

Antiestrogenic Glyceollins Suppress Human Breast
and Ovarian Carcinoma TumorigenesisThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Clin Cancer Res. 2006 (Dec 1); 12 (23): 7159–7164 ~ FULL TEXT
Virgilo A. Salvo1, Stephen M. Boué, Juan P. Fonseca1, Steven Elliott, Cynthia Corbitt, Bridgette M. Collins-Burow, Tyler J. Curiel1,, Sudesh K. Srivastav, Betty Y. Shih, Carol Carter-Wientjes, Charles E. Wood, Paul W. Erhardt, Barbara S. Beckman, John A. McLachlan, Thomas E. Cleveland and Matthew E. Burow
Section of Hematology and Medical Oncology,
Department of Medicine,
Tulane Cancer Center,
New Orleans, LA 70112, USAPURPOSE: We have identified the phytoalexin compounds glyceollins I, II, and III, which exhibit marked antiestrogenic effects on estrogen receptor function and estrogen-dependent tumor growth in vivo. The purpose of this study was to investigate the interactions among the induced soy phytoalexins glyceollins I, II, and III on the growth of estrogen-dependent MCF-7 breast cancer and BG-1 ovarian cancer cells implanted in ovariectomized athymic mice.
EXPERIMENTAL DESIGN: Four treatment groups for each cell line were used: vehicle control, 20 mg/kg/mouse/d glyceollin mixture injection, 0.72 mg estradiol (E2) implant, and E2 implant + 20 mg/kg/mouse/d glyceollin injection.
RESULTS: Treatment with glyceollin suppressed E2-stimulated tumor growth of MCF-7 cells (-53.4%) and BG-1 cells (-73.1%) in ovariectomized athymic mice. These tumor-inhibiting effects corresponded with significantly lower E2-induced progesterone receptor expression in the tumors. In contrast to tamoxifen, the glyceollins had no estrogen-agonist effects on uterine morphology and partially antagonized the uterotropic effects of estrogen.
CONCLUSIONS: These findings identify glyceollins as antiestrogenic agents that may be useful in the prevention or treatment of breast and ovarian carcinoma.
From the FULL TEXT Article:
Discussion:
Antiestrogen therapy has been shown to prevent premenopausal and postmenopausal breast cancer and to be a beneficial adjuvant therapy for women with estrogen receptor (ER)–positive tumors [1–3]. Tumors ultimately develop resistance, however, and certain antiestrogens, such as tamoxifen, can increase the risk of endometrial cancer [3, 4]. Consequently, efforts have been made to develop new antiestrogens from both synthetic and natural sources. Many naturally occurring agents, particularly flavonoids, have shown chemopreventive and anticancer potential in a variety of in vitro and in vivo models [5–12]. The isoflavone genistein has received much attention over the last few years as a potential anticancer agent due to its wide-range effects on a number of cellular processes [5–12]. The potential chemopreventive effects of genistein and other flavonoids have spurred research to discover other naturally occurring flavonoids in soybean and other plants with anticancer activities.
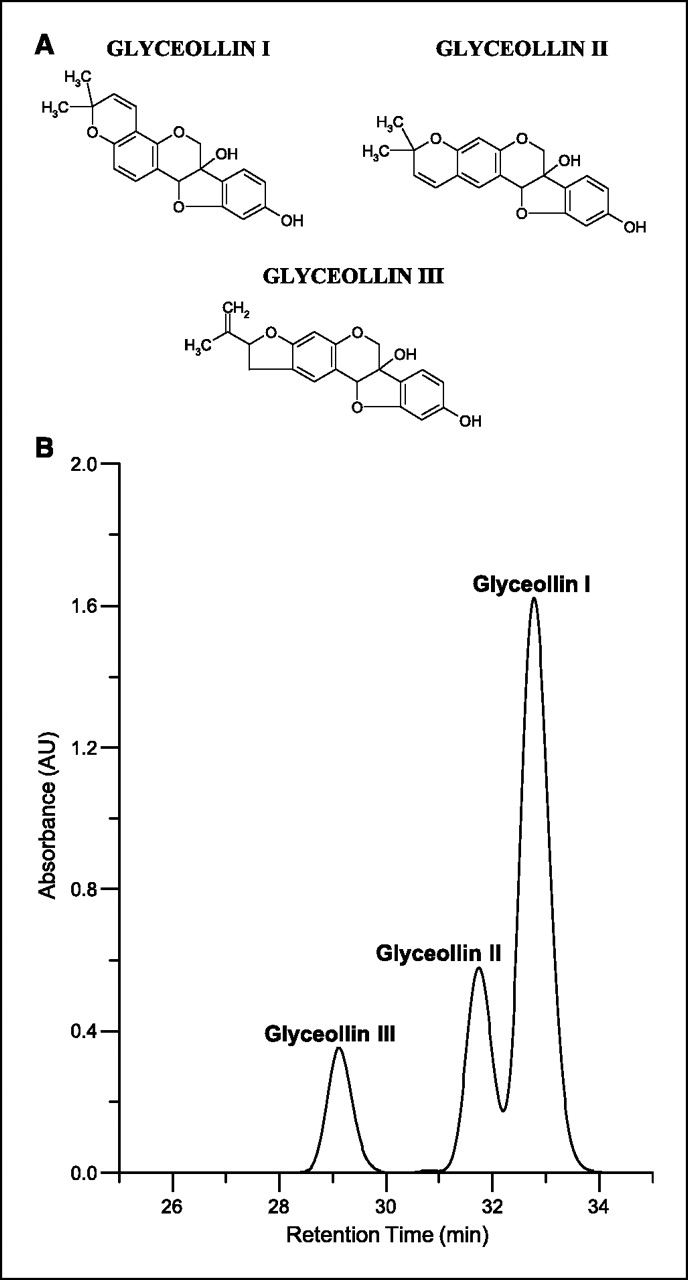
Figure 1 Phytoalexins are low-molecular-weight antimicrobial compounds that are synthesized de novo and accumulate in plants in response to infection or stress [13, 14]. Numerous stress factors and physical stimuli induce phytoalexin accumulation, including wounding, freezing, UV light exposure, and exposure to microorganisms. In addition, compounds referred to as elicitors, either abiotic or biotic, can stimulate the biosynthesis of phytoalexins [13–20]. Glyceollins are one the primary groups of phytoalexins produced in soybeans under conditions of stress [15, 19, 20]. The glyceollins (mixture of glyceollin I, II, and III; Figure 1A) have been produced in high concentrations using several elicitors and have antimicrobial activity against several plant pathogens [13–16, 19]. However, only trace levels of the glyceollins are observed in soybean seeds, and they have not been observed in soy food products.
We showed previously in cell culture studies that glyceollins have a marked antiestrogenic effect on ER signaling, including suppression of direct 17β-estradiol (E2) binding to the ER and inhibition of E2-induced proliferation [21]. No estrogenic activity was observed with the glyceollins in vitro, in contrast to the other major soy isoflavones. In the current study, we sought to extend these findings to an in vivo model. Here, we report the inhibitory effects of the glyceollins on the growth of human ER-positive breast cancer (MCF-7) and ovarian cancer (BG-1) cells injected s.c. in ovariectomized athymic nude mice.
Materials and Methods
Glyceollin mixture. A mixture of glyceollins I, II, and III were isolated using a procedure developed at the Southern Regional Research Center (Agricultural Research Service, U.S. Department of Agriculture, New Orleans, LA). Soybean seeds (1 kg) were sliced and inoculated with Aspergillus sojae. After 3 days, the glyceollins were extracted from the inoculated seeds with 1 liter methanol. The glyceollins were isolated using preparative-scale high-power liquid chromatography (HPLC) using two Waters 25-mm, 10-µm particle size µBondapak C18 radial compression column segments combined using an extension tube. HPLC was done on a Waters 600E System Controller combined with a Waters UV-VIS 996 detector. Elution was carried out at a flow rate of 8.0 mL/min with the following solvent system: A = acetonitrile, B = water; 5% A for 10 minutes, then 5% A to 90% A in 60 minutes followed by holding at 90% A for 20 minutes. The injection volume was 20 mL. The fraction containing the glyceollins was concentrated under vacuum and freeze-dried. The glyceollins were confirmed by UV-VIS spectrophotometry. A mixture of glyceollins I (68%), II (21%), and III (11%) were isolated (see Fig. 1B) and used in animal testing. The solvents acetonitrile (HPLC grade) and methanol were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Water was obtained using a Millipore system and used during sample preparation procedures and HPLC analyses.
Soybean treatment and harvesting. A. sojae (SRRC 1125) cultures were grown at 25°C in the dark on potato dextrose agar. After 5 days, inoculum was prepared by harvesting conidia (3.4 × 107/mL) in 15-mL sterile distilled H2O. Seeds from commercial soybean variety Asgrow 5902 were surface-sterilized for 3 minutes in 70% ethanol followed by a quick deionized water rinse and two 2 minutes rinses in deionized water. Seeds were presoaked in sterile deionized water for 4 to 5 hours and then chopped for 2 minutes in a Cuisinart food processor. A. sojae spore suspension (300 µL) was applied to the cut surface of seeds on each tray. All trays were stored at 25°C in the dark for 3 days, rinsed with water to remove spores, and oven-dried at 40°C for 24 hours. Seeds were ground using a Waring blender before extraction.
Cells and reagents. The MCF-7N cell variant is a subclone of MCF-7 cells from the American Type Culture Collection (Manassas, VA) and has been previously described [21]. The BG-1 cell line has also been previously described and was generously provided by Dr. Diane Klotz (NIEHS, Research Triangle Park, NC; ref. 22). MCF-7 and BG-1 cells were grown in DMEM (pH 7.4; Invitrogen Corp., Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone, Salt Lake City, UT). Cells were incubated at 37°C in an atmosphere of 5% CO2 and air.
Animals. Nu/nu immune-compromised female ovariectomized mice (29-32 days old) were obtained from Charles River Laboratories (Wilmington, MA). The animals were allowed a period of adaptation in a sterile and pathogen-free environment with phytoestrogen-free food and water ad libitum. Mice were divided into four treatment groups of five mice each: control (con), estradiol only (E2), glyceollin mixture only (Gly), and estradiol plus glyceollin mixture (E2 + Gly). Placebo or estradiol pellets (0.72 mg, 60-day release; Innovative Research of America, Sarasota, FL) were implanted s.c. in the lateral area of the neck in the middle point between the ear and shoulder using a precision trochar (10 gauge). MCF-7 and BG-1 cells in the exponential phase of growth were harvested using PBS/EDTA solution and washed. Viable cells (5 × 106) in a 50-µL sterile PBS suspension were mixed with 100 µL Matrigel Reduced Factors (BD Biosciences, Bedford, MA). MCF-7 cells were injected on the right side and the BG-1 cells on the left side of the mammary fat pad through a 5-mm incision at the hypogastrium area, and the incision was closed using staples. All the procedures in animals were carried out under anesthesia using a mix of isofluorane and oxygen delivered by mask.
The glyceollin mixture was suspended in a solution of DMSO (one-third volume) and propylene glycol (two-third volume) and was given s.c. in the dorsal area at 20 mg/kg/mouse/d for 20 days to Gly and E2 + Gly groups starting on the same day of tumor implantation (day 1). Con and E2 groups were injected with vehicle daily for 20 days. Tumor size was measured every 2 days using a digital caliper. The volume of the tumor was calculated using the following formula: 4/3?LM2, where L is the larger radius, and M is the smaller radius. At necropsy on day 21, animals were euthanized by decapitation after exposure to a CO2 chamber. Tumors, uteri, brain, livers, and lungs were removed and either frozen in liquid nitrogen or fixed in 10% formalin for further analysis. All procedures involving these animals were conducted in compliance with State and Federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by the Tulane University Animal Care and Use Committee. The facilities and laboratory animal program of Tulane University are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Immunohistochemistry. Tumor explants were collected at necropsy, fixed in 70% ethanol, embedded in paraffin, and immunostained using a primary monoclonal antibody for human progesterone receptor (PR; NCL-PGR, Novocastra, Newcastle-upon-Tyne, United Kingdom). Expression of PR is up-regulated through ER-mediated pathways and thus serves as a marker of estrogen exposure within target tissues [23]. Staining methods included antigen-retrieval with citrate buffer (pH 6.0), biotinylated rabbit anti-mouse Fc antibody as a linking reagent, alkaline phosphatase–conjugated streptavidin as the label, and Vector Red as the chromogen (Vector Laboratories, Burlingame, CA). Cell staining was quantified by a computer-assisted counting technique, using a grid filter to select cells for counting [24, 25]. At least two microscopic fields were randomly selected for each tumor, and 200 cells were counted at ×20 magnification. Numbers of positively stained cells were expressed as a percentage of the total number examined. All measurements were made blinded to treatment group.
Uterine morphology. Uteri were removed at necropsy, weighed, fixed in 10% formalin for 24 hours, transferred to 70% ethanol, sectioned transversely through each uterine horn, embedded in paraffin, and stained with H&E by routine procedures. Slides were photographed at ×2 and ×40 magnification using a Nikon CoolPix E995 digital camera (Melville, NY). Uterine area, thickness, and epithelial height were measured from digital images using public domain software (NIH Image v1.62; available at http://rsb.info.nih.gov/nih-image/download.html). For epithelial height, three separate measurements were taken, and the average was used for each animal. H&E-stained uteri were also evaluated qualitatively for histologic changes.
Statistics. Longitudinal data for MCF-7 and BG-1 tumor size were analyzed separately using a linear mixed models approach for repeated measure data. The model allowed implementation of random effects in the statistical model and the covariance structure for variances at individual times and correlation between measures at different times on the same tumors. Descriptive results, such as mean, SE, etc., were calculated for all studied variables. The differences between estimated means for all treatments averaged over days and at each day were compared. All statistical analyses were done, assuming 5% level of significance, with the Statistical Analysis Software 9.1 (SAS Institute, Cary, NC).
Data for PR expression and uterine morphology were subjected to one-way ANOVA. Variables were evaluated for their distribution and equality of variances between groups. Overall statistical significance levels were set at P < 0.05, but critical P levels for individual pairwise comparisons were adjusted for the number of comparisons. All data are reported as treatment group means ± SE.
Results
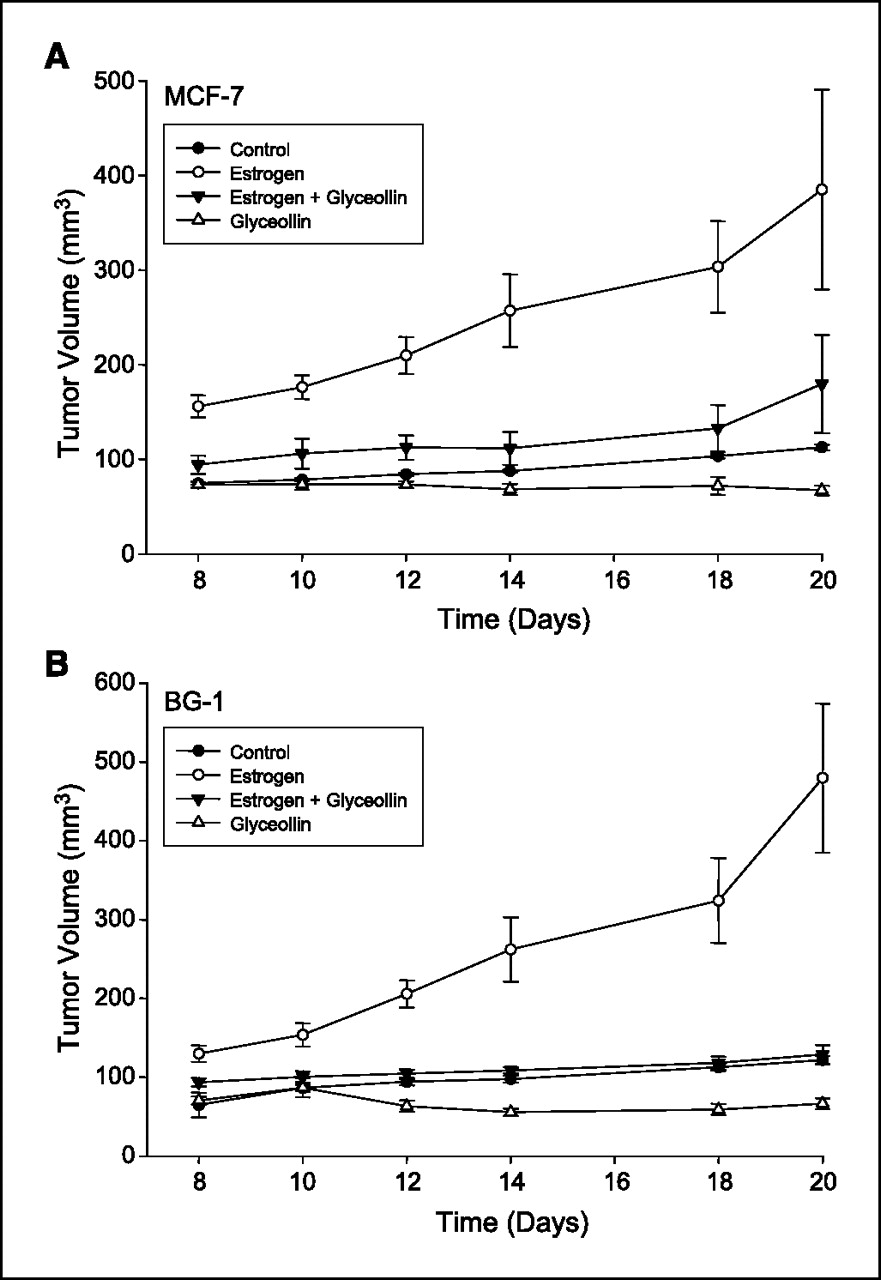
Figure 2 Tumor growth in MCF-7 and BG-1 xenograft mice. Measurable tumors for both MCF-7 cells (Fig. 2A ) and BG-1 cells (Figure 2B) were observed 8 days after tumor implantation in all treatment groups. For tumor size data spanning treatment days 8 to 20, all groups differed significantly from the E2 only group (all comparisons, P < 0.005). The comparison of E2 + Gly versus Gly over the treatment period was close to significantly different for MCF7 tumors (P = 0.0891) and less so for BG1 tumors (P = 0.1504). Other comparisons over treatment days 8 to 20 had Ps as follows: control versus E2 + Gly, P = 0.2416 for MCF7 and P = 0.6486 for BG1; control versus Gly alone, P = 0.5139 for MCF7 and P = 0.3077 for BG1. In the negative control animals (without estrogen pellets or glyceollin mixture), tumors grew slowly with a maximal size of 113 ± 3 mm3 for MCF-7 cells and 122 ± 5 mm3 for BG-1 cells on day 20 of treatment. In contrast, estrogen stimulated the formation of rapidly growing tumors in both MCF-7 and BG-1 tumor cells; tumors in the E2 group were statistically significantly larger than tumors in all other groups on each day measured (usually P < 0.001 on any given measurement day), reaching a maximal size of 385 ± 106 mm3 in MCF-7 and 480 ± 95 mm3 in BG-1 implanted animals by day 20 of treatment. Glyceollin treatment suppressed the growth of MCF-7 and BG-1 tumors in the presence and absence of estrogen. Treatment with glyceollin mixture suppressed estrogen-stimulated tumor growth to be not significantly different from negative control levels with a size of 180 ± 52 mm3 for MCF-7 (control versus E2 + Gly, day 20, P = 0.3649) and 129 ± 12 mm3 for BG-1 (control versus E2 + Gly, day 20, P = 0.9162) on day 20 of treatment. In the absence of E2, glyceollin mixture stabilized growth of tumors to 67 ± 5 mm3 in MCF-7 cells and 67 ± 7 mm3 in BG-1 tumors, in both cases suppressing growth to below negative control levels, although comparisons were not statistically significant.

Figure 3
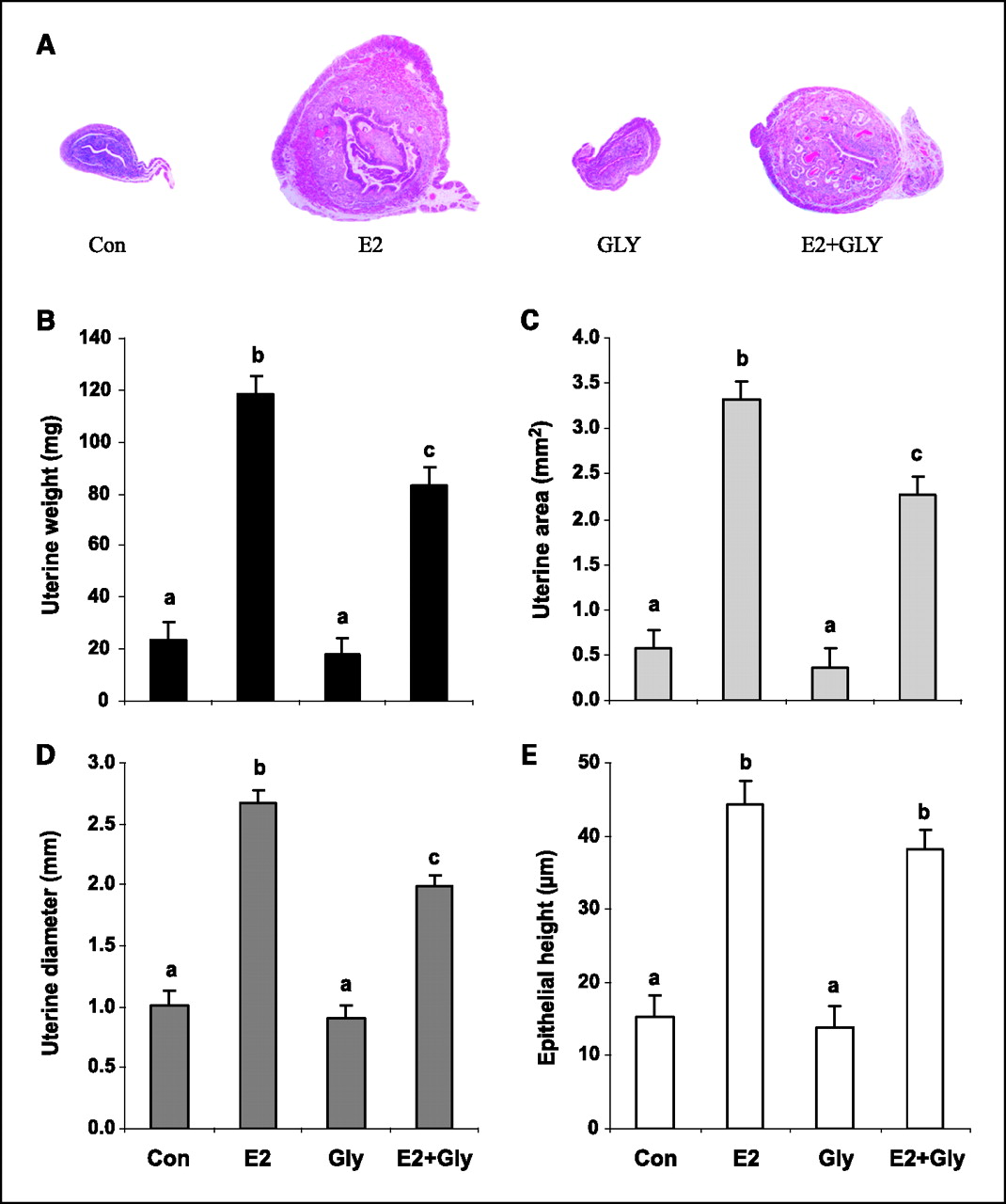
Figure 4 PR expression. PR expression is an estrogen-sensitive marker induced by ER transactivation [23]. In this study, we used PR immunostaining to evaluate whether glyceollin treatment was acting as an estrogen agonist when given alone and/or an estrogen antagonist when given with E2. In MCF-7 and BG-1 xenografts, E2 treatment significantly increased PR expression (Con versus E2, P < 0.05), whereas glyceollin mixture alone did not (Con versus Gly, P = 0.92; Figures 3A and B). When given with E2, treatment with glyceollin mixture completely abolished E2 effects on PR expression in MCF-7 tumors (E2 versus E2 + Gly, P < 0.05) and partially antagonized E2 effects in BG1 tumors (Con versus E2 + Gly, P = 0.16; Fig. 3A).
Uterine morphology. Mouse uteri were also evaluated to assess glyceollin effects on other reproductive tissues. E2 significantly increased uterine weight, area, diameter, and epithelial area (Con versus E2, P < 0.0001 for all), whereas glyceollin treatment had no significant effects on these measures (Con versus Gly, P > 0.4 for all; Figure 4). However, glyceollins partially antagonized the effects of E2 on uterine weight, area, and diameter (E2 versus E2 + Gly, P < 0.01). On histology, uteri from control and glyceollin-treated animals were diffusely atrophic and could not be distinguished morphologically. Superficial epithelial cells were predominantly cuboidal with round to oval nuclei, whereas stromal cells were densely packed with scant cytoplasm. In contrast, uteri from E2-treated mice were markedly larger with epithelial pseudostratification and hyperplasia, stromal edema, and increased numbers of endometrial glands. Uteri from E2 + Gly animals exhibited attenuated E2-induced features (Fig. 4A).
Discussion:
Breast cancer afflicts –1 in 8 women and represents a leading cause of cancer-related mortality. Of the breast cancers diagnosed, –50% to 60% will be positive for ER expression and, therefore, potentially susceptible to endocrine therapy. The development of endocrine therapeutic agents, such as the antiestrogen tamoxifen and fulvestrant, represented a major advance in the treatment of ER+ breast carcinoma. Although tamoxifen exerts antiestrogenic effects upon breast tissue, it exhibits estrogenic activity in the bone, cardiovascular, and endometrial tissues [1–4]. In addition, de novo or developed resistance to current therapeutics represents a major obstacle in the treatment of breast cancer, which diminishes the effectiveness of endocrine therapy. Therefore, there is a critical need to identify novel antiestrogenic agents useful in both the therapy of breast carcinoma as well as potentially chemopreventive agents.
We have previously published evidence that certain naturally occurring flavonoid compounds exhibit antiestrogenic activity [21, 26]. Through these studies we also described the isolation of the glyceollins as a mixture of three isomers (glyceollin I, II, and III) from stressed soybeans [21]. Our functional analysis of these compounds showed that the glyceollins displayed a marked antiestrogenic effect on ER signaling, accompanied by suppression of 17β-estradiol-induced proliferation in MCF-7 cells [21]. From these studies, we hypothesized that the glyceollins represented novel antiestrogenic flavonoids naturally produced by soy that may be relevant to human health.
To examine the effects of glyceollin mixture on both basal and estrogen-stimulated growth, we used the well-established xenograft model of MCF-7 and BG-1 tumor formation in immunocompromised female ovariectomized nu/nu mice [22, 27]. Our results establish the in vivo antiestrogenic activity of the glyceollin mixture. This study is the first demonstration of ability of the glyceollin mix to significantly suppress estrogen-stimulated tumor growth of MCF-7 and BG-1 cells in ovariectomized female nude mice. At day 20 in glyceollin mixture treatments with added 17β-estradiol, MCF-7 tumor volume was reduced 53.4%, and BG-1 tumor volume was reduced 73.1% compared with E2 alone. The ability of glyceollins to antagonize the effects of 17β-estradiol was also observed when analyzing progesterone expression. The glyceollins were able to completely suppress E2-induced PR expression in MCF-7 and partially suppress E2-induced PR expression in BG-1 cells, further exemplifying the antiestrogenic activity of these compounds.
Unlike some phytoestrogens, the glyceollin mixture did not cause the Gly group to display any evidence of weak estrogenic action on tumor growth, PR expression in tumors, or uterine morphology. If anything, the glyceollin mixture alone suppressed tumor growth to below that of the negative control, although this did not reach statistical significance using the linear mixed model for statistical analysis. The mechanism for this result was not tested in this study, but two obvious possibilities are that the glyceollin mixture opposed effects of low levels of non-ovarian estrogen or that it suppressed tumor growth by acting via a non–ER-regulated pathway. Further study is needed to explain the Gly versus Con results.
The uterotrophic assay of ovariectomized mice is one of the principal assays used to evaluate the estrogenic and antiestrogenic properties of estrogenic compounds. Several isoflavones, including genistein, have shown agonist activity in the uterus [5, 11, 28]. In these same animals treated for 20 days with vehicle, estradiol, glyceollin mixture, or estrogen + glyceollin mixture, uterine size and morphology was examined. In the present study, the glyceollin mixture alone showed no agonist uterotrophic activity. However, glyceollin mixture treatment antagonized the 17β-estradiol effects on the uterus, significantly reducing uterine area and diameter, but nonsignificantly reduced overall uterine fixed weight. The ability of the glyceollin mixture to function as an estrogen antagonist in the uteri in mice is a distinct advantage when compared with other phytoestrogens and tamoxifen. In several studies, tamoxifen has increased uterine weight and acts as an agonist in the uteri [3, 29, 30]. These results suggest that the glyceollin mixture may be functioning as phyto–selective estrogen receptor modulators, selectively antagonizing ER function in a tissue type–specific manner.
In summary, here, we describe the in vivo activities of a novel group of antiestrogenic phytochemical glyceollins. Our data indicate that glyceollin mixture functions in vivo to inhibit the growth of human breast and ovarian cancer xenografts and may represent novel chemopreventive or therapeutic agents for the treatment of hormone-dependent cancers.
Acknowledgments
We thank Hermina Borgerink, Lisa O'Donnell, and Beth Phifer for their technical contributions and Prof. Thomas B. Clarkson for advice and support.
References:
Fisher B, Costantino JP, Wickerham DL, et al.
Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study.
J Natl Cancer Inst 1998;90:1371–88.Martino S, Cauley JA, Barrett-Connor E, et al.
Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene.
J Natl Cancer Inst 2004;96:1751–61.Brown K.
Breast cancer chemoprevention: risk-benefit effects of the antiestrogen tamoxifen.
Expert Opin Drug Saf 2002;1:253–67.Jensen EV, Jordan VC.
The estrogen receptor: a model for molecular medicine.
Clin Cancer Res 2003;1:1980–9.Diel P, Smolnikar K, Schulz T, Laudenbach-Leschowski U, Michna H, Vollmer G.
Phytoestrogens and carcinogenesis: differential effects of genistein in experimental models of normal and malignant rat endometrium.
Hum Reprod 2001;16:997–1006.Barnes S.
The chemopreventive properties of soy isoflavonoids in animal models of breast cancer.
Breast Cancer Res Treat 1997;46:169–79.Aronson WJ, Tymchuk CN, Elashoff RM, et al.
Decreased growth of human prostate LNCaP tumors in SCID mice fed a low-fat, soy protein diet with isoflavones.
Nutr Cancer 1999;35:130–6.Hewitt AL, Singletary KW.
Soy extract inhibits mammary adenocarcinoma growth in a syngeneic mouse model.
Cancer Lett 2003;192:133–43.Tham DM, Gardner CD, Haskell WL.
Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence.
J Clin Endocrinol Metab 1998;83:2223–35.Constantinou AI, Krygier AE, Mehta R.
Genistein induces maturation of cultured human breast cancer cells and prevents tumor growth in nude mice.
Am J Clin Nutr 1998;68:1426–30S.Lamartiniere CA, Zhang J, Cotroneo MS.
Genistein studies in rats: potential for breast cancer prevention and reproductive and developmental toxicity.
Am J Clin Nutr 1998;68:1400–5S.Lamartiniere CA, Moore JB, Brown NM, Thompson R, Hardin MJ, Barnes S.
Genistein suppresses mammary cancer in rats.
Carcinogenesis 1995;16:2833–40.Darvill AG, Albersheim P.
Phytoalexins and their elicitors: a defense against microbial infection in plants.
Annu Rev Plant Physiol 1984;35:243–75.Paxton JD.
Biosynthesis and accumulation of legume phytoalexins.
In: Sharma RP, Salunkhe DK, editors.
Mycotoxins and phytoalexins.
Boca Raton (FL): CRC Press; 1991. p. 485–99.Graham TL, Kim JE, Graham MY.
Role of constitutive isoflavone conjugates in the accumulation of glyceollin in soybean infected with Phytophthora megasperma.
Mol Plant Microbe Interact 1990;3:157–66.Daniel O, Meier MS, Schlatter J, Frishknect P.
Selected phenolic compounds in cultivated plants: ecologic functions, health implications, and modulation by pesticides.
Environ Health Perspect 1999;107:109–14.Rivera-Vargas LI, Schmitthenner AF, Graham TL.
Soybean flavonoid effects on and metabolism by Phytophthora sojae.
Phytochem 1993;32:851–7.Rizk AM, Hammouda FM, Ismail SI, Azzam SA, Wood G.
Studies on green beans (Phaseolus vulgaris): 1. Phytoalexins of the pods.
Qualitas Plantarum Pl Foods Human Nutr 1984;34:203–10.Bhattacharyya MK, Ward EWB.
Resistance, susceptibility and accumulation of glyceollins I-III in soybean organs inoculated with Phytophthora megasperma f. sp. Glycinea.
Physiol and Mol Pl Pathol 1986;29:227–37.Graham TL, Graham MY.
Glyceollin elicitors induce major but distinctly different shifts in isoflavonoid metabolism in proximal and distal soybean cell populations.
Mol Plant Microbe Interact 1991;4:60–8.Burow ME, Boue SM, Collins-Burow BM, et al.
Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor ? and β.
J Endocrinol 2001;86:1750–8.Baldwin WS, Curtis SW, Cauthen CA, Risinger JI, Korach KS, Barret JC.
BG-1 ovarian cell line: an alternative model for examining estrogen-dependent growth in vitro.
In Vitro Cell Dev Biol Anim 1998;34:649–54.Petz LN, Ziegler YS, Schultz JR, Nardulli AM.
Fos and Jun inhibit estrogen-induced transcription of the human progesterone receptor gene through an activator protein-1 site.
Mol Endocrinol 2004;18:521–32.Lindholm J, van Diest PJ, Haffner D, Mikuz G, Weger AR.
Amorphometric filter improves the diagnostic value of morphometric analyses of frozen histopathologic sections from mammary tumors.
Anal Cell Pathol 1992;4:443–9.Cline JM, Soderqvist G, von Schoultz B, Skoog L.
Regional distribution of proliferating cells and hormone receptors in the mammary gland of surgically postmenopausal macaques.
Gynecol Obstet Invest 1997;44:41–6.Collins-Burow BM, Burow ME, Duong BN, McLachlan JA.
Estrogenic and antiestrogenic activities of flavonoid phytochemicals through estrogen receptor binding-dependent and -independent mechanisms.
Nutr Cancer 2000;38:229–44.Clarke R, Leonessa F, Welch JN, Skaar TC.
Cellular and molecular pharmacology of antiestrogen action and resistance.
Pharmacol Rev 2001;53:25–71.Whitten PL, Russell E, Naftolin F.
Influence of phytoestrogens diets on estradiol action in the rat uterus.
Steroids 1994;59:443–9.Stygar D, Muravitskaya N, Eriksson B, Eriksson H, Sahlin L.
Effects of SERM (selective estrogen receptor modulator) treatment on growth and proliferation in the rat uterus.
Reprod Biol Endocrinol 2003;1:40–7.Nunez NP, Jelovac D, Macedo L, et al.
Effects of the antiestrogen tamoxifen and the aromatase inhibitor letrozole on serum hormones and bone characteristics in a preclinical tumor model for breast cancer.
Clin Cancer Res 2004;10:5375–80.
Return to SOY PROTEIN
Since 1-15-2007


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |