

The Impact of Pain-related Fear on Neural Pathways
of Pain Modulation in Chronic Low Back PainThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Pain Rep. 2017 (Apr 11); 2 (3): e601 ~ FULL TEXT
OPEN ACCESS Michael Lukas Meiera, Philipp Stampfli, Barry Kim Humphreys,
Andrea Vrana, Erich Seifritz, Petra Schweinhardt
Department of Chiropractic Medicine,
Interdisciplinary Spinal Research,
University Hospital Balgrist,
Zurich, Switzerland.
INTRODUCTION: Pain-related fear plays a substantial role in chronic low back pain (LBP) by amplifying the experienced disability. Related dysfunctional emotions and cognitions may also affect sensory aspects of pain through a modulatory pathway in which the periaqueductal gray (PAG) and the amygdala play key roles.
OBJECTIVES: We therefore hypothesized a differential amygdala-PAG functional connectivity (FC) in patients with chronic LBP that is modulated by the degree of pain-related fear.
METHODS: We used data of a previously reported fMRI study where 20 chronic LBP patients (7 females, mean age = 39.35) and 20 healthy controls (12 females, mean age = 32.10) were asked to observe video clips showing potentially harmful and neutral activities for the back. Pain-related fear was assessed using the Tampa Scale of kinesiophobia (TSK) and Fear Avoidance Beliefs questionnaires (FABQ). Generalized psychophysiological interactions were used to reveal task-based FC.
RESULTS: Compared to controls, patients exhibited a significant decrease in amygdala-PAG-FC (P = 0.022) during observation of harmful activities, but not of neutral activities. Furthermore, amygdala-PAG-FC correlated negatively with Tampa Scale of kinesiophobia scores in patients (R2 = 0.28, P = 0.01) but not with Fear Avoidance Beliefs questionnaires scores.
DISCUSSION: Our findings might indicate a maladaptive psychobiological interaction in chronic LBP patients characterized by a disrupted amygdala-PAG-FC that is modulated by the degree of pain-related fear. These results shed new light on brain mechanisms underlying psychological factors that may have pronociceptive effects in chronic LBP.
KEYWORDS: Amygdala; Chronic low back pain; Fear avoidance beliefs; Functional connectivity; Kinesiophobia; PAG; PPI; fMRI
From the FULL TEXT Article:
Introduction
Chronic low back pain (LBP) accounts for a considerable burden in terms of pain and suffering. [2, 19, 21] Impaired endogenous pain modulation is likely one of the mechanisms contributing to the development and maintenance of chronic pain. [11, 17, 25] The neural circuit underlying emotion and pain modulation comprises 2 key structures that are highly related by sharing functional and structural connections, namely the amygdala and the periaqueductal gray (PAG). [22, 27, 33] The amygdala constitutes an important site for a reciprocal relationship between persistent pain and negative affective states such as fear and anxiety. [4, 10, 23, 24, 31] The PAG is a key region involved in pain modulation and thought to play an important role in the pathogenesis of chronic pain. [5, 14, 33] In support, it has been shown that the resting-state functional connectivity (FC) of the PAG is disrupted in chronic LBP. [33] However, evidence about modulating factors of amygdala- PAG-FC in chronic LBP is sparse. One potential factor might be pain-related fear. Pain-related fear partly predicts LBP chronification, probably via a vicious circle of cognitive dysfunctions that may ultimately lead to physical deconditioning of the musculoskeletal system. [1, 12, 29, 32] Based on the knowledge that cognitions modulate not only emotional functioning but also sensory aspects of pain perception through endogenous pain modulatory mechanisms, [20] we hypothesized that pain-related fear modulates the neural crosstalk between the amygdala and the PAG. Therefore, we used an existing data set to specifically test amygdala-PAG-FC and its relationship with the individual degree of pain-related fear in chronic LBP patients and asymptotic controls.
Methods
Subject recruitment and questionnaires
The study was approved by the Ethics Committee Zurich (Switzerland) and conducted in accordance with the Declaration of Helsinki. Subjects provided written informed consent. The current study involved 20 patients suffering from nonspecific chronic LBP and 20 healthy gender-matched and age-matched controls (HC, Table 1). Pain-related fear was assessed with 2 questionnaires focusing either on fear of movement/(re)injury/ kinesiophobia (Tampa Scale of Kinesiophobia questionnaire, TSK) or fear avoidance beliefs (Fear Avoidance Beliefs questionnaire, FABQ30). Due to abbreviated, validated versions of the original 17-item TSK questionnaire, we additionally calculated the questionnaire scores of the 13-item and 11-item TSK versions. [6, 26] For detailed information about questionnaires, please see supplemental material.
Imaging and experimental protocol
For image preprocessing and detailed description of the experimental protocol, please see supplemental material. Briefly, participants viewed video clips (4 seconds) of activities potentially harmful for the back and control neutral activities in randomized order during fMRI. The activities were adapted from the electronic version of the Photograph Series of Daily Activities that has established a fear hierarchy based on patients’ perceived harmfulness. [13, 16] In addition, our patients rated the clips during fMRI using a visual analog scale, confirming that potentially harmful activities were perceived as being more harmful compared to neutral activities (supplemental material)
Amygdala-PAG-FC analysis
Using the same data set, we previously examined the FC of the amygdala with a whole brain analysis approach and observed that amygdala FC to the anterior insula differed between chronic LBP and controls as a function of the TSK score. [16] Here, we subject the data to a secondary region of interest analysis based on the extensive a priori evidence regarding the involvement of the amygdala and PAG in emotion and pain regulation. [4, 5, 10, 14, 22] To assess the temporal covariance between the amygdala and the PAG on the neural level, generalized psychophysiological interactions were computed. [15] We extracted the deconvolved time course across the bilateral amygdala defined on the Harvard-Oxford Cortical and Subcortical Structural Atlas with a probability threshold of 50% (Fig. 1A). The psychological terms (harmful and neutral video clips), the physiological regressors (time courses of seed region) as well as the interaction terms and the movement parameters were included in the final model. Thus, the variance explained by the interaction term is only that over and above what is explained by the main effects of task and physiological correlation.18 Finally, the amygdala connectivity estimates (contrasts harmful activities > baseline and neutral activities > baseline) were computed for each subject using rfxplot (http://rfxplot.sourceforge. net/)with a study-independentmask of the PAG. The PAG mask consisted of bilateral 6-mm spheres each centered around the average peak location reported in a review of multimodal PAG responses including pain (left MNI xyz: –4, –29, –12/right MNI xyz: 4, –29, –12). [14] The resulting connectivity estimates were analyzed in SPSS (version 23) with a repeated measures ANOVA (within-subject factor “condition” [harmful and neutral] and between-subject factor “group” [patients and controls]), followed by post hoc 2-sample t tests. Condition-specific relationships between questionnaire scores and FC were tested using Pearson’s correlation coefficient and statistically compared using “cocor” (http://comparingcorrelations. org/) based on a modification of Fisher’s Z procedure. [3, 8] Normality of the questionnaire data was assessed by using skewness and kurtosis indices of ±2 [7]
Results
Questionnaire scores

Table 1

Table 2
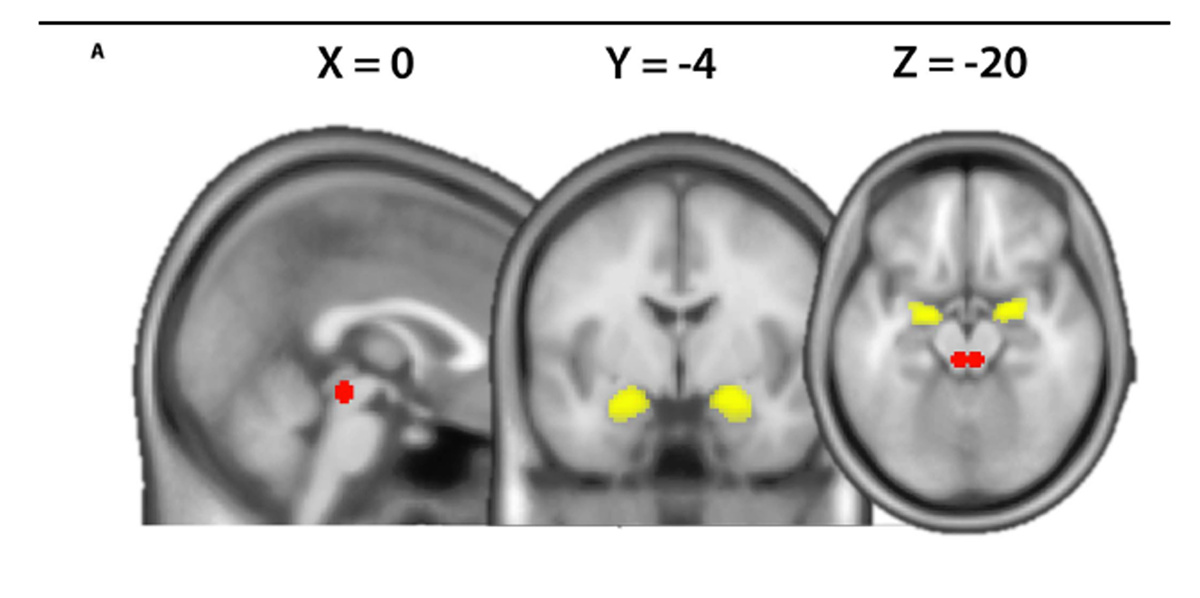
Figure 1 For questionnaire scores Table 1. Several significant correlations between the different pain-related fear questionnaires scores were observed (Table 2).
Amygdala-PAG-FC
There was no significant main effects of “group” or “condition” (P’s > 0.14), whereas the “group 3 condition” interaction showed a trend (F1,38 = 3.41, P = 0.072). Post hoc t tests indicated a significant decrease in amygdala-PAG-FC in patients compared to controls during observation of the harmful activities (patients M = 0.14, SD = 0.38: HCM = 0.44, SD = 0.40, t(38) = -2.385, P = 0.022 [2-tailed]) but not during neutral activities (patients M = 0.27, SD = 0.67, HCM = 0.22, SD = 0.43, t(38) = 0.296, P = 0.769 [Fig. 1B]). Furthermore, during observation of harmful activities, the strength of the amygdala-PAG-FC in patients showed significant negative correlations with the TSK scales with the 13-item version demonstrating the strongest relationship (R2 - 0.28, P = 0.01, Fig. 1C). The FABQ questionnaire and its subscales did not correlate with the amygdala-PAG-FC strength (P’s > 0.45). No significant relationships between amygdala-PAG-FC strength and questionnaire scores were observed during observation of neutral activities (P’s > 0.28). Direct comparison of the different TSK and FABQ correlation coefficients between the harmful and neutral conditions revealed no significant differences (P’s > 0.14).
Discussion
Our results might indicate a maladaptive psychobiological interaction in chronic LBP characterized by an attenuation of amygdala-PAG-FC that is modulated by the degree of pain-related fear. Besides the established role of the PAG in the modulation of nociceptive inputs, our results add further evidence to the involvement of the PAG in negative emotional processing not directly related to nociception. [9, 28] Furthermore, while we have previously shown that pain-related fear is positively correlated with amygdala activity in chronic LBP, [16] enhanced pain-related fear seems to simultaneously dampen the neural crosstalk between the amygdala and the PAG. This decreased information exchange between 2 key pain modulatory structures might ultimately tip the balance of PAG function to facilitation, ie, increased pronociception. [11] Thus, the decreased crosstalk between the amygdala and the PAG, in conjunction with increased amygdala activity, might be the neurobiological basis of how pain-related fear contributes to pain and its chronification. However, indicated by the nonsignificant interaction/correlations (see results section 3.2), it must be noted that the potentially harmful video clips might elicit other reactions than fear such as processes associated with threat and defensive responses, which might impact the specificity of the findings. Interestingly, the TSK and the FABQ seem to capture different concepts of pain-related fear reflected in the differential brain-behavior relationships. More research is needed to increase the construct validity of these commonly used questionnaires including a new elaborate framework for pain-related fear to further enhance the specificity of its neural correlates.
Disclosures
The authors have no conflicts of interest to declare.
Supported by the Foundation for the Education of Chiropractors and the Balgrist Foundation, Switzerland.
Acknowledgements
The authors acknowledge support by the Clinical Research Priority Program “Molecular Imaging” at the University of Zurich. Finally, we would like to thank Dr. Sabina Hotz-Boendermaker.
References:
Barke A, Baudewig J, Schmidt-Samoa C, Dechent P, Kro¨ ner-Herwig B.
Neural correlates of fear of movement in high and low fear-avoidant chronic low back pain patients: an event-related fMRI study.
PAIN 2012; 153:540–52.Bronfort G Haas M Evans RL et al.
Efficacy of Spinal Manipulation and Mobilization for Low Back Pain and Neck Pain:
A Systematic Review and Best Evidence Synthesis
Spine J (N American Spine Soc) 2004 (May); 4 (3): 335–356Diedenhofen B, Musch J.
Cocor: a comprehensive solution for the statistical comparison of correlations.
PLoS One 2015;10: e0121945.Ellingsen DM, Wessberg J, Eikemo M, Liljencrantz J, Endestad T, Olausson H, Leknes S.
Placebo improves pleasure and pain through opposite modulation of sensory processing.
Proc Natl Acad Sci U S A 2013;110:17993–8.Fields H.
State-dependent opioid control of pain.
Nat Rev Neurosci 2004; 5:565–75.Goubert L, CrombezG, van Damme S, Vlaeyen JWS, Bijttebier P, Roelofs J.
Confirmatory factor analysis of the Tampa Scale for Kinesiophobia: invariant two-factor model across low back pain patients and fibromyalgia patients.
Clin J Pain 2004;20:103–10.Gravetter F, Wallnau L.
Essentials of statistics for the behavioral sciences, 8th Edition.
Belmont, NY: Wadsworth, 2014.Hittner JB, May K, Silver NC.
A Monte Carlo evaluation of tests for comparing dependent correlations.
J Gen Psychol 2003;130:149–68.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD.
Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies.
NeuroImage 2008;42: 998–1031.Kryklywy JH, Nantes SG, Mitchell DGV.
The amygdala encodes level of perceived fear but not emotional ambiguity in visual scenes.
Behav Brain Res 2013;252:396–404.Kuner R.
Central mechanisms of pathological pain.
Nat Med 2010;16: 1258–66.LeeuwM,GoossensMEJB, LintonSJ,CrombezG,BoersmaK,VlaeyenJWS.
The fear-avoidance model of musculoskeletal pain: current state of scientific evidence.
J Behav Med 2007;30:77–94.Leeuw M, Goossens MEJB, van Breukelen GJP, Boersma K, Vlaeyen JWS.
Measuring perceived harmfulness of physical activities in patients with chronic low back pain: the Photograph Series of Daily Activities–short electronic version.
J Pain 2007;8:840–9.Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D.
Neuroimaging of the periaqueductal gray: state of the field.
NeuroImage 2012;60:505–22.McLaren DG, Ries ML, Xu G, Johnson SC.
A generalized form of contextdependent psychophysiological interactions (gPPI): a comparison to standard approaches.
NeuroImage 2012;61:1277–86.Meier ML, Stampfli P, Vrana A, Humphreys BK, Seifritz E, Hotz- Boendermaker S.
Neural Correlates of Fear of Movement in Patients with Chronic Low Back Pain vs. Pain-Free Individuals.
Front Hum Neurosci 2016;10:386.Mlekusch S, Neziri AY, Limacher A, Juni P, Arendt-Nielsen L, Curatolo M.
Conditioned pain modulation in patients with acute and chronic low back pain.
Clin J Pain 2016;32:116–21.O’Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, Johansen-Berg H.
Tools of the trade: psychophysiological interactions and functional connectivity.
Social Cogn Affect Neurosci 2012;7:604–9.Peterson CK, Bolton J, Humphreys BK.
Predictors of Improvement in Patients With Acute and Chronic Low Back Pain
Undergoing Chiropractic Treatment
J Manipulative Physiol Ther. 2012 (Sep); 35 (7): 525-533Price DD, Verne GN, Schwartz JM.
Plasticity in brain processing and modulation of pain.
Prog Brain Res 2006;157:333–52.Rapoport J, Jacobs P, Bell NR, Klarenbach S.
Refining the measurement of the economic burden of chronic diseases in Canada.
Chronic Dis Can 2004;25:13–21.Rizvi TA, Ennis M, Behbehani MM, Shipley MT.
Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity.
J Comp Neurol 1991;303:121–31.Seifritz E, Esposito F, Neuhoff JG, Lu¨ thi A, Mustovic H, Dammann G, von Bardeleben U.
Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents.
Biol Psychiatry 2003;54:1367–75.Silvers JA, Wager TD, Weber J, Ochsner KN.
The neural bases of uninstructed negative emotion modulation.
Social Cogn Affect Neurosci 2015;10:10–8.Staud R.
The important role of CNS facilitation and inhibition for chronic pain.
Int J Clin Rheumatol 2013;8:639–46.Tkachuk GA, Harris CA.
Psychometric properties of the Tampa Scale for Kinesiophobia-11 (TSK-11).
J Pain 2012;13:970–7.Veinante P, Yalcin I, Barrot M.
The amygdala between sensation and affect: a role in pain.
J Mol Psychiatry 2013;1:9.Villemure C, Bushnell MC.
Mood influences supraspinal pain processing separately from attention.
J Neurosci 2009;29:705–15.Vlaeyen JW, Linton SJ.
Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art.
PAIN 2000;85:317–32.Waddell G, Newton M, Henderson I, Somerville D, Main CJ.
A Fear- Avoidance Beliefs Questionnaire (FABQ) and the role of fearavoidance beliefs in chronic low back pain and disability.
PAIN 1993;52:157–68.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM.
Placebo-induced changes in FMRI in the anticipation and experience of pain.
Science 2004;303: 1162–7.Wertli MM, Rasmussen-Barr E, Weiser S, Bachmann LM, Brunner F.
The role of fear avoidance beliefs as a prognostic factor for outcome in patients with nonspecific low back pain: a systematic review.
Spine J 2014;14:816–36.e4.Yu R, Gollub RL, Spaeth R, Napadow V, Wasan A, Kong J.
Disrupted functional connectivity of the periaqueductal gray in chronic low back pain.
Neuroimage Clin 2014;6:100–8.
Return to LOW BACK PAIN
Return to BIOPSYCHOSOCIAL MODEL
Return to SPINAL PAIN MANAGEMENT
Since 2-04-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |