Identification of Internal Carotid Artery
Dissection in Chiropractic PracticeThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Can Chiropr Assoc. 2004 (Sep); 48 (3): 206–210 ~ FULL TEXT
OPEN ACCESS Michael T Haneline, DC, MPH and Gary Lewkovich, DC
Palmer College of Chiropractic West,
Palmer Center for Chiropractic Research,
90 E. Tasman Drive,
San Jose, CA 95134.Internal carotid artery dissection (ICAD) is a condition involving separation of the artery's intimal lining from its medial division, with subsequent extension of the dissection along varying distances of the artery, usually in the direction of blood flow. ICAD may produce cerebral ischemia due to occlusion of the involved artery. This occlusion may occur at or near the site of the dissection, or "downstream" as a result of embolization from a dislodged thrombus fragment. The problem any chiropractic physician faces in identifying ICAD patients is that the condition may present without any symptoms or the symptoms may appear benign (e.g., headache, neck pain or cervicogenic dizziness). Consequently, it may be impossible to identify some ICAD patients, especially in the early stages of the pathology. As the ICAD progresses and neural blood flow is compromised, the symptom picture typically manifests more completely. The chiropractic physician must be alert to characteristic findings of a progressing ICAD, since an immediate referral to a medical specialist may be required.
From the FULL TEXT Article:
Introduction
Internal carotid artery dissection (ICAD) is a condition wherein there is a separation of the artery’s intimal lining from its medial division, with subsequent extension of the dissection along varying distances of the artery, usually in the direction of blood flow. [1, 2] It has been suggested that ICAD produces stroke in 36–68% of patients as a result of occlusion of the artery at or near the site of the dissection, or embolization occurring distally from a dislodged fragment of thrombus. [3]
ICAD is not a rare condition, with incidence estimates exceeding 7,000 cases per year in the United States. [4] Accordingly, patients with this disorder may present to DCs for treatment of associated symptoms that often mimic musculoskeletal conditions. Established chiropractic patients may also develop ICAD coincidentally during their course of chiropractic management, with no relationship to manipulation. However, when ICAD is recognized, an urgent referral for a neurological and vascular evaluation is appropriate. This article summarizes the presentation, diagnosis, and proper management of patients with this potentially life-threatening condition.
Discussion
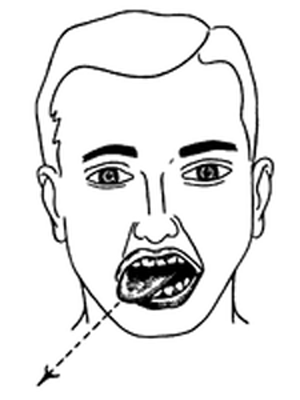
The most common presenting symptoms of ICAD are headache and neck pain, which are typically severe and of sudden onset. There is wide variation in the location of pain, but most commonly it involves the ipsilateral periorbital, frontal, or upper cervical region. [1, 5–10] (Figure 1) One series of 36 ICAD patients reported headache to be present in more than 90% of the cases, [1] with focal cerebral ischemic symptoms in 67%. In a series comprising 135 ICAD cases, headaches were described by 65 patients as being unique, or of different character than what they had previously experienced, while 40 patients indicated that the headaches were familiar. [5] Silverman and Wityk described three cases of ICAD whose presentation suggested migrainous aura, and as a result made diagnosis difficult. [11]
FIGURE 1 Common sites of headache and neck pain
associated with the presenting symptoms
of internal carotid artery dissection.
The second most common clinical manifestations are ischemic signs and symptoms, which include cortical transient ischemic attacks (TIA’s), stroke, or both, and less frequently, transient monocular blindness. Transient monocular loss of vision (amaurosis fugax) has been reported in from 6% to 30% of patients presenting with ICAD. [1, 12, 13] Another common sign of ICAD is an incomplete Horner’s Syndrome that includes miosis and ptosis, but not anhydrosis. [1, 14] The Horner’s Syndrome variant found in ICADs may be painful, and when of sudden onset is strongly suggestive of this condition. [5, 15] (Table 1)
TABLE 1 Most common presenting symptoms of ICAD (In descending order)
Symptom Reported Frequency Character Headache and neck pain >90% Typically severe and of sudden onset, affecting the ispsilateral periorbital, fontal, or upper cervical region. Often unique, or of different character than previously experienced. Ischemic symptoms 50–95% Transient ischemic attacks (TIAs) or stroke. Horner’s Syndrome <52% Incomplete, lacking anhydrosis. May be painful. Visual scintillations 33% Episodic Monocular blindness 6–30% Transient Subjective bruit 25–48% May also manifest as a pulsatile tinnitus. Dysgeusia 10–19% Impairment of taste.
The clinical presentation of ICAD patients has been described as a characteristic triad of symptoms including neck or head pain, Horner’s Syndrome, and focal hemispheric ischemic symptoms. [16] Local symptoms preceded ICAD in 96% of the series of patients described by Biousse et al., [17] and consisted of head or neck pain, Horner’s Syndrome, and tinnitus. Based on another group of 21 ICAD patients, 20 had head pain and 12 had accompanying neck pain. [8] Other common symptoms included oculosympathetic palsy, subjective bruit, dysgeusia, and visual scintillations.
Greater than 10% of ICAD patients experience cranial nerve palsies that almost always involve cranial nerve XII, but IX, X, and XI have also been affected. The cranial nerve palsies are thought to be the result of disruption of their blood supply by way of mechanical compression from the distended ICA or by embolization. [18]
Some authors have indicated that there is a delay between intimal disruption and the development of ischemic signs which usually spans hours to days, but has been reported to occur after years. [17, 19, 20] Jacobs et al. indicated that the latent interval between the presumed initiation of dissection and onset of symptoms is usually hours to days, but may be even weeks, which makes diagnosis difficult. [21] Conversely, the precise pathogenesis of ICAD is not clear in most cases, [22] with the majority of ICADs occurring spontaneously. Indeed, determining the cause of ICAD has been described as being speculative. [2–4]
In general, clinical findings appear to be different when comparing traumatically induced ICAD to spontaneous ICAD. In the traumatic group, focal cerebral ischemic symptoms were the most common manifestations. In the spontaneous group, unilateral heaches were the most commonly experienced symptom, often in association with Horner’s Syndrome. [12, 23, 24] Like spontaneous ICAD, the onset of ischemic signs and symptoms may be delayed in traumatic cases. [25] The longer this delay, the greater the confusion in assigning any causation to the ICAD.
ICAD can be very difficult to diagnose, and is often overlooked, especially in its early stages before ischemic signs are apparent. [20, 26] It may go undiagnosed in cases that only cause mild symptoms or are asymptomatic. [23] Asymptomatic ICAD has occasionally been detected incidentally, for instance during imaging of the opposite carotid or vertebral arteries for suspected dissection. This suggests that many cases are never discovered, and probably heal spontaneously.
Fisher classified the various angiographic presentations of ICAD, noting the typical “string sign” that represents the long tapered lumenal stenosis associated with dissection. [13] Angiography has long been considered to be the gold standard diagnostic test in the identification of ICAD, but is invasive and carries a risk of complication. [27] MRI, along with MR angiography (MRA), however, is a noninvasive method for investigating ICAD, which had 100% sensitivity and 100% specificity in one series of 16 patients. [28] Consequently, MRI/MRA have replaced angiography for the workup of suspected cervical artery dissection in many medical centers.
Several authors have demonstrated the effectiveness of using the different forms of diagnostic ultrasound in the evaluation of dissections. [29] MRI with Duplex examinations were utilized for follow up on two cases of ICAD. [30] The investigators noted that the combination diagnostic protocol would be an efficacious noninvasive method to take the place of serial angiography. Pulsed Doppler ultrasound has been found to be reliable in determining flow abnormalities, but the findings are not specific. For that reason, Doppler can be considered helpful in following up ICAD after it has been initially diagnosed by MRI/ MRA. [27] Steinke et al. were able to detect initial abnormal Doppler signals in all 50 dissections that they evaluated, but with only a moderate degree of sensitivity. [16] Transcranial Doppler (TCD) was helpful in monitoring cerebral microemboli that were present in 59% of a series of ICAD patients. [31] The authors considered the presence of microemboli in ICAD as a risk factor for cerebral ischemia, [32] and that the patients identified through TCD should be provided appropriate treatment.
No treatment protocol has been validated as being successful thus far. [33] However, the traditional medical intervention in ICAD is anticoagulation therapy. Interestingly, up to one third of diagnosed spontaneous ICAD patients have received no treatment. [23, 31] Surgical intervention is not indicated in most cases, but is reserved for a small subset of patients who do not respond to anticoagulation. [33]
The outcome of ICAD is variable. Some patients recover completely, while others experience severe permanent deficits. A small percentage die as a result of complications associated with ICAD. In a series of 36 ICAD patients that were followed by Mokri et al., 22% had residual deficits: 14% had mild deficits, and 8% had moderate-severe residual deficits. [1]
Conclusion
Even though ICAD is uncommon, it is not considered rare and DCs may encounter patients with this condition in their practices. When identified, the chiropractic management of ICAD patients involves immediate referral to an appropriate medical specialist. Unwarranted delay may result in progression of the ICAD even in the absence of any treatment. In addition to urgent referral, it is recommended that any identified ICAD patient not receive cervical manipulation, as a few case reports have correlated worsening of the condition with the intervention. [34, 35] Moreover, any form of excessive or abrupt cervical motion may dislodge an embolus. Unfortunately, ICAD may be completely asymptomatic or symptoms may appear to be benign (e.g., headache, neck pain, or cervicogenic dizziness). Consequently, the condition may be nearly impossible to identify, at least in its early stage. There is currently no credible evidence to support the opinion that cervical manipulation causes ICAD. [4] However, a close temporal relationship between the chiropractic manipulation and the onset of symptoms may give the appearance of a causative relationship. As a result, medical neurologists may report this to patients who develop ICAD subsequent to chiropractic manipulation. [36, 37]
There are no pre-manipulation screening tests available that are capable of reliably identifying patients who are likely to develop cervical artery dissection. Realizing that ICAD is often difficult and sometimes impossible to diagnose, DCs should be aware of the information summarized in Table 1 and consider it in patients who are candidates for cervical manipulation, especially in cases involving new, severe, or unusual headache and/or neck pain. When ICAD is suspected, the patient should be immediately referred to an appropriate medical specialist for evaluation and possible treatment.
Commercial associations that might pose a conflict of interest:
None.
Funding sources supporting the work:
None.References
Mokri B, Sundt TM, Jr, Houser OW, Piepgras DG.
Spontaneous dissection of the cervical internal carotid artery.
Ann Neurol. 1986;19(2):126–138Ozdoba C, Sturzenegger M, Schroth G.
Internal carotid artery dissection: MR imaging features and clinical-radiologic correlation.
Radiology. Apr. 1996;199(1):191–198Desfontaines P, Despland PA.
Dissection of the internal carotid artery: aetiology, symptomatology, clinical and neurosonological follow-up, and treatment in 60 consecutive cases.
Acta Neurol Belg Dec. 1995;95(4):226–234Haneline M, Croft A, Frishberg B.
Association of Internal Carotid Artery Dissection and Chiropractic Manipulation
The Neurologist 2003 (Jan); 9 (1): 35–44Biousse V, D’Anglejan-Chatillon J, Massiou H, Bousser MG.
Head pain in non-traumatic carotid artery dissection: a series of 65 patients.
Cephalalgia. 1994;14(1):33–36Silbert PL, Mokri B, Schievink WI.
Headache and neck pain in spontaneous internal carotid and vertebral artery dissections.
Neurology. 1995;45(8):1517–1522Guillon B, Biousse V, Massiou H, Bousser MG.
Orbital pain as an isolated sign of internal carotid artery dissection. A diagnostic pitfall.
Cephalalgia. 1998;18(4):222–224Fisher CM.
The headache and pain of spontaneous carotid dissection.
Headache. 1982;22(2):60–65Biousse V, Woimant F, Amarenco P, Touboul PJ, Bousser MG.
Pain as the only manifestation of internal carotid artery dissection.
Cephalalgia. 1992;12(5):314–317Bogousslavsky J, Despland PA, Regli F.
Spontaneous carotid dissection with acute stroke.
Arch Neurol. 1987;44(2):137–140Silverman IE, Wityk RJ.
Transient migraine-like symptoms with internal carotid artery dissection.
Clin Neurol Neurosurg. 1998;100(2):116–120Hart RG, Easton JD.
Dissections of cervical and cerebral arteries.
Neurol Clin. 1983;1(1):155–182Fisher CM, Ojemann RG, Roberson GH.
Spontaneous dissection of cervico-cerebral arteries.
Can J Neurol Sci. 1978;5(1):9–19Anson J, Crowell RM.
Cervicocranial arterial dissection.
Neurosurgery. 1991;29(1):89–96Biousse V, Touboul PJ, D’Anglejan-Chatillon J, Levy C, Schaison M, Bousser MG.
Ophthalmologic manifestations of internal carotid artery dissection.
Am J Ophthalmol. 1998;126(4):565–577Steinke W, Rautenberg W, Schwartz A, Hennerici M.
Noninvasive monitoring of internal carotid artery dissection.
Stroke. 1994;25(5):998–1005Biousse V, D’Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG.
Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients.
Stroke. 1995;26(2):235–239Mokri B, Silbert PL, Schievink WI, Piepgras DG.
Cranial nerve palsy in spontaneous dissection of the extracranial internal carotid artery.
Neurology. 1996;46(2):356–359Davis JM, Zimmerman RA.
Injury of the carotid and vertebral arteries.
Neuroradiology. 1983;25(2):55–69Treiman GS, Treiman RL, Foran RF, et al.
Spontaneous dissection of the internal carotid artery: a nineteen-year clinical experience.
J Vasc Surg. 1996;24(4):597–605. discussion 605–597Jacobs A, Lanfermann H, Neveling M, Szelies B, Schroder R, Heiss WD.
MRI- and MRA-guided therapy of carotid and vertebral artery dissections.
J Neurol Sci 20. 1997;147(1):27–34Schievink WI, Mokri B, Piepgras DG.
Spontaneous dissections of cervicocephalic arteries in childhood and adolescence.
Neurology Sep. 1994;44(9):1607–1612Mokri B.
Traumatic and spontaneous extracranial internal carotid artery dissections.
J Neurol. 1990;237(6):356–361Mokri B, Piepgras DG, Houser OW.
Traumatic dissections of the extracranial internal carotid artery.
J Neurosurg. 1988;68(2):189–197Hughes KM, Collier B, Greene KA, Kurek S.
Traumatic carotid artery dissection: a significant incidental finding.
Am Surg. 2000;66(11):1023–1027Lee WW, Jensen ER.
Bilateral internal carotid artery dissection due to trivial trauma.
J Emerg Med. 2000;19(1):35–41Bakke SJ, Smith HJ, Kerty E, Dahl A.
Cervicocranial artery dissection. Detection by Doppler ultrasound and MR angiography.
Acta Radiol. 1996;37(4):529–534Kirsch E, Kaim A, Engelter S, et al.
MR angiography in internal carotid artery dissection: improvement of diagnosis by selective demonstration of the intramural haematoma.
Neuroradiology. 1998;40(11):704–709Guillon B, Tzourio C, Biousse V, Adrai V, Bousser MG, Touboul PJ.
Arterial wall properties in carotid artery dissection: an ultrasound study.
Neurology 12. 2000;55(5):663–666Rothrock JF, Lim V, Press G, Gosink B.
Serial magnetic resonance and carotid duplex examinations in the management of carotid dissection.
Neurology May. 1989;39(5):686–692Srinivasan J, Newell DW, Sturzenegger M, Mayberg MR, Winn HR.
Transcranial Doppler in the evaluation of internal carotid artery dissection.
Stroke. 1996;27(7):1226–1230Babikian VL, Forteza AM, Gavrilescu T, Samaraweera R.
Cerebral microembolism and extracranial internal carotid artery dissection.
J Ultrasound Med. 1996;15(12):863–866Bassi P, Lattuada P, Gomitoni A.
Cervical cerebral artery dissection: a multicenter prospective study (preliminary report)
Neurol Sci. 2003;24 (Suppl 1):S4–7Dragon R, Saranchak H, Lakin P, Strauch G.
Blunt injuries to the carotid and vertebral arteries.
Am J Surg. 1981;141(4):497–500Peters M, Bohl J, Thomke F, et al.
Dissection of the internal carotid artery after chiropractic manipulation of the neck.
Neurology. 1995;45(12):2284–2286Norris JW, Beletsky V, Nadareishvili ZG.
Sudden Neck Movement and Cervical Artery Dissection
CMAJ. 2000 (Jul 11); 163 (1): 38–40Lee KP, Carlini W, McCormick G, Albers G.
Neurologic complications following chiropractic manipulation: a survey of California neurologists.
Neurology. 1995;45(6):1213–1215
Return to STROKE AND CHIROPRACTIC
Since 2-27-2015


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |