Approach to Cervicogenic Dizziness:
A Comprehensive Review of its Aetiopathology and ManagementThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Eur Arch Otorhinolaryngol. 2018 (Oct); 275 (10): 2421–2433 ~ FULL TEXT
K. Devaraja
Department of Otorhinolaryngology,
Kasturba Medical College,
Manipal Academy of Higher Education,
Manipal, Udupi, Karnataka, 576104, India.
deardrdr@gmail.comPURPOSE: Though there is abundant literature on cervicogenic dizziness with at least half a dozen of review articles, the condition remains to be enigmatic for clinicians dealing with the dizzy patients. However, most of these studies have studied the cervicogenic dizziness in general without separating the constitute conditions. Since the aetiopathological mechanism of dizziness varies between these cervicogenic causes, one cannot rely on the universal conclusions of these studies unless the constitute conditions of cervicogenic dizziness are separated and contrasted against each other.
METHODS: This narrative review of recent literature revisits the pathophysiology and the management guidelines of various conditions causing the cervicogenic dizziness, with an objective to formulate a practical algorithm that could be of clinical utility. The structured discussion on each of the causes of the cervicogenic dizziness not only enhances the readers' understanding of the topic in depth but also enables further research by identifying the potential areas of interest and the missing links.
RESULTS: Certain peculiar features of each condition have been discussed with an emphasis on the recent experimental and clinical studies. A simple aetiopathological classification and a sensible management algorithm have been proposed by the author, to enable the identification of the most appropriate underlying cause for the cervicogenic dizziness in any given case. However, further clinical studies are required to validate this algorithm.
CONCLUSIONS: So far, no single clinical study, either epidemiological or interventional, has incorporated and isolated all the constitute conditions of cervicogenic dizziness. There is a need for such studies in the future to validate either the reliability of a clinical test or the efficacy of an intervention in cervicogenic dizziness.
KEYWORDS: Barre–Lieou syndrome; Benign paroxysmal positional vertigo; Bow hunter’s syndrome; Cervical vertigo; Cervicogenic dizziness; Whiplash-associated disorder
From the FULL TEXT Article:
Introduction
Broadly, the dizziness incorporates four descriptive symptoms: namely,‘vertigo’, which is nothing but a false perception of movement of self or surrounding;
‘disequilibrium’ or ‘imbalance’ which is an inability to maintain balance;
‘presyncope’, a sense of losing consciousness; and
‘lightheadedness’, defined as a vague symptom of feeling disconnected from the environment. [1, 2]However, according to the Barany society’s committee for the classification of vestibular disorders, the terms ‘vertigo’ and ‘dizziness’ are non-hierarchical and reflect distinctly separate sets of symptoms. [3] They define ‘vertigo’ as the false sense of self-motion without any motion or the feeling of distorted self-motion with normal movement. Similarly, the dizziness has been defined as a sense of disturbed or impaired spatial orientation without a false or distorted sense of motion. They have further sub-classified vertigo into internal and external, for separating the vestibular sense of false motion from the visual sense of false motion, respectively. [3] Nevertheless, the patients with cervicogenic balance disorder rarely experience true vertigo; instead, they often complain of lightheadedness and disequilibrium which are included under dizziness. [4–7]
Dizziness is said to be cervicogenic when it is closely associated with the neck pain, the neck injury, or the neck pathology, after excluding the other causes of dizziness. [4] Though neurologists, neuro-otologists, physicians, and orthopedicians commonly come across patients with presumptive cervicogenic dizziness in clinics, not many would stake the claim. The dearth in the awareness about the constitute conditions contributes at least partly to this. In addition, most of the existing literature discusses the cervicogenic dizziness, in general, and provides guidelines accordingly. However, cervicogenic dizziness can be caused by many conditions of separate pathophysiological backgrounds. Failure to separate these conditions in clinical practice as well as in research studies may have a negative impact and hamper the further understanding of the disease. Apart from discussing the peculiar features of each condition responsible for the cervicogenic dizziness, this narrative review proposes a simple aetiopathological classification and a sensible management algorithm, to enable the appropriate diagnosis and management in any suspected case of cervicogenic dizziness.
Cervical contribution to the balance and to the dizziness
The sense of balance and orientation of a human being is dependent on the optimal functioning of multisensory perception and the integration in the nervous system. Visual and auditory cues sensing the spatial relationship with the external environment, vestibular organs detecting the internal signals of motion, and muscles and joints involved in the proprioception are the three chief sensory preceptors of one’s sense of orientation. [8] Integration of symmetrical inputs from these afferent systems is essential for the normal orientation and the balance, and any dysfunction in these sensory organs or asymmetry in the afferent inputs would result in sense of imbalance or dizziness. [9, 10] Further discussion of these neural networks seems to be inappropriate for this clinical narrative of cervicogenic dizziness. Nevertheless, the proprioceptive signals of neck muscles and cervical joints play an enormous role in maintaining and fine-tuning the person`s orientation at rest and the balance while motion [11–13], and the alteration in these proprioceptive signals seems to be responsible for the majority of cases of the so-called ‘cervicogenic dizziness’. [14, 15] The diseased cervical joints are shown to have a significantly higher concentration of Ruffini corpuscles [16], which, otherwise, are abundantly found in knee joints [17], and are known to play a major role in proprioception. [18] Some authors use the term ‘cervical proprioceptive vertigo’ to describe this dizziness caused by disharmonic hyperactivity of cervical mechanoreceptors located in joints, ligaments, and muscle spindles. [9]
Classification of cervicogenic dizziness
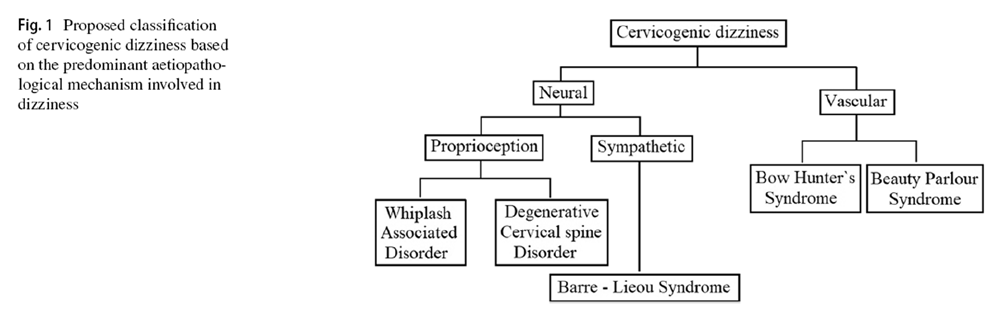
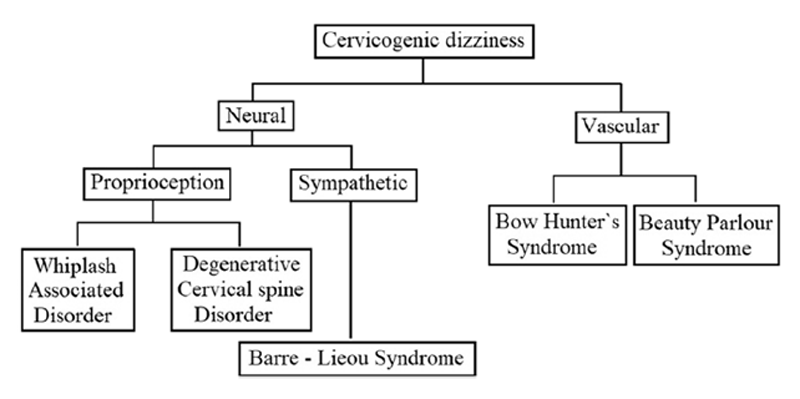
Figure 1 After reviewing the literature thoroughly, the author has classified the cervicogenic dizziness, as shown in Figure 1, based on the predominant aetiopathology responsible for it. It has to be noted, though, that the aetiopathology of some of these conditions could be overlapping, and also, some of the conditions can cause dizziness by several independent mechanisms. Nevertheless, in any given case of cervicogenic dizziness, identifying the appropriate underlying cause is relevant both clinically as well for interpreting the results of the intervention trials. At least differentiating the vascular causes from the neural causes seems sensible as the management principles of these conditions also vary. The aetiopathology of these conditions has been narrated below in the corresponding sections along with their management.
Degenerative cervical spine disorders
Presumptively, the degenerative cervical spine disorder (DCD), also known as the cervical spondylosis (CS), constitutes the most common cause of cervicogenic dizziness. [19, 20] In 1952, the term ‘cervicogenic vertigo’ was introduced by Ryan and Cope, who attributed it to the abnormal signals from the degenerated upper cervical joints to the vestibular nucleus. [21] However, the term ‘cervicogenic dizziness’ is preferred over the ‘cervicogenic vertigo’ owing to the nonspecific dizziness or feeling of lightheadedness complained by DCD patients rather than true vertigo. [19] Growing evidence from the experimental and the human studies support the role of the cervical joints in the maintenance of posture, and also their contribution to the cervicogenic dizziness in a diseased state. [16, 22–24] DCD is commonly seen in older patients. [25] The non-specific dizziness seen in these patients would be mostly episodic, lasting from minutes to hours. [4, 19] In some of the DCD patients, the onset of dizziness can have a temporal relationship to the turning of the neck. [26–29] Neck stiffness, shoulder pain, headache, radiculopathy, or myelopathy are the other features of DCD, which may also be seen in these patients.
Cervical degenerative index (CDI) score is a physicianrated scale used to quantify the radiological changes seen in DCD and has good inter-observer and intra-observer reliability. [30, 31] Disc space narrowing, sclerosis, osteophytes, and olisthesis are the four factors considered in CDI, each one graded from zero to three depending on the severity. [31] However, as far as the cervicogenic dizziness is concerned, correlation with these grades has not been uniformly predictable. [32, 33] In fact, there are no structured clinical studies which have directly evaluated the relationship between severity of CDI scores and the presence or the absence cervicogenic dizziness. There are studies which have correlated the severities of cervical spine degeneration in dizzy patients with the arterial blood flow in their vertebrobasilar system [32–36]. They have reported a significant reduction in the vertebral artery flow velocities on turning the neck in DCD patients with the dizziness, compared to those DCD patients without the dizziness and to the normal controls. Many of the authors have attributed the dizziness seen in these patients of DCD to the dynamic vertebrobasilar insufficiency. [26–29] In other words, at least in a subset of dizzy patients with DCD, the cause of dizziness on turning the neck could be due to the reduced vertebral blood flow. This dynamic compression of the vertebral artery and its mechanism of causation of dizziness have been discussed in detail in the subsequent sections. Interestingly, apart from the abnormal proprioceptive signals from diseased joints and the reduction in vertebrobasilar flow, the DCD can cause dizziness by a third mechanism involving the stimulation of sympathetic system.
Barre–Lieou syndrome
In 1926, Barre and Lieou proposed the neurovascular hypothesis for the cervicogenic dizziness, in which they attributed the vertigo and related symptoms to the transient intracerebral ischemia, secondary to sympathetic fibers’ compression by the diseased cervical joints. [37] However, subsequent experimental studies by Heisted and his colleagues showed that the sympathetic stimulation had little or no effect on cerebral blood flow. [38–41] Many others also have questioned and criticized the existence of BLS. [42, 43] However, the results of some of the recent studies provide certain corroborative pieces of evidence substantiating the involvement of sympathetic nervous system in the aetiopathology of cervicogenic dizziness. Separate studies have reported the association of sympathetic symptoms like vertigo in DCD patients and the relief of these symptoms by the surgical treatment of DCD. [44–49] Both animal and clinical studies have demonstrated that the degenerated cervical vertebral joints can lead to vertigo via the stimulation of sympathetic fibers in the adjacent posterior longitudinal ligament. [44, 50] Researchers have confirmed the distribution of sympathetic fibers in the human posterior longitudinal ligament. [16] Others have identified bidirectional neural networks between the cervical spinal and the sympathetic ganglia re-iterating the possible neuroanatomical hypothesis for this condition. [51] By the critical review of all these studies, it seems improbable at this stage to subvert the role of the sympathetic nervous system in the pathophysiology of cervicogenic dizziness. Clinically, it is hard to differentiate the BLS from the dizziness due to proprioceptive defects of the DCD, and there is no specific diagnostic investigation available either. However, the association of degenerative changes in cervical spine radiograph with sympathetic symptoms and the relief of these symptoms upon the treatment of degenerated joints should suggest the diagnosis of BLS.
Having multiple mechanisms by which it can cause the dizziness, it is understandable that the DCD is responsible for the majority of cases of cervicogenic dizziness, and the management of DCD goes a long way in controlling these symptoms. Muscle relaxants are effective in reducing the neck pain and neck stiffness seen in DCD [52]; however, the effect of these agents on the cervicogenic dizziness is not yet evident. Though a recent retrospective study claims muscle relaxants to be effective in the cervicogenic dizziness [7], there is not enough evidence to support their use to control the dizziness in DCD patients. Similarly, studies evaluating the utility of non-steroidal anti-inflammatory drugs in DCD patients for control of dizziness are non-existent at present. Finally, considering the recent studies’ affirmation of the sympathetic system involvement in DCD, it would not be whimsical to study the role of sympatholytic agents in controlling the dizziness in these patients. On the other hand, many studies have separately evaluated the role of cervical traction and exercise or physiotherapy in control of the cervicogenic dizziness due to DCD, and have found them to be effective. [53] Combination of manual therapy and physiotherapy is shown to be better than physiotherapy alone in this regard. [54] Manual therapy involves the use of therapist’s hands to perform the physiotherapy, whereas physical therapy is nothing but providing passive assistance to the patient to enable him or her to exercise.
Chiropractic is a unique form of manual therapy, popularized by Palmer and Palmer. [55] The primary mode of chiropractic therapy is spinal manipulation, and the cervical spinal manipulation has been shown to be effective in controlling the cervicogenic dizziness. [56] However, this therapy is surrounded by some controversy and is not entirely safe. [57] Acute compression of the vertebral artery [58], dissection of the vertebral artery [59, 60], and bilateral diaphragmatic palsy [61] are some of the reported complications with the chiropractic therapy. Though the cervical manipulation is depicted to be safe in healthy young adults [62], it is preferably to be avoided in elderly patients.
A recent study showed that the surgically treated patients of DCD do much better than conservatively managed patients in terms of both the intensities as well as the frequency of cervicogenic dizziness. [22] In another study, total disc replacement significantly reduced the severity of dizziness and other sympathetic features in DCD. [23] Though both the studies aimed to evaluate the relief of dizziness in DCD patients, the surgery was done for radiculopathy and myelopathy in these studies and not for the dizziness control. In other words, the results of these studies may not imply active surgical intervention for the control of dizziness; nevertheless, the surgical intervention may be an optimal therapeutic option when the cervicogenic dizziness is associated with radiculopathy and/or myelopathy. Many other authors have also reported improved dizziness and other sympathetic symptoms by surgical treatment of degenerated cervical joints or discs. [45, 46, 48, 49]
Whiplash-associated disorders
Whiplash injury refers to acceleration–deceleration injury to the neck resulting commonly from motor vehicle collisions, but could be due to other modes of trauma also. Mechanism of injury involves a sudden movement of the head over the neck or the head and neck over the trunk. This mode of injury may cause a significant damage to the structures in the neck, giving rise to a multitude of signs and symptoms grouped under whiplash-associated disorders (WAD). [63] The Quebec Task Force classified the WAD into four categories and redefined its management in 1995. [64] However, since this classification does not concern the dizziness seen in WAD patients, further discussion on it may deviate the manuscript from its objective. The dizziness-vertigo-imbalance forms one of the significant symptom complexes in the chronic WAD, other predominate manifestations being the neck pain–headache complex, and the paresthesia–sensory disturbances complex. [4, 63, 65, 66]
Dizziness or vertigo in WAD patients could be due to three causes, the labyrinthine concussion and the benign paroxysmal positional vertigo (BPPV) and the cervicogenic cause. [67, 68] The labyrinthine concussion is rare in dizzy patients of an isolated WAD, but it is common when WAD is associated with head trauma. [68, 69] On the other hand, BPPV could be the cause of vertigo in almost one-third of the WAD patients. [70] The clinicopathological characteristics of the BPPV in these patients are similar to that of the idiopathic BPPV, except that the multiple repositioning maneuvers may be required in these WAD associated cases. [70, 71] By and large, the dizziness in most of the symptomatic WAD patients is due to the cervicogenic cause. [68] Nevertheless, in any patient of WAD having dizziness, it is worth performing a positional test before attributing it to the cervicogenic cause. The exact aetiopathology of cervicogenic cause for dizziness in WAD is not fully understood. The abnormal cervical afferent inputs due to mechanoreceptor dysfunction, mismatching with the normal vestibular information has been proposed as the most probable cause for cervical dizziness in WAD patients. [15, 72–74] In another hypothesis, the hypertonicity of cervical and lumbar erector muscles following whiplash injury is thought to affect the balance perception of the central nervous system leading to disequilibrium. [75]
The clinical characteristics of ‘cervicogenic dizziness’ in WAD patients are similar to the dizziness in DCD and include episodic lightheadedness or imbalance lasting minutes to hours. [76] The affected patients would have significantly reduced neck mobility and postural instability compared to the age- and sex-matched normal controls. [77, 78] The postural abnormalities in dizzy patients with WAD can be of diagnostic significance and could help in separating cervicogenic dizziness in WAD from malingering [78, 79] and vestibular causes. [80] One of the clinical signs of the abnormal cervical proprioception is the ‘cervical nystagmus’ or the ‘neck torsion nystagmus’, which is nothing but the nystagmus that arises from a neck rotation without labyrinthine stimulation. [81] This nystagmus is most appreciated in patients with bilateral vestibular loss. [82] To attribute this nystagmus to abnormal cervical signals, one has to examine it using nystagmography in a dark room while rotating the trunk in relation to stationary head, rather than the neck rotation over trunk. [82] Though it is observed in some cases of WAD [83], its pathophysiological mechanism and clinical relevance have been questionable. [63, 81, 82] Some authors have also reported spontaneous nystagmus in 30–60% of the WAD patients with cervicogenic dizziness. [83]
Measuring the postural deficit is one of the ways to confirm cervicogenic cause for dizziness in WAD. In posturography, a computerized stable force platform measures the postural sway and the changes in standing balance, under the altered visual and support conditions. [74] Posturography has demonstrated significant deficiencies in the postural responses among the WAD patients having dizziness compared to the WAD patients not having dizziness and the healthy controls. [73, 74] Since the results of posturography are not influenced by age, medication, vestibular compensation status, or anxiety levels at the time of testing [74], it can be used to differentiate the malingering patients complaining of vertigo after injury with the malicious intent of claiming compensation. [78, 79] It can also be used as a quantitative assessment tool for grading the imbalance in WAD patients with cervicogenic dizziness. [69, 77]
Joint position error (JPE) or head repositioning error (HRE) is another measure to detect the abnormalities in cervical proprioception. In this test, the blindfolded patients are asked to perform neck movement within the comfortable range and to return to starting position as accurately as possible. [15] Simple computer-based algorithms have been found useful and can be of clinical utility. [84] If repeatedly performed (at least six times), the results can be reliable. [85] WAD group would have larger JPE compared to the healthy controls during flexion–extension and repositioning tasks. [86, 87] Among WAD patients, those with dizziness are shown to have significantly greater JPE than those without. [15] Interestingly, the amount of JPE seems to be correlating with the severity of WAD. [87] The vestibular function influences the JPE results more often [88] than not. [89] Moreover, the results of JPE are dependent on the behavior of the subject undergoing the test [86, 88] and have to be interpreted accordingly.
The smooth pursuit neck torsion (SPNT) test has a sensitivity of 90% and specificity of 91% in WAD patients having the cervicogenic dizziness. [72] In this test, the patients are asked to follow the moving object/light as closely as possible by keeping the head still. These smooth pursuit eye movements are checked in the neutral position, in 45° head turned to the right, and then in head turned to the left. [72, 88] In patients of WAD with cervicogenic dizziness, the smooth pursuit gain has been shown to reduce significantly in the torsion positions compared to that in the neutral position. Such differences in smooth pursuit gains were observed neither in normal subjects nor in patients with central or peripheral vestibular pathologies. [72] Though many authors have been able to reproduce the similar SPNT test results [90], some have reported the statistically insignificant differences [91–93], and this is probably because of the methodological differences between these studies. [94] In addition, the results of SPNT test may get influenced by age, presence or absence of neck pain, sedation, and examination conditions like the predictability of the moving target [95, 96] making this a less reliable test in isolation.
In fact, a combination of JPE, posturography, and SPNT test can better predict the possibility of cervicogenic dizziness in WAD. [97] More than 2/3 of WAD patients having cervicogenic dizziness would have an abnormality in two out of three and more than 1/3 would yield abnormal results in all three tests. Abnormal cervical JPE score has a high positive predictive value (88%) for predicting abnormal scores in one or both of the other postural control tests in WAD; however, as an isolated test, it has a low sensitivity (60%) and specificity (54%) to determine the abnormality in balance. [97] The JPE is one of the manifestations of the cervical motor system dysfunction seen in WAD patients. Two other signs of the cervical motor system dysfunction include the restricted neck movement and the increased activity of superficial neck flexors, which can be investigated respectively, by ‘cervico-ocular-reflex (COR) gain measures’ and ‘craniocervical flexion test (CCFT)’. [87, 98] During the rotation of the trunk with a stationary head in the dark, the COR measured by an electronystagmography shows an increased gain in WAD patients. [99, 100]
Similar to ‘the cervical nystagmus’ described previously, this increased COR gain can be attributed to WAD-related cervicogenic dizziness only after negating the influence of the vestibular system. Furthermore, clinicians have to keep in mind that both ‘the cervical nystagmus’ and ‘the increased COR gain’ are apparent signs in bilateral vestibular hypofunction. Accordingly, these signs are of limited value for diagnosing the cervical contribution to dizziness in patients who are also having vestibular deficits. In fact, the workup for diagnosing the cervicogenic dizziness is to be considered only after excluding the vestibular as well as the central causes. In addition, in the course of the diagnostic workup for cervicogenic cases, nystagmography has to be done in a dark room, by making the patient turn his/her trunk against the stationary head to prevent the stimulation of intact vestibular system. Nevertheless, the COR gain also has a potential to be an objective test to diagnose WAD. [100] In CCFT, the patient is asked to progressively increase the craniocervical flexion range, while the contraction of longus coli is monitored by electromyography, and in patients with WAD, instead of the deep flexors like longus coli, the superficial flexors like sternocleidomastoid and trapezius are stimulated. [87, 98] However, this test is not specific for WAD. [101]
The cervicogenic dizziness in WAD patients can be effectively treated by cervical physiotherapy. [69, 102] Reviews have also found enough evidence to support the manual therapy in patients with cervicogenic dizziness. [103, 104] However, the studies included have not categorized the exact cause for cervicogenic dizziness and seem to contain the mixed cohort of DCD as well as WAD; accordingly, the usefulness of manual therapy in specific patients of WAD is still not apparent. Nevertheless, since the physiotherapy is the preferred treatment modality in WAD and the manual therapy is a form of physiotherapy, its role in controlling the cervicogenic dizziness in WAD patients cannot be ruled out. The recent review by Lystad et al. did not find supportive evidence to combine the vestibular rehabilitation with the manual therapy. However, they have highlighted enough rationale to back the vestibular rehabilitation in cervicogenic dizziness. [103] Patient education is another effective treatment modality in WAD. [105] The patient education integrated into exercise programs, and behavioral programs not only reduce the pain and the dizziness but also enhance the recovery and the mobility in chronic WAD patients. [106, 107] One session of patient education and subsequent telephonic support is shown to be as effective as 20 sessions of comprehensive physiotherapy exercise programs. [108]
However, this study does not imply to discontinue the exercise therapy in the chronic WAD, but it reinforces the physiotherapists on the importance of patient education in rehabilitating such patients. [109] There are very few trials on pharmacotherapy in the chronic WAD, and thus, no specific recommendations exist for drug therapy. [110] The role of muscle relaxants and NSAIDs in controlling cervicogenic dizziness due to WAD is yet another potential research question for further studies. Similarly, the utility of surgical procedures in WAD patients is also not apparent, owing to the contradictory evidence in the literature. [111] Among many invasive procedures tried in WAD, the existing evidence supports only the radiofrequency neurotomy in chronic WAD patients, who are not responding to the conventional therapy. [111]
Bow hunter’s syndrome
Sorenson in 1978 named the condition of ‘symptomatic vertebral artery insult with dynamic neck posture’ as ‘Bow hunter’s stroke’ [112], which then became popular as bow hunter’s syndrome (BHS) due to the multitude of possible symptoms in the affected individuals. Sorenson description was related to the mechanical occlusion of vertebral artery at the level of the atlantoaxial joint during neck turning. Though C1–C2 is the most common site of symptomatic compression, followed by C4–C5 [113–115], the vertebral artery narrowing anywhere in the neck can also produce the classical symptoms of BHS [27, 116–119]. BHS is commonly diagnosed in older patients, in their VI or VII decades [115], but can also be seen in young individuals. [119–123] Men are more commonly affected than women. [115] Vertigo is one of the predominant symptoms in BHS, but the clinical manifestations may vary from syncope to posterior circulation stroke [27, 113, 124–128]. Commonly seen accompanying symptoms include tinnitus, headache, vision loss, ataxia, double vision, tinnitus, and/or headache. [27, 113, 129, 130]Typically, the affected individuals present with vertigo and other symptoms on turning the neck anywhere between 45° and 90° [122, 131–133], and these symptoms disappear on bringing the head back to the neutral position.
Vertigo in BHS is most often due to dynamic compression of the dominant vertebral artery, and since the left vertebral artery is dominant in most individuals, it is responsible for BHS in the majority. [113, 114, 121, 129] Rarely compression of the non-dominant vertebral artery can also lead to BHS symptoms [29, 120, 131, 134, 135]. Pre-existing narrowing in the contralateral vertebral artery may predispose to vertigo when the normal ipsilateral vertebral artery gets compressed by neck turn. The direction of neck turn producing the symptoms in BHS can reasonably suggest the side and site of the vertebral artery compression. Vertigo on turning the neck to one side may either be due to the ipsilateral vertebral artery compression at or below C4 [117–119] or due to the contralateral vertebral artery compression at or above C3. [114] Sometimes, turning on to either side can produce symptoms, which is seen in the bilateral vertebral artery compression or stenosis [27, 119, 136]. The symptomology in BHS is attributed to reduced vertebral artery flow resulting in transient hypoperfusion of the ipsilateral labyrinth or the lateral medulla or the inferior cerebellum [135] and resultant neural excitation. [130, 137, 138]
The transient ischemia in these organs can also give rise to typical nystagmus which may accompany the vertigo in some of the BHS patients. [29, 130, 131, 135] The nystagmus is a mixed type, with horizontal and torsional down beating components, and the direction of nystagmus is generally towards the side of the compressed vertebral artery. [139] This nystagmus may also have latency, and it may change the direction on bringing the head back to neutral. [29] As discussed in the earlier paragraphs, many patients of BHS are predisposed by the degenerative joints of the cervical spine. Other predisposing conditions for BHS include malformed vertebrae [116], bony stenosis of vertebral canal [128], accessory osseous canal in the transverse process [119], hypertrophied neck musculature [122], and trauma. [140]
The transcranial Doppler (TCD) or the cervical arterial duplex ultrasonography (CDU) is used to demonstrate the reduction in posterior circulation velocity on turning the patient’s head, corresponding to his or her symptoms. [141, 142] Even the pulsed-wave Doppler of the cervical region could also be sufficient for screening the patients with cervicogenic dizziness. [143] However, as the most common site of vertebral artery compression in BHS is above the level of C3 vertebra, the reduced flow distal to this obstruction is unlikely to be picked up by the cervical Doppler. Nevertheless, neither of these investigations can precisely account for the site and the extent of vertebral artery compression. These can be achieved through dynamic digital subtraction angiogram (d-DSA), which is the current investigation of choice for diagnosing BHS. [144] In all these tests, care should be taken to avoid the sustained end-of-range rotation and quickthrust rotational manipulations. [145] The three dimension computed tomography integrated with angiography would provide the detailed bony as well as soft-tissue anatomy in topographic relation to the course of the vertebral artery. [128] It can also delineate the precise cause for dynamic compression like a fibrous band, osteophyte, or narrowing of the bony canal, and thus may assist in the surgical planning.
BHS can be best treated by surgical intervention and the two most effective surgical procedures are vertebral artery decompression [113, 114, 119, 124, 125, 128, 140, 141] and fusion of the vertebrae [123, 126, 127, 131, 133]. Though both the approaches have shown to be effective, the isolated fusion of the vertebrae is useful primarily in the vertebral instabilities, and these patients may eventually lose up to 50°–70° of rotational movement postoperatively. [121] Accordingly, vascular decompression is the most preferred treatment [115], and it can be done by anterior approach or posterior approach. [113] Considering the ease of accessing the vertebral artery, for lesions at or below C4, the anterior approach is preferred, and the posterior approach is for lesions at or above C3. [113, 114] The posterior approach seems to have relatively higher morbidity, which can be minimized by a novel minimally invasive approach. [113] Though some authors have reported nearly 33% re-occlusion rate after successful vascular decompression [121], this unfavorable outcome is attributed to possibly missed anterior fibrous bands during posterior approach decompression. [128] Nevertheless, surgical intervention needs to be individualized to each patient depending on the site of the occlusion, the extent of the occlusion, and the exact cause of the occlusion. Often, the combination of vascular decompression and intervertebral fusion would be required to achieve the desired clinical outcomes. [27, 29, 116–118, 128, 136, 141]
At times, fusion may be required in the later stage to correct the instability caused by prior decompression. [114] Similar success rates have been reported with isolated decompression and decompression with fusion. [146] Irrespective of the approach and type of surgery, it is imperative to have a sound anatomical understanding and to exercise extreme caution intraoperatively to prevent the surgical morbidity. Any of the dynamic vascular studies like TCD, CDU, or d-DSA can be used for intraoperative assistance in decompression surgeries, and these studies can also be handy for the post-operative follow-up [128, 140, 141, 146, 147]. Some surgeons have tried endovascular treatments like stenting [120, 148] with or without angioplasty [132] of the stenosed segment, but the practical value of such approaches in the long term is not yet proven. Conservative management has also been reported to be useful in BHS, especially when there is no identifiable structural abnormality compressing the vertebral artery. [137] Avoiding the head rotation, use of the cervical collar and use of anticoagulants are some of the conservative maneuvers to have produced favorable outcomes. [112, 122, 149]
Other rare causes
Beauty parlor stroke syndrome
Beauty parlor stroke syndrome (BPSS) was first reported by Weintraub in 1993. [150] This condition is similar to the BHS in many of the aetiopathology as well as the clinical aspects, but probably gets this name due to its occurrence in beauty parlors. However, unlike BHS, the vertebrobasilar insufficiency in BPSS is not always due to the external compression of the arteries and could also be due to dissection of the vertebral artery or some systemic conditions. [151] The onset of symptoms in BPSS is associated with the extension of the neck and is related to the external compression of the posterior neck due to hanging the head behind the headrest while hairdressing or hair massage. [152]
The patients may manifest with varied symptoms ranging from vertigo to evolved stroke. [151–153] Age more than 50 years [154], female sex [151] and hyperextension of the neck [153, 154] are some of the risk factors associated with this syndrome. Color Doppler or angiogram may reveal the vascular pathology, and magnetic resonance imaging may show the area of ischemia in the brain. The treatment varies from case to case depending on the nature of vascular compromise and the volume of stroke. Restriction of neck movements and antiplatelet medications form the basis for the treatment of BPS along with the other supportive measures. [153] Surgical correction of external compression or vertebral arterial wall defect may help in relieving the symptoms in the affected patients. [152]
Cervical myofascial pain syndrome
Cervical myofascial pain syndrome (CMPS) includes a group of disorders of cervical skeletal muscle characterized by the presence of trigger points. Dizziness is seen in 35% of the CMPS patients [155], and it correlates with the symptom of pain. [156] It is commonly seen in young individuals, in fourth decade of life, and females are affected more often. [155] Trapezius muscle would have trigger points in half of the affected patients. [155] There may be associated features like skin flushing, lacrimation, and other autonomic symptoms. CMPS is commonly associated with cervical trauma, fibromyalgia, and joint hypermobility syndrome. [155] Apart from the typical clinical features, the needle electromyography findings can help in diagnosing this condition. The affected muscles fatigue faster than the rest, and this active focus is depicted as spontaneous endplate activity in electromyography. [157]
Exercise therapy is the treatment for cervicogenic dizziness and other complaints in CMPS patients. [158] Along with stretching and strengthening rehabilitation programs, the trigger point injections are also useful in treating the cervicogenic dizziness caused by CMPS. [156, 157] The combination of manual therapy with needle therapy [159] and added vestibular rehabilitation [160] is shown to provide the maximum benefit and even complete regression of dizziness in these patients.
Approach to cervicogenic dizziness
By the above discussion, it is clear that some of the conditions causing cervicogenic dizziness have overlapping aetiopathology, and in addition, they can cause dizziness by several separate mechanisms. Both the DCD and WAD can cause cervicogenic dizziness by affecting the proprioceptive signals, from joints and muscle spindles, respectively. However, the DCD can lead to dizziness also by the vascular compression or by the sympathetic stimulation, and on the other hand, in many of the patients with WAD, the dizziness can be attributed to benign paroxysmal positional vertigo. Separating these clinical conditions, and identifying the exact cause for cervicogenic dizziness in a given case is essential clinically and is relevant for research studies. However, due to the overlapping symptomology and the lack of specific diagnostic investigations [161], many of the high-quality studies and the reviews have not separated these conditions. [103, 104, 107]
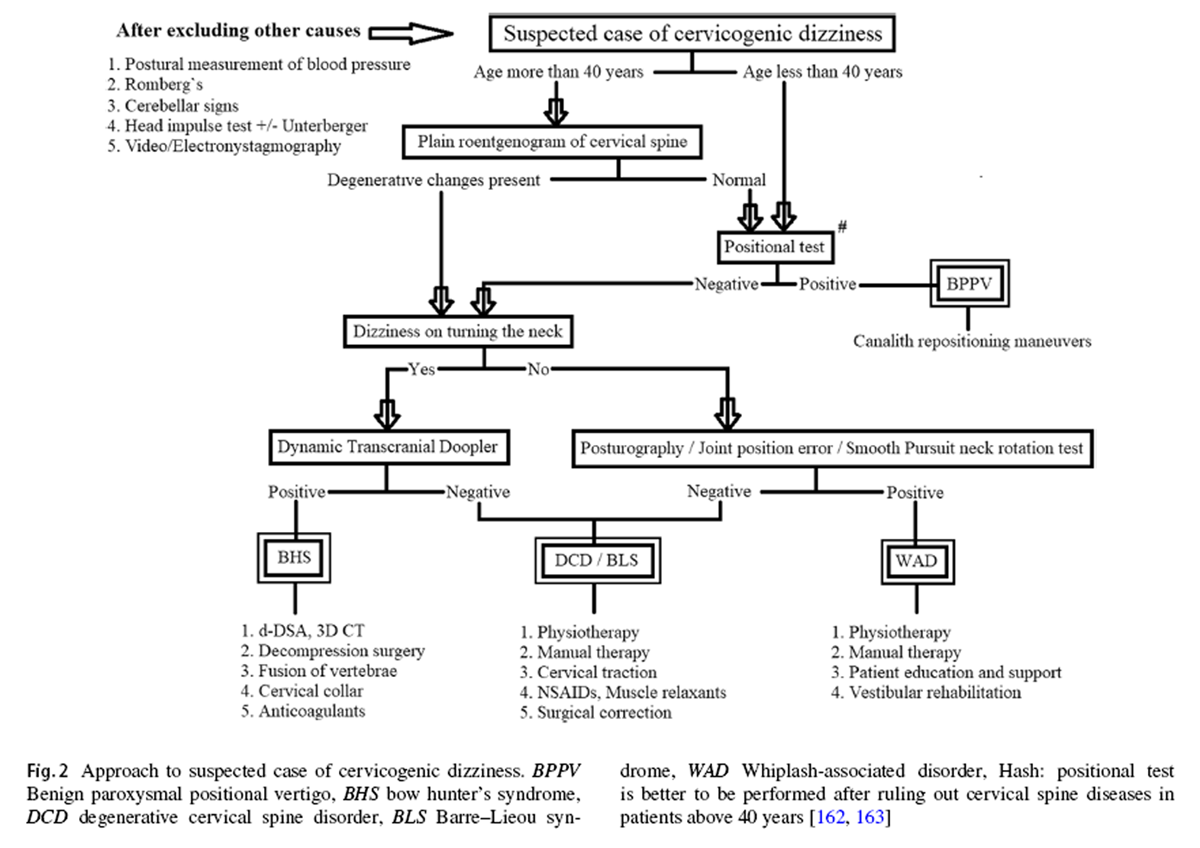
Figure 2 One cannot rely on the universal conclusions of these studies unless the constitute conditions of cervicogenic dizziness are separated and contrasted against each other. So far, no single clinical study, either epidemiological or interventional, has incorporated and isolated all these conditions in its methodology. There is a need for such studies in the future to validate either the reliability of a clinical test or the efficacy of an intervention in cervicogenic dizziness. After a thorough review exercise, an algorithm has been formulated, as shown in Figure 2, on how to approach a patient with suspected cervicogenic dizziness. The objective of this flowchart is to help the clinicians and therapists to identify the most appropriate, if not the actual, underlying cause for the cervicogenic dizziness in any given case. By this approach, each of the above-discussed causes for cervicogenic dizziness can be ruled in or out in a systematic manner. Nevertheless, one can proceed with the given algorithm only after the vestibular, neural, and systemic causes for the dizziness are excluded beyond the reasonable doubt. The algorithm is incorporated with the elements from the history, the examination, and the investigations, in a particular pattern to maximize the clinical utility and to minimize the morbidity as well as the wastage of resources.
Vantage and limitations of the study
In this narrative review, the various conditions causing cervicogenic dizziness have been discussed systematically, incorporating and segregating the existing literature as per the different categories. The classification of cervicogenic dizziness described is simple yet practical. The algorithm provided here for evaluating the suspected cases of cervicogenic dizziness seems appropriate and sensible. Nevertheless, since the algorithm is based on the extrapolation of facts from the literature, it needs further validation by clinical studies. Moreover, in some patients, dizziness could be due to a combination of cervicogenic cause and vestibular or neural deficits, the scenario which has been not addressed in the given algorithm.
Conflict of interest
The authors declare that they have no conflict of interest.
References:
Lee AT (2012)
Diagnosing the cause of vertigo: a practical approach.
Hong Kong Med J 18:327–332Post RE, Dickerson LM (2010)
Dizziness: a diagnostic approach.
Am Fam Physician 82:361–369Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE (2009)
Classification of vestibular symptoms: towards an international classification of vestibular disorders.
J Vestib Res Equilib Orientat 19:1–13Wrisley DM, Sparto PJ, Whitney SL, Furman JM (2000)
Cervicogenic dizziness: a review of diagnosis and treatment.
J Orthop Sports Phys Ther 30:755–766Yacovino DA, Hain TC (2013)
Clinical characteristics of cervicogenic- related dizziness and vertigo.
Semin Neurol 33:244–255Jung FC, Mathew S, Littmann AE, MacDonald CW (2017)
Clinical decision making in the management of patients with cervicogenic dizziness: a case series.
J Orthop Sports Phys Ther 47:874–884Takahashi S (2018)
Importance of cervicogenic general dizziness.
J Rural Med JRM 13:48–56St George RJ, Fitzpatrick RC (2011)
The sense of self-motion, orientation and balance explored by vestibular stimulation.
J Physiol 589:807–813Grgi? V (2006)
Cervicogenic proprioceptive vertigo: etiopathogenesis, clinical manifestations, diagnosis and therapy with special emphasis on manual therapy.
Lijec Vjesn 128:288–295Karnath H-O, Reich E, Rorden C, Fetter M, Driver J (2002)
The perception of body orientation after neck-proprioceptive stimulation.
Exp Brain Res 143:350–358Wyke B (1979)
Cervical articular contribution to posture and gait: their relation to senile disequilibrium.
Age Ageing 8:251–258Maravita A, Spence C, Driver J (2003)
Multisensory integration and the body schema: close to hand and within reach.
Curr Biol CB 13:R531–R539Pettorossi VE, Schieppati M (2014)
Neck proprioception shapes body orientation and perception of motion.
Front Hum Neurosci 8:895Grande-Alonso M, Moral Saiz B, Mínguez Zuazo A, Lerma Lara S, La Touche R (2018)
Biobehavioural analysis of the vestibular system and posture control in patients with cervicogenic dizziness. A cross-sectional study.
Neurol Barc Spain 33:98–106Treleaven J, Jull G, Sterling M:
Dizziness and Unsteadiness Following Whiplash Injury: Characteristic Features and Relationship
with Cervical Joint Position Error
J Rehabil Med 2003 (Jan); 35 (1): 36–43Yang L, Yang C, Pang X, Li D, Yang H, Zhang X et al (2017)
Mechanoreceptors in diseased cervical intervertebral disc and vertigo.
Spine 42:540–546Hirasawa Y, Okajima S, Ohta M, Tokioka T (2000)
Nerve distribution to the human knee joint: anatomical and immunohistochemical study.
Int Orthop 24:1–4Abraira VE, Ginty DD (2013)
The sensory neurons of touch.
Neuron 79:618–639Yahia A, Ghroubi S, Jribi S, Mâlla J, Baklouti S, Ghorbel A et al (2009)
Chronic neck pain and vertigo: is a true balance disorder present?
Ann Phys Rehabil Med 52:556–567Morinaka S (2009)
Musculoskeletal diseases as a causal factor of cervical vertigo.
Auris Nasus Larynx 36:649–654Ryan GM, Cope S (1955)
Cervical vertigo.
Lancet Lond Engl 269:1355–1358Peng B, Yang L, Yang C, Pang X, Chen X, Wu Y (2018)
The effectiveness of anterior cervical decompression and fusion for the relief of dizziness in patients with cervical spondylosis: a multicentre prospective cohort study.
Bone Jt J 100:81–87Sun Y-Q, Zheng S, Yu J, Yan K, Tian W (2013)
Effect of total disc replacement on atypical symptoms associated with cervical spondylosis.
Eur Spine J 22:1553–1557Ren L, Guo B, Zhang J, Han Z, Zhang T, Bai Q et al (2014)
Mid-term efficacy of percutaneous laser disc decompression for treatment of cervical vertigo.
Eur J Orthop Surg Traumatol 24:S153–S158Garfin SR (2000)
Cervical degenerative disorders: etiology, presentation, and imaging studies.
Instr Course Lect 49:335–338Nishikawa H, Miya F, Kitano Y, Mori G, Shimizu S, Suzuki H (2017)
Positional occlusion of vertebral artery due to cervical spondylosis as rare cause of wake-up stroke: report of two cases.
World Neurosurg 98:877.e13–877.e21Fleming JB, Vora TK, Harrigan MR (2013)
Rare case of bilateral vertebral artery stenosis caused by C4–5 spondylotic changes manifesting with bilateral bow hunter’s syndrome.
World Neurosurg 79:799.E1–799.E5Piñol I, Ramirez M, Saló G, Ros AM, Blanch AL (2013)
Symptomatic vertebral artery stenosis secondary to cervical spondylolisthesis.
Spine 38:E1503–E1505Iida Y, Murata H, Johkura K, Higashida T, Tanaka T, Tateishi K (2018)
Bow Hunter’s syndrome by nondominant vertebral artery compression: a case report, literature review, and significance of downbeat nystagmus as the diagnostic clue.
World Neurosurg 111:367–372Gore DR, Sepic SB, Gardner GM (1986)
Roentgenographic findings of the cervical spine in asymptomatic people.
Spine 11:521–524Ofiram E, Garvey TA, Schwender JD, Denis F, Perra JH, Transfeldt EE et al (2009)
Cervical degenerative index: a new quantitative radiographic scoring system for cervical spondylosis with interobserver and intraobserver reliability testing.
J Orthop Traumatol 10:21–26Bayrak IK, Durmus D, Bayrak AO, Diren B, Canturk F, Canturk F (2009)
Effect of cervical spondylosis on vertebral arterial flow and its association with vertigo.
Clin Rheumatol 28:59–64Machaly SA, Senna MK, Sadek AG (2011)
Vertigo is associated with advanced degenerative changes in patients with cervical spondylosis.
Clin Rheumatol 30:1527–1534Cevik R, Bilici A, Nas K, Demircan Z, Tekin RC (2010)
Noninvasive evaluation of vertebral artery blood flow in cervical spondylosis with and without vertigo and association with degenerative changes.
Clin Rheumatol 29:541–546Olszewski J, Majak J, Pietkiewicz P, Luszcz C, Repetowski M (2006)
The association between positional vertebral and basilar artery flow lesion and prevalence of vertigo in patients with cervical spondylosis.
Otolaryngol Head Neck Surg 134:680–684Strek P, Rero? E, Maga P, Modrzejewski M, Szybist N (1998)
A possible correlation between vertebral artery insufficiency and degenerative changes in the cervical spine.
Eur Arch Oto-Rhino-Laryngol 255:437–440Barre J (1926)
On a posterior cervical sympathetic syndrome and its frequent cause: cervical arthritis [in French].
Rev Neurol (Paris) 45:1246–1248Heistad DD, Marcus ML, Gross PM (1978)
Effects of sympathetic nerves on cerebral vessels in dog, cat, and monkey.
Am J Physiol 235:H544–H552Heistad DD, Marcus ML (1978)
Evidence that neural mechanisms do not have important effects on cerebral blood flow.
Circ Res 42:295–302Faraci FM, Heistad DD (1990)
Regulation of large cerebral arteries and cerebral microvascular pressure.
Circ Res 66:8–17Baumbach GL, Heistad DD, Siems JE (1989)
Effect of sympathetic nerves on composition and distensibility of cerebral arterioles in rats.
J Physiol 416:123–140Pearce JMS (2004)
Barré-Liéou “syndrome”.
J Neurol Neurosurg Psychiatry 75:319Foster CA, Jabbour P (2007)
Barré–Lieou syndrome and the problem of the obsolete eponym.
J Laryngol Otol 121:680–683Li J, Gu T, Yang H, Liang L, Jiang D, Wang Z et al (2014)
Sympathetic nerve innervation in cervical posterior longitudinal ligament as a potential causative factor in cervical spondylosis with sympathetic symptoms and preliminary evidence.
Med Hypotheses 82:631–635Hong L, Kawaguchi Y (2011)
Anterior cervical discectomy and fusion to treat cervical spondylosis with sympathetic symptoms.
J Spinal Disord Tech 24:11–14Li H, Ma X, Wu X, Liu F, Yu T, Yue B et al (2014)
Morphological observation of sympathetic nerve fibers in the human posterior longitudinal ligament.
Spine 39:2119–2126Zhong Z, Hu J, Zhai J, Tian Y, Qiu G, Weng X et al (2015)
Therapeutic effect and mechanism of the surgical treatment for cervical vertigo with cervical spondylosis.
Zhonghua Yi Xue Za Zhi 95:2014–2017Muheremu A, Sun Y, Yan K, Yu J, Zheng S, Tian W (2016)
Effect of anterior cervical discectomy and fusion on patients with atypical symptoms related to cervical spondylosis.
J Neurol Surg A Cent Eur Neurosurg 77:395–399Li J, Jiang D-J, Wang X-W, Yuan W, Liang L, Wang Z-C (2016)
Mid-term outcomes of anterior cervical fusion for cervical spondylosis with sympathetic symptoms.
Clin Spine Surg 29:255–260Wang Z, Wang X, Yuan W, Jiang D (2011)
Degenerative pathological irritations to cervical PLL may play a role in presenting sympathetic symptoms.
Med Hypotheses 77:921–923Zuo J, Han J, Qiu S, Luan F, Zhu X, Gao H et al (2014)
Neural reflex pathway between cervical spinal and sympathetic ganglia in rabbits: implication for pathogenesis of cervical vertigo.
Spine J 14:1005–1009Bose K (1999)
The efficacy and safety of eperisone in patients with cervical spondylosis: results of a randomized, double-blind, placebo-controlled trial.
Methods Find Exp Clin Pharmacol 21:209–213Moustafa IM, Diab AA, Harrison DE (2017)
The effect of normalizing the sagittal cervical configuration on dizziness, neck pain, and cervicocephalic kinesthetic sensibility: a 1-year randomized controlled study.
Eur J Phys Rehabil Med 53:57–71Galm R, Rittmeister M, Schmitt E (1998)
Vertigo in patients with cervical spine dysfunction.
Eur Spine J 7:55–58Homola S (2006)
Chiropractic: history and overview of theories and methods.
Clin Orthop 444:236–242Ndetan H, Hawk C, Sekhon VK, et al.
The Role of Chiropractic Care in the Treatment of Dizziness or Balance Disorders:
Analysis of National Health Interview Survey Data
J Evid Based Complementary Altern Med. 2016 (Apr); 21 (2): 138–142Ernst E (2008)
Chiropractic: a critical evaluation.
J Pain Symptom Manag 35:544–562Young Y-H, Chen C-H (2003)
Acute vertigo following cervical manipulation.
Laryngoscope 113:659–662Frumkin LR, Baloh RW (1990)
Wallenberg’s syndrome following neck manipulation.
Neurology 40:611–615Albuquerque FC, Hu YC, Dashti SR, Abla AA, Clark JC, Alkire B et al (2011)
Craniocervical arterial dissections as sequelae of chiropractic manipulation: patterns of injury and management.
J Neurosurg 115:1197–1205John S, Tavee J (2015)
Bilateral diaphragmatic paralysis due to cervical chiropractic manipulation.
Neurologist 9:65–67Quesnele JJ, Triano JJ, Noseworthy MD, Wells GD.
Changes in Vertebral Artery Blood Flow Following Various Head Positions and Cervical Spine Manipulation
J Manipulative Physiol Ther. 2014 (Jan); 37 (1): 22–31Fischer AJ, Verhagen WI, Huygen PL (1997)
Whiplash injury. A clinical review with emphasis on neuro-otological aspects.
Clin Otolaryngol Allied Sci 22:192–201Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, Zeiss E.
Scientific Monograph of the Quebec Task Force on Whiplash-Associated Disorders
Redefining Whiplash and its Management
Spine (Phila Pa 1976). 1995 (Apr 15); 20 (8 Suppl): S1-S73Evans RW (1992)
Some observations on whiplash injuries.
Neurol Clin 10:975–997Claussen CF, Claussen E (1995)
Neurootological contributions to the diagnostic follow-up after whiplash injuries.
Acta Oto-Laryngol Suppl 520:53–56Ernst A, Basta D, Seidl RO, Todt I, Scherer H, Clarke A (2005)
Management of posttraumatic vertigo.
Otolaryngol Head Neck Surg 132:554–558Vibert D, Häusler R (2003)
Acute peripheral vestibular deficits after whiplash injuries.
Ann Otol Rhinol Laryngol 112:246–251Nacci A, Ferrazzi M, Berrettini S, Panicucci E, Matteucci J, Bruschini L et al (2011)
Vestibular and stabilometric findings in whiplash injury and minor head trauma.
Acta Otorhinolaryngol Ital 31:378–389Dispenza F, De Stefano A, Mathur N, Croce A, Gallina S (2011)
Benign paroxysmal positional vertigo following whiplash injury: a myth or a reality?
Am J Otolaryngol 32:376–380Gordon CR, Levite R, Joffe V, Gadoth N (2004)
Is posttraumatic benign paroxysmal positional vertigo different from the idiopathic form?
Arch Neurol 61:1590–1593Tjell C, Rosenhall U (1998)
Smooth pursuit neck torsion test: a specific test for cervical dizziness.
Am J Otol 19:76–81Yu L-J, Stokell R, Treleaven J (2011)
The effect of neck torsion on postural stability in subjects with persistent whiplash.
Man Ther 16:339–343Treleaven J, Jull G, Lowchoy N (2005)
Standing balance in persistent whiplash: a comparison between subjects with and without dizziness.
J Rehabil Med 37:224–229Hinoki M (1985)
Vertigo due to whiplash injury: a neurotological approach.
Acta Oto-Laryngol Suppl 419:9–29Treleaven J (2017)
Dizziness, unsteadiness, visual disturbances, and sensorimotor control in traumatic neck pain.
J Orthop Sports Phys Ther 47:492–502Madeleine P, Prietzel H, Svarrer H, Arendt-Nielsen L (2004)
Quantitative posturography in altered sensory conditions: a way to assess balance instability in patients with chronic whiplash injury.
Arch Phys Med Rehabil 85:432–438Endo K, Suzuki H, Yamamoto K (2008)
Consciously postural sway and cervical vertigo after whiplash injury.
Spine 33:E539–E542Vonk J, Horlings CGC, Allum JHJ (2010)
Differentiating malingering balance disorder patients from healthy controls, compensated unilateral vestibular loss, and whiplash patients using stance and gait posturography.
Audiol Neurootol 15:261–272Karlberg M, Johansson R, Magnusson M, Fransson PA (1996)
Dizziness of suspected cervical origin distinguished by posturographic assessment of human postural dynamics.
J Vestib Res Equilib Orientat 6:37–47Norré ME (1987)
Cervical vertigo. Diagnostic and semiological problem with special emphasis upon “cervical nystagmus”.
Acta Otorhinolaryngol Belg 41:436–452Brandt T, Bronstein AM (2001)
Cervical vertigo.
J Neurol Neurosurg Psychiatry 71:8–12Oosterveld WJ, Kortschot HW, Kingma GG, de Jong HA, Saatci MR (1991)
Electronystagmographic findings following cervical whiplash injuries.
Acta Otolaryngol (Stockh) 111:201–205Basteris A, Pedler A, Sterling M (2016)
Evaluating the neck joint position sense error with a standard computer and a webcam.
Man Ther 26:231–234de Vries J, Ischebeck BK, Voogt LP, van der Geest JN. 2015.
Joint Position Sense Error in People With Neck Pain: A Systematic Review
Man Ther. 2015 (Dec); 20 (6): 736–744Feipel V, Salvia P, Klein H, Rooze M (2006)
Head repositioning accuracy in patients with whiplash-associated disorders.
Spine 31:E51–E58Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R (2003)
Development of motor system dysfunction following whiplash injury.
Pain 103:65–73Jansen GB, Edlund C, Grane P, Hildingsson C, Karlberg M, Link H et al (2008)
Whiplash injuries: diagnosis and early management.
The Swedish Society of Medicine and the Whiplash Commission Medical Task Force.
Eur Spine J 17:S355–S417Malmström E-M, Karlberg M, Fransson P-A, Lindbladh J, Magnusson M (2009)
Cervical proprioception is sufficient for head orientation after bilateral vestibular loss.
Eur J Appl Physiol 107:73–81Gimse R, Tjell C, Bjørgen IA, Saunte C (1996)
Disturbed eye movements after whiplash due to injuries to the posture control system.
J Clin Exp Neuropsychol 18:178–186Dispenza F, Gargano R, Mathur N, Saraniti C, Gallina S (2011)
Analysis of visually guided eye movements in subjects after whiplash injury.
Auris Nasus Larynx 38:185–189Prushansky T, Dvir Z, Pevzner E, Gordon CR (2004)
Electrooculographic measures in patients with chronic whiplash and healthy subjects: a comparative study.
J Neurol Neurosurg Psychiatry 75:1642–1644Kongsted A, Jørgensen LV, Bendix T, Korsholm L, Leboeuf-Yde C (2007)
Are smooth pursuit eye movements altered in chronic whiplash-associated disorders? A cross-sectional study.
Clin Rehabil 21:1038–1049Ischebeck BK, de Vries J, Van der Geest JN, Janssen M, Van Wingerden JP et al (2016)
Eye movements in patients with Whiplash Associated Disorders: a systematic review.
BMC Musculoskelet Disord 17:441Treleaven J, Jull G, LowChoy N (2005)
Smooth pursuit neck torsion test in whiplash-associated disorders: relationship to self-reports
of neck pain and disability, dizziness and anxiety.
J Rehabil Med 37:219–223Janssen M, Ischebeck BK, de Vries J, Kleinrensink G-J, Frens MA (2015)
Smooth pursuit eye movement deficits in patients with whiplash and neck pain are modulated by target predictability.
Spine 40:E1052–E1057Treleaven J, Jull G, LowChoy N (2006)
The relationship of cervical joint position error to balance and eye movement disturbances in persistent whiplash.
Man Ther 11:99–106Jull GA, O’Leary SP, Falla DL (2008)
Clinical assessment of the deep cervical flexor muscles: the craniocervical flexion test.
J Manip Physiol Ther 31:525–533Montfoort I, Van Der Geest JN, Slijper HP, De Zeeuw CI, Frens MA (2008)
Adaptation of the cervico- and vestibulo-ocular reflex in whiplash injury patients.
J Neurotrauma 25:687–693Kelders WP, Kleinrensink GJ, Geest JNVD, Schipper IB, Feenstra L et al (2005)
The cervico-ocular reflex is increased in whiplash injury patients.
J Neurotrauma 22:133–137Jull G, Kristjansson E, Dall’Alba P (2004)
Impairment in the cervical flexors: a comparison of whiplash and insidious onset neck pain patients.
Man Ther 9:89–94Teasell RW, McClure JA, Walton D, Pretty J, Salter K, Meyer M et al (2010)
A research synthesis of therapeutic interventions for whiplash-associated disorder (WAD):
part 4—noninvasive interventions for chronic WAD.
Pain Res Manag 15:313–322Lystad RP, Bell G, Bonnevie-Svendsen M, Carter CV (2011)
Manual therapy with and without vestibular rehabilitation for cervicogenic dizziness: a systematic review.
Chiropr Man Ther 19:21Yaseen K, Hendrick P, Ismail A, Felemban M, Alshehri MA (2018)
The effectiveness of manual therapy in treating cervicogenic dizziness: a systematic review.
J Phys Ther Sci 30:96–102Van Oosterwijck J, Nijs J, Meeus M, Truijen S, Craps J, Van den Keybus N et al (2011)
Pain neurophysiology education improves cognitions, pain thresholds, and movement performance in people with chronic whiplash: a pilot study.
J Rehabil Res Dev 48:43–58Meeus M, Nijs J, Hamers V, Ickmans K, Oosterwijck JV (2012)
The efficacy of patient education in whiplash associated disorders: a systematic review.
Pain Physician 15:351–361Minguez-Zuazo A, Grande-Alonso M, Saiz BM, La Touche R, Lara SL (2016)
Therapeutic patient education and exercise therapy in patients with cervicogenic dizziness: a prospective case series clinical study.
J Exerc Rehabil 12:216–225Michaleff ZA, Maher CG, Lin C-WC, Rebbeck T, Jull G, Latimer J et al (2014)
Comprehensive physiotherapy exercise programme or advice for chronic whiplash (PROMISE): a pragmatic randomised controlled trial.
Lancet Lond Engl 384:133–141Nijs J, Ickmans K (2014)
Chronic whiplash-associated disorders: to exercise or not?
Lancet Lond Engl 384:109–111Sterling M (2014)
Physiotherapy management of whiplash-associated disorders (WAD).
J Physiother 60:5–12Teasell RW, McClure JA, Walton D, Pretty J, Salter K, Meyer M et al (2010)
A research synthesis of therapeutic interventions for whiplash-associated disorder (WAD): part 5—surgical and injection-based interventions for chronic WAD.
Pain Res Manag 15:323–334Sorensen BF (1978)
Bow hunter’s stroke.
Neurosurgery 2:259–261Lu DC, Zador Z, Mummaneni PV, Lawton MT (2010)
Rotational vertebral artery occlusion—series of 9 cases.
Neurosurgery 67:1066–1072Zaidi HA, Albuquerque FC, Chowdhry SA, Zabramski JM, Ducruet AF, Spetzler RF (2014)
Diagnosis and management of bow hunter’s syndrome: 15-year experience at barrow neurological institute.
World Neurosurg 82:733–738Jost GF, Dailey AT (2015)
Bow hunter’s syndrome revisited: 2 new cases and literature review of 124 cases.
Neurosurg Focus 38:E7Tsutsumi S, Ito M, Yasumoto Y (2008)
Simultaneous bilateral vertebral artery occlusion in the lower cervical spine manifesting as bow hunter’s syndrome.
Neurol Med Chir (Tokyo) 48:90–94Buchanan CC, McLaughlin N, Lu DC, Martin NA (2014)
Rotational vertebral artery occlusion secondary to adjacent-level degeneration following anterior cervical discectomy and fusion.
J Neurosurg Spine 20:714–721Miele VJ, France JC, Rosen CL (2008)
Subaxial positional vertebral artery occlusion corrected by decompression and fusion.
Spine 33:E366–E370Lee V, Riles TS, Stableford J, Berguer R (2011)
Two case presentations and surgical management of Bow Hunter’s syndrome associated with bony abnormalities of the C7 vertebra.
J Vasc Surg 53:1381–1385Darkhabani MZ, Thompson MC, Lazzaro MA, Taqi MA, Zaidat OO (2012)
Vertebral artery stenting for the treatment of bow hunter’s syndrome: report of 4 cases.
J Stroke Cerebrovasc Dis 21:908.e1–908.e5Matsuyama T, Morimoto T, Sakaki T (1997)
Comparison of C1–2 posterior fusion and decompression of the vertebral artery in the treatment of bow hunter’s stroke.
J Neurosurg 86:619–623Sarkar J, Wolfe SQ, Ching BH, Kellicut DC (2014)
Bow hunter’s syndrome causing vertebrobasilar insufficiency in a young man with neck muscle hypertrophy.
Ann Vasc Surg 28:1032.e1–1032. e10Saito K, Hirano M, Taoka T, Nakagawa H, Kitauchi T, Tanizawa E et al (2010)
Artery-to-artery embolism with a mobile mural thrombus due to rotational vertebral artery occlusion.
J Neuroimaging 20:284–286Tominaga T, Takahashi T, Shimizu H, Yoshimoto T (2002)
Rotational vertebral artery occlusion from occipital bone anomaly: a rare cause of embolic stroke.
J Neurosurg 97:1456–1459Citow JS, Macdonald RL (1999)
Posterior decompression of the vertebral artery narrowed by cervical osteophyte: case report.
World Neurosurg 51:495–499Inamasu J, Nakatsukasa M (2013)
Rotational vertebral artery occlusion associated with occipitoatlantal assimilation, atlantoaxial subluxation, and basilar impression.
Clin Neurol Neurosurg 115:1520–1523Safain MG, Talan J, Malek AM, Hwang SW (2014)
Spontaneous atraumatic vertebral artery occlusion due to physiological cervical extension: case report.
J Neurosurg Spine 20:278–282Cornelius JF, George B, Oka DN, Spiriev T, Steiger HJ, Hänggi D (2012)
Bow-hunter’s syndrome caused by dynamic vertebral artery stenosis at the cranio-cervical junction—a management algorithm based on a systematic review and a clinical series.
Neurosurg Rev 35:127–135Yenigun A, Ustun ME, Tugrul S, Dogan R, Ozturan O (2016)
Classification of vertebral artery loop formation and association with cervicogenic dizziness.
J Laryngol Otol 130:1115–1119Strupp M, Planck JH, Arbusow V, Steiger HJ, Brückmann H, Brandt T (2000)
Rotational vertebral artery occlusion syndrome with vertigo due to “labyrinthine excitation”.
Neurology 54:1376–1379Matsuyama T, Morimoto T, Sakaki T (1997)
Bow Hunter’s stroke caused by a nondominant vertebral artery occlusion: case report.
Neurosurgery 41:1393–1395Sugiu K, Agari T, Tokunaga K, Nishida A, Date I (2009)
Endovascular treatment for bow hunter’s syndrome: case report.
Minim Invasive Neurosurg MIN 52:193–195Yoshimura K, Iwatsuki K, Ishihara M, Onishi Y, Umegaki M, Yoshimine T (2011)
Bow Hunter's Stroke Due To Instability at the Uncovertebral C3/4 Joint
European Spine Journal 2011 (Jul); 20 Suppl 2: S266–170Yeh J-F, Lin Y-J, Po HL, Wang S-F, Pan P-Y, Cheng S-J et al (2005)
A case of bow hunter’s stroke caused by non-dominant vertebral artery.
Acta Neurol Taiwanica 14:69–73Noh Y, Kwon O-K, Kim H-J, Kim JS (2011)
Rotational vertebral artery syndrome due to compression of nondominant vertebral artery terminating in posterior inferior cerebellar artery.
J Neurol 258:1775–1780Healy AT, Lee BS, Walsh K, Bain MD, Krishnaney AA (2015)
Bow hunter’s syndrome secondary to bilateral dynamic vertebral artery compression.
J Clin Neurosci 22:209–212Choi K-D, Choi J-H, Kim J-S, Kim HJ, Kim M-J, Lee T-H et al (2013)
Rotational vertebral artery occlusion: mechanisms and long-term outcome.
Stroke 44:1817–1824Kim H-A, Yi H-A, Lee C-Y, Lee H (2011)
Origin of isolated vertigo in rotational vertebral artery syndrome.
Neurol Sci 32:1203–1207Choi K-D, Shin H-Y, Kim JS, Kim S-H, Kwon O-K, Koo J-W et al (2005)
Rotational vertebral artery syndrome: oculographic analysis of nystagmus.
Neurology 65:1287–1290Whitmore RG, Simon SL, Hurst RW, Nisenbaum HL, Kasner SE, Zager EL (2007)
Bow hunter’s syndrome caused by accessory cervical ossification: posterolateral decompression and the use of intraoperative Doppler ultrasonography.
Surg Neurol 67:169–171Vilela MD, Goodkin R, Lundin DA, Newell DW (2005)
Rotational vertebrobasilar ischemia: hemodynamic assessment and surgical treatment.
Neurosurgery 56:36–45Iguchi Y, Kimura K, Shibazaki K, Iwanaga T, Ueno Y, Inoue T (2006)
Transcranial doppler and carotid duplex ultrasonography findings in Bow hunter’s syndrome.
J Neuroimaging 16:278–280Shum GL, Cinnamond S, Hough AD, Craven R, Whittingham W (2017)
Test–retest reliability of measuring the vertebral arterial blood flow velocity in people with cervicogenic dizziness.
J Manip Physiol Ther 40:255–262Rastogi V, Rawls A, Moore O, Victorica B, Khan S, Saravanapavan P et al (2015)
Rare etiology of bow hunter’s syndrome and systematic review of literature.
J Vasc Interv Neurol 8:7–16Mitchell J (2009)
Vertebral artery blood flow velocity changes associated with cervical spine rotation: a meta-analysis of the evidence with implications for professional practice.
J Man Manip Ther 17:46–57Strickland BA, Pham MH, Bakhsheshian J, Russin JJ, Mack WJ, Acosta FL (2017)
Bow hunter’s syndrome: surgical management (video) and review of the literature.
World Neurosurg 103:953. e7–953.e12Velat GJ, Reavey-Cantwell JF, Ulm AJ, Lewis SB (2006)
Intraoperative dynamic angiography to detect resolution of Bow Hunter’s syndrome: technical case report.
Surg Neurol 66:420–423Chastain HD, Campbell MS, Iyer S, Roubin GS, Vitek J, Mathur A et al (1999)
Extracranial vertebral artery stent placement: inhospital and follow-up results.
J Neurosurg 91:547–552Horowitz M, Jovin T, Balzar J, Welch W, Kassam A (2002)
Bow hunter’s syndrome in the setting of contralateral vertebral artery stenosis: evaluation and treatment options.
Spine 27:E495–E498Weintraub MI (1993)
Beauty parlor stroke syndrome: report of five cases.
JAMA 269:2085–2086Correia PN, Meyer IA, Eskandari A, Michel P (2016)
Beauty parlor stroke revisited: an 11-year single-center consecutive series.
Int J Stroke 11:356–360Kameda T, Otani K, Tamura T, Konno S (2018)
Beauty parlor stroke syndrome due to a bone fragment from an osteophyte of the atlas: case report.
J Neurosurg Spine 28:389–394Endo K, Ichimaru K, Shimura H, Imakiire A (2000)
Cervical vertigo after hair shampoo treatment at a hairdressing salon: a case report.
Spine 25:632–634Heckmann JG, Heron P, Kasper B, Dorfler A, Maihofner C (2006)
Beauty parlor stroke syndrome.
Cerebrovasc Dis Basel Switz 21:140–141Sahin N, Karata? O, Ozkaya M, Cakmak A, Berker E (2008)
Demographics features, clinical findings and functional status in a group of subjects with cervical myofascial pain syndrome.
Agri 20:14–19Krabak BJ, Borg-Stein J, Oas JA (2000)
Chronic cervical myofascial pain syndrome: Improvement in dizziness and pain with a multidisciplinary rehabilitation program. A pilot study.
J Back Musculoskelet Rehabil 15:83–87Giamberardino MA, Affaitati G, Fabrizio A, Costantini R (2011)
Myofascial pain syndromes and their evaluation.
Best Pract Res Clin Rheumatol 25:185–198Thompson JM (2012)
Exercise in muscle pain disorders.
PM R 4:889–893Aydin T, Dernek B, Sentürk Ege T, Karan A, Aksoy C (2018)
The effectiveness of dry needling and exercise therapy in patients with dizziness caused by cervical myofascial pain syndrome; prospective randomized clinical study.
Pain Med Malden Mass.
https ://doi.org/10.1093/pm/pny07Jaroshevskyi OA, Payenok OS, Logvinenko AV (2017)
Evalution of the effectiveness of multimodal approach to the management of cervical vertigo.
Wiad Lek 70:571–573Reneker JC, Clay Moughiman M, Cook CE (2015)
The diagnostic utility of clinical tests for differentiating between cervicogenic and other causes of dizziness after a sports-related concussion: an international Delphi study.
J Sci Med Sport 18:366–372Rashad UM (2010)
Patients with benign paroxysmal positional vertigo and cervical spine problems: is Epley’s manoeuvre contraindicated, and is a proposed new manoeuvre effective and safer?
J Laryngol Otol 124:1167–1171Humphriss RL, Baguley DM, Sparkes V, Peerman SE, Moffat D (2003)
Contraindications to the Dix–Hallpike manoeuvre: a multidisciplinary review.
Int J Audiol 42:166–173
Return to VERTIGO and/or BALANCE
Since 5-19-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |