

Kinematic Analysis of Dynamic Lumbar Motion in Patients with
Lumbar Segmental Instability Using Digital VideofluoroscopyThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: European Spine Journal 2009 (Nov); 18 (11): 1677–1685 ~ FULL TEXT
Amir Ahmadi, Nader Maroufi, Hamid Behtash, Hajar Zekavat, and Mohamad Parnianpour
Faculty of Rehabilitation,
Iran University of Medical Sciences,
P.O. Box 15875-4391,
Tehran, Iran.The study design is a prospective, case-control. The aim of this study was to develop a reliable measurement technique for the assessment of lumbar spine kinematics using digital video fluoroscopy in a group of patients with low back pain (LBP) and a control group. Lumbar segmental instability (LSI) is one subgroup of nonspecific LBP the diagnosis of which has not been clarified. The diagnosis of LSI has traditionally relied on the use of lateral functional (flexion-extension) radiographs but use of this method has proven unsatisfactory. Fifteen patients with chronic low back pain suspected to have LSI and 15 matched healthy subjects were recruited. Pulsed digital videofluoroscopy was used to investigate kinematics of lumbar motion segments during flexion and extension movements in vivo. Intersegmental linear translation and angular displacement, and pathway of instantaneous center of rotation (PICR) were calculated for each lumbar motion segment. Movement pattern of lumbar spine between two groups and during the full sagittal plane range of motion were analyzed using ANOVA with repeated measures design.
Intersegmental linear translation was significantly higher in patients during both flexion and extension movements at L5-S1 segment (p < 0.05). Arc length of pathway of instantaneous center of rotation (PICR) was significantly higher in patients for L1-L2 and L5-S1 motion segments during extension movement (p < 0.05). This study determined some kinematic differences between two groups during the full range of lumbar spine. Devices, such as digital videofluoroscopy can assist in identifying better criteria for diagnosis of LSI in otherwise nonspecific low back pain patients in hope of providing more specific treatment.
From the FULL TEXT Article:
Introduction
Low back pain is one of the most common problems in industrialized countries and its direct and indirect cost is enormous. Nearly 80% of people over the age of 30 will experience back problems during some periods of their life [1]. Eighty five percent of this population is classified as having ‘nonspecific low back pain’ which contains little specific therapeutic and diagnostic information and refers to a large heterogeneous group of patients suffering from a variety of pathological or pathophysiological conditions [2]. Despite the increased recognition of lumbar segmental instability (LSI) as an identifiable subgroup within this population [3–5], the identification of reliable and valid clinical diagnostic tools has thus far been elusive [4].
The diagnostic standard for LSI has traditionally centered on identifying excessive translational or rotational movements between lumbar vertebrae by using functional (flexion–extension) radiographs [6–19]. There may be other factors as well, such as neuromuscular control of spinal movement and aberrant or abnormal midrange motion characteristics. Pathway of instantaneous center of rotation (PICR) may be affected in presence of segmental instability [20, 21] which has not been considered in vivo for LSI patients. Therefore, it is imperative that for assessing some disorders, such as LSI both quantitative and qualitative characteristics of movement should be considered.
Traditional radiographic assessment has some limitations, such as large variation, being based on static postures at extreme ranges of motion and associated large measurement errors [7, 22–27]. Therefore, there is a need for tools to assess kinematics in vivo in order to measure the motion characteristics in midrange, where aberrant motion and dysfunction have been postulated to occur, based on neutral zone concept put forth by Panjabi et al. [28, 29].
Digital video fluoroscopy (DVF) has been suggested as a tool for reliably evaluating normal and abnormal lumbar motions in vivo [1, 27, 30–35] and it seems that DVF is capable of identifying functional abnormalities in patients with LSI who have no structural abnormalities detectable by X-ray. Hence, the aim of this study was to develop a reliable measurement technique that would allow for the assessment of sagittal plane lumbar spine kinematics using digital video fluoroscopy in a group of patients diagnosed having LSI and a control group.
Methods
Study participants

Table 1 A convenient sample of 15 healthy (12 female and 3 male) and 15 patients (12 female and 3 male) was recruited. Healthy subjects were matched with patients (in weight, height, body mass index, BMI and age) and excluded if they had experienced LBP 1 year before the study (Table 1). Patients were examined by a spine surgeon and diagnosed with LSI according to screening criteria adopted from Hicks et al. [36] which requires having at least 3 of 4 positive predictive variables; (1) any aberrant movement pattern during performance of lumbar range of motion including instability catch, painful arc of motion and Gower’s sign, (2) less than 40 years of age, (3) positive prone instability test at least at one segmental level and (4) average straight leg raise (SLR) test >90°.
The sensitivity of 0.83 (0.61–0.94) and specificity of 0.56 (0.40–0.71) were reported for this prediction rule. The prone instability test is performed in two positions (lying prone with first resting the feet on the floor and then lifting the legs off the floor) with posterior pressure applied to the lumbar spine which is positive when the pain is present in the resting position but subsides with lifting the legs [4]. Patients were excluded if their pain was greater than 3 based on visual analog scale (VAS) during the assessment session. Moreover, the exclusion criteria for both groups included spine surgery, spondylolisthesis and a query of pregnancy in female subjects. All subjects received explanations about the potential risk of radiation exposure, approved by Iran University of Medical Sciences and informed consent was obtained before fluoroscopic investigation.
Instrumentation
Pulsed DVF was collected (Fluoroscopy: DAR-300, Shimadzu, Japan) 5 frame per second and a calibration grid (2 9 1 cm) was used to calibrate each frame during image analysis. The maximum radiograph parameters were 50 KV, 47 MA with 12 inches image intensifier and 1 million pixel CCD camera. The images were captured via a computer directly to random access memory and then saved directly to a hard disc drive. The average fluoroscopy time per study was 30 s. A home made software (CARA) was used to calculate kinematic parameters.
Collection of DVF

Figure 1 As full range of motion was required, subjects were asked to bend forward from standing at 10° lumbar hyperextension and then return from full flexion to starting position (10° lumbar hyperextension). Subjects were instructed to complete this motion within 10 to 15 s. Sagittal plane was selected for this study because of greater range of motion (ROM) and smaller out of plane motion [37, 38]. After resting and walking for 5 min, subjects were reimaged for test–retest reliability (interimage reliability). Teyhen et al. [33] used a lead harness to improve the quality of the image and to prevent “white-out” during flexion. In this study, the special lead harness was designed and placed on the back of each subject which did not limit their ROM because of its axis of rotation and could bend freely in flexion and extension of lumbar spine (Figure 1).
Fluoroscopic intersegmental motion measurements
The sagittal ROM was calculated with reference to the angle between the projected lines of the lower endplate of L1 and upper endplate of sacrum as described by Wong et al. [35] (Table 1). We measured five evenly distributed sampling points (0, 25, 50, 75 and 100% of ROM) between the starting hyperextension position and maximum flexion (flexion movement arc), and 5 sampling points between maximum flexion and hyperextension position (extension movement arc). Each sampling point represents 25% of either the flexion or extension movement arc. Intersegmental linear translation and angular displacement in each sampling point were calculated according to White and Panjabi method [18] using customized software.
Instantaneous center of rotation (ICR) was measured for each lumbar motion segment [39]. Four ICR points were obtained from 5 sampling images in each movement arc for each vertebra. Hence, PICR was measured as the total length of the digital line passing through ICR points.
Reliability procedures
Interimage reliability was assessed to determine the reliability of data obtained from two movement trials separated by a 5 min of rest and intraimage reliability was assessed to determine the reliability of data obtained from two separate analyses from 30 randomly selected frames with 1-month interval by the same observer (AA).
Evaluation of errors
A calibrated board was moved in an arc at varying speed within the range intended in our experiment while images were taken by DVF. Since the landmarks on the calibrated board experienced rigid body rotation in various frames, the distance and angles between them must have remained invariant. The root mean square errors were computed from deviation of measured distances and angles from the reference (gold standard) values in 30 randomly selected frames.
Statistical analyses
A Kolmogorov-Smirnov test was performed to determine normal distribution of each variable. Independent sample t test was used to test if there was any difference between two groups for age, weight, height, BMI and sagittal ROM. The data were coded before analyzing and therefore analyzing process was blinded.
A mixed between–within subjects analysis of variance was conducted to assess the effect of phase of movement (5 levels) for each movement arc and each motion segment’s linear translations and angular displacements. Multiple comparisons were performed using Bonferroni corrections between levels of phase of movement. Independent sample t test was performed to identify the differences of PICR between two groups. Chi-square test was used to identify differences between groups and patterns of movements. Confidence level was set at a B 0.05 for statistical significance. An interclass correlation coefficients (ICC2,1) and standard error of measurement (SEM) were calculated to determine reliability and response stability of each measure, respectively. All statistical analyses were performed using SPSS statistical software version 16.0 (SPSS, Chicago, IL, U.S.A.).
Results
There was no significant difference in variables, such as weight, height, age, BMI and sagittal ROM between two groups (Table 1). In LSI group 14 patients showed instability catch, 5 showed painful arc, 7 showed Gower sign, 10 showed positive prone instability test (at least at 1 segmental level) and 1 showed SLR[90°.
Interimage reliability
The average ICC was 0.95 for intersegmental angular displacement (range 0.89–0.98), 0.92 for intersegmental linear translation (range 0.89–0.96) and 0.95 for PICR (range 0.94–0.98). The average SEM was 1.19° (range 0.77–1.45) for intersegmental angular displacement, 0.19 mm (range 0.11–0.22) for intersegmental linear translation and 5.4 mm (range 2.8–8.16) for PICR.
Intraimage reliability
The average ICC was 0.92 for intersegmental angular displacement (range 0.84–0.96), 0.92 for intersegmental linear translation (range 0.85–0.96) and 0.93 for PICR (range 0.85–0.99). The average SEM was 1.19° (range 0.62–1.97) for intersegmental angular displacement, 0.22 mm (range 0.17–0.28) for intersegmental linear translation and 7.67 mm (range 2.16–17.35) for PICR.
Evaluation of errors
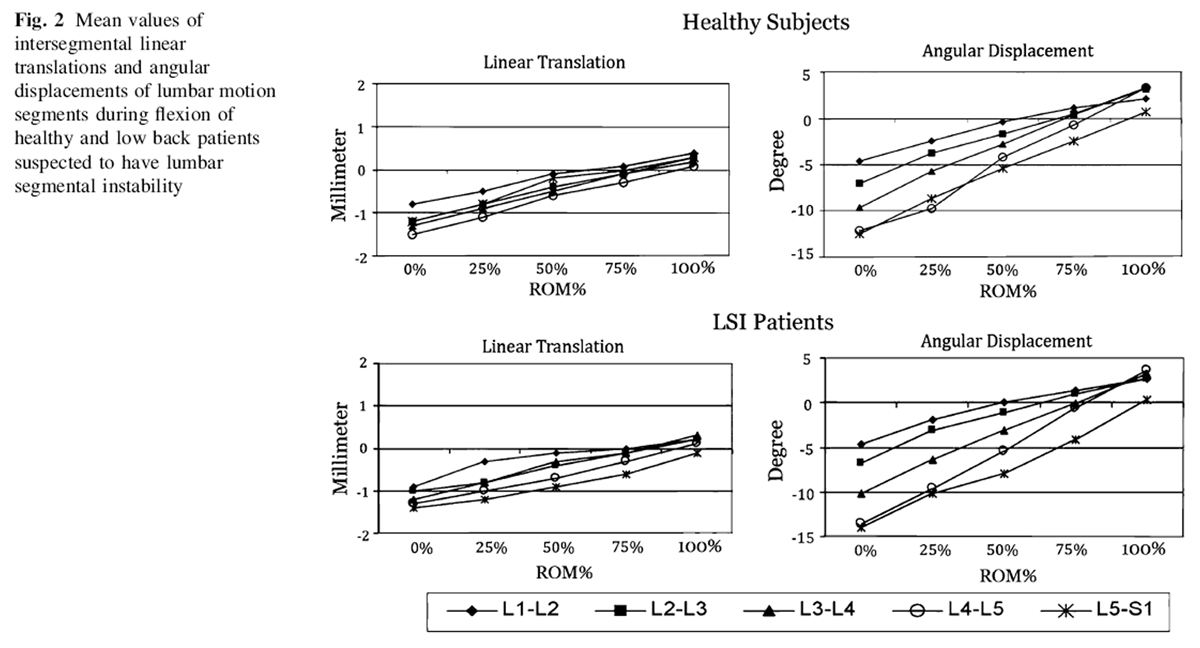
Figure 2
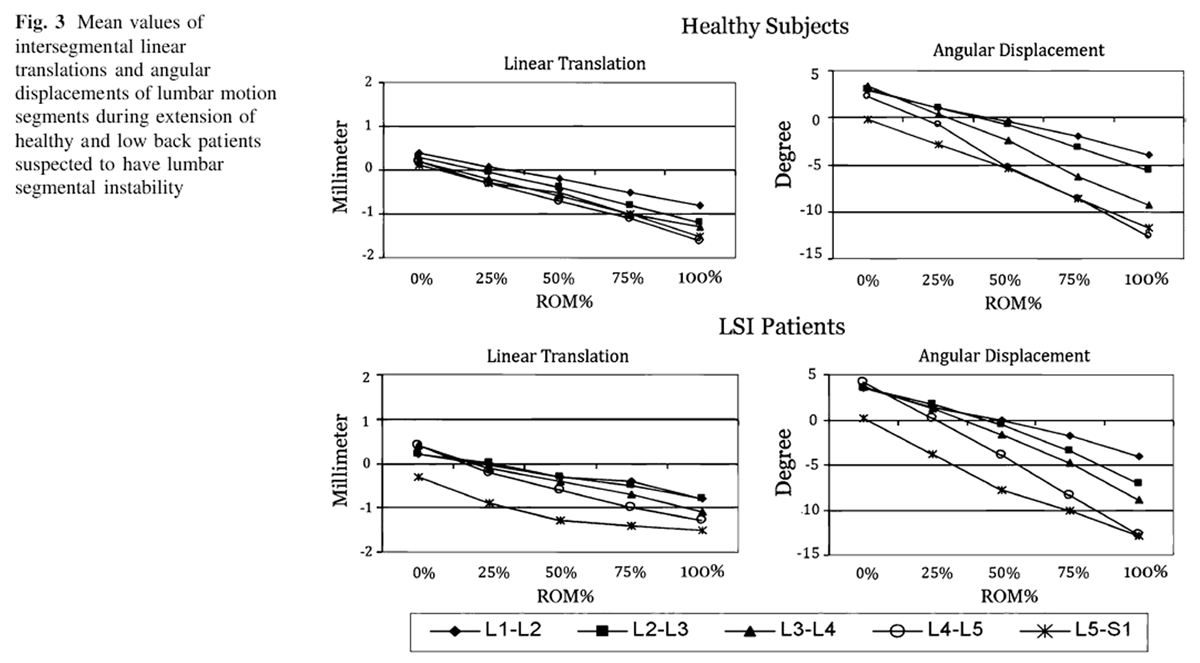
Figure 3
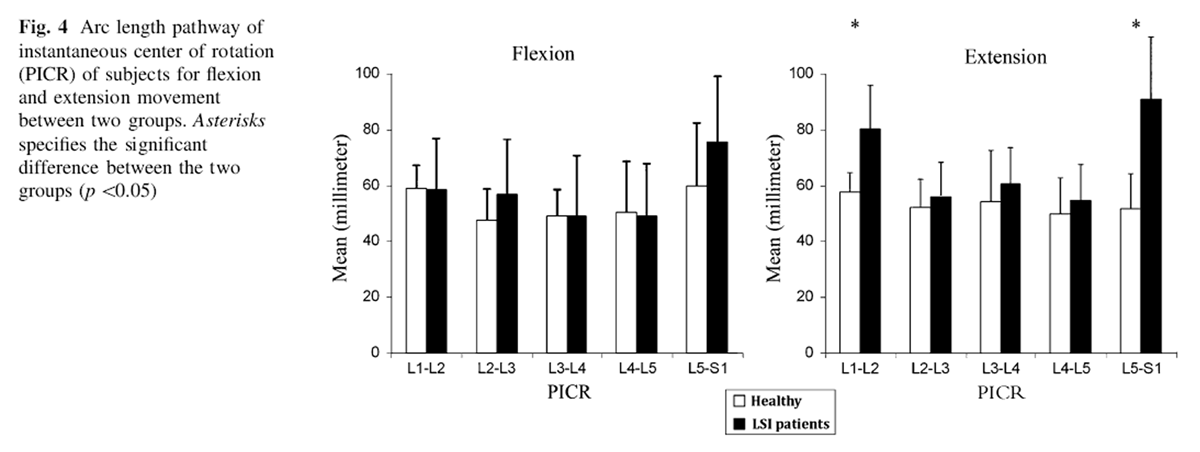
Figure 4
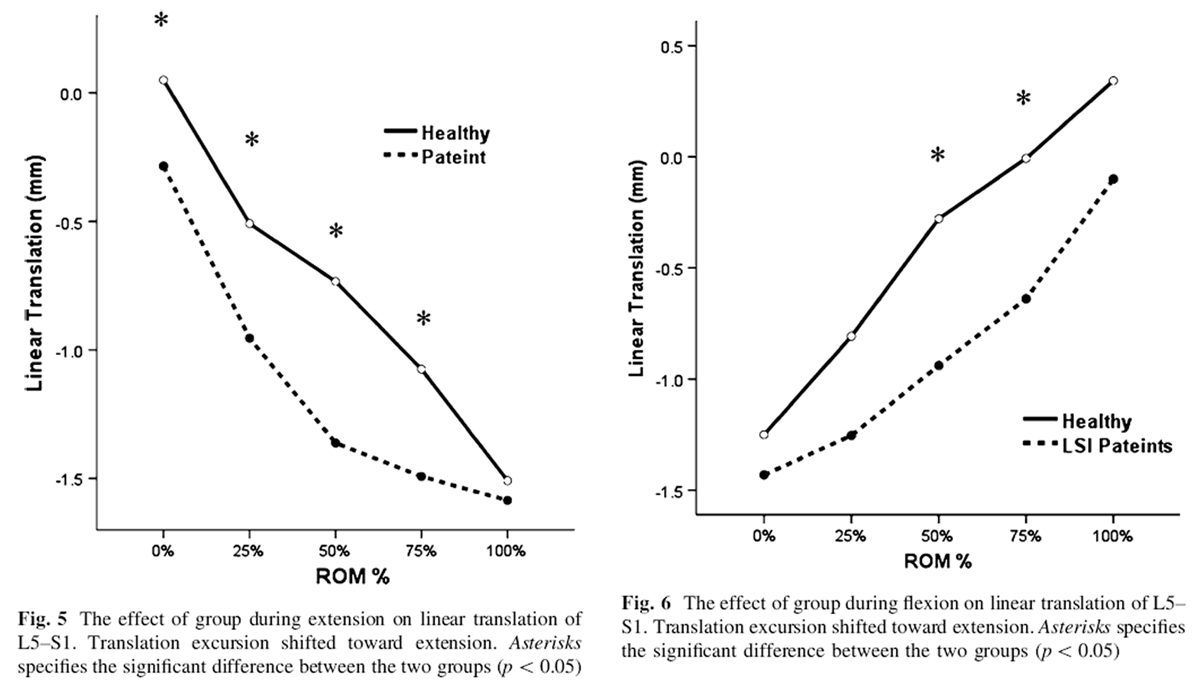
Figure 5, 6 The root mean square error computed from calibrated board in the linear translation and angular displacement were 0.26 mm and 0.41°, respectively.
Assessment of lumbar spinal motion
The mean intersegmental linear translation and angular displacements of lumbar motion segments for both groups in each direction of motion is depicted in Figures 2 and 3. The arc lengths of PICR in both groups in flexion and extension are presented in Figure 4. Average arc length of PICR for each vertebra was 53.2 ± 17.4 mm (range 47.5–59.9) for healthy subjects and 57.8 ± 10.9 mm (range 48.9–75.8) for patients during flexion movement arc. There was statistically significant difference for arc length of PICR for extension movement at L1–L2 and L5–S1 motion segments (p<0.05) (Fig. 4).
The results of ANOVA indicated no significant interaction between group and phase of movement displacements (linear and angular) except for L5–S1 linear translation during extension movement, Wilks Lambda = 0.50, F(4,20) = 4.83, p<0.007 (Figure 5). The main effect of phase of movement was significant for all motion segments in both directions (p<0.0005). Multiple comparison was significant between all phases of movement for all motion segments in both directions (p<0.005). The main effect comparing the two groups was not significant except at midrange of L5–S1 linear translation in flexion and extension movements (p<0.05) (Figures 5, 6).
Motion patterns of both flexion and extension movement arcs were simultaneous in all healthy subjects, but 6 patients at L5–S1 level showed “delayed-sequence” movement pattern (Chi-square = 7.5, p<0.01).
Discussion
Lumbar motion kinematics has been evaluated by a variety of instruments—from functional radiography [8, 9, 12, 13, 26, 40, 41] to cineradiography [42, 43] and videofluoroscopy [27, 30–35, 44] — in both normal and patient subjects. Some studies assessed intervertebral motion only in a few segments (e.g. L3 to S1) [27, 32, 33, 42, 43] and some others considered solely intersegmental angular displacement without any attention to intersegmental linear translation or other parameters, such as PICR [17, 34, 35, 44]. There are some studies which used camera to capture images from monitor of analog fluoroscopy system [30, 31].
In this improved study, flexion and extension movements of lumbar spine were investigated in vivo by DVF. In contrast to some previous studies, the image intensifier of current study was not fixed while subjects wore a lead harness which enabled us to measure intersegmental linear and angular displacements at all vertebral levels within whole range of motion and had better quality of digital images. Furthermore, we used ICR and PICR variables to identify the quality of motion and assessed neuromuscular control of motion segments during lumbar movements [21].
Our study indicated that arc length of PICR was significantly different at L5 vertebra between two groups through the extension movement of lumbar spine. Additionally, independent sample t test was used for linear translation excursion of L5–S1 segment to determine hypo or hyper mobility in patients during flexion and extension. These results showed no significant difference between two groups. On the other hand, significant differences in the midrange of this motion segment (Figs. 5, 6) indicated that, however, linear translation of L5–S1 motion segment in patients tended to extension, the total excursion of this motion segment was similar to the healthy subjects. It seems that the neuromuscular system adopts some strategies to resist the anterior shear of the instable segment in both extension and flexion movements. Therefore, these results imply that the altered quality of movement in LSI patients may be due to altered neuromuscular control. To date, it is unclear that this alteration in neuromuscular control is an adaptive mechanism to prevent further tissue injury or the impairment of motor control system.
Another drawback in some previous studies is that the segmental analysis was measured at certain fixed time points or frame points [31, 32, 34, 35, 43, 44]. Since the speed of lumbar movements and sagittal ROM of each subject may be different, comparison of the results between subjects becomes questionable. In this study, to control variations across subjects in their sagittal ROM, each 25% of total ROM was selected as a sampling point. We used White and Panjabi method [18] for measuring intersegmental linear translation and angular displacement because Dupuis method of measurement [8] did not compute these values for L5–S1 segment which ironically showed the most significant differences in this study between the two groups.
“Normal movement pattern” of lumbar spine during flexion movement is not at as yet determined and there is still some controversy in literature. Kanayama et al. [45] concluded that each lumbar segment started stepwise from the upper to the lower segment with a phase lag but Wong et al. [34, 35] and Lee et al. [44] reported simultaneous pattern for lumbar spine, while Okawa et al. [31] identified both sequential and simultaneous pattern in normal subjects. In this study we observed simultaneous movement pattern in all healthy subjects and nine of the LSI patients which imply that every lumbar segment does move and in each time increment, each motion segment has a specific contribution to the total lumbar movement.
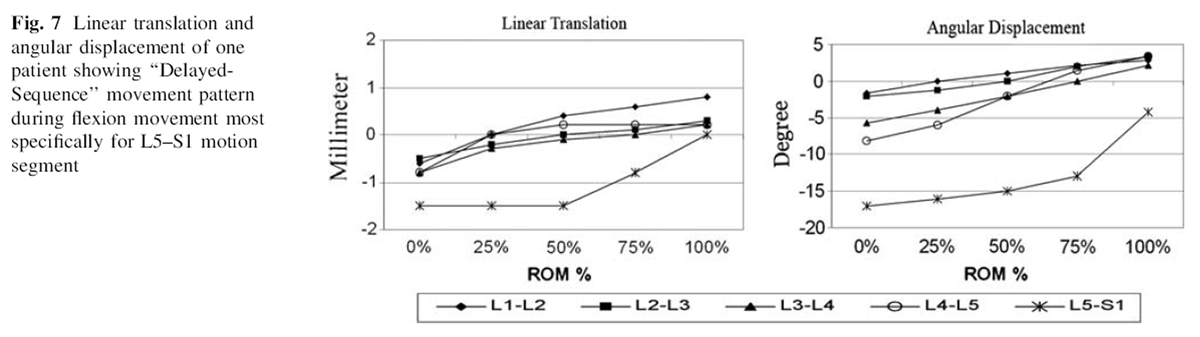
Figure 7 It seems that hip fixation during the test may significantly affect the quality of intersegmental movement patterns. This concept has been warned against during functional evaluation of spine (i.e. strength measurement) [46, 47]. Some previous studies fixed the hip to have better quality of images [27, 31–33, 42, 43]; whereas, in this study the subjects were free to move and we did not use any fixation. Moreover, six patients showed sequential movement pattern at the level of L5–S1 along with hypomobility in the middle range of motion segment movement. In these patients sequential pattern was accompanied with latency which justified the term used as “Delayed-Sequence” pattern. In these patients the impaired segment did not start to move until 50% to 75% of total ROM had occurred. For illustrative purposes, the linear translation and angular displacement of one patient which showed delayedsequence pattern is depicted in Figure 7.
It is known that patients with chronic LBP suffer from episodic pain in their life [48, 49] and since the pain is a confounding factor that may alter movement pattern of lumbar spine [50, 51], researchers should consider the severity of subjects’ pain. While previous fluoroscopybased studies in this field did not consider this factor in selecting their patients, we excluded patients with the pain higher than 3 according to VAS during the assessment session. Hence, in this study the effect of pain on movement pattern was controlled.
Patients with LSI have been proposed as a unique subgroup of LBP patients [36, 52–55] and LSI has been defined as a condition in which there is a loss of stiffness of spinal motion segments, such that normally tolerated external loads result in pain [55]. Diagnosis of LSI have been developed traditionally from studies that have examined intersegmental linear and angular displacement using lateral flexion–extension radiographs and reported some threshold values [6–19] but unfortunately their usefulness is controversial. The use of these criteria for identifying LSI has proven unsatisfactory because of high false-positive rates [7, 12].
Schneider et al. [56] reported that patients with spondylolisthesis— which demonstrate the hallmark of segmental instability—showed reverse linear translation during lumbar movement using functional radiography, while Teyhen et al. indicated that patients with LSI showed hypomobility in both flexion and extension movements [57] and reversed intersegmental linear translation in the middle of flexion movement using video fluoroscopy [27]. The results of current study imply that in both flexion and extension movement arcs, through the middle of total lumbar range of motion there were significant differences in the intersegmental linear translation at level of L5–S1. Therefore, our findings support that in presence of LSI the impaired lumbar motion segment may not exactly follow the other segments and show different behavior. Such abnormal motions lead to abnormal loading of spine and may predispose the discs to degeneration [58–61]. Multiple comparisons were significant between all 5 phases of movements at all motion segments because of the small proportion of population of patients with delayed pattern.
In the current study subjects were asked to finish their movement during 10–15 s. Upper bound of this time period was for refraining from harmful radiation effects and lower bound was because of limitation in sampling rate of fluoroscopy system. Some previous related articles indicated that there is no statistical difference between genders [34, 35, 44]. Future studies should once again use the current protocol for evaluation of the gender effect on patterns of movement. The other limitation in this study was encountering with a nonhomogenous group of patients. Our results imply that proposed screening criteria [36] were not specific enough because nine patients showed similar kinematics to healthy subjects. Much larger multicenter studies are needed before we can develop more accurate criteria for diagnosis of LSI patients groups.
It seems that with using devices, such as digital videofluoroscopy which are capable of assessing kinematics of lumbar motion segments in vivo, clinicians probably could distinguish patients suspected to LSI. Therefore, in near future by using these noninvasive techniques the patients with LSI might be discriminated from other nonspecific LBP, appropriate plan of treatment could be designed and reassessment would be easier.
References:
Zheng Y, Nixon MS, Allen R (2003)
Lumbar spine visualisation based on kinematic analysis from videofluoroscopic imaging.
Med Eng Phys 25:171–179Petersen T, Olsen S, Laslett M (2004)
Inter-tester reliability of a new diagnostic classification system for patients with non-specific low back pain.
Aust J Physiother 50(2):85–94Frymoyer J, Pope M, Wilder D (1990)
Segmental instability. In: Weinstein J, Wiesel S (eds) The lumbar spine.
WB Saunders, Philadelphia, pp 612–636Hicks G, Fritz J, Delitto A (2003)
Interrater reliability of clinical examination measures for identification of lumbar segmental instability.
Arch Phys Med Rehabil 84(12):1858–1864O’sullivan P (2000)
Lumbar segmental ‘instability’: clinical presentation and specific stabilizing exercise management.
Man Ther 5:2–12Abbott JH, Fritz JM, McCane B, Shultz B, Herbison P, Lyons B (2006)
Lumbar segmental mobility disorders: comparison of two methods of defining abnormal displacement kinematics
in a cohort of patients with non-specific mechanical low back pain.
BMC Musculoskelet Disord 7:45Boden S, Wiesel S (1990)
Lumbosacral segmental motion in normal individuals.
Spine 15:571–575Dupuis P, Yong-Hing K, Cassidy J (1985)
Radiologic diagnosis of degenerative lumbar spinal instability.
Spine 10:262–276Dvorak J, Panjabi MM (1991)
Functional radiographic diagnosis of the lumbar spine.
Spine 16:562–571Dvorak J, Panjabi MM, Novotny JE, Chang DG, Grob D (1991)
Clinical validation of functional flexion-extension roentgenograms of the lumbar spine.
Spine 16:943–950Friberg O (1987)
Lumbar instability: a dynamic approach by traction-compression radiography.
Spine 12:119–129Hayes M, Howard T, Gruel C, Kopta J (1989)
Roentgenographic evaluation of lumbar spine flexion-extension in asymptomatic individuals.
Spine 14:327–331Knutsson F (1944)
The instability associated with disc herniation in the lumbar spine.
Acta Radiol 25:593–609Korpi J, Putto E, Poussa M, Heliovaara M (1991)
Radiological translatory mobility between lumbar vertebrae in women and men with low back pain.
J Man Med 6:121–123Pitkanen M, Manninen H, Lindgren K, Sihvonen T, Airaksinen O, Soimakallio S (2002)
Segmental lumbar spine instability at flexionextension radiography can be predicted by conventional radiography.
Clin Radiol 57:632–639Spratt K, Weinstein J, Lehmann T, Woody J, Sayre H (1993)
Efficacy of flexion and extension treatments incorporating braces for low-back pain patients with
retrodisplacement, spondylolisthesis, or normal sagittal translation.
Spine 18:1839–1849Tallroth K, Alaranta H, Soukka A (1992)
Lumbar mobility in asymptomatic individuals.
J Spinal Disord 5:481–484White A, Panjabi MM (1990)
Clinical biomechanics of the spine.
JB Lippincott, PhiladelphiaWood K, Popp C, Transfeldt E (1994)
Radiographic evaluation of instability in spondylolisthesis.
Spine 19:1697–1703Lee S, Draper E, Hughes S (1997)
Instantaneous center of rotation and instability of the cervical spine. A clinical study.
Spine 22:641–647Gertzbein S, Seligman J, Holtby R, Chan K, Kapasouri A (1985)
Centrode patterns and segmental instability in degenerative disc disease.
Spine 10:257–261Danielson B, Frennered K, Irstam L (1988)
Roentgenologic assessment of spondylolisthesis. I. A study of measurement variations.
Acta Radiol 29:345–351Danielson B, Frennered K, Selvik G (1989)
Roentgenologic assessment of spondylolisthesis. II. An evaluation of progression.
Acta Radiol 30:65–68Penning L, Wilmink J, Hv Woerden (1984)
Inability to prove instability. A critical appraisal of clinical-radiological flexionextension studies
in lumbar disc degeneration.
Diagn Imaging Clin Med 53:182–192Polly D, Kilkelly F, McHale K (1996)
Measurement of lumbar lordosis. Evaluation of intraobserver, interobserver, and technique variability.
Spine 21:1530–1535Shaffer W, Spratt K, Weinstein J (1990)
Volvo Award in clinical sciences. The consistency and accuracy of roentgenograms for measuring sagittal
translation in the lumbar vertebral motion segment. An experimental model.
Spine 15:741–750Teyhen DS, Flynn TW, Childs JD, Kuklo TR, Rosner MK, Polly DW, Abraham LD (2007)
Fluoroscopic video to identify aberrant lumbar motion.
Spine 32:E220–E229Panjabi MM (1992)
The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement.
J Spinal Disord 5:383–389Panjabi MM (1992)
The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis.
J Spinal Disord 5:390–396Auerbach JD, Wills BP, McIntosh TC, Balderston RA (2007)
Evaluation of spinal kinematics following lumbar total disc replacement and
circumferential fusion using in vivo fluoroscopy.
Spine 32:527–536Okawa A, Shinomiya K, Komori H, Muneta T, Arai Y, Nakai O (1998)
Dynamic motion study of the whole lumbar spine by videofluoroscopy.
Spine 23:1743–1749Otani K, Okawa A, Shinomiya K, Nakai O (2005)
Spondylolisthesis with postural slip reduction shows different motion patterns with video-fluoroscopic analysis.
J Orthop Sci 10:152–159Teyhen DS, Flynn TW, Bovik AC, Abraham LD (2005)
A new technique for digital fluoroscopic video assessment of sagittal plane lumbar spine motion.
Spine 30:E406–E413Wong KW, Leong JC, Chan MK, Luk KD, Lu WW (2004)
The flexion-extension profile of lumbar spine in 100 healthy volunteers.
Spine 29:1636–1641Wong KW, Luk KD, Leong JC, Wong SF, Wong KK (2006)
Continuous dynamic spinal motion analysis.
Spine 31:414–419Hicks G, Fritz JM, Delitto A, Macgill S (2005)
Preliminary development of clinical prediction rule for determining which patients with low back pain will
respond to a stabilization exercise program.
Arch Phys Med Rehabil 86:1753–1762Harvey S, Hukins D (1998)
Measurement of lumbar spinal flexion- extension kinematics from lateral radiographs:
simulation of the effects of out-of-plane movement and errors in reference point placement.
Med Eng Phys 20:403–409Pearcy M (1985)
Stereo radiography of lumbar spine motion.
Acta Orthop Scand Suppl 212:1–45Panjabi MM, White A (2001)
Biomechanics in the musculoskeletal system.
Churchill Livingstone, New YorkKeessen W, During J, Beeker T (1984)
Recordings of the movement at the intervertebral segment L5–S1: a technique for the determination of
the movement in the L5–S1 spinal segment by using three specified postural positions.
Spine 9:83–90Posner I, White A, Edwards W (1982)
A biomechanical analysis of the clinical stability of the lumbar and lumbosacral spine.
Spine 7:374–389Harada M, Abumi K, Ito M, Kaneda K (2000)
Cineradiographic motion analysis of normal lumbar spine during forward and backward flexion.
Spine 25:1932–1937Takayanagi K, Takahashi K, Yamagata M, Moriya H, Kitahara H, Tamaki T (2001)
Using cineradiography for continuous dynamicmotion analysis of the lumbar spine.
Spine 26:1858–1865Lee SW, Wong KW, Chan MK, Yeung HM, Chiu JL, Leong JC (2002)
Development and validation of a new technique for assessing lumbar spine motion.
Spine 27:E215–E220Kanayama M, Abumi K, Kaneda K, Tadano S, Ukai T (1996)
Phase lag of the intersegmental motion in flexion–extension of the lumbar and lumbosacral spine: an in vivo study.
Spine 21:1416–1422Cox M, Asselin S, Gracovetsky S, Richards M, Newman N (2000)
Relationship between functional evaluation measures and self-assessment in nonacute low back pain.
Spine 25:1817–1826Marriott A, Newman N, Gracovetsky S, Richards M, SA S (1999)
Improving the evaluation of benign low back pain [Clinical Trial]. Comparative study.
Spine 24:952–960Vet Hd, Heymans M, Dunn K, Pope D, Beek Avd, Macfarland G. (2002)
Episodes of low back pain.
Spine 27:2409–2416Waddell G (1987)
1987 Volvo Award in clinical sciences. A new clinical model for the treatment of low-back pain.
Spine 12:632–644Shum G, Crosbie J, Lee R (2007)
Movement coordination of the lumbar spine and hip during a picking up activity in low back pain subjects.
Eur Spine J 16:749–758Sihvonen T, Lindgren K, Airaksinen O, Manninen H (1997)
Movement disturbances of the lumbar spine and abnormal back muscle electromyographic findings in recurrent low back pain.
Spine 22:289–295Grieve G (1982)
Lumbar instability.
Physiotherapy 68:2–9Delitto A, RB RW (1995)
A treatment based classification approach to low back syndrome: identifying and staging patients
for conservative treatment.
Physical Ther 75:470–489Farfan H, Gracovetsky S (1984)
The nature of instability.
Spine 9:714–719Frymoyer J, Selby D (1985)
Segmental instability rationale for treatment.
Spine 10:280–285Schneider G, Pearcy M, Bogduk N (2005)
Abnormal motion in Spondylolytic Spondylolisthesis.
Spine 30:1159–1164Teyhen DS, Flynn TW, Childs JD, Abraham LD (2007)
Arthrokinematics in a subgroup of patients likely to benefit from a lumbar stabilization exercise program.
Phys Ther 87:313–325Anderson P, Haughton V, Iatridis J, Kang J, Lotz J, Natarajan R, Oegema T (2004)
Disc degeneration: summary.
Spine 29:2677–2678Natarajan R, Williams J, Andersson G (2004)
Recent advances in analytical modeling of lumbar disc degeneration.
Spine 29:2733–2741Natarajan R, Williams J, Andersson G (2006)
Modeling changes in intervertebral disc mechanics with degeneration.
J Bone Joint Surg Am 88:36–40Natarajan R, Williams J, Lavender S, Anderson G (2008)
Relationship between disc injury and manual lifting: a poroelastic finite element model study.
J Eng Med 222:195–207
Return to RADIOLOGY
Since 1-22-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |
