Vertebral Artery Strains During High-speed,
Low amplitude Cervical Spinal ManipulationThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Electromyo and Kinesio 2012 (Oct); 22 (5): 740746 ~ FULL TEXT
Walter Herzog, PhD, Tim Leonard, PhD, Symons B, Conrad Tang, DC, Wuest S.
Faculty of Kinesiology,
University of Calgary,
Calgary, Canada.
walter@kin.ucalgary.ca.
FROM: J Electromyogr Kinesiol 2012Spinal manipulative therapy (SMT) has been recognized as an effective treatment modality for many back, neck and musculoskeletal problems. One of the major issues of the use of SMT is its safety, especially with regards to neck manipulation and the risk of stroke. The vast majority of these accidents involve the vertebro-basilar system, specifically the vertebral artery (VA) between C2/C1. However, the mechanics of this region of the VA during SMT are unexplored. Here, we present first ever data on the mechanics of this region during cervical SMT performed by clinicians. VA strains obtained during SMT are significantly smaller than those obtained during diagnostic and range of motion testing, and are much smaller than failure strains. We conclude from this work that cervical SMT performed by trained clinicians does not appear to place undue strain on VA, and thus does not seem to be a factor in vertebro-basilar injuries.
There are more articles like this @ our
STROKE AND CHIROPRACTIC Page
From the FULL TEXT Article:
Introduction
Spinal manipulative therapy (SMT) has been recognized as an effective treatment modality for many back, neck and musculoskeletal problems (Haldeman, 1986; Herzog, 2000a). It has received wide-spread acceptance in a variety of disciplines including chiropractic, physiotherapy, nursing, and mainstream health care. However, despite its surge in popularity as a cost-effective treatment modality, there is little basic research as to the beneficial mechanisms underlying SMT (Herzog, 2010).
Spinal manipulative therapy typically consists of a high-speed, low-amplitude thrust delivered by a practitioner to a specific landmark on a patients body. We and others, have quantified the force time histories of SMTs in a variety of settings and for a variety of clinical problems (Conway et al., 1993; Downie et al., 2010; Forand et al., 2004; Herzog, 2000a; Hessel et al., 1990; Triano, 2000). Several results have emerged from these studies:(i) the forces applied by a given clinician are fairly consistent, but they vary dramatically
across clinicians and location of application with peak forces ranging from approximately
200 N (44 lbs) to values of up to 1400 N (308 lbs);
(ii) the speed of the treatment thrust is consistent (within about 100200 ms for
experienced clinicians) (Herzog, 2000a); and
(iii) the forces applied by male and female clinicians are similar (Forand et al., 2004),
as are the forces applied to patients or non-patients subjects in a laboratory setting
(Symons et al., in Press), but the forces applied on cadaveric specimens (which
have been used to study the potential injurious effects of SMTs) are significantly
greater and are applied significantly faster than the forces applied
to patients and non-patient control subjects (Symons et al., in Press).Forces applied during SMT have been shown to elicit a variety of mechanical, biological and physiological responses including relative movements of the intervertebral joints (Gál et al., 1997a,b), muscle reflex responses (Dishman and Bulbulian, 2000; Dishman and Burke, 2003; Floman et al., 1997; Gibbons et al., 2000; Herzog, 2000b, 2010; Herzog et al., 1999; Lehman et al., 2001; Suter et al., 2005, 2009), changes to the blood biochemistry (Brennan et al., 1991; Triano et al., 1991), and cavitation of joints (Cascioli et al., 2003; Conway et al., 1993).
Although the total forces during SMTs can be very high, these forces are typically distributed across a large contact area which increases with increasing forces because of the soft tissue contacts between patient and clinician (Herzog et al., 2001), thereby reducing the local forces to 510 N (12 lbs) for typical thoracic manipulations. Nevertheless, there has been increased interest over the past three decades to elucidate the possible damaging effects of SMT forces on internal tissues (Cote et al., 1994; Haldeman et al., 2001; Haldeman and Rubinstein, 1992a,b; Ianuzzi and Khalsa, 2005; Paciaroni and Bogousslavsky, 2009; Powell et al., 1993; Rubinstein, 2008; Rubinstein and Haldeman, 2001; Terrett and Kleynhans, 1980). One of the major issues of the use of SMT is its safety, especially with regards to neck manipulation and the risk of stroke (Lee et al., 1995; Paciaroni and Bogousslavsky, 2009; Rubinstein, 2008; Wuest et al., 2010). Although the estimates of stroke associated (but not necessarily caused) by SMT is small about one in a million (Hurwitz et al., 1996) the severity and irreversible nature of such accidents makes this a material risk (Herzog and Symons, 2002; Symons et al., 2002).
The vast majority of these accidents involves the vertebro-basilar system, specifically the vertebral artery (VA) between C2/C1 and the cephalad/ distal loop as the VA exits the C1 foramen transversarium and travels to the foramen magnum (Haldeman et al., 1999). Because of the specific anatomy of the VA in that region, it has been assumed that the VA experiences considerable stretch during extension and rotation of the neck, which may lead to hemodynamic occlusions and damage to the VA, predisposing the patient to stroke (Herzog and Symons, 2002; Symons et al., 2002). However, recent evidence suggests that such damage appears unlikely in the distal extra-cranial loop of the VA (between C1 and the foramen magnum) and the proximal/caudal loop between C6 and VAs origin from the subclavian artery (Austin et al., 2010; Herzog and Symons, 2002; Symons et al., 2002), but the regions between C1 and C6 remain unexplored, except for some preliminary data (Wuest et al., 2010).
Here, we review the results of existing studies on human VA strains during high-speed, low-amplitude SMTs administered by qualified clinicians and compare them to the strains encountered during full range of motion (ROM) tests, and furthermore, add the summarized results of unpublished works from strains measured from all sections of 8 VAs using data from 3 clinicians, resulting in a total of 3034 segment strains obtained during SMTs and 2380 segment strains obtained during full ROM testing.
Methods
Subjects

Table 1 Tests were performed on a total of 12 human cadavers. Two embalmed cadavers were initially used for pilot testing and evaluation of all measurements (not included in the results), and 10 fresh, unembalmed human cadavers were used for measurements of strains in 16 VAs. Five of these specimens and 6 VAs were used for measurements of the extra-cranial loop of the VA (from C1 to the foramen magnum and the proximal/caudal loop (from C6 to the subclavian artery) (Herzog and Symons, 2002; Symons et al., 2002) (Cadavers 15; Table 1), and four of the cadavers and corresponding 8 VAs were used for measurements throughout the VA (from the foramen magnum to the origin of VA at the subclavian artery) (Cadavers 69; Table 1). One specimen and corresponding arteries were excluded from analysis (as were some individual segments) when strain measurements could not be obtained for technical reasons. The details of the cadaveric specimens are given in Table 1.
VA dissection
The VA was approached by blunt dissection using an anterolateral approach as described previously (Symons et al., 2002). Care was taken to leave all structures intact while exposing the VA.
Specifically, no ligaments, muscles or bones were cut to preserve the in situ mechanical behavior of the VAs as much as possible.
Range of motion (ROM) testing and spinal manipulative treatments

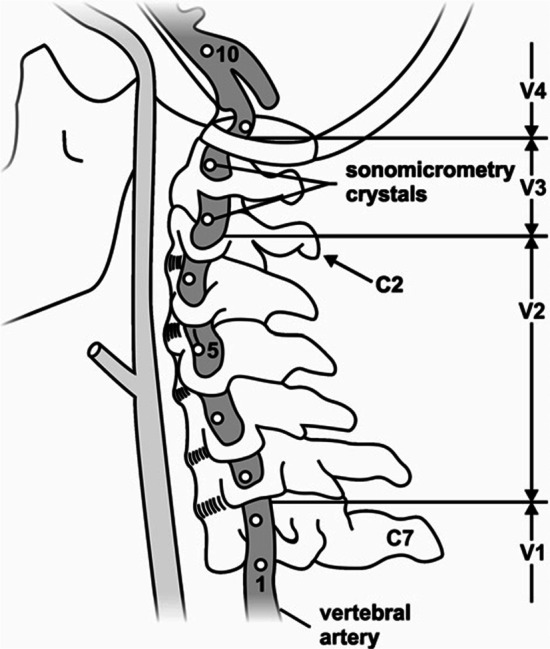
Figure 1

Figure 2 ROM testing was performed in flexion, extension, rotation and lateral bending (Figure 1). ROM was established by moving the head passively from the neutral position (head and neck aligned straight; Figure 1A) to the point where no further movement was possible (Figure 1B end range of rotational movement). Following that, a Houles vertebro basilar insufficiency test was performed by placing the head in a rotated/extended position. All asymmetric tests were performed bilaterally (i.e. rotation to the left and right). The SMTs consisted of a diversified lateral/rotary manipulation with a second metacarpal contact specifically against the articular pillar with the cadaver supine, and also a pure lateral manipulation with the force applied in an essentially lateral direction to the neck (Figure 1C). These SMTs were delivered at levels C1/C2, C3/C4, and C6/C7 while measuring strains in all segments that were instrumented on the side of manipulation and the contralateral side. All ROM testing was repeated three times and was performed bilaterally; all SMTs were repeated three times, on all levels and both sides of the neck. Therefore, for a cadaver instrumented as shown in Figure (2 markers 29) up to 7 strain measurements could be made for each test, for a total of 252 strain measurements (3 repeat tests 3 locations 2 treatment techniques 2 sides of treatments 7 vertebral segments) for the SMTs alone and a single clinician working on a single VA. Testing of the first 6 VAs was performed by a single clinician (Symons et al., 2002), while measurements on the last 8 VAs were performed by 3 clinicians. For the last 8 VAs tested, strain measurements were accompanied by measurements of the contact forces, and the VAs were injected with ultrasound gel to maximize the strain signals (through sonomicrometry) and to restore the VAs to a natural, fluid filled shape (Wuest et al., 2010).
All ROM and SMT testing was performed by three licensed chiropractors. Two of the clinicians were male with 2 and 13 years of experience, and one was female with 5 years of experience.
VA Strain measurements
Strain measurements were made using sonomicrometry, as explained in detail in our earlier works (Herzog and Symons, 2002; Symons et al., 2002; Wuest et al., 2010). Briefly, sonomicrometry crystals (Sonometrics Corporation, London, Ontario, Canada) of 0.51.0 mm diameter were inserted into the wall of the VA at defined locations (4 crystals and 2 segments for the initial 6 VA tests (Figure 2 markers 12 and 910) and 8 crystals and 7 segments typically for the 8 final VA tests) (Figure 2 markers 29). Crystals were placed in the lumen of the artery and then sutured to the VA wall so that they could not move relative to the VA. Each crystal served as a receiver and a transmitter of short (400 ns) ultrasound pulses. Knowing the time required for traveling from one crystal to the next with the head and neck in the neutral position (Figure 1A; defined as the 0% strain position), strains during ROM and SMT testing were calculated by the increase or decrease in time it took to cover the distance from one crystal (transmitter) to the neighboring crystal (receiver). This approach has the advantage that the strains are independent of the actual speed of sound transmission, and that strains can be measured continuously (2000 Hz) during testing. The detailed calculations and the measurements of accuracy and resolution (0.016 mm) can be found in our previous works (Herzog and Symons, 2002; Symons et al., 2002; Wuest et al., 2010). Percentages of strain (elongations were defined as positive) were then calculated for each ROM and each SMT test from the neutral position to the position of maximal VA strain. For example, a strain of 3.4% would indicate that the VA was stretched by 3.4% from its original length at neutral, while a strain of ÿ2.1% would indicate a shortening of the VA of 2.1% from its neutral length.
Strain results were calculated for each segment, chiropractor, and ROM/SMT test, but will be given here as summary results across four identified regions of the VA designated as V1, V2, V3 and V4 (Figure 2). Strains for V1 segments are calculated using markers 1 and 2 (markers are labeled consecutively from inferior to superior as shown in Figure 1), for V2 segments markers 36, V3 markers 7 and 8, and V4 markers 9 and 10.
Force measurements
Forces applied by the clinicians on the neck of the specimens during SMTs were measured using a thin flexible pressure pad as described before by our group (Conway et al., 1993; Forand et al., 2004; Herzog, 1991, 2010; Hessel et al., 1990; Wuest et al., 2010). Briefly, the pressure pad (Novel Inc, Munich, Germany) was sealed for moisture proofing and then held against the neck of the specimen over the target site. The clinician then contacted the pressure pad with her/his 2nd metacarpal, applied a preload force on the target site, and then executed the treatment thrust. Analysis of these forces revealed that treatments on the cadaver specimens, in general, were performed with greater peak forces and higher rates of load application than found in patient and non-patient subjects, as illustrated in this special issue of JEK (Symons et al., in Press). Thus, if anything at all, the strains during SMTs measured here are likely greater than those one would obtain in a patient.
Results

Table 2 Mean strains (and minimal and maximal strains) for the V1, and V4 segments for the ROM testing in our initial study (Symons et al., 2002) were 3.2% (2.04.9%), and 5.9% (1.212.5%), respectively, while the corresponding values for the SMT testing were 6.2% (4.58.0%) and 2.1% (1.42.7%) (Table 2). Note that the ipsilateral values for the SMT testing were not used in this analysis as they had been shown to be affected by direct contact of the clinician with the sonomicrometry markers, and were found unreliable. However, ipsilateral values were considered for the ROM testing as contact with the VA embedded markers was avoided. For example, VA strains for the left VA would have been used for left and right rotations of the head and neck, while strains for the left VA would only have been considered for manipulations on the right side of the neck, which would create the greatest strains (Symons et al., 2002).
In our more recent study (Table 1, cadavers 69) using three clinicians and 8 VAs that were anatomically restored by filling them with ultrasound gel, the mean strains (minimal and maximal strains) for the V1, V2, and V3 segments for the ROM testing were 3.3% (1.014.5%), 4.9% (1.312.7%), and 12.2% (3.622.9%). The corresponding values for the SMT testing were 0.9% (04.3%), 1.4% (0 4.6%), and 3.8% (012.6%) (Table 2).
Mean failure strains of VAs have been reported previously to be 58%, on average (Symons et al., 2002).

Figure 3 An example of raw data of VA length changes (V4 segment) for a full flexion range of motion test (Figure 3; gray trace) and a diversified lateral/rotational SMT (Figure 3; black trace) from the same subject illustrates many typically observed results:
(i) The length change of the VA during SMT (indicated by the two arrows) is much smaller
than that observed during the range of motion testing;
(ii) the length of the VA throughout the entire SMT is smaller than the length in the neutral
head and neck position (about 15.4 mm as seen at the very beginning of the range of
motion test which started at the neutral length); and
(iii) the rate of change in VA length (given by the slope of the distance-time traces), is similar
for the SMT and range of motion testing, even though the range of motion testing was
performed very slowly (a full cycle took approximately 4s, while the displayed SMT took 150 ms).
Discussion
Vertebro basilar accidents associated with high-speed, lowamplitude spinal manipulative treatments have been a concern for clinicians and patients alike. The primary argument for such accidents has been that SMTs stretch vertebral artery segments to such a degree that there is an occlusion of blood flow, and possible stretch-induced damage to the VA. Since the thrust phase of neck SMTs lasts 100150 ms (Herzog and Symons, 2001; Triano, 2000), any occlusion would likely have little to no effect on blood flow to the brain. Therefore, we focused on the stretches that occur during SMTs to the neck by measuring directly the strains of VAs during SMTs applied by clinicians certified in manipulative treatments and compared these strains to those one would expect to occur during normal head and neck movements in everyday life (passive range of motion).
The average failure strain of human VAs has been found to be 58% (Symons et al., 2002), with failure defined as the first softening of the VA in a stretch experiment. All values shown in Table 1 are clearly below the mechanical failure strains of VAs with the largest measured value of 22.9% for ROM testing in the V3 region representing about 39% of the failure strain. However, failure strain might be considered a rude estimate of damage in VAs and it might be argued that micro-structural failures could occur in the absence of gross mechanical failure of the tissue. Furthermore, repeat treatments, as may occur in clinical practice, might cause micro-structural damage that is not visible following a single SMT.
In order to investigate the structural behavior and integrity of arterial tissues exposed to repeat loading cycles, we subjected rabbit descending aortas (which are of similar size to human VAs) to 1000 repeat stretch cycles of 0% strain (controls), 6% strain and 30% strain. Following mechanical loading, the arteries were fixed, embedded and analyzed histologically for microstructural damage by a pathologist blinded to the strain grouping. Exposure to 1000 cycles of 6% strain did not result in increased microstructural damage compared to control tissues, but 1000 cycles of 30% strain caused significant increases in microstructural damage of the arterial tissue (Austin et al., 2010). Mean strains across all SMTs, all clinicians and neck segments ranged from 0.96.2%, suggesting that even 1000 cycles of exposure in a 20 min period (as done in the study by Austin et al. (2010) would not result in micro-structural damage of the VA.
Another way of estimating if SMTs might produce harmful stretches of the VA is to compare the maximal strain values obtained across all clinicians and all manipulations to the maximal strain values obtained for ROM testing, as the ROM tests indicate the range of head and neck movements that a person can perform in everyday life, assuming that everyday life head/neck rotations, extensions, and bending are safe. We have done that by calculating the ratio of the maximal strains obtained by SMT and ROM testing, where values of less than 100% indicate that the SMT strain was smaller than the strain during ROM testing, and thus would be considered safe. For the V1 segment, this ratio is 55% (8%/14.5%). The corresponding values for V2, V3, and V4 are 36%, 55%, and 22%, respectively. In summary, the maximal strain values for the ROM testing at each segmental level were always greater than the corresponding strain values for the SMTs, suggesting that neck SMTs impose less stretch than turning your head, or extending your neck while looking up at the sky.
Limitations
The results of this study suggest that neck SMTs impose stretches on the VA that are maximally 55% of the passive range of motion achieved with normal movements of the head and neck and are maximally 22% of the observed mean mechanical failure strain of human VAs. Therefore, based on the mechanical tests performed here, one should be able to conclude that stretching of VA during neck SMTs does not cause any damage of the VAs. However, these results must be considered in view of experimental and technical limitations, and ultimate proof of the safety regarding stretch of VAs needs to come from live human experiments performed during actual SMTs in patients with accurate strain measurements of the VA, but such experiments are not possible at present and our approach is currently the best to provide information on VA mechanics during neck SMTs. All measurements were performed on human cadavers. It has been argued in the past that strain measurements in cadaveric specimens might differ from live subjects, however that is rather unlikely. The strains of the VA (and for that matter of any tissue) only depend on the distance between origin and insertion points of the specimen (VA in our case), and the proper shape of the test specimen. There is no reason why a specific head/neck position should cause the origin/insertion points to be at a different location in a live compared to a dead subject. In fact, strain measurements in other tissues, such as skeletal muscles, have been used for decades as indications of strains in the live body (Crowninshield et al., 1978; Wickiewicz et al., 1983). After all, geometrical considerations dictate that the strain only depends on the joint configuration (relative location of the vertebral bodies in our case) and the moment arm around the centre of rotation of the joint (two adjacent vertebral bodies, respectively). Thus, there is no a priori reason why strains in the VA should differ between a person that is alive and the same person right after death. However, in our initial study on VA strains, we did not perfuse the VAs and that might have resulted in a systematic error in our strain measurements (Symons et al., 2002). This potential error was rectified in our second study (Table 2) by restoring the proper VA shape by filling the arteries with ultrasound gel. Giving the VAs their in vivo shape resulted in smaller mean strains during SMT application (0.9% strain vs. 6.2% strain), but the ROM strains remained unchanged (3.3% vs. 3.2%).
Another limitation that has been put forward in the past is the idea of failure strains obtained from old cadaveric specimens and their applicability to the average patient. The average failure strain observed in our cadaveric specimens was 58%. However, this is likely an underestimation of the true value, as the age of the specimens, the placing of the piezoelectric markers into the lumen of the VA, the dissection of the neck, and the isolation and clamping of the VA in the materials testing machine, all would have (if anything at all) compromised VA strength, and therefore given failure strains less than those of the intact, in vivo VA. Thus, although failure testing is difficult, compromising the VAs strength as done in our testing would tend to reduce the failure values, thus working against the argument that neck SMTs produce safe VA strains.
Engineering strain of a material is typically defined from a reference length at which stress is just zero, and a small stretch will produce force and thereby stress in the material. Here, the reference length (0% strain) was defined as the length of the VA segments when the head and neck were in a neutral position. We do not know if that is already a strained position. However, in our initial(Herzog and Symons, 2002; Symons et al., 2002) studies, we found that first force in the VA was measured at approximately 12% of strain, which for the segments tested, was approximately the maximal strain achieved in the ROM testing. Therefore, it appears that there is no real strain in the VA until the head and neck are moved to the end range of motion, and any elongation prior to that (that is all the elongations during SMTs) would be within a strain free region (Herzog and Symons, 2002). This would mean that the VA is never really strained during spinal manipulative treatments but that the VA is merely taking up slack as the neck and head are moved during SMT, but that there is no stress and thus no possibility for microstructural damage. Qualitative observation of the VAs in many specimens seem to support the notion that at least some segments in some specimens are slack, and do not reach a length of engineering strain even at the longest lengths during SMT and ROM testing. However, in order to confirm this assertion, careful tests need to be done where individual segments of the VA are removed from the neck in the neutral position, and sensitive forceelongation testing is then performed in these specimens. Such data have not been published to date for human VAs.
Another limitation that has been mentioned in the past is the idea that SMT forces applied to cadaveric specimens are not the same as those applied in patient or non-patient, live human subjects. Force measurements during neck SMTs in cadavers and live subjects are the topic of another paper in this special issue of JEK (Symons et al., in Press). Without taking all the thunder from that publication, it can be stated here that, on average, SMTs performed by the same clinicians on cadaveric specimens were statistically significantly more powerful: that is, the peak forces were greater and the rate of force application higher in the cadaveric specimens than the human patient and non-patient subjects. The possible reasons for this are discussed in the accompanying paper, but the implications of these results are that, if anything at all, the VA strains obtained from the cadaveric subjects reported here, would likely be greater than those obtained in actual patients.
Finally, it has been argued that the rate of stretch of the VA is more important than the actual stretch (strain) magnitude, and that safe strain magnitudes during neck SMTs might still be harmful because of the exceptional rate of strain application. The VA is a visco-elastic structure (Austin et al., 2010), and thus stress would be greater for a given amount of strain when the VA is stretched at a high compared to a low rate. However, the mean peak strain during SMT was 6.2% and occurred over an average time of about 150 ms for a strain rate of about 41% strain/s. Considering the largest ROM strain observed in our study (22.9%), the head movement for that strain (rotation) would produce a similar strain rate if the rotation occurred in about 560 ms, which seems a perfectly achievable movement. Similar considerations could be made for the mean strains during SMT and the peak strains during ROM, and it would become clear that strain rates for the largest head/neck movements are within similar rates of strain application than neck SMTs (e.g. Figure 3). Of course, actual head and neck movements are slower than what they are during neck SMTs, but the peak strains are also much larger, thus producing rates of strain that are actually quite comparable. Nevertheless, in order to determine the rate dependence of stress in VAs, careful stressstrain testing should be performed at different rates of stretch. To our best knowledge, such data are currently not available for human VAs.
Conclusion
The results from this study demonstrate that average and maximal VA strains during high-speed low-amplitude cervical spinal manipulation are substantially less than the strains that can be achieved during ROM testing for all vertebral artery segments. Furthermore, VA strains obtained during SMT and ROM testing are substantially smaller than average failure strains. Therefore, we conclude that cervical spinal manipulations, as tested here, are safe from a mechanical point of view for normal, healthy VA.
Acknowledgements
The Canadian Chiropractic Protective Agency, the Canadian Chiropractic Research Foundation, and the Alberta College and Association of Chiropractors.
References:
Austin, N, DiFrancesco, LM, and Herzog, W.
Microstructural Damage in Arterial Tissue Exposed to Repeated Tensile Strains
J Manipulative Physiol Ther 2010 (Jan); 33 (1): 1419Brennan PC, Kokjohn K, Kaltinger CJ, et al.
Enhanced Phagocytic Cell Respiratory Burst Induced by Spinal Manipulation:
Potential Role of Substance P
J Manipulative Physiol Ther 1991 (Sep); 14 (7): 399408Cascioli V, Corr P, Till Ag AG.
An investigation into the production of intra-articular gas bubbles and increase in joint space in the zygapophyseal joints of the cervical spine in asymptomatic subjects after spinal manipulation.
J Manipulative Physiol Ther 2003;26(6):35664.Conway PJW, Herzog W, Zhang Y, Hasler EM, Ladly K.
Forces required to cause cavitation during spinal manipulation of the thoracic spine.
Clin Biomech 1993;8:2104.Cote P, Mior SA, Vernon H.
The short-term effect of a spinal manipulation on pain/ pressure threshold in patients with chronic mechanical low back pain.
J Manipulative Physiol Ther 1994;17(6):3648.Crowninshield RD, Johnston RC, Andrews JG, Brand RA.
A biomechanical investigation of the human hip.
J Biomech 1978;11:7585.Dishman JD, Bulbulian R.
Spinal reflex attenuation associated with spinal manipulation.
Spine 2000;25(19):251925.Dishman JD, Burke J.
Spinal reflex excitability changes after cervical and lumbar spinal manipulation: a comparative study.
Spine J 2003;3(3):20412.Downie AS, Vemulpad S, Bull PW:
Quantifying the High-velocity, Low-amplitude Spinal Manipulative Thrust: A Systematic Review
J Manipulative Physiol Ther. 2010 (Sep); 33 (7): 542-53Floman Y, Liram N, Gilai AN.
Spinal manipulation results in immediate H-reflex changes in patients with unilateral disc herniation.
Eur Spine J 1997;6:398401.Forand D, Drover J, Suleman Z, Symons B, Herzog W.
The forces applied by female and male chiropractors during thoracic spinal manipulation.
J Manipulative Physiol Ther 2004;27:4956.Gál JM, Herzog W, Kawchuk GN, Conway PJW, Zhang Y.
Movements of vertebrae during manipulative thrusts to unembalmed human cadavers.
J Manipulative Physiol Ther 1997a;20:3040.Gál JM, Herzog W, Kawchuk GN, Conway PJW, Zhang YT.
Measurements of vertebral translations using bone pins, surface markers and accelerometers.
Clin Biomech 1997b;12(5):33740.Gibbons PF, Gosling CM, Holmes M.
Short-term effects of cervical manipulation on edge light pupil cycle time: a pilot study.
J Manipulative Physiol Ther 2000;23(7):4659.Haldeman S.
Spinal manipulative therapy in sports medicine.
Clin Sports Med 1986;5:27793.Haldeman S, Carey P, Townsend M, Papadopoulos C.
Arterial Dissections Following Cervical Manipulation: The Chiropractic Experience
Canadian Medical Association Journal (CMAJ) 2001 2001 (Oct 2); 165: 905906Haldeman S, Kohlbeck FJ, McGregor M.
Risk Factors and Precipitating Neck Movements Causing Vertebrobasilar Artery Dissection
After Cervical Trauma and Spinal Manipulation
Spine (Phila Pa 1976) 1999 (Apr 15); 24 (8): 785794Haldeman S, Rubinstein SM.
Cauda equina syndrome in patients undergoing manipulation of the lumbar spine.
Spine 1992a;17(12):146973.Haldeman S, Rubinstein SM.
Compression fractures in patients undergoing spinal manipulative therapy.
J Manipulative Physiol Ther 1992b;15(7):4504.Herzog W.
Biomechanical studies of spinal manipulative therapy.
The Journal of the CCA 1991;35:15664.Herzog W.
Clinical biomechanics of spinal manipulation.
Philadelphia: Churchill Livingstone; 2000a.Herzog W.
The mechanical, neuromuscular, and physiologic effects produced by spinal manipulation.
In: Herzog W, editor.
Clinical biomechanics of spinal manipulation.
Philadelphia: Churchill Livingstone; 2000b. p. 191207.Herzog W:
The Biomechanics of Spinal Manipulation
J Bodyw Mov Ther. 2010 (Jul); 14 (3): 280286Herzog W, Kats M, Symons B.
The effective forces transmitted by high-speed, lowamplitude thoracic manipulation.
Spine 2001;26(19):210510.Herzog W, Scheele D, Conway PJW.
Electromyographic responses of back and limb muscles associated with spinal manipulative therapy.
Spine 1999;24(2):14652.Herzog W, Symons B.
The biomechanics of spinal manipulation.
Crit Rev Phys Rehabil Med 2001;13(2&3):191216.Herzog, W., Symons, B.,2002.
The Mechanics of Neck Manipulation With Special Consideration of the Vertebral Artery
J Can Chiropr Assoc. 2002 (Sep); 46 (3): 134136Hessel BW, Herzog W, Conway PJW, McEwen MC.
Experimental measurement of the force exerted during spinal manipulation using the Thompson technique.
J Manipulative Physiol Ther 1990;13:44853.Hurwitz EL, Aker PD, Adams AH, Meeker WC, Shekelle P.
Manipulation and Mobilization of the Cervical Spine:
A Systematic Review of the Literature
SPINE (Phila Pa 1976) 1996 (Aug 1); 21 (15): 17461760Ianuzzi A, Khalsa PS.
Comparison of human lumbar facet joint capsule strains during simulated high-velocity, low-amplitude spinal manipulation versus physiological motions.
Spine J 2005;5(3):27790.Lee KP, Carlini WG, McCormick GF, Albers GW.
Neurologic complications following chiropractic manipulation: a survey of California neurologists.
Neurology 1995;45(6):12135.Lehman GJ, Vernon H, McGill SM.
Effects of a mechanical pain stimulus on erector spinae activity before and after a spinal manipulation in patients with back pain: a preliminary investigation.
J Manipulative Physiol Ther 2001;24(6):4026.Paciaroni M, Bogousslavsky J.
Cerebrovascular complications of neck manipulation.
Eur Neurol 2009;61(2):1128.Powell FC, Hanigan WC, Olivero WC.
A risk/benefit analysis of spinal manipulation therapy for relief of lumbar or cervical pain.
Neurosurgery 1993;33(1):739.Rubinstein, S.M.
Adverse Events Following Chiropractic Care for Subjects With Neck or Low-Back Pain:
Do The Benefits Outweigh the Risks?
J. Manip. Physiol. Ther. 2008, 31, 461464.Rubinstein SM, Haldeman S.
Cervical manipulation to a patient with a history of traumatically induced dissection of the internal carotid artery: a case report and review of the literature on recurrent dissections.
J Manipulative Physiol Ther 2001;24(8):5205.Suter E, McMorland G, Herzog W.
Short-term effects of spinal manipulation on Hreflex amplitude in healthy and symptomatic subjects.
In: Huijing PA, Hollander P, Findley TW, Schleip R, editors.
Fascia research II basic science and implications for conventional and complementary health care.
Munich: Elsevier; 2009. p. 2705.Suter E, McMorland GM, Herzog W.
Short-term effects of spinal manipulation on Hreflex amplitude in healthy and symptomatic subjects.
J Manipulative Physiol Ther 2005;28:66772.Symons, B., Leonard, T.R., Herzog, W., 2002.
Internal Forces Sustained by the Vertebral Artery
During Spinal Manipulative Therapy
J Manipulative Physiol Ther 2002 (Oct); 25 (8): 504510Symons B, Wuest S, Leornard T, Herzog W.
Biomechanical characterization of cervical spinal manipulation in living subjects and cadavers.
J Electromyogr Kinesiol. 2012 Oct;22(5):747-51Terrett AGJ, Kleynhans AM.
Cerebrovascular complications of manipulation.
In: Haldeman S, editor.
Principles and practice of chiropractic.
Connecticut: Appleton
& Lange; 1980. p. 57998.Triano JJ. The mechanics of spinal manipulation.
In: Herzog W, editor.
Clinical biomechanics of spinal manipulation.
Philadelphia, PA: Churchill-Livingstone; 2000. p. 92190.Triano JJ, Brennan PC, McGregor M.
A study of threshold response to thoracic manipulation.
Proc 3rd Int Conf Spinal Manip 1991;3:1502.Wickiewicz TL, Roy RR, Powell PL, Edgerton VR.
Muscle architecture of the human lower limb.
Clin Orthop 1983;179:27583.Wuest, S, Symons, B, Leonard, T, and Herzog, W.
Preliminary Report: Biomechanics of Vertebral Artery
Segments C1-C6 During Cervical Spinal Manipulation
J Manipulative Physiol Ther. 2010 (May); 33 (4): 273278
Return to STROKE AND CHIROPRACTIC
Since 11-15-2012


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |