

Effect of High-dose Oral Multivitamins and Minerals
in Participants Not Treated with Statins in the
Randomized Trial to Assess Chelation Therapy (TACT)This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Am Heart J. 2018 (Jan); 195: 7077 ~ FULL TEXT
Omar M. Issa, DO, Rhonda Roberts, MPH, Daniel B. Mark, MD, MPH, Robin Boineau, MD,
Christine Goertz, DC, PhD, Yves Rosenberg, MD, MPH, Eldrin F. Lewis, MD,
Erminia Guarneri, MD, Jeanne Drisko, MD, Allan Magaziner, DO, Kerry L. Lee, PhD,
Gervasio A. Lamas, MD'Correspondence information about the author MD Gervasio A. Lamas
Columbia University Division of Cardiology at Mount Sinai Medical Center,
Miami Beach, FL.IMPORTANCE: In a prespecified subgroup analysis of participants not on statin therapy at baseline in the TACT, a high-dose complex oral multivitamins and multimineral regimen was found to have a large unexpected benefit compared with placebo. The regimen tested was substantially different from any vitamin regimen tested in prior clinical trials.
OBJECTIVE: To explore these results, we performed detailed additional analyses of participants not on statins at enrollment in TACT.
DESIGN: TACT was a factorial trial testing chelation treatments and a 28-component high-dose oral multivitamins and multiminerals regimen versus placebo in post-myocardial infarction (MI) patients 50 years or older.
PARTICIPANTS: There were 460 (27%) of 1,708 TACT participants not taking statins at baseline, 224 (49%) were in the active vitamin group and 236 (51%) were in the placebo group.
SETTING: Patients were enrolled at 134 sites around the United States and Canada.
INTERVENTION: Daily high-dose oral multivitamins and multiminerals (6 tablets, active or placebo).
MAIN OUTCOME: The primary end point of TACT was time to the first occurrence of any component of the composite end point: all-cause mortality, MI, stroke, coronary revascularization, or hospitalization for angina.
RESULTS: The primary end point occurred in 137 nonstatin participants (30%), of which 51 (23%) of 224 were in the active group and 86 (36%) of 236 were taking placebo (hazard ratio, 0.62; 95% confidence interval, 0.44-0.87; P=.006). Results in the key TACT secondary end point, a combination of cardiovascular mortality, stroke, or recurrent MI, was consistent in favoring the active vitamin group (hazard ratio, 0.46; 95% confidence interval, 0.28-0.75; P=.002). Multiple end point analyses were consistent with these results.
CONCLUSION AND RELEVANCE: High-dose oral multivitamin and multimineral supplementation seem to decrease combined cardiac events in a stable, post-MI population not taking statin therapy at baseline. These unexpected findings are being retested in the ongoing TACT2.
From the FULL TEXT Article:
Introduction
More than half of the US population uses dietary supplements. Oral multivitamins and multiminerals (OMVMs), the most commonly used, accounted for 31% of the supplements purchased by consumers in 2011 to 2012. [1, 2] Interestingly, there is no standardized definition for OMVM, and the term can refer to a wide range of products with varied compositions and characteristics. [3] This rapidly growing, nearly $30 billion industry [4], is built on the premise that OMVMs promote health and wellness, particularly with respect to cardiovascular disease.
Despite promising results obtained in some initial trials of individual vitamins such as vitamin E or niacin, [57] no OMVM has demonstrated reductions in cardiovascular events in the statin era. [811] The uniformity of results from most of these studies have led many professional organizations, including the National Institutes of Health [12] and the US Preventive Services Task Force [13] to conclude that there is no evidence to support any form of OMVM supplementation for the prevention of cardiovascular disease. Lacking any strong theoretical model or empirical data to guide the design of trials in this area, past studies used a drug model for vitamin therapy, testing individual supplements, most commonly vitamins A, B12, C, and E, as well as folic acid and selenium, generally at low to moderate doses, [8, 9, 11] whereas the consumer market has moved toward complex mixtures containing dozens of compounds in varying doses. [14, 15]
The TACT vitamin study previously reported 11% and 18% nonsignificant reductions in the composite primary event rate (hazard ratio [HR], 0.89; 95% confidence interval [CI], 0.751.07; P=.21) and key secondary end point of cardiovascular, death, myocardial infarction (MI), or nonfatal stroke (HR, 0.82; 95% CI, 0.611.07; P=.14) associated with the high-dose vitamin regimen relative to placebo. [16, 17] One of the 9 prespecified subgroups, use of statins at baseline, showed a significant interaction with the vitamin treatment: patients on statins at baseline had no effect from the OMVM regimen (HR, 1.03), whereas patients not on statins at baseline had an almost 40% reduction in the primary end point (HR, 0.62). The present report provides additional information and analyses regarding this prespecified (but unexpected) subgroup interaction.
Methods
Design
TACT was the result of a request for applications, issued by the National Center for Complementary and Alternative Medicine, cofunded by the National Heart Lung and Blood Institute, for a trial of edetate disodium chelation treatments in participants with cardiovascular disease. No extramural funding was used to support this work. In 2002, when the trial was being designed, the investigators found that edetate disodium chelation as used by alternative medicine practitioners was routinely accompanied by high doses of OMVM, a potential source of confounding. Thus, TACT used a 2 Χ 2 factorial design that included 4 groups: active chelation infusion plus active OMVM, active chelation infusion plus placebo OMVM, placebo chelation infusion plus active OMVM, and placebo chelation infusion plus placebo OMVM in a 1:1:1:1 ratio. TACT was a double-blind, multicenter investigation that enrolled participants from 134 sites in the United States and Canada. The trial was conducted under the appropriate supervision of institutional review boards, and informed consent was obtained from all participants. The full design and results of TACT have been published previously (https://clinicaltrials.gov/ct2/show/ NCT00044213). [1620]

Table 1 Participants included in the study were 50 years or older, had sustained an MI at least 6 weeks before enrollment, and had serum creatinine <2.0 mg/dL. The treatments consisted of 40 chelation or placebo infusions (30 weekly infusions followed by 10 maintenance infusions spaced 28 weeks apart), and daily high-dose OMVM (6 tablets, active or placebo) for the duration of the trial. The 28component high-dose OMVM used in the study was designed under guidance from chelation experts to represent a typical regimen used in a chelation practice in the early 2000s (Table I). Participants, study staff, and study data managers were blinded to treatment allocation.
Study end points
The primary end point of TACT was time to the first occurrence of any component of the composite end point: all-cause mortality, MI, stroke, coronary revascularization, or hospitalization for angina. The major secondary end point was time to the first occurrence of cardiovascular mortality, stroke, or recurrent MI. These were assessed at quarterly clinic visits and adjudicated, except for revascularizations, by a blinded independent Endpoints Committee at Brigham at Women's Hospital.
Statistical analysis
Statistical power calculations, randomization processes, and allocation concealment have been previously published. [1618] Adverse events were statistically similar in the OMVM group and the placebo group as previously published. [17] Baseline characteristics of participants were descriptively summarized using the median and 25th and 75th percentiles for continuous variables, and frequencies and percentages for categorical variables. The baseline characteristics of participants on statins were compared with those not taking statins using the Wilcoxon rank sum test for continuous variables and the conventional χ2 test for categorical variables. These tests were also used for comparing baseline characteristics between the OMVM and placebo groups in the prespecified subgroup of participants not taking statins. All analyses were by intention-to-treat. Cumulative event rates as a function of time from randomization were calculated using the Kaplan-Meier method. [21] The log-rank test was used to compare outcome differences with respect to(a) the primary end point between groups taking statins versus not,
(b) the primary end point between active OMVM and placebo OMVM among participants not taking statins,
(c) the primary end point between active OMVM and placebo OMVM among participants taking statins, and
(d) all-cause mortality between active OMVM and placebo OMVM among participants not taking statins.Hazard ratios with 95% CIs for summarizing between-group differences were computed using the Cox proportional hazards model. [22] The Cox model was also used for assessing whether the effects of OMVM differed substantially according to whether or not participants were taking statins at the time of enrollment, that is, whether there was an interaction between statin use and OMVM use with respect to the primary and secondary end points. Although the principal analyses were performed using time until the first occurrence of any component of the end points (thus, each patient was counted only once in the treatment comparisons), participants could experience >1 component of the composite primary and secondary end points. Therefore, we performed exploratory analyses using all events that occurred. Two approaches were used, namely,
(1) the method of Anderson and Gill, which is an extension of the Cox proportional hazards model to allow for comparisons using multiple or recurrent events, [23] and
(2) the marginal modeling approach developed by Wie, Lin, and Weissfeld. [24]Two-sided significance testing was used for all statistical tests. Statistical analyses were performed using SAS 9.4 and JMP Pro 12.1 (SAS Institute, Inc, Cary NC). The trial ended based on attainment of the goals of the statistical analysis plan. The analyses presented here must be interpreted as exploratory in nature because of the non-prespecified nature of the analyses, concern with multiplicity (9 prespecified subgroups, of which we are presenting one), and the small number of end points upon which firm conclusions should not be drawn.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Results
Baseline characteristics
TACT patients were enrolled between September 10, 2003, and October 4, 2010, with the final follow-up visit taking place October 31, 2011. The median follow-up was 55 months (interquartile range [IQR], 2660 months). Of the 1708 participants enrolled in the study, 18% were women and the median age was 65 years (IQR, 5972 years). The qualifying MI occurred a median of 4.6 years (IQR, 1.692 years) before enrollment. [16] The overall TACT population consisted of 853 participants randomized to the active OMVM group and 855 randomized to the placebo OMVM group. Treatment was continued for at least 1 year in 76% of patients in each arm and for 3 years in 47% OMVM patients and 50% of placebo patients. The most common reason for the discontinuation of vitamins or placebo was patient preference, 74% and 78%, respectively. Participants also discontinued treatment due to physician preference (5.6% and 4.9%) or adverse events (9.9% and 7.9%).
Statin usage at the time of enrollment in the trial, the focus of these analyses, was a prespecified subgroup. In TACT, 1,248 (73%) were taking a statin medication at baseline, whereas 460 (27%) were not. Participants not taking statins had a lower prevalence of prior revascularization (7/100 fewer for both percutaneous coronary intervention [PCI] and coronary artery bypass grafting [CABG]) and less frequent use of aspirin (21/100), β-blockers (22/100), and blockers of the renin-angiotensin system (23/100). ).

Table 2

Table 3

Table 4
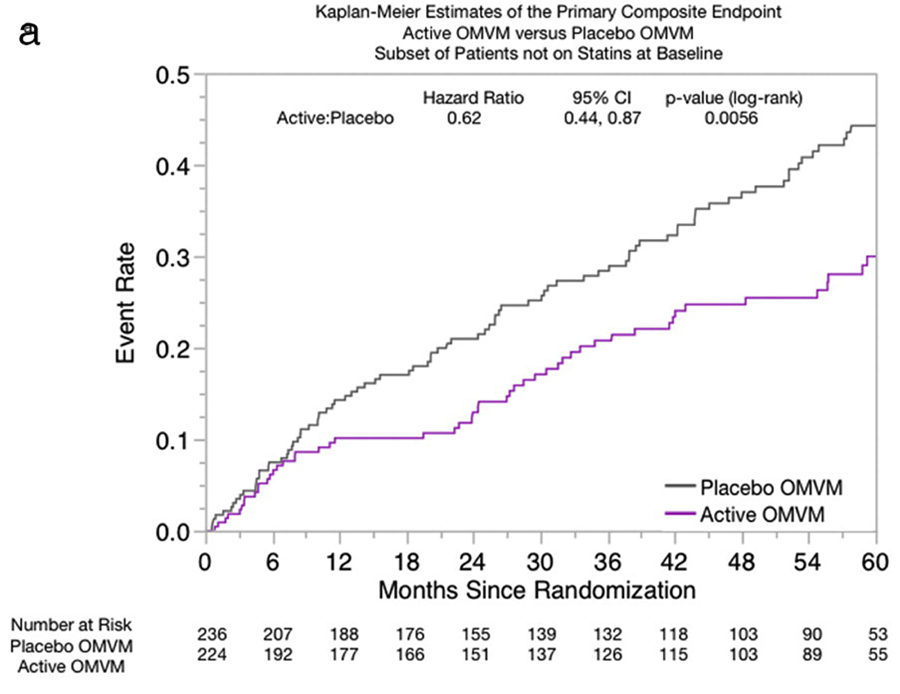
Figure A
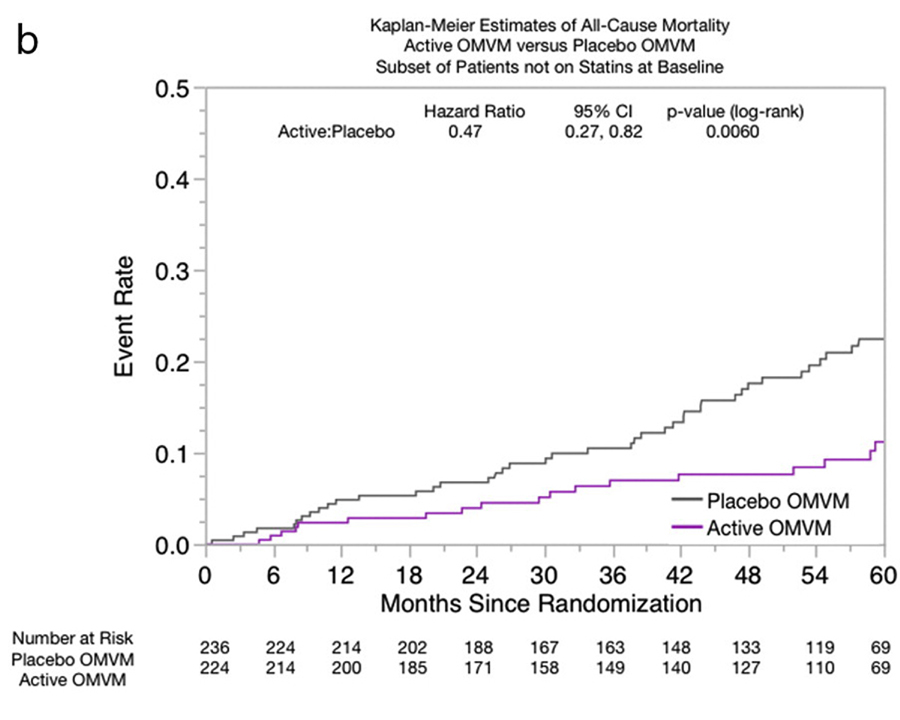
Figure B
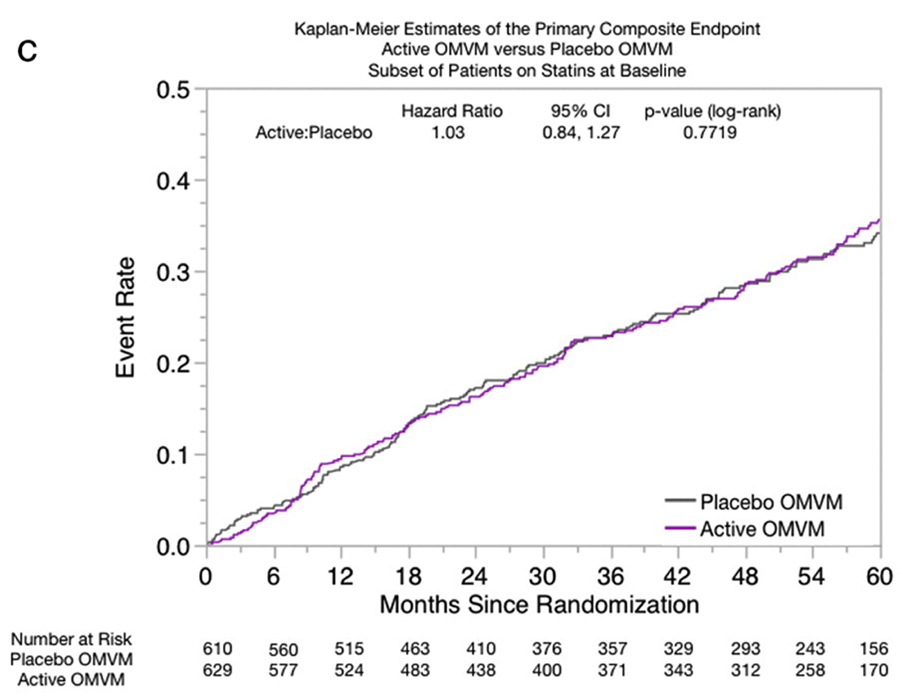
Figure C Total cholesterol and low-density lipoprotein (LDL) cholesterol were both higher in the no-baseline statin therapy group (155198 and 79120 mg/dL, respectively) (Table II). Common reasons participants were not taking statins were patient refusal (32%), physician preference (28%), and other (23%). There were no significant differences in the time to primary end point between the group taking statins and the group that was not (28% vs 30%, P=.27).
Among the 460 participants not taking statins, 224 (49%) were in the active OMVM group and 236 (51%) were in the placebo OMVM group. Nonstatin participants had comparable characteristics when randomized to OMVM or to placebo OMVM, other than less aspirin use (10/100) in the placebo group (Table III).
Clinical eventsNonstatin participants The primary end point of combined major adverse cardiac events occurred in 137 participants (30%), of which 51 (23%) of 224 were in the active OMVM group and 86 (36%) of 236 were in the placebo OMVM group (HR, 0.62; 95% CI, 0.440.87; P=.006) (Table IV, Figure A). There were fewer events in all the individual components of the primary end point in participants in the OMVM group, including mortality, where the difference reached statistical significance (HR, 0.47; 95% CI, 0.270.82; P=.007) (Figure B). ).
In addition, there was a significant difference in the secondary end point, a combination of cardiovascular mortality, stroke, or recurrent MI, in favor of the active OMVM group (HR, 0.46; 95% CI, 0.280.75; P=.002) that was driven by cardiovascular death (HR, 0.39; 95% CI, 0.190.81; P=.009). A multivariable analysis, adjusted for age, sex, history of stroke, history of atrial fibrillation/flutter, history of smoking, aspirin, and warfarin use, and chelation treatment group was conducted demonstrating a similar benefit with OMVM in participants off statins at baseline (HR, 0.65; 95% CI, 0.450.93; P=.019).
The 137 participants not taking statins that suffered from a primary end point event accounted for 205 of the 741 total events from TACT. This included 77 events in the active OMVM arm and 128 in the placebo OMVM arm, giving an HR of 0.28 (95% CI, 0.130.60; P=.001) by the Anderson and Gill method and 0.48 (95% CI, 0.140.83; P ≤ .001) by the Wie, Lin, and Weissfeld model in favor of OMVM.
Statin participants In participants taking statins, there was no beneficial effect of OMVM in the primary end point, secondary end point, or any of their individual components (Figure C). There were no significant differences noted between the active OMVM and placebo arms in total events among participants taking statins (HR of 1.20 [95% CI, 0.801.80; P=.385] by the Anderson and Gill method and 0.94 [95% CI, 0.731.15; P=.542] by the Wie, Lin, and Weissfeld model).
Discussion
The principal finding of this prespecified subgroup analysis is that active OMVM supplementation using a unique formulation created for the TACT trial decreased combined cardiac events in participants not taking statin therapy at baseline. Multivariable analyses and multiple events analyses resulted in congruent statistically significant findings.
These findings were unexpected and have to be viewed with appropriate skepticism. The ongoing TACT2 study, which is currently enrolling 1,200 post-MI diabetic participants into a chelation/OMVM factorial design identical to the TACT1 design, is also using the identical OMVM formulation (XYMOGEN, Orlando, FL) (www.tact2.org). The comparison of OMVM to placebo in the participants unable to take statins will be a prespecified secondary analysis. Results are expected in 2021.
Until TACT2 provides verification that the unexpected benefits of OMVM observed in patients not taking statins can be reproducibly demonstrated, these results should not be used to alter or direct any patient's clinical care. Specifically, participants or physicians cannot use them as a reason to avoid statins for secondary prevention. In such participants, multiple studies demonstrate that the benefits of statin therapy are extraordinarily robust. However, clinicians who care for post-MI participants all have a subset of participants who cannot or will not take statins, and devising effective prognosis modifying alternative management options for these participants has been quite difficult. For this reason, we feel that our unexpected findings regarding a relatively inexpensive therapy that might offer such a benefit should not be dismissed out of hand as simply the play of chance. Whether such is the case can only be determined by attempted replication.
The lack of benefit of oral vitamin supplementation to prevent cardiovascular disease has been reported multiple times. No clinical practice guideline currently recommends such use. Most studies upon which these recommendations are based, however, used moderate doses of small numbers of supplement compounds, usually focusing on vitamins A, C, E, and B12 and folic acid and selenium. The drug therapy model used by these studies is useful for studying single agents to determine their effectiveness for specific clinical applications but do not extend well to the more complex multicomponent therapies increasingly used for self-care by large numbers of the general population together with many patients with diverse illnesses.
TACT used a previously unstudied OMVM preparation composed of 28 components, some in unusually high doses. This regimen was not chosen because of any prior expectations of benefit by the research team but rather to ensure against the potential that such therapy, which was commonly used by the chelation practitioners involved in the study, might confound the ability to clearly identify the treatment effects of the chelation regimen TACT was conducted to study. No evidence of harm was identified in any category of adverse events with this treatment. [17] The use of the TACT OMVM regimen brings with it the potential for very complex pharmacologic and pharmacodynamic interactions that cannot be predicted from either single-agent studies or mechanistic considerations.
The differences in previously studied populations and treatment regimens, when compared with the present study, merit discussion (Table I). The Physician's Health Study II is the only investigation listed that randomized participants to a modern multivitamin regimen (Centrum Silver, Pfizer, formerly Wyeth, 32 components) or placebo. [10] Among the 14,641 men included in the study, only 754 (5%) self-identified as having a prior MI or stroke. Thus, the only prior multivitamin clinical trial included only men and was a primary prevention study. At a median follow-up of 11.2 years, no significant benefit was observed with respect to any major cardiovascular outcomes.
Other recent studies of OMVM do not offer much guidance as to the credibility of the OMVM findings from TACT either because of substantial differences in the regimen used, the population studied, or both. The Supplιmentation en Vitamines et Minιraux Antioxydants (SU.VI.MAX) randomly assigned 13,017 French participants to OMVM, consisting of modest doses of vitamin E, vitamin C, β-carotene, zinc, selenium, or a placebo. [8] There were no significant differences with regard to ischemic heart disease or all-cause mortality in the overall population. The Heart Protection Study, one of very few secondary prevention trials of vitamin therapy, [11] tested a small suite of low-dose supplements and had negative results. Other secondary prevention studies used even smaller regimens, most notably the Heart Outcome Prevention Evaluation (HOPE) studies, and discerned no benefits from supplementation with vitamin E [25, 26] or B vitamins/folic acid. [27, 28]
Caveats
The rationale for including high-dose OMVM in TACT was to prevent confounding of the parent chelation study. The TACT investigators did not expect the OMVM regimen to produce clinically important benefits independent of the chelation treatment. The findings reported here were serendipitously discovered. The relative treatment effect seems quite large and this, in the presence of substantial noncompliance and what we think we already know about OMVM, makes these results seem implausible. However, implausible does not mean wrong. Under these circumstances, it is prudent to view these results skeptically and await TACT2 replication before any serious consideration is given to the potential clinical value of these findings.
References:
Bailey, R.L., Gahche, J.J., Lentino, C.V. et al.
Dietary supplement use in the United States, 2003-2006.
J Nutr. 2011; 141: 261266Kantor, E.D., Rehm, C.D., Du, M. et al.
Trends in dietary supplement use among US adults from 1999-2012.
JAMA. 2016; 316: 14641474Yetley, E.A.
Multivitamin and multimineral dietary supplements: definitions, characterization, bioavailability, and drug interactions.
Am J Clin Nutr. 2007; 85: 269276Anonymous.
NBJ's supplement buisiness report 2006, an analysis of markets, trends, competition and strategy in the U.S. dietary supplement industry.
Nutr Bus J. 2005; : 186200Garg, A., Sharma, A., Krishnamoorthy, P. et al.
Role of niacin in current clinical practice: a systematic review.
Am J Med. 2017; 130: 173187Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico.
Lancet. 1999; 354: 447455Stephens, N.G., Parsons, A., Schofield, P.M. et al.
Randomised controlled trial of vitamin E in patients with coronary disease:
Cambridge Heart Antioxidant Study (CHAOS).
Lancet. 1996; 347: 781786Hercberg, S., Galan, P., Preziosi, P. et al.
The SU.VI.MAX study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals.
Arch Intern Med. 2004; 164: 23352342Chylack, L.T. Jr., Brown, N.P., Bron, A. et al.
The Roche European American Cataract Trial (REACT): a randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract.
Ophthalmic Epidemiol. 2002; 9: 4980Sesso, H.D., Christen, W.G., Bubes, V. et al.
Multivitamins in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial.
JAMA. 2012; 308: 17511760Heart Protection Study Collaborative Group.
MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals:
a randomised placebo-controlled trial.
Lancet. 2002; 360: 2333NIH State-of-the-Science Panel.
National Institutes of Health state-of-the-science conference statement:
multivitamin/mineral supplements and chronic disease prevention.
Ann Intern Med. 2006; 145: 364371Fortmann, S.P., Burda, B.U., Senger, C.A. et al.
Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer:
an updated systematic evidence review for the U.S. Preventive Services Task Force.
Ann Intern Med. 2013; 159: 824834Balk, E.M., Horsley, T.A., Newberry, S.J. et al.
A collaborative effort to apply the evidence-based review process to the field of nutrition:
challenges, benefits, and lessons learned.
Am J Clin Nutr. 2007; 85: 14481456Chung, M., Balk, E.M., Ip, S. et al.
Reporting of systematic reviews of micronutrients and health: a critical appraisal.
Am J Clin Nutr. 2009; 89: 10991113Lamas, G.A., Goertz, C., Boineau, R. et al.
Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial.
JAMA. 2013; 309: 12411250Lamas, G.A., Boineau, R., Goertz, C. et al.
Oral high-dose multivitamins and minerals after myocardial infarction: a randomized trial.
Ann Intern Med. 2013; 159: 797804Lamas, G.A., Goertz, C., Boineau, R. et al.
Design of the Trial to Assess Chelation Therapy (TACT).
Am Heart J. 2012; 163: 712Lamas, G.A., Boineau, R., Goertz, C. et al.
EDTA chelation therapy alone and in combination with oral high-dose multivitamins and minerals for coronary disease: the factorial group results of the Trial to Assess Chelation Therapy. ([e5])
Am Heart J. 2014; 168: 3744Escolar, E., Lamas, G.A., Mark, D.B. et al.
The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT).
Circ Cardiovasc Qual Outcomes. 2014; 7: 1524Kaplan EL, M.P.
Nonparametric estimation from incomplete observations.
J Am Stat Assoc. 1958; 53: 457481Cox, D.R.
Regression models and life-tables.
J R Stat Soc Series B. 1972; 34: 187220Muller, H.G.
Change-points in nonparametric regression analysis.
Ann Stat. 1992; 20: 737761Wei, L., Lin, D., and Weissfeld, L.
Regression analysis of multivariate incomplete failure time data by modeling marginal distributions.
J Am Stat Assoc. 1989; 84: 10651073Lonn, E., Bosch, J., Yusuf, S. et al.
Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial.
JAMA. 2005; 293: 13381347The Heart Outcomes Prevention Evaluation Study Investigators.
Vitamin E supplementation and cardiovascular events.
N Engl J Med. 2000; 342: 154160Lonn, E., Yusuf, S., Pogue, J. et al.
Homocysteine lowering with folic acid and B vitamins in vascular disease.
N Engl J Med. 2006; 354: 15671577Ebbing, M., Bleie, Ψ., Ueland, P.M. et al.
Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial.
JAMA. 2008; 300: 795804
Return to NUTRITION
Since 12-14-2017


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |