Imaging Strategies for Low-back Pain:
Systematic Review and Meta-analysisThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Lancet. 2009 (Feb 7); 373 (9662): 463–472 ~ FULL TEXT
Roger Chou, Rongwei Fu, John A Carrino, Richard A Deyo
Oregon Health and Science University,
Portland, OR, USA.BACKGROUND: Some clinicians do lumbar imaging routinely or in the absence of historical or clinical features suggestive of serious low-back problems. We investigated the effects of routine, immediate lumbar imaging versus usual clinical care without immediate imaging on clinical outcomes in patients with low-back pain and no indication of serious underlying conditions.
METHODS: We analysed randomised controlled trials that compared immediate lumbar imaging (radiography, MRI, or CT) versus usual clinical care without immediate imaging for low-back pain. These trials reported pain or function (primary outcomes), quality of life, mental health, overall patient-reported improvement (based on various scales), and patient satisfaction in care received. Six trials (n=1804) met inclusion criteria. Study quality was assessed by two independent reviewers with criteria adapted from the Cochrane Back Review Group. Meta-analyses were done with a random effects model.
FINDINGS: We did not record significant differences between immediate lumbar imaging and usual care without immediate imaging for primary outcomes at either short-term (up to 3 months, standardised mean difference 0.19, 95% CI -0.01 to 0.39 for pain and 0.11, -0.29 to 0.50 for function, negative values favour routine imaging) or long-term (6-12 months, -0.04, -0.15 to 0.07 for pain and 0.01, -0.17 to 0.19 for function) follow-up. Other outcomes did not differ significantly. Trial quality, use of different imaging methods, and duration of low-back pain did not affect the results, but analyses were limited by small numbers of trials. Results are most applicable to acute or subacute low-back pain assessed in primary-care settings.
INTERPRETATION: Lumbar imaging for low-back pain without indications of serious underlying conditions does not improve clinical outcomes. Therefore, clinicians should refrain from routine, immediate lumbar imaging in patients with acute or subacute low-back pain and without features suggesting a serious underlying condition.
From the FULL TEXT Article:
Introduction
Studies have consistently shown that clinicians vary widely in how frequently they obtain imaging tests for assessment of low-back pain. [1–3] In the absence of historical or clinical features (so-called red flags), suggestive of a serious underlying condition (such as cancer, infection, or cauda equina syndrome), the 1994 Agency for Healthcare Policy and Research (AHCPR) guideline made recommendations against lumbar imaging in the first month of acute low-back pain. [4] These recommendations were based on observational studies that indicated a low frequency of serious conditions in patients without red flags, [5, 6] weak correlation between findings on lumbar imaging studies and clinical symptoms, [7] high likelihood for acute low-back pain to improve, [8] and lack of evidence that imaging is helpful for guiding treatment decisions. [9] Clinical guidelines for acute low-back pain published after 1994 have consistently recommended a similar approach. [10] Some guidelines have also advised against lumbar imaging for chronic low-back pain without red flags.
Some clinicians still do lumbar-spine imaging routinely or without a clear indication,3 possibly because they aim to reassure their patients and themselves, to meet patient expectations about diagnostic tests, to identify a specific anatomical diagnosis for low-back pain, or because reimbursement structures provide financial incentives to image. [11–13] However, imaging can be harmful because of radiation exposure (radiography and CT) and risks of labelling of patients with an anatomic diagnosis that might not be the actual cause of symptoms. [14, 15] Furthermore, imaging studies have high direct and indirect costs. Increased frequency of lumbar MRI is associated with higher rates of spine surgery, without clear differences in patient outcomes. [16, 17]
Most diagnostic imaging studies quantify test accuracy for the identification of the presence or absence of disease compared with an established reference standard. For low-back pain, such studies are difficult to interpret because no reference standard reliably differentiates symptomatic from asymptomatic spinal imaging abnormalities. [14, 18] Furthermore, studies of diagnostic-test accuracy do not investigate effects on clinical decision making or patient outcomes. By contrast, randomised trials that assess clinical outcomes incorporate effects of test results on subsequent treatments and are regarded as the strongest evidence for the assessment of diagnostic tests. [19]
Since the publication of the AHCPR guidelines, several randomised trials of immediate, routine lumbar imaging versus usual clinical care without immediate imaging have been published. [20–24] In some trials, small differences have been reported in favour of routine imaging, but results have not always been significant. In such situations, meta-analyses can be helpful to assess whether a true difference exists, by increasing statistical power. [25] The purpose of this systematic review and meta-analysis was to see whether immediate, routine lumbar-spine imaging is more effective than usual clinical care without immediate lumbar imaging in patients with low-back pain and no features suggesting a serious underlying condition.
Methods
Procedures
We searched Medline (from 1966 to first week of August, 2008) and the Cochrane Central Register of Controlled Trials (third quarter of 2008), with the terms “spine”, “low-back pain”, “diagnostic imaging”, and “randomised controlled trials” (see webpanel for complete search strategy). We reviewed reference lists for additional citations.
We included randomised controlled trials that compared immediate, routine lumbar imaging (or routine provision of imaging findings) versus usual clinical care without immediate lumbar imaging (or not routinely providing results of imaging) for low-back pain without indications of serious underlying conditions.

Table 1 These trials assessed at least one of the following outcomes: pain, function, mental health, quality of life, patient satisfaction, and overall patient-reported improve ment (Table 1). We applied no language restriction. Two reviewers independently assessed potentially relevant citations for inclusion. Disagreements were resolved by consensus. Two independent reviewers abstracted data from trials and assessed quality with modified Cochrane Back Review Group criteria. [31] We excluded criteria for blinding of patients and providers because of lack of applicability to imaging trials, and the criterion needing similarity of co-interventions because a potential effect of different imaging strategies is to alter subsequent treatment decisions. The remaining eight criteria and methods to make the criteria operational are shown in the webtable. We resolved disagreements about quality ratings by discussion and consensus. We classified trials that met at least half (four or more) of the eight criteria “higher-quality”, and those that met three or fewer of the eight criteria “lower-quality”. We categorised duration of symptoms as acute (<4 weeks), subacute (4–12 weeks), and chronic (>12 weeks). We contacted authors for additional data if included outcomes were not published, or if median (rather than mean) outcomes were reported.
Statistical analysis
Primary outcomes were improvement in pain or function. [32] Secondary outcomes were improvement in mental health, quality of life, patient satisfaction, and overall improvement. Other than overall improvement, which was assessed as a dichotomous variable with various scales (Table 1), all other outcomes were assessed as continuous variables. We categorised outcomes as short term (≤3 months), long term (>6 months to ≤1 year), or extended (>1 year).
We calculated pooled estimates and 95% CIs with the DerSimonian-Laird random effects model. [33] We chose this model because trials differed in patient populations (eg, duration of low-back pain and presence of sciatica symptoms), type of imaging intervention (lumbar radiography, MRI, or CT), and other factors. For continuous outcome measures, we calculated standardised mean differences (SMDs, Hedge’s d) of interventions for changes between baseline and follow-up scores. We needed correlations between baseline and follow-up score to calculate corresponding SDs, but these were not reported or calculable in most trials. We used the correlation obtained from one trial [21] to estimate SDs for the other trials. If a study assessed pain or function with more than one method, we used the short-form-36 (SF-36) bodily pain score for pain and the Roland disability questionnaire (RDQ) for primary analyses. We analysed pain and function measures so that lower scores indicated better outcomes. For quality of life and mental health, higher scores indicated improved outcomes. We calculated weighted mean differences (WMDs) for subgroups of trials reporting the same pain or function outcomes. We excluded from the main analysis trials that did not report SDs for included outcomes or suffi cient data to impute [34] them. When a trial reported only median data, we analysed results with the median value instead of the mean, and estimated the SD with the interquartile difference. When both mean and median data were available, we used mean values in the primary analyses. Although one trial reported results adjusted for differences in baseline factors, [21] we calculated unadjusted results to enter into the meta-analyses to be consistent with the other trials.
Statistical heterogeneity was assessed by Cochran’s Q test and the I2 statistic. [35] Because of small numbers of trials that could be pooled (maximum four trials), we did not construct L’Abbé plots [36] or assess for publication bias. [37] For outcomes that could not be pooled, we assessed results qualitatively.
We did meta-regression on primary outcomes (pain and function) to assess whether duration of pain (mainly acute or subacute vs mainly chronic low-back pain), overall trial quality (higher quality vs lower quality), or imaging technique (radiography vs MRI or CT) could explain variation between studies. Because the number of trials was small, we interpreted meta-regression results cautiously. We did sensitivity analyses on the correlation between baseline and follow-up scores by assessing values from 0 to 0·8, substituted primary pain or function outcomes with other reported measures, median with mean data when reported, and adjusted with unadjusted results when available. We also investigated how including trials that did not provide data to impute SDs could aff ect results by assuming various plausible values for SDs (from values one-eighth the mean to equivalent to the mean). All analyses were done with Stata version 10.0 (StataCorp, College Station, TX, USA).
We regarded SMDs of 0·2 to 0·5 as small; 0·5 to 0·8 as moderate; and greater than 0·8 as large. [38] For WMDs, we regarded mean improvements of 5–10 points on a 100–point scale (or equivalent) as small; 10–20 points as moderate; and more than 20 points as large. [39] For the RDQ (the most commonly reported measure of back-specific function), we classified mean improvements of 1–2 points as small and 2–5 points as moderate. [40]
Role of the funding source
The sponsor of the study had no role in the design and conduct of the study; data collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript; or the decision to submit the article for publication. RC had full access to all data in the study, and had final responsibility for the decision to submit for publication.
Results
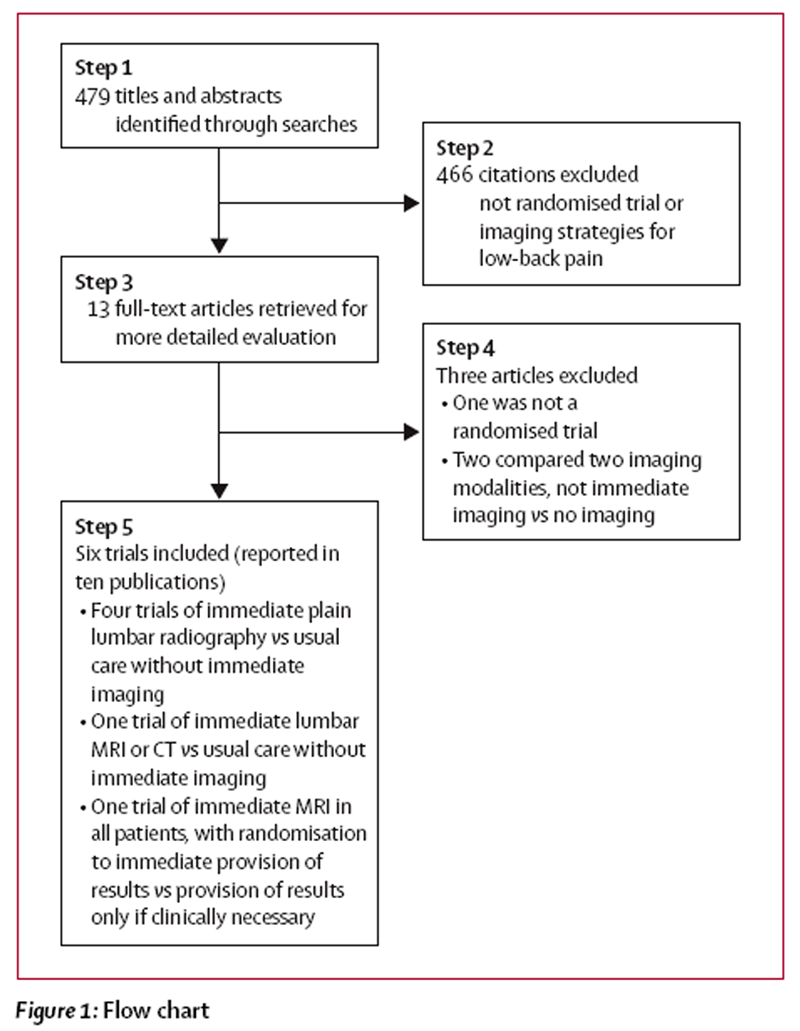
Figure 1 Figure 1 shows the flow chart of studies from initial results of publication searches to final inclusion or exclusion. Of the six trials that met inclusion criteria, four, reported in six publications, assessed lumbar radiography [20, 22, 23, 28–30] and two, reported in four publications, assessed MRI or CT. [21, 24, 26, 27] We excluded two randomised trials that compared rapid MRI with plain radiography [16, 41] and one non-randomised study. [9]
1,804 patients were randomly assigned in six trials. [20–24, 30] Five of the six trials were done in the UK21–23 or USA. [24, 30] Duration of follow-up ranged from 3 weeks [20] to 2 years. [21] One trial excluded patients with sciatica or other symptoms of radiculopathy, [20] and one did not report the proportion of patients with such symptoms. [23] In the other four trials, [21, 22, 24, 30] the proportion of patients with sciatica or radiculopathy ranged from 24% to 44%.
Three trials [20, 22, 23] compared immediate lumbar radiography with usual clinical care without immediate lumbar radiography, and one [30] compared immediate lumbar radiography with a brief educational intervention plus lumbar radiography, if no improvement was seen by 3 weeks. Patients enrolled in these trials had mainly acute or subacute (<12 weeks) low-back pain (Table 1), and all trials were done in primary-care or urgent-care settings.
Two studies21,24 assessed advanced imaging modalities. One study21 compared immediate MRI or CT with usual clinical care without advanced imaging in patients with mainly chronic low-back pain (82% had low-back pain for >3 months) referred to a surgeon, whereas in the other study [24] all patients with low-back pain for less than 3 weeks underwent MRI, with randomisation to routine notifi cation of results within 48 h versus notification of results only if clinically indicated. Patients were recruited from various settings (primary care, spine clinic, or emergency room). In both trials, the proportion of patients who underwent lumbar radiography before enrolment was not reported.

Table 2 Five trials [21–23, 26, 30] met at least four of eight predefined quality criteria, and were classified as higher quality (Table 2). Two investigators agreed on all quality ratings, apart from those about baseline-group similarity for one trial [24] and use of intention-to-treat analysis for another trial. [30] The most frequent methodological shortcoming was lack of (or unclear use of) blinded outcome assessment (five of six trials), followed by inadequate description of randomisation method (four of six trials). All trials excluded patients with features suggestive of a serious underlying condition, but exclusion criteria varied (Table 2) and trials did not indicate the number of patients excluded because of such factors. In one trial, [23] 95 of 506 patients who were not randomly assigned were referred for lumbar radiography, but reasons for imaging were not explained. All trials assessed improvement in pain and function, and four [20, 22, 24, 30] reported overall improvement, but varied in the methods used to assess these and other outcomes (Table 1).
Two trials [20, 22] reported median rather than mean data for outcomes. We obtained unpublished mean outcome data for one [22] of these trials. Five trials [20–24] could be included in the primary meta-analysis on improvement in pain or function at one or more follow-up intervals, and one [30] did not report baseline pain data or provide data to impute SDs.

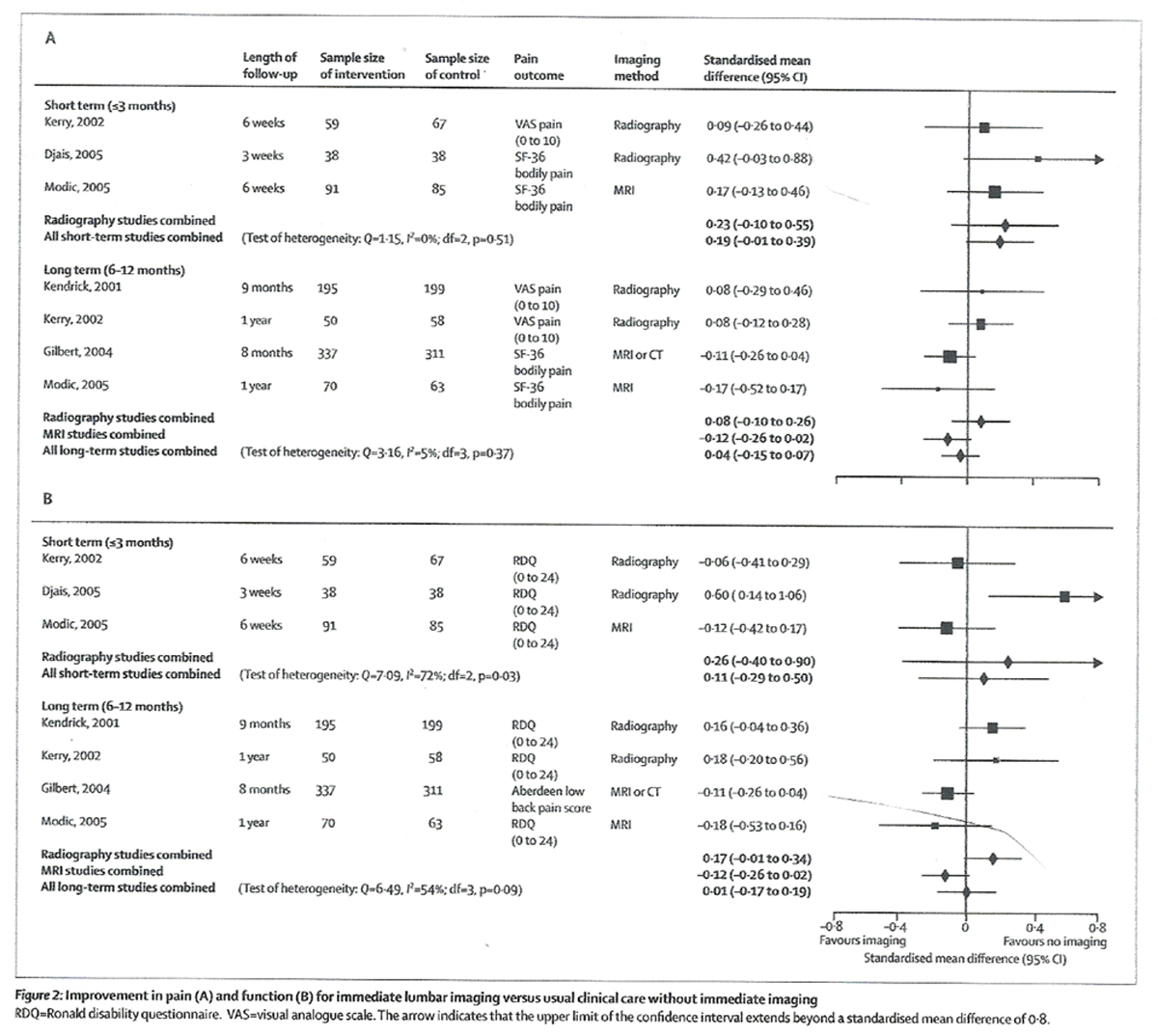
Table 3 We did not note any significant difference between routine, immediate lumbar imaging and usual clinical care without immediate imaging for improvement in pain or function at short-term or long-term follow-up (Table 3 and Figure 2), although several results slightly favoured non-immediate imaging (positive values). Heterogeneity was not present. Improvement (calculated as WMDs) in pain at short-term follow-up slightly favoured no immediate imaging in trials that used a visual analogue (0 to 10) pain scale (WMD 0·62, 95% CI 0·03 to 1·21), [20, 24] but was not significantly different in trials that used the SF-36 bodily pain score (2·99, –2·04 to 8·03).23,24 For long-term pain, immediate imaging and usual clinical care without immediate imaging did not differ in trials reporting either a visual analogue pain scale (0·08, –0·11 to 0·27) [22, 24] or the SF-36 bodily pain score (–2·14, –5·10 to 0·80). [21, 23, 24]
Figure 2 Figure 2 also shows improvement in function for short-term and long-term follow-up. Heterogeneity was present at both short-term and long-term follow-up. For short-term function, heterogeneity seemed due to inclusion of a lower-quality trial that only reported median outcome data. [20] However, the exclusion of this trial did not change the conclusion of no difference between imaging strategies. For long-term follow-up, heterogeneity seemed due to imaging type (p=0·012 for lumbar radiography vs MRI or CT in meta-regression analysis). Results remained statistically and clinically non-significant (Figure 2) after trials were stratified according to whether they assessed lumbar radiography, or MRI or CT, although heterogeneity was no longer present (I2=0% for both analyses).
All three trials [20, 23, 24] included in the analysis for short-term function used the RDQ, with a WMD of 0·48 points (95% CI –1·39 to 2·35) for immediate imaging versus usual clinical care without immediate imaging. In the three trials that reported long-term function with the RDQ, [22–24] the WMD was 0·33 points (–0·65 to 1·32).
Only one trial [21] reported pain or function at extended (2–year) follow-up. On the basis of calculated, unadjusted analysis, immediate lumbar MRI or CT did not differ from usual clinical care without immediate lumbar imaging for improvement in SF-36 bodily pain score (mean difference –2·7, 95% CI –6·19 to 0·79) or the Aberdeen low-back pain score (–1·6, –4·04 to 0·84). Immediate MRI or CT caused small but significant improvements in pain and function when results were adjusted for age, sex, diagnostic category (radiculopathy due to herniated disc or degenerative disease, neurogenic claudication, or other low-back pain), and clinician, although groups did not differ in these factors (adjusted mean difference on the SF-36 bodily pain score –5·14, –8·67 to –1·61, and on the Aberdeen low-back pain score –3·62, –5·92 to –1·32).
In meta-regression analyses, trial quality and duration of low-back pain were not good predictors of differences in estimates for either pain or function. Imaging type (MRI or CT vs lumbar radiography) was not a good predictor of differences in estimates for pain. Estimates were similar when we substituted median with mean outcome data from one trial, [22] adjusted with unadjusted results from another trial, [21] and when we used various plausible values for the correlation between baseline and follow-up scores for trials that did not report these data. Results of short-term function were unchanged when we included a trial in which SDs were not reported, [30] by assuming a broad range of plausible values. For example, with an SD equivalent to half the mean, immediate, routine lumbar imaging and non-immediate lumbar imaging did not differ (SMD 0·08, 95% CI –0·20 to 0·37). [20, 23, 24, 30]
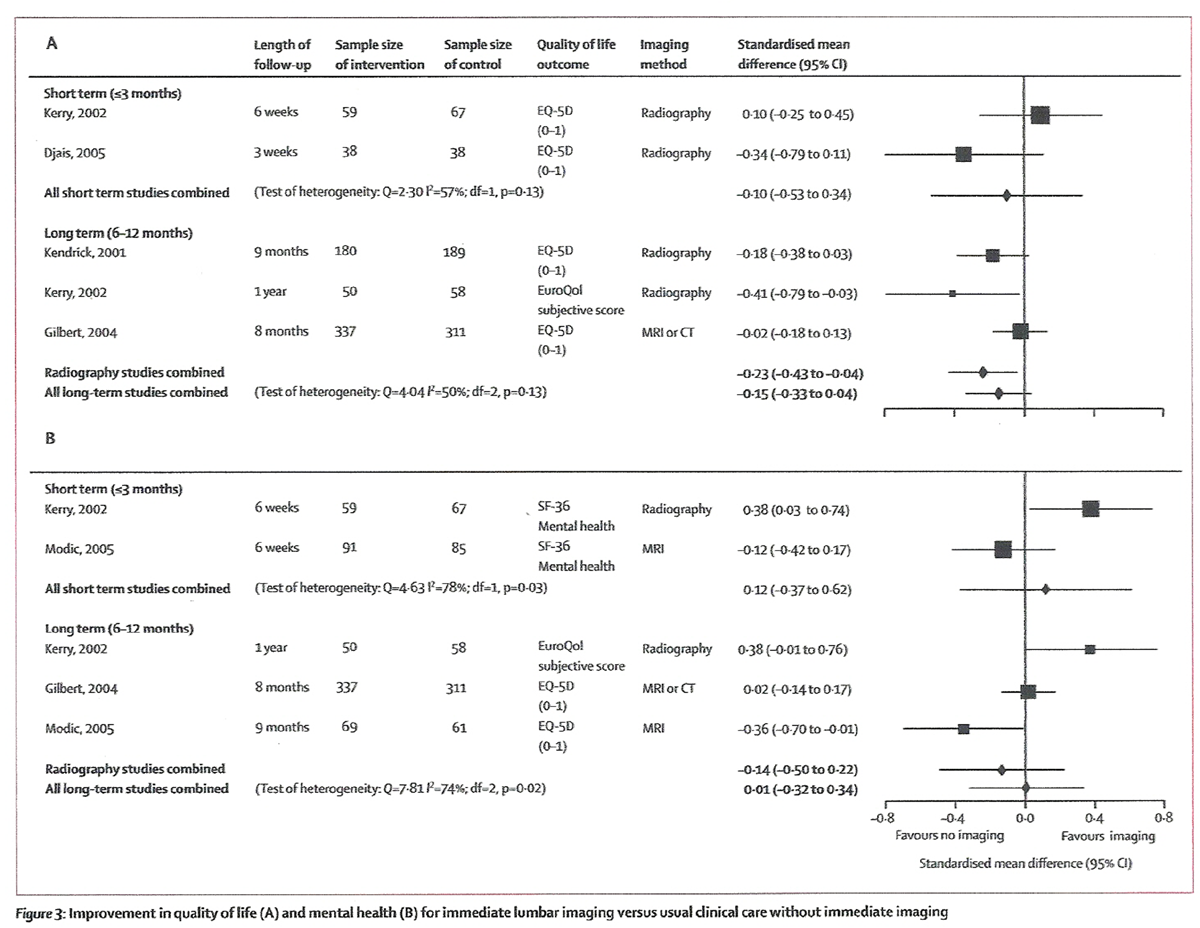
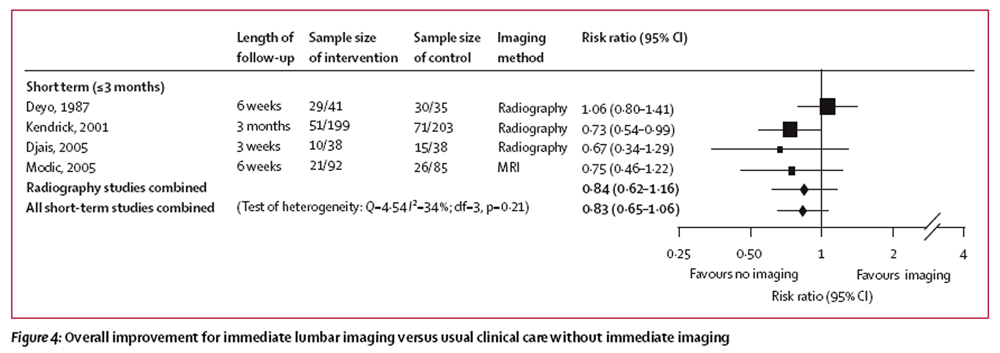
Figure 3 Immediate lumbar imaging and usual clinical care without immediate imaging did not differ for short-term or long-term quality of life, mental health, and overall improvement (Table 3, and Figures 3 and 4). For quality of life, results slightly favoured non-immediate imaging (negative values, Figure 3).
In the trial that reported extended (2–year) follow-up data, immediate MRI or CT was not better than usual clinical care without immediate imaging on either the EuroQol-5D (mean difference 0·02, 95% CI –0·02 to 0·07, 0 to 1 scale) or the SF-36 mental health score (–1·50, –4·09 to 1·09, 0 to 100 scale) in unadjusted analyses. [21] However, results slightly favoured immediate MRI or CT on the EuroQol-5D after adjustment for age, sex, diagnostic category, and clinician (adjusted mean difference 0·06, 0·01 to 0·10).
Figure 4 We were unable to pool data for patient satisfaction from three trials. [22, 23, 30] One trial [23] showed no difference between immediate lumbar radiography and usual clinical care without radiography in the proportion of patients who were satisfied or very satisfied (78% vs 70%). Another trial [22] showed no difference based on the patient satisfaction score (minimum score 9, maximum 27) after 3 months (median 20 vs 21, favouring usual clinical care, p=0·13), but immediate imaging was better after 9 months (median 21 vs 19, p<0·01). Another trial [30] also used the patient satisfaction score, and showed no difference between immediate lumbar radiography and an educational intervention without radiography, but only assessed outcomes after 3 weeks (mean 23·7 vs 24·0).
Four trials (n=399) obtained imaging in all patients [24] or recorded low-back pain diagnoses based on clinical follow-up through at least 6 months of follow-up. [22, 23, 30] No cases of cancer, infection, cauda equina syndrome, or other serious diagnoses were reported in patients randomly assigned to any imaging strategy.
Discussion
Our meta-analysis of randomised controlled trials showed that immediate, routine lumbar-spine imaging in patients with low-back pain and no features suggesting serious underlying conditions did not improve clinical outcomes compared with usual clinical care without immediate imaging. Results were limited by small numbers of trials for some analyses, but seemed consistent for the primary outcomes of pain and function, and for quality of life, mental health, and overall improvement. Data for patient satisfaction could not be pooled, but showed no clear difference. In addition to non-significance, pooled estimates were small or close to zero and, in some cases, slightly favoured the non-imaging strategy. This result suggests that, even if statistical power could be increased by other trials, clinically important benefits from routine lumbar imaging are unlikely, assuming that future results are similar to those currently available. Based on lower limits of 95% CIs, maximum plausible benefits on pain or function with routine imaging would be small (SMD 0·29 for short-term function) or trivial (SMD <0·2).
Several trials also showed no serious underlying conditions in patients without risk factors for these conditions. [22–24, 30] These results should be interpreted cautiously, because identification of serious conditions was not a primary outcome in any trial; most trials relied on routine clinical follow-up to identify these conditions, and the trials enrolled a total of less than 400 people. However, findings are in line with large observational investigations. [5, 9, 42] For example, a prospective study of 1,975 patients in a walk-in clinic showed no cases of cancer in 1,170 patients under the age of 50 with no history of cancer, no weight loss, or other sign of systemic illness, and no history of failure to improve with conservative therapy. [5]
Data for any outcome beyond 1 year of follow-up are sparse. One trial [21] showed that immediate lumbar MRI or CT was better than usual clinical care without immediate imaging for pain, function, and quality of life in patients mainly affected by chronic low-back pain for 2 years. However, benefits averaged only 3–5 points on a 100–point scale, and were only present when results were adjusted for sex, age, diagnostic category, and clinician. The need to adjust results in this trial is unclear, because factors that were adjusted for were similar in the two groups. The recorded discrepancy could be related to reliance on estimated correlations between baseline and follow-up scores to calculate unadjusted results compared with use of direct data in the adjusted analyses. Nonetheless, meta-analyses for shorter-term outcomes that were included in this trial were similar when we substituted adjusted with unadjusted results.
This meta-analysis compared imaging strategies for assessment of low-back pain. In addition to lack of clinical benefit, lumbar imaging is associated with radiation exposure (radiography and CT), [7] may not aff ect diagnostic or treatment plans, [43] increases direct costs, [21–23] and may lead to increased use of expensive but potentially unnecessary invasive procedures. [17] In this study, we assessed effects of different imaging strategies on the basis of randomised controlled trials that reported patient outcomes and not on the basis of trials that assessed intermediate outcomes, such as diagnostic accuracy or effects on clinical decision making. [19] Similar trials that assess patient outcomes could be done to investigate other diagnostic tests with uncertain clinical utility, such as provocative discography and various diagnostic blocks. Our study has several limitations. First, the trials included are clinically diverse, and varied in the type of imaging modality or strategy assessed, the duration of low-back pain in enrollees, and trial quality.
However, other trials [16, 41] have shown no difference between immediate lumbar MRI and radiography, suggesting that pooling trials that investigate these modalities is reasonable. We also used a random effects model, which leads to more conservative estimates (wider CIs) when statistical heterogeneity is present, [25] and did meta-regression analyses, which showed that predefi ned methodological and demographic factors had no major effects on overall estimates or conclusions, although results were based on a small number of trials. Second, we pooled trials that assessed different pain or function measures, which could introduce heterogeneity. However, conclusions were similar when we analysed trials that reported the same outcome measure. Finally, we were unable to assess effects of baseline patient characteristics on estimates because we did not have access to individual patient data. Results of a trial [21] that assessed results stratifi ed according to presence of lumbar-disc herniation or nerve-root entrapment were not different compared with overall trial results.
We identified several factors related to the management and reporting of randomised trials of lumbar imaging that could be improved. First, all trials had methodological shortcomings. Future trials should describe randomisation methods in more detail, use blinded outcome assessors, and report intention-to-treat analyses. [44] Second, assessment and reporting of outcomes was not well standardised. For example, function was measured with three different scales in six trials, and only three trials measured patient satisfaction with two different methods. Availability of scarce and inconsistent data for patient satisfaction is particularly relevant because one study [45] showed that routine lumbar radiography could be cost effective, depending on how highly patient satisfaction is valued. More standardised methods for reporting outcomes based on published recommendations would greatly help future analyses. [32]
Third, assessment of applicability of imaging trials was diffi cult. [46] These trials used different criteria for excluding patients with risk factors, and none explicitly indicated the number excluded because of features suggestive of serious underlying conditions. Improved reporting of the number of patients from initial screening through randomisation would help to clarify how much trial results are likely to relate to general practice. Finally, trials of MRI or CT did not report how many patients had previously undergone lumbar radiography. [21, 24] Whether these trials truly assessed MRI or CT versus no imaging, or the incremental benefit of advanced imaging in patients who were already examined with lumbar radiography, is not clear.
Our study confirmed that clinicians should refrain from routine, immediate lumbar imaging in patients with low-back pain and without features suggesting a serious underlying condition. [47–49] Conclusions mainly apply to patients with acute or subacute, non-specific low-back pain assessed in primary-care settings. Results from one trial [21] suggested that MRI or CT might also not be routinely indicated for chronic low-back pain because of unclear or small benefits. However, more studies are needed to identify best possible imaging strategies in patients with chronic low-back pain, symptoms of radiculopathy or spinal stenosis, patients assessed in referral settings, and other specific subgroups.
Rates of utilisation of lumbar MRI are increasing, [50] and implementation of diagnostic-imaging guidelines for low-back pain remains a challenge. However, clinicians are more likely to adhere to guideline recommendations about lumbar imaging now that these are supported by consistent evidence from higher-quality randomised controlled trials. [51] Patient expectations and preferences about imaging should also be addressed, because 80% of patients with low-back pain in one trial [22] would undergo radiography if given the choice, despite no benefits with routine imaging. Educational interventions could be effective for reducing the proportion of patients with low-back pain who believe that routine imaging should be done. [30] We need to identify back-pain assessment and educational strategies that meet patient expectations and increase satisfaction, while avoiding unnecessary imaging.
Contributors
RC participated in the conception, design, and drafting of the article. All authors participated in analysis and interpretation of data, revision of the article, and gave fi nal approval of the version to be published. RC had responsibility for the integrity of the data and the accuracy of the analysis.
Contributors
RC participated in the conception, design, and drafting of the article. All authors participated in analysis and interpretation of data, revision of the article, and gave fi nal approval of the version to be published. RC had responsibility for the integrity of the data and the accuracy of the analysis.
Confl ict of interest statement
We declare that we have no confl ict of interest.
Acknowledgments
We thank Jayne Schablaske and Michelle Pappas for administrative support, and Laurie Hoyt Huff man for assisting with data abstraction and quality ratings.
References:
Carey TS, Garrett J, and the North Carolina Back Pain Project.
Patterns of ordering diagnostic tests for patients with acute low back pain.
Ann Intern Med 1996; 125: 807–14.Cherkin DC, Deyo RA, Wheeler K, Ciol MA.
Physician variation in diagnostic testing for low back pain. Who you see is what you get.
Arthritis Rheum 1994; 37: 15–22.Di Iorio D, Henley E, Doughty A.
A survey of primary care physician practice patterns and adherence to acute low back problem guidelines.
Arch Fam Med 2000; 9: 1015–21.Stanley J. Bigos, MD, Rev. O. Richard Bowyer, G. Richard Braen, MD, et al.
Acute Lower Back Problems in Adults. Clinical Practice Guideline No. 14.
Rockville, MD: Agency for Health Care Policy and Research, [AHCPR Publication No. 95-0642].
Public Health Service, U.S. Department of Health and Human Services; 1994Deyo R, Diehl A.
Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies.
J Gen Intern Med 1988; 3: 230–38.Deyo R, Diehl A.
Lumbar spine films in primary care: current use and effects of selective ordering criteria.
J Gen Intern Med 1986; 1: 20–25.Jarvik JG, Deyo RA.
Diagnostic evaluation of low back pain with emphasis on imaging.
Ann Intern Med 2002; 137: 586–97.Pengel LHM, Herbert RD, Maher CG, Refshauge KM.
Acute low back pain: systematic review of its prognosis.
BMJ 2003; 327: 323–27.Rockey P, Tompkins R, Wood R, Wolcott B.
The usefulness of x-ray examinations in the evaluation of patients with back pain.
J Fam Pract 1978; 7: 455–65.Koes B, van Tulder M, Ostelo R, Kim Burton A, Waddell G.
Clinical Guidelines for the Management of Low Back Pain in Primary Care:
An International Comparison
Spine (Phila Pa 1976) 2001 (Nov 15); 26 (22): 2504–2513Sox HJ, Margulies I, Sox C.
Psychologically mediated effects of diagnostic tests.
Ann Intern Med 1981; 95: 680–85.Verbeek J, Sengers M, Riemens L, Haafkens J.
Patient Expectations of Treatment for Back Pain: A Systematic Review
of Qualitative and Quantitative Studies
Spine (Phila Pa 1976). 2004 (Oct 15); 29 (20): 2309–2318Wilson IB, Dukes K, Greenfield S, Kaplan S, Hillman B.
Patients’ role in the use of radiology testing for common office practice complaints.
Arch Intern Med 2001; 16: 256–63.Jarvik JJ, Hollingworth W, Heagerty P, Haynor DR, Deyo RA.
The Longitudinal Assessment of Imaging and Disability of the Back (LAIDBACK) study.
Spine 2001; 26: 1158–66.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS.
Magnetic resonance imaging of the lumbar spine in people without back pain.
N Engl J Med 1994; 331: 69–73.Jarvik JG, Hollingworth W, Martin B, Emerson SS, Gray DT, Overman S, et al.
Rapid magnetic resonance imaging vs radiographs for patients with low back pain: a randomized controlled trial.
JAMA 2003; 289: 2810–18.Lurie JD, Birkmeyer NJ, Weinstein JN.
Rates of advanced spinal imaging and spine surgery.
Spine 2003; 28: 616–20.van Tulder MW, Assendelft WJ, Koes BW, Bouter LM.
Spinal radiographic findings and nonspecific low back pain: a systematic review of observational studies.
Spine 1997; 22: 427–34.Sackett DL, Haynes RB.
The architecture of diagnostic research.
BMJ 2002; 324: 539–41.Djais N, Kalim H.
The role of lumbar spine radiography in the outcomes of patients with simple acute low back pain.
APLAR J Rheumatol 2005; 8: 45–50.Gilbert FJ, Grant AM, Gillan MG, et al.
Low back pain: influence of early MR imaging or CT on treatment and outcome—multicenter randomized trial.
Radiology 2004; 231: 343–51.Kendrick D, Fielding K, Bentley E, Kerslake R, Miller P, Pringle M.
Radiography of the lumbar spine in primary care patients with low back pain: randomised controlled trial.
BMJ 2001; 322: 400–05.Kerry S, Hilton S, Dundas D, Rink E, Oakeshott P.
Radiography for low back pain: a randomised controlled trial and observational study in primary care.
Br J Gen Pract 2002; 52: 469–74.Modic MT, Obuchowski NA, Ross JS, et al.
Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome.
Radiology 2005; 237: 597–604.Lau J, Ioannidis JP, Schmid CH.
Quantitative synthesis in systematic reviews.
Ann Intern Med 1997; 127: 820–26.Ash LM, Modic MT, Obuchowski NA, Ross JS, Brant-Zawadzki MN, Grooff PN.
effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain.
Am J Neuroradiol 2008; 29: 1098–103.Gilbert FJ, Grant AM, Gillan MG, et al.
Does early imaging influence management and improve outcome in patients with low back pain? A pragmatic randomised controlled trial.
Health Technol Assess (Engl) 2004; 8: 1–144.Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
Health Technol Assess (Engl) 2000; 4: 1–119.Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
Health Technol Assess (Engl) 2001; 5: 1–69.Deyo RA, Diehl AK, Rosenthal M.
Reducing roentgenography use. Can patient expectations be altered?
Arch Intern Med 1987; 147: 141–45.van Tulder M, Furlan AD, Bombardier C, Bouter L,
the Editorial Board of the Cochrane Collaboration Back Review Group.
Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group.
Spine 2003; 28: 1290–99.Bombardier C.
Outcome assessments in the evaluation of treatment of spinal disorders: summary and general recommendations.
Spine 2000; 25: 3100–03.Dersimonian R, Laird N.
Meta-analysis in clinical trials.
Control Clin Trials 1986; 7: 177–87.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N.
Imputing missing standard deviations in meta-analyses can provide accurate results.
J Clin Epidemiol 2006; 59: 7–10.Higgins JPT, Thompson SG.
Quantifying heterogeneity in a meta-analysis.
Stat Med. 2002; 21: 1539–58.Song F.
Exploring heterogeneity in meta-analysis: is the L’Abbe plot useful?
J Clin Epidemiol 1999; 52: 725–30.Egger M, Smith G, Schneider M, Minder C.
Bias in meta-analysis detected by a simple, graphical test.
BMJ 1997; 315: 629–34.Cohen J.
Statistical power analysis for the behavioral sciences, 2nd edn.
Hillsdale, NJ: Lawrence Earlbaum Associates, 1988.Bombardier C, Hayden JA, Beaton DE.
Minimal clinically important difference. Low back pain: outcome measures.
J Rheumatol 2001; 28: 431–38.Roland M, Fairbank J.
The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire.
Spine 2000; 25: 3115–24.Jarvik JG, Maravilla KR, Haynor DR, Levitz M, Deyo RA.
Rapid MR imaging versus plain radiography in patients with low back pain: initial results of a randomized study.
Radiology 1997; 204: 447–54.Deyo RA, Diehl AK.
Lumbar spine films in primary care: current use and effects of selective ordering criteria.
J Gen Intern Med 1986; 1: 20–25.Gillan MG, Gilbert FJ, Andrew JE, Grant AM, Wardlaw D, Valentine NW, et al.
influence of imaging on clinical decision making in the treatment of lower back pain.
Radiology 2001; 220: 393–99.Hollis S, Campbell F.
What is meant by intention to treat analysis? Survey of published randomised controlled trials.
BMJ 1999; 319: 670–74.Miller P, Kendrick D, Bentley E, Fielding K.
Cost-effectiveness of lumbar spine radiography in primary care patients with low back pain.
Spine 2002; 27: 2291–97.Rothwell PM.
External validity of randomised controlled trials: to whom do the results of this trial apply?
Lancet 2005; 365: 82–93.Airaksinen O, Brox J, Cedraschi C, et al,
on behalf of the Cost B. Working Group on Guidelines for Chronic Low Back Pain.
Chapter 4—European guidelines for the management of chronic nonspecific low back pain.
Euro Spine J 2006; 15 (suppl 2): s192–300.Chou R, Qaseem A, Snow V, et al.
Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society.
Ann Intern Med 2007; 147: 478–91.van Tulder M, Becker A, Bekkering T, et al,
on behalf of the Cost B. Working Group on Guidelines for the Management of Acute Low Back Pain in Primary Care.
Chapter 3—European guidelines for the management of acute nonspecific low back pain in primary care.
Euro Spine J 2006; 15 (suppl 2): s169–91.Maitino AJ, Levin DC, Parker L, Rao VM, Sunshine JH.
Nationwide trends in rates of utilization of noninvasive diagnostic imaging among the Medicare population between 1993 and 1999.
Radiology 2003; 227: 113–17.Grol R, Dalhuijsen J, Thomas S, Velt CI, Rutten G, Mokkink H.
Attributes of clinical guidelines that influence use of guidelines in general practice: observational study.
BMJ 1998; 317: 858–61
Return to RADIOLOGY
Since 3-21-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |