

The Vertebral Subluxation Complex PART 2:
The Neuropathological and Myopathological ComponentsThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Chiropractic Research Journal 1990; 1 (4): 19-38 ~ FULL TEXT
Charles A. Lantz, Ph.D., D.C.
Life Chiropractic College - West
EDITORS' NOTE: This is the second of a four part series on the Vertebral Subluxation Complex and its relevance to chiropractic theory and basic chiropractic research, as well as to the practice of chiropractic. The first part of this series appear ed in CRJ Volume 1, No. 3. It introduced an 8 part model and went on to discuss the kinesiopathological component of the vertebral subluxation complex. This part continues with the descriptions of the neuropathological and myopathological components.
INTRODUCTION
The concept of subluxation has been a cornerstone of the theory and practice of chiropractic since its founding by D.D. Palmer in 1895. It is one of the most controversial concepts in health care today, and finds its supporters and critics both within and outside the chiropractic profession. The original concept of subluxation was that of a slightly misaligned vertebra, not sufficient to be qualified as a true luxation or dislocation but substantial enough to impinge on the segmental nerves associated with it. While this original concept requires some modification in light of current research findings, there has been a wealth of knowledge accumulated in the past two decades that supports the concept of vertebral subluxation as a real entity. It must be stressed that, from the contemporary, scientific chiropractic point of view, the subluxation is a dynamic process involving several tissue levels and integrative components.
AUTHOR'S COMMENTS RELATIVE TO THIS SERIES:
Currently, there appears to be a lack of consensus in chiropractic concerning the exact nature of the subluxation and opinions vary widely as to its existence. Although the term subluxation is in wide use in general chiropractic practice, in the scientific community it is generally agreed that the term is much too imprecise. However, common to all definitions currently in use within the chiropractic profession is the notion of a structural and/or functional disrelationship with some form of neurological involvement. What has been missing from the subluxation concept is the substantive evidence to support the idea of dynamic dysfunction.
It has been suggested that the term "Vertebral Subluxation Concept" (VSC) or "Chiropractic Subluxation Complex" be used in place on the simple noun subluxation. We support this recommendation, as do others, as a means of broadening the idea to encompass all possible etiologies and ramifications of the subluxation concept. What is lacking, however us an organizational structure which relates current knowledge and experience to a common central conceptual model of subluxation.
It is the purpose of these articles to provide a model of the subluxation which is relevant to the theory and practice of chiropractic and consistent with chiropractic clinical experience. Support for the model and the contribution of the individual components to overall subluxation behavior has been drawn from the scientific, chiropractic, medical, and osteopathic literature.
NEUROPATHOLOGY
The neurological component of the Vertebral Subluxation Complex (VSC) is, for many, the cornerstone of chiropractic theory.[1] For those who see beyond the application of chiropractic and other manipulative procedures as merely a means of relieving head ache and low back pain, the nervous system is the mediator of vitality and health to the individual organs and tissues. [2] Today, more than ever before, basic scientific and medical research supports this fundamental concept of chiropractic. [3–7]. In chiropractic clinical practice, the prominence of the nervous system is unquestionable. Pain is by far the most significant factor in a patient's seeking chiropractic care. [8] In the diagnostic evaluation, motor function, reflexes, altered sensation and pain responses are primary indicators in the physical examination [9], and are interpreted as clinical indications of neurological function. A weakened muscle or reflex action, for example, is interpreted as a loss of motor function. Spasticity or pain is indicative of neurological excess, that is a facilitation, or hyperactivity of nerve fibers, as due to irritation or inflammation.
It is safe to say that every aspect of the nervous system's organization and function is relevant to the theory and practice of chiropractic. In this section we shall explore the more pertinent aspects of this relationship. An outline of the nervous sys tem from the chiropractic clinical perspective is given in Table I. Aspects of the neurological structure and function associated with the VSC are outlined in Table II. While it is beyond the scope of this article to deal with every aspect of this outli ne in detail, enough information is will be presented so that the astute reader can not only see the relevance and significance of the nervous system in the subluxation complex, but will also appreciate the many different levels of potential neurological involvement. The interested reader is referred to several excellent reviews on the neurological component of the VSC. [10–13].
IN THE VERTEBRAL SUBLUXATION COMPLEX |
I. Motor
A. Visceromotor
1. Sympathetic
2. Parasympathetic
B. Somatomotor
1. Skeletomotor
Alpha-motor Neurons
2. Fusimotor
Gamma-motor Neurons
C. Neuroendocrine
II. Sensory
A. Visceral Afferents
1. Chemoceptors
2. Baroceptors
B. Proprioception
1. Muscle Spindle Fibers
2. Golgi Tendon Organs
C. Mechanoreceptors
1. Pacinian Corpuscles
2. Ruffinian End Organs
D. Special Sensory
1. Vision
2. Vestibular (Balance)
|
III. Pain
A. Peripheral
1. Radicular
B. Spinal
C. Cerebral
D. Referred
E. Ectopic
F. Vascular
G. Articular
H. Phantom
IV. Internuncial Connections
A. Reflexes
1. Somatovisceral
2. Viscerosomatic
3. Somatosomatic
4. Viscerovisceral
B. Central Integration
1. Craniocervical
Coordination
2. Vestibular
Reflexes
3. Visual-cervical
Reflexes
C. Spinal Pathways
1. Descending
Pathways
2. Ascending
Pathways
|
VERTEBRAL SUBLUXATION COMPLEX |
I. Anatomical
A. CNS
1. Brain
2. Brain stem
3. Cord
a. Tracks
b. Zona
c. Nuclei
4. Mezinger
a. Dura
B. Neurological Organization
1. Rootlets
2. Roots
a. Ventral
b. Dorsal
c. Dorsal Root Ganglion
3. Spinal Nerves
a. Sinuvertebral Nerve
4. Primary Rami
a. Anterior (Ventral)
b. Posterior (Dorsal)
Medial Branch
5. Terminal Branches
C. Associated Structures
1. Intervertebral Canals
2. Neural Investments
D. Associated Structures
1. Anatomical Anomalies
E. Nerve Structures
1. Soma
a. Motor
b. Sensory
c. Internuncial
2. Axons
a. Myelin
3. Synapses
II. Physiological
A. Nerve Conduction
1. Polarization
2. Action Potentials
a. Graded
b. All-or-None
c. Saltatory
3. Axoplasmic Flow
4. Fiber Diameter
B. Inflammation
|
C. Neuromodulation
1. Neurohumoral Transmission
a. Neurotransmitters
b. Neuroendocrine Hormones
2. Neurotrophism
a. Neurochemical
b. Neurofunctional
3. Neuroimmunological
a. Neuroendocrine
b. Neuroinflammatory
D. Blood/Nerve Diffusion Barriers
1. Blood-Brain Barrier
2. Blood-Nerve Barrier
3. Blood-Ganglion Barrier
a. Dorsal Root Ganglia
b. Cranial Nerve Ganglia
c. Autonomic Ganglia
III. Biochemical
A. Neurotransmitter
B. Nutrition
C. Pharmacological Considerations
D. Metabolic Effects
IV. Biomechanical
A. Compression
B. Tension/Stretching
C. Avulsion
D. Laceration
E. Connective Tissue0
V. Integrative
A. Neuroendocrine Relations
B. Neuroimmunological Interactions
C. Neurovascular Reflexes
D. Neuroplanchnic Relationships
1. Somatoautonomic
2. Viscerosomatic
E. Neurosomatic
1. Somatosomatic
F. Cerviocranial Reflexes
1. Cervico-occular
2. Cervico-auditory
3. Cervico-vestibular
VI. Pathological
A. Fibrosis
B. Neuroma
C. Tumors
D. Wallerian Degeneration
E. Infections
F. Trauma
|
SPINAL NERVES
The segmental spinal nerve (Fig 1) is an integral component of each vertebral joint, consisting of the spinal zygapophyseal articulations (SZA) and the intervertebral discs (IVD), the pedicles, portions of the vertebral bodies and associated ligaments. It is reasonable to assume, therefore, that degeneration of the spinal articulations would have an effect on the associated spinal nerves. This is commonly recognized in the case of herniated discs which impinge on segmental nerves and roots [14–15] as well as spurs and osteophytes around the SZA and the joints of Luschka [16]. Nerve impingement due to hypertrophy of the SZA has also been well documented [16].
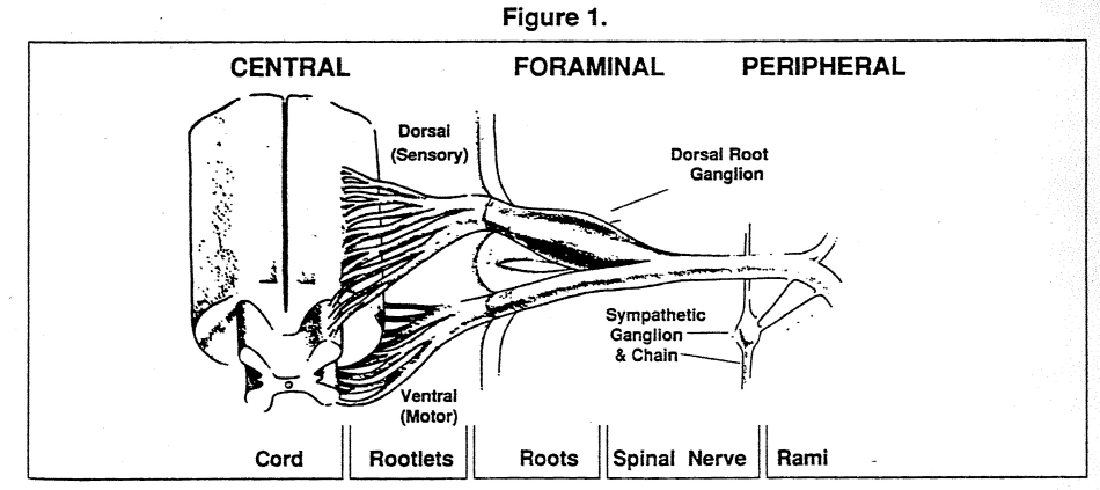
Chiropractic has unequivocally established itself as preeminent in caring for such cases [17–20]. Not all patients who benefit from chiropractic, however, suffer from herniated discs, nor, do all patients with herniated discs suffer with clinical symptoms [21–23]. While cases of herniated discs provide dramatic evidence of the effectiveness of chiropractic treatment programs [24], we must search for more general mechanisms to explain the positive results obtained by chiropractic care. Chiropractic has also shown its value in treatment of facet syndrome [25], cervical syndrome [26], whiplash syndrome [27], cervical trauma [28] and the chiropractic subluxation complex [29], referred to medically as joint dysfunction and osteopathological as "somatic dysfunction" or "segmental dysfunction". Chiropractic has shown its effectiveness in treating infantile colic [30] hyperactivity [31] and may even be effective in the management of sudden cardiac death syndrome or other cardiac related conditions [32] and headache [33].
DORSAL ROOT GANGLIA
The integral relationship between the spinal joints and the dorsal root ganglia (DRG) necessitates that we evaluate the role these structures play in the VSC. An extensive review of this information was provided by Lantz [34], and several other excellent reviews describe more basic aspects of the structure and function of the DRG [35–36]. With the exception of the first two spinal nerves, all DRG lie within the intervertebral canals (IVC), in intimate association with the articular capsule of the SZA. The DRG contain the cell bodies of all sensory neurons, except for those found in the cranial nerves. Their location between adjacent pairs of vertebrae, makes them key elements in the etiology of subluxations, and the focus of chiropractic adjustive procedures.
It is widely postulated in medical literature that compression of DRG as well as nerve roots and spinal nerves can be the source of pain and discomfort. Compression has been documented as resulting from osseous impingement in the lateral recesses of the lumbar and sacral vertebrae [37], osseous constriction of the vertebral canal [38], protrusion of the IVD [37, 39–40], protrusions of the posterior longitudinal ligament [41], fibrosis and sclerosis of the root sleeve [42], or cystic invasion of the DRG with connective tissue hyperplasia or the meninges [43]. Compression or nerve root inflammation (radiculitis) has been described as giving rise to a spectrum of clinical symptoms including otological manifestations [44], claudication [45], spastic paraplegia [46], respiratory problems simulating cardiac asthma [47], renal pain [48], chest pain simulating coronary occlusion [49] and sexual impotence [50]. While most of the literature addresses the issue of nerve and nerve root compression, it is believed that chronic irritation [51], as is thought to occur with intervertebral subluxation, or traction of the nerve roots or DRG by fibrous adhesions [52] can lead to similar pathological profiles.
The ganglia appear to have no blood-nerve barrier; they are richly vascularized [39], but the permeability of ganglionic capillaries is far greater than those of the CNS or of the peripheral nerve [53]. This greater permeability in the ganglionic capillary bed has been implicated as a route of infection by virus and bacteria alike [54–57] and as a site of chemical irritation and inflammation by blood-borne agents [57]. Any compression or arterial sclerosis which might compromise the arterial supply to, or venous drainage from, the ganglia is likely to promote irritability, as has been described for peripheral nerves [58]. Ischemia is known to lead to hyperexcitability of neural tissue, and would likely have a similar effect in the ganglia.
Invasion of DRG by arachnoidal proliferations has been shown by Tarlov [59] to be a source of the radicular pain of sciatica in a significant portion of patients suffering from this condition. Smith [60] did a comprehensive study of the occurrence of such cyst-like formations in the spines of 100 consecutive cadavers, without regard to clinical manifestations. In 9% of these cases there were grossly observable cysts. In all cases they were multiple and usually symmetrically distributed. On later evaluation of clinical records, none of the cases of cysts were associated with symptoms of nerve root compression. Tarlov [59] reported that about 17% of his cases of sciatica showed evidence of sacral cysts, but a casual relation was not established in these cases. Rexed [61] found 8 cases out of 13 (53%) had lumbar cysts, but did not address the issue of sacral cysts. The frequency of occurrence of the cysts increases with age.
DRG are far more sensitive to mechanical stimulation than peripheral nerves, and become even more so when inflamed. Howe et al. [62] demonstrate that "minimal acute compression" or chronic irritation of DRG or dorsal roots lead to periods of repetitive firing which last longer than the stimulus itself. Acute compression of peripheral nerves or nerves roots, on the other hand, does not lead to repetitive or prolonged firing. When inflamed, the ganglia become hyperexcitable, and will even give rise to spontaneous discharges [62]. Sharpless [63] reports that the roots are five time more sensitive to compressive forces than are the peripheral nerves. General consensus is that the roots and the peripheral nerves differ little in their responses to uniform radial compression [62], but this does not appear to be true for other types of irritation and stimulation, such as chemicals [64], eccentric compression [63], chronic irritation or mechanical stimulation [62].
When isolated from the periphery by transecting the ventral roots and the spinal nerve distal to the ganglia, the DRG give rise to spontaneous impulses [65]. It is obvious that afferent information can arise from the ganglion and this is almost certainly interpreted and integrated centrally as sensory input. Spontaneous impulses arising from the sensory neurons might play a physiological role in the maintenance of somatic or visceral tone. Aberrant impulses could lead to clinical signs and symptoms, the actual manifestations of which would depend upon the distribution of neurons within the ganglia, the site of existing lesions and the internal state of the tissue, e.g. normal, inflamed, fibrotic, and the projection of the involved neurons into the CNS.
NEUROTRANSMITTERS
It has long been known that nerves release chemicals, called neurotransmitters, at their synaptic termini and that these substances elicit immediate and dramatic effects in the organs supplied by these nerves. Some examples of neurotransmitters and the functions they mediate are acetylcholine, which stimulates muscle contraction, norepinephrine, which controls arterioles muscles and serotonia, which leads to uterine contraction [66].
The release of the same neurotransmitters in other tissues produce differing effects; acetylcholine in the heart suppresses the intrinsic rate of discharge of the heart's pacemaker, and noradrenalin in the lungs leads to bronchodilation following relaxation of the bronchial smooth muscle. Chemical transmitters are involved in the perception of pain as well as light and sound. A knowledge of the chemical nature of nervous function forms the basis of our understanding of the function of the nervous system and must be included in any comprehensive model of subluxations. It is also the foundation for the use of medicines in the treatment of nervous disorders. Many medicines work by mimicing or interfering with the function of these chemical messengers.
TROPHIC INFLUENCES
It is unclear, even today, whether trophic influences are due to the release of some chemical substances, to the rates of the electrical discharge of fibers, or to some other aspect of nerve function. [67,69]. Chemicals which perform such functions are called trophic substances, and Acetyl choline is often implicated as a primary mediator of trophic influences [70–71]. Trophic influences stimulate more subtle responses in tissue than do neurotransmitters. Such responses include altered growth rate, and change in the characteristics of the people [74–75].
It is suggested that proper vitality, morphology and function of the target tissues is dependent on an adequate degree of trophic stimulation [68]. In muscle, for example, exchanging nerves between "white" muscle and "red" muscle led to white muscle transforming to red and vice versa [75]. This line of research gave considerable impetus to the compression models of subluxation [76]. Trophic substances are felt to be synthesized in the cell body and transported to the synapse by axoplasmic transport [77]. Thus, by compressing the nerve and shutting off the flow of these vital supportive substances, one could explain how the tissues might suffer from degeneration for lack of chemical stimulation.
The problem to this concept, however, is that the amount of force required to cut off axoplasmic flow would lead to serious neurological deficit which would far overshadow any subtle trophic changes predicted by compression models of subluxation. It remains to be seen, however, whether chronic irritation might lead to excessive release of trophic substances that could let to tissue hypertrophy or even pathological degeneration. Certainly there is a role for trophic substances in the pathodynamics of subluxations, but exactly what this may be is, at this time, speculative .
A more likely explanation might be altered rate of synthesis and/or transport of chemical mediators due to inflammatory or irritative stresses on the tissues, such as the DRG or the ventral horns of the spinal cord. these machines will be discussed in the section covering the inflammatory responses.
VISCERO-SOMATIC RELATIONSHIPS
Viscero- somatic relationship (VSR) are much more widely recognized, and correspondingly more readily characterized, than either trophic influences or somato-visceral relationships.These are the well known patterns of referred pain [78]. For example, pain associated with heart attack is often felt in the left shoulder and radiating down the left arm. Kidney degeneration will refer pain to the low back while pancreatic degeneration refers pain to the right shoulder. It has been further demonstrated in the osteopathic profession that skilled examiners can palpate spinal soft tissue changes associated with ischemic heart disease,and even differentiate these changes from those associated with other abnormal heart conditions [78]. While the existence of such reflexes is unquestioned, the mechanism of such responses is completely unknown, and the clinical implications are widely disputed.
SOMATO-VISCERO RELATIONSHIPS
Somato-visceral relationships (SVR) are perhaps the key concept of chiropractic today. The central issue of SVR can be divided into two complementary aspects:
Can spinal or paraspinal neurological dysfunction lead to viscera degeneration in the organs supplied by the involved nerve? If so, under what conditions and which types of degenerative patterns are involved
Can chiropractic intervention prevent degeneration of visceral organs and reverse the degenerative process to restore vitality to degenerating tissues?
If so, for what conditions and how effectively? This is perhaps the more controversial issue in chiropractic theory. The evidence, however, tends to support such a concept.
Sato and Swenson [4] have shown that intervertebral movement can lead to sympathetic reflex responses which results in the discharge of adrenal and the renal sympathetic nerve fiber. The neuronal responses in the nerves to this organs was recorded after lateral flexion of a pair of vertebrae. The presumption is that the sensory component in this reflex arc is the proprioceptor population of the spinal ligaments. This follow from similar studies by Sato et al [79] on somato visceral reflexes involving the effect of stimulation of the articular nerve of the cat knee on cardiac sympathetic activity . The sympathetic response observed by Sato and Swenson was correlated in the rat with alterations in heart rate and blood pressure, but changes in the blood levels hormones known to be released from these organs was not investigated in their study. As these authors are quick to point out, the presence of those reflexes does not mean that abnormal stimulation would result in degenerative changes of the end organs: whether they do or not must be shown by experiment and cannot be left to conjecture.
Clinical studies tend to support these observations, however.In a randomized, controlled trial [7] it was shown that chiropractic adjustments were effective in reducing blood pressure in humans. In another human study [6] it was shown that chiropractic adjustment exert a definite influence on pupillary diameter, another visceral response. It has been shown in animals that sympathectomy of one side of the body leads to an increase in the development of tumors on the denervated side [80]. This suggest that interference with the sympathetic nervous system (SNS) can lead to a compromise of the body's immune system [81–82]. Conversely, an immunological response can alter the response pattern of the sympathetic nervous system. [83]
THE NEURODYSTROPHIC HYPOTHESIS
As presented by Leach [76] the neurodystrophic hypothesis proposes that neural dysfunction is stressful to the viscera and other body structures and leads to "lowered tissue resistance" which can modify the non-specific and specific immune responses and alter the trophic function of the involved nerves. This has often been evoked by chiropractors as a mechanism to explain the positive results obtained in patients suffering from conditions of a more general nature than musculoskeletal pain , such as COPD [84], bronchial asthma, dysmenorrhea and hypertension [85]. Current research provides growing evidence of the presence of a dynamic interaction between the nervous system and the immune system.
In particular, it has been demonstrated that there is an intimate relationship between the SNS and the white blood cells of the immune system. Histological studies have shown that mast cells are innervated directly by sympathetic nerve fibers, and that this relationship appears to serve a regulatory role in immunological response [86]. These observations are consistent with those showing a reduction of norepinephrine in lymphoid tissue following an immunological challenge. [83] The evidence supports the hypothesis that sympathetic innervation exerts and inhibitory effect on the immune system and that changes in tissue levels of norepinephrine can affect immunological responsiveness.
Most of the work relating to neuro-immunological relationships are focused on the sychoneuroendocrine axis [87–88]. It is without question that interference with pituitary and other endocrine function can lead to a compromised immunological response. However, this work does not address the idea that subluxations would exert such an effect. This issue has been addressed in several investigative studies, both directly and indirectly. Chiropractic researchers have shown that spinal adjustments can lead to an increase in immunological response of the patient [89]. One must also consider the work of Sato and Swenson [4] in this regard. Certainly the adrenal gland is involved in the stress response, and the finding that specific spinal intersegmental movement leads to reflex sympathetic input into the adrenal gland and the kidney suggests a fundamental mechanism for chiropractic adjustment in modulating the stress response. We would support the conclusion reached by Leach [76] that there is overwhelming evidence to support the chiropractic neurodystrophic hypothesis. Although a causal connection has not been established, the evidence cited above suggests a plausible link between vertebral lesions (VSC) and immunological competence.
PAIN
The most common clinical characteristic of patients entering chiropractic offices is pain [8]. Due to the largely subjective nature of pain, its evaluation by clinical methods and objective measures is a challenge to clinicians of all professions [90–91]. For the whether or not there are objective clinical findings, and, as in the case of phantom pain[92], regardless of whether or not the body part is present.
There have been numerous theories proposed in the medical profession which attempt to explain pain. [93] One of the more widely discussed of these is the Gate Theory of Pain proposed by Melzak and Wall [94]. In this theory, specific internuncial neurons of the spinal cord control the perception of pain. These interneurons receive input from a large number of sensory (afferent) sources. If input signals are numerous, then the interneuron becomes unresponsive, thereby shutting the gate of pain sensation. According to Melzak, the transmission of pain sensation through the gate is dependent upon the relative input of large (A-beta) and small (A-delta and C) fibers converging on the gate [93]. This is one of the major mechanisms evoked in modern theories of manipulative therapies [9, 95–96]. These and other ideas of pain and reflex mechanisms have been discussed in relation to chiropractic theory. [3, 11, 12, 97] Still, much research is needed in order to have a more complete understanding of the mechanism of effectiveness of chiropractic adjustments. Pain is known to be a significant aspect of cervical spinal degeneration [56] as well as in the lumbar spine and pelvis [91].
The mechanism for such pain is almost certainly related to mechanical or chemical irritation of the spinal nerves or their roots [56], or specific articular nerves [22,91]. Given the success of chiropractic procedures in reducing such pain, one must explore the role which spinal biomechanical factors play in normal neurological function.
ARTICULAR NEUROLOGY
The field of articular neurology is germane to the theory and practice of chiropractic. Wyke [98] has classified the spinal joint receptors into four groups, Types I-IV; three types of mechanoreceptors and the nociceptor (pain) receptor system. The role that each plays in degenerative processes, and particularly in pain [91], is the subject of intensive reserve. Gillet [10] has proposed that co-activation of the articular receptor system and other somatic receptors constitute a major component of the chiropractic adjustment. It is further known that the SZA are involved in the mechanism of referred pain (somato-somatic reflex), but the neurological mechanisms are not well understood [21–22]. Given the significance of the spinal articulations in chiropractic theory, we cannot minimized the importance of articular neurology in understanding the effectiveness of chiropractic procedures.
The afferent discharges derived from articular mechanoreceptors have a three-fold impact when they center the neuroaxis:1) Reflexogenic effects: mobilization or manipulation at one level may have an impact on areas remote from the side of motion;
2) Perceptual effects: influence on postural and kinesthetic senses;
3) Pain Suppression: modulation of the pain gate trough changes in mechanoreceptors located in the joint capsules can result in abnormalities of posture and movement (including gait), impairment of postural and kinesthetic sensation [100] and an increase in pain perception [91].There is a significant correlation between proprioceptive input from the cervical spine and coordination of the extremities [100]. Experimental studies on the knee joint have demonstrated the discharge of afferent fibers following passive movements of the leg [101]. The impulses were particularly prominent when the knee was subjected to noxious movements, such as twisting. It was proposed that this constituted a warning signal which stimulate motor reflex patterns designed to prevent joint damage. Studies performed on cats with inflamed knee joints [102] showed that joint inflammation sensitizes articular nociceptors to fire at rest during normally non-noxious joint movements. The proportion of neurons displaying resting discharges was higher, the frequency of discharges was higher and the receptive field were larger in the inflamed joints than in normal controls.
Studies in humans [103] showed that distension of the joint capsule of the knee by gradual infusion of plasma into the joint led to reflex weakening of the quadriceps muscles. Injection of saline into the lumbar facets resulted in pain and significant increases in the myoelectric activity of the quadriceps [21]. These responses were abolished by injection of local anesthetic. Traction or passive movement of the posterior elements of the vertebrae or of the limbs with the concomitant stimulation of the mechanoreceptors of the joint capsule, can inhibit nociceptor activity or central integration and can thereby significantly reduce the perception of pain by means of the gating mechanism of presynaptic inhibition [91]. These procedures are known to reduce the patient's need for analgesic drugs, thereby avoiding their undesirable and unpleasant side effects. While these studies demonstrate unequivocally the involvement of joint receptors in the generation of clinical symptoms, much more information is needed to integrate these processes in a comprehensive theory of chiropractic.
MYOPATHOLOGY
As with all of the components, there is considerable interaction between this and other components in the model. It is often difficult to distinguish aberrations in muscle function from neuropathology, and muscle degeneration will almost invariably involve alterations in tendon function. However, special situations arise clinically or can be created experimentally which allow us to differentiate the contribution of muscle to joint pathology as distinguished from other components, such as neurological factors [104–107]. Tendons will be considered here in conjunction with muscle function, but will be discussed elsewhere in this series in the context of connective tissue. Similarly, the connective tissue stroma must be considered as an integral component of muscle, contributing to both structure and function [108], but the treatment of connective tissue elements elsewhere [109] applies with equal strength to the muscle stroma.
DISUSE ATROPHY
It is widely known that joint immobilization leads to muscle atrophy [105,110–111], often referred to as "disuse atrophy" [112]. But the changes that follow immobilization, or joint fixation, differ significantly from those of other disuse models [113–114]. The details of this process have been extensively studied [115], but the precise role that changes in muscle structure and function play in joint degeneration is not well understood. In some cases muscle changes are secondary to immobilization [116], or as a reflex response to painful joints [117], but in turn contribute to joint degeneration [118].
In other instances muscle degeneration or pathology can be primary and might also contribute to joint degeneration. Among these are trauma to muscle, congenital anomalies or diseases which affect muscles, such as polio and muscular dystrophy. In some situations, it is not possible to discern the role of muscle in joint, especially spinal joint, pathology. In particular, scoliosis poses an enigma. While muscles tend to differ on the concave versus convex sides of the scoliotic curve [119–123], their contribution to the development of the curve is not understood in the vast majority of cases of scoliosis [124]. Muscular changes in idiopathic scoliosis are considered to be secondary, not causal to the spinal deformity [119]. Therapy with TENS units uses stimulation of muscle activity on the convex side of the curve in an attempt to draw the spine back to a more erect posture [125]. Current trends in scoliosis theory lean toward the idea that there is a loss of unilateral regional control of muscle tone or loss of coordination of the righting (postural) response in the spinal musculature [126–127]. Needless to say, any complete theory of vertebral subluxations must include the spinal musculature as an integral component. Virtually every major aspect of muscle structure and function has been evaluated in the context of degenerative changes following immobilization, and a partial list with references is given in Table III.
In studies on immobilization of the knee it has been shown that in the early stages of joint degeneration, restricted joint mobility was due almost exclusively to the muscle/tendon component [128]. Cutting the muscle away restored movement to normal ranges. This was not true of later stages in joint degeneration where joint mobility appears to be restricted due to capsular and ligamentous stricture [129–130] followed by intra-articular adhesions [131] and ending in bony ankylosis. While the changes in muscle function are often completely reversible [114, 116, 132], the time required for complete restoration of muscle function depends upon the duration of immobilization [114, 116]. These findings are complicated by the different responses to immobilization by different muscle types [112–114, 133–136], as well as by differences in degenerative response related to the position of the joint, and thereby the length of the muscle in the immobilized state [108, 113, 116, 134].
TABLE III Alterations of Muscle Structure and Function following Immobilization Aspect References Gross Structure Weight [112, 113, 134, 165, 166] Length [134] Volume [167] Cross-section [134, 167] Morphology Sacromere number [116, 160] Sacromere length [116] Fiber number [164, 168] Fiber type [164] Fiber cross-section [134, 164, 167] Metabolism Glycolysis/Krebs [136, 166] Glycogen [132] Cytochrome activity [166] NAD-diaphorase activity [136] Protein concentration [132] Myoglobin content [136, 166] Myosin ATPase [136] Protein metabolism [134] Daily urinary loss [165] Calcium balance [165] Protein content [132, 166] Lysosome function [169] Aspect References Connective tissue Collagen content [133] Collagen cross-linkages [133] Tendon length [108] Collagen organization [159] Biomechanical properties Ultimate tensile strength [133] Tangent Modulus [133] Maximum strain [133] Extensibility [112, 160] Contractile Properties Maximal shortening velocity [114, 136] Peak tetanic tension [111, 114, 136] Peak twitch tension [112] Active twitch tension [112, 136] Maximal isometric tension [132] Isometric twitch duration [114] Contraction time [114] Half-relaxation time [114] Maximal dynamic strength [170] Isokinetic strength [170] Isometric strength [170] Isometric endurance [170] Dynamic endurance [170] Passive length-tension curves [112, 116, 160] Aspect References Neurology/Bioelectrical Spindle activity [137-141, 143] Electromyograms [113, 136] Sensitivity [171] Motor end plates [135] Neuromuscular transmission [135]MUSCLE SPINDLES
One extremely important aspect of muscle pathophysiology in regard to the VSC is the effect of immobilization on the structural integrity and the response characteristics of the muscle spindles. It has been shown that the spindles exhibit significant morphological changes following neurogenic atrophy. Tower [137] noted different changes in the spindles upon the ventral rhizotomy compared to dorsal root ganglionectomy. Although changes in the spindles following myogenic atrophy are less marked [138], some alterations do occur, particularly a shortening and thickening of the spindle. When a joint is immobilized, the spindles of the associated muscles show histological signs of degeneration within one week [139]; degeneration of the primary spindle endings, swollen capsules and loss of cross striations.
Following immobilization there is also a change in the physiological response pattern of the spindle afferents showing an increased sensitivity to stretch and an elevation in the resting rate of discharge when the muscle is under no tension[140]. This is in contrast to studies showing a decrease in resting spindle activity in tendotomized muscle preparations [141]. When the severed and control muscles were compared under similar tensions, i.e. severance of the control tendon before recording, the activity of the spindles in the tendotomized muscle was also greater than in the control.
One consequence of such an increase in spindle activity in immobilized muscles would be to feed excessive stimuli into the central reflex pathways resulting in altered efferent (output) response. This could lead to the overstimulation of muscle groups which respond to the stretch reflex, leading in the end state to muscle spasm and tender trigger points. This would constitute a positive feed-back loop (i.e. a vicious cycle) which, if unchecked or uncontrolled, could lead to degeneration.
IMMOBILIZATION DEGENERATION
In other studies in joint immobilization, it was postulated that muscle tension might lead to excessive degeneration of cartilage causing compression of the joint surfaces together [118], thereby contributing to the development of osteoarthritis. On the other hand, it has been noted [142] that thickening of the joint capsule is a regular feature of osteoarthritis. A vicious cycle has been described [143] in which muscle spasticity leads to joint contracture, which in turn leads to more spasticity and muscular contracture; the specified treatment for this condition being to return the joints to their full range of motion and maintain that range through the healing stages. Such a degenerative cycle has been described in spinal cord injuries which are accompanied by a cycle of spasticity, joint contracture and muscle contracture [143]. Alternatively, such abnormal input could lead to reflex inhibition of synergistic or antagonistic muscles of failure of joint musculature upon challenge of the joint [144].
It is known that when a joint is immobilized, the effect on muscles depends upon their length in the immobilized state [145–146] or the angle at which a joint is fixed [105]. Muscles immobilized in the shortened position show a reduction in tension producing capacity, while those which are chronically stretched retain their ability to generate force in direct proportion to changes in cross-sectional area. Changes in muscle dependent upon the length of the muscle during immobilization have been reported for gross morphologic appearance [147] as well as biochemical [148–150] and ultrastructural [151] characteristics. Thus, as with connective tissue discussed below, changes reflect not only the duration and extent of immobilization, but the position of the joint when immobilized.
In immobilization studies on cats [111] it was shown that there is a continually decreasing electrical output from the anterior tibial muscles during immobilization period. The effect was ascribed to both a decrease in voltage of single motor unit potentials and a decrease in the number of motor units responding. In this study it was shown that there were no fibrillation potentials at any time in the course of the study, and evoked potentials had a normal wave form. These observations suggest that the decrease in muscle potential was not due to alterations in the motor nerve or end plate. These authors concluded that the decrease in response potential of the muscle was due to loss of muscle mass with a concomitant reduction of muscle fiber membrane are a and associated ions.
In other studies, it has been shown that there is a reflex stimulation of myoelectric activity of the hamstring muscles when L4–L5 and L5–S1 facet joints were injected with hypertonic saline [143]. This effect was abolished by injection of the facets with xylocaine. In symptomatic patients, depressed deep tendon reflexes could be restored to normal following injection into the spinal articular capsule of local anesthetic. These responses are characteristic of the facet syndrome [143] and are believed to be due to reflex inhibition of the anterior horn cells by noxious stimuli arising from the facet joints. Similar reflex activity of the intrinsic spinal muscles is believed to occur in patients with nerve root involvement, leading to splinting of the involved painful joints in an attempt to reduce their motion. Elsewhere [117], it has been shown that reflex muscle atrophy is a sequel to induced trauma and chronic pain.
In conjunction with the immobilization/degeneration model of vertebral subluxation [109], it has been shown [151] that alteration of the distractive forces applied to the achilles tendon induces extensive cellular and extracellular changes in the musclotendinous junction. Other studies on the effect of mechanical stress on connective tissue morphology have used tendons and support previous observations [152]; the distribution of cell types and architecture of the extracellular matrix depend to a large degree on the type of force applied to the tissue (compressive, distractive, torsional). [153–157] It was noted that adhesions formed in immobilized limbs between the tendon and its sheath, and similar observations have been made in tendotomized animals as a result of surgical procedures [141]. It has also been shown [158] that early mobilization of previously immobilized limbs increased the rate of healing in lacerated flexor tendons. Not only are tendons affected by immobilization, but the connective tissue scaffolding (stroma) of the muscle substance undergoes changes following immobilization as well [108, 133, 159]. These changes have been associated with a decrease in muscle extensibility [108]. On the other hand, decreased extensibility has also been correlated with a reduction in the number of sarcomeres [160]. In neither case, however, was a causal relationship established.
In contrast to the effects of immobilization on muscle structure and function, denervation leads to significantly greater degeneration of both intra- and extrafusal fibers [161]. This effect is strongly dependent upon the muscle fiber type, with Type IIB being more strongly affected than Type IIA fibers. Passive activity appears to retard the atrophy of the Type II fibers but has little affect on Type I [162]. It thus appears that trophic influence of the nerves exerts a substantial control over the response characteristics of Type IIB muscle fibers, but not Type I fibers. Since the trophic influence of nerves is known to affect muscle morphology and function [75], this aspect of spinal integration must be incorporated into the VSC model.
The spinal musculature has been partially characterized with regard to fiber type [163, 119–123], and have been shown to contain about 75% Type I fibers on the right side and about 67% Type I on the left side [119] of the spine. This is consistent with these muscles serving a postural role, but with movement as an aspect of these muscle's function as well[163]. It has been shown that there is a decrease in Type I fibers on the concave side of the scoliotic curve, consistent with the idea that this side experiences less mobility [164].
It is clear, from the above discussion, that muscle degeneration and alterations of muscle function are integrally related to joint degeneration following immobilization. In the immobilization degeneration model of subluxations, it will be important to further characterize and understand the role of spinal musculature in posture and movement, and the intricate interrelationships between the spinal musculature and the articular system of the spine.
REFERENCES:
1. Palmer DD. The science, art and philosophy of chiropractic. Portland: Portland printing House, 1910.
2. Janse J. Principles and practice of chiropractic. Hildebrandt RW, ed. Lombard IL: National College of Chiropractic, 1976.
3. Korr IM. The collected papers of IM Korr. Colorado Springs, CO: Am Acad Osteopathy, 1979.
4. Sato A, Swenson R. Sympathetic nervous system response to mechanical stress of the spinal column in rats. J Manipulative Physiol Ther 1984; 7(3): 141-147.
5. Vernon HT, Dhami MS, Howley TP, Amnett R. Spinal manipulation and beta-endorphin: A controlled study of the effect of spinal manipulation on plasma beta-endorphin levels in normal males. J Manipulative Physiol Ther 1986; 9(2):115-123.
6. Briggs L., Boone W. Effects of a chiropractic adjustment on changes in pupillary diameter: A model for evaluating somatovisceral response. J Manip Phys Ther 1988; 11(3):181-189.
7. Yates G, Lamping D, Abram N, Wright C. Effects of chiropractic treatment on blood pressure and anxiety: A randomized, controlled trial. J Manip Physiol Ther 1988; 11(106):484-488.
8. Phillips R, Butler R. Survey of chiropractic in Dade County, Florida. J Manip Phys Ther 1982; 5(2):83-89.
9. Mazion, J.M. Illustrated manual of neurological reflexes/signs/tests: Orthopedic signs/tests/maneuvers for office procedure. Arizona City, AZ: J.M. Mazion, Pub., 1980.
10. Gillete R. A speculative argument for the coactivation of diversive somatic receptor populations by forceful chiropractic adjustments. Manual Med 1987;3:1-14.
11. Dishman R. Review of the literature supporting a scientific basis for the chiropractic subluxation complex. J Manipul Physiol Therap 1985; 8:163-174.
12. Slosberg M. Effects of altered afferent articular input on sensation, proprioception, muscle tone and symapthetic reflex responses. J Manip Phys Ther 1988; 11(5):400-408.
13. Gunn C., Milbrandt W. Early and subtle signs in low-back sprain. Spine 1978; 3:267-281.
14. Tay E., Chacha P. Midline prolapse of a lumbar intervertebral disc with compression of the cauda equina. J Bone Joint Surg 1979; 61B:43-46.
15. Lindblom K, Rexed B. Spinal nerve injury in dorso-lateral protrusions of lumbar discs. J Neurosurg 1948; 5:413-432.
16. Lyon E. Uncovertebral osteophytes and osteochondrosis of the cervical spine. J Bone Joiny Surg 1945; XXVII(2):248-253.
17. Bronfort G. Chiropractic treatment of low back pain: A Prospective Survey. J Manip Phys Ther 1986; 9(2):99-113.
18. Mierau D, Cassidy J, McGregor M, Kirkaldy-Willis W. A comparison of the effectiveness of spinal manipulative therapy for low back pain patients with and without spondylolisthesis. J Manip Phys Ther 1987; 10(2):49-55.
19. Ingis B, Raser B, Penfold B. Chiropractic in New Zealand Report of the commision of inquiry. Wellington, New Zealand: Government Printer, 1979.
20. Johnson MR, Schultz MK, Ferguson AC. A comparison of chiropractic, medical and osteopathic care for work-related sprains and strains. J Manipulative Physiol Ther 1989;12:335-344.
21. Mooney V, Robertson J. The facet syndrome. Clin Ortho 1976; 115:149-156.
22. Shealy C. Facet denervation in the management of back and sciatic pain. Clin Ortho 1976;115:157-164.
23. DeVilliers P, Booysen E. Fibrous spinal stenosis. Clin Ortho 1976; 115:140-144.
24. Cox J. Low back pain: Mechanism, diagnosis and treatment, 4th ed. Baltimore, MD: Williams & Wilkins, 1985.
25. Hourigan CL, Bassett JM. Facet syndrome: Clinical signs, symptoms, diagnosis and treatment. J Manipulative Physiol Ther 1989; 12:293-297.
26. Lenhart JL. Post-traumatic cervical syndrome. J Manipulative Physiol Ther 1988; 11:409-414.
27. Foreman SM, Croft AC. Whiplash injuries: The cervical acceleration/deceleration syndrome. Baltimore:Williams & Wilkins, 1980.
28. Fitz-Ritson D. The chiropractic management and rehabilitations of cervical trauma. J Manipulative Physiol Ther 1990; 12: 17-25.
29. Jarmel ME. Possible role of spinal dysfunction in the genesis of sudden cardiac death. J Manipulative Physiol Ther 1989; 12:469-477.
30. Klougart N, Nilsson N, Jacobsen J. Infantile colic treated chiropractors: A prospective study of 316 cases. J Manipulative Physiol Ther 1989; 12:281-291.
31. Giesen JM, Center DB, Leach RA. An evaluation of chiropractic manipulation as a treatment of hyperactivity in chil- dren. J Manipulative Physiol Ther 1989; 12:353-363.
32. Mennell J. MCM. The validation of the diagnosis "joint dysfunction" in the synovial joints of the cervical spine. J Manip Physiol Therap 1990; 13:7-12.
33. Vernon HT. Spinal manipulation and headaches of cervical origin. J Manipulative Physiol Ther 1989; 12:455-468.
34. Lantz C. The role of dorsal root ganglia in the development of chiropractic subluxations. A chiropractic theory. Proceedings 3rd Annual Current Topics in Chiropractic. 1986; C2 (1)-C2(12).
35. Svaetichin G. Electrophysiological investigations on single ganglion cells. Acta Phys Scand 1951; 24(1085):5-57.
36. Lieberman A. Sensory ganglia. In: The peripheral nerve. Landon D, ed. New York:John Daily and Sons Inc., 1976:188- 278.
37. Epstein JA, Epstein BS, Rosenthal AD, Carras R, Lavine LS. Sciata caused by nerve root entrapment in the lateral recess: The superior facet syndrome. J Neurosurg 1972; 36: 584-589.
38. Epstein J, Epstein B.S., & Lavine L. Nerve root compression associated with narrowing of the lumbar spinal canal. J Neurol Neurosurg Psychiat 1962; 25:165-176.
39. Bergman L., Alexander L. Vascular supply of the spinal ganglia. Arch Neur Psyc 1941; 46:761-782.
40. O'Connel JAE. Protrusions of the lumbar intervertebral discs. A clinical review based on five hundred cases treated by excision of the protrusion. J Bone Joint Surg 1951; 33B: 8-30.
41. Beatty RA, Lugr O, Fix TA. Protrusion of the posterior longitudinal ligament simulating herniated lumbar intervertebral disc. J Neurol Neurosurg Psychiat 1968;31:61-66.
42. Frykholm R. Cervical nerve root compression resulting from disc degeneration and root-sleeve fibrosis. Acta Chiru Scand 1951; suppl 160:1-149.
43. Rexed BA, Wennstrom KG. Arachnoidal proliferation and cystic formation in the spinal nerve-root pouches of man. J Neurosurg 1959; 16:73-84.
44. Chrisman OD, Gervais EF. Otological manifestations of the cervical syndrome. Clin Orthop 1962; 24:34-39.
45. Morris WT. Spinal nerve compression: A cause of claudication. New Zeal Med J 1978; 88:101-103.
46. Heilbrun MP, Davis DO. Spastic paraplegia secondary to cord constriction by the dura. J Neurosurg 1973; 39:645-647.
47. Davis D. Respiratory manifestations of the dorsal spine radiculitis simulating cardiac asthma. Ann Int Med 1950; 32: 954-959.
48. Smith DR, & Raney FL, Jr. Radiculitis distress as a mimic of renal pain. J Urol 1976; 116:269-276.
49. Davis D. Spinal nerve root pain (radiculitis) simulating coronary occlusion: A common syndrome. Am Heart J 1948; 35: 70-80.
50. LaBan MM, Burk RD, Johnson EW. Sexual impotence in men having low-back syndrome. Arch Phys Med Rehab 1966;:715-723.
51. Badalamente M, Ghillani R, Chien P, Daniels K. Mechanical stimulation of dorsal root ganglia induces increased production of substance P: A mechanism for pain following nerve root compromise? Spine 1987; 12(6):552-555.
52. Spencer DL, Irwin GS, Miller JA. Anatomy and significance of fixation of the lumbosacral nerve roots in sciatica. Spine 1983;8:672-679.
53. Brierley JB. The sensory ganglia: Recent anatomical, physiological and pathological contributions. Acta Psych Neur Scand 1955; 30:553-576.
54. Clements GB, Subak-Sharpe JH. Recovery of Herpes Simplex Virus 1 TS mutants from the dorsal root ganglia of mice. Brain Research 1983; 59:203-207.
55. Sato A, Schiable HG, Schmidt RF. Types of afferents from the knee joint evoking sympathetic reflexes in cat interior cardiac nerves. Neurosci Let 1983; 39:71-75.
56. Wakesman BH. Experimental study of diptheric polyneuritis in the rabbit and guinea-pig. III. The blood nerve barrier in the rabbit. J Neuropain Exp Neurol 1961; 20:35-77.
57. Chang LW, Yip RK. Corn oil-induced changes in dorsal root ganglia of rats. Envir Res 1983; 30:50-58.
58.Porter EL, Wharton PS. Irritability of mammalian nerve following ischemia. J Neurophys 1948; 12:109-116.
59. Tarlov IM. Cysts (perineurial) of the sacral roots. J Amer Med Assoc 1948; 138:740-744.
60. Smith D. Cyst formations associated with human spinal nerve roots. J Neurosurg 1961; 18:6545-660.
61. Rexed B. Arachnoidal proliferations with cystic formation in human spinal roots at their entry into the intervertebral foramen. J Neurosurg 1947; 4:414-421.
62. Howe JF, Loeser JD, Calvin WH. Mechanosensitivity of dorsal root ganglia and chronically injuried axons:A phsyiological basis for the radicular pain of nerve root compression. Pain 1977; 3:25-41.
63. Sharpless S. Compression on spinal roots. In: Goldstein M. 10ed. The research status of spinal manipulative therapy. Bethesda, MD: NINCDS #15, 1975.
64. Marshall LL, Trethewie ER, Curtain CC. Chemical radiculitis. A clinical, physiological and immunological study. Clin Ortho Rel Res 1977; 129:61-67.
65. Kirk EJ. Impulses in dorsal spine nerve rootlets in cats and rabbits arising from dorsal root ganglia isolation from the periphery. J Comp Neur 1975; 115:165-175.
66. Goodman LS, Gilman A. The pharmacological basis of therapeutics. 4th Ed. London: The MacMilan Co., Pub., 1970.
67. Salmons S, Sreter FA. Significance of impulse activity in the transformation of skeletal muscle type. Nature 1976; 263:30-34.
68. Drachman DB, ed. Trophic functions of the neuron Ann NY Acad Sci 1974; 228.
69. Davis HL. Trophic action of nerve extract on denervated skeletal muscle IN VIVO: Dose dependency, species specificity, and timing of treatment. Exp Neurol 1983; 80:383-394.
70. Drachman DB. Is acetylcholine the trophic neuromuscular transmitter? Arch Neurol 1967; 17:206-218.
71. Guth L. "Trophic" influences of nerve on muscle. Physiol Rev 1968; 48:645-687.
72. Davis HL, Heinicke EA. Prevention of denervation atrophy in muscle: Mammalian neurotrophic factor is not transferrin. Brain Research 1984; 309:293-298.
73. Davis HL, Kiernan JA. Neurotrophic effects of sciatic nerve extract on denervated extensor digitorum longus muscle in the rat. Exp Neurol 1980; 69:124-134.
74. Close R. Physiology. The effects of cross-union on motor nerves to fast and slow skeletal muscles. J Physiol 1964; 173:831-832.
75. Close R. Dynamic properties pf fast and slow skeletal muscles of the rat after nerve cross-union. J Physiol 1969; 204: 331-346.
76. Leach RA. The chiropractic theories. A synopsis of scientific research. 2nd ed. Baltimore, MD: Williams & Wilkins, 1980.
77. Fernandez H. Inestrosa N. Role of axoplasmic transport in neurotrophic regulation of muscle end plate acetylcholinesterase. Nature 1976; 262:55-56.
78. Nicholas A, DeBias D, Ehrenfeuchter W, et al. Somatic component to myocardial infarction. J AOA 1987; 87(2):123-129.
79. Sato A, Schiable HG, Schmidt RF. Types of afferents from the knee joint evoking sympathetic reflexes in cat inferior cardiac nerves. Neurosci Let 1983; 39:671-75.(80)
80. Coujard R, Heitz F. Cancerologic: Production de tumeurs malignes consecutives a des lesions des fibres sympathiques du nerf sciatique chez le Cobaye. C R Acad Sci 1957; 244: 409-411.
81. Besedovsky H, DelRey A, Sorkin E, DaPrada M, Keller H. Immunoregulation mediated by the sympathetic nervous system. Cellular Immun 1979; 48:346-355.
82. Stein-Werblowsky R. The sympathetic nervous system and cancer. Exper Neuro 1974; 42:97-100.
83. Delrey A, Besedovsky H, Sorkin E, DaPrada M, Arrenbrecht S. Immunoregulation mediated by the sympathetic nervous system, II. Mol Immunol 1981; 63:329-334.
84. Deal MC, Morlock JW. Somatic dysfunction associated with pulmonary disease. J Am Osteo Assoc 1984; 84:179-183.
85. Wiles M, Diakow P. Chiropractic and visceral disease: A brief survey. J CCA 1982; 26(2):65-68.
86. Bienenstock J, Tomioka M., Matsuda H, et al. The role of mastcells in inflammatory processes: Evidence for nerve/mast cell interactions. Int Archs Allergy appl Immun 1987; 82: 238-243.
87.Jankovic B, Markovic B, Spector N. Neuroimmune interactions. Proceedings of the Second International Workshop on Neuroimmunomodulations. Annals NY Acad Sci 1987; 496:1-3.
88. Ader R. Psychneuroimmunology. Academic Press(1981) New York.
89. Vora GS, Bates HA. The effects of spinal manipulation on the immune system (a preliminary report). ACA J Chiro. 1980; 14:S103-S105.(90)
90. Mooney V. Where is the pain coming from? Spine 1987; 12: 754-759.
91. Wyke B. The neurology of low back pain. In: The lumbar spine and back pain. 2nd ed. Jayson M, ed. Pitman Med. Publ. Co. Ltd. 1976, 1980:265-339.
92. Howe J. Phantom limb pain: A reafferentation syndrome. Pain 1983; 15:101-107.
93. Melzak R. The puzzle of pain. New York: Basic Books, Inc., 1973.
94. Melzak R, Wall PD. Pain mechanisms: A new theory. Science 1965; 150:971.
95. Zusman MA. Theoritical basis for the short-term relief of some types of spinal pain with manipulative therapy. Manual Medicene 1987; 3:54-56.
96. Kempson GE, Muir H, Swanson SAV, Freeman MAR. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochem Biophys Acta 1970; 215: 70-77.
97. Irving R. Pain and the protective reflex generators:Relevance to the chiropractic concept of spinal subluxation. J Manip Phys Ther 1981; 4(2):69-71.
98. Wyke B. The neurology of joints:A review of general principles. Clin Rheum Dis 1981; 7(1):223-239.
99. Lippitt A. The facet joint and its role in spine pain:Management with facet joint injection. Spine 1984; 9(7):746-750.
100. deJong PTVM, deJong JMB, Cohen B, Jogkees LBW. Ataxia and nystagmus induced by injection of local anesthetics in the neck. Ann Neurol 1977; 1:240-246.
101. Schiable H, Schmidt R. Responses of fine medial articular nerve afferents to passive movements of knee joint. J Neurophys 1983; 49(5):1118-1126.
102. DeAndrade J, Grant C, Dixon A. Joint distension and reflex muscle inhibition in the knee. J Bone Joint Surg 1965; 47 (A/2):313-322.
103. Coggeshall R, Hong K, Langford L, Schaible H, Schmidt R. Discharge characteristics of fine medial articular afferents at rest and during passive movements of inflamed knee joints. Brain Research 1983; 272:185-188.
104. Moritani T, DeVries H. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 1979; 58(3):115-130.
105. Tomanek RJ, Lund DD. Degeneration of different types of skeletal muscle fibers II. Immobilization. J Anat 1974;118: 531-541.
106. Tomanek RJ, Lund DD. Degeneration of different types of skeletal muscle fibers I. Denervation. J Anat 1973; 3:395- 407.
107. McLachlan E. Atrophic effects of proximal tendon transection with and without denervation on mouse soleus muscles. Exper Neuro 1983; 81:651-668.
108. Tardieu C, Tabary JC, Tabar C, Tardieu G. Adaptation of connective tissue length to immobilization in the lengthened and shortened positions in cat soleus muscle. J Physiol Paris 1982; 78:214-220.
109. Lantz CA.
Immobilization Degeneration and the Fixation Hypothesis of Chiropractic Subluxation
Chiro Res J 1988; 1 (1) Spring: 21–46
110. Fuglsang-Frederiksen A, Scheel U. Transient decrease in number of motor units after immobilization in man. J Neuro 1978; 41:924-929.
111. Fudema J, Fizzell J, Nelson E. Electromyography of experimentally immobilized skeletal muscles in cats. Am J Physiol 1961; 200:963-967.
112. Witzmann FA, Kim DH, Fitts RH. Hindlimb immobilization: Length-tension and contractile properties of skeletal muscle. J Appl Physiol: Respirat Environ Exercise Physiol 1982; 53(2):335-345.
113. Fournier M, Roy RR, Perham H, Simard CP, Edgerton VR. Is limb immobilization a model of muscle disuse? Experimental Neurology, 1983; 80:147-156.
114. Witzmann FA, Kim DH, Fitts RH. Recovery time course in contractile function of fast and slow skeletal muscle after hindlimb immobilization. J Appl Physiol:Respirat Environ Exercise Physiol 1982; 52(3):677-682.
115. St. Pierre D, Gardiner PF. The effect of immobilization and exercise on muscle function: A review. Physiotherapy Canada 1987; 39:24-36.
116. Tabray JC, Tabray C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in cat soleus muscle due to immobilization at different lengths by plaster casts. J Physiol, 1972; 224:231-244.
117. Hnik P. Holas M, Payne R. Reflex muscle atrophy induced by chronic peripheral nociceptive stimulation. J Physiol Paris 1977; 73:241-250.
118. Thaxter TH, Mann RA, Anderson CE. Degeneration of immobilized knee joints in rats. J Bone Joint Surg 1965; 47A:568.
119. Bylund P, Jansson E, Dahlberg E, Eriksson E. Muscle fiber types in thoracic erector spinae muscle; fiber types in idiopathic and other forms of scoliosis. Clin Ortho Rel Res 1987; 214:222-228.
120. Yarom R, Robin G. Studies on spinal and peripheral muscles from patients with scoliosis. Spine 1979; 4(1):12-21.
121. Zetterberg C, Aniansson A, Grimby G. Morphology of the paravertebral muscles in adolescent idiopathic scoliosis. Spine 1983; 8(5):457-462.
122. Ford DM, Bagnall KM, McFadden KD, Greenhill BJ, Raso VJ. Paraspinal muscle imbalance in adolescent idiopathic scoliosis. Spine 1984; 9(4):373-376.
123. Hosla K, Tredwell SJ, Day B, Shinn SL, Ovalle WK. An ultrastructural study of multifidus muscle in progressive idio- pathic scoliosis. J Neuro Sci 1980; 46:13-31.
124. Cassidy JD, Brandell BR, Nykolation JW, Wedge J. The role of paraspinal muscles in the pathogenesis of idiopathic scoliosis: A preliminary EMG study. J Canad Chiro Assoc 1987; 31:179-184.
125. Axelgaard J, Anders N, Brown JC. Correction of spinal curvatures by transcutaneous electrical muscle stimulation. Spine 1983; 8(5):463-481.
126. Herman R, Mixon J, Fisher A, Maulucci T, Stuyk J. Idiopathic scoliosis and central nervous system: A motor control problem. Spine 1985; 10:1-14.
127. Shalstrand T, Ortengren R, Nachemson A. Postural equilibrium in adolescent idiopathic scoliosis. Acta Orthop Scand 1978; 49:354.
128. Evans DB, Eggers GWN, Butler JK, Blumel J. Experimental immobilization and remobilization of rat knee joints. J Bone Joint Surg 1960; 42-A:737-758.
129. Enneking WF, Horowitz M. The inra-articular effects of immobilization on the human knee. J Bone Joint Surg 1972; 54-A: 973-985.
130. Peacock EE. Some biochemical and biophysical aspects of joint stiffness: Role of collagen synthesis as opposed to altered molecular bonding. Ann Surg 1966; 164:1-12.
131. Harris RI, MacNab I. Structural changes in lumbar intervertebral discs. Their relationship to low back pain and sciatica. J Bone Jt Surg 1954; 36B(2):304-322.
132. Booth FW, Seider MJ. Recovery of skeletal muscle after 3 mo of hindlimb immobilization in rats. J Appl Physiol: Respirat Environ Exercise Physiol 1979: 47(2):435-439.
133. Kovanen V, Suominen H, Heikkinen E. Mechanical properties of fast and slow skeletal muscle with special reference to collagen and endurance training. J Biomechanics 1984; 17: 725-735.
134. Spector SA, Simard CP, Fournier M, Sternlight E, Edgerton, VR. Archintectural alterations of rat hindlimb skeletal muscles immobilized at different lengths. Experimental Neurology 1982; 76:94-110.
135. Duchen LW, Tonge DA. The effects of tetanus toxin on neuro muscular transmission and on the morphology of motor endplates in slow and fast skeletal muscles of the mouse. J Physiol 1973; 228:157-172.
136. Edgerton VR, Barnard RJ, Peter JB, Maier A, Simpson DR. Properties of immobilized hind-limb muscles of the GALAGO SENEGALENSIS. Experimental Neurology 1975; 46:115-131.
137. Tower SS. Atrophy and degeneration in the muscle spindle. Brain 1932; 55:79-80.
138. Yellin H, Eldred E. Spindle activity of the tendotomized gastrocnemius muscle. Exp Neurol 1970; 29:513-533.
139. Esaki K. Morphological study of muscle spindle in atrophic muscle induced by immobilization. Nagoya Med J 1966; 12: 185-201.
140. Maier A, Eldred E, Edgerton V. The effects of spindles on muscle atrophy and hypertrophy. Exper Neuro 1972; 37: 100-123.
141. Yellin H, Eldred E. Spindle activity of tendotomized gastrocnemius muscle in the cat. Exper Neuro 1970; 29:513-533.
142. Videman T, Michelsson JE, Rauhamaki R, Langenskiold A. Changes in 35 S sulphate uptake in different tissues in the knee and hip regions of rabbits during immobilization, mobilization and the development of osteoarthritis. Acta Orthop Scand 1976; 47:290-298.
143. Stauffer ES. Rehabilitation of the spinal cord-injuried patient. In: Rothman, Simeone, eds. The spine, Philadelphia: W.B. Saunders Co., 1982.
144. Ferrell W, Nade S, Newbold P. The interrelation of neural discharge, intra-articular pressure, and joint angle in the knee of the dog. J Physiol 1986; 373:353-365.
145. Simard C, Spector S, Edgerton V. Contractile properties of rat hind limb muscles immobilized at different lengths. Exper Neuro 1982; 77:467-482.
146. Fournier M, Roy R, Perham H, Simard C, Edgerton V. Is limb immobilization a model of muscle disuse? Exper Neuro 1983; 80:147-156.
147. Ralston HJ, Feinstein B, Inman VT. Rate of atrophy in muscles immobilized at different lengths. Fed Proc 1952; 11:127.
148. Kurakami K. Studies on changes of rabbit skeletal muscle components induced by immobilization with plaster cast. Nagoya Med J 1966; 12:165-184.
149. Maier A, Crockett J, Simpson D, Saubert C, Edgerton V. Properties of immobilized guinea pig hind limb muscles. Am J Phys 1976; 231(5):1520-1526.
150. Rifenberick DH, Gamble JG, Max SR. Response of mitochondiral enzymes to decrease in muscular activity. Am J Physiol 1973; 225:1295-1299.
151. Flint M. Interrelationships of mucopolysaccharide amd collagen in connective tissue remodeling. J Embryol Exp Morph 1972; 27(2):481-495.
152. Merrilees MJ, Flint MH. Ultrastructural study of tension and pressure zones in a rabbit flexor tendon. Amer J Anat 1980; 157:87-106.
153. Boyle AC. Color atlas of rheumatology. Year Book Medical Pub., Chicago 1980.
154. Flint MH, Gillard GC, Merilees MJ. The effects of local physical environmental factors on connective tissue organization and glycosaminoglycan synthesis. In:Fibrous proteins: Scientific, industrial and medical aspects. Parry DAD, Crea- mer LK, eds. London: Academic Press, 1979.
155. Reimann I. Experimental osteoarthritis of the knee in rabbits induced by alterations of the load-bearing. Acta Orthop Scand 1973; 44:496-504.
156. Higuchi M, Abe K, Kaneda K. Changes in the nucleus pulposus of the intervertebral disc in bipedal mice. Clin Orthop Related Res 1983; 175:251-257.
157. Castor CW. Regulation of connective tissue metabolism. In McCarty, D.J. Arthritis and allied conditions, a textbook of rheumatology. Philadelphia: Lea & Febiger, 1985:242-256.
158. Gelberman R, Manske P, Akeson W, Woo S, Lundborg G, Amiel D. Flexor tendon repair. J Ortho Res 1986; 4:119-128. .pa
159. Borg TK, Caulfield JB. Morphology of connective tissue in skeletal muscle. Tissue & Cell 1980; 12(1):197-207.
160. Huet De La Tour E. Tardieu C, Tabary JC, Tabary C. Decrease of muscle extensibility and reduction of sarcomere number in soleus muscle following a local injection of tetanus toxin. J Neuro Sci 1979; 40:123-131.
161. Davis H. Kiernan J. Effect of nerve extract on atrophy of denervated or immobilized muscles. Exper Neuro 1981;72:582- 591.
162. Pachter B, Eberstein A. Effects of passive exercise on neurogenic atrophy in rat skeletal muscle. Exper Neuro 1985; 90:467-470.
163. Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles; an autopsy study. J Neuro Sci 1973; 18:111-129.
164. Halkjaer-Kristensen J. Ingemann-Hansen T. Wasting of the human quadriceps muscle after knee ligament injuries. Scan J Rehab Med Suppl 1985; 13:12-20.
165. Musacchia XJ, Deavers DR, Meininger GA, et al. A model for hypokinesia: Effects on muscle atrophy in the rat. J Appl Physiol: Respirat Environ Exercise Physiol 1980; 48(3):479- 486.
166. Booth FW. Time course of muscular atrophy during immobilization of hindlimbs in rats. J Appl Physiol: Respirat Environ Exercise Physiol 1977; 43:656-661.
167. Sargeant AJ, Davies CTM, Edwards RHT, et al. Functional and structural changes after disuse of human muscle. Clin Sci Mol Med 1977; 52:337-342.
168. Cardenas DD, Stolov WC, Hardy R. Muscle fiber number in immobilization atrophy. Arch Phys Med Rehabil 1977; 58:423- 426.
169. Max SR, Mayer RF, Vogelsang L. Lysosmes and disuse atrophy of skeletal muscle. Arch Biochem. Biophysics 1971; 146:227- 232.
170. Halkjaer-Kristensen J, Ingemann-Hansen T. Wasting of the human quadriceps muscle after knee ligament injuries. Scand J Rehab Med Suppl 1985; 13:29-37.
171. Johns TR, Thesleff S. Effects of motor inactivation on the chemical sensitivity of skeletal muscle. Acta Physiol Scand 1961; 51:136-141.
Return to SUBLUXATION THEORY


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |