

A Modern Neuroscience Approach to Chronic Spinal Pain:
Combining Pain Neuroscience Education with
Cognition-targeted Motor Control TrainingThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Phys Ther. 2014 (May); 94 (5): 730–738 ~ FULL TEXT
OPEN ACCESS Jo Nijs, Mira Meeus, Barbara Cagnie, Nathalie A. Roussel, Mieke Dolphens,
Jessica Van Oosterwijck, Lieven Danneels
Pain in Motion Research Group,
Departments of Human Physiology and Physiotherapy,
Faculty of Physical Education & Physiotherapy,
Vrije Universiteit
Brussels, Belgium
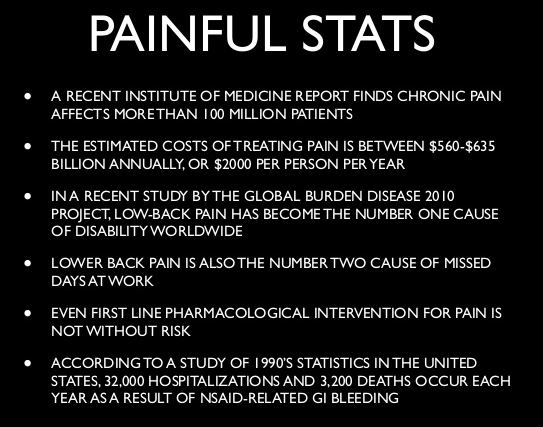
Chronic spinal pain (CSP) is a severely disabling disorder, including nontraumatic chronic low back and neck pain, failed back surgery, and chronic whiplash-associated disorders. Much of the current therapy is focused on input mechanisms (treating peripheral elements such as muscles and joints) and output mechanisms (addressing motor control), while there is less attention to processing (central) mechanisms. In addition to the compelling evidence for impaired motor control of spinal muscles in patients with CSP, there is increasing evidence that central mechanisms (ie, hyperexcitability of the central nervous system and brain abnormalities) play a role in CSP. Hence, treatments for CSP should address not only peripheral dysfunctions but also the brain. Therefore, a modern neuroscience approach, comprising therapeutic pain neuroscience education followed by cognition-targeted motor control training, is proposed. This perspective article explains why and how such an approach to CSP can be applied in physical therapist practice.
From the FULL TEXT Article:
Introduction
Chronic spinal pain (CSP) includes nonrecurrent chronic low back pain, failed back surgery, chronic whiplash-associated disorders, and chronic nontraumatic neck pain, among other conditions, and accounts for a large proportion of the chronic pain population. [1] Chronic spinal pain is a severely disabling disorder characterized by tremendous personal and socioeconomic impact, with long-term sick leave, low quality of life, and very high socioeconomic costs. [2]
Within the context of the management of painful musculoskeletal disorders, it is crucial to consider the concept of pain mechanisms. [3]
Pain mechanisms have been broadly categorized into:(1) input mechanisms, including nociceptive pain and peripheral neurogenic pain;
(2) processing mechanisms, including central pain and central sensitization and the cognitive-affective mechanisms of pain; and
(3) output mechanisms, including autonomic, motor, neuroendocrine, and immune systems. [4]Except for inflammatory pain conditions (eg, rheumatoid arthritis) or noninflammatory sources of ongoing spinal nociception, the stage of real tissue damage or nociception has disappeared in CSP. Within this context, there is increasing evidence that central mechanisms (ie, brain abnormalities [changes in brain structure and function] and hyperexcitability of the central nervous system [sensitization of the brain]) play a tremendous role in patients with CSP.
Brain atrophy, especially decrease in the density of brain gray matter (containing the neural cell bodies), [5–10] has been shown repeatedly in patients with chronic low back pain. Besides brain atrophy, descending pain inhibition or brain-orchestrated analgesia is malfunctioning in people with CSP. [11–14] The latter suggests a cardinal role for hyperexcitability of the central nervous system, or central sensitization, in patients with CSP. Many patients with CSP show features of central sensitization, [2, 11, 13–22] which is operationally defined as “an amplification of neural signalling within the central nervous system that elicits pain hypersensitivity.” [23](pS2)
In addition to the disturbed pain and central brain mechanisms, there is compelling evidence of impaired motor control in patients with CSP. [24–31] Intriguingly, these dysfunctions do not spontaneously resolve when spinal pain dissipates, [32] and our previous accomplishments have shown that these dysfunctions are even observable in patients with recurrent pain during periods of remission. [25, 33, 34] Optimal function of the back muscles is a prerequisite for static and dynamic control of spinal stiffness and movement. This impaired motor control implies that spinal muscles of patients with CSP are no longer able to accurately control body postures and movements.
Much of our current therapy is focused on input mechanisms (treating peripheral elements such as muscles and joints) and output mechanisms (addressing motor control), while there is less attention to processing (central) mechanisms. Although randomized clinical trials have shown that exercise therapy for improving spinal motor control is effective in reducing pain and disability related to CSP, [35–37] the effects are similar to those seen in response to general exercise therapy not addressing spinal motor control. [38, 39] In addition, the effect sizes of exercise therapy for improving spinal motor control in patients with CSP are rather small, [38, 39] limiting its socioeconomic impact.
In order to adopt the treatment for the brain abnormalities seen in patients with CSP and increase the effect sizes and socioeconomic impact of treatment for CSP, it seems to be mandatory to address the central mechanisms in CSP as well. This mandate asks for a modern neuroscience approach to CSP using a comprehensive rehabilitation program comprising pain neuroscience education followed by cognition-targeted motor control training. At present, physical therapy for patients with CSP is either based on a pure biomedical model (eg, neuromuscular training) or is biopsychosocially driven (eg, graded exposure in vivo, graded activity, multidisciplinary pain treatment). This perspective article argues to combine both approaches in an approach that addresses peripheral dysfunctions (here narrowed to impaired motor control of spinal muscles) in a broader biopsychosocially driven framework.
In the first part of this article, the theoretical rationale for applying the modern neuroscience approach to CSP is presented. In the second part, the application in clinical practice is explained.
Abnormal Brain Structure and Function in Patients With CSP
The interplay between the brain and the spinal muscles is crucial for accurate control of body movements and postures, suggesting that the function and structure of the brain may be affected in patients with CSP. This reasoning has been confirmed by several neuroscience studies. Brain atrophy, especially brain gray matter density and volume decrease, has been shown repeatedly in patients with chronic low back pain. [5–10] These studies demonstrated a loss of gray matter volume in patients with chronic low back pain compared with healthy controls, more specifically in the dorsolateral prefrontal cortex, thalamus, brain stem, and somatosensory cortex, which was strongly correlated with pain duration and pain intensity. Yet, studies examining brain gray matter density and volume in patients with acute spinal pain are essentially lacking. Such studies, including longitudinal studies examining the transition from acute spinal pain to CSP, are needed for assessing the true meaning of the observed changes in brain gray matter density and volume in patients with CSP. Longitudinal studies should unravel whether brain changes are the cause or the consequence of pain.
One study examined the transition from subacute to chronic low back pain and showed that when pain persisted (in contrast to patients recovering from low back pain and healthy controls), brain gray matter density decreased. [40] Recent studies that investigated the effect of surgical interventions demonstrated that many of the gray matter changes observed in patients with pain subsided with cessation of pain. [41–43] It is suggested, therefore, that the gray matter abnormalities found in people with CSP do not reflect brain damage but rather a reversible consequence of chronic pain, which normalizes when the pain is adequately treated. However, until now, no brain imaging studies have evaluated how physical therapy can influence gray matter volume.
Brain atrophy is not the only brain abnormality observed in patients with CSP. Impaired motor control of spinal muscles in patients with recurrent pain and CSP implies maladaptive brain plasticity of motor control-related brain areas. [24, 44–46] In other words, in patients with CSP, the brain regions involved in spinal motor control are altered, which influences the brain's capacity to accurately control body movements and postures. Hence, the brain of patients with CSP appears to undergo changes not only with regard to structure but also in function, especially of regions involved in spinal motor control. These changes often are referred to as reorganization of motor control–related brain areas or smudging of the motor brain. [24, 44–46] Thus, there is no wonder that patients with CSP have difficulty in fine-tuning body movements during activities of daily living.
Interestingly, the brain abnormalities in patients with CSP are reversible. One uncontrolled study showed that effective medical pain treatment (surgery or infiltrations) is accompanied by restoration of brain atrophy (gray matter volume) and function (brain activity during cognitive tasks) in humans with chronic low back pain, [5] but further studies are needed to confirm these preliminary findings in other treatments with known long-term benefits such as exercise and behavioral therapies. In another study, it was shown that motor control training, and not unskilled general exercise, can reverse reorganization of the motor cortex in patients with CSP (ie, low back pain). [47] The observed relationship between cortical reorganization and changes in motor coordination following motor training stresses the potential mechanisms of this specific approach.
The Sensitized Brain of Patients With CSP
With respect to the altered brain function, not only are motor control–related brain areas involved, but brain-orchestrated pain processing also is a malfunctioning in people with CSP. [11–14] Briefly, the brain controls 2 major pain systems: a facilitatory system (the accelerator) and an inhibitory system (the brake). Malfunctioning of brain-orchestrated analgesia in people with CSP implies that the brake is not working properly, contributing to the process of central sensitization, which is thought to play an important role in different chronic pain populations.
Central sensitization, characterized by generalized hypersensitivity of the somatosensory system, [48] is due to a dominance of the facilitatory system over the inhibitory system. More specifically at the brain level, central sensitization encompasses altered sensory processing, [49] malfunctioning descending inhibition, [50] increased activity of descending pain facilitatory pathways, [49] and an increased efficacy in processing of incoming nociceptive stimuli (temporal summation of second pain or wind-up [49] and long-term potentiation [51, 52]). In addition, the brain regions and circuits activated during pain (ie, the pain neuromatrix) differ: patients with central sensitization and CSP show more brain activity in response to painful stimuli and have brain activity in regions normally not involved in pain sensations. [53] It is important to highlight that central sensitization is not only seen in patients with chronic pain but also has been demonstrated to occur soon after injury [17] and is dependent upon the context of the injury (environmental influences surrounding the injurious event).
A brain that is constantly processing a pain experience does not have the opportunity to maintain circuitry for fine motor control, postural control, language, and even emotions. [54] These changes are observed as maladaptive “output mechanisms” in these patients, whereby they become incapable of isolating a particular muscle in a motor control exercise. [54] In this respect, it is important to highlight the impact of unhelpful emotions, such as emotional distress, and the neurobiological evidence suggesting they generate central nervous system pain sensitization. [55]
Clinically, central sensitization implies that patients show decreased pain thresholds all over their body, as well as increased sensitivity for nonmechanical stimuli such as bright light, sound or noise, stress, odors, and medication. Patients typically experience disproportionate pain, implying that the severity of pain and related disability (eg, intolerance to activities of daily living) are disproportionate to the nature and extent of injury or pathology (ie, tissue damage).
Many patients with CSP, including those with chronic traumatic neck pain, [14–18] chronic pelvic pain, [19] chronic low back pain, [11, 13, 20] osteoarthritis, [200, 56] and rheumatoid arthritis, [57] show features of central sensitization. Our group has contributed to this understanding, [48, 57, 58] including several studies in patients with CSP. [13, 14, 21, 22, 59] The studies that provided evidence favoring central sensitization in patients with CSP mainly include brain imaging studies, psychophysical testing studies, and cerebral metabolism studies. [11–14] Given the increased awareness that central sensitization provides an evidence-based explanation for many cases of CSP, rehabilitation of such patients should target, or at least take into account, the process of central sensitization. [58]
A Modern Neuroscience Approach for the Treatment of CSP
As studies have demonstrated that patients with CSP demonstrate both changes in peripheral dysfunctions and alterations in brain structure and function, treatments for CSP should address not only the peripheral dysfunctions of spinal muscles and joints but also the brain. Therefore, it is our belief that an accurate approach has to tackle both, which means that pain neuroscience education is followed by a more specific treatment of the movement dysfunction. The second part can consist of different treatment components accounting for our current understanding of spinal pain (eg, hands-on manual therapy, graded activity, exercise therapy with different therapeutic goals [circulation, sensorimotor control, mobility, endurance, strength, and so on]), depending on what emerges from the clinical reasoning as the most dominant peripheral dysfunction. This article intends to focus on motor control dysfunctions as a dominant peripheral dysfunction in CSP and puts forward the “modern neuroscience approach.”
This approach to CSP entails pain neuroscience education followed by cognition-targeted motor control training. For clarity, the treatment is divided into 3 consecutive phases. However, in clinical practice, the 3 phases will naturally merge together.
Phase 1: Therapeutic Pain Neuroscience Education
The presence of central sensitization implies that the brain produces pain, fatigue, and other “warning signs” even when there is no real tissue damage or nociception. This issue can be addressed by explaining to patients with CSP the mechanism of central sensitization with evidence from modern neuroscience, a strategy known as “therapeutic pain neuroscience education.”
Therapeutic pain neuroscience education enables patients with CSP to understand the controversy surrounding their pain, including the lack of objective biomarkers or imaging findings. One of the main goals of therapeutic pain neuroscience education is changing pain beliefs through the reconceptualization of pain. The focus is convincing patients that pain does not, in itself, result from tissue damage. Pain neuroscience has taught us that pain is often present without tissue damage, that pain is often disproportionate to tissue damage, and that tissue damage (and nociception) does not, in itself, result in the feeling of pain. To some extent, this finding relates to the clinical feature of “hurting versus harming,” for which there is much scientific support, for instance with the fear-avoidance model [60–62] that can be placed in a multidimensional framework. [63] Therapeutic pain neuroscience education intends to transfer this knowledge to patients with CSP. This transfer of knowledge enables applying a time-contingent approach (“Perform the exercise for 5 minutes, regardless of the pain”) instead of a symptom-contingent approach (“Stop the exercise once it hurts”) to exercises and physical activity.
Why is a time-contingent approach preferred over a symptom-contingent approach? Central sensitization implies that the brain can produce pain and other “warning signs” even when there is no real tissue damage. A symptom-contingent approach may facilitate the brain in its production of nonspecific warning signs such as pain, and a time-contingent approach may deactivate brain-orchestrated top-down pain facilitatory pathways. This view is supported by findings of reduced central nervous system hyperexcitability [64] and an increase in prefrontal cortical volume [65] in response to time-contingent therapy in patients with chronic pain.
Therapeutic pain neuroscience education is acceptable to patients [66, 67] and was found to be effective for changing pain beliefs and improving health status in patients with various chronic pain disorders, [66, 67] including those with CSP. [68–73] However, the effects are small, and education is insufficient as a sole treatment. [67]
Practice guidelines for therapeutic pain neuroscience education were presented previously [66, 67]; this approach encompasses 2 to 3 individual sessions spread over at least 2 weeks. Detailed pain neuroscience education is needed to reconceptualize pain and to convince the patient that hypersensitivity of the central nervous system rather than local tissue damage may be the cause of the presenting symptoms. The content of the education sessions can be based on the book Explain Pain, [84] covering the characteristics of acute versus chronic pain, the purpose of acute pain, how acute pain originates in the nervous system (nociceptors, ion gates, neurons, action potential, nociception, peripheral sensitization, synapses, synaptic gap, inhibitory and excitatory chemicals, spinal cord, descending and ascending pain pathways, role of the brain, pain memory, and pain perception), and how pain becomes chronic (plasticity of the nervous system, modulation, modification, central sensitization, the pain neuromatrix theory).
One of the common pitfalls of this approach is that it implies the patient's misunderstanding the neuroscience education message and believing that he or she is being told “the pain is all in your head.” This pitfall can be prevented by in-depth explanation of the neurophysiology of pain and chronic pain, before discussing the potential sustaining factors of central sensitization such as emotions, stress, illness perceptions, pain cognitions, and pain behavior. Acute nociceptive mechanisms are typically explained first and are then contrasted with central sensitization processes (ie, in the case of CSP). Illustrations, examples, and metaphors are frequently used. [75] Explaining pain neuroscience to patients with pain can become a challenge, particularly to patients of modest intellectual capability or those distracted by strong emotion. Therefore, the general messages need to be delivered in a language and at a pace that take into account the patient's level of intellectual ability and health literacy.
Hence, it is clear that the boundary between education and therapeutic intervention is hard to define precisely. Much more than an educational framework is required for providing effective therapeutic pain neuroscience education. The wording therapeutic pain neuroscience communication is applicable here, and such communication can open the avenue for a behavioral change (including adherence to exercise therapy). Therapeutic pain neuroscience communication should be regarded as an inherent part of the treatment program.
Therapeutic pain neuroscience education prepares patients for a time-contingent, cognition-targeted approach to daily physical activity and exercise therapy. Therapeutic pain neuroscience education is a continuous process initiated during educational sessions prior to and continuing into active treatment and followed-up during the longer-term rehabilitation program [67] through specific exercise therapy. Before moving on to the next phase, it often is helpful to determine whether the patient has adopted adaptive pain beliefs. This step can be done by thorough questioning of the patient's illness perceptions, use of self-reported measures such as the Pain Catastrophizing Scale , [76] or asking the patient to explain the nature of his or her pain.
NOTE: You may review the Pain Catastrophizing Scale at our Outcome Assessment Section
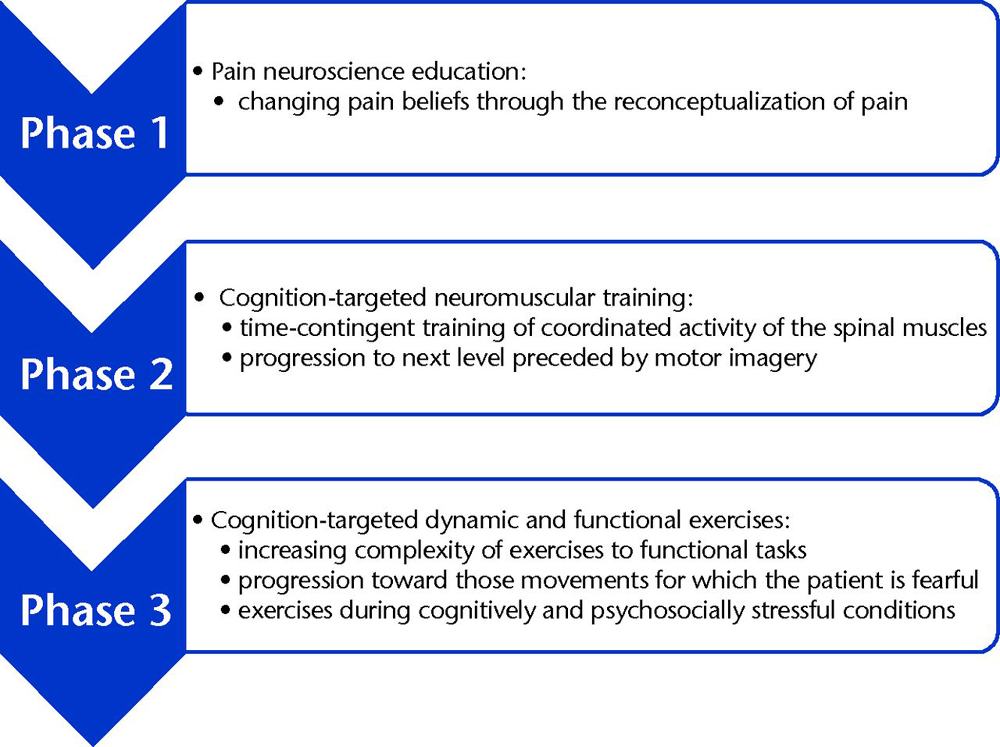
Figure 1 Once the patient has adopted adaptive beliefs regarding CSP, the next step can be taken: exercise therapy with specific emphasis on spinal motor control training. In the treatment of CSP, it is crucial not to initiate motor control training before the patient has adopted adaptive pain beliefs. Thus, therapeutic pain neuroscience education precedes motor control training [71] (Figure).
The cognition-targeted motor control exercise program is divided into 2 stages (ie, phases 2 and 3). The exercises can be introduced using motor imagery, and they can be integrated with increasing complexity using a time-contingent progression and practiced in different environments and contexts in order to maximize transfer to daily situations. This approach is detailed below.
Phase 2: Cognition-Targeted Neuromuscular Training
The training consists of a proprioceptive, coordination, and sensorimotor control training program based on the principles and ideas published in the work of many innovative researchers and clinicians such as Richardson and Jull, [77] Comerford and Mottram, [78] and Sahrmann. [79] The exercises are designed to improve function of specific muscles of the spinal region and control of posture and movement. The aim of this phase is to restore an optimal balance among the different muscles, which often means that the deeper muscles need to be facilitated by independent activation while overactive superficial muscles need to be inhibited in an individualized manner. [77] In patients with low back pain, this phase of the exercise program involves retraining of the deep muscles surrounding the lumbopelvic region (eg, multifidus, transversus abdominis, psoas, pelvic-floor muscles), whereas retraining of the deep cervical flexors and extensors and scapular muscles is proposed for patients with neck pain.
However, within a modern neuroscience approach to CSP, it is mandatory that motor control training be cognition targeted.
This approach includes the following modifications to the original motor control training program:
All exercises are performed in a time-contingent rather than in a symptom-contingent way.
Progression to the next level of more difficult exercises can be preceded by an intermediate phase of motor imagery (ie, the patients are imagining that they are performing the exercise or activity) for training or retraining the brain circuitry responsible for successful execution of the targeted movement. [71]
The treating physical therapist is advised to address the patients' cognitions about their problems as well as their perceptions about the outcome of the exercises during the cognition-targeted motor control training so that they will have positive perceptions regarding their illness and treatment outcome.
The treating therapist is advised to take the time required to discuss the patients' perceptions about each exercise. This modification includes discussion of the anticipated consequences of the exercises (eg, pain increase, further damage to the spine) and challenging the patients' cognitions in relation to the exercises. The pre-exercise communication facilitates the application of the principles learned during the preparatory phase of therapeutic pain neuroscience education during exercise interventions.
Phase 3: Cognition-Targeted Dynamic and Functional Exercises
The purpose of this phase is to implement precision of the desired coordination, train these skills in static tasks, and incorporate them into dynamic tasks and functional positions. It involves increasing the complexity of the exercises by progressing through a range of functional tasks and exercises targeting coordination of trunk and limb movements, maintenance of optimal trunk stability, and improvement of posture and movement patterns.
Progression of exercises is targeted and developed toward those movements and activities for which the patient is fearful (eg, forward bending in case of low back pain). [71] Indeed, especially those movements and activities that are fearful should be exercised, meaning that the exercise program is individually tailored. The Photograph Series of Daily Activities (PHODA) scale can be used to obtain a hierarchy of fearful movements and activities. [80] In addition to the PHODA, other practical tools such as the the Fear of Daily Activities Questionnaire [81] are available for assessing specific fear of activities. The Fear of Daily Activities Questionnaire generates reliable data and appears responsive to therapeutic change. [81] Final progression can include exercising during physically demanding tasks, exposure to the feared movements and activities, and exercising during cognitively and psychosocially stressful conditions. [71] In addition, the participants should be instructed to perform a daily set of home exercises.
During the exercise program, the therapist may review the principles learned during the therapeutic pain neuroscience education, as it is known that patients with CSP who are fear avoidant show larger correlations between pain expectancies for movements depicted in the PHODA and their ratings of predicted and experienced pain during exercises. [82] As is the case during phase 2, progression to a next level of exercises can be preceded by mentally imaging the task (motor imagery) in order to train the brain. [71] In such cases, therapists should try to decrease the anticipated danger (threat level) of the exercises by challenging the nature of, and reasoning behind, their fears, ensuring the safety of the exercises, and increasing confidence in a successful accomplishment of the exercise.
Clinical trials have shown that both pain neuroscience education [68, 69, 72] and exercise therapy for improving spinal motor control [35–37] are effective sole treatments for people with CSP, but small-scale studies that combined both suggest a strong synergistic effect. These studies reported large effect sizes and small numbers needed to treat. [70, 71, 73] Still, the proof of concept requires confirmation in a larger, multicenter trial with appropriate evidence-based control intervention. Evidence of its specific clinical efficacy in comparison with cognitive and behavioral approaches for CSP is currently lacking and warrants further study as well.
Selecting Patients for the Modern Neuroscience Approach
It needs to be considered that not all patients with CSP require motor control training. Depending on what emerges from the clinical reasoning as the most dominant peripheral dysfunction, therapeutic approaches will be chosen. Some may benefit more from grading daily physical activity levels or using aerobic exercise therapy. It is relevant to mention that in such patients, the same principles as explained here can be applied: therapeutic pain neuroscience education preceding cognition-targeted grading of daily physical activity levels (ie, graded activity) or aerobic exercise therapy (ie, graded exercise therapy). In addition, some patients still hold catastrophic beliefs about pain and movement, including irrational fear of movement, even after intensive pain neuroscience education. This finding represents a pitfall, as it will be inappropriate to progress with these patients toward the phase of cognition-targeted motor control training. In such patients, more educational time might be warranted, together with slow progression in low-grade and cognition-targeted exercises for altering the patient's beliefs about the interplay between pain and movement.
In addition, it should be recognized that the general approach may not be suitable for all patients with CSP. For instance, special mental health expertise may be needed independently or conjointly to help patients with CSP showing high levels of pain-associated distress or psychologically mediated disability. In general, large-scale studies are needed to identify treatment predictors or subgroups that benefit most. Such work could help to contextualize the overall clinical value of the approach and perhaps assist in the further development of an individualized, patient-centered approach.
Conclusions
The brain of patients with CSP differs from the “healthy” brain in structure and function: besides impaired motor control of spinal muscles, patients with CSP also exhibit hyperexcitability of the central nervous system and brain abnormalities such as decreased brain matter density. Still, the exact nature of the brain abnormalities remains to be established, as brain gray matter density and volume in patients with acute spinal pain warrant in-depth study. In order to adopt the treatment for the brain abnormalities seen in patients with CSP, a treatment comprising therapeutic pain neuroscience education followed by cognition-targeted motor control training can be applied. Therapeutic pain neuroscience education is ongoing throughout exercise therapy and motor re-education. Given the evidence that novel motor skill training is associated with rapid changes in cortical excitability as well as cortical reorganization, [47] this training type is considered relevant for treating patients with chronic musculoskeletal pain. [83]
Dr Meeus is awardee of the 2012 Early Research Career Grant of the International Association for the Study of Pain (IASP). Dr Van Oosterwijck is a postdoctoral research fellow funded by the ME Association's Ramsay Research Fund (United Kingdom). Dr Nijs is holder of the Chair on Exercise Immunology and Chronic Fatigue in Health and Disease funded by the European College for Decongestive Lymphatic Therapy, the Netherlands.
REFERENCES:
Manchikanti L, Singh V, Datta S, et al.
Comprehensive review of epidemiology, scope, and impact of spinal pain.
Pain Physician. 2009;12:E35–E70.Chronische Aandoeningen.
Available at:
https://his.wiv-isp.be/nl/Gedeelde%20%20documenten/MA_NL_2008.pdf
Accessed February 6, 2014.Gifford L, Butler D.
The integration of pain sciences into clinical practice.
J Hand Ther. 1997;10:86–95.Gifford L.
Pain, the tissues and the nervous system: a conceptual model.
Physiotherapy. 1998;84:27–36.Seminowicz DA, Wideman TH, Naso L, et al.
Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function.
J Neurosci. 2011;31:7540–7550.Schmidt-Wilcke T, Leinisch E, Ganssbauer S, et al.
Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients.
Pain. 2006;125:89–97.Apkarian AV, Sosa Y, Sonty S, et al.
Chronic back pain is associated with decreased prefrontal and thalamic gray matter density.
J Neurosci. 2004;24:10410–10415.Buckalew N, Haut MW, Morrow L, Weiner D.
Chronic pain is associated with brain volume loss in older adults: preliminary evidence.
Pain Med. 2008;9:240–248.Wood PB.
Variations in brain gray matter associated with chronic pain.
Curr Rheumatol Rep. 2010;12:462–469.Ung H, Brown JE, Johnson KA, et al.
Multivariate classification of structural MRI data detects chronic low back pain.
Cereb Cortex. 2012 Dec 17 [Epub ahead of print]. doi: 10.1093/cercor/bhs378.Giesecke T, Gracely RH, Grant MA, et al.
Evidence of augmented central pain processing in idiopathic chronic low back pain.
Arthritis Rheum. 2004;50:613–623.Siddall PJ, Stanwell P, Woodhouse A, et al.
Magnetic resonance spectroscopy detects biochemical changes in the brain associated with chronic low back pain: a preliminary report.
Anesth Analg. 2006;102:1164–1168.Roussel NA, Nijs J, Meeus M, et al.
Central sensitization and altered central pain processing in idiopathic chronic low back pain: fact or myth?
Clin J Pain. 2013;29:625–638.Van Oosterwijck J, Nijs J, Meeus M, Paul L.
Evidence for central sensitization in chronic whiplash: a systematic literature review.
Eur J Pain. 2013;17:299–312.Herren-Gerber R, Weiss S, Arendt-Nielsen L, et al.
Modulation of central hypersensitivity by nociceptive input in chronic pain after whiplash injury.
Pain Med. 2004;5:366–376.Jull G, Sterling M, Kenardy J, Beller E.
Does the presence of sensory hypersensitivity influence outcomes of physical rehabilitation for chronic whiplash? A preliminary RCT.
Pain. 2007;129:28–34.Sterling M, Jull G, Vicenzino B, Kenardy J.
Sensory Hypersensitivity Occurs Soon After Whiplash Injury and Is Associated With Poor Recovery
Pain. 2003 (Aug); 104 (3): 509–517Sterling M, Treleaven J, Edwards S, Jull G.
Pressure pain thresholds in chronic whiplash associated disorder: further evidence of altered central pain processing.
J Musculoskelet Pain. 2002;10:69–81.Yang CC, Lee JC, Kromm BG, et al.
Pain sensitization in male chronic pelvic pain syndrome: why are symptoms so difficult to treat?
J Urol. 2003;170:823–826; discussion 6–7.Staud R.
Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia.
Curr Rheum Rep. 2011;13:513–520.Van Oosterwijck J, Nijs J, Meeus M, et al.
Lack of endogenous pain inhibition during exercise in people with chronic whiplash associated disorders: an experimental study.
J Pain. 2012;13:242–254.Daenen L, Nijs J, Roussel NA, et al.
Dysfunctional pain inhibition in patients with chronic whiplash-associated disorders: an experimental study.
Clin Rheumatol. 2013;32:23–31.Woolf CJ.
Central sensitization: implications for the diagnosis and treatment of pain.
Pain. 2011;152:S2–S15.Tsao H, Danneels LA, Hodges PW.
ISSLS prize winner: smudging the motor brain in young adults with recurrent low back pain.
Spine (Phila Pa 1976). 2011;36:1721–1727.D'hooge R, Hodges P, Tsao H, Hall L, et al.
Altered trunk muscle coordination during rapid trunk flexion in people in remission of recurrent low back pain.
J Electromyogr Kinesiol. 2013;23:173–181.Hodges PW.
The role of the motor system in spinal pain: implications for rehabilitation of the athlete following lower back pain.
J Sci Med Sport. 2000;3:243–253.Cagnie B, Dirks R, Schouten M, et al.
Functional reorganization of cervical flexor activity because of induced muscle pain evaluated by muscle functional magnetic resonance imaging.
Man Ther. 2011;16:470–475.Roussel NA, De Kooning M, Schutt A, et al.
Motor control and low back pain in dancers.
Int J Sports Med. 2013;34:138–143.Roussel NA, Nijs J, Truijen S, et al.
Altered breathing patterns during lumbopelvic motor control tests in chronic low back pain: a case-control study.
Eur Spine J. 2009;18:1066–1073.Danneels LA, Vanderstraeten GG, Cambier DC, et al.
CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects.
Eur Spine J. 2000;9:266–272.Danneels LA, Coorevits PK, Cools AM, et al.
Differences in multifidus and iliocostalis lumborum activity between healthy subjects and patients with subacute and chronic low back pain.
Eur Spine J. 2002;11:13–19.Hides JA, Richardson CA, Jull GA.
Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain.
Spine (Phila Pa 1976). 1996;21:2763–2769.D'hooge R, Cagnie B, Crombez G, et al.
Lumbar muscle dysfunction during remission of unilateral recurrent nonspecific low-back pain: evaluation with muscle functional MRI.
Clin J Pain. 2013;29:187–194.D'hooge R, Cagnie B, Crombez G, et al.
Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral recurrent low back pain.
Man Ther. 2012;17:584–588.Macedo LG, Latimer J, Maher CG, et al.
Effect of motor control exercises versus graded activity in patients with chronic nonspecific low back pain: a randomized controlled trial.
Phys Ther. 2012;92:363–377.Unsgaard-Tondel M, Fladmark AM, Salvesen O, Vasseljen O.
Motor control exercises, sling exercises, and general exercises for patients with chronic low back pain: a randomized controlled trial with 1-year follow-up.
Phys Ther. 2010;90:1426–1440.Falla D, O'Leary S, Farina D, Jull G.
The change in deep cervical flexor activity after training is associated with the degree of pain reduction in patients with chronic neck pain.
Clin J Pain. 2012;28:628–634.Ask T, Strand LI, Skouen JS.
The effect of two exercise regimes: motor control versus endurance/strength training for patients with whiplash-associated disorders: a randomized controlled pilot study.
Clin Rehabil. 2009;23:812–823.Wang XQ, Zheng JJ, Yu ZW, Bi X, Lou SJ, Liu J, Cai B et. al.
A Meta-analysis of Core Stability Exercise versus General Exercise for Chronic Low Back Pain
PLoS One. 2012 (Dec 17); 7 (12): e52082Baliki MN, Petre B, Torbey S, et al.
Corticostriatal functional connectivity predicts transition to chronic back pain.
Nat Neurosci. 2012;15:1117–1179.Obermann M, Nebel K, Schumann C, et al.
Gray matter changes related to chronic posttraumatic headache.
Neurology. 2009;73:978–983.Gwilym SE, Keltner JR, Warnaby CE, et al.
Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients.
Arthritis Rheum. 2009;61:1226–1234.Rodriguez-Raecke R, Niemeier A, Ihle K, et al.
Brain gray matter decrease in chronic pain is the consequence and not the cause of pain.
J Neurosci. 2009;29:13746–13750.Flor H, Braun C, Elbert T, Birbaumer N.
Extensive reorganization of primary somatosensory cortex in chronic back pain patients.
Neurosci Lett. 1997;224:5–8.Massé-Alarie H, Flamand VH, Moffet H, Schneider C.
Corticomotor control of deep abdominal muscles in chronic low back pain and anticipatory postural adjustments.
Exp Brain Res. 2012;218:99–109.Tsao H, Galea MP, Hodges PW.
Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain.
Brain. 2008;131:2161–2171.Tsao H, Galea MP, Hodges PW.
Driving plasticity in the motor cortex in recurrent low back pain.
Eur J Pain. 2010;14:832–839.Nijs J, Van Houdenhove B, Oostendorp RA.
Recognition of central sensitization in patients with musculoskeletal pain: application of pain neurophysiology in manual therapy practice.
Man Ther. 2010;15:135–141.Staud R, Craggs JG, Robinson ME, et al.
Brain activity related to temporal summation of C-fiber evoked pain.
Pain. 2007;129:130–142.Millan MJ.
Descending control of pain.
Prog Neurobiol. 2002;66:355–474.Zhuo M.
A synaptic model for pain: long-term potentiation in the anterior cingulate cortex.
Mol Cells. 2007;23:259–271.Suarez-Roca H, Leal L, Silva JA, et al.
Reduced GABA neurotransmission underlies hyperalgesia induced by repeated forced swimming stress.
Behav Brain Res. 2008;189:159–169.Seifert F, Maihofner C.
Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies.
Cell Mol Life Sci. 2009;66:375–390.Puentedura EJ, Louw A.
A neuroscience approach to managing athletes with low back pain.
Phys Ther Sport. 2012;13:123–133.Lumley MA, Cohen JL, Borszcz GS, et al.
Pain and emotion: a biopsychosocial review of recent research.
J Clin Psychol. 2011;67:942–968.Mease PJ, Hanna S, Frakes EP, Altman RD.
Pain mechanisms in osteoarthritis: understanding the role of central pain and current approaches to its treatment.
J Rheumatol. 2011;38:1546–1551.Meeus M, Vervisch S, De Clerck LS, et al.
Central sensitization in patients with rheumatoid arthritis: a systematic literature review.
Semin Arthritis Rheum. 2012;41:556–567.Nijs J, Van Oosterwijck J, De Hertogh W.
Rehabilitation of chronic whiplash: treatment of cervical dysfunctions or chronic pain syndrome?
Clin Rheumatol. 2009;28:243–251.Kaya S, Hermans L, Willems T, et al.
Central sensitization in urogynecological chronic pelvic pain: a systematic literature review.
Pain Physician. 2013;16:291–308.Crombez G, Vlaeyen JW, Heuts PH, Lysens R.
Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability.
Pain. 1999;80:329–339.Vlaeyen JW, Kole-Snijders AM, Boeren RG, van Eek H.
Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance.
Pain. 1995;62:363–372.Leeuw M, Goossens ME, Linton SJ, et al.
The fear-avoidance model of musculoskeletal pain: current state of scientific evidence.
J Behav Med. 2007;30:77–94.Wideman TH, Asmundson GG, Smeets RJ, et al.
Rethinking the fear avoidance model: toward a multidimensional framework of pain-related disability.
Pain. 2013;154:2262–2265.Ang DC, Chakr R, Mazzuca S, et al.
Cognitive-behavioral therapy attenuates nociceptive responding in patients with fibromyalgia: a pilot study.
Arthritis Care Res. 2010;62:618–623.de Lange FP, Koers A, Kalkman JS, et al.
Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome.
Brain. 2008;131:2172–2180.Louw A, Diener I, Butler DS, Puentedura EJ.
The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain.
Arch Phys Med Rehabil. 2011;92:2041–2056.Nijs J, van Wilgen CP, Van Oosterwijck J, et al.
How to explain central sensitization to patients with “unexplained” chronic musculoskeletal pain: practice guidelines.
Man Ther. 2011;16:413–418.Van Oosterwijck J, Nijs J, Meeus M, et al.
Pain neurophysiology education improves cognitions, pain thresholds, and movement performance in people with chronic whiplash: a pilot study.
J Rehabil Res Rev. 2011;48:43–58.Moseley GL.
Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain.
Eur J Pain. 2004;8:39–45.Moseley GL.
Widespread brain activity during an abdominal task markedly reduced after pain physiology education: fMRI evaluation of a single patient with chronic low back pain.
Aust J Physiother. 2005;51:49–52.Moseley GL.
Joining forces—combining cognition-targeted motor control training with group or individual pain physiology education: a successful treatment for chronic low back pain.
J Man Manip Ther. 2003;11:88–94.Moseley GL, Nicholas MK, Hodges PW.
A randomized controlled trial of intensive neurophysiology education in chronic low back pain.
Clin J Pain. 2004;20:324–330.Moseley GL.
Combined physiotherapy and education is efficacious for chronic low back pain.
Aust J Physiother. 2002;48:297–302.Butler D, Moseley GL.
Explain Pain.
Adelaide, Australia: NOI Group Publishing; 2003.van Wilgen CP, Keizer D.
The sensitization model to explain how chronic pain exists without tissue damage.
Pain Manage Nurs. 2012;13:60–65.Sullivan MJL, Bishop SR, Pivik J.
The Pain Catastrophizing Scale: development and validation.
Psychol Assess. 1995;7:524–532.Richardson CA, Jull GA.
Muscle control-pain control: what exercises would you prescribe?
Man Ther. 1995;1:2–10.Comerford MJ, Mottram SL.
Functional stability re-training: principles and strategies for managing mechanical dysfunction.
Man Ther. 2001;6:3–14.Sahrmann SA.
Does postural assessment contribute to patient care?
J Orthop Sports Phys Ther. 2002;32:376–379.Leeuw M, Goossens ME, van Breukelen GJ, et al.
Measuring perceived harmfulness of physical activities in patients with chronic low back pain: the Photograph Series of Daily Activities—short electronic version.
J Pain. 2007;8:840–849.George SZ, Valencia C, Zeppieri GJr, Robinson ME.
Development of a self-report measure of fearful activities for patients with low back pain: the Fear of Daily Activities Questionnaire.
Phys Ther. 2009;89:969–979.Trost Z, France CR, Thomas JS.
Examination of the Photograph Series of Daily Activities (PHODA) scale in chronic low back pain patients with high and low kinesiophobia.
Pain. 2009;141:276–282.Boudreau SA, Farina D, Falla D.
The role of motor learning and neuroplasticity in designing rehabilitation approaches for musculoskeletal pain disorders.
Man Ther. 2010;15:410–414.
Return to LOW BACK PAIN
Return to CHRONIC NECK PAIN
Return to SPINAL PAIN MANAGEMENT
Since 2-21-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |