
| PMC full text: |
|
Figure 6.
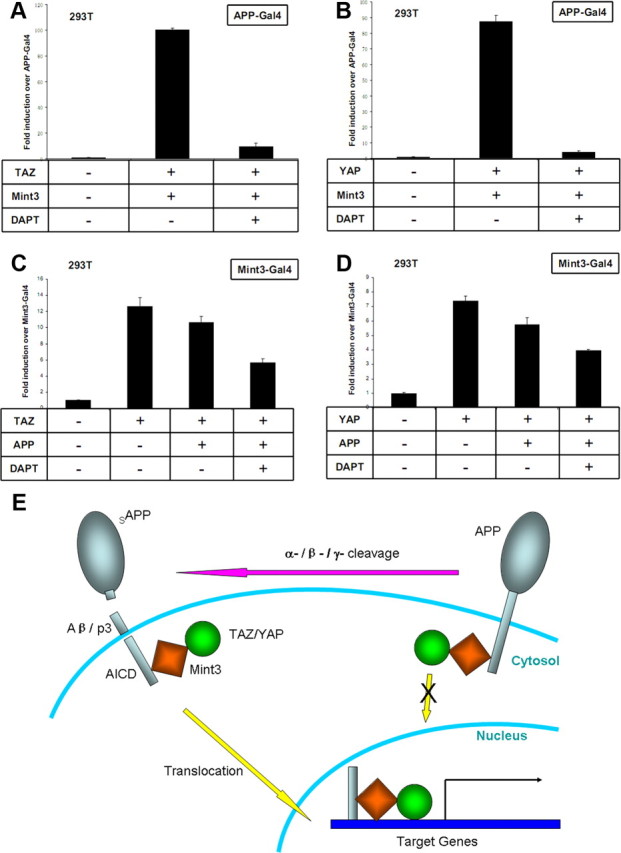
APP processing regulates TAZ/YAP- and Mint3-directed APP signaling. A, B, TAZ- and YAP-directed APP-Gal4/Mint3 transactivation was inhibited by the γ-secretase inhibitor DAPT. Potent transactivation of transcription was achieved with APP fused to the Gal4 DNA binding domain when both Mint3 and TAZ (A)/YAP (B) were present, and the transactivations were abolished by DAPT treatment. C, D, TAZ- and YAP-directed Gal4-Mint3 transactivation was inhibited by coexpression of intact APP. Potent transactivation of transcription was achieved with Mint3 fused to Gal4 DNA binding domain in the presence of TAZ (C)/YAP (D). Coexpression of full-length APP inhibited the transactivation, whereas DAPT treatment further decreased the transactivation. Diagrams exhibit representative experiments in which cells were cotransfected with a Gal4-luciferase reporter plasmid (to measure transactivation), a β-galactosidase plasmid (to normalize for transfection efficiency), and the test plasmids identified below the bars. The normalized luciferase activity is expressed as fold induction over transcription by APP-Gal4 alone (A, B), or as fold induction over Mint3-Gal4 alone (C, D). Error bars indicate SD. E, Schematic model indicating that full-length APP molecules trap Mint3 and TAZ/YAP in the cytoplasm, whereas γ-secretase cleavage releases the triple protein complexes of AICD–Mint3–TAZ/YAP and allows them to translocate into the nucleus and induce transcription of target genes.