Figure 1
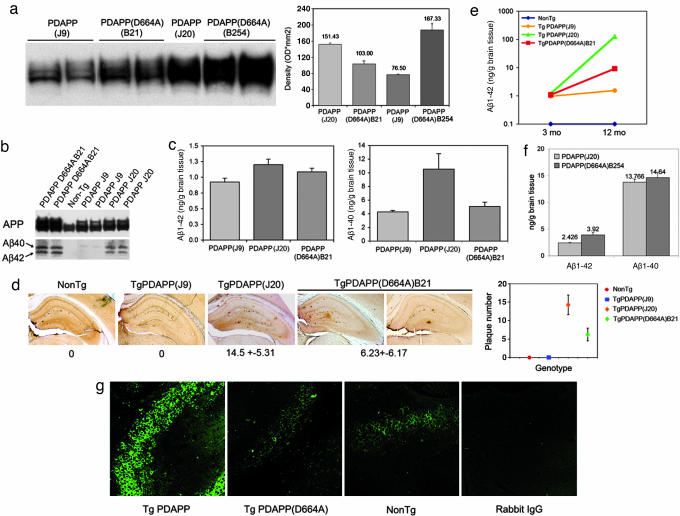
Characterization of PDAPP and PDAPP(D664A) mice. (a) APP expression. (a Left) Human and mouse APP were detected in brain homogenates by using the anti-APP CT15 antibody. (a Right) Densitometric quantitation of immunoreactivity. (b) Detection of soluble Aβ peptide. Aβ peptides in 3- to 4-mo transgenic mouse brains were detected by immunoprecipitation, followed by Western blotting with 26D6 antibody. [Note that, although Western blots suggested similar levels of expression of Aβ1–40 and Aβ1–42 by PDAPP(J20) and PDAPP(D664A)(B21), ELISA quantitations (Fig. 1c) reproducibly demonstrated that expression by PDAPP(J20) was greater than that of PDAPP(D664A)(B21)]. (c) Quantitation of soluble Aβ. Aβ1–40 and Aβ1–42 were determined at 3–4 months by ELISA as described in Methods (n = 26). (d) Quantitation of Aβ deposits. (d Left) Fifty-micrometer vibratome brain sections of transgenic 12-mo mice were stained with 3D6 antibody. (d Right) Total hippocampal Aβ plaques were counted by investigators blinded to strain and genotype (n = 18); means ± SEM. (e and f) Quantitation of soluble Aβ. ELISA assays were as described in Methods. (g) Cleavage of APP at Asp-664 in vivo. An antibody specific for the neoepitope generated by cleavage of APP at Asp-664 (refs. 4 and 6; see also Supporting Text and Fig. 5, which are published as supporting information on the PNAS web site) was used to demonstrate an increase in cleavage in PDAPP in comparison with both controls and PDAPP(D664A) mice.