

Neuromechanical Responses to Spinal Manipulation
and Mobilization: A Crossover Randomized Clinical TrialThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Manipulative Physiol Ther 2022 (Jan); 45 (1): 1–8 ~ FULL TEXT
OPEN ACCESS Arnaud Lardon, DC, PhD, Isabelle Page, DC, PhD, Francois Nougarou, PhD, and Martin Descarreaux, DC, PhD
Department of Human Kinetics,
University of Québec, Trois-Rivières,
Québec, Canada;
Franco-European Institute of Chiropractic,
Ivry-sur Seine, France.
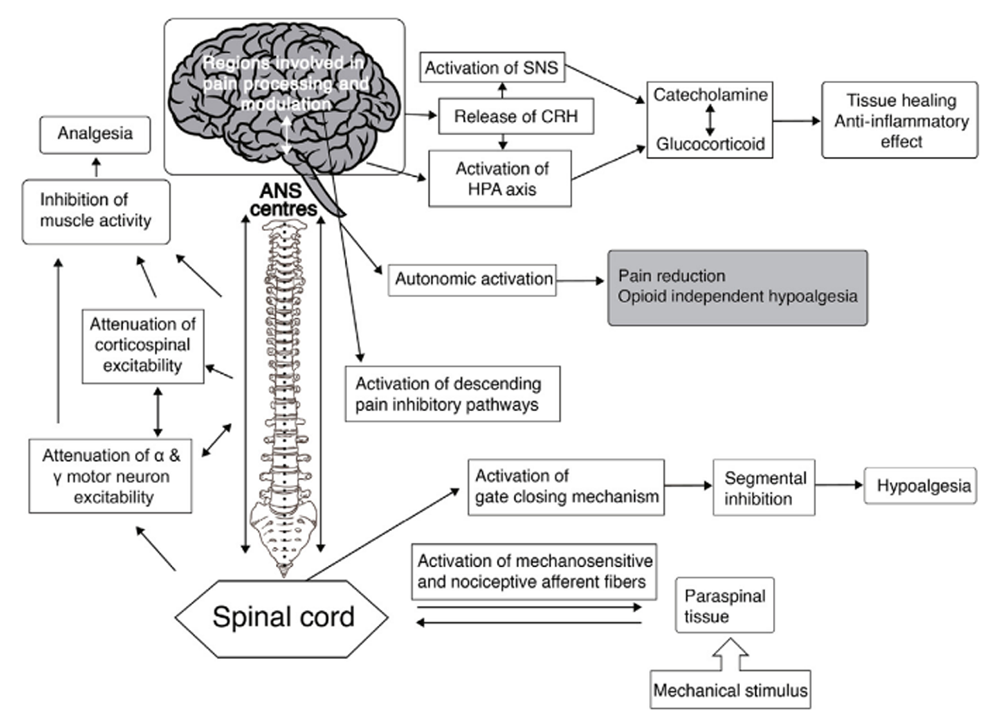
Neurophysiological effects of spinal manipulation
FROM: J Integrative Medicine 2019Objective: The purpose of this study was to compare the mmediate effect of spinal manipulation (SMa) and spinal mobilization (SMo) on muscular responses, spinal stiffness, and segmental spinal pressure evoked pain in a population of participants with chronic middle back pain (MBP).
Methods: In a crossover randomized trial, 2 experienced chiropractors assessed whether volunteers were eligible for the protocol according to a list of specific inclusion and exclusion criteria. Individuals with MBP participated in 2 experimental sessions within 72 hours. During the first session, participants randomly received a SMa or SMo delivered by an apparatus using a servolinear motor. During the second session, the other modality was delivered. Spinal stiffness and pressure-provoked pain intensity outcomes were assessed before and after each therapy, and muscular responses were recorded during the treatment using surface electromyographic sensors. Signed-rank Wilcoxon tests for muscular responses and generalized model for repeated measure for spinal stiffness and pressure-provoked pain were used for statistical analyses.
Results: Among the 32 potential participants, 26 (mean age 29.9 [±9.14], 15 women) completed both sessions. Between-group differences were observed for the muscular response amplitude (P < .001), and indeed the normalized RMS muscular response was found to be higher during SMa than SMo. Similar results were observed for pressure-provoked pain intensity at the level of therapeutic modality application (P = .002) as a higher decrease in pain was found after SMa (47.9 [±22.8] to 36.6 [±23.7]) compared with SMo (47.2 [±23.2] to 45.5 [±24.3]). No between-group differences were found for spinal stiffness change, nor for terminal (P = .08) and global spinal stiffness (P = .06).
Conclusion: In a controlled environment, spinal manipulation and mobilization generated different muscle responses and had different immediate effects on pressure-provoked pain intensity for participants with MBP.
Keywords: Chiropractic; Electromyography; Manipulation, Spinal; Musculoskeletal Manipulations; Pain.
From the Full-Text Article:
Introduction
Although spinal research focuses on low back pain and neck pain, nonspecific middle back pain (MBP) represents a common problem in the general population, with a 1–year prevalence estimated at 13%. [1] MBP also leads to important individual and economic consequences. For instance, a cross-sectional population-based study of 34,902 individuals showed that 6% of the people with MBP seek health care providers to manage their pain, 4% report reduced daily activity, and 2% report sick leaves in the past year. [2] MBP occurs early, during childhood or adolescence, [3] and its persistence or recurrence (ie, development of a new episode) is estimated to be between 13% and 45%. [4] Furthermore, a recent systematic review investigating the effectiveness of noninvasive interventions for musculoskeletal thoracic spine and chest wall pain critically appraised only 2 studies. [5] The authors concluded that manual therapy compared with placebo and acupuncture is associated with a small and clinically unimportant reduction in pain intensity.
Manual therapy, which includes spinal manipulation (SMa) and spinal mobilization (SMo), represents interesting therapeutic tools in the management of spinal pain to reduce pain intensity and disability related to back pain. [5–7] However, pain relief outcomes of SMa and SMo are mainly the same [8] and are often reported in clinical research under the same broad category of spinal manipulative therapy. [9, 10] This lack of clear distinction may lead to misinterpretation of the evidence concerning the different clinical responses when these therapeutic modalities are used. [11] Consequently, manual therapists currently have insufficient data to support their choice of therapeutic modality between SMa and SMo for patients with spine-related pain.
From a biomechanical standpoint, differences exist between SMa and SMo. [12] SMa is defined as a high-velocity, low-amplitude force technique including a thrust, whereas SMo is defined as a nonthrust technique with a low-velocity passive oscillatory force repeated 3 to 4 times to a vertebral level. [8, 10, 12, 13] Besides these differences in force, the rate of force applied by the therapist also differs for these 2 procedures. The range of force applied on the vertebral level during a SMa can vary across the spinal localization between 200 and 1600 N, [13] whereas the range of force for SMo is notably lower, ranging from 10 to 240 N. [10]
It was previously shown that differences in peak force, thrust duration, and rate of force affect spinal neuromechanical responses. [14–16] Indeed, increases in force are associated with increased segmental displacements, whereas decreases in thrust duration led to increases in muscular responses. It is therefore plausible that neuromuscular response differences exist when these therapeutic modalities are being used in clinical practices by health practitioners. However, previous literature comparing SMa and SMo involved clinicians who delivered the therapies and did not record the biomechanical parameters of the delivered therapy. Consequently, it is not known to which extent the compared therapies differed from each other in terms of their biomechanical characteristics, as well as the variability in therapy execution. Considering that these parameters modify spinal neuromechanical responses, it seems useful to explore whether each modality, when performed using a standardized and controlled protocol, can reveal physiological and clinical differences that were not previously described.
Therefore, the objective of the present study was to compare the immediate neuromechanical and clinical effects between SMa and SMo in patients with chronic nonspecific MBP. We hypothesized that clinical and mechanical responses to SMa and SMo would differ in patients with chronic nonspecific MBP.
Methods
Design
This crossover randomized clinical trial was part of a broader research program that aimed to investigate the effects of chronic MBP on spinal stiffness value and its reliability, as well as to explore the association between spinal stiffness pain and muscle activity during assessment. [17]
Participants
Volunteers with chronic, nonspecific MBP were recruited within the local community. Two experienced chiropractors assessed whether volunteers were eligible for the protocol according to a list of specific inclusion and exclusion criteria. Inclusion criteria included chronic, nonspecific MBP for at least 3 months (constant or recurrent) and an age of 18 to 60 years. Participants were excluded if they presented at least 1 of the following criteria: contraindications for SMa/SMo, spinal surgery history, thoracic scoliosis, thoracic herniated disk, radiculopathy, myelopathy neurologic disease, osteoporosis, uncontrolled hypertension, aortic aneurysm, inflammatory or infectious disease, and pregnancy.
Ethics
The study protocol was registered in ClinicalTrials.gov (NCT02660801) and approved by the University of Québec at Trois-Rivières research ethics committee (CER-16-220-07.04). All participants provided their written informed consent to participate in this study.
Experimental Protocol
Baseline Information
The protocol consisted of 2 experimental sessions within 72 hours. Before the experimentation, participants completed various questionnaires to document demographic data (age, sex, weight, and height), baseline MBP (0 to 100 on visual analog scale for pain) and to assess disability (Quebec Back Pain Disability questionnaire), [18] kinesiophobia (Tampa Scale for Kinesiophobia, TSK), [19] and risk of poor prognosis (STarT Back Screening Tool). [20]
Outcome Variables
Spinal Stiffness Assessment
Spinal stiffness is the relationship between spinal displacement and the resistive force to that movement. [21] All participants received information about the basic functioning and main security features of the experimental apparatus. Once baseline information was collected, spinal stiffness was assessed at T5, T6, T7, and T8 using a custom-made apparatus that uses a servocontrolled linear actuator motor (Linear Motor Series P01-48 × 360; LinMot, Inc, Zurich, Switzerland). It was initially developed to simulate SMa [22] and allow the simulation of manual therapy procedures. During the spinal stiffness assessment, the apparatus, using a single-tip padded rod, vertically displaces a slider directly applied over the targeted spinous process (ie, T5, T6, T7, or T8). Two trained chiropractors, with respectively 5 and 9 years of practice, identified T5, T6, T7, and T8 spinous and transverse process using a standardized procedure. [23–25] At the end of the first session, small pieces of adhesive tape were affixed over the transverse processes targeted by the apparatus and over marked spinous processes. This approach ensured that each participant received the modality of the second session at the same spinal level than the modality of the first session. During this procedure, a force is progressively applied to reach a peak force of 45 N (rate of force application: 18 N/s) that is held for 1 second before being released. Instructions were given to the participants to hold their breath at the end of exhalation before the apparatus contacted the spinous process for the time of the assessment. This procedure was repeated 4 times for each spinous process before and after the therapeutic modality application. The order at which the 4 spinal levels were assessed was randomly generated by an online scheme generator. [26]
Pressure-Provoked Pain Intensity
Pressure-provoked pain intensity was assessed immediately after each spinal stiffness assessment using a 0 to 100 visual analog scale. [27] Spinal stiffness assessment and pressure-provoked pain intensity levels were assessed before and after the interventions at T5, T6, T7, and T8 levels.
Muscular Responses
Muscular activity was recorded using surface electromyography (sEMG) electrodes during the modalities (Trigno EMG systems, Delsys Inc, Natick, Massachusetts). This activity was recorded at 2K Hz using 4 Trigno Wireless electromyographic sensors, which were applied bilaterally, just above and below the area contacted by the apparatus (≈2 cm from midline), where the therapeutic modality was executed. Skin impedance was reduced by shaving body hair, abrading the skin with sandpaper, and cleaning it with alcohol. To reduce electromyographic signal variability, a normalization trial was performed at the beginning of the protocol. Participants were asked to perform an active extension of thoracic spine from a prone position and to hold this position for 4 seconds.
Therapeutic Modalities
The therapeutic modality was applied on transverse processes of the most painful vertebral level reported by the participants during the spinal stiffness assessment during the pressure-provoked pain intensity assessment. SMa and SMo were applied to the spine by the apparatus through a twin-tip padded rod. The modality (SMa or SMo) applied at the first session was randomly determined and generated by a computer. [26] Participants were not aware if SMa or SMo would be applied by the device at the first session. If SMa was delivered at the first session, SMo was applied at the second one and vice versa. The apparatus was programmed to execute a SMa characterized by a preload force of 70 N for 500 ms leading to a peak force of 260 N in 125 ms (rate of force application: 1520 N/s). For SMo, 3 oscillatory cycles of 85 N in 800 ms were applied after a 5 N preload force (rate of force application: 106 N/s).
Data Analysis
Spinal Stiffness Calculation
Global and terminal stiffness coefficients were calculated from the force-displacement data of each spinal stiffness assessment using the same methods presented in a previous study. [17] Since the first trial of spinal stiffness assessment has been reported to differ from the subsequent ones, only the second, third, and fourth assessments were included in the analyses. Global stiffness was defined as the slope of the straight line best fitting the force-displacement data between 10 and 45 N, and terminal stiffness was defined as the ratio of the variation of force and displacement between 10 and 45 N. The average value of the second to fourth assessments of each spinal level was used in further analyses. Only data for spinal stiffness at the level of the therapeutic modalities’ application were used in statistical analysis.
Pressure-Provoked Pain Intensity
As explained previously, pressure-provoked pain intensity was recorded immediately after each spinal stiffness measurement. The mean of the 4 trials was calculated. Only data for pressure-provoked pain intensity at the level of the therapeutic modalities’ application were used in statistical analysis.
Muscular Response
To assess the muscular response during therapeutic modalities, the resulting bipolar sEMG signals were first digitally band-pass filtered using a frequency bandwidth of 20 to 450 Hz (second-order Butterworth filter). For SMa, the peak root mean square (RMS) value was computed for each electrode using a 250–ms window (125 ms before and 125 ms after the peak force). The RMS values obtained for each electrode were then normalized (nRMS) to the respective RMS value calculated during the sEMG normalization trial. Because no differences were found between the left and right electrodes’ nRMS at both levels (superior and inferior) using the Wilcoxon rank-sum test (P > .05), the mean between the 2 electrodes (left and right) at the upper and at lower levels was calculated to obtain 2 different outcome variables (superior and inferior level muscular response). For SMo, 3 peak forces were identified and a 250–ms window was also used to obtain a mean RMS value for each peak. Because no difference was found between the 3 peak forces RMS using the Friedman analysis of variance (ANOVA) test (P > .05), the mean between these 3 peaks was calculated. Once these results were obtained, the Wilcoxon rank-sum test was used to determine if any difference existed between the left and right electrodes for both the upper and lower levels. Because no difference (P >.05) was found between left and right electrodes’ nRMS at both levels (superior and inferior), the mean between the 2 electrodes (left and right), respectively at the upper and at lower levels, was calculated to obtain 2 different outcome variables (superior and inferior level muscular response). Statistical Analysis
Descriptive statistics were used for baseline characteristics (sex, age, height, weight, baseline MBP, Quebec Back Pain Disability questionnaire, STarT Back Screening Tool, and TSK) and clinical characteristics of participants (spinal stiffness, pressure-provoked pain, and muscular response).
Normality was assessed using the Shapiro-Wilk. The Wilcoxon signed-rank test was used to compare muscular responses during SMa and SMo. A mixed-model 2–way repeated measures ANOVA was performed to assess treatment (SMa vs. SMo) and time (pretreatment vs. post-treatment) main effects, as well as the interaction effect (treatment × time) for spinal stiffness and pressure-provoked pain. If a variable was not normally distributed, a mathematical transformation was performed to comply with ANOVA assumptions. Whenever ANOVA yielded a significant effect, a Tukey post-hoc test was computed. The level of statistical significance (2–tailed hypothesis) was set at P < .05 for all analyses, and the STATISTICA statistical package version 7.1 (Statsoft) was used to conduct analyses.
Results
Baseline Description
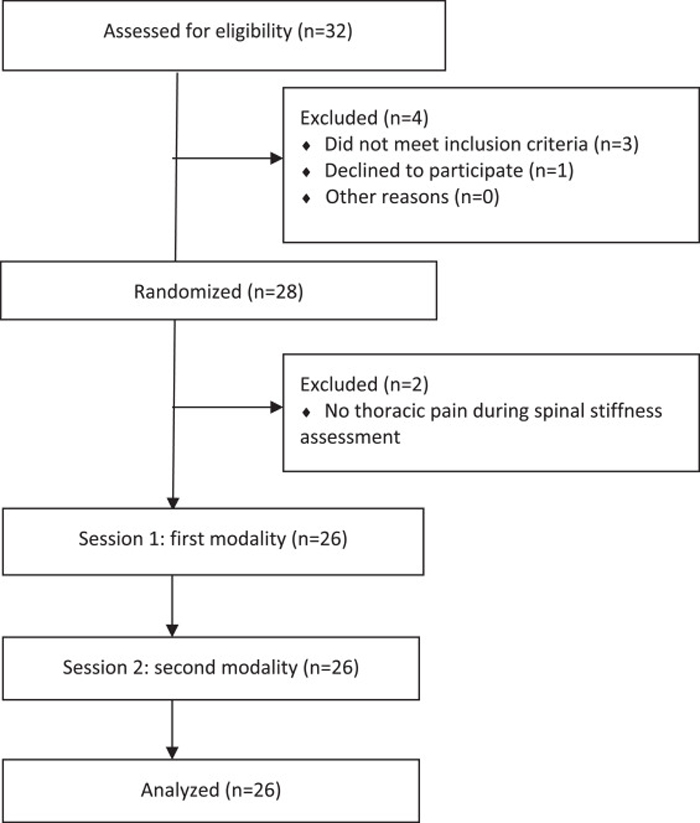
Figure 1

Table 1

Table 2 Thirty-two participants initially volunteered for the study. However, 2 participants presented a thoracic scoliosis, 1 presented contraindications for SMa during the initial examination, and 1 decided to stop his participation before the first therapeutic modality application. Twenty-eight participants were therefore assessed for spinal stiffness, but 2 participants reported no pain during the spinal stiffness assessment and were excluded. Overall, 26 participants completed both experimental sessions (Figure 1). Characteristics of these participants are presented in Table 1. No between-group difference in baseline characteristic (ie, age, height, weight, baseline MBP, Quebec Back Disability Scale, STarT Back Screening Tool, Tampa scale for kinesiophobia, baseline pressure-provoked pain intensity, terminal and global spinal stiffness) were identified (P > .05).
Spinal Stiffness
As this outcome variable was not normally distributed, a square root transformation was performed in order to conduct generalized linear models for repeated-measure ANOVA. The statistical analysis yielded no main effect of treatment or time for the terminal (F[1,25] = 0.022; P = .88, ηp2 = 0.0002 m and F[1,25] = 0.05; P =.82, ηp2 = 0.002) and global (F[1,25] = 0.004; P = .95, ηp2 = 0.0002 and F[1,25] = 0.001; P = .99, ηp2 < 0.001) spinal stiffness. Moreover, no interaction effect (treatment × time) was observed for the terminal (F[1,25] = 3.2; P = .08, ηp2 = 0.11) and global (F[1,25] = 3.7; P = .06, ηp2 = 0.13) spinal stiffness. The results indicate that there was no difference in spinal stiffness after application of both the SMa and SMo. Table 2 reports these results.
Pressure-Provoked Pain Intensity
As this outcome variable was not normally distributed, transformation in square root was performed to use generalized linear models for repeated-measure ANOVA. The statistical analysis yielded a significant main effect of time (F[1,25] = 12.42; P = .002, ηp2 = 0.33) but a nonsignificant main effect of treatment (F[1,25] = 2.5; P = .127, ηp2 = 0.09). This test also highlighted a statistically significant treatment × time interaction effect for pressure-provoked pain intensity (F[1,25] = 11.34; P = .002, ηp2 = 0.31). Tukey post-hoc analyses showed no difference in pressure-provoked pain intensity before the application of both therapeutic modalities (P = .99). It also revealed that a statistically significant decrease of pressure-provoked pain was present after the SMa application (from 47.9/100 [±22.8] to 36.6/100 [±23.7]) (P < .001) and not after SMo application (from 47.2/100 [±23.2] to 45.5/100 [±24.3]) (P = .79) (Table 2).
Muscular Response
Owing to technical difficulties, sEMG data from 2 participants had to be excluded from the analysis. The Wilcoxon signed-rank test showed a statistically significant difference in paraspinal muscular responses (P < .001). Indeed, the muscular activity (normalized RMS value) recorded during SMa was higher than the muscular activity recorded during SMo. This difference was significant for both above (0.78 [.56] vs. 0.16 [.09]) and below (0.79 [.60] vs. 0.16 [.10]) the contact level. Table 2 reports these results.
Discussion
Main Results
To our knowledge, this study is the first to compare the immediate effect of SMa and SMo on thoracic spinal stiffness and pressure-provoked pain intensity and muscle responses. The results of this study showed that a SMa of 260 N (1520 N/s) yields an immediate decrease in pressure-provoked pain but has no effect on spinal stiffness, whereas SMo of 3 oscillatory cycles of 85 N (106 N/s) has no immediate effect on these 2 outcomes. Moreover, higher muscular responses were recorded during the SMa compared with muscular responses recorded during SMo.
Current evidence suggests that a decrease in lumbar spinal stiffness after a lumbopelvic spinal manipulation may help to identify patients with low back pain more likely to rapidly respond to this therapeutic modality. [28, 29] The current study results failed to show a significant change in spinal stiffness after both SMa and SMo. Previous studies conducted in the thoracic spine also did not reveal a significant change in spinal stiffness after manually delivered thoracic SMa in participants without MTP. [30, 31] In addition to the possibility that thoracic SMa/SMo do not result in a change in spinal stiffness, these results also raised the possibility that the lack of significant effect is due to the average mild clinical status of the participants. Future studies should target participants with MDP who present a moderate to severe clinical status.
In the present study, both SMa and SMo triggered a muscular response. However, the responses triggered during SMa were higher, which could be explained by the fact that the rate of force application (1520 N/s) and peak force (260 N) were higher during this therapeutic modality than SMo (106 N/s; 85 N). It was previously shown that the increase in the muscle response amplitude to SMa is closely related to the increase in the peak force and thus to the rate of force application. [15] Although the SMa and the SMo used in the current study yielded different magnitudes of muscular responses, it is not possible to determine if these responses are linked to the different effect of these modalities on the pressure-provoked pain intensity.
Current evidence suggests that both SMa and SMo can have an analgesic effect. A recent systematic review reported that 5 of the 7 appraised studies showed that SMo results in an immediate increase in local pressure pain thresholds. [32] A similar analgesic effect on experimental pressure pain was described in another systematic review (19 of 27 articles) in which the effect of SMa on experimental induced pain was assessed. [33] However, no studies comparing the 2 approaches were identified in the literature on this topic. The present study assesses the spinal pressure-provoked pain induced during stiffness evaluation. This procedure is commonly used by clinicians during spinal manual evaluation of patients in order to identify painful vertebral level and after the intervention to analyze the patient's progress. [34]
As explained by Wong and Kawchuk, [35] manual therapists apply manual posteroanterior forces along the spine of their patient in a prone position to assess segmental spinal stiffness and reproduce the patient's pain. In the present study, a short-term decrease in pain was observed only for the SMa intervention, but the underlying mechanisms are not yet established. Bialosky et al [36] suggested that SMa efficacy could be explained by a mechanical effect on spinal stiffness associated with neurophysiological effects such as hypoalgesia and muscular activity facilitation. Other authors suggested that immediate spinal stiffness decreases and muscular recruitment changes may play a role in the clinical benefits of SMa. [28, 29, 37]
Limitations and Future Research
A few limitations must be considered when interpreting the results of this study. First, although pressure-provoked pain intensity reduction after SMa has both statistical and clinical significance, only short-term effects were assessed and the duration of the effect cannot be established. Moreover, the statistical analyses were not conducted on clinical pain (ie, midthoracic pain intensity just before the beginning of each experimental session) but only on the pressure-provoked pain intensity because no follow-up occurred after the second experimental session.
Another limitation concerns spinal stiffness assessments, as the combined physiological effects of SMa and SMo are unknown. The goal of this study was to identify differences in physiological responses between SMa and SMo. It was therefore not designed as a randomized clinical trial to identify the best therapeutic modality for patients with MBP. Secondly, other physiological variables such as vertebral displacement and acceleration were not considered.
Third, the same forces were applied, without consideration to the patient's anthropometric or preferences, and such standardization may not reflect current practice, limiting the overall generalizability of results.
Finally, even if the participants were not aware of the first modality applied during the first session, the non-naïve participants could deduct the modality for the second time. However, 2 of our outcome variables are “nonsubjective” (stiffness and muscular response). Although the evaluation of pressure-provoked pain can be considered subjective, participants were not aware when the spinal level at which the therapeutic modality was delivered was assessed.
Future research should investigate these 2 therapeutic tools separately and clearly distinguish these terms. It would also be useful to determine which parameters represent good predictors of chronic MBP clinical improvement after a few treatments of spinal manipulative therapy.
Conclusion
In a controlled environment, the delivery of a thoracic spinal manipulation in participants with chronic midback pain resulted in an immediate decrease in thoracic pressure-provoked pain intensity but not spinal stiffness. Spinal mobilization has no effect on these 2 outcomes and generates lower thoracic muscle response than a spinal manipulation.
Practical Applications
Thoracic spinal manipulation in participants with chronic midback pain resulted in an immediate decrease in thoracic pressure-provoked pain intensity.
Thoracic spinal mobilization generated lower thoracic muscle response than a spinal manipulation.
Future research should investigate these therapeutic tools separately to clearly distinguish these terms.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): A.L., I.P., M.D.
Design (planned the methods to generate the results): A.L., I.P., M.D.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): M.D.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): A.L., I.P.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): A.L., I.P., F.N., M.D.
Literature search (performed the literature search): A.L., I.P. Writing (responsible for writing a substantive part of the manuscript): A.L., I.P.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): A.L., I.P., F.N., M.D.
References:
Leboeuf-Yde C Nielsen J Kyvik KO Fejer R Hartvigsen J.
Pain in the lumbar, thoracic or cervical regions:
do age and gender matter? A population-based study
of 34,902 Danish twins 20-71 years of age.
BMC Musculoskelet Disord. 2009; 10: 39Leboeuf-Yde C Fejer R Nielsen J Kyvik KO Hartvigsen J.
Consequences of Spinal Pain: Do Age and Gender Matter?
A Danish Cross-sectional Population-based Study
of 34,902 Individuals 20-71 Years of Age
BMC Musculoskelet Disord. 2011 (Feb 8); 12: 39Kjaer P, Wedderkopp N, Korsholm L, Leboeuf-Yde C.
Prevalence and Tracking of Back Pain From Childhood to Adolescence
BMC Musculoskelet Disord. 2011;12:98Johansson MS Jensen Stochkendahl M Hartvigsen J Boyle E Cassidy JD
Incidence and prognosis of mid-back pain in the general population:
a systematic review.
Eur J Pain. 2017; 21: 20-28Southerst D Marchand AA Cote P et al.
The effectiveness of noninvasive interventions for musculoskeletal
thoracic spine and chest wall pain: a systematic review by the Ontario
Protocol for Traffic Injury Management (OPTIMa) collaboration.
J Manipulative Physiol Ther. 2015; 38: 521-531Wong JJ Cote P Sutton DA et al.
Clinical Practice Guidelines for the Noninvasive Management
of Low Back Pain: A Systematic Review by the Ontario
Protocol for Traffic Injury Management (OPTIMa) Collaboration
European J Pain 2017 (Feb); 21 (2): 201–216Sutton DA Cote P Wong JJ et al.
Is multimodal care effective for the management of patients with
whiplash-associated disorders or neck pain and associated
disorders? A systematic review by the Ontario Protocol
for Traffic Injury Management (OPTIMa) Collaboration.
Spine J. 2016; 16: 1541-1565Bronfort, G, Haas, M, Evans, RL, and Bouter, LM.
Efficacy of Spinal Manipulation and Mobilization for Low Back Pain
and Neck Pain: A Systematic Review and Best Evidence Synthesis
Spine J (N American Spine Soc) 2004 (May); 4 (3): 335–356Assendelft WJ Morton SC Yu EI Suttorp MJ Shekelle PG.
Spinal manipulative therapy for low back pain. A meta-analysis
of effectiveness relative to other therapies.
Ann Intern Med. 2003; 138: 871-881Snodgrass SJ Rivett DA Robertson VJ.
Manual forces applied during posterior-to-anterior spinal mobilization:
a review of the evidence.
J Manipulative Physiol Ther. 2006; 29: 316-329Kotoulas M.
The use and misuse of the terms manipulation and mobilization in
the literature establishing their efficacy in the
treatment of lumbar spine disorders.
Physiother Can. 2002; 54: 53-61Cleland JA Fritz JM Kulig K et al.
Comparison of the effectiveness of three manual physical therapy
techniques in a subgroup of patients with low back pain
who satisfy a clinical prediction rule:
a randomized clinical trial.
Spine (Phila Pa 1976). 2009; 34: 2720-2729Herzog W Conway PJ Kawchuk GN Zhang Y Hasler EM.
Forces exerted during spinal manipulative therapy.
Spine (Phila Pa 1976). 1993; 18: 1206-1212Nougarou F Page I Loranger M Dugas C Descarreaux M.
Neuromechanical response to spinal manipulation therapy:
effects of a constant rate of force application.
BMC Complement Altern Med. 2016; 16: 161Nougarou F Dugas C Deslauriers C Page I Descarreaux M.
Physiological responses to spinal manipulation therapy:
investigation of the relationship between electromyographic
responses and peak force.
J Manipulative Physiol Ther. 2013; 36: 557-563Page I Nougarou F Dugas C Descarreaux M.
The effect of spinal manipulation impulse duration on
spine neuromechanical responses.
J Can Chiropr Assoc. 2014; 58: 141-148Page I Nougarou F Lardon A Descarreaux M.
Changes in spinal stiffness with chronic thoracic pain:
correlation with pain and muscle activity.
PLoS One. 2018; 13e0208790Kopec JA Esdaile JM Abrahamowicz M et al.
The Quebec Back Pain Disability Scale:
conceptualization and development.
J Clin Epidemiol. 1996; 49: 151-161Lundberg M Grimby-Ekman A Verbunt J Simmonds MJ.
Pain-related fear: a critical review of the related measures.
Pain Res Treat. 2011. 2011; 494196Bruyere O Demoulin M Beaudart C et al.
Validity and reliability of the French version of the STarT Back
screening tool for patients with low back pain.
Spine (Phila Pa 1976). 2014; 39: E123-E128Snodgrass SJ Haskins R Rivett DA.
A structured review of spinal stiffness as a kinesiological outcome
of manipulation: its measurement and utility in diagnosis,
prognosis and treatment decision-making.
J Electromyogr Kinesiol. 2012; 22: 708-723Descarreaux M Nougarou F Dugas C.
Standardization of spinal manipulation therapy in humans:
development of a novel device designed to measure dose-response.
J Manipulative Physiol Ther. 2013; 36: 78-83Page I Descarreaux M Sobczak S.
Development of a new palpation method using alternative landmarks
for the determination of thoracic transverse processes: An in vitro study.
Musculoskelet Sci Pract. 2017; 27: 142-149Stonelake PS Burwell RG Webb JK.
Variation in vertebral levels of the vertebra prominens
and sacral dimples in subjects with scoliosis.
J Anat. 1988; 159: 165-172Cooperstein R Haneline MT Young MD.
The location of the inferior angle of the scapula in relation
to the spinal level of prone patients.
J Can Chiropr Assoc. 2009; 53: 121-128Randomization.com. Available at:
http://www.randomization.com/
Accessed February 2016.Maher C.
Responsiveness of visual analogue and McGill Pain Scale measures.
J Manipulative Physiol Ther. 2001; 24: 501-504Fritz JM Koppenhaver SL Kawchuk GN Teyhen DS Hebert JJ Childs JD.
Preliminary investigation of the mechanisms underlying the effects
of manipulation: exploration of a multivariate model including
spinal stiffness, multifidus recruitment, and clinical findings.
Spine (Phila Pa 1976). 2011; 36: 1772-1781Wong AYL, Parent EC, Dhillon SS, Prasad N, Kawchuk GN.
Do Participants with Low Back Pain who Respond to Spinal
Manipulative Therapy Differ Biomechanically From
Nonresponders, Untreated Controls
or Asymptomatic Controls?
Spine (Phila Pa 1976). 2015 (Sep 1); 40 (17): 1329–1337Lee M Latimer J Maher C.
Manipulation: investigation of a proposed mechanism.
Clin Biomech (Bristol, Avon). 1993; 8: 302-306Campbell BD Snodgrass SJ.
The effects of thoracic manipulation on posteroanterior spinal stiffness.
J Orthop Sports Phys Ther. 2010; 40: 685-693Lascurain-Aguirrebena I Newham D Critchley DJ.
Mechanism of action of spinal mobilizations: a systematic review.
Spine (Phila Pa 1976). 2016; 41: 159-172Millan M, Leboeuf-Yde C, Budgell B, Amorim MA.
The Effect of Spinal Manipulative Therapy on Experimentally
Induced Pain: A Systematic Literature Review
Chiropractic & Manual Therapies 2012 (Aug 10); 20 (1): 26Tuttle N.
Is it reasonable to use an individual patient's progress after
treatment as a guide to ongoing clinical reasoning?
J Manipulative Physiol Ther. 2009; 32: 396-403Wong AYL Kawchuk GN.
The clinical value of assessing lumbar posteroanterior segmental
stiffness: a narrative review of manual and instrumented methods.
PM R. 2017; 9: 816-830Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ.
The Mechanisms of Manual Therapy in the Treatment of
Musculoskeletal Pain: A Comprehensive Model
Manual Therapy 2009 (Oct); 14 (5): 531–538Koppenhaver SL Fritz JM Hebert JJ et al.
Association between changes in abdominal and lumbar multifidus
muscle thickness and clinical improvement after spinal manipulation.
J Orthop Sports Phys Ther. 2011; 41: 389-399
Return to SUBLUXATION
Since 9-21-2022


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |