

Changes in Biochemical Markers of Pain Perception
and Stress Response After Spinal ManipulationThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Orthop Sports Phys Ther. 2014 (Apr); 44 (4): 231–239 ~ FULL TEXT
Gustavo Plaza-Manzano, PT, Francisco Molina, PT, PhD, Rafael Lomas-Vega, PT, PhD, Antonio Martínez-Amat, PhD, Alexander Achalandabaso, PT, Fidel Hita-Contreras, MD, PhD
Department of Physical Medicine and Rehabilitation,
Faculty of Medicine,
Universidad Complutense de Madrid,
Madrid, Spain.

STUDY DESIGN: Controlled, repeated-measures, single-blind randomized study.
OBJECTIVES: To determine the effect of cervical or thoracic manipulation on neurotensin, oxytocin, orexin A, and cortisol levels.
BACKGROUND: Previous studies have researched the effect of spinal manipulation on pain modulation and/or range of movement. However, there is little knowledge of the biochemical process that supports the antinociceptive effect of spinal manipulation.
METHODS: Thirty asymptomatic subjects were randomly divided into 3 groups: cervical manipulation (n = 10), thoracic manipulation (n = 10), and nonmanipulation (control) (n = 10). Blood samples were extracted before, immediately after, and 2 hours after each intervention. Neurotensin, oxytocin, and orexin A were determined in plasma using enzyme-linked immuno assay. Cortisol was measured by microparticulate enzyme immuno assay in serum samples.
RESULTS: Immediately after the intervention, significantly higher values of neurotensin (P<.05) and oxytocin (P<.001) levels were observed with both cervical and thoracic manipulation, whereas cortisol concentration was increased only in the cervical manipulation group (P<.05). No changes were detected for orexin A levels. Two hours after the intervention, no significant differences were observed in between-group analysis.
There are more articles like this @ our: SPINAL PAIN MANAGEMENT Page CONCLUSION: The mechanical stimulus provided by spinal manipulation triggers an increase in neurotensin, oxytocin, and cortisol blood levels. Data suggest that the initial capability of the tissues to tolerate mechanical deformation affects the capacity of these tissues to produce an induction of neuropeptide expression.
Keywords cortisol, neurotensin, orexin A, oxytocin, spinal manipulation
From the FULL TEXT Article:
Background
Spinal manipulation (SM) is a common treatment approach for pain reduction in low back and neck disorders. [37, 38, 41] The effectiveness of SM to treat musculoskeletal pain, such as spinal pain, has been summarized in recent Cochrane reviews. [32, 56]
Overall, the evidence suggests that SM provides improvements in pain relief, though similar results have been described in other competing treatments, such as general practitioner management, medication, and exercise, in patients with musculoskeletal pain. [6, 7] It has been shown that the presence of pain induces changes in the anatomy and function of the central and peripheral nervous systems. [20, 46, 53] Therefore, research on an asymptomatic population may be important to accurately determine the antinociceptive mechanism of SM. Several studies in asymptomatic subjects have shown that SM techniques induce changes in physiological reflexes, [28] increase neuromuscular excitability, [22] and modify sensitivity. [30]
The mechanisms through which SM alters musculoskeletal pain are still unknown. However, current evidence suggests an interaction between the mechanical stimulus and the associated neurophysiological responses, [6, 51] including rapid hypoalgesia with concurrent sympathetic nervous system and motor system excitation, similar to those generated by direct stimulation of the periaqueductal gray matter. [61, 68] Recent animal studies show that the analgesia produced by joint mobilization involves serotonin and noradrenaline receptors in the spinal cord, thereby performing a supporting role for central mechanisms of pain modulation. [60] Several neuropeptides, such as neurotensin, [23] oxytocin, [29] or orexin A, [3] have been associated with hypoalgesia and pain modulation, and it is well known that cortisol plays an analgesic role related to stress responses. [4, 44] Recent theories have also suggested that chronic pain could be partly maintained by maladaptive physiological responses of the organism facing a recurrent stressor, a situation related to high cortisol levels. [45, 66] To our knowledge, there is a lack of studies analyzing changes in these nociception-related biochemical markers in response to manual therapy.
There are controversial opinions regarding the antinociceptive effects of SM according to the site of application. Some authors have reported that cervical manipulation may produce better analgesic effects than thoracic manipulation, [52] and other authors have not detected differences in pain relief between the 2 techniques. [43] To make better therapeutic decisions, professionals would profit from knowing whether one type of SM is better than others in terms of antinociceptive effects. Taking these data into account, our purpose was to determine whether cervical and thoracic manipulation would induce differences in neuropeptide production or have a similar biochemical response. The aim of this study was to evaluate the effects of cervical and thoracic SM on the plasmatic concentration of biochemical markers (neurotensin, orexin A, oxytocin, and cortisol). This study represents a preliminary step in advancing the understanding of the underlying mechanisms of SM treatment and its effects.
Methods
Subjects
The sample population consisted of graduate students who responded to advertisements placed in the University of Jaén (Spain). All subjects signed an informed consent form approved by the University of Jaén Institutional Review Board prior to participating in the study. Participants were verbally screened for their history of neck pain and for current use of any drug. Those who had 1 or more of the following conditions were excluded from the study: contraindication to manipulation, history of whiplash or cervical surgery, pain related to cervical spine or arm in the previous month, headache in the previous days, spinal manipulative therapy in the previous 2 months, or loss of standing balance. The study was approved by the Ethical Committee in Clinical Research of the University of Jaén, and the protocol was performed following the Ethical Principles for Medical Research in Humans of the Declaration of Helsinki.
Interventions
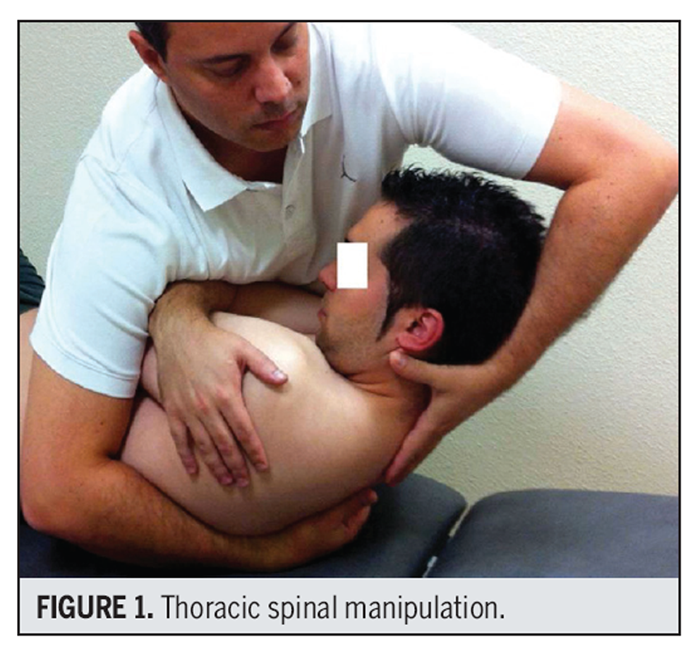
Figure 1

Figure 2 SM procedures consisted of standard techniques performed as described by Gibbons and Tehan. [31] The thoracic SM technique involved a high-velocity, end-range, anterior-posterior force through the elbows to the middle thoracic spine (T3-4) on the lower thoracic (T4-5) spine in a supine position, with the patient’s arms crossed (FIGURE 1). The cervical manipulation involved a high-velocity, midrange, left rotational force to the mid cervical spine (C4) on the lower cervical spine (C5) in supine, with left rotation and right sidebending (FIGURE 2).
Blood samples and active cervical rotation movement were obtained from all subjects before, immediately after, and 2 hours after the intervention. Confounding factors such as time of day (circadian rhythms), prior diet, and activity patterns were controlled in the 2-hour period prior to reassessment. All interventions were performed at the same time of day for each participant.
Outcome MeasuresStatistical AnalysisExtracting Blood Samples and Obtaining Serum/Plasma Serum samples were extracted by venipuncture of the cephalic vein, according to a standardized protocol36 that used a Vacutainer system (Becton, Dickinson and Company, Franklin Lakes, NJ). Blood was collected in a tube for serum (Vacutainer SST II Advance, model 367953) and a tube for plasma (Vacutainer PST II Advance, model 367374) separation. After blood extraction, tubes stood at room temperature for 1 hour until the blood clotted. Afterward, the tubes were centrifuged for 10 minutes at 2000g (Avanti J-30I; Beckman Coulter, Inc, Brea, CA). Supernatant was collected, aliquoted, and kept at –80°C until used.
Neuropeptide Quantification It has been shown that neurotensin is implicated in analgesia via its actions within central and peripheral pain modulatory circuits, [23] oxytocin plays an antinociceptive role in the central nervous system, [2] and orexin is involved in nociceptive sensory processes. [3, 24] Neuropeptides were determined by a Luminex (Luminex Corporation, Austin, TX) assay (Milliplex; EMD Millipore Corporation, Billerica, MA). This kit allows the simultaneous quantification of neurotensin, orexin A, and oxytocin (Milliplex HNP-35K; EMD Millipore Corporation). Plasma samples were thawed at room temperature and processed following recommendations from the manufacturer. Neuropeptide data were normalized with the total protein concentration of each sample, which was calculated using the Bradford assay. [10] Cortisol has been found to correlate inversely with pain intensity, and in this sense, a specific increase of cortisol has been proven to have an antinociceptive effect.1 Cortisol concentration was determined in serum samples using the microparticulate enzyme immuno assay in the AxSYM analyzer (Abbott Laboratories, Abbott Park, IL) following the manufacturer’s recommendations.
Data for continuous variables were expressed as mean ± SD. Categorical data were expressed as frequencies and percentages. The Kolmogorov-Smirnov and Levene tests were performed to assess normality and homoscedasticity, respectively. A 3-by-3, mixed-model analysis of variance (ANOVA) was performed to test the effect of the factor (control, thoracic manipulation, and cervical manipulation) on the dependent variables (range of motion and concentration of neurotensin, orexin A, oxytocin, and cortisol). The hypothesis of interest was the group-by-time interaction. Additionally, to find out if there was any significant interaction, a Bonferroni pairwise comparison was performed. Pearson correlation coefficients were used to analyze the relations between continuous variables. Eta-square and adjusted R2 were used for measuring effect sizes. Management and data analysis were performed using the statistical package SPSS for Windows Version 17.0 (SPSS Inc, Chicago, IL) and MedCalc Version 12.5 (MedCalc Software bvba, Ostend, Belgium). The level of statistical significance was set at P<.05.
Results
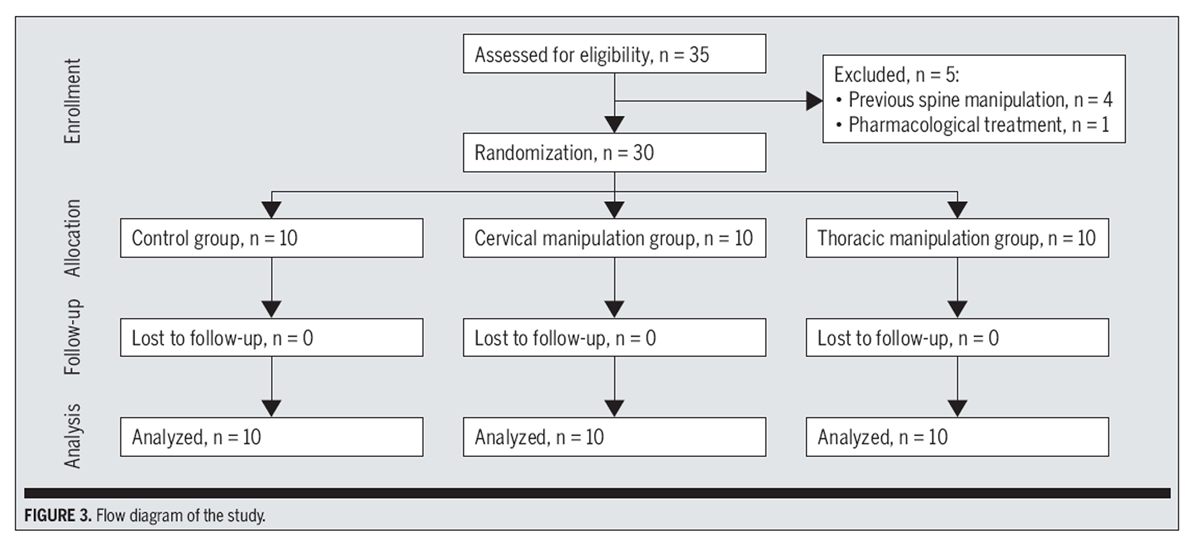
Figure 3

Table 1

Table 2

Table 3

Figure 4

Table 4 Out of the 35 participants screened, 30 subjects (46.7% women; mean ± SD age, 27.8 ± 4.2 years) satisfied the eligibility criteria, agreed to participate, and were randomized by sealed-envelope selection into the cervical SM group (n = 10), the thoracic SM group (n = 10), or a control group that did not receive any treatment (n = 10) (FIGURE 3). Baseline characteristics of participants are shown in TABLE 1.
Neurotensin Concentration in Blood Samples
The 3-by-3, mixed-model ANOVA revealed a significant interaction of time by group for neurotensin concentration (P = .029), with an eta-square value of 18% (TABLE 2). On the other hand, within-group comparisons in cervical and thoracic manipulation groups showed a significant increase in neurotensin levels immediately postintervention compared with preintervention levels (P<.05) (TABLE 3). For the between-group analysis (FIGURE 4A), statistically significant differences were found on posttreatment measurements between the control group and the thoracic manipulation group (mean difference, –3.87; 95% confidence interval [CI]: –6.00, –0.74; P = .012) and between the control and the cervical manipulation groups (mean difference, –4.86; 95% CI: –7.99, –1.74; P = .001).
Orexin A Concentration in Blood Samples
A 3-by-3, mixed-model ANOVA did not show a significant interaction of group by time for orexin A concentration in blood samples (P = .210) (TABLE 2). The effect size, measured by eta-square, was 10%. At the descriptive level, an important decrease in orexin A concentration was detected after the intervention in the thoracic SM group in comparison with the control group (mean difference, 47.16; 95% CI: –4.78, 99.10; P = .085), although this decrease did not reach statistical significance (FIGURE 4B).
Oxytocin Concentration in Blood Samples
The group-by-time interaction was significant for oxytocin plasma concentration (P<.001). The effect size, measured by eta-square, was 62% (TABLE 2). An increase in oxytocin concentration was detected just after the intervention in both the cervical SM group (mean difference, –200.58; 95% CI: –271.03, –130.12; P<.001) and the thoracic SM group (mean difference, –96.42; 95% CI: –166.87, –25.96; P = .005) when compared with the control group. In the same way, the cervical SM group showed increased oxytocin values when compared with the thoracic SM group immediately postintervention (mean difference, –104.16; 95% CI: –174.62, –33.71; P<.002) (FIGURE 4C).
Likewise, in the within-group analysis, an increase in oxytocin plasma concentration levels was detected in both the cervical manipulation and thoracic manipulation groups immediately postintervention (P<.001) compared to preintervention levels (TABLE 3). At 2 hours after the intervention, an increase was found only in the cervical SM group (P<.05) when compared with preintervention levels (TABLE 4).
Cortisol Concentration in Blood Samples
Using a mixed-model ANOVA, the group-by-time interaction for cortisol as a dependent variable was significant (P<.001). Eta-square analysis yielded a 32% effect size (TABLE 2).
Blood samples extracted from the cervical SM group showed a significant increase in cortisol plasma concentration immediately postintervention compared with baseline values (P<.001) (TABLE 3). On the other hand, a significant decrease was detected at 2 hours postintervention in the thoracic SM group when compared with the preintervention values (P<.05) (TABLE 4).
A significant increase in the between-group analysis was found immediately posttreatment in the cervical manipulation group compared with the control group (mean difference, 4.60; 95% CI: 0.65, 8.55; P = .018) and the thoracic manipulation group (mean difference, 4.10; 95% CI: 0.15, 8.05; P<.040) (FIGURE 4D).
Discussion
Several studies currently support the idea that the analgesic effect of manual therapy is mediated by central mechanisms of pain modulation through the modulation of neuropeptide production. [5, 27, 60] To our knowledge, this is the first work to analyze neurotensin, oxytocin, orexin A, and cortisol levels after a cervical or a thoracic manipulation in asymptomatic subjects.
Neurotensin is a 13-amino acid produced in several regions of the central nervous system, such as the substantia nigra, amygdala, hypothalamus, prefrontal cortex, periaqueductal gray matter, and the spinal cord, [62] and it has several actions, including analgesia. [14, 23] Our data indicate an increase in neurotensin plasmatic concentration after an SM, suggesting that the mechanical stimulus provided by SM is enough to modulate the liberation of this neuropeptide. In this sense, neurotensin has long been known to include analgesia among its actions. [9, 16, 23] The analgesic actions of neurotensin are readily distinct from those of the opioids, based on their insensitivity to the highly opioid-selective antagonist naloxone, thus ruling out an opioid mechanism. [55] Neurotensin acts as part of the peripheral and central mechanisms of pain modulation, [23] because the antinociceptive effect of neurotensin has been reported after the injection of the peptide in many brain areas. [62] There are anatomical data suggesting an interaction between neurotensin and serotonergic neurons.
As a matter of fact, neurons of the rostral part of the raphe synthesize neurotensin, whereas neurotensin receptors are widely expressed in most of the raphe. [18, 40, 57] The functional role of neurotensin in the raphe remains to be determined, but it may participate in the modulation of some of the known functions of the serotonergic system, including nociception [13] and stress-related responses. [19] It may also play a role in mediating stress-induced analgesia, as neurotensin knockout mice and rats pretreated with neurotensin antagonists show no increase in pain tolerance after stress. [34] Recent studies with neurotensin antagonists and knockout mice lacking neurotensin or neurotensin receptors have revealed that the neurotensinergic system plays a pivotal role in the nonopioid form of stress-induced analgesia. [34, 42, 58] In summary, the antinociceptive effect of neurotensin after SM may increase the mechanical stress threshold that cervical tissues can tolerate.
It is well established that neurotensin affects the activity of oxytocin-positive cells in the supraoptic nucleus. [39] Oxytocin is a nonapeptide that plays a major neuroendocrine role, modulating several physiological functions in mammals, like somatosensory transmission, nociception, and pain. [2, 64, 65] Oxytocin is synthesized and secreted by a subpopulation of the paraventricular and supraoptic nuclei of the hypothalamus. [64] In fact, several studies now support the idea that oxytocin exerts a potent antinociceptive control after its release in the spinal cord from hypothalamo-hypophysal descending projections. [17, 59, 73] Breton et al [12] have shown that this antinociceptive action is mediated, in part, by an increase in synaptic inhibition within the most superficial layers of the spinal cord. In addition, Robinson et al [54] showed that oxytocin inhibits sensory glutamatergic transmission between afferent fibers and dorsal horn neurons. Along the same lines, Petersson et al [50] hypothesized that an increase of oxytocin might possibly result in a greater synthesis of endogenous opioids, because the antinociception observed after repeated injections of oxytocin was temporarily reversed by the opioid antagonist naloxone.
In studies involving human subjects, pain relief was reported in central neurogenic pain and in low back pain [82] after the intracerebroventricular and intrathecal administration of oxytocin. No previous study has evaluated whether SM has an effect on oxytocin plasmatic concentration. Our results suggest that the increase of the plasmatic concentration of oxytocin following an SM could be partly responsible for the analgesic effect linked to manual therapy techniques due to the activation of descending pain-inhibitory pathways. Orexins are known to be a hypothalamic peptide critical for feeding and normal wakefulness. Orexin A and B are distributed throughout the spinal cord, and orexin fibers are concentrated in lamina I of the dorsal horn and in lamina X surrounding the central canal. [71] Orexinergic projections were identified in periaqueductal gray matter, the rostral ventral medulla, the dorsal horn, and the dorsal root ganglion. [21, 33, 67] Emerging evidence shows that the central nervous system administration (intracranial ventricle or intrathecal injection) of orexin A can suppress mechanical allodynia and thermal hypersensitivity in multiple pain models, suggesting the regulation of nociceptive processing via spinal and supraspinal mechanisms. [8, 70]
In addition, orexins showed antinociceptive effects on models of pain, such as neuropathic pain, carrageenan test, and postoperative pain. [47, 48] There is a lack of literature that analyzes the effect of physical therapy techniques on orexin A expression. A recent study using a rat model reported a significant increase of orexin A following electroacupuncture therapy after a laparotomy. [25] In contrast, our results did not show a statistically significant change in orexin A levels after a thoracic or cervical SM.
One of the actions of orexin A in stress situations is the activation of glucocorticoid production from adrenocortical cells. [11] Cortisol is therefore one of the biochemical factors delivered in stress situations [35] that acts to decrease local edema and pain by blocking early stages of inflammation. In addition, it is also believed that high cortisol levels promote wound healing by stimulating gluconeogenesis. [69] The response to stress is triggered by the stimulation of the hypothalamus-pituitary-adrenal axis. It has been proven that a subject’s level of stress can be correlated with secreted cortisol levels. [41] Our results suggest that no plasmatic concentration changes of cortisol follow a thoracic SM, which agrees with the results of a recent review using massage therapy. [49] Those authors [49] reported that no change in salivary cortisol followed massage therapy in symptomatic and asymptomatic subjects. A study by Whelan et al [69] examined the effect of SM on salivary cortisol levels and found no effect in asymptomatic subjects.
Nevertheless, we found a significant increase of cortisol plasmatic concentration following cervical manipulation, which does not agree with previous results. [15, 69] A possible explanation for this could be the use of venipuncture to obtain blood samples. Venipuncture is thought to be a stress factor that may increase circulating cortisol levels. [26] The use of blood testing for cortisol analysis should be questioned due to the possible increase in cortisol levels due to the invasive nature of the vein puncture required for blood sampling, and to the anticipatory stress experienced by the knowledge of the subject of the impending needle. [63] Even so, all 3 groups were exposed to the vein puncture, and changes were only observed in response to cervical SM. In our opinion, the use of a control group provides a certain degree of confidence that the results of cortisol plasmatic concentration are related to the technique.
Conclusion
Taken together, the results of this study show that cervical and thoracic manipulation resulted in an increase in neurotensin, oxytocin, and plasmatic cortisol concentration in asymptomatic individuals. These neuropeptides are related to the modulation of nociception and stress-induced analgesia. These findings suggest that descending inhibitory pathway mechanisms may be involved in the physiological effects that follow SM. In addition, the effect size for the cervical manipulation group was larger than that for the thoracic manipulation group. This suggests an increase in the activation of the possible descending inhibitory pathway mechanisms after cervical manipulation compared to thoracic manipulation. Further studies with larger sample sizes of both asymptomatic and symptomatic neck-pain populations are required to determine the effect of SM on antinociceptive neuropeptide levels more accurately.
KEY POINTS
FINDINGS: Mechanical stimuli derived from SM can modify neuropeptide expression in asymptomatic subjects.
IMPLICATIONS: The findings of this study suggest that some of the beneficial effects of SM may relate to neurochemical changes.
CAUTION: The small sample size of the population of asymptomatic subjects is the main limitation of this study. The biochemical response after SM in symptomatic subjects may not be extrapolated from the results of the present study.
References:
al’Absi M, Nakajima M, Grabowski J.
Stress response dysregulation and stress-induced analgesia in nicotine dependent men and women.
Biol Psychol. 2013;93:1-8. http://dx.doi.org/10.1016/j.biopsycho.2012.12.007Arletti R, Benelli A, Bertolini A.
Influence of oxytocin on nociception and morphine antinociception.
Neuropeptides. 1993;24:125-129.Azhdari Zarmehri H, Semnanian S, Fathollahi Y, et al.
Intra-periaqueductal gray matter microinjection of orexin-A decreases formalin-induced nociceptive
behaviors in adult male rats.
J Pain. 2011;12:280-287. http://dx.doi.org/10.1016/j.jpain.2010.09.006Bassett JR, Marshall PM, Spillane R.
The physiological measurement of acute stress (public speaking) in bank employees.
Int J Psychophysiol. 1987;5:265-273.Bello D, White-Traut R, Schwertz D, Pournajafi-Nazarloo H, Carter CS.
An exploratory study of neurohormonal responses of healthy men to massage.
J Altern Complement Med. 2008;14:387-394. http://dx.doi.org/10.1089/acm.2007.0660Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ.
The Mechanisms of Manual Therapy in the Treatment of Musculoskeletal Pain:
A Comprehensive Model
Man Ther. 2009 (Oct); 14 (5): 531–538Bialosky JE, Bishop MD, Robinson ME, Barabas JA, George SZ.
The Influence of Expectation on Spinal Manipulation Induced Hypoalgesia:
An Experimental Study in Normal Subjects
BMC Musculoskelet Disord. 2008 (Feb 11); 9: 19Bingham S, Davey PT, Babbs AJ, et al. Orexin-A,
an hypothalamic peptide with analgesic properties.
Pain. 2001;92:81-90.Boules M, Fredrickson P, Richelson E.
Bioactive analogs of neurotensin: focus on CNS effects.
Peptides. 2006;27:2523-2533. http://dx.doi.org/10.1016/j.peptides.2005.12.018Bradford MM.
A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding.
Anal Biochem. 1976;72:248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3Brennan PC, Kokjohn K, Kaltinger CJ, et al.
Enhanced Phagocytic Cell Respiratory Burst Induced by Spinal Manipulation:
Potential Role of Substance P
J Manipulative Physiol Ther 1991 (Sep); 14 (7): 399–408Breton JD, Poisbeau P, Darbon P.
Antinociceptive action of oxytocin involves inhibition of potassium channel currents in lamina II neurons
of the rat spinal cord.
Mol Pain. 2009;5:63. http://dx.doi.org/10.1186/1744-8069-5-63Buhler AV, Choi J, Proudfit HK, Gebhart GF.
Neurotensin activation of the NTR1 on spinally-projecting serotonergic neurons in the rostral
ventromedial medulla is antinociceptive.
Pain. 2005;114:285-294. http://dx.doi.org/10.1016/j.pain.2004.12.031Cheng JK, Chou RC, Hwang LL, Chiou LC.
Antiallodynic effects of intrathecal orexins in a rat model of postoperative pain.
J Pharmacol Exp Ther. 2003;307:1065-1071. http://dx.doi.org/10.1124/jpet.103.056663Christian GF, Stanton GJ, Sissons D, et al.
Immunoreactive ACTH, beta-endorphin, and cortisol levels in plasma following spinal manipulative therapy.
Spine (Phila Pa 1976). 1988;13:1411-1417.Clineschmidt BV, McGuffin JC.
Neurotensin administered intracisternally inhibits responsiveness of mice to noxious stimuli.
Eur J Pharmacol. 1977;46:395-396.Condés-Lara M, Rojas-Piloni G, Martínez-Lorenzana G, Rodríguez-Jiménez J.
Paraventricular hypothalamic influences on spinal nociceptive processing.
Brain Res. 2006;1081:126-137. http://dx.doi.org/10.1016/j.brainres.2006.01.050Cooper PE, Fernstrom MH, Rorstad OP, Leeman SE, Martin JB.
The regional distribution of somatostatin, substance P and neurotensin in human brain.
Brain Res. 1981;218:219-232. http://dx.doi.org/10.1016/0006-8993(81)91302-0Corley KC, Phan TH, Daugherty WP, Boadle-Biber MC.
Stress-induced activation of median raphe serotonergic neurons in rats is potentiated by the
neurotensin antagonist, SR 48692.
Neurosci Lett. 2002;319:1-4.Crosby ND, Weisshaar CL, Winkelstein BA.
Spinal neuronal plasticity is evident within 1 day after a painful cervical facet joint injury.
Neurosci Lett. 2013;542:102-106. http://dx.doi.org/10.1016/j.neulet.2013.03.019Date Y, Mondal MS, Matsukura S, Nakazato M.
Distribution of orexin-A and orexin-B (hypocretins) in the rat spinal cord.
Neurosci Lett. 2000;288:87-90.Dishman JD, Ball KA, Burke J.
First prize: central motor excitability changes after spinal manipulation:
a transcranial magnetic stimulation study.
J Manipulative Physiol Ther. 2002;25:1-9.Dobner PR.
Neurotensin and pain modulation.
Peptides. 2006;27:2405-2414. http://dx.doi.org/10.1016/j.peptides.2006.04.025Erami E, Azhdari-Zarmehri H, Ghasemi-Dashkhasan E, Esmaeili MH, Semnanian S.
Intra-paragigantocellularis lateralis injection of orexin-A has an antinociceptive effect on
hot plate and formalin tests in rat.
Brain Res. 2012;1478:16-23. http://dx.doi.org/10.1016/j.brainres.2012.08.013Feng XM, Mi WL, Xia F, et al.
Involvement of spinal orexin A in the electroacupuncture analgesia in a rat model of post-laparotomy pain.
BMC Complement Altern Med. 2012;12:225. http://dx.doi.org/10.1186/1472-6882-12-225Ferriani RA, Silva de Sá MF.
Effect of venipuncture stress on plasma prolactin levels.
Int J Gynaecol Obstet. 1985;23:459-462.Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C.
Cortisol decreases and serotonin and dopamine increase following massage therapy.
Int J Neurosci. 2005;115:1397-1413. http://dx.doi.org/10.1080/00207450590956459Fryer G, Pearce AJ.
The effect of lumbosacral manipulation on corticospinal and spinal reflex excitability on asymptomatic participants.
J Manipulative Physiol Ther. 2012;35:86-93. http://dx.doi.org/10.1016/j.jmpt.2011.09.010Gao L, Yu LC.
Involvement of opioid receptors in the oxytocin-induced antinociception in the central nervous system of rats.
Regul Pept. 2004;120:53-58. http://dx.doi.org/10.1016/j.regpep.2004.02.011George SZ, Bishop MD, Bialosky JE, Zeppieri G, Jr., Robinson ME.
Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study.
BMC Musculoskelet Disord. 2006;7:68. http://dx.doi.org/10.1186/1471-2474-7-68Gibbons P, Tehan P.
Manipulation of the Spine, Thorax and Pelvis: An Osteopathic Perspective.
Edinburgh, UK: Churchill Livingstone; 2000.Gross A, Miller J, D’Sylva J, Burnie SJ, Goldsmith CH, Graham N, et al.
Manipulation or Mobilisation For Neck Pain: A Cochrane Review
Manual Therapy 2010 (Aug); 15 (4): 315–333Guan JL, Wang QP, Shioda S.
Immunoelectron microscopic examination of orexin-like immunoreactive fibers in the dorsal horn
of the rat spinal cord.
Brain Res. 2003;987:86-92.Gui X, Carraway RE, Dobner PR.
Endogenous neurotensin facilitates visceral nociception and is required for stress-induced
antinociception in mice and rats.
Neuroscience. 2004;126:1023-1032. http://dx.doi.org/10.1016/j.neuroscience.2004.04.034Hellhammer DH, Wüst S, Kudielka BM.
Salivary cortisol as a biomarker in stress research.
Psychoneuroendocrinology. 2009;34:163-171. http://dx.doi.org/10.1016/j.psyneuen.2008.10.026Himberger JR, Himberger LC.
Accuracy of drawing blood through infusing intravenous lines.
Heart Lung. 2001;30:66-73. http://dx.doi.org/10.1067/mhl.2001.110535Huisman PA, Speksnijder CM, de Wijer A.
The effect of thoracic spine manipulation on pain and disability in patients with non-specific neck pain:
a systematic review.
Disabil Rehabil. 2013;35:1677-1685. http://dx.doi.org/10.3109/09638288.2012.750689Hurwitz EL.
Epidemiology: Spinal Manipulation Utilization
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 648–654Johnstone LE, Leng G, Brown CH.
Effects of neurotensin on the organization of activity in supraoptic nucleus cells
in virgin and lactating rats.
J Neuroendocrinol. 2004;16:605-611. http://dx.doi.org/10.1111/j.1365-2826.2004.01208.xKessler JP, Moyse E, Kitabgi P, Vincent JP, Beaudet A.
Distribution of neurotensin binding sites in the caudal brainstem of the rat:
a light microscopic radioautographic study.
Neuroscience. 1987;23:189-198. http://dx.doi.org/10.1016/0306-4522(87)90282-XKuczynski JJ, Schwieterman B, Columber K, Knupp D, Shaub L, Cook CE.
Effectiveness of physical therapist administered spinal manipulation for the treatment of
low back pain: a systematic review of the literature.
Int J Sports Phys Ther. 2012;7:647-662.Lafrance M, Roussy G, Belleville K, et al.
Involvement of NTS2 receptors in stress-induced analgesia.
Neuroscience. 2010;166:639-652. http://dx.doi.org/10.1016/j.neuroscience.2009.12.042Martínez-Segura R, de-la-Llave-Rincón AI, Ortega-Santiago R, Cleland JA.
Immediate changes in widespread pressure pain sensitivity, neck pain, and cervical range of motion
after cervical or thoracic thrust manipulation in patients with bilateral chronic mechanical neck pain:
a randomized clinical trial.
J Orthop Sports Phys Ther. 2012;42:806-814. http://dx.doi.org/10.2519/jospt.2012.4151Matousek RH, Dobkin PL, Pruessner J.
Cortisol as a marker for improvement in mindfulness-based stress reduction.
Complement Ther Clin Pract. 2010;16:13-19. http://dx.doi.org/10.1016/j.ctcp.2009.06.004Melzack R.
Pain and the neuromatrix in the brain.
J Dent Educ. 2001;65:1378-1382.Melzack R, Coderre TJ, Katz J, Vaccarino AL.
Central neuroplasticity and pathological pain.
Ann N Y Acad Sci. 2001;933:157-174.Mobarakeh JI, Takahashi K, Sakurada S, et al.
Enhanced antinociception by intracerebroventricularly administered orexin A in histamine H1 or
H2 receptor gene knockout mice.
Pain. 2005;118:254-262. http://dx.doi.org/10.1016/j.pain.2005.08.024Mobarakeh JI, Takahashi K, Sakurada S, et al.
Enhanced antinociception by intracerebroventricularly and intrathecally-administered orexin A and
B (hypocretin-1 and -2) in mice.
Peptides. 2005;26:767-777. http://dx.doi.org/10.1016/j.peptides.2005.01.001Moyer CA, Seefeldt L, Mann ES, Jackley LM.
Does massage therapy reduce cortisol? A comprehensive quantitative review.
J Bodyw Mov Ther. 2011;15:3-14. http://dx.doi.org/10.1016/j.jbmt.2010.06.001Petersson M, Alster P, Lundeberg T, Uvnäs-Moberg K.
Oxytocin increases nociceptive thresholds in a long-term perspective in female and male rats.
Neurosci Lett. 1996;212:87-90. http://dx.doi.org/10.1016/0304-3940(96)12773-7Pickar JG.
Neurophysiological Effects of Spinal Manipulation
Spine J (N American Spine Society) 2002 (Sep); 2 (5): 357–371Puentedura EJ, Landers MR, Cleland JA, Mintken PE, Huijbregts P, Fernández-de-las-Peñas C.
Thoracic spine thrust manipulation versus cervical spine thrust manipulation in patients with acute neck pain:
a randomized clinical trial.
J Orthop Sports Phys Ther. 2011;41:208-220. http://dx.doi.org/10.2519/jospt.2011.3640Quinn KP, Dong L, Golder FJ, Winkelstein BA.
Neuronal hyperexcitability in the dorsal horn after painful facet joint injury.
Pain. 2010;151:414-421. http://dx.doi.org/10.1016/j.pain.2010.07.034Robinson DA, Wei F, Wang GD, et al.
Oxytocin mediates stress-induced analgesia in adult mice.
J Physiol. 2002;540:593-606.Rossi GC, Matulonis JE, Richelson E, Barbut D, Pasternak GW.
Systemically and topically active antinociceptive neurotensin compounds.
J Pharmacol Exp Ther. 2010;334:1075-1079. http://dx.doi.org/10.1124/jpet.109.165282Rubinstein SM, van Middelkoop M, Assendelft WJ, de Boer MR, van Tulder MW.
Spinal manipulative therapy for chronic low-back pain: an update of a Cochrane review.
Spine (Phila Pa 1976). 2011;36:E825-E846. http://dx.doi.org/10.1097/BRS.0b013e3182197fe1Sarret P, Perron A, Stroh T, Beaudet A.
Immunohistochemical distribution of NTS2 neurotensin receptors in the rat central nervous system.
J Comp Neurol. 2003;461:520-538. http://dx.doi.org/10.1002/cne.10718Seta KA, Jansen HT, Kreitel KD, Lehman M, Behbehani MM.
Cold water swim stress increases the expression of neurotensin mRNA in the lateral hypothalamus
and medial preoptic regions of the rat brain.
Brain Res Mol Brain Res. 2001;86:145-152.Shiraishi T, Onoe M, Kojima T, Sameshima Y, Kageyama T.
Effects of hypothalamic paraventricular nucleus: electrical stimulation produce marked analgesia in rats.
Neurobiology (Bp). 1995;3:393-403.Skyba DA, Radhakrishnan R, Rohlwing JJ, Wright A, Sluka KA.
Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not
opioid or GABA receptors in the spinal cord.
Pain. 2003;106:159-168.Sterling M, Jull G, Wright A.
Cervical mobilisation: concurrent effects on pain, sympathetic nervous system activity and motor activity.
Man Ther. 2001;6:72-81. http://dx.doi.org/10.1054/math.2000.0378St-Gelais F, Jomphe C, Trudeau LE.
The role of neurotensin in central nervous system pathophysiology: what is the evidence?
J Psychiatry Neurosci. 2006;31:229-245.Tuchin PJ.
The effect of chiropractic spinal manipulative therapy on salivary cortisol levels.
Australas Chiropr Osteopathy. 1998;7:86-92.Uvnäs-Moberg K, Bruzelius G, Alster P, Bileviciute I, Lundeberg T.
Oxytocin increases and a specific oxytocin antagonist decreases pain threshold in male rats.
Acta Physiol Scand. 1992;144:487-488. http://dx.doi.org/10.1111/j.1748-1716.1992.tb09327.xUvnäs-Moberg K, Petersson M.
[Oxytocin, a mediator of anti-stress, well-being, social interaction, growth and healing].
Z Psychosom Med Psychother. 2005;51:57-80.Vachon-Presseau E, Roy M, Martel MO, et al.
The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans.
Brain. 2013;136:815-827. http://dx.doi.org/10.1093/brain/aws371van den Pol AN.
Hypothalamic hypocretin (orexin): robust innervation of the spinal cord.
J Neurosci. 1999;19:3171-3182.Vicenzino B, Collins D, Benson H, Wright A.
An investigation of the interrelationship between manipulative therapy-induced hypoalgesia and sympathoexcitation.
J Manipulative Physiol Ther. 1998;21:448-453.Whelan TL, Dishman JD, Burke J, Levine S, Sciotti V.
The effect of chiropractic manipulation on salivary cortisol levels.
J Manipulative Physiol Ther. 2002;25:149-153.Yamamoto T, Nozaki-Taguchi N, Chiba T.
Analgesic effect of intrathecally administered orexin-A in the rat formalin test and in the rat hot plate test.
Br J Pharmacol. 2002;137:170-176. http://dx.doi.org/10.1038/sj.bjp.0704851Yan JA, Ge L, Huang W, Song B, Chen XW, Yu ZP.
Orexin affects dorsal root ganglion neurons: a mechanism for regulating the spinal nociceptive processing.
Physiol Res. 2008;57:797-800.Yang J.
Intrathecal administration of oxytocin induces analgesia in low back pain involving
the endogenous opiate peptide system.
Spine (Phila Pa 1976). 1994;19:867-871.Yirmiya R, Ben-Eliyahu S, Shavit Y, Marek P, Liebeskind JC.
Stimulation of the hypothalamic paraventricular nucleus produces analgesia not mediated by
vasopressin or endogenous opioids.
Brain Res. 1990;537:169-174.

Return to SPINAL PAIN MANAGEMENT
Since 5-29-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |