

Deconstructing Chronic Low Back Pain in the Older Adult -
Step by Step Evidence and Expert-Based Recommendations
for Evaluation and Treatment.
Part VIII: Lateral Hip and Thigh PainThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Pain Med. 2016 (Jun 21); [Epub ahead of print] ~ FULL TEXT
OPEN ACCESS Monica Rho MD, Alejandra Camacho-Soto MD, Abby Cheng MD,
Mark Havran DPT, LAT, CSCS, Natalia E. Morone MD, MS,
Eric Rodriguez MD, Joseph Shega MD, Debra K. Weiner MD
Department of Physical Medicine and Rehabilitation,
Rehabilitation Institute of Chicago/McGaw Medical Center
of Northwestern University, Chicago, Illinois.OBJECTIVE: This article presents an evidence-based algorithm to assist primary care providers with the diagnosis and management of lateral hip and thigh pain in older adults. It is part of a series that focuses on coexisting pain patterns and contributors to chronic low back pain (CLBP) in the aging population. The objective of the series is to encourage clinicians to take a holistic approach when evaluating and treating CLBP in older adults.
METHODS: A content expert panel and a primary care panel collaboratively used the modified Delphi approach to iteratively develop an evidence-based diagnostic and treatment algorithm. The panelists included physiatrists, geriatricians, internists, and physical therapists who treat both civilians and Veterans, and the algorithm was developed so that all required resources are available within the Veterans Health Administration system. An illustrative patient case was chosen from one of the author's clinical practices to demonstrate the reasoning behind principles presented in the algorithm.
RESULTS: An algorithm was developed which logically outlines evidence-based diagnostic and therapeutic recommendations for lateral hip and thigh pain in older adults. A case is presented which highlights the potential complexities of identifying the true pain generator and the importance of implementing proper treatment.
CONCLUSIONS: Lateral hip and thigh pain in older adults can contribute to and coexist with CLBP. Distinguishing the true cause(s) of pain from potentially a myriad of asymptomatic degenerative changes can be challenging, but a systematic approach can assist in identifying and treating some of the most common causes.
KEYWORDS: Chronic Low Back Pain; Diagnostic Algorithm; Elderly; Greater Trochanteric Pain Syndrome; Hip Osteoarthritis; Iliotibial Band Pain; Lateral Hip Pain; Lumbar Radiculitis; Lumbar Radiculopathy; Lumbar Spinal Stenosis; Meralgia Paresthetica; Thigh Pain
From the FULL TEXT Article:
Introduction
Many physicians assume that an older adult with low back pain (LBP) and concomitant lateral hip/thigh pain has lumbar spinal stenosis. However, in reality there are myriad causes of lateral hip/thigh pain in older adults and the diagnosis of this pain can be challenging due to pain referral patterns. First, the hip and nearby lumbopelvic structures share innervation from common nerve roots, so pain referral patterns from pathology of these structures overlap. [1, 2] Second, faulty mechanics of the lumbar spine and/or hip can lead to compensatory movement patterns and eventually result in multiple pain generators. These challenges are illustrated in a study by Sembrano and colleagues. In a sample of 200 patients presenting for evaluation by a spine surgeon, only 65% had isolated spine pain, whereas 17.5% had a combination of hip, spine, and/or sacroiliac (SI) joint pain. [3] Lastly, diagnosing the etiology of hip and lumbopelvic pain in older adults is challenging in that many people have structural abnormalities on imaging studies that are asymptomatic. For instance, 93% of asymptomatic people 60–80 years old have MRI evidence of disc degeneration, 36% have a herniated disc, and 21% have spinal stenosis. [4] Additionally, only 46.5% of women ages 65 years and older who have radiographic evidence of hip osteoarthritis (OA) report hip pain “on most days for at least 1 month”. [5]
This article is part of a series that addresses coexisting conditions and contributors to chronic low back pain (CLBP) with and without leg pain in older adults. [6] We present a diagnostic and therapeutic evidence-based algorithm designed for primary care clinicians to approach lateral hip and thigh pain in older adults. This is followed by a case that illustrates the challenges of identifying the cause of lateral hip pain and/or thigh pain in older adults and highlights the importance of a proper diagnosis to initiate appropriate management. Greater trochanteric pain syndrome (GTPS) and iliotibial band syndrome (ITBS) are two local causes of lateral hip and thigh pain; therefore, the management of these syndromes will be discussed in detail. Furthermore, because as many as one in four people with hip OA present with lateral hip pain [7] and because lumbar radicular pain and spinal stenosis affecting the lumbar nerve roots can also refer pain to this region, the algorithm will describe how to identify pain from these referred sources, as well. Finally, while meralgia paresthetica (MP) is less common and its clinical presentation is often clearly distinct from the conditions listed above, we will also briefly review its presentation, diagnosis, and management. Pathology of many other lumbopelvic structures, such as the sacroiliac joints and lumbar facets, also refers pain to the lateral hip and thigh. Discussion of these conditions is beyond the scope of this paper, and if a patient does not respond in a timely manner to management outlined in the algorithm in Figure 1, referral should be made to a musculoskeletal specialist who can evaluate for these conditions, as well.
Methods
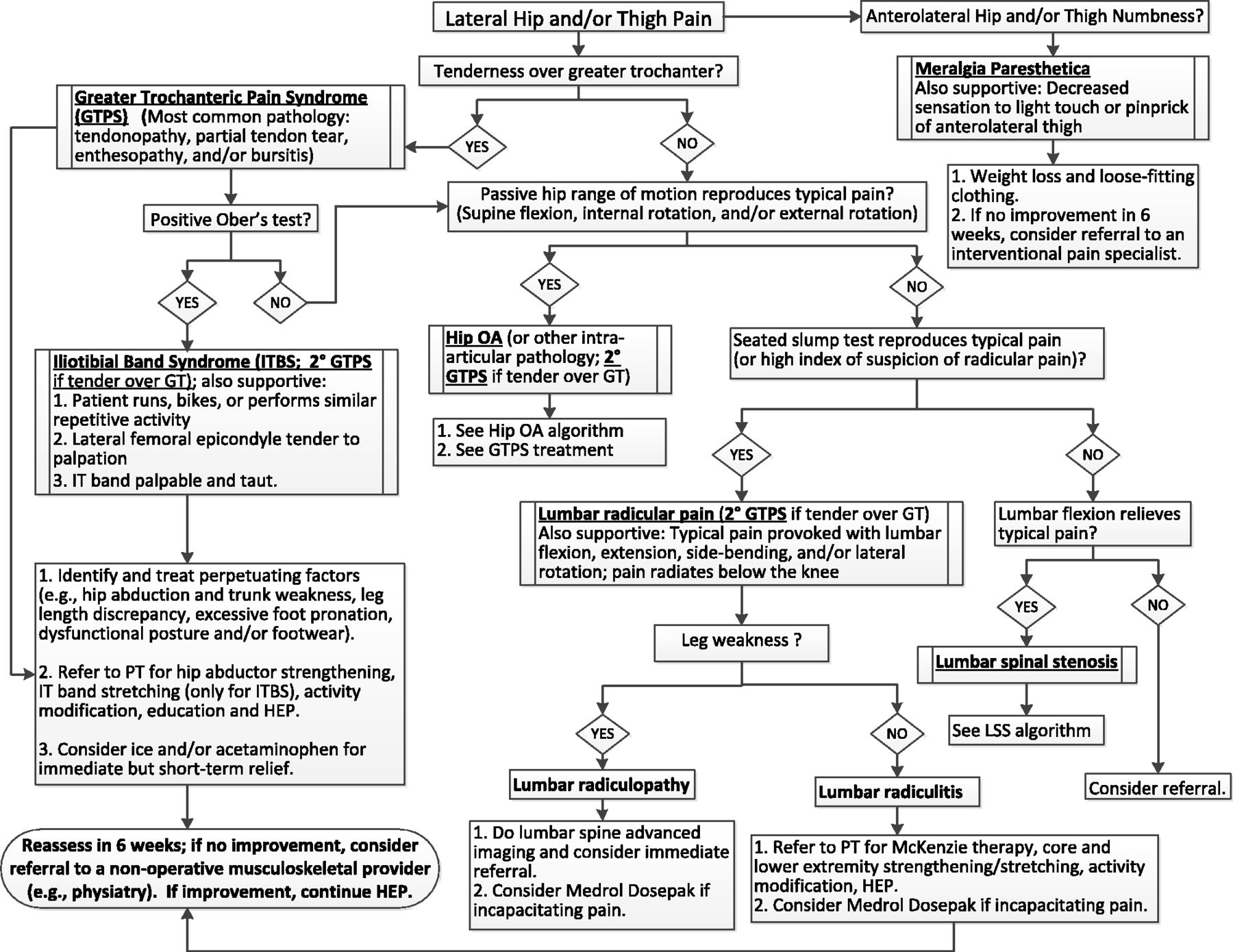
Figure 1

Table 1 As described in-depth in the series introduction [6], a modified Delphi approach was used by a content expert panel comprised of geriatric and physiatry specialists and physical therapists, in collaboration with a primary care panel. An evidence-based diagnostic and treatment algorithm (Figure 1) was developed iteratively. A corresponding table outlining the supporting evidence is presented in Table 1. The panelists included clinicians who treat both civilians and Veterans, and the algorithm was developed so that all required resources are available within the Veterans Health Administration system.
Case Presentation
Relevant History
The patient is a 90–year-old female with past medical history including a left sacral insufficiency fracture, thoracic vertebral compression fracture, and degenerative left medial meniscus tear, who presented with stabbing left lateral hip pain. The pain was chronic but had been progressing in severity over the past month. There was no inciting event or fall. The pain was exacerbated by lying on her left side and was affecting her sleep. At baseline, she ambulated with a rollator walker, but the pain was starting to limit her ability to walk. She had a history of low back and posterior left buttock pain, but she denied current pain in these regions. She also denied weakness or pain radiation into her lower extremities.
Relevant Physical Examination
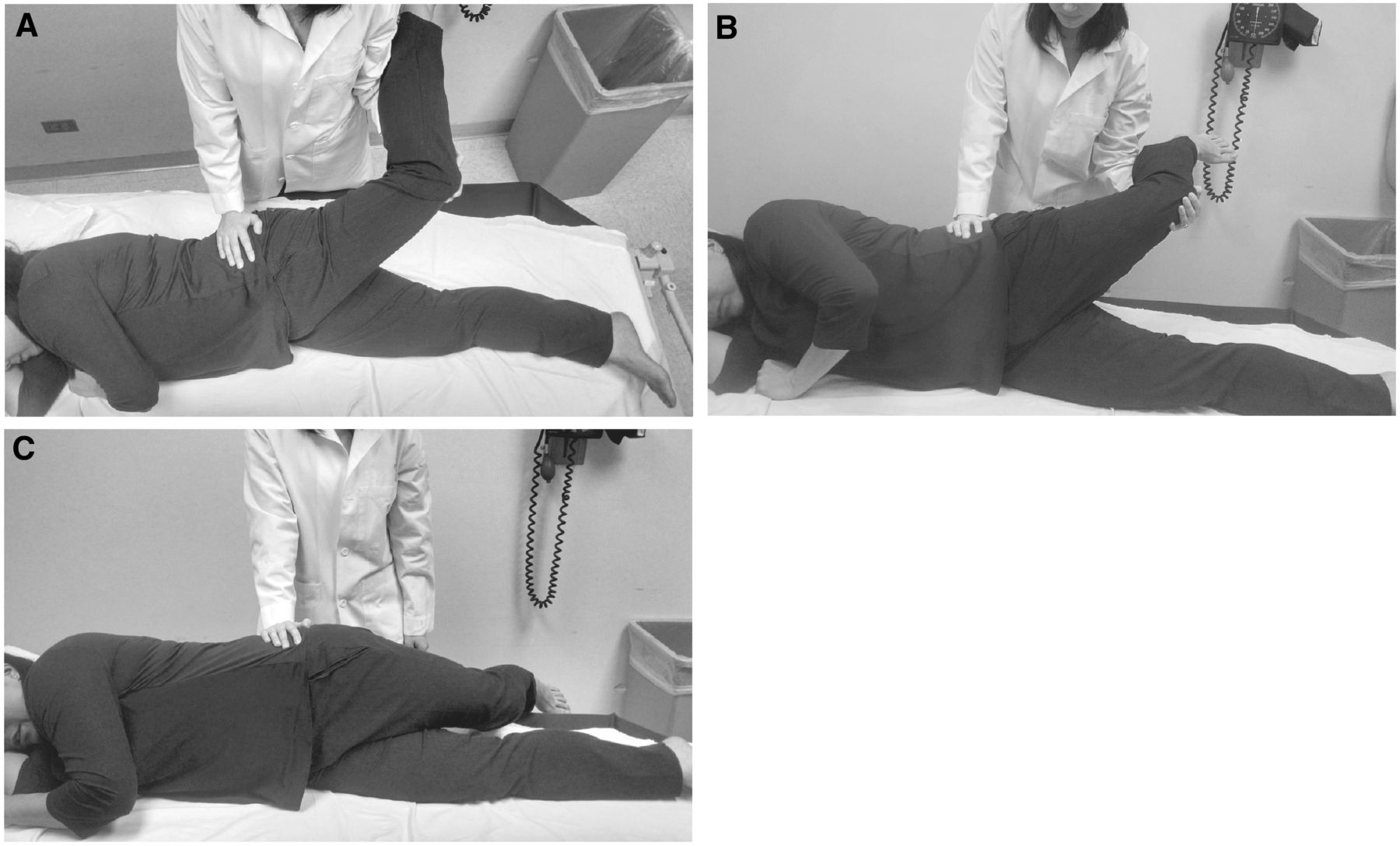
On examination, she had no pain with lumbar or hip range of motion. She had full, symmetric, and pain-free lower extremity strength, and deep tendon reflexes and sensation to light touch were intact and symmetric in her lower extremities. She had a positive Trendelenburg sign with single-leg stance bilaterally, and she was tender to palpation over the left greater trochanter but not the right. Ober’s, seated slump, and straight leg raise tests were negative. (See Figures 2 and 4 for descriptions of Ober’s and the seated slump test.)
Figure 2. Ober’s test:
1) The patient is positioned side-lying, and the leg to be tested is on top;
the pelvis is stabilized perpendicular to the exam table by the examiner’s hand;
2) The knee of the affected leg is passively flexed to 90°, and then the hip
is passively stretched through abduction and extension (to position the
iliotibial band behind the greater trochanter) by the examiner;
(A) shows the view from above and (B) shows the anterior perspective;
3) The knee of the affected leg is allowed to slowly drop by gravity until reaching
its final resting angle.
If the final angle is greater than 0° when compared to the plane of the table
(i.e., the hip is still abducted), Ober’s test is positive; (C) highlights a negative Ober’s test.
Imaging
Focused ultrasound performed by a musculoskeletal physiatrist with ultrasound training revealed evidence of bursitis of the left greater trochanter, which correlated with the patient’s point of maximal tenderness.
Clinical Course
The patient was diagnosed with greater trochanteric pain syndrome (GTPS) that included an inflammatory component as evidenced by bursitis seen on ultrasound. She was educated on hip abductor strengthening exercises, but she was not interested in performing the exercises regularly. Instead she requested a corticosteroid injection, so an ultrasound-guided greater trochanteric bursa injection was performed by the ultrasound-trained physiatrist.
Approach to Evaluation and Management
Optimal management of patients presenting with lateral hip and/or thigh pain requires a systematic approach for accurate diagnosis of underlying pathology contributing to symptoms. This section describes evidence-based diagnostic and therapeutic approaches for the most common causes of lateral hip and thigh pain in older adults.
Greater Trochanteric Pain Syndrome (GTPS)
GTPS is defined as pain and tenderness to palpation over the greater trochanter (GT). [8] Until the early 2000s this entity was most frequently called “greater trochanteric bursitis;” however, ultrasound and magnetic resonance imaging (MRI) studies have shown that approximately 20% of people with tenderness over the GT have imaging-proven bursitis. Non-inflammatory pathology is often seen on imaging, such as gluteal tendonosis, gluteal tendon tears, and/or enthesopathy at the GT. [9, 10] GTPS is often caused by relative weakness of the hip abductor muscles, especially the gluteus medius, which leads to excessive gluteal tendon shearing over the GT and eventual tendonopathy. [11]

Table 2

Table 3 GTPS often coexists with other musculoskeletal lumbopelvic and hip conditions. Additionally, it is often a secondary pain generator resulting from suboptimal biomechanics. In fact, in people with low back pain, GTPS has a prevalence of up to 45% [12–14], and it commonly occurs in people with knee OA, as well. [8] GTPS is more common in women, possibly because they have a wider pelvis structure. [14, 15] It may be associated with nighttime pain when lying on the affected side, pain with prolonged standing, and pain or paresthesias radiating down the lateral thigh along the course of the iliotibial band. [13, 15] Table 2 reviews additional common pain characteristics which can be elicited during the patient history to help distinguish GTPS from other conditions.
Because GTPS is a clinical syndrome, the diagnosis can be confirmed with physical examination. Table 3 describes an efficient method to perform several exam maneuvers to assess for GTPS and other common lumbopelvic and hip conditions. To localize the GT, palpate the proximal lateral thigh of the limb being tested while the patient is standing on both feet and pivoting the leg being tested into hip internal and external rotation. The bony protuberance that is most prominent to the examiner’s touch is the GT. Focal tenderness over this bony protuberance is the definition of GTPS. Another common finding in GTPS is a positive Trendelenburg sign because hip abductor weakness is often the leading biomechanical cause of GTPS. If the patient is able, a positive Trendelenburg can be determined by asking the patient to stand on one leg. If the pelvis contralateral to the stance leg drops, there is weakness of the stance leg hip abductor muscles. This is a positive Trendelenburg sign. If the patient intentionally shifts his/her trunk over the stance leg (thereby elevating the contralateral pelvis) in order to perform the task, this is considered a positive compensated Trendelenburg sign. This finding also corroborates hip abductor weakness of the stance leg. The presence of a Trendelenburg or compensated Trendelenburg sign can help a provider identify biomechanical issues to help direct treatment. However, a Trendelenburg’s sign is positive in 54% of all people with CLBP [12] and it is not a finding specific to the diagnosis of GTPS, so it was not included in the diagnostic algorithm.
In the past, GTPS was commonly treated with corticosteroid injection; however, a recent study suggests that the most effective long-term treatment is hip abductor strengthening [16] in addition to management of any contributing underlying musculoskeletal conditions, such as gluteal tendinopathy. As discussed earlier, only 20% of GTPS cases have inflammatory pathology [9, 10], thus corticosteroids would be expected to provide relief to this small subset of patients. Even in these patients the effect will likely only be temporary if the underlying biomechanical etiology is not also addressed. Furthermore, corticosteroids should be used with caution in older adults because of their well-known systemic side effects such as hypertension, hyperglycemia, increased appetite, edema, immune suppression, behavior and sleep alterations, and, with frequent repeated use, hormonal and bone density effects. Evidence has demonstrated that corticosteroid injections are also toxic to local tenocytes and can potentially contribute to progressive tendonopathy and partial tears. [17–20]
A hip-strengthening program is considered to be first-line treatment for patients with GTPS since it directly addresses the biomechanical cause of pain. If a patient is resistant to engaging in a strengthening program and/or is severely limited by pain from GTPS, if possible, we prefer referral to a musculoskeletal specialist prior to considering treatment with a corticosteroid injection for two reasons. First, using ultrasound or MRI to identify whether bursitis (i.e., active inflammation) is actually present helps the clinician determine how likely the patient is to benefit from an anti-inflammatory medication such as a corticosteroid. (When available and in the hands of an experienced ultrasonographer, ultrasound is superior to MRI because it is faster, cheaper, and provides real-time imaging which can also be used for visualization during injection.) Second, cadaveric studies have demonstrated that there are seven bursae surrounding the GT. Therefore, in order to maximize a clinical response to a corticosteroid injection, it should be performed under ultrasound guidance by an experienced provider into the appropriate bursa demonstrating inflammation. An experienced specialist can use ultrasound to evaluate for bursitis and accurately administer any indicated injection during a regular office visit, without the need for delay to obtain an MRI to confirm inflammation. Also, there is often concomitant gluteal tendinopathy in the form of tendon tears or tendinosis. It is possible that decreasing the inflammation of the bursa will result in incomplete pain relief. Of course, patients receiving a corticosteroid injection should still be encouraged to pursue physical therapy for hip abductor (especially gluteus medius) strengthening in order to minimize the chance for pain recurrence.
Iliotibial Band Syndrome (ITBS)
ITBS usually presents as distal lateral thigh pain, caused by friction from the iliotibial band (ITB), which rubs repeatedly over the lateral femoral epicondyle. The condition most commonly occurs in people who perform repetitive unidirectional activities such as running and cycling, have recently increased their training intensity, and who have relative hip abductor weakness. [21, 22] The gluteus medius muscle is the primary stabilizer of the ITB during foot-strike, therefore weakness of the muscle can result in compensation and overuse of the tensor fascia latae. This, in turn, can lead to a shortened ITB and ITBS. Since similar suboptimal biomechanics predispose to both ITBS and GTPS, the two conditions can co-exist. [8] As a result of the location of the origin and insertion of the ITB, ITBS can also mimic lumbar radicular pain by presenting as lateral thigh pain radiating to the knee. Despite the increasing age of runners [23], it is important to note that ITBS more commonly occurs in younger runners and the syndrome is not commonly described in older adults. Older runners tend to be more limited by calf, Achilles, and hamstring injuries rather than knee injuries. [21]
In the context of pain and tenderness over the lateral femoral epicondyle, a positive Ober’s test is supportive of ITBS. The maneuver has a high inter-rater reliability of 97% and intra-rater reliability of 90%. [24, 25] To perform the test, as demonstrated in Figure 2, the patient is positioned in side-lying with the affected leg on top, and the pelvis is stabilized perpendicular to the exam table by the examiner’s hand. Next, the hip and knee of the affected leg are passively flexed to 90°, and then the examiner passively stretches the hip through abduction and extension in order to position the ITB behind the GT. Finally, the knee of the affected leg is allowed to slowly drop under the weight of gravity until it reaches its final resting angle. If the final angle is greater than 0°, meaning the hip is still abducted, Ober’s test is positive and indicates the patient has a tight ITB. [26] Oftentimes in patients with ITBS, the examiner will also be able to palpate a taut ITB while the patient is standing or supine. Because the diagnosis of this syndrome is clinical, imaging is not necessary unless coexisting lumbar radicular and/or intra-articular hip pathology is suspected.
Similar to the treatment for GTPS, hip abductor strengthening is essential for long-term resolution of ITBS. [27] ITB stretching, in addition to correction of other contributing factors such as core weakness, suboptimal biomechanics, and improper footwear can be helpful, as well. Relative rest, ice, and acetaminophen are appropriate for acute management, but similar to the limitation of corticosteroid injections for GTPS, these treatments are unlikely to provide lasting relief unless the underlying biomechanical imbalance is also addressed.
Hip OA
Symptomatic hip OA is classically thought to present as anterior groin pain, but intra-articular hip pathology can also cause pain in the lateral hip, anterior thigh, knee, low back, buttock, and lower leg [7]. The diagnosis and management of hip OA is discussed in depth in another article in this series [28], but it is still important to consider this diagnosis when older adults present with lateral hip and/or thigh pain. In a study of 369 patients (443 hips) with symptomatic hip OA proven by pain relief after total hip arthroplasty, 27% of these patients presented with lateral hip pain. [7] Pain with passive hip range of motion while the patient is supine should prompt the clinician to consider the possibility of hip OA or other intra-articular hip pathology contributing to the patient’s pain. If the patient is also tender to palpation over the GT, secondary GTPS is likely present and should be managed as detailed above.
Lumbar Radicular Pain
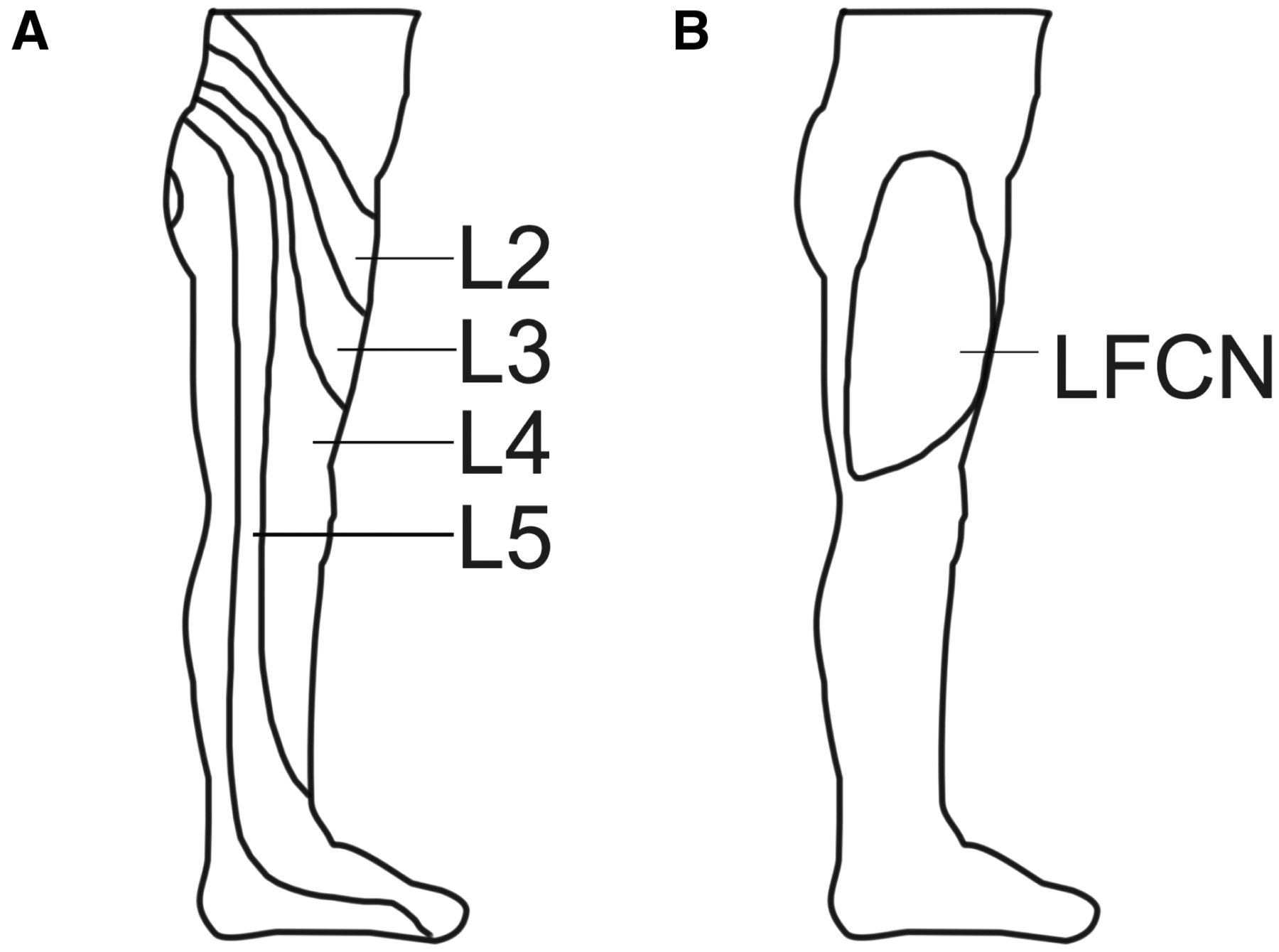
Lateral hip and thigh pain may be referred from irritation of the lumbar nerve roots. [29] Most commonly the L2 through L5 nerve roots refer pain to this distribution, but the L4 and L5 nerve roots are more likely to also refer pain below the knee (see Figure 3A). Based on the path of nerve roots, disc herniations or bulges of any of the L1–2 through L5–S1 intervertebral discs can potentially refer pain to this area. When a patient is describing the location of his/her pain, radiation below the knee is also suggestive, but not definitive for, lumbar radicular pain.
Figure 3. Sensory innervation of the lateral hip and thigh:
Dermatomes L2–L5 (A) and the peripheral lateral femoral
cutaneous nerve (LFCN) (B) provide the primary cutaneous
innervation to the lateral hip and thigh.
Physical examination supportive of lumbar radicular pain involves provocation of pain with isolated movements of the lumbar spine elements, especially the lumbar nerve roots. As an initial screen for lumbar radicular pain, the examiner should ask the patient to perform active lumbar flexion, extension, side-bending, and lateral rotation from a standing position to evaluate whether any of these movements reproduce and/or exacerbate the patient’s lateral hip/thigh pain. For a more specific test, the seated slump test is designed to put selective tension on the lumbar nerve roots. To perform this maneuver, while seated the patient is instructed to slump into cervical, thoracic, and lumbar flexion. The examiner then extends the knee and dorsiflexes the ankle of the affected leg to the patient’s end range of motion (see Figure 4). Of note, older adults often have stiff joints and muscle contractures which limit their passive range of motion, but the test can still be performed effectively. The test is positive if the patient’s typical pain is reproduced with this maneuver and is subsequently relieved with neck extension. [30] Neck extension should result in less tension on the nerve roots but does not affect lumbar vertebral position or lower extremity muscle length, so if the patient’s pain does not abate with neck extension, the pain may be due to myofascial low back pain or lower extremity muscle tightness rather than nerve irritation.
Figure 4. Seated slump test:
1) While seated, the patient is instructed to place her hands behind her back
and then slump into cervical, thoracic, and lumbar flexion;
2) The examiner then extends the knee and dorsiflexes the ankle of the affected leg (A);
3) The test is positive if the patient’s typical pain is reproduced with this maneuver
and is subsequently relieved with neck extension (B).

For lumbar disc herniations, the seated slump test has a sensitivity of 0.84 and specificity of 0.83, which is overall superior to the straight leg raise test (sensitivity of 0.52 and specificity of 0.89. [31] The predictive value of the seated slump test for lumbar radicular pain is not ideal. In patients with a negative seated slump test but high clinical suspicion for radicular pain, conservative management can be pursued despite the negative test. Additionally, the patient can be referred to a non-operative musculoskeletal specialist for further evaluation. Of note, thorough discussion of the femoral nerve stretch test is beyond the scope of this article, but this test has also been shown to have a high likelihood ratio for mid-lumbar nerve root impingement. [32–34]
Management of lumbar radicular pain depends on whether neurologic deficit is present. The definition of lumbar radiculopathy is radicular pain in the setting of focal weakness and/or asymmetrically diminished deep tendon reflexes. Objective sensory impairment may be evident on examination, but it is not a criterion for defining radiculopathy. Electrodiagnostic studies, although not necessary to make the diagnosis, are abnormal in lumbar radiculopathy. Deep tendon reflexes are commonly diminished bilaterally in older adults; therefore, in our algorithm we only recommend pursuing management of radiculopathy if the patient has objective evidence of asymmetric weakness on exam.
Patients with radiculopathy are at risk for progressive and permanent neurologic damage. Therefore, if a clinician is concerned for lumbar radiculopathy based on history and physical exam, the patient would benefit from urgent imaging and referral. Advanced imaging such as lumbar spine MRI (or CT if MRI is contraindicated) is useful to plan interventions such as epidural corticosteroid injections and/or surgical decompression. When referring the patient for specialist care, we recommend initial evaluation by a non-operative musculoskeletal provider if available, because not all radiculopathy requires surgical management. Seventy percent of patients with lumbar radiculopathy will improve with conservative care within 4 weeks of the onset of symptoms [25], and up to 90% of patients with lumbar radiculopathy from a herniated disc (in the absence of significant lumbar spinal stenosis) treated with core stabilization exercises and education eventually achieve good or excellent outcomes. [36, 37] Especially in the aging population, avoiding unnecessary surgical procedures can help minimize associated morbidity and peri-operative complications. Nevertheless, this population will require close follow-up for patients who do not improve and in those with functionally significant weakness that may benefit from surgical decompression within the first 8 weeks of symptom onset. [35]
Contrary to lumbar radiculopathy, lumbar radiculitis describes nerve root irritation without objective findings of nerve root damage (i.e., weakness or reflexes changes). Patients may complain of altered sensation (i.e., pain or pins and needles), but electrodiagnostic testing is normal and therefore not a recommended part of the work-up for lumbar radiculitis. For patients with lumbar radiculitis, we recommend a longer trial of conservative management prior to considering referral because, unlike in radiculopathy, these patients are not at immediate risk of progressive, permanent neurologic damage. For first-line treatment of radiculitis, we recommend McKenzie physical therapy, which is a standardized approach to the assessment and management of radicular pain. Also called Mechanical Diagnosis and Therapy (MDT), in this method McKenzie-certified therapists help patients identify a “directional preference” of lumbar spine motion (i.e., extension, flexion, and/or side-bending) which helps reduce radicular pain, centralize the pain to the back, and minimize pain altogether. McKenzie therapy teaches simple exercises which the patient should perform several times throughout the day, and it does not emphasize passive modalities such as manual massage or manipulation. [38] Patients who respond well to McKenzie therapy are likely to be able achieve adequate pain relief without operative intervention. [39]
For radicular pain, whether from radiculitis or radiculopathy, we only recommend a short course of oral corticosteroids (such as a 6-day methylprednisolone taper) if a patient has incapacitating pain interfering with functioning and/or engaging in physical therapy. For lumbar disc herniation, oral corticosteroids have been shown to offer only modest improvement in function and have not been proven to reduce pain. [40] In older adults, the systemic side effects are often limiting and should not be prescribed without carefully considering the risks and benefits and involving the patient and/or caregiver in the decision-making process.
Another treatment option for patients with incapacitating pain limiting function is fluoroscopically-guided epidural corticosteroid injections. Full discussion of the indications and expected efficacy of these injections is beyond the scope of this paper, but it is important to appreciate that these injections must be utilized thoughtfully because in an improperly selected older patient, there is a risk for significant side effects from the procedure itself and from epidural corticosteroid administration, potentially without a high likelihood for clinical improvement. Based on a modified Delphi method, our expert panel believes that best practice is for primary care clinicians who are considering the use of an injection to first refer the patient to a non-operative musculoskeletal specialist (such as a physiatrist, anesthesia pain physician, or sports medicine physician), prior to ordering the injection, in order to evaluate for proper patient selection.
Lumbar Spinal Stenosis (LSS)
LSS is another common condition in older adults and can cause positional-dependent nerve root irritation due to degenerative changes resulting in narrowing of the spinal canal. The most common presenting symptom of LSS is progressive pain down the leg with continued walking that improves with sitting. In a study by Kalichman et al., up to 20% of 60–69–year-olds had CT-evidence of LSS, and while not everyone with the finding was symptomatic, people with imaging-diagnosed LSS were over three times more likely to have LBP. [41] When symptomatic, 42% of patients with LSS present with lateral thigh pain. [29] The diagnosis of LSS is important to consider when older adults present with lateral hip or thigh pain that improves by functionally increasing the spinal canal area with lumbar flexion. [42] Management of LSS is discussed in Part VI of this series. [43]
Meralgia Paresthetica (MP)
In meralgia paresthetica, the lateral femoral cutaneous nerve (LFCN) is compressed, which results in sensory abnormalities along the lateral and anterolateral thigh. Since there is no motor component to the nerve, MP does not cause weakness. MP has been associated with metabolic factors such as diabetes mellitus and alcoholism, in addition to mechanical factors such as obesity, tight-fitting clothing around the waist (e.g., uniforms, jeans, belts, etc.), and lumbopelvic or lower extremity surgery as a result of direct trauma or positioning during surgery. [44]
Clinically, MP is less common and tends to present much differently than the other conditions discussed thus far. Patients with MP often complain of numbness, tingling, and/or itching, more so than pain, and this is reflected in the algorithm in Figure 1. Additionally, whereas patients with lumbar radicular pain usually have difficulty describing the exact region of sensory symptoms, patients with MP can often precisely trace the affected cutaneous distribution of the LFCN (see Figure 3B). On physical exam, MP may cause dysesthesias over the entire anterolateral thigh, but it does not cause frank tenderness over the GT like GTPS does. It is important to note that as the prevalence of obesity rises, MP can co-exist with the other conditions that cause lateral hip and thigh pain.
To confirm the diagnosis of MP, patients can be referred for a nerve conduction study, but this test is technically difficult, particularly in obese individuals. Ultrasound, when performed by an experienced ultrasonographer [45], and magnetic resonance neurography (MRN) can also be useful diagnostically by detecting morphologic changes to the LFCN in people with MP. [44]
Conservative management of MP involves losing weight, loosening clothing around the waist, and avoiding positions such as excessive hip extension, which can compress the LFCN under the inguinal ligament or elsewhere along its course. Additionally, KinesioTaping can be considered. [44] If conservative measures do not result in symptom relief, patients can be referred to an interventional pain specialist for possible LFCN diagnostic nerve block, neurolysis, and/or neurectomy. [46]
Resolution of Case
The patient experienced significant pain relief from the corticosteroid injection for a few weeks. However, 5 weeks after the injection her lateral hip pain returned, now with pain radiating into her left lateral thigh, knee, and lateral lower leg. On repeat exam, she now had tenderness over her left ITB, mild pain with passive hip range of motion and lumbar extension, and a positive seated slump test on the left. At this time, she was felt to have GTPS and ITBS with superimposed lumbar radicular pain. Ultrasound confirmed the recurrence of bursitis in the GT. She continued to be resistant to physical therapy, and 1 month later her pain had not improved so she requested a repeat ultrasound-guided greater trochanteric bursa corticosteroid injection.
The clinical course of this case highlights that corticosteroid injections for GTPS, even when an inflammatory bursitis is present, usually only provide temporary relief, and more definitive management of hip abductor strengthening must be strongly encouraged. In the setting of true bursitis and severe pain, a corticosteroid injection can help facilitate active engagement in a rehabilitation program. However, in older adults who are at risk for co-morbidities such as osteoporosis, hypertension, diabetes, and delirium, minimizing the use of corticosteroids is advantageous. This case also demonstrates that evaluation of lateral hip and thigh pain is often not straightforward. In this particular patient, she likely developed secondary lumbar pain, possibly in part due to compensatory mechanical changes in an attempt to relieve pain from her sub-optimally treated GTPS.
Summary
The evaluation of lateral hip and thigh pain in an older adult can be challenging. Potential etiologies include local and referred causes, and clinicians must consider a myriad of lumbopelvic and hip conditions such as GTPS, ITBS, hip OA, lumbar radicular pain, and LSS. Identifying a patient’s true pain generator(s) enables initiation of proper treatment and avoidance of unnecessary treatments and their potential adverse effects. Therefore, if a patient is not responding adequately to the conservative measures described in the algorithm, we recommend considering referral to a non-operative musculoskeletal specialist, who has expertise in evaluating the entire lumbopelvic region. We do not recommend prescribing opioids before the patient has completed a trial of conservative management and has been evaluated by a specialist as these medications often place the patient at risk of adverse drug reactions. These side effects may be avoided if the underlying etiology of the pain is addressed.
Perhaps the most common local cause of lateral hip and thigh pain in older adults is GTPS. Gluteus medius weakness is often a significant contributor to the development of GTPS. As a result, hip abductor strengthening exercises should be the first-line treatment of this syndrome in conjunction with medication. While medications and modalities may provide temporary symptomatic relief, pain will likely recur if the underlying weakness is not addressed. Additionally, since GTPS and other lumbopelvic conditions often coexist, it is important to perform a thorough history and physical examination to evaluate for the possibility of multiple pain generators, as well.
Key Points
Lateral hip and/or thigh pain in the older adult with CLBP can be from
a number of causes, with those most common being greater
trochanteric pain syndrome (GTPS), iliotibial band syndrome
(ITBS), hip osteoarthritis, lumbar radiculopathy/radiculitis,
and lumbar spinal stenosis. Meralgia paresthetica also should
be considered in the differential diagnosis. Often lateral hip
and/or thigh pain is multifactorial.Greater trochanteric pain syndrome (GTPS) is a common cause of lateral
hip pain and is usually due to weakness of the gluteus medius muscle.
Hip abductor strengthening is the first-line treatment for GTPSTrue GT bursitis causing GTPS occurs in a minority of cases; therefore,
corticosteroid injections should be used sparingly, and ideally only
when there is imaging evidence of bursitis. Injection should always be
accompanied by a hip abductor strengthening program.In older adults with CLBP, iliotibial band syndrome (ITBS) is less commonly
described than GTPS. However, hip abductor strengthening is part of
the first-line treatment for both GTPS and ITBS.Lumbar radiculopathy (i.e., pain?+?weakness) requires aggressive evaluation
and treatment to avoid progressive and permanent neurologic damage.
Lumbar radiculitis (pain without weakness) can be evaluated and
managed more conservatively.
Acknowledgments
The authors wish to thank Dave Newman for his valuable assistance in preparing the manuscript.
Funding sources:
This material is based on work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service.
Disclosure and conflicts of interest:
The contents of this report do not represent the views of the Department of Veterans Affairs or the United States Government. The authors have no conflicts of interest to report.
References:
Birnbaum K, Prescher A, Hessler S, Heller K. The sensory innervation of the hip joint–an anatomical study. Surg Radiol Anat 1997;19(6):371–5
Clohisy JC, Knaus ER, Hunt DM, et al. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res 2009;467(3):638–44
Sembrano JN, Polly DW. Jr.. How often is low back pain not coming from the back? Spine 2009;34(1):E27–32
Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990;72(3):403–8
Lane NE, Nevitt MC, Hochberg MC, Hung YY, Palermo L. Progression of radiographic hip osteoarthritis over eight years in a community sample of elderly white women. Arthritis Rheum 2004;50(5):1477–86
Weiner DK.
Deconstructing Chronic Low Back Pain in the Older Adult -
Shifting the Paradigm from the Spine to the Person
Pain Med 2015; 16 (5): 881–885Nakamura J, Inuma K, Ohtori S, et al. Distribution of hip pain in osteoarthritis patients secondary to developmental dysplasia of the hip. Mod Rheumatol 2013;23(1):119–24
Segal NA, Felson DT, Torner JC, et al. Greater trochanteric pain syndrome: Epidemiology and associated factors. Arch Phys Med Rehabil 2007;88(8):988–92
Klontzas ME, Karantanas AH. Greater trochanter pain syndrome: A descriptive MR imaging study. Eur J Radiol 2014;83(10):1850–5
Long SS, Surrey DE, Nazarian LN. Sonography of greater trochanteric pain syndrome and the rarity of primary bursitis. AJR Am J Roentgenol 2013;201(5):1083–6
Klauser AS, Martinoli C, Tagliafico A, et al. Greater trochanteric pain syndrome. Semin Musculoskelet Radiol 2013;17(1):43–8
Cooper NA, Scavo KM, Strickland KJ, et al. Prevalence of gluteus medius weakness in people with chronic low back pain compared to healthy controls. Eur Spine J 2016;25(4):1258–65
Collee G, Dijkmans BA, Vandenbroucke JP, Rozing PM, Cats A. A clinical epidemiological study in low back pain. Description of two clinical syndromes. Br J Rheumatol 1990;29(5):354–7
Tortolani P, Carbone J, Quartararo L. Greater trochanteric pain syndrome in patients referred to orthopedic spine specialists. Spine 2002;4:251–4.
Mulligan EP, Middleton EF, Brunette M. Evaluation and management of greater trochanter pain syndrome. Phys Ther Sport 2015;16(3):205–14
Rompe JD, Segal NA, Cacchio A, Furia JP, Morral A, Maffulli N. Home training, local corticosteroid injection, or radial shock wave therapy for greater trochanter pain syndrome. Am J Sports Med 2009;37(10):1981–90
Dean BJ, Lostis E, Oakley T, et al. The risks and benefits of glucocorticoid treatment for tendinopathy: A systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum 2014;43(4):570–6
Wong MW, Tang YN, Fu SC, Lee KM, Chan KM. Triamcinolone suppresses human tenocyte cellular activity and collagen synthesis. Clin Orthop Relat Res 2004;421:277–81
Scutt N, Rolf CG, Scutt A. Glucocorticoids inhibit tenocyte proliferation and Tendon progenitor cell recruitment. J Orthop Res 2006;24(2):173–82
Wong MW, Tang YY, Lee SK, Fu BS. Glucocorticoids suppress proteoglycan production by human tenocytes. Acta Orthop 2005;76(6):927–31
McKean KA, Manson NA, Stanish WD. Musculoskeletal injury in the masters runners. Clin J Sport Med 2006;16(2):149–54
Fredericson M, Weir A. Practical management of iliotibial band friction syndrome in runners. Clin J Sport Med 2006;16(3):261–8
Fields KB. Running injuries—Changing trends and demographics. Curr Sports Med Rep 2011;10(5):299–303
Ferber R, Kendall KD, McElroy L. Normative and critical criteria for iliotibial band and iliopsoas muscle flexibility. J Athl Train 2010;45(4):344–8
Reese NB, Bandy WD. Use of an inclinometer to measure flexibility of the iliotibial band using the Ober test and the modified Ober test: Differences in magnitude and reliability of measurements. J Orthop Sports Phys Ther 2003;33(6):326–30
Wang TG, Jan MH, Lin KH, Wang HK. Assessment of stretching of the iliotibial tract with Ober and modified Ober tests: An ultrasonographic study. Arch Phys Med Rehabil 2006;87(10):1407–11
Fredericson M, Cookingham CL, Chaudhari AM, et al. Hip abductor weakness in distance runners with iliotibial band syndrome. Clin J Sport Med 2000;10(3):169–75
Weiner DK Fang M Gentili A , et al.
Deconstructing Chronic Low Back Pain in the Older Adult - Step by Step
Evidence and Expert-Based Recommendations for Evaluation and Treatment.
Part I: Hip osteoarthritis
Pain Med 2015; 16 (5): 886–897Rainville J, Lopez E. Comparison of radicular symptoms caused by lumbar disc herniation and lumbar spinal stenosis in the elderly. Spine 2013;38(15):1282–7.
Cleland J., Netter’s Orthopaedic Clinical Examination: An Evidence-Based Approach. 2nd edition. Philadelphia: Saunders/Elsevier; 2011.
Majlesi J, Togay H, Unalan H, Toprak S.. The sensitivity and specificity of the slump and the straight leg raising tests in patients with lumbar disc herniation. J Clin Rheumatol 2008;14(2):87–91
Suri P, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine 2011;36(1):63–73
Kobayashi S, Suzuki Y, Asai T, Yoshizawa H. Changes in nerve root motion and intraradicular blood flow during intraoperative femoral nerve stretch test. J Neurosurg 2003;99(suppl 3):298–305.
Christodoulides AN. Ipsilateral sciatica on femoral nerve stretch test is pathognomonic of an L4/5 disc protrusion. J Bone Joint Surg Br 1989;71(1):88–9.
Alentado VJ, Lubelski D, Steinmetz MP, Benzel EC, Mroz TE. Optimal duration of conservative management prior to surgery for cervical and lumbar radiculopathy: A literature review. Global Spine J 2014;4(4):279–86
Saal JA, Saal JS. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy. An outcome study. Spine 1989;14(4):431–7
Saal JS, Saal JA. Physical medicine and rehabilitation: Lumbar stabilizing exercises for the nonoperative treatment of disc lesions. West J Med 1990;153(4):432
McKenzie R., The Lumbar Spine: Mechanical Diagnosis and Therapy. Waikanae, New Zealand: Spinal Publications; 1981.
Wetzel FT, Donelson R. The role of repeated end-range/pain response assessment in the management of symptomatic lumbar discs. Spine J 2003;3(2):146–54
Goldberg H, Firtch W, Tyburski M, et al. Oral steroids for acute radiculopathy due to a herniated lumbar disk: A randomized clinical trial. JAMA 2015;313(19):1915–23.
Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: The Framingham Study. Spine J 2009;9(7):545–50
Inufusa A, An HS, Lim TH, et al. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine 1996;21(21):2412–20
paresthetica: A review of the literature. Int J Sports Phys Ther 2013;8(6):883–93
Suh DH, Kim DH, Park JW, Park BK. Sonographic and electrophysiologic findings in patients with meralgia paresthetica. Clin Neurophysiol 2013;124(7):1460–4
de Ruiter GC, Kloet A. Comparison of effectiveness of different surgical treatments for meralgia paresthetica: Results of a prospective observational study and protocol for a randomized controlled trial. Clin Neurol Neurosurg 2015;134:7–11

Return to LOW BACK PAIN
Return to McKENZIE METHOD
Since 8-30-2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |