

Cervical Musculoskeletal Impairment in Frequent
Intermittent Headache. Part 1: Subjects
with Single HeadachesThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Cephalalgia 2007 (Jul); 27 (7): 793–802 ~ FULL TEXT
OPEN ACCESS G Jull 1 M Amiri, J Bullock-Saxton, R Darnell, C Lander
Division of Physiotherapy,
The University of Queensland,
St Lucia, Australia
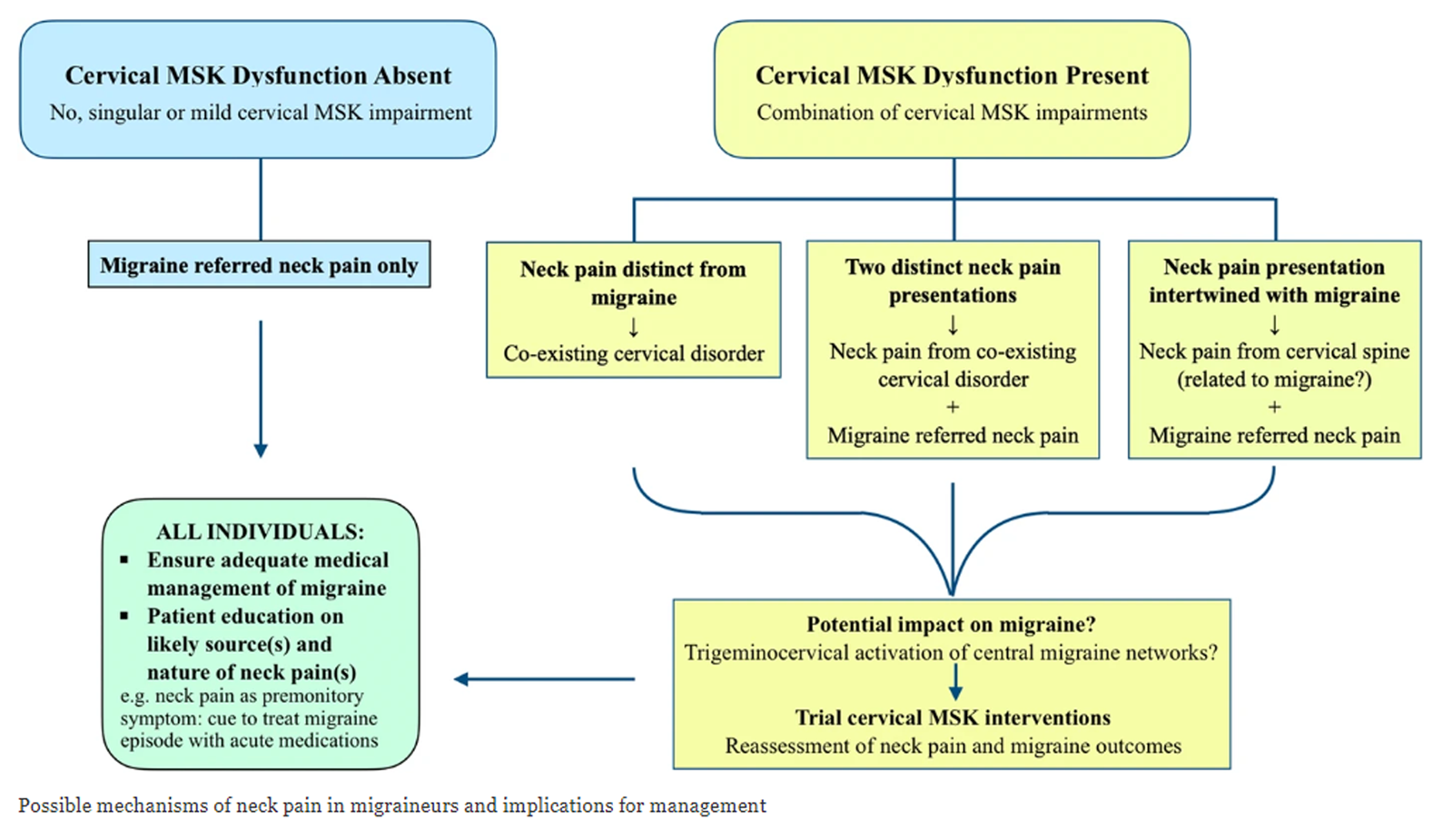
FROM: Archives of Physiotherapy 2021Musculoskeletal disorders are considered the underlying cause of cervicogenic headache, but neck pain is commonly associated with migraine and tension-type headaches. This study tested musculoskeletal function in these headache types. From a group of 196 community-based volunteers with headache, 73 had a single headache classifiable as migraine (n = 22), tension-type (n = 33) or cervicogenic headache (n = 18); 57 subjects acted as controls. Range of movement, manual examination of cervical segments, cervical flexor and extensor strength, the cranio-cervical flexion test (CCFT), cross-sectional area of selected extensor muscles at C2 (ultrasound imaging) and cervical kinaesthetic sense were measured by a blinded examiner. In all but one measure (kinaesthetic sense), the cervicogenic headache group were significantly different from the migraine, tension-type headache and control groups (all P < 0.001). A discriminant function analysis revealed that collectively, restricted movement, in association with palpable upper cervical joint dysfunction and impairment in the CCFT, had 100% sensitivity and 94% specificity to identify cervicogenic headache. There was no evidence that the cervical musculoskeletal impairments assessed in this study were present in the migraine and tension-type headache groups. Further research is required to validate the predictive capacity of this pattern of impairment to differentially diagnose cervicogenic headache.
From the FULL TEXT Article:
Introduction
The role of disorders of cervical spine in frequent intermittent headache has historically been a controversial subject. [1] Cervical musculoskeletal disorders are considered to be the underlying cause of cervicogenic headache. However, there are bidirectional interactions between trigeminal afferents and afferents from the three upper cervical nerves in the trigeminocervical nucleus. [2, 3] In consequence, neck pain and hypersensitivity, features of cervicogenic headache, are not uncommon accompaniments of migraine and tension-type headaches. [1–6] Hagen et al. [6], for example, in a large cross-sectional, population-based study of 51,050 persons, reported a frequent association between headache and upper body musculoskeletal symptoms. The incidence of neck pain associated with migraine headache was twice that for persons with non-migrainous headache. There was a four times higher prevalence of chronic headache in individuals with musculoskeletal symptoms than in subjects without such symptoms. The need is evident for a better understanding of the possible role of cervical musculoskeletal dysfunction in the common frequent intermittent headache types, particularly migraine and tension-type headache, and for more discriminating clinical features of cervicogenic headache, as highlighted in the revised International Headache Society Classification (ICHD-II). [1] Such knowledge would better assist proper identification and treatment of cervical musculoskeletal causes or components of benign frequent intermittent headaches.
With respect to cervicogenic headache, the physical examination of the neck is regarded as a critical part of the diagnosis. [7] Yet the cervical musculoskeletal impairments listed in current classification criteria are either non-specific [1] or limited to a restricted range of cervical movement only. [8] There are certainly other measurable features of cervical musculoskeletal impairment in cervicogenic headache. Studies have confirmed that range of motion is reduced [9, 10], muscle function is impaired [11], there is tenderness to palpation over the upper cervical joints [12, 13] and there is asymmetry of tenderness and skin-fold thickness. [14] However, the spread in values in these features, when compared with control subjects or those with other headache types, means that no one feature is necessarily unique to cervicogenic headache. Thus, when taken singularly, their diagnostic significance is questionable. [1, 14, 15]
Investigations into the reliability of various symptomatic criteria for cervicogenic headache have likewise shown that there is no singular diagnostic feature for this headache type. [16, 17] However, Vincent [18] has shown that if seven or more criteria documented by Sjaastad et al. [19] were present collectively, then cervicogenic headache could be distinguished from migraine and tension-type headache with a high level of sensitivity and a moderate level of specificity. We questioned whether there was similarly a pattern of musculoskeletal impairment that could distinguish cervicogenic headache from other frequent intermittent headache types, in particular migraine and tension-type headache.
This study employed a set of clinical tests of the cervical articular and muscle systems, previously found to be impaired in cervical disorders, to measure cervical musculoskeletal function in a heterogeneous group reporting frequent intermittent headache as well as an asymptomatic control group. The aims of this study were: to identify the presence or absence of cervical musculoskeletal pain and impairment in frequent intermittent headache (migraine, tension-type and cervicogenic headache); and to investigate if there was a specific pattern of musculoskeletal impairment that differentiated headaches classifiable as cervicogenic from these other common frequent intermittent headaches with symptomatic overlap.
Materials and methods
Subjects
Volunteers from the community (age range 18–55 years) reporting frequent intermittent headache as well as non-headache control subjects of a similar age range and gender were sought for the study through advertisement in local media. A priori, no attempt was made to recruit subjects with a particular frequent intermittent headache type. The criterion was that volunteers were suffering from frequent intermittent headache that was not associated with a diagnosed pathological process or structural change. [20] To be included in the headache group, volunteers had to have a minimum 1–year history of headache with a frequency of one per month or greater. Participants in the non-headache group were either headache free or experienced no more than occasional mild headache (less than five times per year) for which they had never sought any medical treatment.
Sample size was based on the difference in cervical range of rotation between cervicogenic headache and control subjects as found by Zwart. [10] Ten subjects were required in each group in order to detect a 14% (24.4°) difference with a standard deviation of 20.5° at 80% power, and 95% confidence. The estimate for the total number of subjects was based on an expected proportion of headache types in any group of 10 frequent intermittent headache subjects (i.e. migraine, three; tension-type headache, five; cervicogenic headache, two). The minimal number of subjects in each group should not be less than five times the estimated proportional numbers for each headache type [R statistical analysis (n = 10)]. Thus a minimum of 50 headache subjects was required. It was expected that some subjects would be rejected with unclassifiable headaches or headaches classified as other than migraine, tension-type and cervicogenic. In addition, it could be expected that up to half the population could report more than one headache type. [5, 21] As we were interested in subjects with either single or, for the second part of the overall study, concurrent multiple headache types, it was decided not to compromise power and to overestimate required subject numbers. Thus, approximately 200 headache subjects as well as 50 control subjects were sought.
Volunteers (n = 336) who responded to the advertisements were initially screened by telephone interview. This identified 304 eligible subjects, the remainder being outside the age limits. A further 51 subjects failed to attend their scheduled testing session. Thus, 253 subjects entered the study (196 headache subjects and 57 non-headache control subjects). Ethical approval for the study was granted by the Institutional Medical Research Ethics Committee and all procedures were conducted according to the Declaration of Helsinki. Written informed consent was provided before participation.
MeasurementsQuestionnaire
A questionnaire was developed from which headache types were classified. Its construction was based on and included the symptomatic criteria used in the classification of the common frequent intermittent types of migraine with and without aura, tension-type headache (episodic or chronic), medication overuse headache [1] and cervicogenic headache as established by the Cervicogenic Headache International Study Group. [8] Subjects with more than one headache completed questionnaires for each headache type.
Tests of the cervical musculoskeletal systemCervical range of motion. Range of motion was measured in each primary plane using the Fastrak, an electromagnetic, motion-tracking device (Polhemus; Kaiser Aerospace, Palo Alto, CA, USA). Previously established methodology was used to document cervical range of motion in the directions of flexion, extension, lateral flexion and rotation. [22, 23] Three trials were performed in each direction and the mean used in analysis.
Symptomatic joint dysfunction. Manual examination was used to determine the presence or absence of painful cervical segmental joint dysfunction in the headache and non-headache control subjects. It is a clinical method of examination, which has proven reliability in the examination of neck pain and headache subjects. [12, 24] In the clinical manual examination, the assessor applies a gentle oscillatory manual force to each cervical segment and a decision of normality or abnormality is made on the perceived presence of abnormal displacement, abnormal tissue resistance to displacement and the provocation of pain by the testing procedure. [12] A categorical scale was used to grade qualitatively the tissue resistance to joint movement (normal, slight, moderate, marked resistance). A joint was rated as symptomatic if the subject rated any local or referred pain provoked on palpation as >2 on a 0–10 visual analogue scale and the trained examiner judged the presence of moderate or marked abnormal segmental tissue compliance.
Cervical muscle strength. Maximal isometric force in the neck extensor and flexor muscles was measured by utilizing a strain gauge (PM4-SG-240-5E-A; Amalgamated Instrument Co. Pty Ltd, Hornsby, Australia), which had 0.01% full-scale accuracy. A custom-designed steel frame with an attachment arm and padded head block was built for measuring isometric neck muscle strength. The analogue signals from the load cell were converted to digital data using a data acquisition system (Labview; National Instruments, Austin, TX, USA). The maximal reading of a 1–s contraction was extracted from a 4–s maximal voluntary contraction (MVC) for each repetition. Three trials were conducted for cervical flexor and extensor strength and the average used for analysis.
Measurement of cross-sectional area of selected cervical extensor muscles. Measurement of the cross-sectional area (CSA) of the muscle is one approach to evaluate muscle atrophy. [25] Magnetic resonance imaging is the gold standard for imaging, but its costs were a deterrent for its use in this community-based study. Real-time diagnostic ultrasound imaging (Diasonics Ultrasound Inc., Santa Clara, CA, USA) was used to measure the CSA of the semispinalis capitis (SC), longissimus capitis (LC) and trapezius (T) muscles at the level of C2. The measurements were made using a 7.5–MHz linear array transducer. The subject was positioned prone lying on a plinth with a specially designed head support attached to one end. This support maintained and controlled head position in all planes of motion. Once a clear image of each particular muscle was identified, this image was captured in freeze frame mode. Orientation with the C2 lamina was maintained for all muscle images. The CSA measurements were taken via automated planimetry by following the contours of muscles. Three images were taken for each CSA measurement on each side and the mean value was calculated for data analysis.
Cranio-cervical flexion muscle test. Cranio-cervical flexion is the anatomical action of the deep neck flexors, the longus capitis/colli. The laboratory version of the craniocervical flexion test (CCFT) uses a surface electrode inbuilt into a nasopharyngeal tube to access the deep cervical flexors and conventional surface electrodes to measure activity in the superficial flexors [sternocleidomastoid (SCM) and anterior scalenes (AS)] during the test. [26] The impairment identified in patients with neck disorders is less activity in the deep neck flexors associated with higher levels of SCM and AS activity when compared with control subjects. [26] Impaired performance in clinical versions of the test has been demonstrated in subjects with cervicogenic headache [11] as well as those with idiopathic neck pain and neck pain following a whiplash injury. [27–29] The test is performed in supine lying and consists of a five-staged performance of progressively inner range of cranio-cervical flexion. Subjects are guided to each stage by feedback provided by an air-filled pressure sensor (Stabiliser, Chattanooga, TN, USA), which is positioned behind the neck to monitor the slight flattening of the cervical lordosis which occurs with the contraction of the longus colli/capitus muscles. [30] The modified version of the test which measures activity in the superficial flexors (SCM) with surface electromyography (EMG) only was used in this study. Pairs of Ag/AgCl surface electrodes (Conmed, Utica, NY, USA) were positioned over the lower one-third of the SCM bellies bilaterally. [31] A 10–s recording was made during each of the five stages of the test. The raw EMG signals were amplified to 20 000 units using an AMLAB data acquisition system (Associated Measurement Pty Ltd, Sydney, Australia). The signal was sampled at a frequency of 1000 Hz and bandpass filtered between 10 and 500 Hz. Raw EMG signals were converted to root mean square (RMS) values. The 1 s of maximum RMS in the superficial neck flexor muscles was selected during the 10–s recording of each of the five stages of the CCFT. The maximum RMS was standardized against 1 s maximum RMS during a head lift task.
Cervical kinaesthetic sense. Altered kinaesthetic sense [joint position error (JPE)] has been found in cervical disorders. [32, 33] JPE was measured according to the method of Revel et al. [34] using the Fastrak system and custom software developed in our laboratory. [33] The subject's ability to relocate the head to a natural head posture, whilst blindfolded, was measured following performance of active cervical extension, left and right rotation. The difference between the starting (zero) and the position on return (JPE) was calculated in absolute degrees for each movement tested. Three trials were performed in each direction and the mean JPE was used in analysis.Procedure
A research assistant recruited all subjects and provided subjects with the questionnaire. The physical measures were conducted by an examiner blinded to the headache or non-headache status of the subject. Prior to the main study, between-day repeatability tests of the physical measures were performed on 15 subjects. The intraclass correlation coefficient values ranged between 0.88 and 0.98, indicating a high level of repeatability. Classification of headaches from the questionnaires was undertaken as the final stage of the study following completion of all measurements on all subjects to ensure no bias during the physical assessments. Two researchers (G.J., M.A.) classified the headache types on the symptomatic characteristics and pattern of the headache history. In the cases of disagreement, headaches were classified by a third examiner, a neurologist (C.L.). Agreement was necessary between two of three examiners in these cases, and, if not, the data were excluded from analysis. Prior to the main study, interexaminer agreement in classification was undertaken using questionnaires of 11 persons with frequent intermittent headache. The two researchers and the neurologist independently classified the headache types. Overall, there was 82% agreement (9/11) between examiners, which reflects the level of agreement determined by other researchers. [35]
Data management and statistical analysis
To identify the presence or not of cervical musculoskeletal impairment in the common frequent intermittent headache types, only subjects reporting a single headache type were considered for this part of the study. Those classified as migraine with or without aura were grouped into one migraine category for the purposes of this study, as were episodic and chronic tension-type headache. Migraine type, tension-type and cervicogenic headaches were the prime headache types for consideration. Headache types not fitting these categories, headaches unable to be diagnosed or those where there was examiner disagreement were rejected for this analysis. Preliminary analysis of the control population revealed that age and gender had significant effects on several physical measures. In consequence, a series of one-way analyses of covariance were used in the main analysis to investigate any differences between measures of physical impairments between headache and control groups with age and gender as covariates. Post hoc analysis (Bonferroni) was applied to determine where any differences lay. χ2 analysis was used to analyse the categorical data of the manual examination. Discriminant analysis was used to investigate whether a pattern of physical impairment distinguished cervicogenic headache from other headache types. An α level of 0.05 was chosen and data were analysed using the SPSS statistical package (Version 11.0.1; SPSS Inc., Chicago, IL, USA).
Results

Table 1 Of the 196 headache subjects, 88 reported one headache type. Data from 15 cases were discarded because of disagreement between examiners regarding classification (n = 13) and two headaches were unclassifiable. These cases were removed from further analysis, leaving 73 subjects in the single headache group. These headaches were classified on a symptomatic basis as; migraine [with (4) and without aura (18)] (n = 22), tension-type [episodic (18), chronic (15)] (n = 33) and cervicogenic (n = 18). The demographics of all subjects retained for analysis are presented in Table 1. Headache history was prolonged in each group and the average intensity of headache reported was similar, although highest for the migraine group. Approximately one-third of subjects in each group reported a headache at the time of testing, although its intensity at that time did not preclude testing. All subjects in the cervicogenic headache group reported neck pain with their headache. Neck pain was reported in 59.1% of subjects with migraine and 57.6% of those with tension-type headaches.
Measures of physical impairmentCervical range of movement

Figure 1 Analysis revealed a significant difference in range of movement between the groups in the directions of extension, rotation to the left and right (all P < 0.001). Post hoc analysis revealed that the cervicogenic headache group had lesser motion in these directions and that there was no difference between the migraine, tension-type headache and control groups (Figure 1).
Symptomatic joint dysfunction
χ2 analyses of the frequency of symptomatic cervical joints, determined by manual examination, revealed significant differences between the groups for the upper cervical levels of C0–C4 (all P < 0.001). Subjects with cervicogenic headache had a significantly higher prevalence of palpably painful upper cervical joint dysfunction in comparison with the migraine, tension-type headache and control groups. No significant between-group differences were found for the C4–C5 to C7–T1 levels.
Muscle function
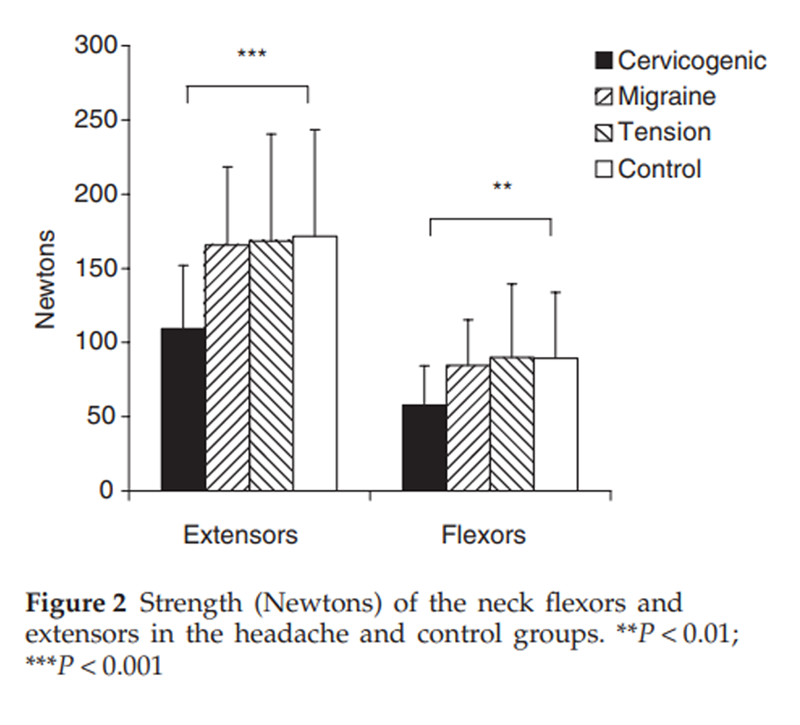
Figure 2
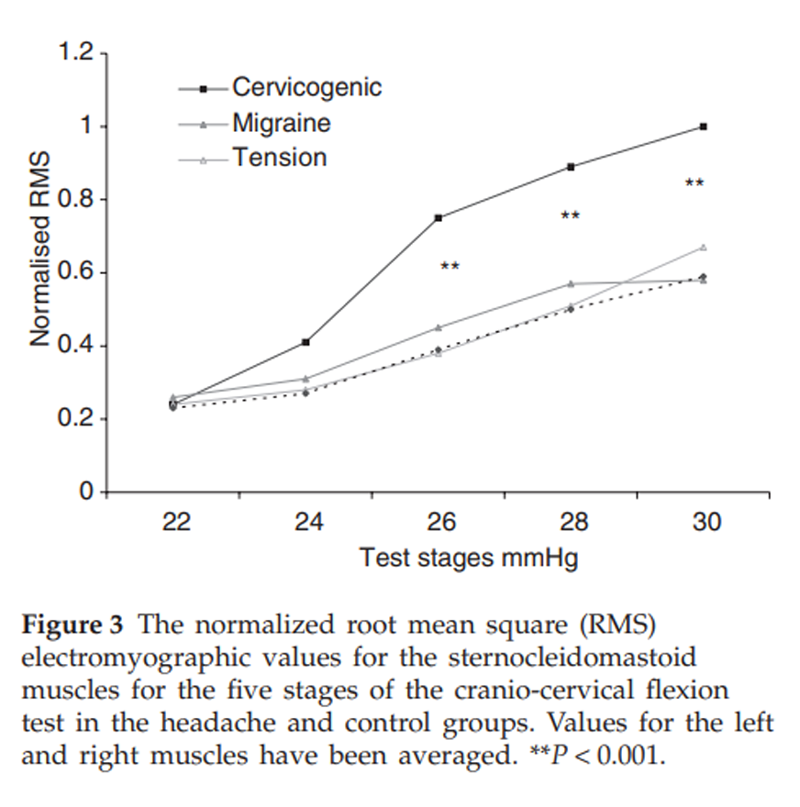
Figure 3

Table 2

Table 3 A significant between-group difference was found for cervical flexor (P < 0.001) and extensor (P < 0.001) muscle strength with post hoc analyses revealing that the cervicogenic headache group had lesser strength than other groups and there was no difference in strength between the migraine, tension-type headache and control groups (Figure 2). Analysis of extensor muscle CSA measures revealed that for migraine, tension-type headache and control groups, a systematic difference between sides existed, with right-sided muscles being slightly larger than the left muscles. For the cervicogenic headache group, muscles were grouped for symptomatic vs. non-symptomatic headache side. Analysis revealed that there was a significantly reduced CSA on the symptomatic side for the SC in comparison with the non-symptomatic side (P < 0.0001). There were no differences for the LC and upper trapezius muscles. The normalized RMS EMG values for the sternocleidomastoid muscles during the five stages of the CCFT are presented in Figure 3. As can be observed, there was an increase in EMG amplitude of SCM with progressive stages of the test for all groups, but there was significantly greater activity in the cervicogenic headache group for the final three stages of the test (P < 0.001).
Kinaesthetic sense
No differences were found between the groups for the JPE measured from rotation or extension. A discriminant analysis was undertaken to identify those physical measures that discriminated cervicogenic headache from other intermittent chronic headache forms. The results revealed three physical measures that were most important in the discrimination between the three headache categories: palpably painful joint dysfunction at the C0–C1, C1–C2, C2–C3 and C3–C4 cervical segments, the range of movement of cervical spine extension and sternocleidomastoid activity in the CCFT (Table 2). To test the sensitivity and specificity of the group of physical measures to classify cervicogenic headache from other headache types and non-headache subjects, the discriminant coefficients were used to predict the group membership in terms of a cervicogenic headache vs. non-cervicogenic headache (i.e. inclusive of migraine, tension-type headache and control subjects). The external validity was tested in a cross-validation analysis. The results of these analyses are presented in Table 3. Most notably, the cross-validation analysis indicated 100% sensitivity and 94% specificity for the pattern of physical impairment to differentiate cervicogenic headache.
Discussion
The recent ICHD-II classification [1] suggests that cervicogenic headache could be diagnosed on clinical signs that implicate a source of pain in the neck, but this and other seminal publications on cervicogenic headache [36] have called for proof of the reliability and validity of such signs. The current study investigated in the first instance whether or not there was a pattern of cervical musculoskeletal impairment which characterized cervicogenic headache or whether such clinical signs lacked specificity, in that they could be found to be present in other frequent intermittent headache types where neck pain is frequently reported.
This study has established that the cervical musculoskeletal impairments measured in this study were not associated features of migraine and tension-type headache, whereas they were characteristic of cervicogenic headache. In this study population, neck pain was reported in approximately 60% of subjects with migraine and tension-type headache. However, in all measures of physical impairment of the cervical spine the values of the measures from these groups were not different from those of the control group.
The musculoskeletal impairment recorded in the cervicogenic headache group in this study was in accordance with that found in previous studies. Restricted range of movement was confirmed as a classification criterion. [8] Both this study and that of Zwart [10] determined that the major loss was in the sagittal (extension in this study) and horizontal planes (axial rotation). This loss of active motion was accompanied by the presence of palpably painful cervical joints principally within the upper three cervical segments, which has been documented in other studies of neck pain and cervicogenic headache. [12, 24, 37–39] The predominant involvement of these segments in cervicogenic headache is in accordance with the convergence of afferents from the cervical and trigeminal nerves on second-order neurons in the trigeminocervical nucleus. [4, 15]
Not unexpectedly, and as well recognized and visible in extremity joint arthropathies, this joint dysfunction was associated with impairment in the muscle system in the cervicogenic headache group. There was a loss of cervical flexor and extensor muscle strength that was not apparent in the migraine and tension-type headache groups when compared with the control group. This is in accordance with the loss of strength and endurance previously documented in cervicogenic headache. [40, 41] The impairment in muscle control via the higher measured level of activity in the SCM in the CCFT in the cervicogenic headache group has been recorded in a number of studies of patients with cervical disorders. [26, 28, 29] Again, there was no difference in performance of the CCFT between the migraine, tension-type headache and control groups. In another variation of the clinical test, in which the stage achievable in the test was assessed (i.e. surface EMG was not used), deficiencies have been found in neck pain patients including those with cervicogenic headache. [11, 27] Atrophy (reduced CSA) was measured in the SC at the level of C2 on the side of headache in the cervicogenic headache group. This was not found for the upper trapezius and LC and the explanation for such findings may be reflected in the muscles' nerve supply. SC in the upper cervical region is innervated wholly or in part from the dorsal rami of the upper cervical nerves [42], consistent with the segmental levels which were symptomatic in the cervicogenic headache subjects. The motor supply to the upper trapezius is the accessory nerve and LC is innervated by the dorsal rami of the lower cervical nerves. [42] The lower cervical joints did not exhibit a substantial incidence of dysfunction in the cervicogenic headache subjects of this study. No differences were found in measures of kinaesthetic sense (JPE) between the headache and control groups.
Neck pain is not an uncommon feature of migraine and tension-type headache [4–6], but, as demonstrated in this study, this neck pain was not associated with cervical musculoskeletal impairment. There were no significant differences in any of the musculoskeletal features measured in this study between the migraine, tension-type headache and control groups. Consistent with this, there are no indications that the pathogenesis of migraine is related to cervical musculoskeletal disorders and equally there is no evidence that the trigeminovascular system is involved in cervicogenic headache. [43] Neck pain associated with migraine and tension-type headache seems consistent with the neurophysiological occurrence of convergence-projection involving the trigeminocervical complex [2, 3]It is also to be noted that the length of history of headache in these migraine and tension-type headache groups was prolonged (Table 1), suggesting that pain perceived in the neck from a non-musculoskeletal cause does not, with time, result in any secondary musculoskeletal impairment.
Diagnosis of frequent intermittent headache by a pattern of symptoms as used to classify headaches in this current study has limitations because of the potential overlap in symptoms of these headache types. [1] Diagnostic blocks are a recommended standard for diagnosis of cervicogenic headache [1, 8], but it could be argued that these invasive diagnostic techniques are unsuitable as a first-line diagnostic method in general practice. The recent ICHD-II classification [1] suggests that cervicogenic headache could be diagnosed on clinical signs that implicate a source of pain in the neck but more evidence was required. The diagnostic significance of an isolated feature of musculoskeletal impairment has rightly been questioned because of the potential normal variability in a single measure such as range of movement. [14, 15] To date, there has been no clinical sign or set of signs that has been helpful for diagnosis. [44] Classically, musculoskeletal disorders are characterized by a pattern of coexisting impairments in the articular and muscular systems. The notable, but logically not unexpected finding of this study was that cervicogenic headache was characterized by such a pattern of musculoskeletal impairment, and not a single clinical sign. The discriminant coefficients revealed that three physical measures collectively could discriminate cervicogenic headache from migraine, tension-type headache and control groups. In other words, a pattern of palpably painful upper cervical joint dysfunction associated with a restriction in range of movement (extension) and with muscle impairment (CCFT) distinguished this headache type. The presence of this pattern had 100% sensitivity and 94% specificity to differentiate cervicogenic headache type from migraine and tension-type headache.
The physical examination of the neck is an important aspect of the differential diagnosis of headache [7] and this study has provided evidence of a sensitive and specific pattern of cervical musculoskeletal impairment which characterizes cervicogenic headache and differentiates it from migraine and tension-type headache. Such physical characterization of cervical musculoskeletal impairment is important, as current imaging still fails to identify relevant cervical pathology to assist in differential diagnosis. [45]
The identification of specific features of cervical musculoskeletal impairment, namely the presence of palpably painful upper cervical joint dysfunction, in association with restricted cervical motion as well as specific muscle impairment as in evidence in the CCFT, provides a more comprehensive description of the musculoskeletal impairment than is currently available in classification criteria. In a clinical setting, albeit with clinically available measurement instruments, assessment of these musculoskeletal impairments can be undertaken within 15 min. These features augment the information gained from the history and symptomatic pattern of headache for diagnosis and also provide directions for specific conservative management methods for cervicogenic headache, the efficacy of which has been proven. [46]
Further research is required to validate the capacity of this pattern of cervical musculoskeletal impairment, which enables a clinical diagnosis of cervicogenic headache to be made, against the current gold standard of diagnostic blockades. If this clinical method can be validated then it will facilitate a reduction in the need for invasive diagnostic techniques in sufferers of frequent intermittent headache with neck pain and help answer the challenge posed in the ICHD-II for the clinical diagnosis of cervicogenic headache.
References:
Headache Classification Subcommittee of the International Headache Society.
The International Classification of Headache Disorders, 2nd Edition.
Cephalalgia 2004; 24 (Suppl. 1):1–151.Bartsch T, Goadsby P.
Stimulation of the greater occipital nerve induces
increased central excitability of dural afferent input.
Brain 2002; 125:1496–509.Bartsch T, Goadsby P.
Increased responses in trigeminocervical nociceptive neurons
to cervical input after stimulation of the dura mater.
Brain 2003; 126:1801–3.Bartsch T, Goadsby P.
The trigeminocervical complex and migraine. Current concepts and synthesis.
Curr Pain Headache Rep 2003; 7:371–6.Fishbain D, Cutler R, Cole B, Rosomoff H, Rosomoff R.
International Headache Society headache diagnostic
patterns in pain facility patients.
Clin J Pain 2001; 17:78–93.Hagen K, Einarsen C, Zwart J, Svebak S, Bovim G.
The co-occurrence of headache and musculoskeletal symptoms
amongst 51,050 adults in Norway.
Eur J Neurol 2002; 9:527–33.van Suijlekom H, de Vet H, van den Berg S, Weber W.
Interobserver reliability in physical examination of
the cervical spine in patients with headache.
Headache 2000; 40:581–6.Sjaastad O, Fredriksen TA, Pfaffenrath V.
Cervicogenic headache: diagnostic criteria.
Headache 1998; 38:442–5.Hall T, Robinson K.
The flexion-rotation test and active cervical mobility—
a comparative measurement study in cervicogenic headache.
Man Ther 2004; 9:197–202.Zwart JA.
Neck mobility in different headache disorders.
Headache 1997; 37:6–11.Jull G, Barrett C, Magee R, Ho P.
Further characterisation of muscle dysfunction in cervical headache.
Cephalalgia 1999; 19:179–85.Jull G, Bogduk N, Marsland A.
The Accuracy of Manual Diagnosis for Cervical
Zygapophysial Joint Pain Syndromes
Med J Aust 1988 (Mar 7); 148 (5): 233–236Zito G, Jull G, Story I.
Clinical tests of musculoskeletal dysfunction in the
diagnosis of cervicogenic headache.
Manual Ther 2006; 11:118–29.Sjaastad O, Fredriksen T, Petersen H, Bakketeig L.
Features indicative of cervical abnormality. A factor to
be reckoned with in clinical headache work and research?
Funct Neurol 2003; 18:195–203.Bogduk N.
The neck and headaches.
Neurol Clin N Am 2004; 22:151–71.Leone M, D’Amico D, Grazzi L, Attanasio A, Bussone G.
Cervicogenic headache: a critical review of current diagnostic criteria.
Pain 1998; 78:1–5.Leone M, D’Amico D, Moschiano F, Farinotti M, Filippini G, Bussone G.
Possible identification of cervicogenic headache among
patients with migraine: an analysis of 374 headaches.
Headache 1995; 35:461–4.Vincent M.
Validation of criteria for cervicogenic headache.
Funct Neurol 1998; 13:74–5.Sjaastad O, Fredriksen TA, Pfaffenrath V.
Cervicogenic headache: diagnostic criteria.
Headache 1990; 30:725–6.Lance JW.
Mechanism and management of headache, 6th edn.
Oxford: Butterworths-Heinemann Ltd 1998.Pfaffenrath V, Kaube H.
Diagnostics of cervicogenic headache.
Funct Neurol 1990; 5:159–64.Dall’Alba P, Sterling M, Treleaven J, Edwards S, Jull G.
Cervical range of motion discriminates between
asymptomatic and whiplash subjects.
Spine 2001; 26:2090–4.Trott P, Pearcy M, Ruston S, Fulton I, Brien C.
Threedimensional analysis of active cervical motion:
the effect of age and gender.
Clin Biomech 1996; 11:201–6.Jull G, Zito G, Trott P, Potter H, Shirley D, Richardson C.
Inter-examiner reliability to detect painful upper cervical joint dysfunction.
Aust J Physiother 1997; 43:125–9.Rankin G, Stokes M, Newham D.
Size and shape of the posterior neck muscles measured by ultrasound
imaging: normal values in males and females of different ages. Man Ther 2005; 10:106–15.Falla D, Jull G, Hodges P.
Neck pain patients demonstrate reduced activity of the deep neck flexor
muscles during performance of the craniocervical flexion test.
Spine 2004; 29:2108–14.Chiu T, Ey EL, Chiu T.
Performance of the craniocervical flexion test
in subjects with and without chronic neck pain.
J Orthop Sports Phys Ther 2005; 35:567–71.Jull G, Kristjansson E, Dall’Alba P.
Impairment in the cervical flexors: a comparison of
whiplash and insidious onset neck pain patients.
Man Ther 2004; 9:89–94.Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R.
Development of motor system dysfunction following whiplash injury.
Pain 2003; 103:65–73.Mayoux-Benhamou MA, Revel M, Vallee C, Roudier R, Barbet JP, Bargy F.
Longus colli has a postural function on cervical curvature.
Surg Radiol Anat 1994; 16:367–71.Falla D, Dall’Alba P, Rianoldi A, Merletti R, Jull G.
Location of innervation zones of sternocleidomastoid and scalene muscles—
a basis for clinical and research electromyography applications.
Clin Neurophysiol 2002; 113:57–63.Kristjansson E, Dall’Alba P, Jull G.
A study of five cervicocephalic relocation tests
in three different subject groups.
Clin Rehabil 2003; 17:768–74.Treleaven J, Jull G, Sterling M:
Dizziness and Unsteadiness Following Whiplash Injury:
Characteristic Features and Relationship
with Cervical Joint Position Error
J Rehabil Med 2003 (Jan); 35 (1): 36–43Revel M, Andre-Deshays C, Minguet M.
Cervicocephalic kinesthetic sensibility in patients with cervical pain.
Arch Phys Med Rehabil 1991; 72:288–91.van Suijlekom JA, de Vet HC, van den Berg SG, Weber WE.
Interobserver reliability of diagnostic criteria
for cervicogenic headache.
Cephalalgia 1999; 19:817–23.Pöllman W, Keidel M, Pfaffenrath V.
Headaches and the cervical spine: a critical review.
Cephalalgia 1997; 17:801–16.Gijsberts TJ, Duquet W, Stoekart R, Oostendorp R.
Pain-provocation tests for C0–4 as a tool in the diagnosis of cervicogenic headache.
Cephalalgia 1999; 19:436 (Abstract).Lord SM, Barnsley L, Wallis BJ, Bogduk N.
Third occipital nerve headache: a prevalence study.
J Neurol Neurosurg Psych 1994; 57:1187–90.Sandmark H, Nisell R.
Validity of five common manual neck pain provoking tests.
Scand J Rehabil Med 1995; 27:131–6.Dumas JP, Arsenault AB, Boudreau G, Magnoux E, Lepage Y, Bellavance A et al.
Physical impairments in cervicogenic headache:
traumatic vs. nontraumatic onset.
Cephalalgia 2001; 21:884–93.Watson D, Trott P.
Cervical Headache: An Investigation of Natural Head Posture
and Upper Cervical Flexor Muscle Performance
Cephalalgia 1993 (Aug); 13 (4): 272—284Strandring S, editor.
Grays anatomy, 38th edn.
Amsterdam: Elsevier 2005.Frese A, Schilgen M, Edvinsson L, Frandsen E, Evers S.
Calcitonen gene-related peptide in cervicogenic headache.
Cephalalgia 2005; 25:700–3.Bogduk N.
Distinguishing primary headache disorders from cervicogenic
headache: clinical and therapeutic implications.
Headache Curr 2005; 2:27–36.Coskun O, Ulcer S, Karakurum B, Atasoy H, Yildirim T, Ozkan S et al.
Magnetic resonance imaging of patients with cervicogenic headache.
Cephalalgia 2003; 23:842–5.Jull G, Trott P et al.
A Randomized Controlled Trial of Exercise and
Manipulative Therapy for Cervicogenic Headache
Spine (Phila Pa 1976) 2002 (Sep 1); 27 (17): 1835—1843
Return to HEADACHE
Since 11-02-2022


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |