

Best-Practice Recommendations for
Chiropractic Management of Patients With Neck PainThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Manipulative Physiol Ther. 2019 (Nov); 42 (9): 635–650 ~ FULL TEXT
OPEN ACCESS Wayne Whalen, DC • Ronald J. Farabaugh, DC • Cheryl Hawk, DC, PhD
Amy L. Minkalis, DC, MS • William Lauretti, DC • Louis S. Crivelli, DC, MS
Larry Wyatt, DC • Michael Sheppard, DC • Sheryl A. Walters, MLS
Texas Chiropractic College,
Pasadena, Texas.
cherylkhawk@gmail.com
Thanks to JMPT for permission to reproduce this Open Access article! OBJECTIVE: The purpose of this study was to develop best-practice recommendations for chiropractic management of adults with neck pain.
METHODS: A steering committee of experts in chiropractic practice, education, and research drafted a set of recommendations based on the most current relevant clinical practice guidelines. Additional supportive literature was identified through targeted searches conducted by a health sciences librarian. A national panel of chiropractors representing expertise in practice, research, and teaching rated the recommendations using a modified Delphi process. The consensus process was conducted from August to November 2018. Fifty-six panelists rated the 50 statements and concepts and reached consensus on all statements within 3 rounds.
RESULTS: The statements and concepts covered aspects of the clinical encounter, ranging from informed consent through diagnosis, assessment, treatment planning and implementation, and concurrent management and referral for patients presenting with neck pain.
CONCLUSIONS: These best-practice recommendations for chiropractic management of adults with neck pain are based on the best available scientific evidence. For uncomplicated neck pain, including neck pain with headache or radicular symptoms, chiropractic manipulation and multimodal care are recommended.
KEYWORDS: Chiropractic; Neck Pain; Spinal Manipulation; Spine
From the FULL TEXT Article:
Introduction
Neck pain is one of the leading causes of disability. [1] In a 2012 report of US health statistics by the Centers for Disease Control and Prevention, 14% of adults had experienced neck pain in the 3 months before being interviewed. [2] Most people with neck pain are likely to continue to have neck pain, with a clinically variable course in pain intensity and disability. [3, 4] Neck pain is a common complaint in ambulatory care and comprises approximately 18% to 23% of chief complaints in chiropractic practice. [5–8]
Because there is variation in diagnosis, assessment, and treatment approaches to neck pain in chiropractic practice, developing a consistent approach based on the best available evidence is important to provide optimal patient care. [9] Clinical practice guidelines (CPGs) help to improve care.
Advances in medical, biomedical and health services research have reduced the level of uncertainty in clinical practice. Clinical practice guidelines (CPG) complement this progress by establishing standards of care backed by strong scientific evidence. CPG are statements that include recommendations intended to optimize patient care. These statements are informed by a systematic review of evidence and an assessment of the benefits and costs of alternative care options. [10]
Prior published CPGs relied on the highest level of evidence for their treatment recommendations. [3, 11, 12] However, because some procedures and practices commonly used by doctors of chiropractic (DCs) have lower-level evidence, those practices were either not addressed by the CPG or had conflicts in the recommendations between the CPGs. As a consequence, several procedures and modalities commonly used in chiropractic practice were not clearly addressed, which leaves gaps in evidence-based recommendations. Although reliance on the highest level of evidence is noteworthy, this evidence may not be synonymous with the highest possible level of evidence.
As a result:(1) some treatment modalities used commonly are given no recommendation or conflicting recommendations, [3, 11, 12]
(2) controversy remains about use or overuse of diagnostic imaging in chiropractic practice, [13] and
(3) treatment frequency and duration are not clear because they are not fully addressed in the CPG. [12, 14]To address these gaps, a consensus process is needed to provide clinicians and payers with the highest-level evidence available regarding these issues to assist them in making clinical and coverage decisions. Therefore, the purpose of this project was to develop a set of recommendations addressing the chiropractic management of adults with neck pain based on the best available scientific evidence, and expert opinion where the literature was either lacking or contradictory, in a format that may be usable by clinicians, payers, and policy makers.
Methods
Project Overview
The project was designed to make recommendations on the most beneficial approach to chiropractic management of adult patients with neck pain. Management refers to the entire clinical encounter, including history, examination, assessment and diagnosis, informed consent, interventions within the scope of American chiropractic practice, and comanagement and referral.
Methods included(1) examination of the current neck pain CPG related to management;
(2) identification of gaps in the CPG that represent a barrier to standardization of care and to maximum utility to practitioners;
(3) a literature search to identify the highest available evidence on the gap topics;
(4) formulation of a set of recommendations on a comprehensive approach to management based on the best available evidence; and
(5) conduct of a consensus process with a panel composed of a representative group of experienced practitioners, faculty members, and researchers.Ethics
The project was approved by Texas Chiropractic College’s institutional review board before the conduct of the consensus process (IRB No: 2018-US-Hawk.Delphi-1-01-V1). Participants signed an informed consent to participate and only were acknowledged by name in any publication if they provided an additional written consent to be acknowledged.
Steering Committee
The steering committee (SC) included 9 DCs and 1 health sciences librarian. All have held or currently hold leadership positions in chiropractic professional organizations, education, or research. Two members of the SC work in research and have practice experience. Five of the DCs are full-time practitioners; 2 are full-time clinical faculty in a chiropractic program. All are members of the Scientific Council of the Clinical Compass (previously known as Council on Chiropractic Guidelines and Practice Parameters). The SC was responsible for identifying, reviewing, and evaluating initial draft documents that formed the seed documents; developing the initial seed statements; revising per panelist input; and preparing the final document.
Literature Searches
Literature searches using PubMed were conducted by a health sciences librarian (S.W.) using Medical Subject Headings for each topic, restricted to English language. The literature search was conducted in 2 phases. Before developing the seed statements, we identified the most recent clinical practice guidelines for each aspect of the clinical encounter for chiropractic management of neck pain. We restricted the search to CPGs published later than 2015 because a large proportion of CPGs are updated approximately every 3 years. [15] If no CPG was published on the topic, we then searched for systematic reviews. These were also used to identify gaps in recommendations. The SC conducted targeted searches for additional guidelines or systematic reviews on the specific topics of these gaps. If higher-level evidence (CPG and systematic review) was not available, randomized controlled trials and large observational studies were considered; lower-level studies such as pilot studies were considered in the absence of higher levels.
Foundational Documents and Seed Statements
The foundational documents were 3 recent CPGs, which represent the current most comprehensive analyses of conservative management of patients with neck pain with relevance to chiropractic practitioners: the Canadian Chiropractic Guideline Initiative (2016), [12] the American Physical Therapy Association (2017), [3] and the Royal Dutch Society for Physical Therapy (2018). [11]
Based on these 3 CPGs, the SC drafted a set of seed statements and concepts, covering the chief aspects of the chiropractic clinical encounter, including informed consent, diagnosis, treatment, concurrent care, and referral. The original draft document was dissected into smaller statements and concepts, which were then organized and sent to the Delphi panel for rating. References supporting the seed statements were placed directly underneath each statement section to facilitate the raters’ assessment of the evidence.
Modified Delphi Panel
The panelists were selected based on(1) experience with treating patients with neck pain (a minimum of 5 years in practice),
(2) geographic diversity within the United States (because other countries have differing practice parameters and insurance issues, we confined our recommendations to the United States), and
(3) ability to respond in a timely manner to a process conducted wholly electronically.Because our recommendations apply specifically to chiropractic practice, we restricted the panel to DCs, although some were cross-trained in other health care disciplines. We invited experts who had participated in previous projects and put out a general call for nominations through the Clinical Compass board, which includes representatives of the Congress of Chiropractic State Associations. The SC reviewed nominations. All volunteers who met the above selection criteria were invited to participate.
Delphi Rounds, Rating System Including Data Analysis
We conducted the consensus process electronically. Panelists were anonymous during the rating process, identified only by assigned numbers. They did not know other panelists’ identities during the process to avoid possible bias. We used the RAND-University of California, Los Angeles methodology to conduct the consensus process. [16] Raters in this method use an ordinal scale of 1 to 9 (highly inappropriate to highly appropriate) to evaluate each seed statement. In the RAND/University of California, Los Angeles method, “appropriate” means that the patient’s expected health benefits are greater than the expected negative effects by a large enough margin that the recommended action is worthwhile without considering costs. [16]
After a round, data were entered into a Statistical Package for the Social Sciences file, and the project director and coordinator provided the medians, percentages, and comments for each statement (as a Word table) to the SC. They reviewed all comments and, guided by the panelists’ comments, revised those statements that did not reach consensus. The project coordinator then sent the revised statements, along with a set of the deidentified comments, to the panel for the next round. We divided the seed statements into 3 sets to avoid overwhelming the panelists. Each statement was immediately followed by citations supporting it, along with either a link to the article abstract or, if it was an open-access article, a link to the full text. We considered consensus on appropriateness to be present if the median rating for a statement was 7 to 9 and 80% or more of panelists rated it at least 7. Once consensus was reached, all statements were combined into a single document.
Results
Delphi Panel
A total of 56 DCs were included on the panel. Eighty-eight percent (49) were men and 12% (7) women, consisting of practicing chiropractors, research faculty, or educators. In addition to their DC, 20 (36%) had other degrees: 13 had a master’s degree; 2 had master’s degrees in acupuncture or oriental medicine; 1 a master’s in education; 1 a master’s in public health; 1 a doctor of philosophy; 1 a doctor of physical therapy; and 1 in nursing. Sixty-six percent had additional certifications: 9 were certified in acupuncture; other certifications included Certified Health Education Specialist, manipulation under anesthesia, orthopedics, pain management, neurology, sports injury, strength, and conditioning. Twenty-three percent (13) were current chiropractic college faculty, either full-time or part-time; 18% (10) were current non-chiropractic college faculty, either full-time or part-time.
Panelists had been in the profession a mean of 23.6 years (range 6–48). They reported a mean number of patient visits per week of 96 (range 30–200), and a mean percentage of patients with neck complaints of 37% (range 20%–85%). Participants represented 27 US states (Arkansas, Arizona, California, Hawaii, Iowa, Illinois, Indiana, Kansas, Massachusetts, Maryland, Maine, Michigan, Minnesota, Missouri, Mississippi, North Carolina, New Jersey, New York, Ohio, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, and Washington) and 1 Canadian province (Quebec).
Modified Delphi Rounds
The initial consensus process was conducted from August to November 2018. On each of the rounds, at least 54 of the 56 panelists participated (96%). For the 50 seed statements, 44 reached at least 80% agreement on the first round. Five of the 6 remaining statements reached consensus on the second round, and 1 on the third. When viewing the panelists’ final ratings (that is, when consensus was reached) for all 50 statements, there were no panelists who consistently disagreed with most of the statements.
The resulting set of statements, all achieving #8805;80% consensus, were further revised as per external reviewer comments and then returned to the Delphi panel to achieve consensus on 11 revised and expanded statements. In this additional Delphi round, 96% (54) of the original panelist group responded. Over 90% agreement was reached on 10 statements and 85% on 1, so consensus was reached in 1 round. Panelists’ suggestions for rewording for clarity were incorporated in the final statements because these changes were not substantive.
These final recommendations are presented below.
Best-Practice Recommendations for Chiropractic Management of Neck Pain
Summary of Key Aspects of a Best-Practice Approach to Neck Pain
The process of care includes the following benchmarks in the continuum of patient or condition treatment [12, 14]:
Begin care management with a thorough history.
Follow the history with a condition-specific examination. It is the duty of the provider to perform an examination consistent with the complexity of the case, based initially on history, which includes the mechanism of injury.
Evaluate patients with complaints of neck pain for potentially serious red flags.
Consider referral for diagnostic imaging or other studies based on established clinical practice guidelines (see “Diagnostic Imaging” later).
Develop a care plan based on the history and examination. The care plan includes appropriate diagnostic tests. Sometimes referred to as a report of findings, the history, examination findings, plan of care, and prognosis should be reviewed with the patient through a process of shared decision-making and with their consent to proceed obtained. [17]
Document factors that may delay recovery.
Develop a working diagnosis and, when clinically indicated, consider differential diagnoses.
Reassure the patient regarding the generally benign nature of minor neck pain and encourage activity and movement. With moderate to severe neck pain, emphasize the importance of treatment plan compliance.
Determine whether to (a) manage the patient exclusively, (b) co-manage, or (c) refer to another provider.
Begin treatment with a brief trial of care, 6 to 12 visits, followed by evaluation for treatment effectiveness. The initial trial is not the same as a limit or cap on care.
Evaluate the patient briefly during each encounter, pre- and post-treatment. Conduct a more focused condition-specific evaluation after each benchmark in the treatment plan. Examples: Every 6 to 12 visits, or in 30–60–90-day intervals.
Some patients’ responses to treatment may not follow a predictable pattern, or they may not respond. In this case, consider a modification to the treatment plan that may include, but may not be limited to (a) change in technique and/or modality, (b) referral to another provider within the same discipline for a second opinion, (c) referral to another provider outside the discipline for a second opinion and consideration of other treatment approaches, or (d) referral for diagnostic tests (eg, X-ray, magnetic resonance imaging [MRI], computed tomography scan, neurodiagnostic or blood studies)
Refer patients with new or worsening symptoms or evidence of psychological issues to providers with expertise in those areas (eg, behavioral health).
Determine at each visit and/or evaluation if the patient is improving, is worsening, or has plateaued, and discharge if appropriate.
Encourage and provide home and self-care approaches.
Document the history, clinical examination, treatments performed, rationale for and response to care, and any referrals.
Patient Recovery or Maximum Therapeutic Benefit
Clinical experience suggests that, excluding patients who do not respond or worsen with care, a patient either reaches a point of full recovery and can be discharged or reaches a state of maximum therapeutic benefit (MTB), with some degree of ongoing neck pain. For patients who have reached MTB, the question then becomes: What is the best course of care to help control the ongoing pain? In general, patients unable to reach full recovery fall into one of these categories:
No physician/provider intervention is necessary. The patient has residual minor neck pain but can manage it with self-care strategies: ice, nonsteroidal anti-inflammatory drugs, home-based exercise.
Physician/provider intervention is necessary in periodic episodes of care. The patient experiences pain that exceeds his or her ability to self-manage and must return for care in an episodic fashion.
Physician/provider intervention is necessary on an ongoing basis. The patient experiences pain that exceeds his or her ability to self-manage, and in the absence of care the condition deteriorates. These patients often benefit from 1 to 2 visits per month to providers of nonpharmacologic conservative care who use spinal manipulation, to be reevaluated every 6 to 12 visits. [18–23]
History
A thorough history and examination based on evidence-informed procedures are critical to appropriate chiropractic management of patients with neck pain. Depending on the complexity of the case, components of the history may include(1) assessment of red flags and yellow-flag risk factors;
(2) onset of current neck pain/mechanism of injury;
(3) pain parameters;
(4) provocative and relieving factors;
(5) past history of injury, previous treatment, and response;
(6) history of diagnostic tests with results;
(7) surgical history;
(8) medications and nutraceuticals; and
(9) social/family history.
Informed Consent
Informed consent combines legal, ethical, and administrative duties, which underlie a patient’s right to determine what is done to his or her body. It is not static but involves active communication between the patient and clinician. The clinician should explain the diagnosis, treatment options (which may also include no treatment), and benefits and risks of the proposed treatment in terms the patient understands. [14, 24–26] The clinician may also wish to advise the patient of any substantive risks that might be present from the evaluation and physical examination procedures that will be performed, and if the procedure or therapy is to be provided by someone other than the clinician obtaining consent, the patient can be so advised. The patient must understand this information to make an informed decision. [17] The clinician should ask the patient if he or she has any questions and then answer them before proceeding.14 These discussions and the patient’s agreement to proceed should be documented in the patient’s medical record.
Most health care practices today include a written consent form as part of their intake paperwork. Although such a form is extremely important, it does not in and of itself constitute informed consent. Informed consent is a process in which the clinician provides relevant information specific to the patient’s condition and potential concerns and unique risk factors, if any. In addition, the patient may withdraw consent at any time, and consent to treat one body part does not necessarily confer that consent to other body parts. Different legal jurisdictions may have different specific legal requirements, and clinicians are urged to contact their malpractice carrier, state association, or attorney for specific advice regarding their area. Detailed information is available through the Association of Chiropractic Colleges policy on informed consent (http://www.chirocolleges.org/resources/informed-consent-guideline/).
Red Flags
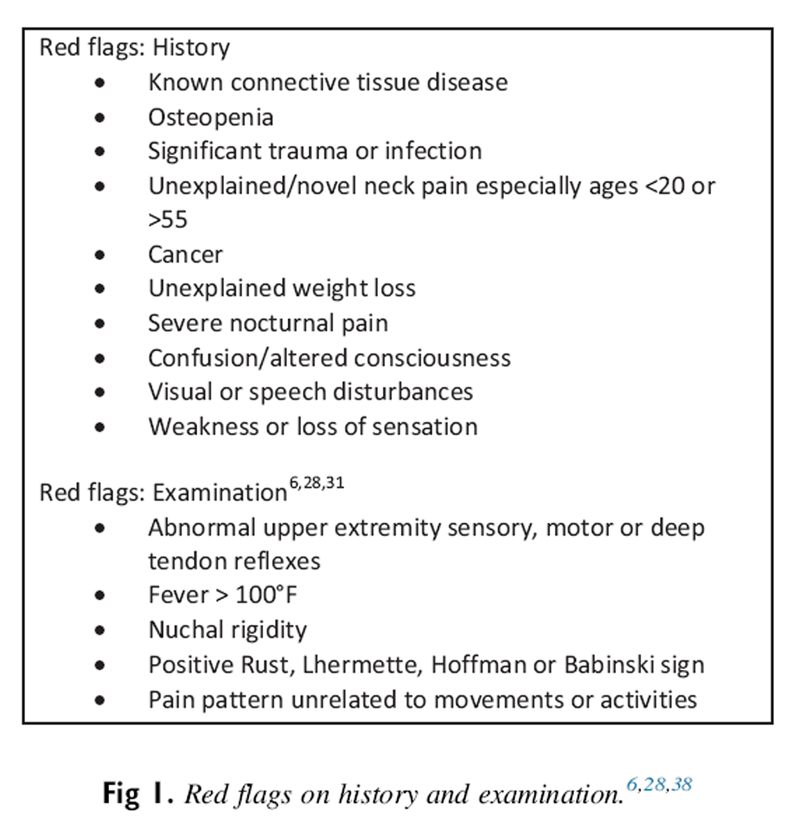
Figure 1 Red flags suggest the possibility of a more serious underlying illness that may need immediate referral, additional evaluation, or comanagement. [6, 27] The history and clinical examination findings are the primary source used to rule out serious potential pathology in patients presenting with neck pain. See Figure 1 for a list of red flags.
Patients presenting with signs suggestive of potential evolving stroke, such as a patient reporting “the worst headache ever,” may require emergent referral to a hospital for definitive evaluation and care. [27–30]
Most DCs, and some patients, are aware of the potential for vertebral artery dissection (VAD) and potential stroke. Cassidy et al [31] concluded that the association between chiropractic care and vertebral basilar artery–related stroke found in previous studies was likely explained by presenting symptoms attributable to evolving VAD and found similar risks between primary care physicians and chiropractors, which might be explained by the fact that patients with evolving VAD typically have headaches and neck pain. Several recent papers have associated VAD with a variety of common activities, [32–36] and others failed to find any high-quality evidence to support a causal link between chiropractic manipulation and cervical artery dissection. [35] Doctors of chiropractic need to be aware of possible signs and symptoms of potential VAD and stroke, and there are simple, clinically relevant questions and examination findings DCs should be looking for. [36]
Other red flags do not necessarily require referral or present a contraindication to spinal manipulation or other chiropractic procedures. [37, 38] These depend on the findings of the additional evaluation. Although some red flags represent contraindications to use of high-velocity low-amplitude manipulation, other approaches using less biomechanical force may be used to address the musculoskeletal disorders while the red-flag issues are being addressed via further diagnostic testing, referral, or interdisciplinary care coordination.
Yellow Flags
The term yellow flag refers to psychosocial factors that might predict poorer outcomes of musculoskeletal pain. Optimal treatment frequency and duration must be determined on an individual basis, considering barriers to recovery such as yellow flags, comorbidities, or other factors. Yellow flags are considered to relate mainly to psychological issues in the categories of beliefs, emotional responses, and pain behavior. [39] These may include factors such as stress, anxiety, pain behavior, and job dissatisfaction. Presence of yellow flags can affect the speed of recovery and compliance with, and success of, treatment protocols. [40]
Examples of yellow flags include [39, 40](1) belief that pain is harmful,
(2) belief that activity should be avoided,
(3) negative attitude or depression,
(4) work-related stress,
(5) lack of social support, and
(6) current compensation and claims issues related to neck pain.Clinicians may want to use an outcome assessment tool such as a Fear Avoidance Behavior Questionnaire to evaluate patients for psychological factors that might delay recovery. Fear-avoidance behavior is a risk factor for poorer outcomes for patients with musculoskeletal pain. [41, 42]
If psychological factors, including fear-avoidance behavior, catastrophizing, or other behaviors, appear to be present, evaluation by a psychologist may be appropriate, and consideration of a trial of cognitive behavioral therapy may be warranted. [43, 44]
Onset and Mechanism of Injury
Record precipitating factors (particularly injury or non-injury onset) and the date of onset or duration of pain. Temporal factors associated with neck pain including onset of pain may provide clues about the nature of the condition. [6]
Pain Parameters
Patients with neck pain may present with localized neck pain only, headache alone or in combination with neck pain, radicular symptoms, thoracic spine pain, weakness, overlapping radicular patterns, a dermatomal distribution, or some combination of symptoms. Forming a working or differential diagnosis may include the following [30]:
Severity: Often recorded using a numeric rating scale of 0 to 10 or stratification (mild, moderate, or severe)
Frequency (timing and duration): Is it constant or intermittent? Is there a relationship between the pain and activities?
Location/distribution—often recording using a pain diagram
Radiation and pattern of referral
Character of the pain such as dull, achy, stiff, sharp, numbing, tingling
Associated symptoms, such as headache, dizziness or vertigo, arm or back pain
Relieving and aggravating factors
In the presence of radicular or other upper-extremity symptoms, similar inquiries should be made:
Location, referral, and timing of pain often suggest the sources of pain.
Is it constant or intermittent?
Quality? (ie, pain, numbness, reduced or increased sensation)
Location?
Relieving and aggravating factors?
Does it follow a known dermatomal, peripheral nerve, or trigger point pattern? [7]
Other Important History Factors
For patients presenting with neck pain, a history and physical exam appropriate to the nature, severity, and risks associated with the presenting problem should be conducted. Clinicians must exercise judgment regarding the extent to which other factors should be evaluated, including(1) review of systems;
(2) previous similar conditions, treatments, and response to care;
(3) relevant past medical history;
(4) occupational history and relevance to presenting complaints;
(5) sleep position and quality;
(6) lifestyle issues, such as smoking, diet, exercise, use of drugs, alcohol, or tobacco, repetitive texting or computer use, and other factors; and
(7) behavioral health issue/stress/depression.
Patient-Based Outcome Measurement

Table 1 Clinicians should assess baseline status and monitor changes in pain, function, disability, and psychosocial functioning. They may use validated self-report questionnaires. [3] The Neck Disability Index questionnaire is the most commonly used [45]; however, there are a number of questionnaires that have been validated for assessment of the cervical spine. See Table 1 for examples of reliable and valid outcome assessment tools (OATS) relevant to neck pain patient care. [44, 46–48]
Patient-based outcome measures are useful when administered at the initial examination and during reevaluation at regular intervals during treatment to evaluate for patient improvement and treatment effectiveness. The use of reliable and valid OATS can serve to establish and benchmark functional goals within a patient treatment plan and to establish medical necessity for ongoing care. Thus, the proper use of OATS can address the growing emphasis of third-party payers on outcome-based systems for reimbursement. [49, 50] Table 1 provides several commonly used OATs, but is by no means exhaustive, and other instruments may prove useful for specific patients.
Physical Examination
The provider should conduct a condition-specific examination that may include assessments of function, which can be used to establish a baseline, monitor changes over time, and provide information, which can be used to clarify diagnostic alternatives.
Examples of commonly used tests include [30]:
Vital signs, including age, height, and weight
Observation/inspection of posture, musculature, pain behaviors, and distress [51, 52]
Active and passive cervical range of motion (valid tools include cervical range of motion device, goniometer, or inclinometer) [3, 6]
Palpatory findings: tissue tone, spasm, tenderness, and trigger points [6, 30]
Upper- and lower-extremity motor strength
Reflexes
Sensation
Orthopedic tests with good reliability and validity, such as Spurling, Valsalva, neurodynamic testing, cervical distraction, and the cervical flexion-rotation test [3]
Pressure/pain threshold testing using an algometer [3]

Figure 2 Evaluation of orientation, general cognitive function, cranial nerve testing, cerebellar tests, fund of knowledge, and other general assessments of brain function may also be warranted, particularly in the context of potential or documented concussion, whiplash, or suspected or potential vertebral artery insult. [53–55]
Evaluating the Site of Care Several approaches to determining the site of treatment for spinal manipulation are widely used in the chiropractic profession. Procedures that are recommended and not recommended, based on a 2013 systematic review, are summarized in Figure 2. [56]
Diagnostic Imaging: General Considerations
Similar to the history and examination procedures, consideration of imaging must also be condition specific. It should not be based on philosophy, office policy, or financial considerations. Clinical examinations have low sensitivity for some conditions such as stenosis, and therefore imaging studies such as MRI may be indicated if these conditions are suspected. [3]
The skill, training, and experience of the provider are important components of clinical decision-making and should be considered when evaluating the medical necessity of any diagnostic test, including plain film radiography. Not every patient presenting for care requires x-ray, and it is important for the provider to consider established medical guidelines such as the American College of Physicians and the National Emergency X-Radiography Utilization Study low-risk criteria. [57, 58]Acute Neck Pain: Indications for Diagnostic Imaging Imaging is indicated in the initial assessment of patients with acute neck pain when myelopathy, suspicion of significant ligamentous injury, or presence of other red flags is noted. [59] In those cases, computed tomography or MRI without contrast are procedures of choice. [57] Plain film radiography is indicated by the National Emergency X-Radiography Utilization Study criteria. [60] Some of these limitations may not be appropriate for patients over age 65. [31]
Chronic Neck Pain: Indications for Diagnostic Imaging The American College of Radiology reports that it is usually appropriate to perform anteroposterior and lateral views of the cervical spine as a first study in patients(1) with chronic neck pain with or without a history of trauma,
(2) with a history of malignancy, or
(3) with a history of neck surgery in the distant past. [57, 61]Diagnostic imaging for the purpose of identifying spinal degeneration is not recommended. Spinal degenerative changes are often present in pain-free individuals. [62]
Follow-Up Radiography There is no high-quality evidence to suggest that serial radiography of the cervical spine is a useful tool with high clinical yield. [56, 63]
Diagnostic Codes

Figure 3
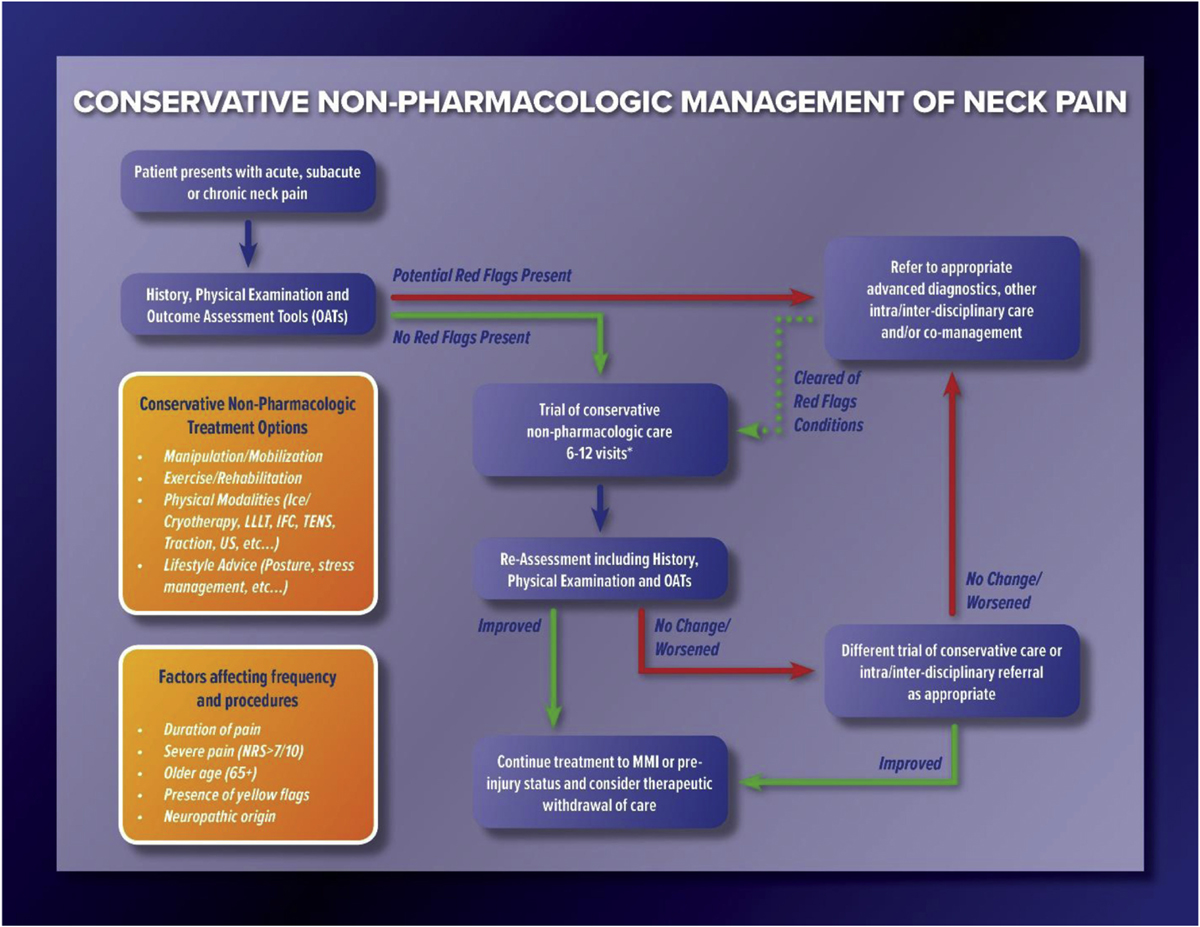
Figure 4 Figure 3 lists commonly used diagnostic codes in chiropractic practice for cervical spine-related conditions.
General Care Pathway for Chiropractic Treatment of Neck Pain
Figure 4 shows the care pathway for the best-practice approach to chiropractic management of neck pain.
General Treatment Recommendation Principles
Avoid basing treatment recommendations on philosophy, habitual practice procedures, or financial considerations.
Frequency and duration of treatment should be consistent with severity of the presenting complaint, history, and examination findings.
Treatment should include an initial trial of care, 6 to 12 visits, to determine the success or failure of treatment and the possible need for additional diagnostic tests or referral, including multidisciplinary, multimodal care.
In general, there should be diminishing reliance on passive care and a shift toward active care and patient self-reliance.
Although the current set of recommendations focuses on the duration of symptoms (acute, subacute, and chronic), it is important to consider other parameters, including symptoms such as radicular complaints. Radiculopathy is often treated conservatively, and an initial trial of conservative care including spinal manipulation may be appropriate, as long as there is no evidence of progressive neurologic deficit. [64, 65]
Current literature indicates that although most patients with acute neck pain experience significant relief within 2 months, up to 50% continue to have pain or frequent flare-ups for the first year. [5, 6] Clinically meaningful reduction in pain and disability of 20% may take 6 months to achieve, and clinical recovery varies in success. [3]
Acute and Subacute (0–3 Months) Neck Pain—Initial Course of Treatment
Multimodal treatment [12] consisting of manual therapy (joint manipulation/mobilization and/or other soft tissue techniques), education, and exercise is recommended.
Emphasize education, advice, reassurance, activity, and encouragement. Consider watchful waiting and clinical monitoring as evidence-based therapeutic options during the acute phase. [3]
Spinal manipulation or mobilization of cervical and thoracic spine
Supervised graded strengthening exercises (mobility, strengthening, and stability exercises combined with a home exercise program, typically 2×/week for 6 weeks)
Supervised group exercises
Supervised yoga (Iyengar, 9 sessions over 9 weeks)
For patients with more severe pain, or with signs of potential neurological involvement, consider early comanagement with a neurologist, orthopedist, pain management specialist, or physical medicine and rehabilitation specialist.
Patients with evidence of progressive neurologic deficits (weakness, reflex changes, stroke symptoms, myelopathy, balance issues, etc.) should be referred for further evaluation. [65–69]
Throughout the care episode and at discharge, reassure the patient regarding the natural history of neck pain-associated disorders (NADs) or whiplash-associated disorders (WADs); advise resuming normal activities as much as possible; and provide appropriate self-care strategies, including strengthening exercises. Discourage catastrophizing and overdependence on physicians and other providers.
Additional Passive Modalities for Acute and Subacute Neck Pain In addition to multimodal treatment consisting of joint manipulation/mobilization, soft tissue techniques, education, and exercise, some patients may benefit from additional modalities, depending on chronicity, grade of neck pain, and comorbid conditions [3, 12, 70, 71]:
(1) low-level laser therapy (LLLT),
(2) transcutaneous electrical nerve stimulation (TENS),
(3) cervical traction, or
(4) interferential current therapy. [72, 73]Continued use beyond an initial brief trial should be predicated on demonstration of significant improvement in functional status or clinically meaningful relief of pain.
Optimal Dosages for Manual Therapies
Definitive evidence on optimal dosages for manual therapies, including spinal manipulation, is currently lacking. However, studies on treatment parameters for similar conditions, such as cervicogenic headaches, suggest better outcomes with more intensive care, typically 3× per week initially. [22]
Initial Treatment Frequency for NAD/WAD

Table 2 An initial trial of care for acute and chronic uncomplicated NAD and WAD typically consists of multimodal treatment 3× per week for up to 4 weeks. Some acute conditions may require fewer treatments to achieve resolution (see Table 2).
Optimal treatment dosage depends on the specific neck condition (such as WAD, chronic NAD, radicular disorders, and instability), severity and chronicity, and individual patient characteristics (age, severity, comorbidities, activity, motivation). [74] Given the heterogeneous nature of this patient population, multiple contributing factors, and lack of definitive research-based guidance, specific clinical cookbook treatment regimens are impractical and unrealistic.Treatment Frequency and Duration Treatment may be initially provided more frequently and tapered as the patient improves. Continuing treatment should be predicated on demonstration of improvement in functional capacity and not only temporary reduction in subjective complaints. A small population of patients with chronic pain with more complex problems may require ongoing care after a plateau in subjective and functional status has been reached.
Patients With Severe Pain Patients with severe pain (numeric rating scale >7 of 10) and findings consistent with moderate to severe functional limitations may warrant daily treatment for up to 1 week to manage pain and improve function. These patients may also benefit from multimodal and multidisciplinary pain management that includes medications. Patients with more complex presentations, significant comorbidities, or chronic NAD or WAD may require longer periods to demonstrate subjective, objective, or functional improvement.
Interim Assessment Conduct an interim assessment of improvement after approximately 6 to 12 visits. In some cases, a certain percentage of patients seem to respond favorably, based on subjective reporting of pain levels and activities of daily living, within a shorter duration (6–8 visits). For these patients, reexamine/reassess at 8 visits rather than 12.
Use of a Different Approach If no clinically meaningful improvement is noted after 8 to 12 visits, a different multimodal approach (using different modalities or schedule) is warranted. If no further clinical improvement is noted after an additional 6 to 12 visits, assuming appropriate diagnostic studies including imaging have been obtained, the patient should be referred to a provider of a different discipline or be deemed to have reached MTB.
Trials of Therapeutic Withdrawal and MTB When further improvement in clinical status, particularly functional status, appears to be tapering or stabilizing, a trial of therapeutic withdrawal may be warranted. Current literature does not define a specific length of time for adequate therapeutic withdrawal. This process remains patient specific.
If the patient’s clinical status appears to have resolved or remains stable, a final examination is indicated. In the presence of ongoing pain or functional deficit, when all reasonable, evidence-based clinical interventions and diagnostic studies have been tried or considered and no further significant functional improvement is anticipated from additional care, the patient is deemed to have achieved MTB or maximum medical improvement and should be discharged from care. If care from a different health care discipline might provide further benefit, the patient should be appropriately comanaged or referred.
Recommended Initial Course of Treatment for Chronic (>3 Months) Neck Pain
General recommendations for chronic pain are essentially the same as those for acute pain.
Individualized multimodal care is recommended, including [3, 12](1) manual therapy (manipulation, mobilization, muscle energy/other soft tissue techniques, massage, traction);
(2) acupuncture/dry needling;
(3) home exercise (cervical retraction, deep neck flexor strengthening, cervical rotation range of motion, stretching);
(4) postural advice, including encouragement to maintain neck motion and daily activities;
(5) stress self-management (relaxation, balance/body awareness exercises, self-management lectures);
(6) intermittent traction, particularly for neck pain with radiating pain;
(7) high-dose massage (60–minute sessions 3×/wk for 4 weeks; lower dose not effective) [12]; and
(8) strength training (3 sessions/wk for 20 minutes).Additional Passive Modalities for Chronic Neck Pain A trial of care for passive modalities such as heat, TENS, traction, LLLT, ultrasound, and electric muscle stimulation, as part of a multimodal approach to treatment, may be warranted to provide some short-term relief, particularly because they are considered low-risk procedures. [3, 60, 72, 73, 75, 76]
Safety of Passive Modalities Passive modalities are generally considered low risk and associated with minor, transient, or self-limiting increases in pain or stiffness. [3, 12, 76–79] Therefore, these modalities may benefit some patients in the context of a multimodal treatment approach primarily geared toward a transition to active care. Clinicians may wish to include these modalities on a short-term basis, with documentation of patient-reported or functional improvement.
Whiplash-Associated Disorders (WADs)
Overall, recommendations for recent and persistent WADs are similar to those for acute/subacute and chronic NAD patients (manipulation/mobilization, multimodal care, and supervised exercise and multidisciplinary care, respectively.) For both NAD and WAD chronic patients, intermittent mechanical traction may be considered. [3]
Reassessment
Although the doctor should monitor the patient at each encounter for clinical gains, it is also necessary to observe for clinical decline, including untoward effects of treatment and the emergence of clinical red flags such as progressive loss of neurologic function, which would warrant referral (diagnostic testing and/or an intra- or interdisciplinary second opinion) for further evaluation and treatment.+
Interval Reassessments
Reassessments should be conducted at regular intervals to document clinical status. Typically, during the acute, intensive treatment phase, these should be performed every 6 to 8 visits and may include(1) subjective complaints including numerical pain scales,
(2) a relevant condition severity-based detailed or focused physical examination,
(3) appropriate outcome assessment tools, and
(4) barriers to recovery.
Goals of Interval Assessments
The goal of the interval assessments and reexaminations is to determine whether the treatment being provided is achieving clinically meaningful improvement, particularly in functional improvement and reductions in perceived pain level. For example, on an 11–point numerical pain scale (0–10), a 2–point reduction in pain (eg, from 8 of 10 to 6 of 10) is considered clinically meaningful. [80] Additionally, an evaluation helps to determine whether the patient has reached a therapeutically stable plateau or would likely benefit from additional care or referral.
Providers should assess pain and functional status, using established measures.
Referral and Comanagement
Patients with moderate to severe initial or recurrent pain may benefit from concurrent pharmacologic interventions directed by a medical physician.
Patients who fail to demonstrate significant improvement may also benefit from consults or comanagement with orthopedists, family physicians, physical medicine and rehab professionals, pain specialists, psychologists, or neurologists, depending on their symptoms and clinical findings.
Patients with clinical red flags including progressive neurologic deficits require appropriate referral.
Patients who may benefit from practices/modalities not available in the treating chiropractor’s office may be referred to the appropriate provider, such as a colleague, or physical therapist, acupuncturist, or massage therapist.
Continuing Course of Treatment
The natural history of NAD/WAD suggests many patients may never fully recover, and exacerbations are common. The goal of care for patients with remaining functional deficits who have reached MTB is to help them become as self-sufficient as possible.
Goals for ongoing chronic pain management are different than those related to acute pain management. Chronic pain management goals may include but are not limited to the following:
Control pain.
Maximize the highest levels of function and ability to engage in activities of daily living.
Keep the patient employed or functional in his or her daily life.
Minimize the need for pain medications.
Periodic Return for Care
Some patients may require periodic care when they experience exacerbations/flare-ups with recurrence of previously improved functional deficits. Under such circumstances, the clinician should document subjective and objective findings and the capacity to perform daily activities while providing care appropriate to returning them to the MTB baseline. The frequency and duration of such care will depend on the clinical presentation: Some may require an acute care approach, while others may only need a few visits. Periodic reevaluations are warranted after short trials of care, typically 6 to 8 visits. The need for additional care should be predicated on the ability to demonstrate significant positive response and the likelihood of further improvement.
Discharge
Some patients will demonstrate a progressive return to the pre-injury baseline, and once that is achieved the patient should be released. Others will demonstrate improvement to a point and then begin to plateau in their response. In such cases, ongoing care should be tapered until they reach maximum medical improvement or MTB, indicating further improvement is not anticipated. Patients who do not show meaningful improvement or those who plateau with significant pain and functional limitations should be offered alternative evidence-based care, including a referral to providers from other disciplines.
Therapeutic Withdrawal
Once the patient demonstrates likely MTB, a trial of therapeutic withdrawal is appropriate to determine whether therapeutic gains will be maintained, and a final discharge examination documented. The patient should also be educated in appropriate self-management approaches post-discharge. An important goal of care is to allow the patient to become self-sufficient and independent in the management of his or her chronic condition.
Scheduled Ongoing Care
For a small population of patients with chronic pain with more complex problems who fail to maintain therapeutic gains, who tend to deteriorate in the total absence of care, and for whom home-directed management is not sufficient to control pain and maintain function, scheduled ongoing care may be beneficial. One to 2 visits per month may be necessary to be reevaluated at a minimum of every 6 to 12 visits. Periodic trials of therapeutic withdrawal may be required to demonstrate the need for ongoing chronic care.
For a video description of this best-practice recommendation,
please view this video.
Discussion
Clinical practice guidelines are used by clinicians to determine the best treatment approach to patient care. They are also widely used by payers, regulators, and patients. All parties want to ensure that the treatment plan under consideration has the highest likelihood of success and is a reasonable approach based on our current level of scientific evidence. Although it would be ideal if we had definitive evidence indicating exactly which treatment would benefit each patient, this is not the current clinical reality for any health care profession. Some treatments work well for many patients and yet may not work at all for others. In 2008, when asked which treatments for neck pain helped, Binder reported, “The evidence about the effects of individual interventions for neck pain is often contradictory because of poor quality RCTs [randomized controlled trials], the tendency for interventions to be given in combination, and for RCTs to be conducted in diverse groups. This lack of consistency in study design makes it difficult to isolate which intervention may be of use in which type of neck pain.” [69](p2)
Investigators have made some progress in the 11 years since his review, but there continue to be many gaps in our knowledge. High-quality CPGs on neck pain still do not provide definitive recommendations about certain treatments, such as LLLT or TENS, or may make recommendations that conflict with other well-done CPGs [3, 11, 12] Clinicians are left with a quandary: if they rely exclusively on the highest-level evidence, based on high-quality RCTs, must they abandon other widely used treatments that may be helpful but simply do not have the same level of evidence? Other medical specialties have cautioned against automatically making such judgments.81
Regardless, clinicians and payers want to know the best approach to take for patients with neck pain. The literature regarding conservative treatment of neck pain supports multimodal care, including manipulation, mobilization, and exercise. However, the literature is less helpful in providing direction on how often and for how long to provide manipulation/mobilization and exercise. Addressing that issue requires reliance on less robust evidence and on expert opinion. Similarly, other aspects of evaluation and treatment rely on a mix of the conclusions supported by existing CPGs and evidence-based expert opinion.
There are a number of high-quality CPGs regarding neck pain [3, 11, 12] that help direct care pathways and decisions, but they are somewhat limited by the number of high-quality studies upon which to make recommendations. In some cases, these CPGs either failed to make a recommendation owing to an absence of highest-level evidence (such as an RCT/systematic review evidence) or they provided conflicting recommendations. Examples include traction, LLLT, informed consent, the process of care, phases of care, red flags, and other issues affecting practice decisions. [3, 11, 12]
This study reviewed the most current CPGs and filled gaps in those recommendations by reviewing the best available evidence and providing it to a panel of experienced DCs. The result is a broad-spectrum review of how DCs should evaluate and treat patients presenting with neck pain. This may include treatments with the highest level of evidence (multimodal care including manipulation/mobilization with exercise) and other acceptable treatment approaches with less robust but still appropriate treatment options, such as TENS, LLLT, or other approaches. To our knowledge, this is the most comprehensive and current review of these issues and should help clinicians, payers, and regulators identify appropriate chiropractic care of people who present with neck pain.
Limitations
We relied on the Canadian Chiropractic Guideline Initiative and American Physical Therapy Association (APTA) CPG [3, 12] and to a lesser degree on the Royal Dutch Society for Physical Therapy CPG11 in developing our best-practice recommendations. We were unable to simply adopt them for a variety of reasons, however. As noted, the Canadian guideline, with its strong reliance on making recommendations based only on the highest levels of evidence, left many widely used treatment approaches without any clear recommendation. Examples include LLLT, TENS, and cervical traction, among others. [12] The Dutch guideline posed similar problems. [11] Again, to bridge this gap we relied on the best available evidence and the Delphi expert consensus process to provide guidance to clinicians.
There are many areas of common practice between DCs and physical therapists, and the APTA CPG was useful in addressing a number of clinical practice questions on areas of commonality. However, there were conflicts between the recommendations of the Canadian chiropractic and APTA guidelines, particularly regarding some commonly used modalities, such as LLLT and traction. Additionally, physical therapists are generally not licensed to obtain imaging studies such as radiographs or MRIs, and accordingly these issues also warranted additional review and consensus.
The chief limitation of this study is that for some aspects of chiropractic management of neck pain, high-quality evidence was scarce, and we therefore relied on expert opinion. This limitation was most pronounced for the topic of diagnostic imaging, where there is still a lack of literature specifically addressing the use of imaging for the purposes of manual care. [82, 83] We expect this gap to be increasingly addressed in the future; in fact, an article addressing the topic was published just after we completed the consensus process for this project. [83]
Another limitation may be perceived as the composition of our Delphi panel, which was exclusively DCs, although some were cross-trained in other disciplines. However, the composition was planned this way because we intended the recommendations to be used by DCs. Chiropractic practice, although it shares the use of various diagnostic and treatment methods with other professions (such as medical physicians, osteopathic physicians, and physical therapists), has a unique history and education. We felt our recommendations would be more useful and better accepted if they were tailored specifically to typical chiropractic practice. Furthermore, because medicolegal issues and scopes of practice differ in various countries, we made the conscious decision to focus the recommendations on chiropractic practice in the United States.
A strength of this project is, in fact, the choice to target US chiropractic practitioners. Since our purpose is to facilitate evidence-based chiropractic practice, developing a document geared for maximum user-friendliness and relevance was of greatest importance. Another strength of this project was that we used the highest available evidence, thus enabling clinicians to use evidence-informed pragmatic approaches to the care of patients with neck pain with evidence of efficacy, reasonable costs, and low risk.
Conclusion
A set of best-practice recommendations for chiropractic management of patients with neck pain based on the best available evidence reached a high level of consensus by a large group of experienced chiropractors. The recommendations indicate that manipulation and mobilization as part of a multimodal approach are front-line approaches to patients with uncomplicated neck pain.
Funding Sources and Conflicts of Interest
Acknowledgments The authors thank Cathy Evans for coordination of project communications and data management. The authors thank the Delphi panelists for their contribution of time and expertise:
Kris R. Anderson, DC, MS; Michael T. Anderson, DC; Shery Assal, DC; Craig R. Benton, DC; Charles L. Blum, DC; Gina M. Bonavito-Larragoite, DC; Michael S. Calhoun, DC; Wayne H. Carr, DC; Jeffrey R. Cates, DC, MS; Jason B. Cook, DC; Matthew C. Coté, DC, MS; John Curtin, MSS, DC; Clinton Daniels, DC, MS; Vincent DeBono, DC; Mark D. Dehen, DC; C. Michael DuPriest, DC, PT, DPT; Marc Dupuis, DC; Paul M. Ettlinger, DC; Drew Fogg, DC, MS; David Folweiler, DC; Bill Gallagher, DC; Jason Guben, DC; Nathan Hinkeldey, DC; Renée Tocco Hunter, DC; Jeffrey M. Johnson, DC; Robert E. Klein, DC; Rick Louis LaMarche, DC; William M. Lawson, MS, DC; Robert A. Leach, DC, MS, CHES; Marvin C. Lee, DC; Scott A. Mooring, DC; Mark Mulak, DC, MBA; Brett A. Myers, DC; Marcus Nynas, DC; Juli Olson, DC, MAOM, Lac; David Paris, DC; David B. Parish, MS, DC; Kendall Payne, DC; Colette Peabody, DC; Mariangela Penna, DC; Roger Kevin Pringle, DC, MEd; Mario D. Roybal, DC; Todd S. Rubley, MS, DC; Vern Saboe, Jr, DC; Mark Sakalauskas, DC; Chris Sherman, DC, MPH; Scott M. Siegel, DC; Charles A. Simpson, DC; Dean L. Smith, DC, PhD, Albert Stabile, Jr, DC; David N. Taylor, DC; Jason Weber, DC; Susan Wenberg, MA, DC; John S. Weyand, DC; and Clint Williamson, DC.
Funding Sources and Conflicts of Interest
Funding for the study was provided by the Council on Chiropractic Guidelines and Practice Parameters. No conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): C.H., A.M., W.W., R.F.
Design (planned the methods to generate the results): C.H., A.M.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): C.H., A.M.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): C.H., A.M., W.W., R.F.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): C.H., W.W., R.F., A.M., L.C., W.L., L.W., M.S., S.W.
Literature search (performed the literature search): C.H., A.M., W.W., R.F., S.W.
Writing (responsible for writing a substantive part of the manuscript): W.W., R.F., C.H., A.M.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): C.H., W.W., R.F., A.M., L.C., W.L., L.W., M.S., S.W.
References:
Global Burden of Disease 2015 Disease and Injury Incidence and Prevalence Collaborators.
Global, Regional, and National Incidence, Prevalence, and Years
Lived with Disability for 310 Diseases and Injuries, 1990-2015:
A Systematic Analysis for the Global Burden of Disease Study 2015
Lancet. 2016 (Oct 8); 388 (10053): 1545–1602Blackwell D.L. Lucas J.W. Clarke T.C.
Summary health statistics for U.S. adults: national health interview survey, 2012.
Vital Health Stat 10. 2014; : 1-161Blanpied P.R. Gross A.R. Elliott J.M. et al.
Neck pain: revision 2017.
J Orthop Sports Phys Ther. 2017; 47: A1-A83Haldeman S, Carroll L, Cassidy JD, Schubert J, Nygren A.
The Bone and Joint Decade 2000–2010 Task Force on Neck Pain
and Its Associated Disorders: Executive Summary
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S5–7Cohen S.P.
Epidemiology, diagnosis, and treatment of neck pain.
Mayo Clin Proc. 2015; 90: 284-299Cohen S.P. Hooten W.M.
Advances in the diagnosis and management of neck pain.
BMJ. 2017; 358: j3221National Board of Chiropractic Examiners
Practice Analysis of Chiropractic, 2015
National Board of Chiropractic Examiners, Greeley, CO2015Beliveau PJH, Wong JJ, Sutton DA, Simon NB, Bussieres AE, Mior SA, et al.
The Chiropractic Profession: A Scoping Review of Utilization Rates,
Reasons for Seeking Care, Patient Profiles, and Care Provided
Chiropractic & Manual Therapies 2017 (Nov 22); 25: 35Bussieres AE, Al Zoubi F, Stuber K, French SD, Boruff J, Corrigan J, et al.
Evidence-based Practice, Research Utilization, and Knowledge Translation
in Chiropractic: A Scoping Review
BMC Complement Altern Med. 2016 (Jul 13); 16 (1): 216Graham R. MM Wolman D.M. Greenfield S. Steinberg E.
Clinical Practice Guidelines We Can Trust.
Institute of Medicine, National Academies Press, Washington, DC2011Bier J.D. Scholten-Peeters W.G.M. Staal J.B. et al.
Clinical practice guideline for physical therapy assessment and treatment in patients with nonspecific neck pain.
Phys Ther. 2018; 98: 162-171Bussières, AE, Stewart, G, Al Zoubi, F et al.
The Treatment of Neck Pain-Associated Disorders and
Whiplash-Associated Disorders: Clinical Practice Guideline
J Manipulative Physiol Ther. 2016 (Oct); 39 (8): 523–564Jenkins H.J. Moloney N.A. French S.D. et al.
Using behaviour change theory and preliminary testing to develop an implementation intervention to reduce imaging for low back pain.
BMC Health Serv Res. 2018; 18: 734Globe, G, Farabaugh, RJ, Hawk, C et al.
Clinical Practice Guideline: Chiropractic Care for Low Back Pain
J Manipulative Physiol Ther. 2016 (Jan); 39 (1): 1–22Vernooij R.W. Sanabria A.J. Sola I. Alonso-Coello P. Martinez Garcia L.
Guidance for updating clinical practice guidelines: a systematic review of methodological handbooks.
Implement Sci. 2014; 9: 3Fitch K. Bernstein S.J. Aguilar M.D. et al.
The RAND UCLA Appropriateness Method User’s Manual.
RAND Corporation, Santa Monica, CA2003Spatz E.S. Krumholz H.M. Moulton B.W.
The new era of informed consent: getting to a reasonable-patient standard through shared decision making.
JAMA. 2016; 315: 2063-2064Descarreaux M, Blouin JS, Drolet M, Papadimitriou S, Teasdale N:
Efficacy of Preventive Spinal Manipulation for Chronic Low-Back Pain and Related Disabilities:
A Preliminary Study
J Manipulative Physiol Ther 2004 (Oct); 27 (8): 509–514Eklund, A., I. Jensen, M. Lohela-Karlsson, J. Hagberg, C. Leboeuf-Yde, et al. (2018).
The Nordic Maintenance Care Program: Effectiveness of Chiropractic Maintenance Care Versus
Symptom-guided Treatment for Recurrent and Persistent Low Back Pain -
A Pragmatic Randomized Controlled Trial
PLoS One. 2018 (Sep 12); 13 (9): e0203029Hawk C, Cambron JA, Pfefer MT.
Pilot Study of the Effect of a Limited and Extended Course of Chiropractic Care
on Balance, Chronic Pain, and Dizziness in Older Adults
J Manipulative Physiol Ther. 2009 (Jul); 32(6): 438–447Senna M.K., Machaly S.A.
Does Maintained Spinal Manipulation Therapy for Chronic Non-specific Low Back Pain
Result in Better Long Term Outcome?
Spine (Phila Pa 1976) 2011 (Aug 15); 36 (18): 1427–1437Haas M., Bronfort G., Evans R., et al.
Dose-Response and Efficacy of Spinal Manipulation for Care of Cervicogenic Headache:
A Dual-Center Randomized Controlled Trial
Spine J. 2018 (Oct); 18 (10): 1741–1754Haas M, Vavrek D, Peterson D, Polissar N, Neradilek MB.
Dose-response and Efficacy of Spinal Manipulation for Care of Chronic Low Back Pain:
A Randomized Controlled Trial
Spine J. 2014 (Jul 1); 14 (7): 1106–1116Winterbottom M. Boon H. Mior S. Facey M.
Informed consent for chiropractic care: comparing patients’ perceptions to the legal perspective.
Man Ther. 2015; 20: 463-468Lehman J.J. Conwell T.D. Sherman P.R.
Should the chiropractic profession embrace the doctrine of informed consent?.
J Chiropr Med. 2008; 7: 107-114Hall D.E. Prochazka A.V. Fink A.S.
Informed consent for clinical treatment.
CMAJ. 2012; 184: 533-540Vijiaratnam N. R Williams D. L Bertram K.
Neck pain: what if it is not musculoskeletal?.
Aust J Gen Pract. 2018; 47: 279-282Mattox R., Smith L.W., Kettner N.W.
Recognition of Spontaneous Vertebral Artery Dissection Preempting Spinal Manipulative Therapy:
A Patient Presenting With Neck Pain and Headache for Chiropractic Care
Journal of Chiropractic Medicine 2014 (Jun); 13 (2): 90-95Tarola G. Phillips R.B.
Chiropractic Response to a Spontaneous Vertebral Artery Dissection
J Chiropractic Medicine 2015 (Sep); 14 (3): 183–190Alexander E.P.
History, physical examination, and differential diagnosis of neck pain.
Phys Med Rehabil Clin N Am. 2011; 22: 383-393, viiCassidy JD, Boyle E, Cote P, et al.
Risk of Vertebrobasilar Stroke and Chiropractic Care: Results of a
Population-based Case-control and Case-crossover Study
Spine (Phila Pa 1976) 2008 (Feb 15); 33 (4 Suppl): S176–183Dittrich R. Rohsbach D. Heidbreder A. et al.
Mild mechanical traumas are possible risk factors for cervical artery dissection.
Cerebrovasc Dis. 2007; 23: 275-281McCrory P.
Vertebral Artery Dissection Causing Stroke in Sport
J Clin Neurosci 2000 (Jul); 7 (4): 298–300Rubinstein S. Cote P.
Mild mechanical traumas are possible risk factors for cervical artery dissection.
Cerebrovasc Dis. 2007; 24: 319Church EW, Sieg EP, Zalatimo O, Hussain NS, Glantz M, Harbaugh RE.
Systematic Review and Meta-analysis of Chiropractic Care and Cervical Artery Dissection:
No Evidence for Causation
Cureus 2016 (Feb 16); 8 (2): e498Murphy D.R.
Current Understanding of the Relationship Between Cervical Manipulation and Stroke:
What Does It Mean for the Chiropractic Profession?
Chiropractic & Osteopathy 2010 (Aug 3); 18 (1): 1–9Gottesman R.F. Sharma P. Robinson K.A. et al.
Clinical characteristics of symptomatic vertebral artery dissection: a systematic review.
Neurologist. 2012; 18: 245-254Nordin M, Carragee EJ, Hogg-Johnson S, Weiner SS, Hurwitz EL, Peloso PM, et al.
Assessment of Neck Pain and Its Associated Disorders:
Results of the Bone and Joint Decade 2000–2010 Task Force on
Neck Pain and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S101–S122Nicholas M.K. Linton S.J. Watson P.J. Main C.J.
“Decade of the Flags” Working Group Early identification and management of psychological risk factors (“yellow flags”) in patients with low back pain: a reappraisal.
Phys Ther. 2011; 91: 737-753Dziedzic K. Huckfield L. Larkin T. et al.
Neck pain: management in primary care.
in: Keele University Arthritis Research UK Primary Care Centre,
Keele University. Vol Series 6.
Reports on the Rheumatic Diseases, Chesterfield, UK2011Waddell G. Newton M. Henderson I. Somerville D. Main C.J.
A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability.
Pain. 1993; 52: 157-168Jay K. Thorsen S.V. Sundstrup E. et al.
Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain.
Pain Res Treat. 2018; 2018: 8347120Monticone M. Ambrosini E. Vernon H. et al.
Efficacy of two brief cognitive-behavioral rehabilitation programs for chronic neck pain: results of a randomized controlled pilot study.
Eur J Phys Rehabil Med. 2018; 54: 890-899Marco Monticone, Howard Vernon, Roberto Brunati, Barbara Rocca, Simona Ferrante
The NeckPix(©): Development of an Evaluation Tool for Assessing Kinesiophobia in Subjects
with Chronic Neck Pain
European Spine Journal 2015 (Jan); 24 (1): 72-79MacDermid J.C. Walton D.M. Avery S. et al.
Measurement properties of the neck disability index: a systematic review.
J Orthop Sports Phys Ther. 2009; 39: 400-417Cleland J.A. Childs J.D. Whitman J.M.
Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain.
Arch Phys Med Rehabil. 2008; 89: 69-74Cleland J.A. Fritz J.M. Whitman J.M. Palmer J.A.
The reliability and construct validity of the Neck Disability Index and patient specific functional scale in patients with cervical radiculopathy.
Spine (Phila Pa 1976). 2006; 31: 598-602McCarthy MJ, Grevitt MP, Silcocks P, Hobbs G.
The Reliability of the Vernon and Mior Neck Disability Index, and its Validity
Compared With the Short Form-36 Health Survey Questionnaire
European Spine Journal 2007;16(12):2111–7Centers for Medicare & Medicaid Services (CMS)
2016 Physician Quality Reporting System (PQRS): Implementation Guide.
Centers for Medicare & Medicaid Services, 2016Kuhn J.E.
Why measure outcomes?.
Instr Course Lect. 2016; 65: 583-586Moore M.K.
Upper Crossed Syndrome and Its Relationship to Cervicogenic Headache
J Manipulative Physiol Ther 2004 (Jul); 27 (6): 414—420Smith L. Louw Q. Crous L. Grimmer-Somers K.
Prevalence of neck pain and headaches: impact of computer use and other associative factors.
Cephalalgia. 2009; 29: 250-257McCrory P. Meeuwisse W. Dvorak J. et al.
Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016.
Br J Sports Med. 2017; 51: 838-847Scorza K.A. Raleigh M.F. O’Connor F.G.
Current concepts in concussion: evaluation and management.
Am Fam Physician. 2012; 85: 123-132Matuszak J.M. McVige J. McPherson J. Willer B. Leddy J.
A practical concussion physical examination toolbox.
Sports Health. 2016; 8: 260-269Triano J, Budgell B, Bagnulo A, Roffey B, Bergmann T, Cooperstein R.
Review of Methods Used by Chiropractors to Determine
the Site for Applying Manipulation
Chiropractic & Manual Therapies 2013 (Oct 21); 21 (1): 36American College of Radiology
ACR Appropriateness Criteria.
2013Paykin G. O’Reilly G. Ackland H.M. Mitra B.
The NEXUS criteria are insufficient to exclude cervical spine fractures in older blunt trauma patients.
Injury. 2017; 48: 1020-1024Moser N, Lemeunier N, Southerst D, Shearer H, Murnaghan K, Sutton D, Cote P (2017)
Validity and Reliability of Clinical Prediction Rules used to Screen for Cervical Spine Injury
in Alert Low-risk Patients with Blunt Trauma to the Neck: Part 2. A Systematic Review
from the Cervical Assessment and Diagnosis Research Evaluation
(CADRE) Collaboration
European Spine Journal 2018 (Jun); 27 (6): 1219–1233Guzman J, Haldeman S, Carroll LJ, et al.
Clinical Practice Implications of the Bone and Joint Decade 2000-2010
Task Force on Neck Pain and Its Associated Disorders:
From Concepts and Findings to Recommendations
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S199–S212Matsumoto M. Okada E. Toyama Y. Fujiwara H. Momoshima S. Takahata T.
Tandem age-related lumbar and cervical intervertebral disc changes in asymptomatic subjects.
Eur Spine J. 2013; 22: 708-713Brinjikji W. Luetmer P.H. Comstock B. et al.
Systematic literature review of imaging features of spinal degeneration in asymptomatic populations.
AJNR Am J Neuroradiol. 2015; 36: 811-816Bussieres A.E., Peterson C., Taylor J.A.
Diagnostic Imaging Practice Guidelines for Musculoskeletal Complaints in Adults — An Evidence-Based Approach:
Part 3: Spinal Disorders
J Manipulative Physiol Ther 2008 (Jan); 31 (1): 33–88Zhu L. Wei X. Wang S.
Does Cervical Spine Manipulation Reduce Pain in People with Degenerative Cervical Radiculopathy?
A Systematic Review of the Evidence, and a Meta-analysis
Clinical Rehabilitation 2016 (Feb); 30 (2): 145–155Childress M.A. Becker B.A.
Nonoperative management of cervical radiculopathy.
Am Fam Physician. 2016; 93: 746-754de Oliveira Vilaca C. Orsini M. Leite M.A. et al.
Cervical spondylotic myelopathy: what the neurologist should know.
Neurol Int. 2016; 8: 6330Arce D. Sass P. Abul-Khoudoud H.
Recognizing spinal cord emergencies.
Am Fam Physician. 2001; 64: 631-638Ghogawala Z. Benzel E.C. Riew K.D. Bisson E.F. Heary R.F.
Surgery vs conservative care for cervical spondylotic myelopathy: surgery is appropriate for progressive myelopathy.
Neurosurgery. 2015; 62: 56-61Binder A.I.
Cervical spondylosis and neck pain.
BMJ. 2007; 334: 527-531Graham N. Gross A.R. Carlesso L.C. et al.
An ICON overview on physical modalities for neck pain and associated disorders.
Open Orthop J. 2013; 7: 440-460Resende L. Merriwether E. Rampazo E.P. et al.
Meta-analysis of transcutaneous electrical nerve stimulation for relief of spinal pain.
Eur J Pain. 2018; 22: 663-678Fuentes J.P. Armijo Olivo S. Magee D.J. Gross D.P.
Effectiveness of interferential current therapy in the management of musculoskeletal pain: a systematic review and meta-analysis.
Phys Ther. 2010; 90: 1219-1238Yesil H. Hepguler S. Dundar U. Taravati S. Isleten B.
Does the use of electrotherapies increase the effectiveness of neck stabilization exercises for improving pain, disability, mood, and quality of life in chronic neck pain? A randomized, controlled, single blind study.
Spine (Phila Pa 1976). 2018; 43: E1174-E1183Jette A.M.
The importance of dose of a rehabilitation intervention.
Phys Ther. 2017; 97: 1043Bussieres A.E., Peterson C., Taylor J.A.
Diagnostic Imaging Practice Guidelines for Musculoskeletal Complaints in Adults — An Evidence-Based Approach:
Part 3: Spinal Disorders
J Manipulative Physiol Ther 2008 (Jan); 31 (1): 33–88Kroeling P. Gross A. Graham N. et al.
Electrotherapy for neck pain.
Cochrane Database Syst Rev. 2013; 8: CD004251Gross A.R. Dziengo S. Boers O. et al.
Low level laser therapy (LLLT) for neck pain: a systematic review and meta-regression.
Open Orthop J. 2013; 7: 396-419Jensen I. Harms-Ringdahl K.
Strategies for prevention and management of musculoskeletal conditions. Neck pain.
Best Pract Res Clin Rheumatol. 2007; 21: 93-108Swedish Council on Health Technology Assessment
Laser Treatment of Neck Pain.
Swedish Council on Health Technology Assessment, Stockholm, Sweden2014Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole MR.
Clinical Importance of Changes in Chronic Pain Intensity
Measured on an 11-point Numerical Pain Rating Scale
Pain 2001 (Nov); 94 (2): 149-158Burns P.B. Rohrich R.J. Chung K.C.
The levels of evidence and their role in evidence-based medicine.
Plast Reconstr Surg. 2011; 128: 305-310Jenkins H.J. Downie A.S. Maher C.G. Moloney N.A. Magnussen J.S. Hancock M.J.
Imaging for low back pain: is clinical use consistent with guidelines? A systematic review and meta-analysis.
Spine J. 2018; 18: 2266-2277Jenkins H.J. Downie A.S. Moore C.S. French S.D.
Current evidence for spinal X-ray use in the chiropractic profession: a narrative review.
Chiropr Man Therap. 2018; 26: 48
Return to CHRONIC NECK PAIN
Return to NECK DISORDER GUIDELINES
Return NON-PHARMACOLOGIC THERAPY
Return to BEST PRACTICES IN CHIROPRACTIC
Since 12-31-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |