

Is Activation of the Back Muscles Impaired
by Creep or Muscle Fatigue?This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Spine (Phila Pa 1976) 2010 (Mar 1); 35 (5): 517525 ~ FULL TEXT
Daniel Sanchez-Zuriaga, PhD Michael A. Adams, PhD, and Patricia Dolan, PhD
Department of Anatomy and Human Embryology,
University of Valencia,
Valencia, Spain
HEREStudy design: Intervention study on healthy human subjects.
Objective: To determine whether reflex activation of the back muscles is influenced by muscle fatigue or soft tissue creep in the spine.
Summary of background data: Reflex contraction of the back muscles normally acts to limit spinal flexion, and hence protect the underlying spine from injury. However, repeated flexion allows bending moments on the spine to increase. Impaired reflexes as a result of fatigue or soft tissue creep may be contributing factors.
Methods: A total of 15 healthy volunteers (8 females/7 males aged 23-55 years) underwent 2 interventions, on separate days: (a) sitting flexed for 1 hour to induce creep and (b) performing the Biering-Sorensen test to induce back muscle fatigue. Before and after each intervention, reflex activation of the erector spinae in response to sudden trunk flexion (initiated by a Kin-Com dynamometer) was monitored bilaterally at T10 and L3 using surface electromyography (EMG) electrodes. These recordings indicated the onset latency of reflex activation, the peak EMG, and time to peak, at each site. Measurements before and after each intervention and between muscle sites were compared using a 2-way repeated measures Analysis of Variance.
Results: Spinal creep was confirmed by an increase in maximum flexion of 2.3 degrees +/- 2.5 degrees (P = 0.003), and fatigue by a significant fall in median frequency at one or more sites. Following creep, onset latency increased from 60 +/- 12 milliseconds to 96 +/- 26 milliseconds (P < 0.001) but there was no change in peak EMG or time to peak EMG. Differences between sites (P = 0.004) indicated greater latencies in lumbar compared to thoracic regions, especially after creep. Muscle fatigue had no significant effects on any of the measured parameters.
Conclusion: Prolonged spinal flexion can impair sensorimotor control mechanisms and reduce back muscle protection of the underlying spine. The effect is due to time-dependent "creep" in soft tissues rather than muscle fatigue.
Key words: reflex activation, spinal creep, back muscle fatigue, sensorimotor control
From the FULL TEXT Article:
Introduction
Frequent and heavy lifting is associated with a high-risk of low back pain and disc prolapse, especially when combined with bending and twisting movements. [13] Cadaver experiments have demonstrated that repeated flexion and compression can initiate radial fissures, disc protrusion, and in some cases extrusion of nucleus pulposus from the disc, [4, 5] and mathematical models have suggested how altered tissue stresses enable these processes to occur. [6] In life, reflex contractions of the back muscles normally act to control spinal movements [7] in order to prevent excessive loading and to protect the underlying tissues from injury. However, the high-risk of back pain associated with repetitive bending and lifting suggests that these protective mechanisms may not always be adequate.
Reflex control of spinal movements depends on sensorimotor mechanisms that are initiated and modulated by mechanoreceptor afferents. Such afferents are found in a variety of spinal tissues including paraspinal [8] and intervertebral [9] muscles, supraspinous, interspinous and flaval ligaments, [10]thoracolumbar fascia, [11] apophyseal joint capsules, [12] and intervertebral discs. [8, 9] Afferents in the disc [8] and joint capsule [13] have a high mechanical threshold and are activated only under severe loading conditions, suggesting a nociceptive role, whereas afferents in the muscles, ligaments, and fascia are activated at lower thresholds and are thought to have a proprioceptive role. [8, 14] The small segmental muscles of the back have a particularly high density of muscle spindles compared with the long polysegmental muscles, [15] which suggests they play an important role in the control of spinal movements.
In recent years, animal models have helped to elucidate how sensorimotor control mechanisms operate in the spine. These studies have shown that reflex contractions of multifidus and longissimus muscles can be elicited by electrical stimulation of afferents in the discs, capsules, and ligaments, and that the reflex response is heightened when afferents in several tissues are stimulated simultaneously. [16, 17] In some cases, reflex activation of multifidus could be initiated simply by stretching the supraspinous ligament, suggesting the presence of a discrete ligamentomuscular reflex. [18] Other experiments demonstrated the existence of an intersegmental paraspinal reflex where stimulation of muscle afferents at one spinal level caused efferent activity in motor neurons at neighboring levels. [19] These sensorimotor control systems operate alongside muscle spindle and Golgi tendon organ reflexes to co-ordinate the activation of trunk muscles in order to limit movement and prevent excessive loading. However, when the spine is subjected to sustained or repetitive flexion, this reflex protection may become impaired.
Biomechanical studies have shown that repetitive bending and lifting leads to a change in lifting strategy so that subjects adopt a more stooped posture as lifting continues. [2023] This causes increased lumbar flexion and increased bending stresses (bending moments) on the underlying osteoligamentous spine. [20] These changes could be due to muscle fatigue, which reduces force output, [24, 25] alters afferent input, [25, 26] and impairs proprioception. [28, 28] Alternatively, they may be caused by soft tissue creep that occurs in human spines after only a short period of sustained [29, 30] or repetitive [20] flexion. Creep arises from time-dependent expulsion of water from spinal tissues, especially the intervertebral discs, and it causes a loss of disc height, slack in the posterior ligaments, and hence a reduced resistance to bending by the osteoligamentous spine. [31] Animal studies have shown that the resulting ligament laxity alters afferent feedback from ligamentous receptors and impairs reflex activation of the back muscles. [32]
The above findings suggest that both muscle fatigue and soft tissue creep may influence the sensorimotor mechanisms that protect the spine from excessive flexion. However, the functional importance of these different effects in humans remains uncertain. The aim of the present study was to determine how physiologic levels of creep and fatigue affect reflex activation of the back muscles in response to sudden loading. We hypothesize that reflex activation will be altered by both creep and fatigue, but the latter will have the greater effect because of its expected influence on both sensory and motor components of the reflex.
Materials and Methods
Ethical Approval
The study was approved by the Research Ethics Committee of the Faculty of Medical and Veterinary Sciences at the University of Bristol, and conformed to the standards set by the latest revision of the Declaration of Helsinki. All subjects received an information sheet that described the purpose of the study and the experiments involved, and their written informed consent to participate in the study was obtained.
Experimental Protocol
Healthy volunteers were subjected to 1 of 2 interventions on separate days. The first required subjects to sit with the lumbar spine flexed in order to induce creep in the soft tissues of the lumbar spine. The second required subjects to perform the Biering-Sorensen test in order to induce fatigue in the main trunk extensor muscles. The order of the interventions was randomized, and interventions were carried out several days apart to allow full recovery. Subjects were asked to refrain from any vigorous exercise before testing, and all tests were carried out at least 2 hours after the subjects had risen from bed to minimize the effects of diurnal variations in spinal mechanics. [33, 34] Before and after each intervention, reflex activation of the main trunk extensor muscles (the erector spinae muscles) was assessed using surface electromyography (EMG), which indicated the latency and amplitude of muscle activation in response to a sudden perturbation of the trunk.
Subjects

Table 1 A total of 15 healthy subjects (7 males/8 females) with no history of low back pain that required medical attention or time off work took part in the study. The physical characteristics of the subjects are summarized in Table 1.
Surface EMG
Subjects adopted a moderately flexed position to enable the spinous processes of thoracic and lumbar vertebrae to be palpated. Marks were made using a skin pencil at the midpoint of the spinous processes at the level of the 10th thoracic (T10), first lumbar (L1), third lumbar (L3), and first sacral (S1) vertebrae. To ensure reproducible placement of EMG electrodes and movement sensors (described later) on the second day of testing, an anatomic map was drawn, documenting the distance of the markings from the seventh cervical vertebra (C7) when the subject stood upright. Other permanent surface landmarks (moles/scars) were also noted. Four pairs of disposable skin-surface Ag/AgCl electrodes (MSB, UK) were then attached bilaterally to the skin surface overlying the erector spinae muscles at T10 and L3. Electrodes were placed with their centers approximately 5 cm from the midline of the spine with an interelectrode spacing, in erect standing, of 20 mm for each pair. Electrodes were orientated along the muscle fiber direction, in the middle of the muscle belly, to ensure the maximum amplitude signal was obtained. [7] A reference electrode was positioned over the sternum. Before attaching the electrodes, the skin was shaved, if necessary, and abrasive skin preparation pads and alcohol swabs were used to abrade and clean the skin reduce impedance. If the impedance between any recording electrode and the reference was greater than 5k Ω, then the skin preparation procedures were repeated until the impedance fell below this level. This helped to ensure a high signal-to-noise ratio.
EMG signals were acquired at a sampling rate of 500 Hz or 1024 Hz as described later. The raw signal was amplified with a differential EMG amplifier (Biodata PA400, UK), filtered with a band-pass of 5 to 300 Hz, analogue-digital converted, and recorded on a computer for subsequent analysis.
Measurement of Reflex Activation
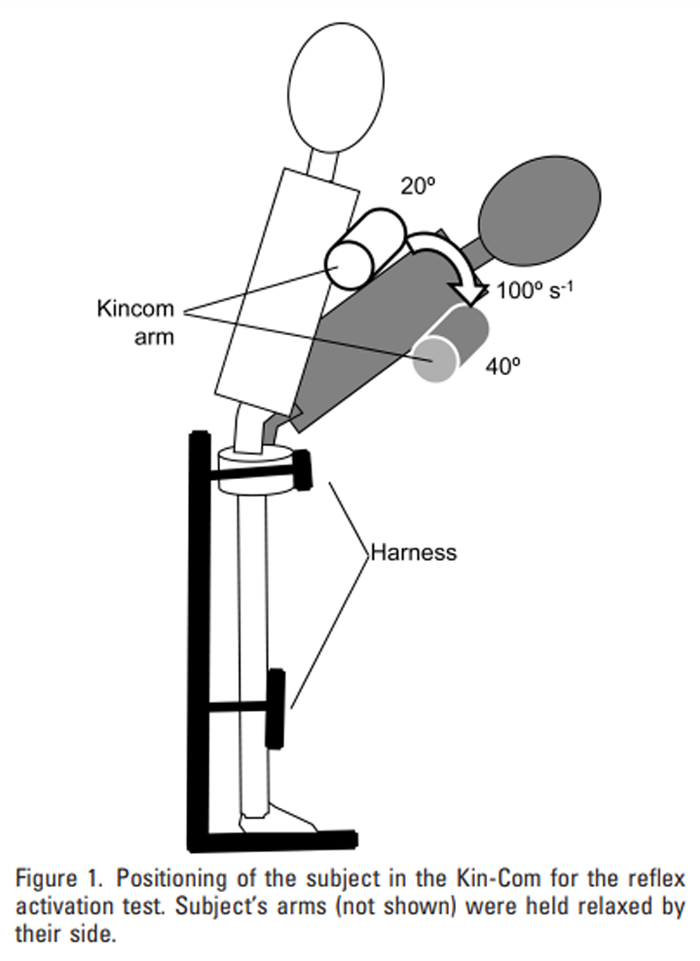
Figure 1 This was carried out before and after each intervention. Subjects were positioned in a Kin-Com isokinetic dynamometer (Chattanooga Corp., Hixson, TN) in an upright standing position with their hips and knees supported by restraining devices, and their chest resting against the cushioned movable arm of the machine. In this position, the hips and knees were restrained but the trunk could move freely. Subjects were blindfolded and given headphones with relaxing music in order to remove visual and auditory cues. The arm of the Kin-Com was then moved manually in order to place the trunk in 20° of flexion (Figure 1), and subjects were asked to relax their back muscles as much as possible so that their upper body rested completely on the cushioned arm of the machine. In this relaxed position, baseline EMG activity was recorded for at least 4 seconds, and after a variable time period, the arm of the machine was then activated to move the trunk from 20° to 40° of flexion at a velocity of 100°/sec (Figure 1), resulting in a sudden and unexpected displacement of the trunk. EMG activity was recorded at 500 Hz, full-wave rectified and averaged (with a time constant of 0.02 sec) using BioWare 3.20 software (Kistler Corp., Winterthur, Switzerland).
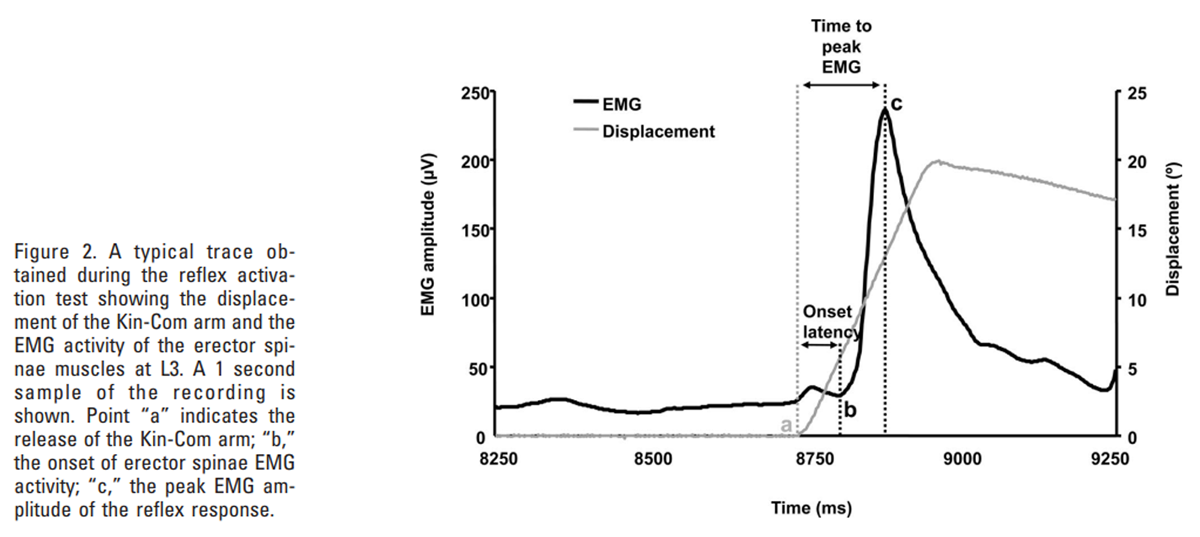
Figure 2 During each test, the onset of movement was identified from the abrupt movement of the Kin-Com arm from its baseline starting position (point a in Figure 2). From the EMG recordings, 3 parameters were determined for each muscle site:
(i) the onset latency determined as the time between the sudden displacement of the Kin-Com arm and the start of reflex activity (time difference between points a and b in Figure 2),
(ii) the peak EMG amplitude, identified as the maximum EMG signal during the initial burst of reflex activity, shortly after the perturbation (point c in Figure 2), and
(iii) the time to peak EMG determined as the time between the sudden displacement of the Kin-Com arm and the peak amplitude of reflex EMG activity (time difference between points a and c in Figure 2).These parameters were identified by visual inspection of the EMG recordings, and the onset of reflex activity was defined as the start of the first peak in EMG activity, following the perturbation, that exceeded the peak amplitude of the baseline activity.
Before each intervention, reflex activation was assessed in 2 repeated trials several minutes apart in order to assess the within-day reliability of the measurements. After each intervention, reflex activation was reassessed during a single further test which was performed within 90 seconds of completing the fatigue or creep protocol.
Fatigue Intervention
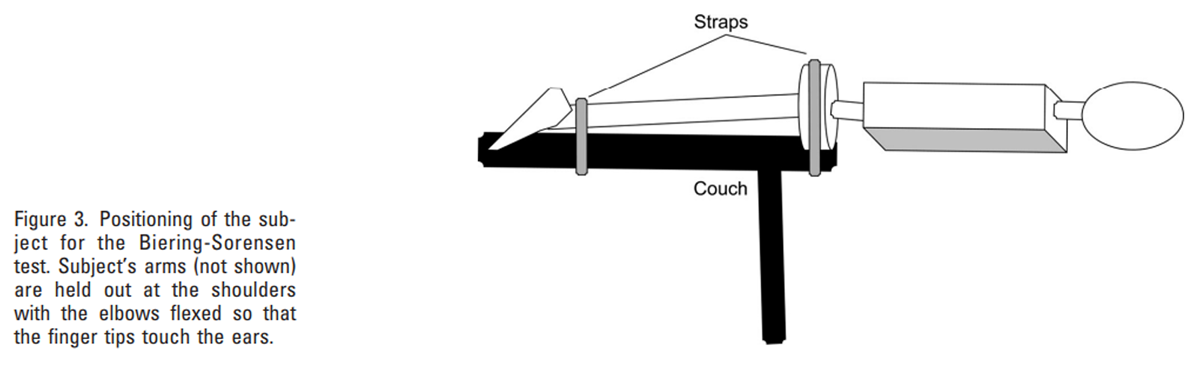
Figure 3 To induce fatigue in the erector spinae muscles, subjects were strapped to an examination couch with their upper body (above the iliac crest) overhanging the edge and their hands placed next to the head (Figure 3). They were asked to maintain their upper body horizontal for as long as possible until back muscle fatigue prevented them from continuing. [36, 37] During this test, the EMG activity of the erector spinae was recorded at 1024 Hz. A Fast Fourier Transform algorithm was used to calculate EMG power spectra for 1 second samples of EMG activity recorded at 1 second intervals throughout the test, and from these data the change in median frequency over time was estimated at each recording site using linear regression analysis to determine its rate of decline. [36, 37]
Creep Intervention
To induce creep in the soft tissues of the spine, subjects were seated in a low easy chair for 1 hour so that their lumbar spine was flexed by approximately 70% of their range of flexion. During this time, the trunk was supported enabling the back muscles to relax. [38] Range of flexion of the lumbar spine was measured before the intervention using a 3-dimensional electromagnetic movement analysis device, the 3-Space Fastrak (Polhemus, VT), which consists of a source (transmitter) of electromagnetic waves and several sensors (receivers). Sensors were attached to the skin overlying the spinous processes at L1 and S1. The sensors were positioned with the subject in a semiflexed posture and were fixed in place using small perspex baseplates that were attached to the skin using Hypafix (Smith & Nephew, London, UK) and double-sided tape. [39] The baseplates provided a flat surface for sensor attachment, and the method of attachment helped to minimize displacement of sensors relative to the spinous processes during subsequent movements.40 In order to obtain accurate measures of lumbar flexion, the source was placed on a height-adjustable stand so that it was as close as possible to the subject and approximately level with the sacral sensor during testing. [39]
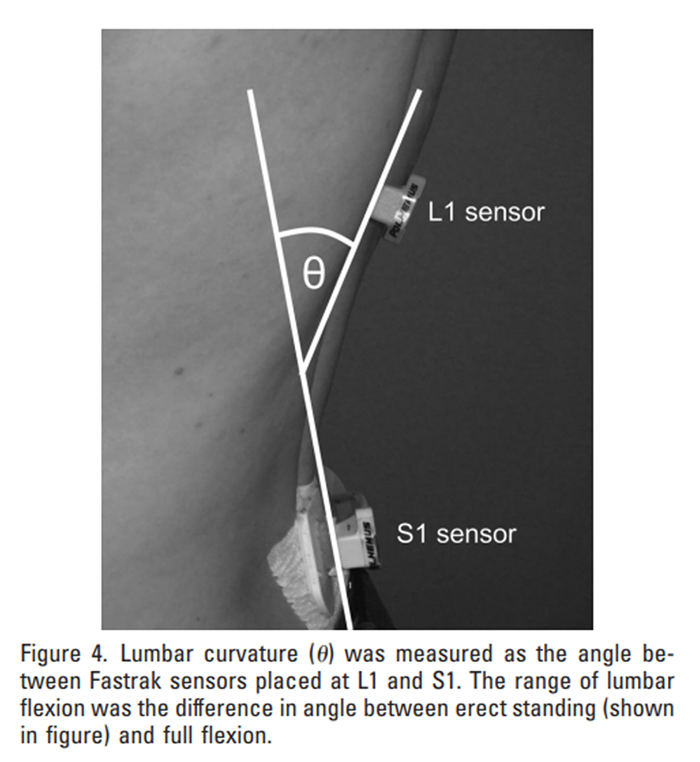
Figure 4 To measure range of flexion, lumbar curvature (the angle subtended between the L1 and S1 sensors in the sagittal plane: Figure 4) was measured in erect standing and full flexion.40 To achieve full lumbar flexion, subjects were instructed to bend forwards as far as possible in an attempt to touch their toes or touch their knees with their forehead. This test was performed in standing and with subjects seated on the floor with their legs straight in front of them, and the greater of the 2 values was recorded as maximum flexion. The range of flexion was then calculated as the difference in lumbar curvature between maximum flexion and erect standing. During the one hour sitting period, lumbar curvature was measured several times to ensure that approximately 70% flexion was maintained throughout. At the end of the 1 hour, maximum flexion was immediately remeasured to indicate the amount of creep achieved by the intervention.
Statistical Analysis
Reliability of the parameters used to assess reflex activation (onset latency, peak EMG amplitude, and time to peak EMG) was determined using repeated measures Analysis of Variance to calculate the intraclass correlation coefficient (ICC). Withinday reliability was determined by comparing values obtained in the 2 tests carried out before each intervention. Day-to-day reliability was determined by comparing the initial preintervention measurement on each testing day.
Creep was confirmed using a matched-pair Student t test to compare maximum lumbar flexion before and after prolonged sitting. Fatigue was assessed using linear regression analysis to indicate the rate of decline in median frequency during the Biering-Sorensen test.
Changes in reflex activation, as a result of each intervention, were determined using a 2-factor repeated measures Analysis of Variance to compare between muscles and between pre- and postintervention measurements. Where a main effect of muscle was identified, pairwise comparisons (least significant difference) were performed to compare between specific sites. SPSS for Windows (version 14.0) was used for all statistical analyses, and significance was accepted at an alpha level of 0.05.
Results
Reliability
For most measures of reflex activation, ICC values were between 0.60 and 0.94, indicating fair to excellent reliability. The only exceptions were the day-to-day values for the time to peak EMG, where the ICC for 3 of the 4 muscle sites was between 0.41 and 0.43. ICC values were highest for onset latency, where they ranged from 0.70 to 0.94 for within-day comparisons and from 0.60 to 0.85 for day-to-day comparisons.
Creep Intervention
Peak lumbar flexion increased after prolonged sitting with maximum lumbar curvature (Figure 4) increasing from 25.2° (standard deviation [SD], 9.4°) before the intervention to 27.5° (SD, 9.2°) afterwards (P 0.003). The average increase was 2.3° (SD, 2.5°) which corresponds to 4.1% of the average range of flexion.
Fatigue Intervention

Table 2

Table 3 Mean endurance time during the fatigue test was 172 (SD, 60) seconds. Muscle fatigue was induced in all subjects, indicated by a significant fall in median frequency at one or more of the recording sites. [36] Mean values of the median frequency gradient were as follows: T10 left, 0.18 (SD, 0.08) Hz/sec; T10 right, 0.16 (SD, 0.11) Hz/ses; L3 left, 0.24 (SD, 0.12) Hz/sec; L3 right, 0.26 (SD, 0.11) Hz/sec. Larger gradients at lumbar levels indicate a higher rate of fatigue in the lumbar erector spinae.
Effects of Creep and Fatigue on Reflex Muscle Activation
Tables 2 + 3 show the pre- and postintervention values for onset latency, time to peak EMG, and peak EMG amplitude. Following creep, peak EMG amplitude and the time to peak EMG remained unchanged. However, onset latency was increased (P 0.001, Table 2) with mean values rising from 57 to 65 milliseconds before creep to 89 to 106 milliseconds after creep. A main effect of muscle site on onset latency was also observed (P 0.004) and pairwise comparisons showed that lumbar regions demonstrated longer latencies than thoracic on both left (P 0.043) and right (P 0.002) sides of the spine. Differences were greatest after creep where average values were 103 milliseconds in the lumbar region and 90 milliseconds in the thoracic region (Table 2). Following the fatigue intervention, there were no significant changes in any of the measured parameters, and no main effects were observed between muscle sites.
Discussion
Summary of Findings
The results only partly support our hypothesis: they show that reflex muscle activation of the thoracic and lumbar erector spinae in response to a sudden perturbation becomes delayed as a result of creep but is not significantly altered by fatigue. Furthermore, no significant changes were observed, following either intervention, in the timing or amplitude of peak EMG activity associated with the reflex response.
Strengths and Weaknesses of the Study
This is the first study on human subjects to compare the effects of spinal creep and fatigue on reflex muscle activation. The increase in maximum lumbar flexion after creep, and the decrease in median frequency during the fatigue test, confirmed that the interventions were effective. Some recovery from fatigue may have occurred in the short period following the intervention before reflex activation was remeasured. However, the fatiguing protocol was fairly vigorous, requiring moderately high levels of isometric force to be sustained to the endurance limit, [36] so any effects should still have been evident at the time of reassessment. [41] Isometric contractions were used in preference to dynamic contractions to avoid inducing creep in the soft tissues. However, it is unlikely that repeated dynamic contractions would have elicited a greater fatigue response because isometric contractions at 60% MVC occlude the circulation to the muscle, thereby accelerating the fatigue process.
Reliability of the parameters used to assess reflex activation was generally good, with onset latency showing the highest ICCs. In this study, peak EMG amplitude was measured as the maximum EMG within the initial burst of activity following the perturbation in an attempt to represent reflex rather than voluntary activation. However, the Time to peak EMG showed much greater variability than the Onset latency as demonstrated by the much higher standard deviations for the former compared to the latter measurements (Tables 2, 3). This raises the possibility that, in some cases, the peak EMG may have included a component of voluntary activation incurred slightly after the initial reflex response. These findings highlight the difficulties involved in attempting to separate reflex and voluntary responses under normal physiologic conditions. They also suggest that, under such conditions, onset latency may be the most reliable measure of reflex activation to assess.
Relationship to Other Studies
Onset latencies were similar to those reported previously for the trunk muscles, [4244]and were sufficiently short to suggest that the initial muscle activation was reflex in nature. [45, 46]
Muscle fatigue is a commonly cited cause of musculoskeletal injuries, and is often held responsible for the high-risk of back pain in occupations that involve frequent bending and lifting. Some support for this assumption is provided by experimental studies, which have shown that repetitive bending and lifting is associated with an increase in spinal flexion [20, 22, 23] that leads to increased bending stresses on the osteoligamentous spine. [20] Altered motion patterns were previously attributed to fatigue in the back or leg muscles, although current findings suggest that other factors such as soft tissue creep may have contributed.
Studies on anesthetized cats have shown that ligamentous creep, induced by repeated stretching of the lumbar supraspinous ligament, causes reflex activation of multifidus to diminish due to desensitization of mechanoreceptors following laxity in the viscoelastic tissues of the spine. [32] In the present study, reflex activation of the back muscles was delayed significantly when creep was induced by sustained lumbar flexion. The consequences of this delayed response, during normal unconstrained movements, would be a slower deceleration of the trunk that would allow increased lumbar flexion and increased bending stresses on the spine, as reported previously. [20] To our knowledge, this is the first direct evidence that physiologic levels of creep in human spines can impair reflex activation of the back muscles in vivo.
The effects of delayed muscle responses could be widereaching. Sudden and unexpected loading generates high forces on the spine [47, 48] and is associated with a high-risk of low back injury, [49, 50] so an inability to respond rapidly to a sudden perturbation may increase injury risk. Longer trunk muscle latencies have been reported in people with back pain compared to healthy control subjects, [42, 51, 52] suggesting that pain may impair reflex activation, or that people with slower reflexes are at greater risk of developing back pain. Some evidence to support the latter suggestion comes from a recent prospective study which assessed trunk muscle response times in college athletes. [53] This study found that people who sustained a back injury during the 2 to 3 year follow-up had trunk muscle latencies at baseline that were 14 milliseconds longer, on average, than those who remained injury-free. In the present study, muscle activation after creep was delayed by 36 milliseconds on average, which represented a 60% increase in onset latency. These findings suggest that the risk of back injury may be increased considerably by activities that involve sustained or repeated spinal flexion.
The absence in the current study of any significant changes following the fatigue intervention suggests that any effects of fatigue on reflex muscle activation were slight or of short duration. Previous studies in human knee extensors [54] and back muscle extensors [44] have similarly found no change in the latency of muscle reflexes with fatigue. However, the effects on reflex amplitude are more variable with some studies reporting an increase in fatigued muscle [44, 54, 55] and others a decrease. [5658] These apparently conflicting findings may reflect true differences between different muscles or they may simply reflect different methodologies. The only other study to examine reflexes in the back muscles used a dynamic protocol to fatigue the muscles, and found increases in reflex EMG amplitude even after normalizing EMG for load, suggesting that force generated by the reflex was increased. [11]
In the present study, the amplitude of the reflex response remained unchanged following sustained isometric contraction of the back muscles, suggesting that any force decrement in fatigued muscle fibers was not compensated for by increasing reflex activation of the muscle. These contrasting findings may reflect differences in the extent of fatigue induced by the different fatigue protocols, or they may be due to other factors, such as increased muscle temperature or the variable responses of muscle spindles to different types of contraction. As mentioned previously, dynamic contractions involving repeated flexion and extension of the trunk have the capacity to cause creep as well as fatigue and this may have influenced the different responses reported. The present results suggest that fatigue induced in the absence of creep, for example by repeated back muscle contractions when working in an upright posture, would have only a small effect on back muscle reflexes.
Explanation of Results
The marked delay in reflex muscle activation following creep may have been mediated via a number of afferent pathways because tendon, muscles, ligaments, and fascia were all subjected to sustained stretching that may have caused adaptation or sensitization of the receptors within them.
There is evidence from both animal [59, 60] and human [61, 62] studies that activation of muscle spindles is influenced by their recent load-history. Shortening of muscle before test ing increases spindle sensitivity resulting in increased firing in response to a subsequent stretch. However, prior stretching of the muscle desensitizes the spindles so that a greater degree of stretch is then required to initiate spindle firing. Such after-effects are observed regardless of whether the muscle is active or relaxed during the movements. [62, 63] However, they may be of short duration. [64] In the present study, sustained stretching of the back muscles during creep may have caused some desensitization of the spindles located within them, and this may have contributed to the delayed reflex activation observed after creep. Reduced spindle sensitivity might also occur following dynamic trunk flexion and this could contribute to delayed muscle responses and increased spinal flexion during repetitive bending and lifting tasks.
Loading history also appears to influence afferent responses in ligamentous receptors. Previous studies on anesthetized patients and animals18 have demonstrated the existence of a ligamento-muscular reflex where activation of multifidus is initiated by stretching the supraspinous ligament. However, cyclic [32] or sustained [65] loading of the ligament attenuates the reflex response. In these studies, the fall in reflex activation of multifidus over time was attributed to stress-relaxation in the ligament, and this was confirmed by the reappearance of the reflex during subsequent loading cycles where an additional preload was applied to the ligament to offset the effects of creep. [32] In the present study, the onset of reflex activity was delayed following creep although the amplitude of the response was unaffected, indicating that the reflex was simply initiated later in the flexion movement when tissue strains would be greater. Since a greater degree of ligament and soft tissue deformation would be required to generate the same tensile stress after creep, these findings support those of the animal studies and suggest that reflex activation depends on tissue stress rather than strain.
In the present study, fatigue in the absence of creep had no significant effect on either the onset or amplitude of reflex muscle activation. These findings suggest that the reflex response initiated by the sudden perturbation could have been mediated largely by mechanoreceptors in ligaments, fascia, tendons, and joint capsules, which were little affected by metabolic changes in the fatigued muscle. Evidence to support this is provided by the onset latency of the reflex which, at 60 milliseconds, is longer than the 30 to 50 milliseconds latency expected for monosynaptic stretch reflexes mediated by Group Ia spindle afferents. [45, 66] However, polysynaptic reflexes with longer onset latencies have been recorded from Group II [67, 68] and III [69] muscle afferents following sudden perturbations, suggesting that these muscle proprioceptors may also have contributed to the reflex response in the present study.
Animal studies have shown that different muscle afferents show varying responses during sustained contractions, with nonspindle group II and group III afferents showing a late increase in firing that is enhanced by ischemia and lactic acid build-up. [70] However, other muscle afferents appear to become desensitized under these conditions, and studies on human subjects suggest that during sustained contractions at moderate load, firing of Ia and II spindle afferents reduces over time after an initial burst of activity in the early stages of the contraction. [26] These findings suggest that the effects of fatigue on reflex activation will be largely determined by the afferents involved and that heightened sensitivity in some muscle afferents may act to compensate for decreased sensitivity in others, in order to preserve reflex responses under varying contraction conditions. Variable adaptations in mechanoreceptor firing in response to fatigue may explain the lack of any notable effect in the current study.
Unanswered Questions and Future Research
This study has shown that creep leads to delayed reflex activation of the back muscles in response to sudden flexion, and during normal activities this may lead to an increased risk of bending injury. However, voluntary muscle activation may compensate to some extent for impaired reflexes so the functional consequences of creep during everyday activities remain to be determined. Fatigue responses may be influenced by muscle temperature, type of contraction (eccentric, isometric, or concentric) and even the method used to evaluate the reflexes, so future studies should be designed to evaluate the importance of these effects. Changes in central drive as a result of fatigue may also confound the interpretation of results if such changes significantly alter the relationship between EMG and force, so future studies should take account of this effect in order to determine whether the force associated with the reflex response is increased, decreased, or unaffected by fatigue.
Conclusion
Soft tissue creep causes delayed activation of the back muscles in response to sudden loading. These findings suggest that impaired reflexes in the back muscles as a result of prolonged or repeated flexion could increase the risk of bending injuries to the spine
Key Points
Sustained or repetitive spinal flexion is known to increase bending moments acting on the osteoligamentous spine, but the reasons for this are unclear.
Experiments on 15 healthy subjects showed that reflex activation of the back muscles, in response to a sudden perturbation, was delayed following sustained flexion, which induced creep in the soft tissues, but was not significantly affected by back muscle fatigue.
Impaired reflexes in the back muscles as a result of prolonged flexion could increase the risk of bending injuries to the spine.
Acknowledgment
The authors thank Clare Costigan for technical assistance.
References:
Kelsey JL, Githens PB, White AA, et al.
An epidemiologic study of lifting and twisting on the job and
risk for acute prolapsed lumbar intervertebral disc.
J Orthop Res 1984;2:616.Marras WS, Lavender SA, Leurgans SE, et al.
The role of dynamic three-dimensional trunk motion in occupationally-related
low back disorders. The effects of workplace factors, trunk position,
and trunk motion characteristics on risk of injury.
Spine 1993;18:61728.Hartvigsen J, Bakketeig LS, Leboeuf-Yde C, et al.
The association between physical workload and low back pain
clouded by the healthy worker effect: population-based
cross-sectional and 5- year prospective questionnaire study.
Spine 2001;26:178892.Adams MA, Hutton WC.
Gradual disc prolapse.
Spine 1985;10:52431.Gordon SJ, Yang KH, Mayer PJ, et al.
Mechanism of disc rupture. A preliminary report.
Spine 1991;16:4506.Schmidt H, Kettler A, Rohlmann A, et al.
The risk of disc prolapses with complex loading in different
degrees of disc degenerationa finite element analysis.
Clin Biomech 2007;22:98898.Dolan P, Adams MA.
The relationship between EMG activity and extensor moment generation
in the erector spinae muscles during bending and lifting activities.
J Biomech 1993;26:51322.Yamashita T, Minaki Y, Oota I, et al.
Mechanosensitive afferent units in the lumbar
intervertebral disc and adjacent muscle.
Spine 1993;18:22526.Roberts S, Eisenstein SM, Menage J, et al.
Mechanoreceptors in intervertebral discs.
Morphology, distribution, and neuropeptides.
Spine 1995;20: 264551.Rhalmi S, Yahia LH, Newman N, et al.
Immunohistochemical study of nerves in lumbar spine ligaments.
Spine 1993;18:2647.Yahia L, Rhalmi S, Newman N, et al.
Sensory innervation of human thoracolumbar fascia.
An immunohistochemical study.
Acta Orthop Scand 1992; 63:1957.McLain RF, Pickar JG.
Mechanoreceptor endings in human thoracic and lumbar facet joints.
Spine 1998;23:16873.Cavanaugh JM, Ozaktay AC, Yamashita T, et al.
Mechanisms of low back pain: a neurophysiologic and neuroanatomic study.
Clin Orthop Relat Res 1997:16680.Yamashita T, Cavanaugh JM, Ozaktay AC, et al.
Effect of substance P on mechanosensitive units of
tissues around and in the lumbar facet joint.
J Orthop Res 1993;11:20514.Nitz AJ, Peck D.
Comparison of muscle spindle concentrations in large and
small human epaxial muscles acting in parallel combinations.
Am Surg 1986; 52:2737.Stubbs M, Harris M, Solomonow M, et al.
Ligamento-muscular protective reflex in the lumbar spine of the feline.
J Electromyogr Kinesiol 1998;8:197204.Holm S, Indahl A, Solomonow M.
Sensorimotor control of the spine.
J Electromyogr Kinesiol 2002;12:21934.Solomonow M, Zhou BH, Harris M, et al.
The ligamento-muscular stabilizing system of the spine.
Spine 1998;23:255262.Kang YM, Choi WS, Pickar JG.
Electrophysiologic evidence for an intersegmental reflex
pathway between lumbar paraspinal tissues.
Spine 2002;27: E5663.Dolan P, Adams MA.
Repetitive lifting tasks fatigue the back muscles and
increase the bending moment acting on the lumbar spine.
J Biomech 1998; 31:71321.Sparto PJ, Parnianpour M, Reinsel TE, et al.
The effect of fatigue on multijoint kinematics and
load sharing during a repetitive lifting test.
Spine 1997; 22:264754.van Dieen JH, van der Burg P, Raaijmakers TA, et al.
Effects of repetitive lifting on kinematics:
inadequate anticipatory control or adaptive changes?
J Motor Behav 1998;30:2032.Trafimow JH, Schipplein OD, Novak GJ, et al.
The effects of quadriceps fatigue on the technique of lifting.
Spine 1993;18:3647.Bigland-Ritchie BR, Dawson NJ, Johansson RS, et al.
Reflex origin for the slowing of motoneurone firing rates
in fatigue of human voluntary contractions.
J Physiol 1986;379:4519.Taylor JL, Butler JE, Gandevia SC.
Changes in muscle afferents, motoneurons
and motor drive during muscle fatigue.
Eur J Appl Physiol 2000;83: 10615.Macefield G, Hagbarth KE, Gorman R, et al.
Decline in spindle support to alpha-motoneurones
during sustained voluntary contractions.
J Physiol 1991;440:497512.Lattanzio PJ, Petrella RJ, Sproule JR, et al.
Effects of fatigue on knee proprioception.
Clin J Sport Med 1997;7:227.Pedersen J, Ljubisavljevic M, Bergenheim M, et al.
Alterations in information transmission in ensembles of primary muscle
spindle afferents after muscle fatigue in heteronymous muscle.
Neuroscience 1998;84:9539.McGill SM, Brown S.
Creep response of the lumbar spine to prolonged full flexion.
Clin Biomech 1992;7:436.Solomonow M, Baratta RV, Banks A, et al.
Flexion-relaxation response to static lumbar flexion in males and females.
Clin Biomech (Bristol, Avon) 2003;18:2739.Adams MA, Dolan P.
Time-dependent changes in the lumbar spines resistance to bending.
Clin Biomech 1996;11:194200.Solomonow M, Zhou BH, Baratta RV, et al.
Biomechanics of increased exposure to lumbar injury caused by cyclic loading:
part 1. Loss of reflexive muscular stabilization.
Spine 1999;24:242634.Adams MA, Dolan P, Hutton WC.
Diurnal variations in the stresses on the lumbar spine.
Spine 1987;12:1307.Adams MA, Dolan P, Hutton WC, et al.
Diurnal changes in spinal mechanics and their clinical significance.
J Bone Joint Surg Br 1990;72:26670.Biering-Sorensen F.
Physical measurements as risk indicators for low-back
trouble over a one-year period.
Spine 1984;9:10619.Mannion AF, Dolan P.
Electromyographic median frequency changes during isometric
contraction of the back extensors to fatigue.
Spine 1994;19: 12239.Dolan P, Mannion AF, Adams MA.
Fatigue of the erector spinae muscles. A quantitative assessment
using frequency banding of the surface electromyography signal.
Spine 1995;20:14959.Dolan P, Adams MA, Hutton WC.
Commonly adopted postures and their effect on the lumbar spine.
Spine 1988;13:197201.Swinkels A, Dolan P.
Regional assessment of joint position sense in the spine.
Spine 1998;23:5907.Dolan P, Adams MA.
Influence of lumbar and hip mobility on the bending
stresses acting on the lumbar spine.
Clin Biomech 1993;8:18592.Sahlin K, Ren JM.
Relationship of contraction capacity to metabolic
changes during recovery from a fatiguing contraction.
J Appl Physiol 1989;67:64854.Radebold A, Cholewicki J, Polzhofer GK, et al.
Impaired postural control of the lumbar spine is associated with
delayed muscle response times in patients with chronic
idiopathic low back pain.
Spine 2001;26:72430.Vera-Garcia FJ, Brown SH, Gray JR, et al.
Effects of different levels of torso coactivation on trunk muscular
and kinematic responses to posteriorly applied sudden loads.
Clin Biomech (Bristol, Avon) 2006;21:44355.Herrmann CM, Madigan ML, Davidson BS, et al.
Effect of lumbar extensor fatigue on paraspinal muscle reflexes.
J Electromyogr Kinesiol 2006;16:63741.Dietz V.
Human neuronal control of automatic functional movements:
interaction between central programs and afferent input.
Physiol Rev 1992;72: 3369.Wilder DG, Aleksiev AR, Magnusson ML, et al.
Muscular response to sudden load.
A tool to evaluate fatigue and rehabilitation.
Spine 1996; 21:262839.Mannion AF, Adams MA, Dolan P.
Sudden and unexpected loading generates high forces on the lumbar spine.
Spine 2000;25:84252.Marras WS, Rangarajulu SL, Lavender SA.
Trunk loading and expectation.
Ergonomics 1987;30:55162.Andersson GB.
Epidemiologic aspects on low-back pain in industry.
Spine 1981;6:5360.Magora A.
Investigation of the relation between low back pain and occupation. IV.
Physical requirements: bending, rotation, reaching and sudden maximal effort.
Scand J Rehabil Med 1973;5:18690.Hodges PW, Richardson CA.
Delayed postural contraction of transversus abdominis in
low back pain associated with movement of the lower limb.
J Spinal Disord 1998;11:4656.Magnusson ML, Aleksiev A, Wilder DG, et al.
Unexpected load and asymmetric posture as etiologic factors in low back pain.
Eur Spine J 1996;5:2335.Cholewicki J, Silfies SP, Shah RA, et al.
Delayed trunk muscle reflex responses increase the risk of low back injuries.
Spine 2005;30:261420.Hortobagyi T, Lambert NJ, Kroll WP.
Voluntary and reflex responses to fatigue
with stretch-shortening exercise.
Can J Sport Sci 1991;16:14250.Hakkinen K, Komi PV.
Electromyographic and mechanical characteristics of human skeletal
muscle during fatigue under voluntary and reflex conditions.
Electroencephalogr Clin Neurophysiol 1983;55:43644.Balestra C, Duchateau J, Hainaut K.
Effects of fatigue on the stretch reflex in a human muscle.
Electroencephalogr Clin Neurophysiol 1992;85:4652.Garland SJ, McComas AJ.
Reflex inhibition of human soleus muscle during fatigue.
J Physiol 1990;429:1727.Hagbarth KE, Bongiovanni LG, Nordin M.
Reduced servo-control of fatigued human finger extensor and flexor muscles.
J Physiol 1995;485(pt 3): 86572.Morgan DL, Prochazka A, Proske U.
The after-effects of stretch and fusimotor stimulation on
the responses of primary endings of cat muscle spindles.
J Physiol 1984;356:46577.Gregory JE, Morgan DL, Proske U.
After effects in the responses of cat muscle spindles and
errors of limb position sense in man.
J Neurophysiol 1988;59:122030.Gregory JE, Wise AK, Wood SA, et al.
Muscle history, fusimotor activity and the human stretch reflex.
J Physiol 1998;513(pt 3):92734.Avela J, Kyrolainen H, Komi PV.
Altered reflex sensitivity after repeated and prolonged passive muscle stretching.
J Appl Physiol 1999;86:128391.Hagbarth KE, Hagglund JV, Nordin M, et al.
Thixotropic behaviour of human finger flexor muscles with
accompanying changes in spindle and reflex responses to stretch.
J Physiol 1985;368:32342.Ge W, Pickar JG.
Time course for the development of muscle history in lumbar paraspinal
muscle spindles arising from changes in vertebral position.
Spine J 2008;8:3208.Solomonow M, Zhou B, Baratta RV, et al.
Neuromuscular disorders associated with static lumbar flexion:
a feline model.
J Electromyogr Kinesiol 2002;12:8190.Sinkjaer T, Andersen JB, Nielsen JF, et al.
Soleus long-latency stretch reflexes during walking
in healthy and spastic humans.
Clin Neurophysiol 1999;110: 9519.Lundberg A, Malmgren K, Schomburg ED.
Reflex pathways from group II muscle afferents.
3. Secondary spindle afferents and the FRA: a new hypothesis.
Exp Brain Res 1987;65:294306.Matthews PB.
Evidence from the use of vibration that the human longlatency stretch
reflex depends upon spindle secondary afferents.
J Physiol 1984;348:383415.Hasan Z, Stuart DG.
Mammalian muscle receptors.
In: Davidoff RA, ed. Handbook of the Spinal Cord.
New York, NY: Dekker; 1984:559607.Hayward L, Wesselmann U, Rymer WZ.
Effects of muscle fatigue on mechanically sensitive afferents
of slow conduction velocity in the cat triceps surae.
J Neurophysiol 1991;65:36070
Return to LOW BACK PAIN
Since 4-17-2024


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |