

Mechanisms of Chiropractic Spinal Manipulative Therapy
for Patients with Chronic Primary Low Back Pain:
Protocol for a Mechanistic Randomized
Placebo-controlled TrialThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: BMJ Open 2023 (Feb 10); 13 (2): e065999 ~ FULL TEXT
OPEN ACCESS Carlos Gevers-Montoro, Arantxa Ortega-De Mues, Mathieu Piché
Real Centro Universitario Escorial Maria Cristina,
San Lorenzo de El Escorial, Spain.

Introduction: Chronic low back pain (CLBP) is a highly prevalent and disabling condition. Identifying subgroups of patients afflicted with CLBP is a current research priority, for which a classification system based on pain mechanisms was proposed. Spinal manipulative therapy (SMT) is recommended for the management of CLBP. Yet, little data are available regarding its mechanisms of action, making it difficult to match this intervention to the patients who may benefit the most. It was suggested that SMT may influence mechanisms associated with central sensitisation. Therefore, classifying patients with CLBP according to central sensitisation mechanisms may help predict their response to SMT.
Methods and analysis: This protocol describes a randomised placebo-controlled trial aiming to examine which variables linked to central sensitisation may help predict the clinical response to SMT in a cohort of patients with CLBP. One hundred patients with chronic primary low back pain will be randomised to receive 12 sessions of SMT or placebo SMT over a 4–week period. Pain intensity and disability will be assessed as primary outcomes after completing the 4–week treatment (primary endpoint), and at 4–week and 12–week follow-ups. Baseline values of two pain questionnaires, lumbar pressure pain thresholds, concentrations of an inflammatory cytokine and expectations of pain relief will be entered as predictors of the response to SMT in a multiple regression model. Changes in these variables after treatment will be used in a second multiple regression model. The reference values of these predictors will be measured from 50 age and sex-matched healthy controls to allow interpretation of values in patients. Mixed analyses of variance will also be conducted to compare the primary outcomes and the predictors between groups (SMT vs placebo) over time (baseline vs post-treatment).
Ethics and dissemination: Ethical approval was granted by the Fundación Jiménez Díaz Clinical Research Ethics Committee.
Trial registration number: Registered at ClinicalTrials.gov @: NCT05162924.
Keywords: Back pain; COMPLEMENTARY MEDICINE; Clinical trials; IMMUNOLOGY.
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study will expand our understanding of the relevance of clinical, psychological, psychophysical and inflammatory variables in predicting the response of patients with chronic low back pain to manual therapy.
The design, including a control group with healthy participants, will allow confirming the usefulness of a classification system for patients with chronic primary low back pain according to the underlying pain mechanisms.
The blinding of outcome assessors, statistician, laboratory technician and of the investigator providing care to the patients’ progress will contribute to reduce bias.
A high degree of similarity between the sham and real manipulations increases the odds of successfully blinding participants. However, the sham intervention may produce clinical effects.
Clinical trials on manual therapy, including the present study, are limited by the impossibility of blinding the investigator providing care to the intervention.
From the FULL TEXT Article:
Introduction
Low back pain (LBP) is the single most important cause of disability globally, [1] with a high proportion of patients whose pain persists or recurs. [1–4] Aiming to identify patient profiles that respond more favourably to specific treatments and their prognosis, recent investigations highlight the importance of identifying subgroups among people with chronic LBP (CLBP). One of the proposed classification systems stratifies patients into specific subgroups according to pain mechanisms (nociceptive, neuropathic or central sensitisation). [5–10] It has been suggested that a large proportion of patients with CLBP presents chronic primary pain, which has been linked to altered nociceptive processing. [11, 12] Among the phenomena that may underlie this aberrant processing, central sensitisation (CS) is likely the predominant mechanism, [12, 13] and its involvement in CLBP deserves further research. [14]
One of the currently recommended interventions for the management of CLBP is spinal manipulative therapy (SMT). [15, 16] However, not all patients have an identical response. [17] There are insufficient data to determine which CLBP subgroups respond better to this intervention. [18, 19] This may be so because the analgesic mechanisms are still largely unknown. It was proposed that the pain-relieving effects of SMT partly rely on segmental pain inhibition processes. [20] These processes influence temporal summation of pain, [21, 22] primary and secondary hyperalgesia, [23, 24] which may be measured to identify patients with a CS phenotype. Further, emerging data from animal and human studies support the hypothesis that SMT modulates the inflammatory response, influencing inflammatory cytokines. [25–28] Cytokines can induce neuroinflammation, which may mediate the development of CS [29, 30] in the transition towards chronic pain. [8, 31] SMT may thus relieve CLBP by impacting mechanisms linked to CS. [24, 32–34]
Altered pain sensitivity in a specific musculoskeletal region may indicate nociplastic pain, [12, 35, 36] likely reflecting CS. [13] Abundant studies have reported that a subgroup of patients with CLBP demonstrate segmental mechanical hyperalgesia, assessed via lower pressure pain thresholds (PPTs) in low back or lower extremity areas, when compared with healthy controls. [37–42] Changes in pain sensitivity are not confined to lumbar segments but rather may be present in remote anatomical locations. [14, 38, 43–45] Increased pain sensitivity is a clinical indicator possibly reflecting CS not just at the spinal level, but potentially implicating supraspinal structures. [8, 14, 31] Thus, it is plausible that mechanical pain sensitivity may play an important role in defining a CS phenotype in CLBP. [35]
Pain catastrophising has been described as a psychological trait and pain cognition linked to the development of CLBP with an altered pain sensitivity profile and a CS phenotype. [46–48] Patients with CLBP with higher pain sensitivity often demonstrate higher levels of catastrophising and other negative psychological traits. [32, 49–51] Similarly, higher pain catastrophising was associated with higher central sensitisation inventory (CSI) scores. [52] The CSI and a clinical presentation suggestive of CS mechanisms have been proposed to identify a specific CLBP subgroup. [5, 6, 53, 54]
Currently, the mechanisms leading to CS are still unknown; however, recent data suggest an important role for neuroinflammation. [29] Neuroinflammation may act at multiple levels, from the periphery [50] to the brain, [55] including the dorsal horn of the spinal cord. [56] The release of inflammatory cytokines, including the proinflammatory tumour necrosis factor alpha (TNF-α), was identified as a potential mechanism supporting this phenomenon. [29, 30, 57, 58] Studies have shown an association between proinflammatory cytokines and CLBP, [59–62] suggesting that these may serve as a reliable biomarker to identify patients with a CS phenotype.
The classification of mechanism-based pain phenotypes is a complex and controversial task, [35, 63, 64] for which a variety of clinical, inflammatory, psychological and psychophysical constructs must be considered. [9, 65] Although CS may influence changes in pain sensitivity induced by SMT, [32] pain phenotyping has been scarcely applied to manual therapy research. [66] Therefore, the response of this subgroup of patients to SMT has yet to be assessed. The aim of this clinical trial is to investigate whether variables associated with a CS phenotype may help predict the response to SMT.
The specific objectives are:(1) to identify the clinical, psychological, psychophysical and inflammatory variables linked to CS in a cohort of patients with CLBP; and
(2) to examine which of these variables predict the clinical response to SMT.
Methods
Experimental design and setting
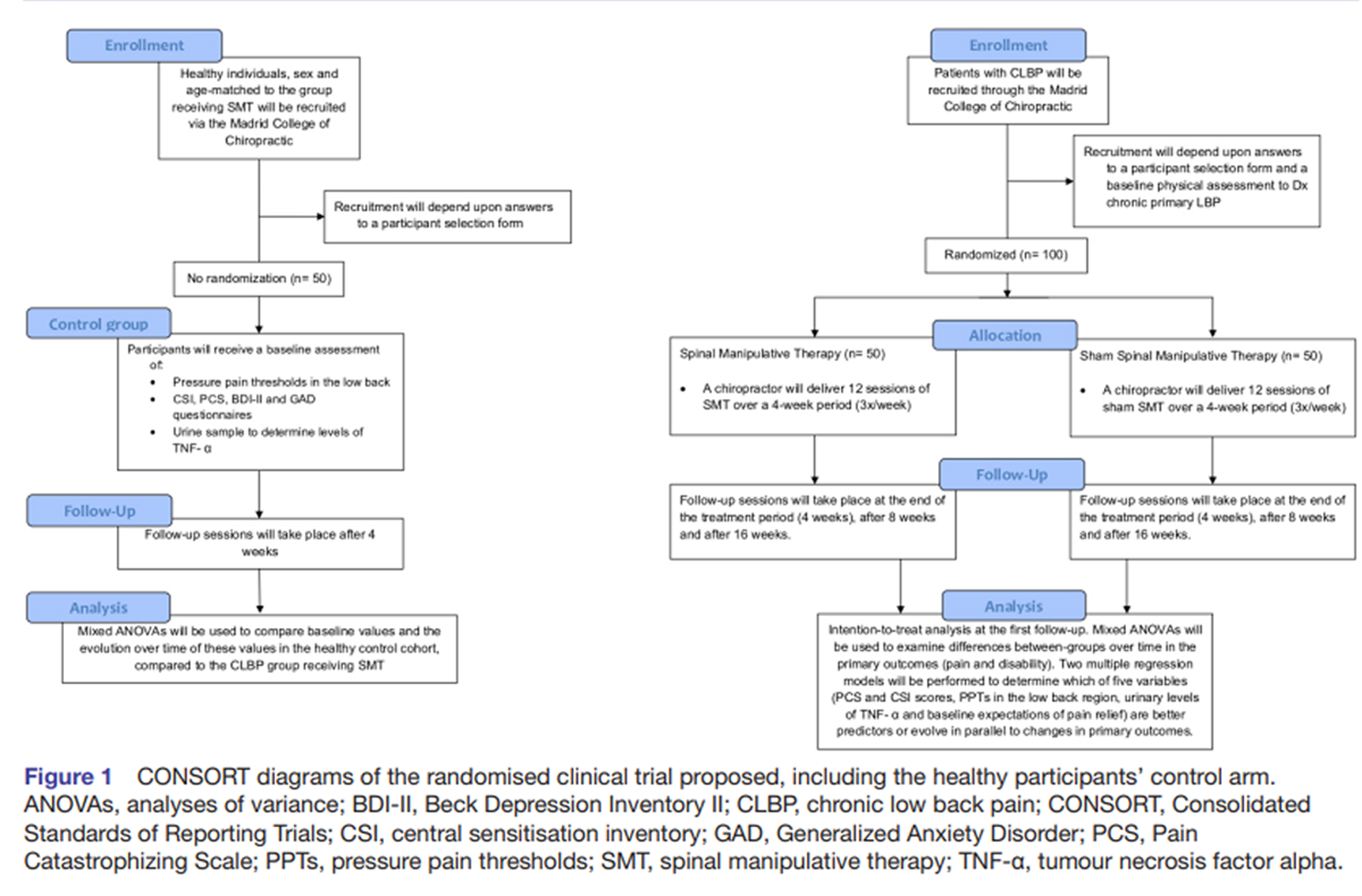
Figure 1 The study consists of a mechanistic randomised placebo-controlled clinical trial with a mixed experimental design, whose objective is to assess which variables linked to CS in patients with chronic pain can predict the response of patients with CLBP to SMT (Figure 1). This protocol is reported according to the guidelines for clinical trial protocols Standard Protocol Items: Recommendations for Interventional Trials. [67] Starting in November 2021, 150 participants will be recruited through the Madrid College of Chiropractic (MCC) teaching clinic in San Lorenzo de El Escorial (Spain). This includes 100 patients with CLBP and 50 healthy participants. The MCC clinic is a primary care setting specialised in spine care, including chiropractic and physical therapy services. Clinical, psychological, psychophysical and inflammatory variables will be measured in patients with CLBP, which will be exposed to either SMT or a placebo SMT for 12 visits over a 4–week period. A group made up of 50 age and sex-matched healthy volunteers will be used to determine the reference values of the same psychological, psychophysical, and inflammatory variables in a healthy population and compare them with the clinical population, before and after exposure.
Selection criteria
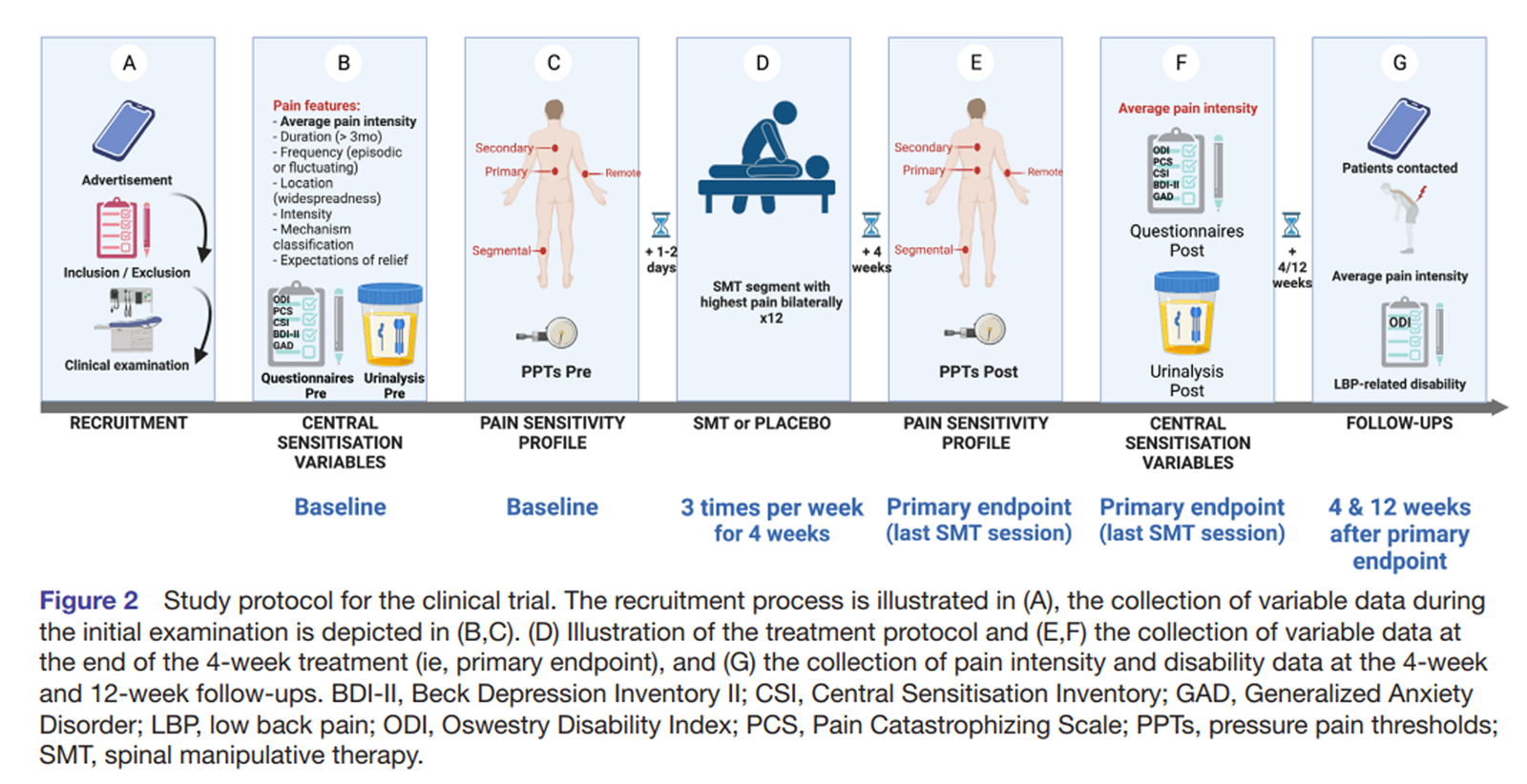
Figure 2 An investigator with over 20 years of clinical experience will be responsible for the selection of participants. To be eligible to participate in the study, patients must be 18–70 years old, receive a diagnosis of chronic primary LBP of at least 3–month duration, with or without leg pain (according to a clinical examination carried out at the MCC; see figure 2A). If pain affecting the low back or lower limb is suspected to be predominantly of neuropathic origin, the patient will be excluded. [12] Additionally, patients will be excluded from the study if they present any of the following criteria: evidence of specific pathology as the cause of their CLBP, diagnosis of mental illness (with the exception of anxiety and depression, as these conditions are frequently comorbid with CLBP [58, 69] and may suggest a CS phenotype [5, 49]), presence of pain of equal or higher intensity affecting any other body region, use of corticosteroids, opiates or anti-cytokine medication, pregnancy, lumbar fusion surgery or recent laminectomy, and having received chiropractic SMT in the 12 months prior to the beginning of the study. [5, 50, 51]
A cohort of healthy volunteers will be recruited to be used as a reference for the psychological, psychophysical and inflammatory variables collected in the sample of patients with CLBP. They will be age- and sex-matched to the patients allocated to the group receiving SMT. Individuals meeting the following criteria are eligible to participate: being 18–70 years old; presenting no current or chronic pain condition, as well as not having received any diagnosis of a systemic, inflammatory, neurological or psychiatric condition.
Randomisation, concealed allocation and blinding
A computer application (random-number generator) will be used to generate a balanced randomisation sequence. Participants will be allocated in a 1:1 ratio to the intervention (SMT) or placebo arms following the chronological order of recruitment. Patients, outcome assessors and statistician will be blinded to group allocation. To confirm the efficacy of the patients’ blinding, participants will respond in three occasions to the questions: ‘Do you think that the treatment you have received is a real chiropractic treatment for back pain?’; and ‘On a Numerical Rating Scale of 0–100, please rate the degree of certainty for having received a real chiropractic treatment’ (with 0 being total uncertainty and 100 being absolute certainty). [70]
Additionally, to avoid biases in the reporting of patient-reported outcome measures and to blind the investigator delivering the interventions, participants will provide these data via electronic questionnaires without the presence or interference of any investigator.
Interventions

Figure 3 Both real and placebo SMT will be delivered by a chiropractor with 20 years of experience who is part of the research team (CG-M). Two real SMTs will be performed with the patient positioned in the lateral decubitus position (once on each side), by applying a high-velocity, low-amplitude force on the manipulated segment, with the aim of generating at least one joint cavitation (associated with an audible sound). For this, the chiropractor will use the hypothenar surface or the last phalanx of the second and/or third fingers of the hand to contact the spinous process of the vertebral segment with the most intense clinical pain (see online supplemental figure 1A), as identified in the initial patient examination. In case of not perceiving a cavitation or satisfactory joint movement, SMT may be repeated once on each side. Therefore, all participants in the SMT arm will receive a minimum of two and a maximum of four SMT thrusts. Participants in the placebo arm will receive a validated sham SMT that is very similar to SMT. [70] The patient is positioned in the same lateral decubitus position, with the lower leg in extension and the upper leg in flexion, and an unintended force is applied bilaterally to the gluteal region (online supplemental figure 1B). The number of real or placebo SMT attempts resulting in joint cavitation will be recorded. Participants in both groups will receive three treatment sessions per week for 4 weeks (see figure 2). Healthy volunteers will receive no intervention during the same time frame of 4 weeks (see Figure 3).
Outcome variables
Primary outcomes Patients will rate their current CLBP intensity, as well as the average, minimum and maximum pain throughout the preceding 7 days or since the time of the previous session, once the study is underway, [71, 72] using a Numerical Rating Scale between 0 (no pain) and 100 (maximum pain imaginable). Average pain intensity will be used as a primary outcome for all statistical analyses. The primary endpoint will be the change from baseline at the completion of the 12 sessions of SMT. For the follow-up, average pain intensity will be assessed 4 and 12 weeks after the completion of the trial.
Disability caused by CLBP will also be assessed as a primary outcome. After completing the case history, patients will fill out the Oswestry Low Back Disability Index Questionnaire. [73] The questionnaire will also be completed after the 12th treatment session (primary endpoint), and at subsequent 4–week and 12–week follow-ups.
Secondary outcomes Five topics were identified to discriminate pain mechanisms between groups of patients, including CS mechanisms: clinical examination, questionnaires, quantitative sensory testing, laboratory tests and imaging tests. [9] For the present study, all categories will be considered except the last one, which will only be used to rule out pain of suspected neuropathic or nociceptive aetiology. Variables belonging to these categories will be assessed for exploratory purposes and five of them will be examined as predictors of the response to SMT (two questionnaires, one quantitative sensory testing variable, one laboratory test variable and the expectations of pain relief).
Clinical examination variables
Data on the characteristics of the patients’ CLBP will be collected at baseline for exploratory purposes: CLBP trajectory (duration and frequency) and localisation. The duration of CLBP will be calculated as the number of months since the onset of the first episode of LBP. As for pain frequency, participants’ CLBP trajectory will be classified as either fluctuating or episodic, depending on whether they recall asymptomatic periods of at least 4 weeks (episodic) or not (fluctuating). [74] For pain localisation, patients will also draw the area affected by their pain on a tablet, using an application (Symptom Mapper) that will allow to calculate the degree of pain widespreadness. [75]
Additionally, CLBP will be classified as either proportionate or disproportionate to the degree or nature of the injury or pathology, with a discrete or diffuse distribution, according to criteria that were defined in the literature. [5, 6] A diffuse rather than a discrete pain distribution was identified as a key criterion of a CS phenotype. [5, 12] Also, classifying symptoms as proportionate (or not) was proposed to differentiate nociceptive pain from CS mechanisms. [35] The pattern of pain distribution and the provocation and response to aggravating and palliative factors will be assessed during case history and physical examination. This will be complemented with information provided by diagnostic imaging when available. [9]
Finally, other variables will be reported such as the intake of pain medication compatible with the selection criteria, both at baseline and after treatment. Similarly, whether the patient regularly smokes will be documented, since smoking has been associated with increased serum levels of proinflammatory cytokines. [76] The average number of hours of sleep will also be recorded, as it may help predict pain patterns. [77] Additionally, the presence of any chronic condition (including pain) that is comorbid with the CLBP will be recorded for exploratory purposes.
Questionnaire variables
The Pain Catastrophizing Scale (PCS) and CSI will be completed before the beginning of the treatment (baseline) and at a single follow-up after the 12th treatment session (see figure 2B and F). [78, 79] The PCS will be used to identify specific pain cognitions that are frequently present in patients with a CS phenotype; this measure will be used to evaluate the association of CLBP with psychosocial factors described by Smart et al. [5] When combined with a clinical presentation suggestive of CS, [35] the CSI is a useful tool to identify patients with certain pain mechanisms compatible with CS, particularly when using the cut-off value of 40 points. [80] Both these scores will be examined as predictors due to their intrinsic association with a CS phenotype.
In addition, the Beck Depression Inventory II (BDI-II) and the Generalized Anxiety Disorder Scale (GAD) Questionnaires will be used to screen and quantify symptoms of depression and anxiety. [81, 82] The scores in these questionnaires will be measured both at baseline and after the 12th treatment session for exploratory purposes. We will examine whether these variables are associated with the primary outcomes. Pre/post-reference values of all questionnaires (PCS, CSI, BDI-II and GAD) will be taken from the healthy control participants in the same time frame (figure 3).
Quantitative sensory testing variables
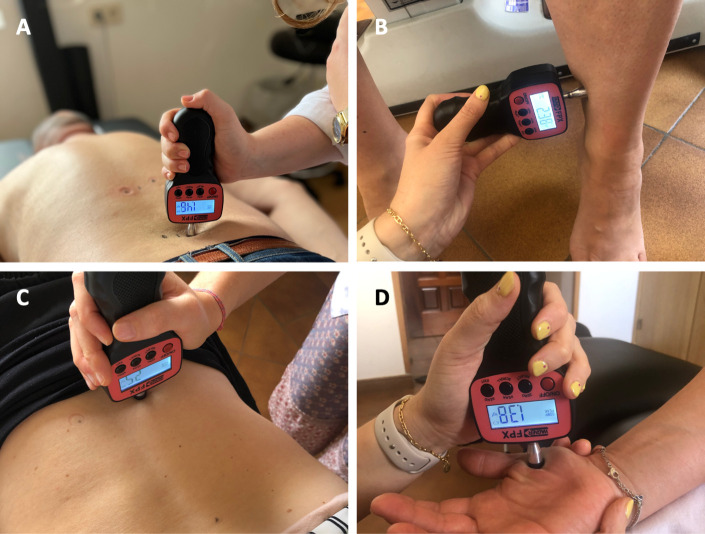
Figure 4 Quantitative sensory testing based on the German protocol [83, 84] will be performed with the aim of evaluating pain thresholds and sensitivity (see figure 2C). Testing will consist of the exploration of the PPTs in deep tissues (Figure 4), using an algometer (Wagner Force Dial FPX, Greenwich, Connecticut, USA). In addition, patients will rate the intensity of the first stimulus above threshold, using a Numerical Rating Scale 0–100. [85] PPTs will be assessed by two interns completing their Master’s in Chiropractic degree, after 3 months of training and pilot data collection. One of the two outcome assessors will be randomly assigned to each patient to perform both baseline and follow-up measurements. Two measurements will be taken bilaterally at a rate of about 50 kPa/s, and the arithmetic mean of both the thresholds and sensitivities reported calculated. Two consecutive measurements provide excellent reliability when assessing both populations with and without LBP, [86, 87] while performing two repetitions per side of the lower back was proposed to optimise intersession reliability. [88]
PPTs will be performed over muscle tissue in four different locations bilaterally. Primary pain will be assessed 2.5 cm lateral to the spinous process in the erector spinae [85] of the vertebral segment with the highest clinical pain intensity indicated by the patient and verified by palpation (figure 4). Manual palpation will be performed to confirm that the selected segment either reproduces clinical pain or is the closest to the area (or to the centre) of CLBP symptoms. This will allow to assess the area of primary pain or hyperalgesia (segmental sensitivity). In addition, PPTs will be measured on both lower limbs in the dermatome corresponding to the segment of highest clinical pain intensity (dermatomal sensitivity), in the erector spinae four to six segments cranial to the most painful lumbar segment (heterosegmental sensitivity in a non-symptomatic segment: secondary hyperalgesia) and in a remote location in both thenar eminences (widespread sensitivity). PPTs will be assessed during the initial examination for baseline and after the final treatment session (see figure 2C and E). Reference values will be taken in healthy volunteers in the same locations as the participants with CLBP receiving SMT (lumbar segmental, dermatomal, heterosegmental, widespread) at baseline and after 4 weeks (figure 3).
Laboratory test variables: TNF-α as an inflammatory biomarker in urine
Before initiating the first treatment session and on the day of the last treatment session, urine samples will be collected (first morning micturition) and stored at –20°C (see figure 2B and F). Additionally, the first morning micturition will be collected twice from healthy individuals in the same time frame (two samples with a 4–week delay; see figure 3). [62] Samples will be deidentified by using only the participant’s ID code, and the laboratory technicians will be blinded to group allocation. Urine concentrations of TNF-α will be quantified for each sample using specific ELISA for TNF-α following manufacturer’s instructions. The cytokine-to-creatinine ratio will be calculated to correct for differences in urine volumes. [89] TNF-α values, including urinary concentrations, were found to be elevated in patients with CLBP and may respond to a treatment based on SMT. [25, 27, 59, 62, 90]
Expectations
Before initiating treatment, each participant will be asked to rate their expectations of pain relief upon completion of the study. To do this, a verbal evaluation will be provided using a Visual Analogue Scale with the descriptors –100, equivalent to ‘total pain relief’; 0, equivalent to ‘no change’; up to +100, equivalent to ‘maximum pain increase’. Such an assessment of patients’ expectations allows to identify their contribution as part of the placebo response, which was found to predict the response to treatment for chronic pain. [91]
Adverse events reporting
At the beginning of every SMT or placebo treatment sessions, patients will inform whether they have suffered any adverse effects that they feel could be related to the treatment received via an electronic questionnaire. Adverse effects will be classified into four categories most frequently reported after lumbar SMT as identified in a clinical trial: muscle stiffness, increased pain, radiating discomfort and others.92 In addition, patients will indicate whether they were triggered immediately, up to 24 hours, or more than 24 hours after the previous session, whether their duration was of minutes, hours (<24 hours), between 24 and 48 hours, or longer than 48 hours, [92] and according to their intensity (very mild, mild, moderate, severe, very severe). The reporting of adverse events will be monitored by an investigator not involved in clinical care or examination. A 30–point increase in pain intensity or the reporting of moderate-to-severe adverse events in three consecutive visits will raise the alarm and the patient will be interviewed to determine whether care should be interrupted.
Healthy volunteers will be contacted 1 week prior to the follow-up appointment to rule out any of the following criteria that would exclude them from the follow-up: presence of pain or other symptoms for >7 days, trauma or injury, initiating a new treatment or receiving a diagnosis compatible with the exclusion criteria. In addition, if the participant reports any pain or taking any pain medication within 24 hours of the follow-up, this session will be postponed for up to 1 week.
Procedures Candidates interested in participating in the study will initially complete a form with the selection criteria (online supplemental appendix 1). If the criteria are met, patients will schedule an appointment at the MCC clinic where they will read and sign a participant information sheet and the informed consent (online supplemental appendices 2 and 3). Subsequently, patients will undergo a clinical examination (consisting of a case history and physical examination) to confirm the diagnosis of chronic primary LBP, during which all outcomes will be collected, except for the urine sample that will be provided before the first treatment session. Patients will then participate in 12 treatment sessions divided into three weekly sessions for 4 weeks. All outcome measures will be reassessed at the 12th and last treatment session (ie, the primary endpoint). After completing data collection at the primary endpoint, patients allocated to the placebo arm will be offered the possibility of receiving the ‘real’ SMT, free of charge, at the MCC. In addition, all patients will be contacted for the follow-up of CLBP intensity and disability, 4 and 12 weeks after the primary endpoint (figure 2G). Meanwhile, healthy volunteers will participate in two visits (baseline and follow-up after 4 weeks) when all relevant outcomes will be assessed (figure 3). The study will have total estimated duration of 1 year.
Sample size calculation To determine the ideal number of participants, the second aim to identify the variables linked to a CS phenotype that could help predict the response to treatment based on SMT for CLBP was considered. A multiple regression analysis will be performed using five independent variables described in the Statistical analysis section as predictors. These variables include baseline values of local PPTs, urinary concentrations of TNF, scores in PCS and CSI questionnaires, and a priori expectations of pain relief. For each predictor variable, it is recommended estimating about 10 sample elements; therefore, we predict that a sample size of 50 patients per group will be necessary.93 A total of 110 patients will be recruited, accounting for an estimated dropout rate of 5%–10%.
Regarding the primary outcome variables, a reduction in pain intensity and disability after 1–month in patients who receive 12 sessions of SMT compared with placebo will be expected. We aim to detect small-to-moderate effects since it is a 1–month intervention in patients with chronic pain unresolved by other treatments over at least 3 months. Therefore, based on an effect size of f=0.175, an alpha of 0.05, a power of 0.8 for two groups and two repeated measures (baseline and primary endpoint), and a correlation between the repeated measures of 0.5, the size of the necessary sample is 34 patients per group, thus a total of 68 patients to detect statistically significant changes in clinical pain and disability. Therefore, the analysis based on the regression model to predict the clinical course provides with a large enough size for identifying small between-group differences.
Statistical analysis
The normal distribution of the data will be verified using the Kolmogorov-Smirnov test. Data deviating from normality will be transformed to obtain a normal distribution before being entered into the data analysis. In order to interpret the values in outcomes measured in patient groups, these will be compared with reference values obtained from the healthy controls to the CLBP group receiving SMT. This will allow characterising the patients’ groups (aim 1) to determine whether they show increased psychological symptoms, pain sensitivity and hyperalgesia as well as increased TNF-α levels compared with a reference healthy population. A series of mixed analyses of variance (ANOVAs) will be performed to examine differences in PPTs, urinary TNF-α levels, PCS, CSI, BDI-II and GAD scores before and after the 4–week treatment period between the three groups (control, SMT and placebo). To test a priori hypotheses, significant effects will be decomposed using planned comparisons. For the rest of the effects, Tukey’s honest significance test (HSD) will be used for testing any pairwise comparisons between group means.
Pearson’s product-moment correlation analyses will be carried out to examine the association between the primary and secondary variables that demonstrate significant effects between groups over time. Subsequently, two multiple regression models will be used to examine the predictors of improvement in clinical pain and disability over time in patients who have received SMT (aim 2). The variables used as predictors for this analysis will be: baseline PCS and CSI score, baseline PPTs in the primary pain region, baseline TNF-α levels and (baseline) expectations of pain relief. In addition, in another regression model, the changes (delta) in these variables (except expectations of pain relief, which are only measured a priori) after 4 weeks of treatment will be used as predictor variables. This is done to identify the variables most associated with clinical evolution to answer the mechanistic question.
The primary outcome variables (clinical pain intensity and disability) will be compared between groups (SMT vs placebo) over time at the primary endpoint using mixed ANOVAs. Average pain intensity since the last treatment visit and in the 7 days prior to the initial visit will be the pain variables used for statistical analyses. With an exploratory objective, the secondary variables (PCS, CSI, BDI-II, GAD scores, PPTs, degree of pain widespreadness, urinary cytokine levels, number and severity of reported adverse effects, presence of leg pain, pain medication use) will be compared between groups (SMT vs placebo) over time (baseline and post-treatment) using mixed ANOVAs. To test a priori hypotheses, significant effects will be decomposed using planned comparisons. For the rest of the effects, Tukey’s HSD will be used for testing any pairwise comparison between group means.
As recommended by White et al, efforts will be directed towards following up all participants for every time point. [94] An intention-to-treat analysis including all randomised study participants with a baseline endpoint assessment will be performed. The use of mixed-model ANOVA allows to include all study participants with a lower attrition bias [95] while handling missing data using maximum likelihood estimations. Further, a per-protocol analysis will be also performed excluding study participants who voluntarily drop out from the study, develop a severe adverse reaction (increase in >30 points average pain intensity associated with treatment) or fail to attend three consecutive visits, or more than 2 treatment weeks. Finally, in order to test whether the data are not missing at random, a sensitivity analysis will be conducted to explore the effect of attrition. [94]
Data management and monitoring
All data will be collected at the MCC teaching clinic of the Real Centro Universitario María Cristina. The clinic uses a password-protected computer app that generates a patient file number linked to their clinical and personal data. This file number will be connected to a unique participant ID code made up of three numbers and a letter. This ID code will be used to deidentify all clinical trial data. Only the investigator involved in delivering care will have knowledge of which clinic file number corresponds to which study ID code. The participants’ selection, information, consent forms and outcome measures collected in paper format will be securely stored in a file cabinet at the MCC clinic. Patient-reported outcome measures will be collected electronically using the study ID code to complete a Google form (Google LLC, Mountain View, CA, USA). Both paper and online data will be transferred to a password-protected spreadsheet, only accessible to the principal investigator. Data will be stored deidentified for 25 years after final publication. The dataset will be made available after publication of the trial, upon request to the corresponding author.
Patient and public involvement
The local chiropractic patient and professional associations (Asociación Española de Usuarios de Quiropráctica and Asociación Española de Quiropráctica) have been involved throughout the study in the recruitment process and in promoting the trial. Upon completion of the study, the results will be disseminated to the patient community in the general assembly of the patient association, as per a formal agreement with the investigators.
Ethics and dissemination
This clinical trial obtained ethical approval from the Fundación Jiménez Díaz Clinical Research Ethics Committee. All participants in the study will sign an informed consent form. Any amendment to the protocol will be communicated to the ethics review board and the clinical trial registry. The results of the study will be submitted for publication in peer-reviewed journals and disseminated via scientific conferences and presentations directed to the professional and patient associations.
Discussion
The stratification of patients with CLBP is essential to better understand the needs of individual patients and provide targeted treatment. A mechanism-based classification is a promising avenue to match patients with the care that is best suited with their CLBP mechanism. However, there is an ongoing debate regarding the definition of these subgroups and the best available tools to diagnose them. [6, 12, 35, 63, 64] The most recent guidelines for the management of CLBP in both a primary care and a physiotherapy setting recommend SMT as one of the first options for care. [96, 97] Nonetheless, it is not yet possible to identify which patients may benefit the most. The current study describes a protocol for a mechanistic randomised placebo-controlled trial that may contribute to unveil the CS-related mechanisms involved in CLBP relief by SMT. The main objective of the proposed trial is to provide some insight on potential mechanisms of SMT that may be particularly relevant for a subgroup of patients with CLBP. Grasping these mechanisms may help better guide conservative care for patients with CLBP by assessing clinical, neurophysiological, cognitive and/or biochemical variables at baseline.
Strengths and limitations
The main strength of the current study is the robust design using a validated placebo and assessing the blinding of participants, while ensuring the blinding of outcome assessors, statistician and laboratory technician. Moreover, the investigator delivering care will be blinded to the patients’ progress. This will reduce biases that are typically introduced in manual therapy trials. Additionally, the use of a control group will help determine reference values and their stability in a healthy population, which has not been readily reported, particularly concerning urinary levels of inflammatory cytokines. [62] Further to this, the multidimensional approach to defining central sensitisation and the mechanisms leading to it may render relevant data in better defining pain mechanisms involved in CLBP.
Regarding potential limitations, having only one clinician may limit the generalisability of the SMT effects. However, it also has the advantage of standardising the interventions and reducing variability in the procedures. It should also be noted that although blinding the investigator providing care is desirable, it is impossible in manual therapy trials, [98] including the present study. As the sham and real SMT have a high degree of similarity, effective blinding of participants is feasible. [70] The inability to distinguish the placebo from the real treatment is desirable to limit interpretation bias, particularly in a mechanistic trial as in the present study. [99] However, the sham SMT may rely on specific mechanisms that overlap with those of real SMT, leading to treatment effects. [20, 99] Accordingly, the sham SMT should not be considered as an inert placebo and the lack of between-group differences should be interpreted with caution, with a potential risk for type II errors.
Supplementary Material
Online Supplemental Figure 1A + 1B
Online Supplemental Appendix 1
Online Supplemental Appendix 2
Online Supplemental Appendix 3
Reviewer Comments: (240K, pdf)
Author's Manuscript (8.0M, pdf)Acknowledgments
Figures 2 and 3 were created with biorender.com.
Contributors:
All authors contributed to the design of this protocol. CG-M and MP conceptualised and designed the protocol, except for every aspect related to laboratory analyses, which were conceptualised by AO-DM. The protocol was drafted by CG-M, and revised by MP and AO-DM. The statistical analysis was designed by MP. CG-M was responsible for ethical committee approval.
Funding:
This work was supported by the Chaire de Recherche Internationale en Santé Neuromusculosquelettique. CG-M’s work was supported by the Fonds de Recherche du Québec en Nature et Technologies (FRQNT), the Asociación Española de Quiropráctica (AEQ) and the European Centre for Chiropractic Research Excellence (ECCRE). AO-DM’s work was supported by ECCRE. MP’s work was supported by the Fondation de Recherche en Chiropratique du Québec and the Fonds de Recherche du Québec en Santé (FRQS).
Competing interests:
None declared.
Supplemental material
This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References:
Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J et al.
What Low Back Pain Is and Why We Need to Pay Attention
Lancet. 2018 (Jun 9); 391 (10137): 2356–2367
This is the second of 4 articles in the remarkable Lancet Series on Low Back PainItz CJ, Geurts JW, van Kleef M, Nelemans P.
Clinical Course of Non-specific Low Back Pain:
A Systematic Review of Prospective Cohort Studies Set in Primary Care
European Journal of Pain 2013 (Jan); 17 (1): 5–15Axen I, Leboeuf-Yde C.
Trajectories of Low Back Pain
Best Pract Res Clin Rheumatol. 2013 (Oct); 27 (5): 601–612Kongsted, A, Kent, P, Hestbaek, L, and Vach, W.
Patients With Low Back Pain Had Distinct Clinical Course Patterns
That Were Typically Neither Complete Recovery Nor Constant Pain.
A Latent Class Analysis of Longitudinal Data
Spine J. 2015 (May 1); 15 (5): 885–894Smart KM, Blake C, Staines A, et al.
Mechanisms-based classifications of musculoskeletal pain: Part 1 of 3:
symptoms and signs of central sensitisation in patients
with low back (± leg) pain.
Man Ther 2012;17:336–44.
10.1016/j.math.2012.03.013Nijs J, Apeldoorn A, Hallegraeff H, et al.
Low Back Pain: Guidelines for the Clinical Classification of
Predominant Neuropathic, Nociceptive, or Central Sensitization Pain
Pain Physician. 2015 (May); 18 (3): E333–346O’Sullivan P, Waller R, Wright A, et al.
Sensory characteristics of chronic non-specific low back pain:
a subgroup investigation.
Man Ther 2014;19:311–8.
10.1016/j.math.2014.03.006Nijs J, George SZ, Clauw DJ, et al.
Central sensitisation in chronic pain conditions:
latest discoveries and their potential for precision medicine.
Lancet Rheumatology 2021;3:e383–92.
10.1016/S2665-9913(21)00032-1Shraim MA, Massé-Alarie H, Hodges PW.
Methods to discriminate between mechanism-based categories of pain
experienced in the musculoskeletal system: a systematic review.
Pain 2021;162:1007–37.
10.1097/j.pain.0000000000002113Vardeh D, Mannion RJ, Woolf CJ.
Toward a mechanism-based approach to pain diagnosis.
J Pain 2016;17:T50–69.
10.1016/j.jpain.2016.03.001Nicholas M, Vlaeyen JWS, Rief W, et al.
The IASP classification of chronic pain for ICD-11:
chronic primary pain.
Pain 2019;160:28–37.
10.1097/j.pain.0000000000001390Kosek E, Clauw D, Nijs J, et al.
Chronic nociplastic pain affecting the musculoskeletal system:
clinical criteria and grading system.
Pain 2021;162:2629–34.
10.1097/j.pain.0000000000002324Shraim MA, Massé-Alarie H, Hall LM, et al.
Systematic Review and Synthesis of Mechanism-based
Classification Systems for Pain Experienced
in the Musculoskeletal System
Clinical J Pain 2020 (Oct); 36 (10): 793–812den Bandt HL, Paulis WD, Beckwée D, et al.
Pain Mechanisms in Low Back Pain: A Systematic Review with
Meta-analysis of Mechanical Quantitative Sensory Testing
Outcomes in People With Nonspecific Low Back Pain
J Orthop Sports Phys Ther. 2019 (Oct); 49 (10): 698–715de Zoete A, Rubinstein SM, de Boer MR, Ostelo R, Underwood M, Hayden JA (2021)
The Effect of Spinal Manipulative Therapy on Pain Relief and Function
in Patients with Chronic Low Back Pain: An Individual
Participant Data Meta-analysis
Physiotherapy 2021 (Mar 17); 112: 121–134Rubinstein SM, De Zoete A, Van Middelkoop M, Assendelft WJJ, De Boer MR, Van Tulder MW.
Benefits and Harms of Spinal Manipulative Therapy for the Treatment of
Chronic Low Back Pain: Systematic Review and Meta-analysis
of Randomised Controlled Trials
British Medical Journal 2019 (Mar 13); 364: 1689Wirth B, Riner F, Peterson C, et al.
An Observational Study on Trajectories and Outcomes of Chronic Low
Back Pain Patients Referred From a Spine Surgery
Division for Chiropractic Treatment
Chiropractic & Manual Therapies 2019 (Feb 5); 27: 6Axén I, Leboeuf-Yde C.
“Typical” chiropractic patients-
can they be described in terms of recovery patterns?
Chiropr Man Therap 2017;25:23.
10.1186/s12998-017-0152-0de Zoete A, de Boer MR, Rubinstein SM, et al.
Moderators of the effect of spinal manipulative therapy on pain relief
and function in patients with chronic low back pain:
an individual participant data meta-analysis.
Spine (Phila Pa 1976) 2021;46:E505–17.
10.1097/BRS.0000000000003814Gevers-Montoro C, Provencher B, Descarreaux M, et al.
Neurophysiological Mechanisms of Chiropractic
Spinal Manipulation for Spine Pain
European Journal of Pain 2021 (Aug); 25 (7): 1429–1448Randoll C, Gagnon-Normandin V, Tessier J, et al.
The mechanism of back pain relief by spinal manipulation
relies on decreased temporal summation of pain.
Neuroscience 2017;349:220–8.
10.1016/j.neuroscience.2017.03.006Bialosky JE, Bishop MD, Robinson ME, Zeppieri G, Jr, George SZ.
Spinal Manipulative Therapy Has an Immediate Effect on Thermal Pain
Sensitivity in People With Low Back Pain:
A Randomized Controlled Trial
Phys Ther. 2009 (Dec); 89 (12): 1292–1303Gevers-Montoro C, Provencher B, Northon S, et al.
Chiropractic spinal manipulation prevents secondary hyperalgesia
induced by topical capsaicin in healthy individuals.
Front Pain Res (Lausanne) 2021;2:702429.
10.3389/fpain.2021.702429Nim CG, Kawchuk GN, Schiøttz-Christensen B, et al.
The effect on clinical outcomes when targeting spinal manipulation
at stiffness or pain sensitivity: a randomized trial.
Sci Rep 2020;10:14615.
10.1038/s41598-020-71557-yTeodorczyk-Injeyan JA, Triano JJ, Gringmuth R, et al.
Effects of Spinal Manipulative Therapy on Inflammatory
Mediators in Patients with Non-specific Low Back Pain:
A Non-randomized Controlled Clinical Trial
Chiropractic & Manual Therapies 2021 (Jan 8); 29 (1): 3Roy RA, Boucher JP, Comtois AS.
Inflammatory Response Following a Short-term Course of Chiropractic
Treatment in Subjects with and without Chronic Low Back Pain.
Journal of Chiropractic Medicine, 2010 (Sep); 9 (3): 107-114Teodorczyk-Injeyan, JA, Injeyan, HS, and Ruegg, R.
Spinal Manipulative Therapy Reduces Inflammatory Cytokines
but Not Substance P Production in Normal Subjects
J Manipulative Physiol Ther 2006 (Jan); 29 (1): 14–21Song, X.-J., Huang, Z.-J., Song, W. B., Song, X.-S., Fuhr, A. F., Rosner, A. L., Ndtan, H., & Rupert, R. L. (2016).
Attenuation Effect of Spinal Manipulation on Neuropathic and Postoperative
Pain Through Activating Endogenous Anti-Inflammatory
Cytokine Interleukin 10 in Rat Spinal Cord
J Manipulative Physiol Ther. 2016 (Jan); 39 (1): 42–53Ji R-R, Nackley A, Huh Y, et al.
Neuroinflammation and central sensitization in chronic
and widespread pain.
Anesthesiology 2018;129:343–66.
10.1097/ALN.0000000000002130Kawasaki Y, Zhang L, Cheng J-K, et al.
Cytokine mechanisms of central sensitization: distinct and overlapping role
of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in
regulating synaptic and neuronal activity in the superficial spinal cord.
J Neurosci 2008;28:5189–94.
10.1523/JNEUROSCI.3338-07.2008Woolf CJ.
Central sensitization: implications for the diagnosis and treatment of pain.
Pain 2011;152:S2–15.
10.1016/j.pain.2010.09.030Nim CG, Weber KA, Kawchuk GN, et al.
Spinal manipulation and modulation of pain sensitivity in persistent
low back pain: a secondary cluster analysis of a randomized trial.
Chiropr Man Therap 2021;29:10.
10.1186/s12998-021-00367-4Boal RW, Gillette RG.
Central neuronal plasticity, low back pain and
spinal manipulative therapy.
J Manipulative Physiol Ther 2004;27:314–26.
10.1016/j.jmpt.2004.04.005Zafereo JA, Deschenes BK.
The role of spinal manipulation in modifying central sensitization.
J Appl Biobehav Res 2015;20:84–99.
10.1111/jabr.12033Nijs J, Lahousse A, Kapreli E, et al.
Nociplastic pain criteria or recognition of central sensitization?
Pain phenotyping in the past, present and future.
J Clin Med 2021;10:3203.
10.3390/jcm10153203Graven-Nielsen T.
Mechanisms and manifestations in musculoskeletal pain:
from experimental to clinical pain settings.
Pain 2022;163:S29–45.
10.1097/j.pain.0000000000002690Corrêa JB, Costa LOP, de Oliveira NTB, et al.
Central sensitization and changes in conditioned pain modulation in people
with chronic nonspecific low back pain: a case-control study.
Exp Brain Res 2015;233:2391–9.
10.1007/s00221-015-4309-6O’Neill S, Manniche C, Graven-Nielsen T, et al.
Generalized deep-tissue hyperalgesia in patients with chronic low-back pain.
Eur J Pain 2007;11:415–20.
10.1016/j.ejpain.2006.05.009Imamura M, Chen J, Matsubayashi SR, et al.
Changes in pressure pain threshold in patients with
chronic nonspecific low back pain.
Spine (Phila Pa 1976) 2013;38:2098–107.
10.1097/01.brs.0000435027.50317.d7Imamura M, Alfieri FM, Filippo TRM, et al.
Pressure pain thresholds in patients with
chronic nonspecific low back pain.
J Back Musculoskelet Rehabil 2016;29:327–36.
10.3233/BMR-150636Farasyn A, Meeusen R.
The influence of non-specific low back pain on
pressure pain thresholds and disability.
Eur J Pain 2005;9:375–81.
10.1016/j.ejpain.2004.09.005Blumenstiel K, Gerhardt A, Rolke R, et al.
Quantitative sensory testing profiles in chronic back pain
are distinct from those in fibromyalgia.
Clin J Pain 2011;27:682–90.
10.1097/AJP.0b013e3182177654Giesbrecht RJ, Battié MC.
A comparison of pressure pain detection thresholds in people
with chronic low back pain and volunteers without pain.
Phys Ther 2005;85:1085–92.
10.1093/ptj/85.10.1085Giesecke T, Gracely RH, Grant MAB, et al.
Evidence of augmented central pain processing in
idiopathic chronic low back pain.
Arthritis Rheum 2004;50:613–23.
10.1002/art.20063Clauw DJ, Williams D, Lauerman W, et al.
Pain sensitivity as a correlate of clinical status
in individuals with chronic low back pain.
Spine (Phila Pa 1976) 1999;24:2035–41.
10.1097/00007632-199910010-00013Owens MA, Bulls HW, Trost Z, et al.
An examination of pain catastrophizing and endogenous pain
modulatory processes in adults with chronic low back pain.
Pain Med 2016;17:1452–64.
10.1093/pm/pnv074Christensen KS, O’Sullivan K, Palsson TS.
Conditioned pain modulation efficiency is associated with pain
catastrophizing in patients with chronic low back pain.
Clin J Pain 2020;36:825–32.
10.1097/AJP.0000000000000878Roussel NA, Nijs J, Meeus M, et al.
Central sensitization and altered central pain processing
in chronic low back pain: fact or myth?
Clin J Pain 2013;29:625–38.
10.1097/AJP.0b013e31826f9a71Aoyagi K, He J, Nicol AL, et al.
A subgroup of chronic low back pain patients with central sensitization.
Clin J Pain 2019;35:869–79.
10.1097/AJP.0000000000000755Klyne DM, Moseley GL, Sterling M, et al.
Are signs of central sensitization in acute low back pain
a precursor to poor outcome?
J Pain 2019;20:994–1009.
10.1016/j.jpain.2019.03.001Gerhardt A, Eich W, Treede R-D, et al.
Conditioned pain modulation in patients with nonspecific chronic back
pain with chronic local pain, chronic widespread pain, and fibromyalgia.
Pain 2017;158:430–9.
10.1097/j.pain.0000000000000777Huysmans E, Ickmans K, Van Dyck D, et al.
Association between symptoms of central sensitization and cognitive
behavioral factors in people with chronic nonspecific low
back pain: a cross-sectional study.
J Manipulative Physiol Ther 2018;41:92–101.
10.1016/j.jmpt.2017.08.007Goubert D, Danneels L, Graven-Nielsen T, et al.
Differences in pain processing between patients with chronic low
back pain, recurrent low back pain, and fibromyalgia.
Pain Physician 2017;20:307–18.
10.36076/ppj.2017.318Roldán-Jiménez C, Pérez-Cruzado D, Neblett R, et al.
Central sensitization in chronic musculoskeletal pain
disorders in different populations: a cross-sectional study.
Pain Med 2020;21:2958–63.
10.1093/pm/pnaa069Torrado-Carvajal A, Toschi N, Albrecht DS, et al..
Thalamic neuroinflammation as a reproducible and
discriminating signature for chronic low back pain.
Pain 2021;162:1241–9.
10.1097/j.pain.0000000000002108Gonçalves Dos Santos G, Delay L, Yaksh TL, et al.
Neuraxial cytokines in pain states.
Front Immunol 2019;10:3061.
10.3389/fimmu.2019.03061Nicol GD, Lopshire JC, Pafford CM.
Tumor necrosis factor enhances the capsaicin sensitivity
of rat sensory neurons.
J Neurosci 1997;17:975–82.
10.1523/JNEUROSCI.17-03-00975.1997Andrade P, Visser-Vandewalle V, Hoffmann C, et al.
Role of TNF-alpha during central sensitization in preclinical studies.
Neurol Sci 2011;32:757–71.
10.1007/s10072-011-0599-zLim YZ, Wang Y, Cicuttini FM, et al.
Association between inflammatory biomarkers and nonspecific
low back pain: a systematic review.
Clin J Pain 2020;36:379–89.
10.1097/AJP.0000000000000810Klyne DM, Barbe MF, Hodges PW.
Systemic inflammatory profiles and their relationships with demographic,
behavioural and clinical features in acute low back pain.
Brain Behav Immun 2017;60:84–92.
10.1016/j.bbi.2016.10.003Li Y, Liu J, Liu Z-Z, et al.
Inflammation in low back pain may be detected from
the peripheral blood: suggestions for biomarker.
Biosci Rep 2016;36:e00361.
10.1042/BSR20160187Gevers-Montoro C, Romero-Santiago M, Losapio L, et al.
Presence of tumor necrosis factor-alpha in urine samples of patients
with chronic low back pain undergoing chiropractic care:
preliminary findings from a prospective cohort study.
Front Integr Neurosci 2022;16:879083.
10.3389/fnint.2022.879083Hoegh M, Schmid AB, Hansson P, et al.
Not being able to measure what is important, does not
make things we can measure important.
Pain 2022;163:e963.
10.1097/j.pain.0000000000002662Shraim MA, Sluka KA, Sterling M, et al.
Features and methods to discriminate between mechanism-based categories
of pain experienced in the musculoskeletal system:
a Delphi expert consensus study.
Pain 2022;163:1812–28.
10.1097/j.pain.0000000000002577Holm LA, Nim CG, Lauridsen HH, et al.
Convergent validity of the central sensitization inventory
and experimental testing of pain sensitivity.
Scand J Pain 2022;22:597–613.
10.1515/sjpain-2021-0090Damian K, Chad C, Kenneth L, et al.
Time to evolve: the applicability of pain phenotyping
in manual therapy.
J Man Manip Ther 2022;30:61–7.
10.1080/10669817.2022.2052560Chan A-W, Tetzlaff JM, Gøtzsche PC, et al.
Spirit 2013 explanation and elaboration:
guidance for protocols of clinical trials.
BMJ 2013;346:e7586.
10.1136/bmj.e7586Wong JJ, Tricco AC, Côté P, et al.
Association between depressive symptoms or depression and health outcomes
for low back pain: a systematic review and meta-analysis.
J Gen Intern Med 2022;37:1233–46.
10.1007/s11606-021-07079-8Gore M, Sadosky A, Stacey BR, et al.
The Burden of Chronic Low Back Pain: Clinical Comorbidities,
Treatment Patterns, and Health Care Costs in Usual Care Settings
Spine (Phila Pa 1976). 2012 (May 15); 37 (11): E668–677Chaibi A, Saltyte Benth J, Bjorn Russell M.
Validation of Placebo in a Manual Therapy Randomized Controlled Trial
Sci Rep. 2015 (Jul 6); 5: 11774de Andrés Ares J, Cruces Prado LM, Canos Verdecho MA, et al.
Validation of the short form of the brief pain inventory
(BPI-SF) in Spanish patients with non-cancer-related pain.
Pain Pract 2015;15:643–53.
10.1111/papr.12219Tan G, Jensen MP, Thornby JI, et al.
Validation of the brief pain inventory for chronic nonmalignant pain.
J Pain 2004;5:133–7.
10.1016/j.jpain.2003.12.005Alcántara-Bumbiedro S, Flórez-García MT, Echávarri-Pérez C, et al.
Escala de incapacidad POR dolor lumbar de oswestry.
Rehabilitación 2006;40:150–8.
10.1016/S0048-7120(06)74881-2Kongsted A, Hestbaek L, Kent P.
How Can Latent Trajectories of Back Pain be
Translated into Defined Subgroups?
BMC Musculoskelet Disord. 2017 (Jul 3); 18 (1): 285Ellingsen D-M, Beissner F, Moher Alsady T, et al.
A picture is worth a thousand words: linking fibromyalgia pain
widespreadness from digital pain drawings with pain
catastrophizing and brain cross-network connectivity.
Pain 2021;162:1352–63.
10.1097/j.pain.0000000000002134Petrescu F, Voican SC, Silosi I.
Tumor necrosis factor-alpha serum levels in healthy smokers and nonsmokers.
Int J Chron Obstruct Pulmon Dis 2010;5:217–22.
10.2147/copd.s8330Edwards RR, Almeida DM, Klick B, et al.
Duration of sleep contributes to next-day pain report in the general population.
Pain 2008;137:202–7.
10.1016/j.pain.2008.01.025García Campayo J, Rodero B, Alda M, et al.
Validation of the Spanish version of the pain
catastrophizing scale in fibromyalgia.
Med Clin (Barc) 2008;131:487–92.
10.1157/13127277Cuesta-Vargas AI, Roldan-Jimenez C, Neblett R, et al.
Cross-cultural adaptation and validity of the spanish central sensitization inventory.
Springerplus 2016;5:1837.
10.1186/s40064-016-3515-4Scerbo T, Colasurdo J, Dunn S, et al.
Measurement properties of the central sensitization inventory:
a systematic review.
Pain Pract 2018;18:544–54.
10.1111/papr.12636Sanz J, García-Vera MP, Espinosa R, et al..
Adaptación española del inventario para la depresión de beck-II (BDI-II): 3.
propiedades psicométricas en pacientes con trastornos psicológicos
[Spanish adaptation of the beck depression inventory-II (BDI-II): 3.
psychometric features in patients with psychological disorders].
Clínica y Salud 2005;16:121–42.García-Campayo J, Zamorano E, Ruiz MA, et al.
Cultural adaptation into Spanish of the generalized anxiety
disorder-7 (GAD-7) scale as a screening tool.
Health Qual Life Outcomes 2010;8:8.
10.1186/1477-7525-8-8Rolke R, Baron R, Maier C, et al.
Quantitative sensory testing in the German research network on
neuropathic pain (DFNS): standardized protocol and reference values.
Pain 2006;123:231–43.
10.1016/j.pain.2006.01.041Starkweather AR, Heineman A, Storey S, et al.
Methods to measure peripheral and central sensitization using
quantitative sensory testing: a focus on individuals
with low back pain.
Appl Nurs Res 2016;29:237–41.
10.1016/j.apnr.2015.03.013Pfau DB, Krumova EK, Treede R-D, et al.
Quantitative sensory testing in the German research network on neuropathic
pain (DFNS): reference data for the trunk and application
in patients with chronic postherpetic neuralgia.
Pain 2014;155:1002–15.
10.1016/j.pain.2014.02.004Balaguier R, Madeleine P, Vuillerme N.
Intra-session absolute and relative reliability of pressure pain thresholds
in the low back region of vine-workers: ffect of the number of trials.
BMC Musculoskelet Disord 2016;17:350.
10.1186/s12891-016-1212-7Balaguier R, Madeleine P, Vuillerme N.
Is one trial sufficient to obtain excellent pressure pain threshold
reliability in the low back of asymptomatic individuals?
A test-retest study.
PLoS One 2016;11:e0160866.
10.1371/journal.pone.0160866Liew B, Lee HY, Rügamer D, et al.
A novel metric of reliability in pressure pain threshold measurement.
Sci Rep 2021;11:6944.
10.1038/s41598-021-86344-6Ortega A, Olea-Herrero N, Arenas MI, et al.
Urinary excretion of parathyroid hormone-related protein correlates
with renal function in control rats and rats with cisplatin nephrotoxicity.
Am J Physiol Renal Physiol 2019;317:F874–80.
10.1152/ajprenal.00091.2019Morris P, Ali K, Merritt M, et al.
A systematic review of the role of inflammatory biomarkers
in acute, subacute and chronic non-specific low back pain.
BMC Musculoskelet Disord 2020;21:142.
10.1186/s12891-020-3154-3Cormier S, Lavigne GL, Choinière M, et al.
Expectations predict chronic pain treatment outcomes.
Pain 2016;157:329–38.
10.1097/j.pain.0000000000000379Walker, BF, Hebert, JJ, Stomski, NJ et al.
Outcomes of Usual Chiropractic.
The OUCH Randomized Controlled Trial of Adverse Events
Spine (Phila Pa 1976). 2013 (Sep 15); 38 (20): 1723–1729Ortega Calvo M, Cayuela Domínguez A.
Unconditioned logistic regression and sample size:
a bibliographic review.
Rev Esp Salud Publica 2002;76:85–93.
10.1590/S1135-57272002000200002White IR, Horton NJ, Carpenter J, et al.
Strategy for intention to treat analysis in randomised trials
with missing outcome data.
BMJ 2011;342:d40.
10.1136/bmj.d40Bell ML, Kenward MG, Fairclough DL, et al.
Differential dropout and bias in randomised controlled trials:
when it matters and when it may not.
BMJ 2013;346:e8668.
10.1136/bmj.e8668Kirkwood J, Allan GM, Korownyk CS, et al.
Peer simplified decision aid: chronic back pain
treatment options in primary care.
Can Fam Physician 2021;67:31–4.
10.46747/cfp.670131George SZ, Fritz JM, Silfies SP, et al.
Interventions for the management of acute and chronic
low back pain: revision 2021.
J Orthop Sports Phys Ther 2021;51:CPG1–60.
10.2519/jospt.2021.0304Hohenschurz-Schmidt D, Draper-Rodi J, Vase L, et al.
Blinding and sham control methods in trials of physical, psychological,
and self-management interventions for pain (article I):
a systematic review and description of methods.
PAIN 2022;2022:10.
10.1097/j.pain.0000000000002723Hohenschurz-Schmidt D, Draper-Rodi J, Vase L, et al.
Blinding and sham control methods in trials of physical, psychological,
and self-management interventions for pain (article II):
a meta-analysis relating methods to trial results.
PAIN 2022;2022:10.
10.1097/j.pain.0000000000002730
Return to LOW BACK PAIN
Return to SPINAL PAIN MANAGEMENT
Since 2-21-2023


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |