Potential Importance of Leucine in Treatment
of Obesity and the Metabolic Syndrome
of Obesity and the Metabolic Syndrome
Send all comments or additions to: Frankp@chiro.org




FROM: J Nutr. 2006 (Jan); 136 (1 Suppl): 319S–323S ~ FULL TEXT
Donald K. Layman and Denise A. Walker
Division of Nutritional Sciences,
University of Illinois,
Urbana, IL 16801, USA.
dlayman@uiuc.eduDiets with total protein intake >1.5 g.kg–1.d–1 and carbohydrate intake <150 g/d are effective for treatment of obesity, type 2 diabetes, and the Metabolic Syndrome. These diets improve body composition and enhance glycemic control. During weight loss, protein-rich diets reduce loss of lean tissue and increase loss of body fat. Specific mechanisms to explain each of these clinical outcomes remain to be fully elucidated. We propose that keys to understanding the relationship between dietary protein and carbohydrates are the relationships between the branched-chain amino acid leucine and insulin and glucose metabolism. Leucine is known to interact with the insulin signaling pathway to stimulate downstream signal control of protein synthesis, resulting in maintenance of muscle protein during periods of restricted energy intake. Leucine also appears to modulate insulin signaling and glucose use by skeletal muscle. Whereas total protein is important in providing substrates for gluconeogenesis, leucine appears to regulate oxidative use of glucose by skeletal muscle through stimulation of glucose recycling via the glucose-alanine cycle. These mechanisms produce protein sparing and provide a stable glucose environment with low insulin responses during energy-restricted periods.
From the FULL TEXT Article:
Introduction
Evidence is accumulating that diets with higher protein and reduced carbohydrates are beneficial for weight loss (1–7). These studies report that diets with reduced ratios of carbohydrates to protein increase weight loss (1–3,5,6), increase loss of body fat (2,3,5), and reduce loss of lean tissue (1,3,5,7). We proposed that the beneficial effects of a higher protein diet included the roles of leucine in sparing muscle protein loss and enhancing glycemic control (8,9). This article will review metabolic roles of leucine and examine some of the clinical trials with higher protein diets and weight loss.
The BCAA leucine plays multiple roles in metabolism beyond the minimum requirement as an essential substrate for synthesis of new proteins (8,10). These roles include a key regulator of translation initiation of protein synthesis in skeletal muscle (11), a modulator of insulin/PI3-kinase signaling (12,13), a fuel for skeletal muscle (14), and a primary nitrogen donor for production of alanine and glutamine in skeletal muscle (15). The potential for leucine to impact protein synthesis, insulin signaling, and production of alanine and glutamine is dependent on dietary intake and increasing leucine concentration in skeletal muscle (8,10,13).
The multiple roles of leucine are, at least in part, associated with absence of the branched-chain aminotransferase enzyme in liver, resulting in an enriched supply of the BCAA appearing in blood (8,10,16). Dietary BCAAs reach the blood virtually unaltered from levels in the diet, allowing leucine to reach skeletal muscle in direct proportion to dietary intakes. This is a striking metabolic difference for these amino acids, which account for >20% of total dietary protein. Using the traditional thinking that dietary protein requirements should be defined by efficiency of nitrogen handling, we are left to ponder why the body evolved to metabolize 20% of total amino acids (and total nitrogen) in peripheral tissues? We hypothesized that this unique treatment of the BCAA and specifically leucine provides an important signal of dietary quality for skeletal muscle (8,10).
One of the first reports that leucine metabolism was optimized at dietary intakes greater than the minimum recommended dietary allowance (RDA) was provided by the MIT group (17). Using stable isotopes and measurement of whole-body leucine metabolism, daily leucine usage was determined to be >6 g/d and ≈3 times higher than recommended dietary allowance values based on nitrogen balance. This difference highlights the important conceptual difference between requirements based on minimum levels to prevent deficiencies versus optimum levels for metabolic balance. These findings were confirmed by Pencharz and Ball (18,19), who determined rates of leucine oxidation and estimated daily leucine needs at ≈8 g/d.
Leucine regulation of muscle protein synthesis
Unique metabolic roles for leucine were reported first for regulation of skeletal muscle protein synthesis (20). During catabolic periods, such as fasting or energy restriction, supplementation with leucine or a complete mixture of the three BCAAs leucine, isoleucine, and valine stimulates muscle protein synthesis (8,11). Likewise, leucine supplementation stimulates recovery of muscle protein synthesis after exercise (21,22). The molecular mechanisms for the actions of leucine in protein synthesis are now known to involve regulation of phosphorylation events and components of the insulin signaling pathway in translational control of muscle protein synthesis (11,23).
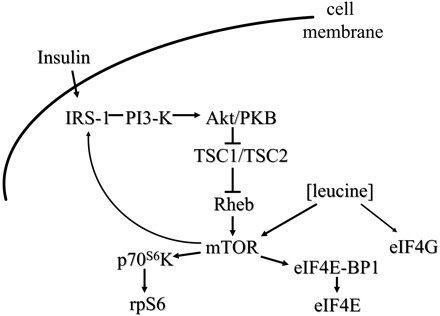
One site for leucine action is a kinase in the insulin signaling cascade previously identified as mammalian target of rapamycin (mTOR)5 (8,11,23). Increases in leucine concentration stimulate mTOR kinase activity for phosphorylation control of the eukaryotic initiation factor 4 complex and the S6 ribosomal protein (Fig. 1). Leucine stimulates phosphorylation of the inhibitory binding protein 4E-BP1, causing the binding protein to dissociate from the eukaryotic initiation factor-4E (eIF4E) translational initiation factor (11). Leucine stimulates activation of a second initiation factor eukaryotic initiation factor-4G (eIF4G) through an mTOR-independent kinase (11). Phosphorylated eIF4G is available to bind with free eIF4E to form the active initiation complex. Furthermore, leucine via mTOR activates p70S6 kinase, leading to phosphorylation of the S6 ribosomal protein (Fig. 1) (11,23). Mechanisms for translational regulations by leucine have been reviewed previously (11).
Figure 1
The signal cascade from phosphatidylinositol-3 kinase to mTOR serves to integrate information about dietary content of carbohydrates and protein represented as insulin and leucine concentrations for regulation of translation initiation of protein synthesis at initiation factors eIF4E, eIF4G, and ribosomal protein S6. Abbreviations used are as follows: 4E-BP1, inhibitory binding protein; IRS-1, insulin receptor substrate 1; PI3-K, phosphatidylinositol-3 kinase; p70S6K, p70S6 kinase; PKB, protein kinase B; rpS6, ribosomal protein S6; TSC, tuberous sclerosis complex. .
Molecular mechanisms for leucine stimulation of protein synthesis are supported by human studies (24–26). Short-term (2 to 4 h) intravenous infusions of large doses (3 to 7 g) of leucine produce anabolic changes in protein turnover and nitrogen balance (25,26). Likewise, leucine stimulates protein synthesis during catabolic conditions produced by short-term food deprivation or exhaustive exercise (24,27). After an overnight fast or intense exercise, protein synthesis is reduced compared with the rate of protein breakdown, producing a net breakdown of muscle protein. This catabolic period continues until adequate protein or specifically leucine is consumed to increase plasma and intracellular leucine concentrations (28). Oral intake of 2.5 g of leucine stimulates muscle protein synthesis after exercise or an overnight fast (24,27). These studies support the role of leucine as a key amino acid for reversing catabolic conditions.
Although leucine stimulates muscle protein synthesis in acute studies, the efficacy of prolonged administration is less clear. Human clinical trials with liver disease or sepsis have not produced consistent long-term benefits with leucine administration (25,26). Although these studies are complicated by the disease states, a sustained anabolic effect of leucine should produce a change in lean tissue mass.
Contrary to the acute clinical conditions, we predicted that regulatory roles of leucine would be more important during catabolic periods when plasma and intracellular concentrations of leucine are reduced, such as during fasting or energy restriction for weight loss. To test this hypothesis, one approach would be to use a low protein diet control and test the effects of supplemental leucine. This approach would mimic protocols of acute studies. Unfortunately, prolonged feeding of a low protein diet supplemented with leucine increases catabolism of valine and isoleucine, producing an amino acid imbalance among the BCAAs (10).
A second experimental approach would be to feed a low protein diet and provide a balanced supplement of the three BCAAs. This approach stimulates short-term increases in protein synthesis but depletes plasma concentrations of other essential amino acids (29,30). Because of the limitations of these approaches, we elected to test the long-term potential of leucine to reduce muscle wasting during weight loss using diets with mixtures of high-quality proteins that provided >8 g/d of leucine (5).
Protein-rich diets for treatment of obesity
An initial concept of protein sparing during weight loss was demonstrated in a short-term study by Bistrian et al. (31). They utilized very low energy diets providing 1.5 g.kg–1.d–1 of protein to produce rapid weight loss and found high protein diets reduced urinary nitrogen losses. Conclusions were reached that high protein intake was beneficial in minimizing wasting of lean tissue during weight loss for obesity.
In another short-term study, leucine infusion was shown to reduce nitrogen loss in obese subjects fasting to obtain weight loss (32). Over a 4-h period, ≈3.5 g of leucine was infused, resulting in reduction of 24-h nitrogen losses. These studies suggest a higher protein diet can be beneficial during weight loss and provide evidence that protein sparing effects are largely derived from leucine.
Using these findings, we conducted two weight loss trials using diets designed to provide 10 g/d of leucine (≈125 g/d of dietary protein) with a minimum of 2.5 g of leucine at each of three meals (5,33). In order to maintain equal energy intake and minimize postprandial insulin response, protein was increased proportional to the reduction in dietary carbohydrates. In comparisons with subjects following the USDA Food Guide Pyramid, subjects consuming the protein-rich diets lost more weight and were more effective in correcting body composition during weight loss (5,33). Consumption of the protein-rich diet resulted in greater loss of body fat and attenuated loss of lean tissue consistent with a protein sparing mechanism for leucine.
We also observed changes in glucose regulations with obese subjects during weight loss (34). Subjects consuming the high carbohydrate diet (ratio of carbohydrates:protein >3.5) maintained blood glucose within the normal physiological range; however, they had a progressive decline in fasting blood glucose and increase in postprandial insulin response during the 10-wk study. These data suggest that obese subjects challenged with a high carbohydrate diet exhibit a progressive decline in glycemic control. Subjects consuming the high protein diet (carbohydrate:protein <1.5) maintained stable blood glucose with minimal changes in blood glucose or insulin from fasting to meal periods.
Similar findings have been reported by other research groups. Diets with total protein intake > 1.5 g.kg–1.d–1 and carbohydrate intake <150 g/d increased weight loss (1–3,6), increased loss of body fat (2,3), attenuated loss of lean tissue (1,3,7), improved glycemic control (1,3,34), reduced serum triacylglyceride levels (5,6,33,35), and reduced blood pressure (36). These diets show efficacy in catabolic conditions, such as weight loss, resulting in these clinical outcomes. The spectrum of metabolic changes seen with weight loss also have specificity for the condition known as the Metabolic Syndrome (9,36,37).
Protein-rich diets for treatment of the metabolic syndrome
Metabolic Syndrome or Syndrome X is a chronic disease affecting >1 in 5 adults in the U.S. It is a condition defined by glucose intolerance and compensatory hyperinsulinemia that is often observed with obesity. Specific criteria for screening and diagnosis of Metabolic Syndrome are elevated fasting blood glucose, elevated triglycerides, low HDL, abdominal obesity, and hypertension (Table 1). Presence of three of these characteristics is diagnostic for Metabolic Syndrome and highly predictive of risk for type 2 diabetes mellitus and coronary heart disease (36).
Table 1 Criteria for Screening and Diagnosis of Metabolic Syndrome [1].
Fasting blood glucose ≥110 mg/dL (6.1 mmol/L) Serum triglycerides ≥150 mg/dL (1.69 mol/L) Serum HDL cholesterol ≤40 mg/dL in men (1.04 mmol/L) ≤50 mg/dL in women (1.29 mmol/L) Waist circumference ≥40 inches for men (102 cm) ≥35 inches for women (88 cm) Blood pressure ≥135/85 mm Hg
Presence of three or more of these symptoms is diagnostic (36).
Although Metabolic Syndrome is often associated with obesity, the defining characteristic is abnormal glycemic control observed as glucose intolerance and insulin resistance (36). Stability of blood glucose requires balance between hepatic glucose release and peripheral glucose disposal. The liver regulates the rate of glucose appearance in blood by balancing absorption of dietary glucose with endogenous production of glucose from gluconeogenesis and glycogen breakdown (38,39). Use of blood glucose by peripheral tissues occurs through insulin-dependent and insulin-independent transporters (40,41). Glycemic control requires precise balance across each of the components of glycemic regulation.
Reasons for enhanced glycemic control with use of higher protein diets remain to be fully elucidated. We proposed that high-protein low-carbohydrate diets achieve glycemic control by shifting regulation from insulin-mediated peripheral glucose disposal to hepatic regulation of glucose appearance (9). Diets with reduced levels of carbohydrates minimize postprandial glucose and insulin responses and minimize the role of liver glycogen in maintaining fasting blood glucose (38,42). Increased dietary protein contributes amino acids to de novo synthesis of glucose via gluconeogenesis, and BCAAs increase recycling of glucose carbon via the glucose-alanine cycle.
Impact of amino acids on glycemic control
Interactions of amino acids with carbohydrate metabolism have been recognized for years; however, research literature is unclear whether dietary protein has a positive or negative impact on glycemic control. Some reports suggest that amino acids increase fasting blood glucose (43–45), cause hyperinsulinemia (44), inhibit peripheral insulin action (44,45), and reduce glucose transport (44). By contrast, diets high in protein and low in carbohydrates reduce postprandial glucose and insulin and appear to stabilize blood glucose for individuals with type 2 diabetes (46) or obesity (1,3,34).
Dietary amino acids contribute to glucose homeostasis through hepatic glucose production. Jungas et al. (47) reported that amino acids serve as a primary fuel for the liver and the primary carbon source for hepatic gluconeogenesis. Other investigators found that gluconeogenesis provides >70% of fasting hepatic glucose release, with amino acids serving as the principal carbon source (38,42). Estimates of the contribution of amino acid carbon from 1 g of dietary protein to de novo glucose synthesis range from 0.6 to 0.7 g of glucose (46,48).
In addition to the direct conversion of amino acid carbon to gluconeogenesis precursors, there is also the contribution of the BCAA to glucose recycling via the glucose-alanine cycle (15,49). There is a continuous flux of BCAA from visceral tissues through the blood to skeletal muscle where transamination of the BCAA provides the amino group for production of alanine from pyruvate with a corresponding movement of alanine from muscle to liver to support hepatic gluconeogenesis. Although the impact of the glucose-alanine cycle has been debated, Ahlborg et al. (49) reported that alanine accounted for 40% of endogenous glucose production during prolonged exercise. Under normal conditions, alanine arising from BCAA nitrogen likely accounts for ≈25% of gluconeogenesis from amino acids (8). These studies provide evidence for the linkage between dietary protein and glucose homeostasis.
Beyond roles of amino acids as direct carbon substrates for gluconeogenesis, leucine serves as a metabolic signal for fuel choices. As discussed above, leucine stimulates muscle protein synthesis through modulation of downstream elements of the insulin/PI3-kinase signal pathway (Fig. 1). Parallel with mTOR actions on translational initiation factors, mTOR stimulates upstream phosphorylation of IRS-1 reducing PI3-kinase activity (12,13). The significance of this feedback loop to the insulin receptor is unknown. We have shown that modulation of the PI3-kinase activity does not alter glucose uptake into muscle tissue, leading us to propose that mTOR phosphorylation of IRS-1 is a component of normal feedback regulation perhaps limiting the duration of the insulin signal (13).
Intracellular leucine concentration also serves as a primary regulator of the branched-chain ketoacid dehydrogenase (BCKD), the rate-limiting step in oxidation of the BCAA (16). An increase in leucine increases the concentration of its keto-analogue α-ketoisocaproate, a potent inhibitor of the BCKD kinase that is responsible for inactivation of the BCKD by phosphorylation. Inhibition of the BCKD kinase results in dephosphorylation and activation of the BCKD. The BCKD stimulates decarboxylation of the three BCAAs and commits them to oxidation. At the same time, the increase in leucine concentration also inhibits pyruvate dehydrogenase–limiting pyurvate oxidation (50). Thus, when intracellular levels of leucine are elevated, muscles use glucose derived from either the blood or glycogen stores as a glycolytic fuel and then trap the pyruvate carbon as alanine via transamination with amino acid nitrogen derived from BCAA. These mechanisms appear to be particularly important during periods of low energy intake or endurance exercise when BCAAs are increased in muscle, insulin is low, and sparing of blood glucose is important (8,14,22).
The relative importance of reducing dietary carbohydrates versus increased dietary protein for glycemic control is difficult to assess using complete diets. Both carbohydrates and protein are known to affect stability of blood glucose (51–53). Diets high in carbohydrates increase the rate of appearance of glucose into the blood and inhibit gluconeogenesis (48,54). These diets reduce the role of gluconeogenesis in management of fasting blood glucose and shift glycemic regulation to glycogenolysis and glycogen stores (48,54). Opposite of this regulation, diets with reduced carbohydrates and increased protein increase rates of gluconeogenesis (43,48,54). Rossetti et al. (43) reported that increases in dietary protein were essential for increases in gluconeogenesis.
Clinical studies with normal and obese subjects (7,52) and individuals with type 2 diabetes (55,56) report similar findings. Using isoenergtic meals, these studies demonstrated that substituting dietary protein for carbohydrates reduced meal responses of both plasma glucose and insulin (52,55,56). Persons with type 2 diabetes reduce postprandial glucose and insulin levels, reduce glycosylated hemoglobin (HbA1C), and increase insulin sensitivity (55,56). Likewise, obese subjects reduce glucose and insulin response after meals and specifically decrease Phase II insulin release (7). Although these results are intuitively obvious, they directly contradict reports that protein is hyperinsulinemic and hyperglycemic.
In summary, use of diets with increased protein and reduced carbohydrates appears to enhance weight loss with increased loss of body fat and reduced loss of lean body mass. Beneficial effects of high-protein diets appear to be associated with sparing of muscle protein loss and enhanced glycemic control. Specific mechanisms to explain each of the observed outcomes remain to be fully elucidated. We suggest that a key to understanding the relationship between dietary protein and carbohydrates is the relationship between the intakes of leucine and glucose. Leucine is now known to interact with the insulin-signaling pathway with apparent modulation of the downstream signal for control of protein synthesis, resulting in maintenance of muscle protein during periods of restricted energy intake. Leucine also appears to modulate the insulin signal and glucose use by skeletal muscle. While total protein is important in providing substrates for gluconeogenesis, leucine appears to regulate oxidative use of glucose by skeletal muscle through stimulation of glucose recycling via the glucose-alanine cycle. These mechanisms appear to provide a stable glucose environment with low insulin responses during energy-restricted periods.

Return to the LEUCINE Page
Since 1-21-2015


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |