi DM, Stark KD. 2012.
Biomarker and dietary validation of a Canadian food frequency questionnaire to measure eicosapentaenoic and docosahexaenoic acid intakes from whole food, functional food, and nutraceutical sources.
J. Acad. Nutr. Diet. 112(7): 1005-1014Pawlosky RJ, Hibbeln JR, Novotny J, Salem N. 2001.
Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans.
J. Lipid Res. 42(8): 1257-1265Rahmawaty S, Charlton K, Lyons-Wall P, Meyer B. 2013.
Dietary intake and food sources of EPA, DPA and DHA in Australian children.
Lipids 48(9): 869-877Rodriguez-Bernal CL, Ramon R, Quiles J,
Murcia M, Navarrete-Munoz EM, Vioque J, et al. 2013.
Dietary intake in pregnant women in a Spanish Mediterranean area: as good as it is supposed to be?
Public Health Nutr. 16(8): 1379-1389Sioen I, Devroe J, Inghels D,
Terwecoren R, De Henauw S. 2010.
The influence of n-3 PUFA supplements and n-3 PUFA enriched foods on the n-3 LC PUFA intake of Flemish women.
Lipids 45(4): 313-320Sontrop J, Avison WR, Evers SE,
Speechley KN, Campbell MK. 2008.
Depressive symptoms during pregnancy in relation to fish consumption and intake of n-3 polyunsaturated fatty acids. Paediatr. Perinat.
Epidemiol 22(4): 389-399Sun Q, Ma J, Campos H, Rexrode KM,
Albert CM, Mozaffarian D, Hu FB. 2008.
Blood concentrations of individual long-chain n-3 fatty acids and risk of nonfatal myocardial infarction.
Am. J. Clin. Nutr. 88(1): 216-223Vatanparast, H., Adolphe, J.L., and Whiting, S.J. 2010.
Mineral Supplement Use in Canada.
Statistics Canada, Ottawa, Ont., CanadaWilliams CM, Burdge G. 2006.
Long-chain n-3 PUFA: plant v. marine sources.
Proc. Nutr. Soc. 65(1): 42-50Yang H, Xun P, He K. 2013.
Fish and fish oil intake in relation to risk of asthma: a systematic review and meta-analysis.
PLoS ONE 8(11): e80048
Return to OMEGA-3 FATTY ACIDS
Since 3-30-2015


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |