

Neural Response During a Mechanically Assisted
Spinal Manipulation in an Animal Model:
A Pilot StudyThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Nov Physiother Phys Rehabil. 2015 (Sep); 2 (2): 2027 ~ FULL TEXT
OPEN ACCESS William R. Reed, DC, PhD, Michael A.K. Liebschner, PhD,
Randall S. Sozio, BS, LATG, Joel G. Pickar, DC, PhD,
Maruti R. Gudavalli, PhD
Palmer Center for Chiropractic Research,
Davenport, IA, USA.INTRODUCTION: Mechanoreceptor stimulation is theorized to contribute to the therapeutic efficacy of spinal manipulation. Use of mechanically-assisted spinal manipulation (MA-SM) devices is increasing among manual therapy clinicians worldwide. The purpose of this pilot study is to determine the feasibility of recording in vivo muscle spindle responses during a MA-SM in an intervertebral fixated animal model.
METHODS: Intervertebral fixation was created by inserting facet screws through the left L5-6 and L6-7 facet joints of a cat spine. Three L6muscle spindle afferents with receptive fields in back muscles were isolated. Recordings were made during MA-SM thrusts delivered to the L7 spinous process using an instrumented Activator IV clinical device.
RESULTS: Nine MA-SM thrusts were delivered with peak forces ranging from 68-122N and with thrust durations of less than 5ms. High frequency muscle spindle discharge occurred during MA-SM. Following the MA-SM, muscle spindle responses included returning to pre-manipulation levels, slightly decreasing for a short window of time, and greatly decreasing for more than 40s.
CONCLUSION: This study demonstrates that recording in vivo muscle spindle response using clinical MA-SM devices in an animal model is feasible. Extremely short duration MA-SM thrusts (<5ms) can have an immediate and/or a prolonged (> 40s) effect on muscle spindle discharge. Greater peak forces during MA-SM thrusts may not necessarily yield greater muscle spindle responses. Determining peripheral response during and following spinal manipulation may be an important step in optimizing its' clinical efficacy. Future studies may investigate the effect of thrust dosage and magnitude.
KEYWORDS: Cat; Manual therapy; Muscle spindle; Neurons afferent; Neurophysiology; Spinal fixation; Spinal manipulation; Zygapophyseal joint
From the Full-Text Article:
Introduction
Spinal manipulation is a form of manual therapy commonly used by clinicians and therapists for conservative treatment of musculoskeletal complaints. Spinal manipulation is typically distinguished from spinal mobilization by the presence of a short duration mechanical thrust applied to the spinal column using either direct hand contact (≤150ms) or one of several commercially available mechanical devices (≤10ms) [14]. Among chiropractic clinicians, use of mechanically-assisted spinal manipulation (MA-SM) is growing rapidly with reports that 4060% of practitioners in the United States, Britain, Belgium, Canada, Australia, and New Zealand use MA-SM in some capacity of patient care [510].
Spinal manipulation has been shown to be effective in the treatment of neck and low back pain and is recommended by clinical guidelines and evidence reports [1116]. Several reviews regarding the clinical efficacy, safety, usage, and mechanical effects of MA-SM have recently been published [1720]. A majority of the MA-SM reviews have noted study weaknesses such as small sample size, non-randomization, and/or lack of a placebo or control group. Despite these limitations, great strides have recently been made in determining the mechanical characteristics and/or biological effects of MA-SM [14, 2131]. These studies may provide a foundation for larger randomly controlled trials of MA-SM therapy. One distinct advantage MA-SM offers over manually delivered manipulative thrusts in a research setting is that the thrust velocity and thrust magnitude can be standardized. This feature is of particular importance in efficacy and mechanistic studies investigating the biomechanical and/or neurophysiological effects of spinal manipulation. In addition, MA-SM devices can be mechanically altered to provide an adequate sham spinal manipulation (no force delivered) which is more difficult to accomplish with manually delivered manipulative thrusts.
Spinal manipulation by its very nature is a mechanical stimulus typically applied at clinically identified sites of intervertebral joint fixation or joint hypomobility. Theorized mechanisms for its therapeutic effects include breaking of joint adhesions and/or alteration of sensory input from primary afferents of paraspinal tissues which subsequently act to influence spinal cord reflexes and/or other central neural mechanisms [32, 33]. MA-SM has been shown to result in oscillatory intervertebral movements [4, 24, 29, 34, 35] and neurophysiological responses in the form of bilateral compound action potentials in both in vivo animal [24, 36] and human [21, 23, 29] studies. The compound action potentials from spinal nerve roots have been attributed to the simultaneous activation of mechano-sensitive afferents innervating viscoelastic spinal tissues such as muscles, ligaments, facet joints, and discs, but the exact sources of neural activity were not identified [23, 29, 37]. Muscle spindles are likely among the mechanoreceptors stimulated by MA-SM. They provide the central nervous system with sensory information regarding both changes in muscle length and the velocity at which those length changes occur.
Using a feedback motor control system, we have previously shown that manipulative thrust durations between 25 and 150ms elicit high frequency discharge from paraspinal muscle spindles [3840]. However to our knowledge, recordings of muscle spindle response associated with short manipulative thrust durations (≤10ms) as generated with clinical MA-SM devices, have never been recorded. It is unclear whether the noise artifact or high frequency mechanical perturbation associated with use of short thrust duration MA-SM devices would prohibit, obscure, or otherwise interfere with dorsal root recordings in a cat preparation. Therefore, the primary goal of this pilot study was to determine the feasibility of recording primary afferent muscle spindle responses in dorsal rootlets using a commercially available MA-SM device in an in vivo feline model of intervertebral joint fixation.
Materials and Methods
The experimental preparation and procedures used in this study have been described in greater detail elsewhere [3942] and are therefore presented here only briefly. Electrophysiological recordings were made from 3 back muscle spindle afferents traveling in the dorsal roots of a single Nembutal-anesthetized (35 mg/kg, iv; Oak Pharmaceuticals, Lake Forest, IL) adult male cat (4.5 kg). All experimental procedures were approved by the Institutional Animal Care and Use Committee (#20120601). This pilot data using a MA-SM device was collected from an experimental preparation associated with a separate study investigating the relationship between intervertebral fixation and L6 spinal manipulation delivered by a computer controlled feedback motor.

Figure 1 Catheters were placed in the common carotid artery and external jugular vein to monitor blood pressure, introduce fluids and/or supplemental anesthesia if the arterial pressure rose above 120mm Hg or if a withdrawal reflex became present. The trachea was intubated and the cat was artificially ventilated. Since our focus was on back afferents, the right sciatic nerve was cut to reduce afferent input from the hindlimb. An L5 laminectomy was performed exposing the right L6 dorsal rootlets which were cut close to their entrance to the spinal cord and placed on a platform. Thin filaments were teased with fine forceps until action potentials from a single neuron were identified that responded to both mechanical pressure applied directly to the paraspinal back muscles (multifidus or longissimus) and a fast vibratory stimulus (~70 Hz; mini-therapeutic massage vibrator; North Coast Medical, Morgan Hill CA, USA). Afferent fibers remained positioned on the recording electrode while facet screws (10mm titanium endosteally anchored miniscrews; Dentaurum, Ispringen, Germany) were inserted through the left articular pillars of L56 and L67 vertebra in similar fashion to that previously described [40]. An x-ray of the L56 and L67 facet fixation is shown in Figure 1. Neural activity was passed through a high-impedance probe (HIP511, Grass, West Warwick, RI), amplified (P511 K, Grass) and recorded using a CED 1401 interface and Spike 2 data acquisition software (Cambridge Electronic Design, Cambridge, England).
MA-SM Device
The Activator IV (Activator IV, Activator Methods Int. Ltd., Phoenix, AZ) is a hand-held clinical device comprised of a rubber-tipped spring-loaded hammer with 4 device settings that produce relative increases in thrust magnitude. Its thrust duration is<10ms and can deliver a maximum force of 212N when tested directly on a load cell [1]. For the current study, the device was modified by attaching an impedance head under the rubber tip (Figure 1). The impedance head included a dynamic load cell (Model 208C04; PCB, NY) and a tri-axial accelerometer (Model 356A01, PCB, NY).
Once a single back afferent had been isolated, the Activator IV device was placed by hand directly onto the exposed fascia overlying the cats L7 spinous process (one segment caudal to the level of afferent recording) and a small preload was applied. The L7vertebra was chosen to receive the MA-SM thrust due to the increased risk of tearing the L6 afferent fiber off the recording electrode during an L6 manipulation. The Activator IV device requires that a preload force be applied in order to completely retract the instrument tip prior to triggering the manipulative thrust. We used the two lowest device settings (1 and 2) which can deliver a force of 123N when tested directly on a load cell [1] but substantially less force (79N) when tested on polymer spinal tissue analog blocks [3]. MA-SM thrusts were applied in a dorsal-ventral direction and separated by a minimum period of 5 minutes. Electronic signals obtained from the force transducer and accelerometer were each sampled at 12,800 Hz and recorded in a binary file format on a computer using Lab View (National Instruments, Austin, TX).
Results

Table 1 Three muscle spindle afferents with receptive fields located in the longissimus back muscle were recorded during 9 L7 MA-SMs in a single cat preparation with intervertebral joint fixation. All afferents responded to mechanical movement of the spine and had sustained responses to fast vibratory stimuli (~70 Hz). All 3 afferents received MA-SM thrusts at a device setting of 1, whereas 2 afferents also received MA-SM thrusts at a device setting of 2. Individual MA-SM thrust profiles are reported in Table 1. All thrust durations were <5ms in duration and applied MA-SM peak forces ranged from 78.2 to 121.8N.
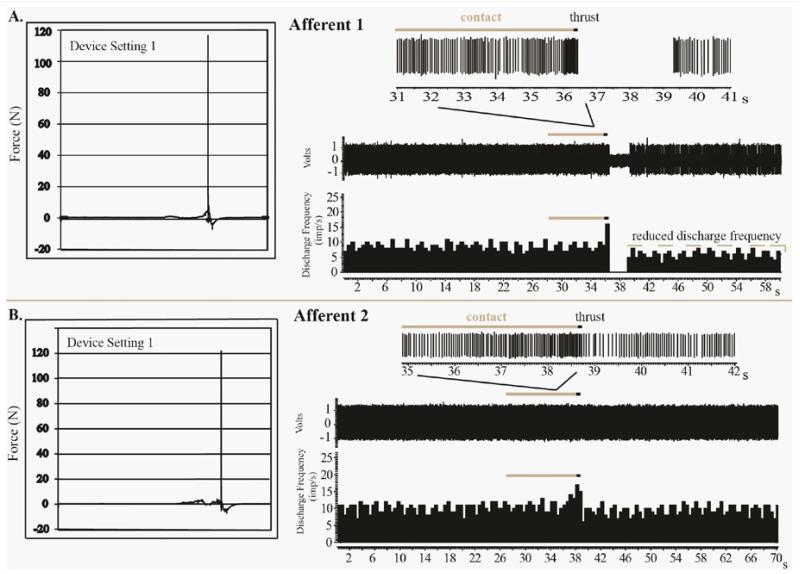
Figure 2 Examples of spindle responses to MA-SM thrusts from Afferent 1 and 2 are shown in Figure 2. For afferent 1 at a device setting of 1, the combined preload and MA-SM peak thrust force was 116.5N and the thrust duration was 2.0ms. The MA-SM thrust resulted in a high frequency spindle discharge during preload and thrust. Immediately following the thrust there was a 2.89s cessation of spindle discharge followed by the resumption of resting discharge but at a mean frequency slightly less than that prior to the thrust and lasting for the remaining 20s of recording (Figure 2A). For afferent 2 at a device setting of 1, the combined preload and peak MA-SM thrust force was 121.8N and the thrust duration was 2.0ms (Figure 2B, Table 1). Unlike Afferent 1, Afferent 2 exhibited no cessation of discharge following the MA-SM thrust and rapidly resumed resting discharge.
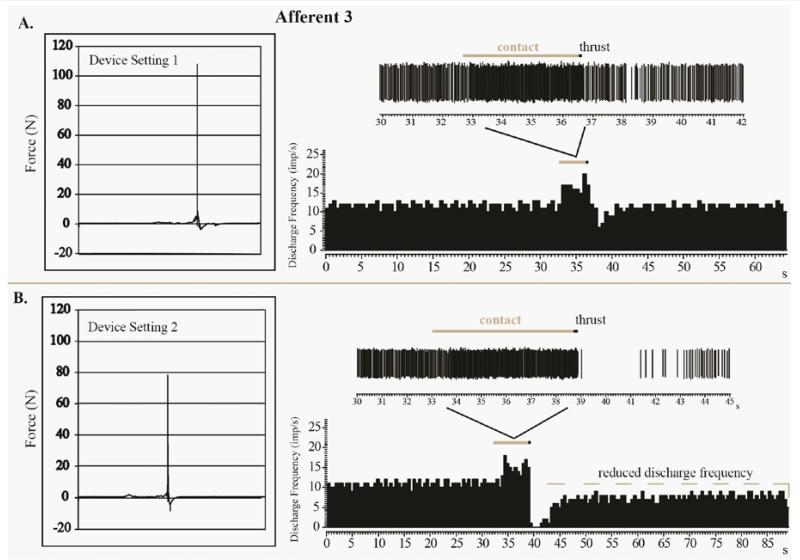
Figure 3 The four MA-SM thrusts using device setting 1 delivered to Afferent 3 had a mean peak force of 109N and mean thrust duration of 3.0ms (Table 1). Similar to Afferents 1 and 2, there was an increase of spindle discharge as a result of preload and MA-SM thrust at the L7 spinous process (Figure 3A). Following the thrust there was a decrease (but not a cessation) in spindle discharge lasting approximately 2.47s before there sumption of pre-thrust resting discharge frequency. For Afferent 3, mean peak force for the two MA-SM thrusts at device setting 2 was 81 N and mean thrust duration was 3.0ms. Afferent 3s response to one of thrusts at device setting 2 is shown in Figure 3B. There was an increase in spindle discharge with preload and thrust similar to that when the device was set at 1. However, unlike with setting 1 post-thrust activity was further reduced and more prolonged (~4.13s) at device setting 2. Despite the lower peak force (78.2N) delivered on device setting 2 compared to device setting 1 (107.9 N), there is a prolonged period (>40s) during which resting discharge did not return to pre-thrust levels (Figure 3A, 3B). It should be noted that mean Afferent 3 resting discharge frequency prior to the MA-SM thrust delivered at device setting 1 or 2 were similar (Figure 3A, 3B). Although the precise time is not known, Afferent 3 returned to its resting discharge frequency at some point within 5 min after the setting 2 thrust delivery depicted in Figure 3B. Afferent 3 also exhibited increased afferent discharge to a fast vibratory stimulus (70 Hz) after the thrust suggesting that no fiber damaged had occurred as a result of this MA-SM.
Discussion
To our knowledge, this study is the first to record muscle spindle response evoked by a mechanically-assisted spinal manipulation device that is used in clinical practice. Because spinal manipulation is typically delivered at sites of clinically determined biomechanical joint dysfunction and/or pain provocation, the relationship between intervertebral joint fixation/hypomobility and sensory signaling elicited from paraspinal mechanoreceptors during spinal manipulation is of particular interest to manual therapy researchers and clinicians alike. The purpose of the facet fixation model was to produce a moderate degree of segmental dysfunction that might be similar to that encountered by manual therapy clinicians in practice. It will likely be through a combination of both basic and clinical research that the underlying physiological mechanisms of manual therapy will be elucidated and its clinical efficacy optimized.
Although this pilot study contained a limited number of afferents, it demonstrated some important findings and will help to inform future studies. First with regards to the preparation, we demonstrated the feasibility of recording muscle spindle responses in an in vivo animal model using a clinical MA-SM device. The afferent fiber was wrapped around the recording electrode and withstood the perturbation associated with the mechanical delivery of 78 to 122 N forces over extremely short durations (< 5ms). Evidence for a lack of damage to the afferent fiber is in part provided through the return of pre-thrust resting spindle discharge following MA-SM. The risk of potential afferent fiber damage during MA-SM delivery in this preparation is real, but can be minimized by using dorsal rootlets that are longer in length. Although noise artifacts were encountered during the experiments, this appeared due in large part to movement of the device while the operator delivered the thrust. This issue can be remedied by non-manually triggering the MA-SM device attached to a rigid frame or perhaps using newer electrically powered (non-spring-loaded) MA-SM devices [3].
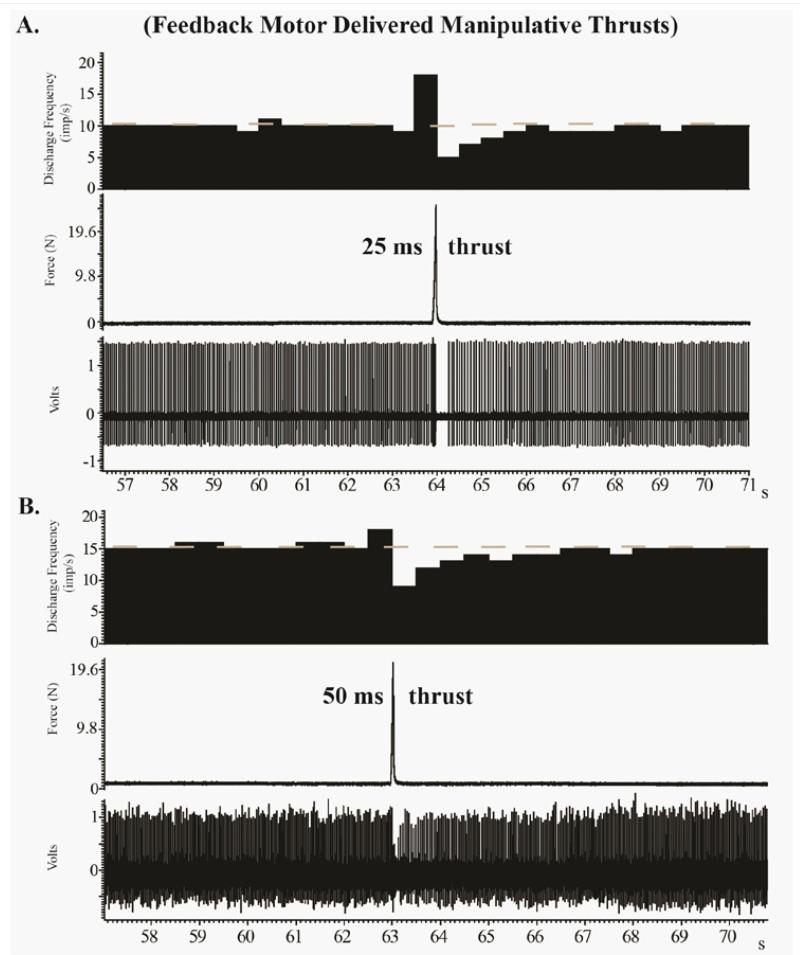
Figure 4 We found that the extremely short MA-SM thrust durations elicited high frequency discharge from paraspinal muscle spindle afferents. This response appears similar that which occurs during 25150ms thrust durations delivered by a computer-controlled feedback motor [3840] (Figure 4), but direct comparisons are difficult due to the presence of preload forces and a lack of controlled preload durations in the current study. This pilot study clearly demonstrated that muscle spindle afferents can respond differently to similar MA-SM thrust forces (Figures 13). Afferents 13 exhibited post-thrust responses ranging from limited diminution of discharge (Afferent 2), to a mild decrease (Afferent 3) or complete cessation of discharge for nearly 3s (Afferent 1). It is not known, whether these differences in post-MA-SM thrust response are due to inherent differences related to muscle spindle intrafusal fiber types (e.g. bag vs chain fibers; for greater discussion in this regard see [43, 44]), the anatomical proximity of the afferents receptive field to the L7 spinous process thrust site, and/or other biological factors. In a previous study investigating the effects of L6 and L7 anatomical thrust delivery sites on L6 muscle spindle discharge, we found that segmental contact sites distant to the muscle spindles receptive field were just as effective at increasing spindle discharge as contact sites close to the receptive field [45].
We found it interesting that the lower force delivered at setting 2 (78.2N) versus the higher peak force delivered at setting 1 force (107.9N) had a greater impact on Afferent 3s discharge post-thrust (Figure 3). It is reasonable to think that greater forces delivered into the spine over the same duration would create greater vertebral displacement and thereby evoke a greater response from paraspinal muscle spindle afferents. However, several variables and conditions in the current experiment may affect this rationale including the use of extremely short thrust durations (<5 ms), a thrust site 1 segment caudal to afferent recording level, the presence of intervertebral fixation, and/or the greater inherent flexibility of the cat spine. Colloca and colleagues in a sheep model found that as the applied force increased vertebral displacements also increased [24, 31]. However, they also found that a constant thrust force of 80N at L3 produced larger adjacent vertebral motions at shorter thrust durations (10ms) compared to longer thrust durations (100 and 200ms) [24, 31]. It is thought that the mechanical principles of resonant frequency may apply to the human spine. If so, lower manipulative forces applied at resonance frequencies of the spine may accomplish similar vertebral motions as greater forces applied at nonresonant frequencies [17]. However, since settings 1 and 2 thrust durations are nearly equivalent, this particular explanation of differences in muscle spindle response is unlikely.
Limitations
Preload forces and preload durations were not standardized in the current study as the Activator IV device was operated by hand as is performed clinically. Applied preload forces are required to retract the tip of Activator IV device, but we consciously attempted to limit the magnitude of applied preload forces since the preload duration was not standardized. We used thrust force magnitudes in our animal model that were the same or similar to those used in human studies in the human cervical spine. In humans, mean peak forces during manually applied cervical manipulation has been reported to be 118N [46]. Although direct circumference measurements were not performed in this study, the actual trunk size of adult male cats appears to be similar to the anatomical size of the human neck. While, we acknowledge that the thrust forces used in the current study were up to 2.7Χ the cats body weight we must also be mindful that the whole lumbar spine stiffness of the cat spine has been shown to be 27Χ less than that of human spines. Species differences in spinal stiffness have been clearly demonstrated in that unlike human cadaveric specimens, structural failure did not occur in the cadaveric cat spines with flexion/extension biomechanical testing [47]. Figure 4 demonstrates that much smaller forces (24.5 N and 19.6 N) have similar effects on paraspinal muscle spindle response suggesting a plateau effect of thrust magnitude. In addition, previous studies have indicated that Activator devices produce a maximum of 0.3 J of kinetic energy which is far below the energies required to produce tissue injury [36, 48]. As is the case clinically, the Activator IV device is commonly used on much smaller human body parts than the human neck such as the wrists, elbows or ankles [49].
Conclusion
This pilot study demonstrates feasibility of recording in vivo muscle spindle response during spinal manipulation using clinical mechanically-assisted spinal manipulation devices. It also demonstrates that extremely short duration manipulative thrusts (<5ms) of equivalent forces to that delivered to the human cervical spine can have an immediate and/or perhaps a prolonged effect (> 40s) on paraspinal muscle spindle discharge. While the clinical relevance of how mechanoreceptor stimulation or inhibition related to spinal manipulation modulates central nervous system activity remains to be clarified, determining how various mechanoreceptors respond during and following spinal manipulative thrusts in a clinically relevant fashion is an important step toward achieving this goal.
Acknowledgments
This work was supported by the NIH National Center for Complementary and Alternative Medicine (K01AT005935) to WRR and was conducted in a facility with support from the NIH National Center for Research Resources under Research Facilities Improvement Grant Number C06RR15433. The authors would like to thank Activator Methods International for providing the Activator IV instrumented device, Dr. Robert Vining for radiology assistance, and Dr. Robert Cooperstein for his helpful editorial comments.
References:
Colloca CJ, Keller TS, Black P, Normand MC, Harrison DE, et al.
Comparison of Mechanical Force of Manually Assisted
Chiropractic Adjusting Instruments
J Manipulative Physiol Ther 2005 (Jul); 28 (6): 414422Kawchuk GN, Herzog W.
Biomechanical characterization (fingerprinting) of five novel methods of cervical spine manipulation.
J Manipulative Physiol Ther. 1993;16:573577Liebschner MA, Chun K, Kim N, et al.
In Vitro Biomechanical Evaluation of Single Impulse and
Repetitive Mechanical Shockwave Devices Utilized for
Spinal Manipulative Therapy
Annals of Biomedical Engineering 2014 (Dec); 42 (12): 25242536Keller ST, Colloca CJ, Moore RJ, Gunzburg R, Harrison DE, Harrison DD.
Three-dimensional Vertebral Motions Produced by Mechanical Force Spinal Manipulation
Journal of Manipulative and Physiological Therapeutics 2006 (Jul); 29 (6): 425436National Board of Chiropractic Examiners .
Job Analysis of Chiropractic 2005
National Board of Chiropractic Examiners;
Greeley, Colorado, USA: 2005.Read DT, Wilson FJ, Gemmell HA.
Activator as a therapeutic instrument: Survey of usage and opinions amongst members of the British Chiropractic Association.
Clin Chiropr. 2006;9:7075.National Board of Chiropractic Examiners .
Job Analysis of Chiropractic: a project report, survey analysis and summary of the practice of chiropractic within the United States.
National Board of Chirorpactic Examiners;
Greeley, Colorado, USA: 1994.Ailliet L, Rubinstein SM, de Vet HC.
Characteristics of chiropractors and their patients in Belgium.
J Manipulative Physiol Ther. 2010;33:618625National Board of Chiropractic Examiners .
Job analysis of Chiropractic:
a project report, survey analysis and summary of the practice of chiropractic in Canada.
National Board of Chiropractic Examiners;
Greeley, Colorado, USA: 1993.Gleberzon BJ.
Chiropractic name techniques in Canada: A continued look at demographic trends and their impact on issues of jurisprudence.
J Can Chiropr Assoc. 2002;46:241256.Bronfort, G, Haas, M, Evans, R, Kawchuk, G, and Dagenais, S.
Evidence-informed Management of Chronic Low Back Pain with Spinal Manipulation and Mobilization
Spine J. 2008 (Jan); 8 (1): 213225Bronfort G, Haas M, Evans R, Leiniger B, Triano J.
Effectiveness of Manual Therapies: The UK Evidence Report
Chiropractic & Osteopathy 2010 (Feb 25); 18 (1): 3Childs JD, Cleland JA, Elliott JM, Teyhen DS, Wainner RS, et al.
Neck pain: Clinical practice guidelines linked to the International Classification of Functioning, Disability, and Health from the Orthopedic Section of the American Physical Therapy Association.
J Orthop Sports Phys Ther. 2008;38:A1A34Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr., Shekelle P, Owens DK:
Diagnosis and Treatment of Low Back Pain: A Joint Clinical Practice Guideline
from the American College of Physicians and the American Pain Society
Annals of Internal Medicine 2007 (Oct 2); 147 (7): 478491Chou R, Huffman LH; American Pain Society.
Nonpharmacologic Therapies for Acute and Chronic Low Back Pain:
A Review of the Evidence for an American Pain Society/
American College of Physicians Clinical Practice Guideline
Annals of Internal Medicine 2007 (Oct 2); 147 (7): 492504Dagenais S, Tricco AC, Haldeman S.
Synthesis of Recommendations for the Assessment and Management of Low Back Pain
From Recent Clinical Practice Guidelines
Spine J. 2010 (Jun); 10 (6): 514529Fuhr AW, Menke JM.
Status of activator methods chiropractic technique, theory, and practice.
J Manipulative Physiol Ther. 2005;28:e1e20Huggins T, Boras AL, Gleberzon BJ, Popescu M, Bahry LA.
Clinical effectiveness of the activator adjusting instrument in the management of musculoskeletal disorders: a systematic review of the literature.
J Can Chiropr Assoc. 2012;56:4957Taylor SH, Arnold ND, Biggs L, Colloca CJ, Mierau DR, et al.
A Review of the Literature Pertaining to the Efficacy, Safety, Educational Requirements,
Uses and Esage of Mechanical Adjusting Devices: Part 1 of 2
J Can Chiropr Assoc. 2004;48:74108Taylor SH, Arnold ND, Biggs L, Colloca CJ, Mierau DR, et al.
A Review of the Literature Pertaining to the Efficacy, Safety, Educational Requirements,
Uses and Esage of Mechanical Adjusting Devices: Part 2 of 2
J Can Chiropr Assoc. 2004;48:152161Colloca CJ, Keller TS, Gunzburg R, Vandeputte K, Fuhr AW:
Neurophysiologic Response to Intraoperative Lumbosacral Spinal Manipulation
J Manipulative Physiol Ther. 2000 (Sep); 23 (7): 447457Colloca CJ, Keller TS.
Stiffness and neuromuscular reflex response of the human spine to posteroanterior manipulative thrusts in patients with low back pain.
J Manipulative Physiol Ther. 2001;24:489500Colloca CJ, Keller TS, Gunzburg R.
Neuromechanical Characterization Of In Vivo Lumbar Spinal Manipulation.
Part II. Neurophysiological Response
J Manipulative Physiol Ther. 2003 (Nov); 26 (9): 579591Colloca CJ, Keller TS, Harrison DE, Moore RJ, Gunzburg R, et al.
Spinal manipulation force and duration affect vertebral movement and neuromuscular responses.
Clin Biomech. 2006;21:254262Kawchuk GN, Prasad NG, McLeod RC, Liddle T, Li T, et al.
Variability of force magnitude and force duration in manual and instrument-based manipulation techniques.
J Manipulative Physiol Ther. 2006;29:611618Keller TS, Colloca CJ, Fuhr AW.
Validation of the force and frequency characteristics of the activator adjusting instrument: effectiveness as a mechanical impedance measurement tool.
J Manipulative Physiol TherSymons BP, Herzog W, Leonard T, Nguyen H.
Reflex responses associated with activator treatment.
J Manipulative Physiol Ther. 2000;23:155159Colloca CJ, Keller TS.
Electromyographic reflex responses to mechanical force, manually assisted spinal manipulative therapy.
Spine. 2001;26:11171124Colloca CJ, Keller TS, Gunzburg R:
Biomechanical and Neurophysiological Responses to Spinal Manipulation in Patients
With Lumbar Radiculopathy
J Manipulative Physiol Ther. 2004 (Jan); 27 (1): 115Colloca CJ, Keller TS, Moore RJ, Gunzburg R, Harrison DE.
Effects of disc degeneration on neurophysiological responses during dorsoventral mechanical excitation of the ovine lumbar spine.
J Electromyogr Kinesiol. 2007;18:82937Colloca CJ, Keller TS, Moore RJ, Gunzburg R, Harrison DE.
Intervertebral disc degeneration reduces vertebral motion responses.
Spine. 2007;32:E544E550Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ.
The Mechanisms of Manual Therapy in the Treatment of Musculoskeletal Pain:
A Comprehensive Model
Man Ther. 2009 (Oct); 14 (5): 531538Pickar JG.
Neurophysiological Effects of Spinal Manipulation
Spine J (N American Spine Society) 2002 (Sep); 2 (5): 357371Keller TS, Colloca CJ, Gunzburg R.
Neuromechanical Characterization of in vivo Lumbar Spinal Manipulation.
Part I. Vertebral Motion
J Manipulative Physiol Ther. 2003 (Nov); 26 (9): 567578Nathan M, Keller TS.
Measurement and analysis of the in vivo posteroanterior impulse response of the human thoracolumbar spine: a feasibility study.
J Manipulative Physiol Ther. 1994;17:431441Smith DB, Fuhr AW, Davis BP.
Skin accelerometer displacement and relative bone movement of adjacent vertebrae in response to chiropractic percussion thrusts.
J Manipulative Physiol Ther. 1989;12:2637Colloca CJ, Keller TS, Gunzburg R, Vandeputte K, Fuhr AW.
Neurophysiologic response to intraoperative lumbosacral spinal manipulation.
J Manipulative Physiol Ther. 2000;23:447457Pickar JG, Sung PS, Kang YM, Ge W.
Response of lumbar paraspinal muscles spindles is greater to spinal manipulative loading compared with slower loading under length control.
Spine J. 2007;7:583595Reed, W. R., Cao, D. Y., Long, C. R., Kawchuk, G. N., & Pickar, J. G.
Relationship between Biomechanical Characteristics of Spinal Manipulation and Neural Responses
in an Animal Model: Effect of Linear Control of Thrust Displacement versus Force,
Thrust Amplitude, Thrust Duration, and Thrust Rate
Evidence-Based Complementary and Alternative Medicine 2013 (Jan 20); 492039Reed, W. R., Long, C.R., & Pickar, J. G.
Effects of Unilateral Facet Fixation and Facetectomy on Muscle Spindle Responsiveness
During Simulated Spinal Manipulation in an Animal Model
J Manipulative Physiol Ther. 2013 (Nov); 36 (9): 585594Pickar JG.
An in vivo preparation for investigating neural responses to controlled loading of a lumbar vertebra in the anesthetized cat.
J Neurosci Methods. 1999;89:8796Reed WR, Long CR, Kawchuk GN, Pickar JG.
Neural responses to the mechanical parameters of a high velocity. low amplitude spinal manipulation: Effect of preload parameters.
J Manipulative Physiol Ther. 2014;37:6878Reed WR, Cao DY, Ge W, Pickar JG.
Using vertebral movement and intact paraspinal muscles to determine the distribution of intrafusal fiber innervation of muscle spindle afferents in the anethesized cat.
Exp Brain Res. 2013;225:205215Taylor A, Ellaway PH, Durbaba R.
Why are there three types of intrafusal muscle fibers?
Prog Brain Res. 1999;123:121131.Reed WR, Long CR, Kawchuk GN, Pickar JG.
Neural responses to the mechanical parameters of a high velocity, low amplitude spinal manipulation: effect of specific contact site.
Man Ther. 2015 Mar 27; 2015.Herzog W, Conway PJ, Kawchuk GN, Zhang Y, Hasler EM.
Forces exerted during spinal manipulative therapy.
Spine. 1993;18:12061212Ianuzzi A, Pickar JG, Khalsa PS.
Determination of torque-limits for human and cat lumbar spine specimens during displacement-controlled physiological motions.
Spine J. 2009;9:7786Fuhr AW, Smith DB.
Accuracy of piezoelectric accelerometers measuring displacement of a spinal adjusting instrument.
J Manipulative Physiol Ther. 1986;9:1521Fuhr AW.
The Activator Method.
Mosby Elsevier; St. Louis: 2009
Return to INSTRUMENT ADJUSTING
Return to CHIROPRACTIC ADJUSTING
Since 6242017


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |