

Peripheral Metabolism
of Thyroid Hormones:
A ReviewThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Alternative Medicine Review 2000 (Aug); 5 (4): 306–333 ~ FULL TEXT
Gregory S. Kelly, ND
Associate Editor,
Alternative Medicine Review;
Private Practice
2009 Summer Street,
Stamford, CT 06905.Peripheral metabolism of thyroid hormones is a critical component of the impact these hormones have on intracellular function. Thyroid hormones can be metabolized in peripheral tissue by deiodination, conjugation, deamination, and decarboxylation enzyme reactions. Therefore, alterations in these metabolic pathways might significantly impact the quantity of specific thyroid hormone metabolites influencing function at the cellular level. Available evidence also suggests that, under some circumstances, the activity of hepatic antioxidant enzyme systems and lipid peroxidation might influence the peripheral metabolism of thyroid hormones. Several syndromes, such as "euthyroid sick syndrome" and "low T3 syndrome," have been classified within the medical literature. The common feature of these disorders is a low level of circulating T3, with generally normal to slightly elevated blood T4 levels and either normal or slightly suppressed TSH levels. This pattern of altered thyroid hormone levels is generally agreed to be a result of impairment in extra-thyroidal peripheral metabolism. Hepatic and renal pathology, as well as catabolic states such as those induced subsequent to severe injury, illness, or trauma result in consistent shifts in the thyroid hormone profile, secondary to their impact on peripheral enzyme pathways. Lifestyle factors, such as stress, caloric restriction, and exercise, influence peripheral metabolism of thyroid hormones. Exposure to toxic metals, chemical poisons, and several drugs can also influence the peripheral fate of thyroid hormones. While the role of vitamins, minerals, and botanical extracts in thyroid hormone metabolism requires further elucidation, current evidence supports a role for selenium in the hepatic 5'-deiodination enzyme.
From the FULL TEXT Article:
Introduction
Peripheral metabolism of thyroid hormones is a critical component of the impact these hormones have on intracellular function. Primary hypothyroidism, which manifests as elevated thyroid stimulating hormone (TSH) and low T4 levels, and secondary hypothyroidism, manifesting as a combination of low T4 levels and low TSH secondary to pituitary dysfunction, are both well defined. However, perturbations in thyroid hormone levels secondary to alterations in peripheral metabolism have received far less clinical attention. Several syndromes, such as “euthyroid sick syndrome” (ESS) and “low T3 syndrome,” have been classified within the medical literature. The common feature of these disorders is a low level of circulating T3, with generally normal to slightly elevated blood T4 levels, and either normal or slightly suppressed TSH levels. This pattern of altered thyroid hormones is now generally agreed to be a result of impairment in extra-thyroidal peripheral metabolism.
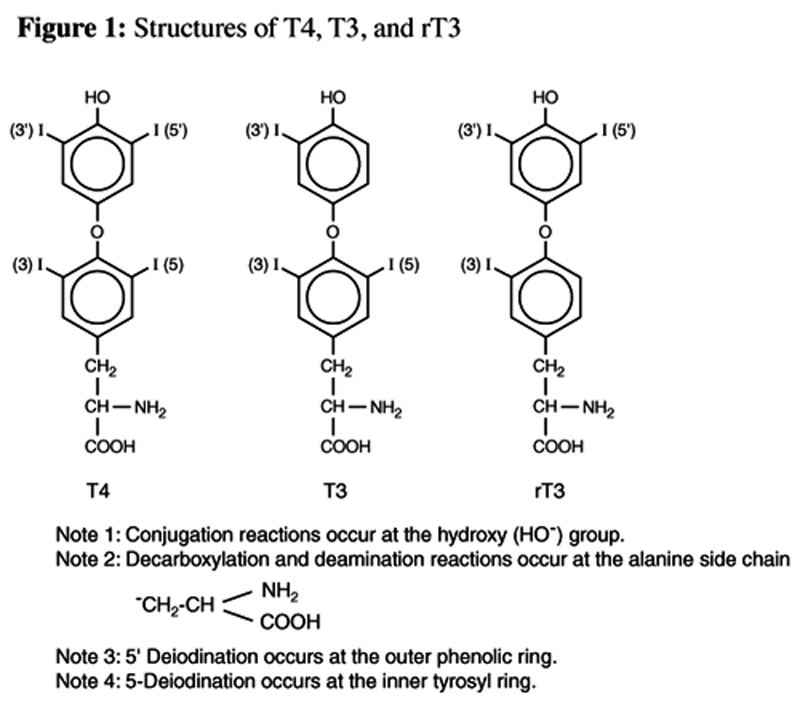
Figure 1 The liver, and to a lesser degree the kidneys, play a dominant, although often under- discussed role in the metabolism of thyroid hormones. The majority of the most metabolically active thyroid hormone, 3,5,3'-triiodothyronine (T3) (Figure 1), is generated in peripheral tissue. Similarly, the preponderance of its competitive inhibitor, 3,3',5'-triiodothyronine (rT3; reverse T3) (Figure 1) is generated outside the thyroid gland. Further transformations to T2 and T1 isomers also occur almost exclusively in peripheral tissue. These transformations are all catalyzed by deiodination enzymes which remove iodine atoms from the inner tyrosyl or outer phenolic benzene rings. This stepwise deiodination is the major route of thyroid hormone metabolism and results in both active and inactive metabolites. [1]
A second pathway of thyroid hormone metabolism involves the conjugation of the phenolic hydroxyl group of the outer phenolic ring with sulfate or glucuronic acid. These conjugation reactions occur primarily in the liver, and to a lesser degree in the kidney, and result in biotransformation of T4 and T3. The resultant metabolites are primed for elimination and are considered relatively inactive. [1] It is thought that partially deiodinated thyroid hormone metabolites are preferred substrates for these conjugation reactions. [2, 3]
Thyroid hormones can also undergo deamination and decarboxylase reactions in the liver, resulting in the formation of so-called acetic acid analogues. These reactions occur at the alanine side-chain of the inner tyrosyl ring. Although these analogues are thought to be metabolically active, little is known about the quantities produced or their contribution to hormone activity in animals or humans. [1]
The role of lipid peroxidation and other antioxidant enzyme systems has also received some attention with respect to thyroid hormone metabolism. Currently, the contribution of these metabolic pathways to thyroid hormone metabolism is not clear in humans; however, the current associations in animal models will be discussed.
Review of Thyroid Hormones
The thyroid gland, in response to stimulation by TSH, produces 3,5,3',5'- tetratiodothyronine (T4) (Figure 1), T3, and rT3. The synthesis of these hormones requires the amino acid tyrosine and the trace mineral iodine. Within the cells of the thyroid gland, iodide is oxidized to iodine by hydrogen peroxide, a reaction termed the “organification” of iodide. Iodine then binds to the number 3 position in the tyrosyl ring in a reaction catalyzed by thyroid peroxidase enzyme, a reaction yielding 3-monoiodotyrosine (MIT). A subsequent addition of another iodine to the number 5 position of the tyrosyl residue on MIT creates 3,5-diiodotyrosine (DIT). T4 is created by the condensation or coupling of two DIT molecules. Within the thyroid, smaller amounts of DIT can also condense with MIT to form either T3 or rT3.
Completed thyroid hormones consist of two benzene rings. An inner tyrosyl ring, often also called the alpha ring, and an outer phenolic or beta ring. After the thyroid hormones are formed, lysosomal proteases sever T4 (as well as any T3 or rT3 formed) from the thyroglobulin molecule, and the hormones are released into circulation. At the cellular level, the activity of thyroid hormones is mediated by interactions with nuclear T3 receptors. Metabolic effects of thyroid hormones result when the hormones occupy specific receptors, evoking subsequent effects on intracellular gene expression. It is estimated a cell needs 5- 7 times more T4 to bind to the nuclear receptors to have a physiological effect comparable to T3. [4]
The biosynthetic processes resulting in generation of thyroid hormones within the thyroid gland are controlled by feedback mechanisms within the hypothalamic-pituitary-thyroid axis. The hypothalamus produces thyroid releasing hormone (TRH) which stimulates the pituitary to release TSH, thus signaling the thyroid gland to upregulate its synthetic machinery.
Although T4, T3, and rT3 are generated within the thyroid gland, T4 is quantitatively the major secretory product. All T4 found in circulation is generated in the thyroid unless exogenously administered. Production of T3 and rT3 within the thyroid is relegated to very small quantities and is not considered significant compared to peripheral production. [1–3]
When T4 is released from the thyroid, it is primarily in a bound form with thyroid binding globulin (TBG), with lesser amounts bound to thyroxine-binding prealbumin (TBPA). It is estimated that only 0.03–0.05 percent of T4 within the circulatory system is in a free or unbound form; this unbound T4 is called free-T4 (fT4). In peripheral tissues, T4 is either converted to T3 or rT3, or eliminated by conjugation, deamination, or decarboxylation reactions. It is estimated that more than 70 percent of T4 produced in the thyroid is eventually deiodinated in peripheral tissues, either at the outer phenolic ring to form T3 or at the inner tyrosyl ring to form rT3. [2]
T3 is considered to be the most metabolically active thyroid hormone. Although some T3 is produced in the thyroid, approximately 80–85 percent is generated outside the thyroid, primarily by conversion of T4 in the liver and kidneys. [5, 6] The pituitary and nervous system are capable of converting T4 to T3, so are not reliant on T3 produced in the liver or kidney. Within the liver and kidney, the enzyme responsible for peripheral production of T3 is a selenium-dependent enzyme called 5'- deiodinase. This enzyme removes iodine from T4’s outer phenolic ring.
Similar to T4, the majority of circulating T3 is in a bound form; however, TBPA and albumin (not TBG) are the binding molecules with highest affinity for T3. The free form of T3 (fT3) found in circulation is more than an order of magnitude greater than fT4, with estimates suggesting fT3 is approximately 8–10 percent of circulating T3. [1]
Small amounts of rT3 are made within the thyroid; however, peripheral generation from T4 is estimated to account for 95 percent of all rT3 produced.5 The enzyme responsible for this conversion is 5-deiodinase and is not believed to be dependent on selenium for its activity. This enzyme acts on the nonphenolic ring of T4 (the inner tyrosyl ring) to produce the hormonally inactive rT3. Under normal conditions, 45–50 percent of the daily production of T4 is transformed into rT3. Substantial individual variation in these percentages can be found secondary to a range of environmental, lifestyle, and physiological influences. [5] Although an adequate understanding of the metabolic role of rT3 is somewhat limited, it is thought to be devoid of hormonal activity and to act as the major competitive inhibitor of T3 activity at the cellular level. [1] Experimental data also suggests rT3 has inhibitory activity on 5'-deiodinase, [2] suggesting it might also directly interfere with the generation of T3 from T4.
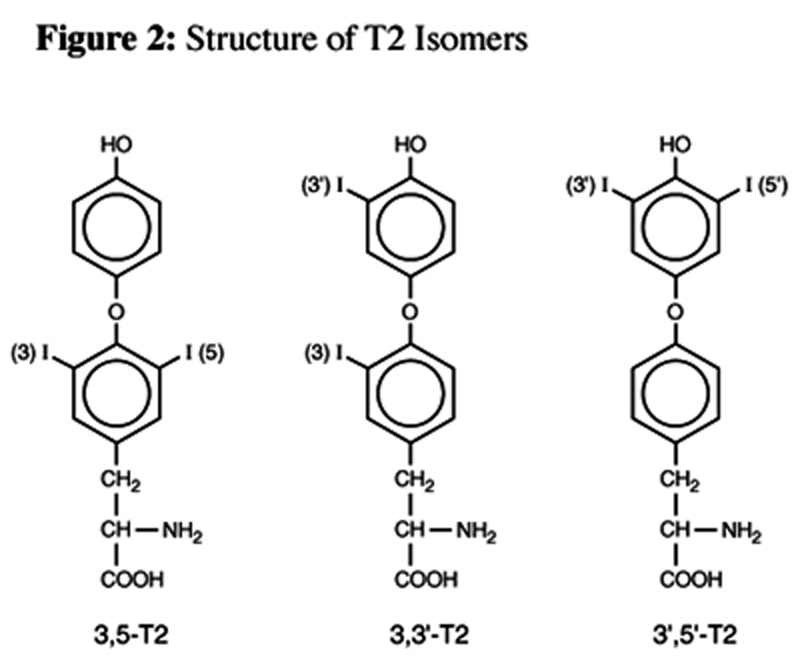
Figure 2

Table 1 Further degradation of rT3 and T3 results in the formation of several distinct diiodothyroxines: 3,5-diiodothyronine (3,5- T2), 3,3'-diiodothyronine (3,3'-T2), and 3',5'- diiodothyronine (3',5'-T2) (Figure 2).
Although the metabolic role of the T2 isomers is poorly understood and the absolute contribution of these hormones to physiological function in humans is unclear, experimental data raises doubts whether the various effects of thyroid hormones in different tissues can all be attributed to T3. The isomer 3,5-T2 has selective thyromimetic activity and has an ability to suppress TSH levels. [7] In animals, the 3,3'-T2 and 3,5-T2 isomers induce a dose-dependent increase in resting metabolic rate (RMR), an increase accompanied by a parallel increase in the oxidative capacity of metabolically active tissues such as liver, skeletal muscle, brown adipose tissue, and heart. In these experiments, 3,5-T2 exerted its greatest stimulatory effect on brown adipose tissue (BAT), while 3,3'-T2 had its greatest effect on muscle oxidative capacity. Although T3 is generally considered to be the metabolically active thyroid hormone, in contrast to these T2 isomers, T3 has only a small metabolic and oxidative effect on skeletal muscle and no significant stimulatory effect in heart and BAT, irrespective of dose. [8, 9] (See Table 1 for a summary of T2 isomer activity.)
Alterations in serum concentrations of 3,3'-T2 have been reported for humans under certain conditions. As a rule this isomer declines significantly with advancing age. Hyperthyroidism is characterized by an expected increase and hypothyroidism with a decrease in 3,3'-T2 concentrations. [10] Of the T2 isomers, 3,5-T2 is presumed to be the most metabolically active and can only be formed from further deiodination of T3 by 3'- deiodinase. The isomer 3,3'-T2 can be formed from the deiodination of either T3 by 5- deiodinase or from rT3 through the same 5'- deiodinase enzyme responsible for the formation of T3 from T4. rT3 can also be degraded to an inactive isomer, 3',5'-T2, by a 3-deiodinase enzyme.
Alterations in Peripheral Metabolism:
The “Euthyroid Sick Syndrome” and “Low T3 Syndrome”
“Euthyroid sick syndrome” (ESS) and “low T3 syndrome” are often used synonymously to describe non-thyroidal illness characterized by low circulating T3 and fT3 levels despite normal thyroid gland function. In both of these syndromes, T4 levels are generally normal (although occasionally they will be slightly elevated), fT4 is normal, and TSH is either normal or slightly suppressed.
Slight differences are implied by these two overlapping terms. In the strictest sense, ESS refers to a condition of normal thyroid gland activity with a reduced peripheral 5'- deiodination of T4 to T3 related to hepatic or renal disease. In ESS a reciprocal increase in rT3 is a consistent finding. Although a similar pattern of thyroid hormones is found in “low T3 syndrome,” technically this term does not imply any underlying illness or pathology. Despite the technical differences, both ESS and low T3 syndrome are used interchangeably to describe a situation characterized by lowered T3 levels with normal thyroid gland activity. [11]
Both syndromes are thought to be a result of impaired or decreased peripheral conversion of T4 to T3; however, either an increased conversion of T4 to rT3, and/or a decrease in the ability to degrade rT3 could result in ESS or low T3 syndrome. Since the formation of T3 from T4 and the degradation of rT3 both require 5'-deiodinase, an impairment in the function of this enzyme would result in a decreased ability to form T3 and a reduced ability to further deiodinate rT3.
Many non-thyroidal illnesses are associated with the pattern of thyroid hormones found in these syndromes. ESS is more prevalent with aging, and in an elderly population undergoing surgery for acute medical conditions, ESS has been associated with longer hospital stays and higher post-operative death rates. [12]
Laboratory abnormalities have been observed in ESS, including high cortisol and epinephrine levels; [13–15] low serum albumin levels among the elderly; [12] and a compromised selenium status associated with both ESS and low T3 syndrome. [16, 17]
Alterations in cytokine profiles appear to have a strong association with ESS. [12, 18–20] A correlation between increased rT3 and elevated levels of interleukin-6 (IL-6) has been reported in elderly patients undergoing emergency surgery. 12 Elevated levels of IL-6, tumor necrosis factor-alpha (TNF-±), and interferon-alpha (IFN-±) have also been reported to have a strong association with the reduced T3 and increased rT3 found under stressful conditions. [19, 20] Administration of IL-6 to healthy subjects results in a thyroid hormone profile similar to ESS and low T3 syndrome: T3 levels are decreased, rT3 levels are increased, and TSH levels are slightly suppressed. IL-6 administration also results in a significant increase in cortisol levels, so it is unclear whether this cytokine or the elevation in cortisol contributed to the alteration in thyroid hormone levels. [21] Because of these observations, it has been suggested these cytokines might act to impair hepatic type I 5'-monodeiodination; [19, 20] however, any absolute role in the regulation of the enzyme systems responsible for peripheral metabolism of thyroid hormones by the various cytokines awaits clarification.
Metabolic Pathways Influencing Thyroid Hormone Peripheral Metabolism
As the liver, and to a lesser extent kidneys, have primary influence on the circulating levels of thyroid hormone metabolites, the health and function of these organs play a critical and under-appreciated role in thyroid hormone function. [22] Deiodination of T4 to form T3 or rT3 and the subsequent disposal of rT3 occurs in the liver and kidneys. The deiodination enzymes are also responsible for formation and elimination of T2 and T1 isomers. Conjugation reactions utilizing glucuronidation, or sulfation pathways leading to the irreversible elimination of thyroid hormones are also primarily mediated by liver microsomal enzyme activity. Available evidence suggests that, under some circumstances, the activity of hepatic antioxidant enzyme systems and lipid peroxidation might influence the peripheral metabolism of thyroid hormones. Hepatic decarboxylation and deamination enzyme reactions are also capable of influencing the formation and elimination of specific thyroid hormone metabolites.
Deiodination
Tissue-specific deiodination of thyroid hormones determines, to a large degree, the fate of these hormones. The majority of the activation of the prohormone T4 to the more metabolically active T3 occurs through nonthyroidal deiodination. The inactivation of T3 to T2 isomers, the inactivation of T4 to yield rT3, and the eventual degradation of rT3 to T2 isomers are also catalyzed by the deiodinase family of enzymes. This stepwise removal of iodine from the benzene ring of the inner tyrosyl and outer phenolic benzene ring is currently thought to be the major route of peripheral thyroid hormone metabolism. [1–3]

Table 2 Currently, three deiodinase families are recognized and are termed isoforms type I, II, and III (Table 2). These three families differ in terms of their tissue distribution, reaction kinetics, efficiency of substrate utilization, and sensitivity to inhibitors. [23] Type I deiodinase is the major enzyme in the liver, kidneys, and skeletal muscle; it can carry out both 5'- and 5-deiodination of T4 to produce either T3 or rT3. Type I 5'-deiodinase is a selenium-dependent enzyme, with selenocysteine at the active site of the enzyme; however, type I 5- deiodinase enzyme does not require selenium for activity. Type II enzyme is the major deiodinase in the brain, pituitary, and brown adipose tissue. This isoform appears to carry out only 5'-deiodination; however, unlike type I 5'-deiodinase, this enzyme does not require selenium for its activity. Since tissue equipped with type II isoforms are relatively independent of circulating T3 for their metabolic demands, type II 5'-deiodinase is particularly important for providing the T3 required to stimulate the pituitary to synthesize and secrete TSH. [2] Type III deiodinase isoform is also found in the central nervous system and catalyzes the 5-deiodination of T4, resulting in generation of rT3. [1–3, 23, 24]
Bioactivity of thyroid hormone is determined to a large extent by the hepatic monodeiodination of the prohormone T4. The best-characterized activities of the liver with respect to thyroid hormone metabolism involve deiodinase reactions. Within the liver, type I deiodinase activity may either result in formation of T3, subsequent to the removal of an iodine from the outer phenolic ring by a selenium- dependent hepatic 5'-deiodinase enzyme, or it can remove iodine from the inner tyrosyl ring by 5-deiodinase resulting in the formation of rT3.
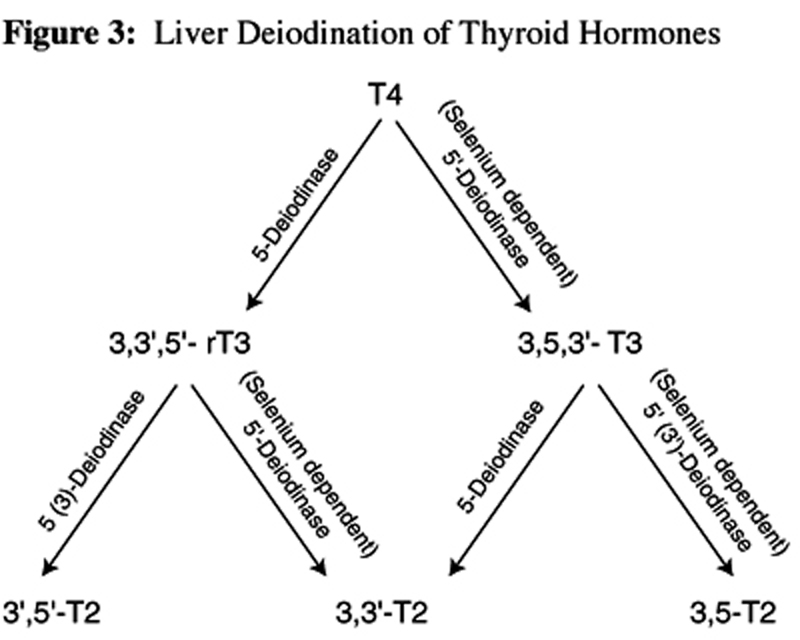
Figure 3

Table 3 The same 5'-deiodinase is responsible for degradation of rT3 and subsequent formation of 3,3'-T2. Of the T2 isomers, 3,5-T2 is presumed to be the most metabolically active and can only be formed from further deiodination of T3 by 3'-deiodinase. The isomer 3,3'-T2 appears to have some metabolic activity in specific tissues and can be formed from the deiodination of either T3 by 5- deiodinase, or from rT3 through the same 5'- deiodinase enzyme responsible for the formation of T3 from T4. Further degradation of rT3 can also be achieved by 3-deiodinase resulting in the formation of an inactive metabolite, 3',5'-T2. (See Figure 3 for a summary of liver deiodination of thyroid hormones.)
In a very simplified sense, T3 and rT3 levels are reciprocally related due to the influence 5'-deiodination has on their formation and degradation, respectively. Current evidence suggests the reciprocal increase in rT3 and decrease in T3 found in many conditions and circumstances shown in Table 3 is primarily a result of an alteration in the 5'-deiodination pathway. This alteration results in a simultaneous decrease in the production of T3 and a decrease in the degradation of rT3. [1] The net result is a decrease in T3 and an increase in rT3 in circulation.
It is generally accepted that type I 5'- deiodinase has higher affinity for rT3 than it does for T4. [5, 25] Because of this, enzyme saturation will shift metabolic pathways away from the generation of T3 and toward the elimination of rT3. Inhibition of the hepatic type I 5'-deiodinase enzyme, whether due to selenium deficiency or some other influence, might also act in a similar manner, preferentially degrading rT3 to 3,3'-T2 at the expense of the generation of T3 from T4. Kinetic data from experimental models suggest 5-deiodinase has a substrate preference for T3 inactivation over T4 conversion to rT3. [5]
Changes in 5'-deiodination occur in a number of situations, such as stress, poor nutrition, illness, selenium deficiency, and drug therapy. Toxic metals such as cadmium, mercury, and lead, have been associated with impaired hepatic 5'-deiodination in animal models. [26–29] Results suggest free radicals are also involved in inhibition of 5'-monodeiodinase activity; however, a positive relationship between total sulfhydryl group availability and 5'-monodeiodinase activity in the presence of free radical scavengers has been observed. [30] In the course of chronic liver disease such as hepatic cirrhosis, alterations in hepatic deiodination resulting in increased rT3 and a simultaneous decrease in T3 levels have also been observed. [31]
Glucuronidation
Enzyme activity can be modulated by numerous foreign compounds, such as common chemicals and drugs. [35] Many of these enzyme inducers can increase the glucuronidation of T4, resulting in a decrease of serum T4 and a subsequent increase in TSH. [36] Animal models suggest these glucuronidation-inducing drugs and toxins act to preferentially eliminate T4 and rT3, with limited effect on T3 indices. [37]
Some of the compounds known to increase T4 glucuronidation in animal models, resulting in decreased serum concentrations of T4, include phenobarbital, [34] chlorinated paraffins, 38 polychlorinated biphenyl, [37] hexachlorobenzene, [32, 39] 3-methylcholanthrene, 32,37 3,3',4,4'-tetrachlorobiphenyl, [32] 2,3,7,8-tetrachloro-p-dioxin, [32] and clofibrate. [32] The latter five compounds also substantially increase rT3 glucuronidation activity. [32]
The impact of these compounds on glucuronidation of thyroid hormones in animals has generally been followed for only short-term exposure; hence, the long-term consequences of exposure on thyroid hormone metabolism are currently unknown. In animals, glucuronidation is a major thyroid hormone metabolism pathway; however, there are species- [33] and gender-dependent [40] variations in glucuronidation enzyme activity and specificity for thyroid hormones. Therefore, it is unclear to what extent animal kinetic data is relevant to humans.
Sulfation
Since it strongly facilitates the degradation of thyroid hormone by type I hepatic deiodinase enzymes, sulfation is an intriguing, although poorly understood, pathway for thyroid hormone metabolism. Based on kinetic data, a substrate preference of 3,3'-T2 > rT3 > T3 > T4 has been postulated. [41]
Sulfation of the phenolic hydroxyl group blocks the outer ring deiodination of T4 to T3, while it strongly stimulates the inner ring deiodination of both T4 to rT3 and T3 to T2. [42] Since the net affect of activation of the sulfation pathway appears to preferentially inhibit the formation of T3 and increase its degradation, and since it can catalyze the degradation of T4 to rT3, it would seem capable of potentially exerting a profound effect on thyroid hormone metabolites.
Specific drugs and environmental toxins have been shown to influence the sulfotransferase activity responsible for thyroid hormone metabolism. As examples, hydroxylated metabolites of polychlorinated biphenyls [43] and phenol derivatives are potent inhibitors of thyroid hormone sulfotransferase activity. [44, 45]
While the role of most nutrients on sulfation of thyroid hormones is unknown, in rats selenium deficiency significantly increases the mean serum concentrations of sulfated T4, T3, and rT3 (T4S, T3S, rT3S) secondary to reduction in 5'-deiodinase activity. [46]
Similar to glucuronidation, the preponderance of information available on the sulfation of thyroid hormones was taken from animal experiments; however, since even among rats, species- and gender-dependent differences in sulfation have been observed, [45, 47] the absolute relevance of animal models to humans is questionable. Current data supports a perspective that, under normal circumstances, sulfation is not as important a mechanism for T3 disposal in humans as it is in rats, possibly suggesting differences in kinetics substrate preference. [48]
In humans, evidence suggests that in healthy subjects the role of sulfation of T3 is probably insignificant since serum T3S is virtually undetectable. However, this process is increased by drugs which inhibit type I deiodinase activity; making elimination of T3 by sulfation a more important metabolic pathway under these circumstances. [48]
Evidence also indicates the sulfation pathway for T4 increases substantially following exogenous T4 therapy in premenopausal women. Although only low T4S levels are detectable in serum both pre- and post-T4 treatment, urinary T4S values increase significantly. [49] However, unlike T4, significant increases in both serum and urine T3S levels are observed following T4 therapy. [50]
Antioxidant Enzyme Systems and Lipid Peroxidation
As mentioned, the roles of lipid peroxidation and antioxidant enzyme systems have also received some attention with respect to thyroid hormone metabolism. An association between hepatic lipid peroxidation and peripheral conversion of T4 to T3 has been observed in animal experiments. This has led some researchers to speculate that changes in antioxidant status might influence functional aspects of thyroid hormone metabolism. Because of the liver’s role in thyroid hormone metabolism, it is quite possible this might be compromised if the liver is subjected to chemicals/ toxins.
Exposure to toxic metals such as cadmium or lead can result in an alteration in peripheral metabolism of thyroid hormones. A substantial reduction in T3 without any significant alteration of serum T4 concentrations has consistently been observed in animal models. [26–28] Activity of hepatic 5'-deiodinase has decreased by as much as 90 percent subsequent to exposure to toxic metals. [26–28] Antioxidant enzyme systems, including superoxide dismutase [26, 28] and catalase,28 are also reduced subsequent to exposure. Coupled with the decline in T3 and hepatic 5'-deiodinase activity and the impairment in antioxidant enzyme systems, a concomitant increase in lipid peroxidation by as much as 200 percent has also been reported. [26–28] This has led researchers to conclude that heavy metal-induced alterations in antioxidant enzyme systems and lipid peroxidation might lead to dysfunction in membrane integrity and that an intact membrane structure might be critical for optimal 5'-deiodinase activity. [26, 28]
Carbon tetrachloride poisoning induces significant alterations in the hepatic antioxidant enzyme systems with a resultant elevation of lipid peroxidation. Levels of T3 are significantly lowered subsequent to carbon tetrachloride exposure. [51]
Associations between hepatic antioxidant enzyme system activity, lipid peroxidation, and peripheral conversion of T4 to T3 in animal models secondary to administration of specific botanical extracts suggest a possible interaction between this aspect of liver function and thyroid hormone metabolism. [51–54] Currently, the contribution of these metabolic pathways to thyroid hormone metabolism is not clear in humans.
Acetic Acid Analogues
Thyroid hormones can undergo deamination and decarboxylase reactions in the liver resulting in the formation of so-called acetic acid analogues of thyroid hormones. These reactions occur at the alanine side-chain of the inner tyrosyl ring. Although these analogues are thought to be metabolically active, little is known about the quantities produced or their contribution to hormone activity in animals or humans.
The effects of tetraiodothyroacetic and triiodothyroacetic acids on thyroid function have been investigated in euthyroid and hyperthyroid subjects. At a sufficient dose, triiodothyroacetic and tetraiodothyroacetic acids can cause a significant reduction in serum T3. [55]
Although 3,3',5-triiodothyroacetic acid (triac), the acetic acid analogue of T3, is bound at least twice as avidly to nuclear receptors as T3, it has limited potency in human peripheral tissues because of its short half-life. [56] Triac, when administered exogenously at high doses, suppresses endogenous thyroid hormone secretion, as evidenced by decreased T4, fT4, fT3 and rT3 levels. [57, 58] Triac also temporarily suppresses serum TSH concentrations [57, 59] However, even high doses of triac did not change basal metabolic rate, an expected effect of thyroid hormones. [57, 60] Triac appears to induce hepatic 5'-deiodinase with about the same activity as T3, [61] and with a markedly stronger effect than treatment with T4. [62]
In human liver, both triac and tetraiodothyroacetic acid are conjugated by glucuronidated reactions about 1500 and 200 times faster than T3 and T4, respectively. This preference of conjugation reactions for the acetic acid analogues might partially explain their short half-life in the body. [56]
Lifestyle Factors Influencing Peripheral Conversion of Thyroid Hormones
Specific lifestyle factors can have a significant impact on peripheral metabolism of thyroid hormones. In addition to these factors, a number of physiological and pathological events perturb the deiodination pathway, leading to a decrease in T3 peripheral genesis and reciprocal changes in the circulating level of T3 (which decreases) and rT3 (which increases). The biological effects resulting from these changes are not currently completely understood but are potentially important in the body’s adjustment to stressful or catabolic states. Several of these circumstances are listed in Table 3.

Table 4 Several drugs have been associated with alterations in thyroid hormone metabolites. These drugs and their effects on T3 and rT3 are summarized in Table 4.
In addition to the circumstances and drugs listed in Tables 3 and 4, elevations in cortisol, [13, 14] catecholamines, [15] and some cytokines (IL-6, TNF-±, and IFN-±), [18–20] and low serum albumin levels12 have been associated with ESS or low T3 syndrome.
Elevated Cortisol and Stress
In virtually any type of situation characterized by increased endogenous secretion of cortisol, a predictable pattern of altered thyroid hormone metabolism occurs. The generalized pattern is characterized by a trend toward lowered TSH production and a blunted TSH response to TRH, a decline in T3, and an increase in rT3. [13, 14, 67, 75–79, 86] Even changes in serum cortisol levels within the normal range can cause significant alterations in thyroid hormone parameters. [87]
Dexamethasone-induced increase in corticosteroids has been found to reduce T3 and increase rT3, probably as a result of impaired 5'-deiodination. [81] When endogenous hypersecretion of cortisol was induced by ACTH, T4 to T3 monodeiodination decreased, T4 to rT3 conversion increased, and TSH decreased due to blunting of TRH response. [14, 86] Among critically ill men, low levels of fT4, T4, and T3 and elevated levels of rT3 and cortisol have been consistent findings. It has been suggested that high levels of cortisol might be responsible for the altered T4 peripheral metabolism to T3 and rT3 in these patients. [76]
While evidence is limited, it appears a relative deficiency in cortisol might have the opposite effect on thyroid hormone values. Observation of patients with chronic ACTH deficiency with low and normal cortisol levels indicated that thyroid hormone values were influenced during the periods of cortisol deficiency. The pattern emerged as follows: T4 was lower, T3 was higher, and rT3 decreased significantly. The observed changes led the researchers to hypothesize there might be “increased peripheral conversion of T4 to T3 during cortisol deficiency.” [88]
Since cortisol appears capable of influencing thyroid hormone metabolism, it would make sense that stress, no matter how induced, might have a similar effect. This appears to be the case based on available evidence. People subjected to cold exposure-induced stress responded with an increase in serum rT3. [68] Examination anxiety is commonly used to measure the stress response. Twenty-two male and 27 female medical students were monitored before and after an academic examination. Both male and female students experienced an increase in rT3 levels on examination days. Although the reason is unclear, the increase among female students was substantially higher than observed in the male students. [77]
Since the stress response is non-specific in nature and can be elicited or exacerbated by a range of external or internal factors, it is not surprising to see other variables induce similar functional influences on thyroid hormone parameters. An expected derangement in cortisol and thyroid hormone parameters was reported among soldiers exposed to chemical weapons containing sulfur mustard. These individuals responded with a predictable increase in cortisol concentrations that did not normalize until the fifth week following the chemical insult. Concomitant with the sulfur mustard-induced impact on cortisol, both fT4 and fT3 decreased while rT3 increased. Similar to cortisol values, serum thyroid concentrations did not normalize until the fifth week after exposure. [67]
Researchers reported a connection between stress of surgery and derangement in thyroid hormone values. Post-surgical decreases in serum T3 and fT3 values and increase in serum rT3 values suggest altered peripheral conversion. Surgery-induced hypersecretion of cortisol was also observed. [78, 79]
Combining several stressful situations appears to have a synergistic and dramatic effect on altering thyroid hormone metabolism. Military cadets subjected to a combination of sleep deprivation, calorie deficiency, and intense physical activity during a training exercise demonstrated significant and lasting changes in endocrine function. Cortisol levels increased along with abolition of circadian rhythm. In parallel with this increase, T4, fT4, and T3 consistently declined. While T4 and fT4 returned to normal levels within 4–5 days following the cessation of the training exercise, T3 and fT3 remained depressed. [89]
Calorie Restriction
Calorie restriction and energy expenditure appear to be capable of substantially influencing thyroid hormone peripheral conversion. There is a wide range of variation between individuals, with factors like genetics, obesity, and gender, as well as macronutrient content of the hypocaloric diet likely intermingling to influence response. Nutritional status and caloric expenditure influences thyroid function centrally at the level of TSH secretion, at the level of monodeiodination, and possibly elsewhere.
Since an increase of rT3 is found at the expense of T3 during caloric restriction, it is possible the hepatic peripheral pathways play a substantial role in metabolic control during energy balance. It appears the fastinginduced increase in rT3 might be a result of increased production of and decreased clearance of this metabolite. [90] The increase in T4 and rT3 seen with caloric restriction might also be influenced by the duration of energy restriction. Evidence suggests that, when caloric restriction is longer than three weeks, T4 and rT3 levels return to normal values. [91]
The ratio of T3/rT3 was found to be extremely sensitive to the balance between intake and expenditure of energy. In men, serum thyroid hormones were sensitive to marginal changes in energy intake and expenditure. A marginally negative (–15%) energy balance shifted the ratios of T4/T3 and T3/rT3 in a manner suggestive of impaired peripheral conversion. [64]
A hypocaloric, low carbohydrate diet consisting of 800 kcal daily for four days resulted in a striking decrease in both T3 and fT3 and an increase in rT3. [65] However, the percentage of calories available as carbohydrates influenced the set point, below which alterations in thyroid peripheral metabolism were significantly impacted. In a study of obese individuals, an energy-restricted diet consisting of 800 kcal with greater than 200 kcal as carbohydrates, sustained higher T3 levels. When carbohydrate content of the diet dipped below 200 kcal, T3 levels fell substantially. Irrespective of carbohydrate content of the diet, when caloric content of the diet dropped to 600 kcal/day, T3 levels were compromised. Reverse T3 was not significantly influenced by carbohydrate content of the diet and seemed to be most impacted by caloric content. [91] Similarly, in non-obese subjects, T3 levels decreased and rT3 levels increased when carbohydrate intake was dramatically reduced. [92] Overall, it appears both calorie and carbohydrate restriction can negatively impact thyroid hormone metabolism.
Fasting also exerts a powerful influence on peripheral metabolism of thyroid hormones, and studies suggest mild elevations in endogenous cortisol levels might be partly responsible. [87] Following a 30–hour fast, healthy male subjects experienced a decrease in serum TSH levels with a concurrent disruption of its rhythm. Fasting serum T3 levels were significantly lower than in the non-fasting state, while rT3 levels increased during fasting. [66] Although the decline in TSH could be partially responsible for the observed decline in T3 concentrations, the increase in rT3 clearly demonstrates an alteration in peripheral metabolism of T4. Even after re-feeding, although TSH tended to increase and rT3 to decrease slightly, these values remained perturbed when compared with values observed prior to fasting. [66]
The effect of a longer period of total energy deprivation produced a similar alteration in thyroid hormone metabolism. Following ten days of fasting, marked reductions in serum levels of T3 and increases in rT3 were found in healthy male subjects; reductions of blood levels of TSH and gradual increases in the blood levels of cortisol were also observed. 93 Fasting for 31 +/– 10 days also decreased T3 and increased rT3 in obese subjects. Although introduction of an 800 kcal diet following the fasting period increased T3, this amount of calories did not influence the fasting- induced elevations in rT3. [94]
Ketones generated from calorie deprivation did not appear to have a suppressive effect on T3 generation and hepatic type I 5'- deiodinase activity. [95, 96] It is not clear whether ketones would have a similar impact in a calorie- sufficient diet.
The role of calories and energy balance might also influence peripheral thyroid hormone metabolism under circumstances of increased caloric consumption. Although on a low-calorie diet elimination of rT3 by 5'- deiodination is decreased, the clearance of rT3 by 5'-deiodination was actually increased with a high calorie diet. An increase in the clearance rate of 3,5-T2 was also found during high calorie consumption. [97] It was not clear to the researchers whether the reported increase in deiodination of rT3 associated with an increase in calories has any substantial metabolic advantages, since it appeared to be at the expense of alternate rT3 disposal pathways. [97]
Exercise
While the role of a hypocaloric diet in producing alterations in thyroid hormones has been consistently demonstrated, the role of exercise in thyroid hormone metabolism is slightly ambiguous, with factors such as prior conditioning making it difficult to predict the influence of exercise. In one study, untrained subjects experienced reductions in cortisol and rT3, and an increase in T3 after exercise. However, trained subjects had an increase in cortisol and rT3 and a decrease in T3 with exercise; concentrations of T4 were unchanged in both groups.98 The confounding results of thyroid hormone levels seen following exercise might be mediated by elevated cortisol levels; however, additional research is required to establish this connection.
Compelling evidence also suggests that, if exercise-related energy expenditure exceeds calories consumed, a low T3 syndrome may be induced. In female athletes, four days of low energy availability reduced T3, fT3, increased rT3, and slightly increased T4. Since an adequate amount of the prohormone T4 was available throughout the study, an alteration in the peripheral metabolism of T4 was likely. The increase in rT3 and decrease in T3 are consistent with a decreased activity of hepatic 5'-deiodinase activity, since this enzyme is responsible for the production of T3 and the clearance of rT3. These alterations in thyroid hormones could be prevented solely by increasing dietary caloric consumption without any alteration in the quantity or intensity of exercise. [96, 99]
Sleep Deprivation
Short-term sleep deprivation can influence thyroid hormone metabolism. The effects of sleep deprivation appear to be both centrally and peripherally mediated. The reported pattern of thyroid hormone response to a night of sleep deprivation included significant increases in T4, fT4, T3, and rT3. This pattern was observed in individuals diagnosed with depression, [100] as well as among healthy, physically fit males and females. [101] The implications of these changes and the impact of longer-term sleep deprivation on peripheral metabolism of thyroid hormones have not been established.
Alcohol Intake
In animal models, ethanol intake was associated with impaired hepatic 5'- deiodination. [102] Among patients with alcoholinduced liver cirrhosis, low T3 and T4, elevated rT3, and normal TSH values have been observed. In these subjects an abolished circadian rhythm and elevated cortisol levels have frequently been observed. [69] While extreme alcohol- induced liver damage is apparently detrimental to the peripheral modulation of thyroid hormones, it is unclear whether moderate alcohol intake has an impact.
Nutrients with Potential Influence on Thyroid Hormone Metabolism: Trace Minerals
Iodine
It is estimated the thyroid gland must capture approximately 60 mcg iodide (the ionic form of iodine) daily to ensure an adequate supply for thyroid hormone synthesis. [103] Because of the critical role of iodine in this synthetic process, this trace mineral has received the most attention historically with respect to thyroid disorders; however, available evidence does not suggest a role for iodine in peripheral metabolism of thyroid hormones. In fact, it has been demonstrated the decreases in T3 and fT3 and increase in rT3 subsequent to a low carbohydrate, 800 calorie diet cannot be corrected by iodine supplementation. [65]
Selenium
Since selenium, as selenocysteine, is a cofactor for type I hepatic 5'-deiodinase, this trace mineral has received the most attention with respect to peripheral metabolism of thyroid hormones. If selenium were deficient, the deiodinase activity would theoretically be impaired, resulting in a decreased ability to deiodinate T4 to T3 and a decreased ability to degrade rT3.
In animal experiments, deficiencies of selenium were associated with impaired type I 5'-deiodinase activity in the liver and kidney and reduced T3 levels. [104, 105] In an animal model a 47–percent reduction in the activity of this deiodinase enzyme has been reported secondary to selenium-deficiency. [106] The 20–fold increase in deiodinase activity secondary to acute cold exposure in rats was impaired in selenium deficiency. [107] Conversely, administration of selenium completely reversed the effects of its deficiency on thyroid-hormone metabolism in animals. [104]
Low T3 syndrome and ESS have been correlated with a decrease in serum selenium. [16, 17] Evidence suggests a strong linear association between lower T3/T4 ratios and reduced selenium status, even among individuals considered to be euthyroid based on standard laboratory parameters. This association is particularly strong in older subjects and is thought to be a result of impaired peripheral conversion. [108, 109] An inverse relationship between T3 and breast cancer associated with decreased selenium status has been reported (although plasma T4 and TSH concentration were similar in cases and controls). [110] This combination of factors strongly suggests that low T3 is secondary to the expected perturbation in T4 conversion to T3 expected in selenium deficiency.
Among individuals with acute renal failure, a reduction of circulating thyroid hormone concentrations without evidence of primary or secondary hypothyroidism frequently occurs. The changes in thyroid hormone concentrations may be due to a compromised peripheral conversion in the kidneys and liver. Current evidence also suggests that, in patients with multiple organ failure, plasma selenium concentrations are substantially reduced compared to normal controls. [72] It is not clear whether selenium supplementation would prove therapeutically beneficial under these circumstances.
Selenium administration has been found to positively influence metabolism of thyroid hormones under certain circumstances. Selenium supplementation (1 mcg/kg/day for 3 weeks) decreased rT3 levels in subjects with phenylketonuria and a low selenium status. [111] In a prospective, randomized clinical trial of 24 critically-ill patients, an expected diminished fT3 level was observed in all patients prior to selenium supplementation. Following parenteral selenium supplementation (sodium selenite 500 mcg twice daily during week 1, 500 mcg daily during week 2, and 100 mcg three times daily during week 3) a gradual restoration of fT3 was observed. [112] A doubleblind, placebo-controlled trial of selenium supplementation among elderly euthyroid subjects resulted in an improvement of selenium indices, a decrease in T4, and a trend toward normalization of the T3/T4 ratio in seleniumtreated subjects. [109]
Sodium selenite therapy administered to cystic fibrosis patients resulted in a significant increase in selenium status and a parallel increase in serum T3. A highly significant decrease in the serum T4/T3-ratio was also observed in these individuals subsequent to selenium administration, suggesting improved peripheral T4 to T3 conversion. [113]
Available animal and human evidence strongly supports a relationship between altered thyroid hormone metabolism and selenium deficiency, although evidence also suggests high intakes of selenium might exert a detrimental influence on thyroid hormone metabolism. In animals fed a diet supplemented with high amounts of selenium (600 or 3,000 mcg/kg), serum T3 concentrations are actually reduced by 50 percent, while T4 and fT4 remain unaffected when compared to animals fed a selenium-adequate diet. [114] Although human subjects exposed to high dietary levels of selenium typically have normal levels of fT4, fT3 and TSH, a significant inverse correlation has been found between fT3 and selenium. Some researchers have hypothesized the activity of hepatic type I iodothyronine 5'- deiodinase might become depressed after a high dietary intake of selenium,115 suggesting a safe level of dietary selenium at or below 500 mcg daily. [115]
Zinc
The role of zinc in thyroid hormone peripheral metabolism is still being elucidated; however, preliminary evidence suggests this nutrient might play an important role. In animal experiments, zinc deficiency, although having no impact on T4 concentrations, resulted in an approximately 30–percent decrease in levels of serum T3 and fT4. The activity of type I 5'-deiodinase was also reduced by 67 percent in zinc-deficient animals.106 Inhibition of conversion of T4 to T3 was similarly demonstrated in an independent animal experiment. [116]
In humans, zinc supplementation reestablished normal thyroid function in hypothyroid disabled patients treated with anticonvulsants. In a study 9 of 13 subjects with low free T3 and normal T4 had mild to moderate zinc deficiency. After oral supplementation with zinc sulfate (4–10 mg/kg body weight for 12 months), levels of serum fT3 and T3 normalized, serum rT3 decreased, and the TRH-induced TSH reaction normalized. [117]
This study suggests zinc status influences peripheral metabolism of thyroid hormones. Since zinc is not a cofactor in hepatic type I-deiodinase enzyme, the nature of zinc’s influence on aspects of peripheral metabolism in animals and humans remains unclear.
Nutrients with Potential Influence on Thyroid Hormone Metabolism: Vitamins
Niacin
Evidence suggests niacin supplementation can influence thyroid hormone levels in at least some individuals. One author reported cytopenia and hypothyroxinemia with a concomitant decrease in thyroxine-binding globulin in two patients receiving niacin. All thyroid function tests returned to normal after niacin supplementation was discontinued. [118]
The impact of sustained supplementation with niacin (mean daily dose of 2.6 grams for an average duration of 1.3 years) was observed in one female and four male subjects with hyperlipidemia. Before and after thyroid function studies revealed significant decreases in serum levels of T4, T3, and TBG, with no significant alterations in fT4 and TSH levels. Discontinuation of niacin resulted in a return to pretreatment levels of these parameters of thyroid function. [119]
While results suggest niacin can influence serum thyroid hormone concentrations, it is currently not known whether this is a centrally- mediated result, a direct result of a decrease in TBG, or a niacin-induced alteration in some aspect of peripheral conversion. However, since TSH was unaltered, evidence suggests an influence outside the hypothalamicpituitary- thyroid axis.
Vitamin B12
In animals, a tissue vitamin B12 deficiency was associated with a slight reduction of type I 5'-deiodinase activity in liver and with a significant reduction of the T3 level in serum. [120] Studies in human subjects have not been conducted; however, it is possible that a vitamin B12 deficiency might provoke a similar detrimental influence on peripheral activation of T3 from T4.
Lipoic Acid
Lipoic acid appears to influence the metabolic fate of T4 when co-administered with T4 therapy. Administration of T4 for 22 days resulted in a substantial increase in serum T3 concentrations; however, when lipoic acid was given in conjunction with T4 therapy for nine days a 56–percent suppression of the expected T4-induced increase in generation of T3 was observed (although T3 levels were elevated above control levels). Continuous supplementation of lipoic acid during T4 treatment resulted in a continued lower production of T3 than would have been expected from T4 therapy. [121]
While the authors suggest lipoic acid might exert an influence on peripheral tissue deiodinase activity, it is also possible this nutrient might have influenced conjugation reactions. It is currently not known whether lipoic acid supplementation influences thyroid hormone metabolism in normal individuals who are not receiving T4 therapy. Since it is usually not a therapeutic advantage to decrease peripheral activation of T3 subsequent to T4 therapy, use of this supplement in hypothyroid patients receiving exogenous hormone therapy should be approached with caution.
Antioxidant Vitamins: E and C
Administration of toxic metals such as cadmium chloride or lead have resulted in reductions in T3 and hepatic 5'-deiodianse activity, with virtually no alteration of T4 levels, [26–28] suggesting the observed alterations are mediated through impaired peripheral metabolism. After exposure to heavy metals, decreases in a variety of hepatic antioxidant enzyme systems and concomitant increases in lipid peroxidation have been observed. [26–28]
In an attempt to determine whether antioxidants such as vitamins E or C might be capable of preventing heavy-metal induced perturbation in thyroid hormones, lipid peroxidation, and hepatic antioxidant enzyme systems, several intervention studies were conducted in animals. Available evidence suggests vitamins C and E had no effect on thyroid hormone levels, antioxidant enzyme systems, and lipid peroxidation under normal circumstances; however, these nutrients were able to partially restore thyroid function to normal in cadmium- or lead-intoxicated animals. [26–28]
Vitamin E at a dose of 5 mg/kg of body weight on alternate days sustained hepatic 5'- deiodinase activity, partially prevented increases in lipid peroxidation, and improved cadmium-induced decreases in T3. [26] Simultaneous administration of vitamin E (5 mg/kg body weight) with lead maintained T3 levels and hepatic 5'-deiodinase activity within normal levels, and partially prevented increased lipid peroxidation and alterations in antioxidant enzyme systems. [28]
Ascorbic acid has also been shown to be effective in preventing cadmium-induced decreases in T3 and hepatic 5'-deiodination. Administration of this antioxidant is thought to function in a manner similar to vitamin E since it was able to act as an antioxidant buffer against lead-induced increased lipid peroxidation. [27]
Since vitamins E and C appear to positively influence hepatic 5'-deiodination only under circumstances associated with increased lipid peroxidation and decreased liver antioxidant- enzyme activity, they have potential therapeutic application.
Botanicals with Potential Influence on Thyroid Hormone Metabolism
An adequate understanding of the influence of botanical extracts on thyroid hormone metabolism and function is lacking for human subjects. However, under experimental conditions in animal models, botanical preparations are known to influence the metabolic fate of thyroid hormones. One of the primary axes of influence for most plants studied appears to be through alterations in peripheral conversion and hepatic antioxidant enzyme systems, although plant extracts may affect other avenues of thyroid hormone metabolism. For example, plants such as Lithospermum officinale and Lycopus europaeus exert some influence on the hypothalamic- pituitary-thyroid axis. [122]
Several plants appear to be capable of influencing peripheral conversion of thyroid hormones in a manner consistent with decreasing the conversion of T4 to T3, or in effect inducing a low T3-like syndrome. An abstract from an untranslated Chinese article found four plants (Aconite, Cinnamon, Cistanches, and Epimedium) used in Traditional Chinese Medicine (TCM) when administered independently or in combination, reduced plasma T3 concentrations; the mechanism for this effect was unclear. [123] Animal experiments indicated that after oral administration of an ethanolic extract of Lycopus europaeus, T3 levels were decreased and remained low for a period of more than 24 hours. Although reductions of T4 and TSH were also reported, since reductions in T4 tended to occur substantially after the observed reductions in T3, this finding was presumably as a consequence of reduced peripheral T4 deiodination. [124] Similar to Lycopus europaeus, Lithospermum officinale appeared to inhibit peripheral T4-deiodination. [125] Administration of an aqueous extract from the leaf of Moringa oleifera (horseradish tree) to female animals resulted in a decrease in T3 and a concomitant increase in T4, suggestive of an inhibitory effect on peripheral conversion. In male rats no significant changes were observed subsequent to administration of the extract. [126] Animal evidence found fenugreek seed extract impaired peripheral conversion of thyroid hormones. Administration of 0.11 g/kg body weight daily for 15 days to male mice and rats resulted in a significant decrease in serum T3, with a concomitant increase in T4 levels. Hepatic lipid peroxidation was unchanged, suggesting the observed impairment in peripheral conversion was not peroxidation-mediated. [127]
The effects of Withania somnifera (ashwagandha) root extract (1.4 g/kg) in the regulation of thyroid function with special reference to type- I iodothyronine 5'-monodeiodinase activity in mice liver have been investigated. Increases in T3 and T4 concentrations were observed; however, hepatic iodothyronine 5'- monodeiodinase activity did not change significantly. In this same experiment, ashwagandha root extract reduced hepatic lipid peroxidation and increased the activity of the superoxide dismutase and catalase antioxidant enzyme systems. [54] In a subsequent animal experiment, daily administration of a similar dose of ashwagandha root extract to female mice resulted in an increase exclusively in T4 concentrations, with no alterations observed in T3 levels. The root extract’s effect of decreasing hepatic lipid peroxidation and increasing the activity of antioxidant enzyme systems was again observed in this experiment. [53] These findings seem to suggest the effect of this plant on thyroid hormone levels is independent of increasing 5'-deiodinase activity.
Administration of an extract of Bauhinia purpurea bark extract (2.5 mg/kg body wt.) resulted in significant increases in serum T3 and T4 in female mice. This plant also demonstrated antiperoxidative effects as indicated by a decrease in hepatic lipid peroxidation and an increase in the activity of antioxidant enzyme systems. [53] Commiphora muku (gugulu) also impacted thyroid hormone conversion in animal models. While administration of 0.2 g/ kg body weight for 15 days produced no significant change in serum T4 levels, serum T3 concentrations were increased. Since a concomitant decrease in lipid peroxidation was observed in the liver, the authors suggested the increased peripheral generation of T3 might be mediated by this plant’s antiperoxidative effects. [52]

Table 5 Liv-52, a proprietary herbal formulation consisting of a variety of herbs from the Ayurvedic medical tradition (Table 5), has been found to ameliorate carbon tetrachloride-induced alterations in thyroid hormone levels and liver antioxidant enzyme system activities. Carbon tetrachloride administration results in a decrease in the levels of T3 found in circulation and a profound disruption in hepatic antioxidant enzyme system activity and a concomitant increase in the amount of lipid peroxidation. These effects are believed to be secondary to the severe liver damage this compound causes. When rats were co-administered Liv-52 in combination with carbon tetrachloride, T3 levels were slightly decreased but were sustained within normal levels. A similar positive trend was seen in the case of lipid peroxidation and liver antioxidant enzyme systems with Liv-52 treatment. [51]
Animal evidence suggests a potential dose-dependent dual action of Piper betel (betel leaf) on thyroid hormone metabolism. Betel leaf extract at a dose of 0.10 g/kg daily for 15 days decreased serum T4 and increased serum T3 concentrations. A concomitant decrease in hepatic lipid peroxidation was observed, suggesting the potential for a peroxidation-mediated effect on increased peripheral deiodination. However, at a dose of either 0.8 or 2.0 g/kg daily for the same interval of time, an opposite effect was observed. At these higher doses, T4 and hepatic lipid peroxidase were increased and hepatic catalase and super oxide dismutase antioxidant enzyme systems and T3 concentrations decreased. [128] This observed dose-dependent, bidirectional impact of betel leaf opens a Pandora’s box of questions regarding other botanicals and the potential for dose-dependent differences in their influences on thyroid hormone metabolism.
As mentioned in the discussion of selenium, acute renal failure has been associated with a reduction of circulating thyroid hormones. 72 Decreased thyroid hormone levels have also been reported in patients with chronic glomerulonephritis. [73] Commentary in an untranslated abstract utilizing Fu shen Decoction, a combination of herbs from TCM, is suggestive of a benefit of this combination for individuals with chronic glomerulonephritis. Administration of this herbal combination apparently increased both T3 and T4 levels; however, levels remained lower than those of healthy individuals. [73] Although the primary herb in this combination is likely to be Poria (Fu shen is the Chinese name given to Poria), the other herbal ingredients, ratio of specific herbal constituents, dose, method and duration of administration are not specified in the abstract.
Potential Influence of Soy on Thyroid Hormone Metabolism
The effect of soy products on thyroid hormone function and metabolism in humans is still being researched; however, animal evidence is suggestive of an impact on aspects of peripheral conversion. In animal experiments soy protein elevated plasma T4 concentrations. [129, 130] This may have been due to an increased glandular production of thyroid hormones or to an elevation of T4 subsequent to inhibition of the peripheral conversion of T4 to T3. Considering the latter, evidence has demonstrated that consumption by animals of roasted soy beans can result in reduced plasma T3, possibly because of an effect on peripheral T4 deiodination.131 Findings also indicated T3 was higher among casein-fed animals and lower among animals fed an equivalent amount of soy protein concentrate. [130] Soy protein consumption also was found to contribute to agerelated alterations in thyroid hormone in animals. [132] These alterations included a decline in T4, fT4, T3, and 2,3’-T2 and an increase in rT3. [132]
The animal study findings indicate soy protein consumption might be capable of generating a thyroid hormone profile similar to that found in low T3 syndrome and ESS; in other words, soy protein consumption might cause a shift in thyroid hormone profiles toward unchanged or increased T4 and rT3 at the expense of T3 production. Human data with respect to the possible influence of soy products on peripheral conversion is currently lacking. However, a diet high in soy isoflavones (128 mg/day) has been reported to induce a modest decrease in fT3 levels during the early follicular phase of the cycle in premenopausal women. [133] Whether the soy is affecting peripheral metabolism or increasing binding to TBPA, albumin, or TBG is unclear.
The effects of 30 grams of soybeans fed daily for 1–3 months to 37 healthy adults was investigated. Soybean consumption resulted in a significant increase in TSH levels, although levels remained within normal limits. Other measured parameters of thyroid function were unchanged; however, hypometabolic symptoms suggestive of a functional thyroid hormone deficiency (malaise, constipation, sleepiness) and goiters appeared in half the subjects who consumed soybeans for three months. Symptoms disappeared one month after cessation of soybean ingestion. [134]
While research has not determined the exact effect of soy products and soy isoflavones on the peripheral metabolic fate of thyroid hormones, excessive soy consumption might be best approached cautiously among subjects with suspected impairment of peripheral metabolic pathways.
Potential Influence of Flavonoids on Thyroid Hormone Metabolism
Flavonoids, both naturally occurring and synthetic derivatives, have the potential to disrupt thyroid hormone metabolism in vitro. Synthetic flavonoid derivatives have been shown to decrease serum T4 concentrations and inhibit both the conversion of T4 to T3 and the metabolic clearance of rT3 by the selenium-dependent type I 5'-deiodinase. [2, 135, 136] Naturally occurring flavonoids appear to have a similar inhibitory effect. Of the naturally occurring flavonoids, luteolin was the most active inhibitor of 5'-deiodinase activity when tested in vitro; however, quercetin, myricetin, and flavonoids with chalcone, aurone and flavone structures have also been shown to have in vitro inhibitory activity. [2, 137]
It is not known whether similar effects occur in vivo or whether, if such effects do occur, they are restricted to specific flavonoid compounds or dosages. Since isolated or concentrated flavonoids are increasingly utilized as therapeutic interventions, more research on the potential influence of these substances on thyroid hormone metabolism is desirable.
Conclusion
Thyroid hormones are metabolized in peripheral tissues by conjugation, deamination, decarboxylation, and a cascade of monodeiodination enzyme reactions. With the exception of deiodination reactions, the current contribution of these pathways to health and disease is relatively poorly understood.
Since conjugation reactions appear to be influenced by exposure to some pharmacological and environmental influences, as well as the quantity of available thyroid hormones, it is quite possible that alterations in activity of sulfation and glucuronidation pathways will influence the specific thyroid hormone metabolites available at the cellular level. Overall, very little is known about how derangements in these pathways might influence an individual at a functional level. Nutrient and lifestyle interactions with these metabolic pathways with respect to thyroid hormone metabolic fate have not been determined.
While certain acetic analogues of the thyroid hormones appear to have limited thyromimetic activity, the overall contribution of deamination and decarboxylase reactions to cellular function in health and disease are poorly understood. Currently, no data is available to determine a role of nutritional status and lifestyle factors for these enzyme pathways with respect to the metabolic fate of thyroid hormones.
The influence of deiodination enzyme reactions with respect to thyroid hormone metabolism are relatively well understood. In addition to liver disease, a wide range of variables can have an effect on thyroid hormone function secondary to their impact on peripheral conversion. These include aspects such as excessive stress and high levels of the stress hormone cortisol, caloric restriction, excessive physical activity, exposure to chemicals, surgery, injury, and systemic illness.
Since type I 5'-deiodinase processes both T4 to T3 and rT3 to 3,3'-T2, it is possible to deplete the enzyme, interfering with the conversion of T4 to T3 and the degradation of rT3. This potential mechanism might explain the observed derangement of thyroid hormones following chronic stress or after a sufficiently large acute stress. In effect, thyroid hormone conversion gets stuck in this cycle with elimination of the excess rT3 at the expense of T3 formation becoming a self-perpetuating problem. The role of the deiodinase enzymes in the formation and elimination of the T2 isomers, particularly since some of these isomers appear to have capability for metabolic activity independent of T3, might also play an important metabolic role in some tissues.
Hepatic antioxidant enzyme systems and lipid peroxidation have shown a consistent association with peripheral metabolism of thyroid hormones in animal models. Exposure to environmental toxins and drugs can influence these pathways. Many vitamin, mineral, and nutritional cofactors, as well as many botanical extracts can also influence the activity of these antioxidant enzymes. Although information in human subjects is currently unavailable, it is possible that many of the routinely utilized supplements might exert an influence on these enzyme systems under specific circumstances.
Because of its role as a cofactor for type I 5'-deiodinase, selenium’s influence on deiodination is the best characterized of any nutrient. While a selenium deficiency does not seem to result in a decrease in the production of T4 or T3 within the thyroid gland, deficiency substantially alters the conversion of T4 to T3 in peripheral tissues such as the liver and kidney. This is generally accompanied by reduced T3 and an increased rT3 in the circulation. Zinc deficiency appears to strongly inhibit type I 5'-deiodinase in animal models; however, the mechanism for this effect is not understood and it is currently not clear if a similar role for zinc exists in humans. The absolute role of other nutritional and botanical agents is still not characterized; however, available data suggests some supplements might have a potential to influence deiodination in specific individuals under some circumstances.
It currently is not clear whether ESS and low T3 syndrome represent protective physiological mechanisms, or are in themselves a damaging maladaptive response. It is certainly possible these syndromes are reflective of an adaptation to stress which acts to protect the body against exaggerated catabolism. However, nutritional approaches to thyroid disorders should not only consider the nutrients and substances that can alter thyroid hormone synthesis within the gland, but also nutrients and factors which might influence the peripheral conversion of thyroid hormones.
REFERENCES:


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |