

Complementary, Integrative, and Nondrug Therapy Use for
Pain Among US Military Veterans on Long-term OpioidsThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Medical Care 2020 (Sep); 58 Supp l 2 9S: S116–S124 ~ FULL TEXT
OPEN ACCESS Elizabeth S. Goldsmith, MD, MS, Richard F. MacLehose, PhD, Agnes C. Jensen, MPH,
Barbara Clothier, MS, Siamak Noorbaloochi, PhD, Brian C. Martinson, PhD,
Melvin T. Donaldson, PhD, and Erin E. Krebs, MD, MPH
Center for Care Delivery and Outcomes Research (CCDOR),
Minneapolis VA Health Care System.

Background: Long-term opioid therapy for chronic pain arose amid limited availability and awareness of other pain therapies. Although many complementary and integrative health (CIH) and nondrug therapies are effective for chronic pain, little is known about CIH/nondrug therapy use patterns among people prescribed opioid analgesics.
Objective: The objective of this study was to estimate patterns and predictors of self-reported CIH/nondrug therapy use for chronic pain within a representative national sample of US military veterans prescribed long-term opioids for chronic pain.
Research design: National two-stage stratified random sample survey combined with electronic medical record data. Data were analyzed using logistic regressions and latent class analysis.
Subjects: US military veterans in Veterans Affairs (VA) primary care who received ≥6 months of opioid analgesics.
Measures: Self-reported use of each of 10 CIH/nondrug therapies to treat or cope with chronic pain in the past year: meditation/mindfulness, relaxation, psychotherapy, yoga, t'ai chi, aerobic exercise, stretching/strengthening, acupuncture, chiropractic, massage; Brief Pain Inventory-Interference (BPI-I) scale as a measure of pain-related function.
Results: In total, 8,891 (65%) of 13,660 invitees completed the questionnaire. Eighty percent of veterans reported past-year use of at least 1 nondrug therapy for pain. Younger age and female sex were associated with the use of most nondrug therapies. Higher pain interference was associated with lower use of exercise/movement therapies. Nondrug therapy use patterns reflected functional categories (psychological/behavioral, exercise/movement, manual).
There is more like this at our
CHIROPRACTIC CARE FOR VETERANS PageConclusions: Use of CIH/nondrug therapies for pain was common among patients receiving long-term opioids. Future analyses will examine nondrug therapy use in relation to pain and quality of life outcomes over time.
Key Words: chronic pain, veterans, opioids, nondrug therapy, complementary and integrative health
From the FULL TEXT :
Background
Long-term opioid therapy (LTOT) is associated with high morbidity and mortality [1–3] and little evidence supports its effectiveness for chronic pain. [4] Current guidelines for many chronic pain conditions recommend avoiding opioids for chronic pain and using nondrug therapies as first-line treatment. [5–9] For patients already on LTOT, guidelines recommend working with patients to reduce opioids and incorporate nondrug terapies. [10, 11] In practice, however, opioids remain overprescribed and nondrug therapies underused for chronic pain. Overprescribing of opioids for chronic pain in the United States and Canada has been described as a marker of inadequate pain management resources overall. [12]
Many nondrug therapies have demonstrated effectiveness in improving functional outcomes in chronic pain, though evidence varies for specific therapies and conditions, and multimodal care may be most effective. [13–15] Nondrug therapies comprise functional categories such as psychological or behavioral (eg, cognitive behavioral therapy), movement-based (eg, exercise therapy), or manual (eg, spinal manipulation), and can be either self-directed or practitioner-delivered. [16] Nondrug therapies include many complementary and integrative health (CIH) therapies such as acupuncture, yoga, and t’ai chi. Although “complementary” initially described health practices with origins in other health traditions outside the biomedical health system, [17] CIH therapies are increasingly integrated into mainstream health practice in the United States. [18–20] Little is known about the extent and predictors of nondrug therapy use for pain among people currently prescribed LTOT.
The US Veterans Health Administration (VA) continues to develop novel approaches to interdisciplinary pain therapy, with emphasis on nondrug modalities including behavioral therapy, physical rehabilitation, and CIH therapies. [16, 20] Understanding how veterans use nondrug therapies is an important step toward understanding demand and opportunities to improve access within VA, especially for veterans on LTOT who may benefit from more effective and safe treatment options. The purpose of this study is to identify patterns of CIH and nondrug therapy use within a national sample of VA primary care patients on LTOT for chronic pain and to evaluate associations of patient characteristics with type of nondrug therapy use.
METHODS
Study Population and Sampling
Survey and administrative data were collected as part of the Evaluating Prescription Opioid Changes in Veterans (EPOCH) study, a prospective longitudinal cohort of VA primary care patients prescribed LTOT for chronic pain in 2016. The EPOCH study used a two-stage stratified random sampling design. Eligibility criteria for patients included current LTOT and at least 1 primary care clinic appointment during the 12 months preceding the most recent opioid dispensing date. Current LTOT was defined as: (1) a qualifying opioid analgesic dispensed within the prior 30 days; and (2) ≥150 days’ supply of a qualifying opioid in the 180 days before the most recent dispensing date with no betweenfill gaps >40 days. Qualifying opioid analgesics were on the VA formulary and indicated for pain, not including tramadol or buprenorphine. Eligibility criteria were assessed using monthly extractions of administrative data from the VA Corporate Data Warehouse (CDW).
To limit the sample to patients receiving LTOT for chronic pain rather than other reasons (such as opioid use disorder or palliative care with limited life expectancy), exclusion criteria included any of the following over the preceding year: oncology, radiation oncology, hospice, or palliative care visit; dementia diagnosis or dementia clinic visit; adult day care; nursing home stay; or opioid addiction treatment program visit.
Selected patients were contacted and invited to participate. Data were collected from participants via mailed paper or telephone annual surveys. Patients were contacted in 7 waves (total contacted N=14,160), including an initial embedded pilot survey wave (pilot contacted N=500). Minor changes to the questionnaire were made after the embedded pilot was completed. Because these changes involved measures that are the focus of this report, we used data for the main 6 survey waves, excluding pilot data. The response rate was 65% for the overall survey cohort (9,53 responded/14,160 contacted) and for the main cohort excluding the pilot (8,891 responded/13,660 contacted). Additional details of EPOCH study design, methods, and survey response have been described elsewhere. [21] The study was approved by the Institutional Review Board of the Minneapolis VA Health Care System.
Patient-reported Survey Measures

Table 1 Nondrug and Complementary and Integrative Health Therapy Measures EPOCH survey questions about CIH and nondrug therapy use were modified from the Pain Management Inventory (PMI). [22] The PMI, a checklist of nondrug conventional and complementary therapies used in pain management, was designed to facilitate standardized self-reported therapy use comparable across studies. PMI therapy descriptions were based on items used in the National Health Interview Survey (NHIS) complementary medicine supplement [23] and refined through cognitive interviews with patients and key informant interviews with pain clinicians. EPOCH survey descriptions of therapies are outlined in Table 1. Questionnaires included questions about each of 10 therapies and practices they “may have used to treat or cope with pain in the past year.” For each therapy or practice, participants were asked to “choose the one answer that best describes your use in the past year” from 5 categories: did not use in past year, a few times, monthly, weekly, or most days.
Pain-related Functional Interference Pain-related functional interference was assessed with the Brief Pain Inventory-Interference (BPI-I) scale. [24, 25] The BPI-I assesses pain-related functional interference over the preceding week in 7 domains rated on a scale of 0 (does not interfere) to 10 (completely interferes). An overall BPI-I score was calculated as the mean of the 7 item scores (range: 0–10). For analyses, BPI-I was categorized as “mild” (0–3), “moderate” (4–6) or “severe” (7–10).
Administrative Data MeasuresDemographic and Health-related Measures Patient demographic information including age, sex, and race were obtained from the VA CDW. Mental health diagnoses were assessed through inpatient and outpatient International Classification of Diseases (ICD) Ninth-Revision, Clinical Modification (ICD-9-CM) [26] and Tenth Revision, Clinical Modification (ICD-10-CM) [27] codes obtained from the CDW and categorized into diagnostic groups including depressive disorders, posttraumatic stress disorder (PTSD), and non-PTSD anxiety disorders. The Charlson Comorbidity Index [28] of chronic disease burden was calculated by extracting included diagnoses from the CDW.
Opioid Dose
Opioid doses were calculated using dispensing data from VA outpatient pharmacies and converted to morphine equivalent (ME) milligrams per day via conversion factors recommended by the Centers for Disease Control and Prevention. [29] To determine average daily dose for each participant, the total milligrams for all opioids dispensed in 6 months (including the index prescription) was calculated then divided by the number of days from the first opioid dispensed to the end of the most recent prescription days’ supply. Categorical analyses considered daily dosage in ME milligram per day according to the following conventional research categories: low (<20), moderate (20–<50), high (50–<100), or very high (≥100). [30–32 ]
Statistical Analysis
To allow comparison across therapies, frequency of use for each individual nondrug therapy was dichotomized as any pastyear use or no past-year use. Therapies were categorized within functional categories cited in the literature: psychological/behavioral, exercise/movement, and manual therapies (Table 1). [16] Frequencies of past year use of any nondrug therapy, each functional category of nondrug therapy, and each individual nondrug therapy were calculated and presented as observed unweighted counts. Percentages were calculated with survey weights as appropriate for the sampling design. [21]
To estimate associations between individual characteristics and use of individual nondrug therapies, multivariable logistic regressions were estimated separately for each therapy. Past-year use versus nonuse of an individual therapy for pain was the dependent variable in each regression. Independent variables included age (categorical: below 55, 55–64, 65–74, 75+), sex (male, female), race (White, Black, other), BPI-I category (mild, moderate, severe), opioid daily dose in ME milligram per day (<20, 20–<50, 50–<100, 100+), Charlson Comorbidity Index (continuous), depressive disorder diagnosis (present vs. absent), anxiety disorder diagnosis (present vs. absent), and PTSD diagnosis (present vs. absent). As past-year use was common for several therapies, results of the logistic models were used to estimate adjusted risk differences and 95% confidence intervals. [33]
To explore patterns of nondrug therapy use, we used latent class analysis (LCA)34 to identify groups with similar past-year use patterns. Past-year use of individual nondrug therapies were the only indicators used to determine latent classes in LCA models. Yoga and t’ai chi were not included in the LCA models because the low prevalences created estimability problems. We explored 3, 4, 5, and 6-class solutions to the model. The final class solution was determined based on interclass heterogeneity, fit statistics, class size, and clinical interpretability. [34, 35] After a final LCA model was identified, participants were assigned to a single latent class based on highest posterior probability of membership. A multinomial logistic regression model was estimated with class membership as the dependent variable. Independent variables were dichotomized for ease of model interpretation: age (below 65, 65+), sex (male, female), race (white, not white), BPI-I (not severe, severe), opioid daily dose in ME milligram per day (<100, 100+), Charlson Comorbidity Index (0–2, 3+), depressive disorder diagnosis (present vs. absent), anxiety disorder diagnosis (present vs. absent), and PTSD diagnosis (present vs. absent). Risk differences were calculated from multinomial logistic regression results.
Survey weights were applied to analyses to account for the two-stage stratified sampling approach. [21] As LCA models could not be fit using survey weights, survey weights were applied at the phase of multinomial logistic regressions assessing posterior probability of class membership. We did not adjust alpha levels for multiple comparisons because this was an exploratory analysis not intended to test specific hypotheses.
All analyses were performed in Stata Version 15.36
RESULTS
Prevalence of Nondrug and Complementary and Integrative Health Therapy Use

Table 2 Use of at least 1 of 10 nondrug therapies was reported by 80.1% (N=6,978) of the cohort. Table 2 presents frequencies of self-reported past-year use of any functional category of nondrug therapy and of each of the 10 nondrug therapies individually. The most commonly used individual therapies were stretching/strengthening exercise (55.9%), aerobic exercise (38.2%), and relaxation techniques (35.7%). All nondrug therapy use variables had <4% missing data.
Factors Associated With Individual Nondrug and Complementary and Integrative Health Therapy
Use Table 3 presents factors associated with past-year use of individual nondrug therapies for pain in multivariable logistic regression analyses. Measures presented have an absolute value range of 0–1 and represent probability differences of reported therapy use as compared with the noted reference group. For example, people aged 75 years or older had 0.18 (18%) lower probability of reporting past-year use of psychotherapy for pain than people aged under 55. Patients aged 65 and older were less likely than patients under 55 to report using every nondrug therapy except t’ai chi and chiropractic care. Women were more likely than men to report use of therapies other than t’ai chi and chiropractic care, most notably meditation, relaxation, stretching/strengthening, aerobic exercise, yoga, and massage. Patients reporting severe pain interference were less likely than patients reporting mild pain interference to report use of stretching/strengthening or aerobic exercise, and more likely than patients reporting mild pain interference to report use of acupuncture or massage. Patients with opioid doses of 100 ME milligram per day or higher were more likely than patients with opioid doses under 20 ME milligram per day to report use of meditation and relaxation. Veterans diagnosed with PTSD were more likely to report use of any psychological/behavioral therapy.
Latent Classes of Nondrug Therapy Use Patterns
We determined that a 5-class model was the best latent class solution based on interclass heterogeneity, fit statistics, and clinical impressions of relevant classes. Classes represented distinct patterns of nondrug therapy use, which we named according to how we perceived their unique characteristics: low-use, manual/strength, exercise/movement, psychological/behavioral, and high use. The 5-class solution offered additional classes with distinct therapy use patterns over those available in the 3- and 4-class solutions, in addition to gains in fit statistics. Use patterns appeared somewhat robust, as similar use classes were evident across multiple latent class solutions. The low-use, exercise/movement and high-use classes were apparent in the 3- and 4-class solutions, and the 4-class solution yielded an additional class analogous to the manual/strength class. The 6-class solution did not identify a substantively meaningful different additional class beyond those available in the 5-class solution.
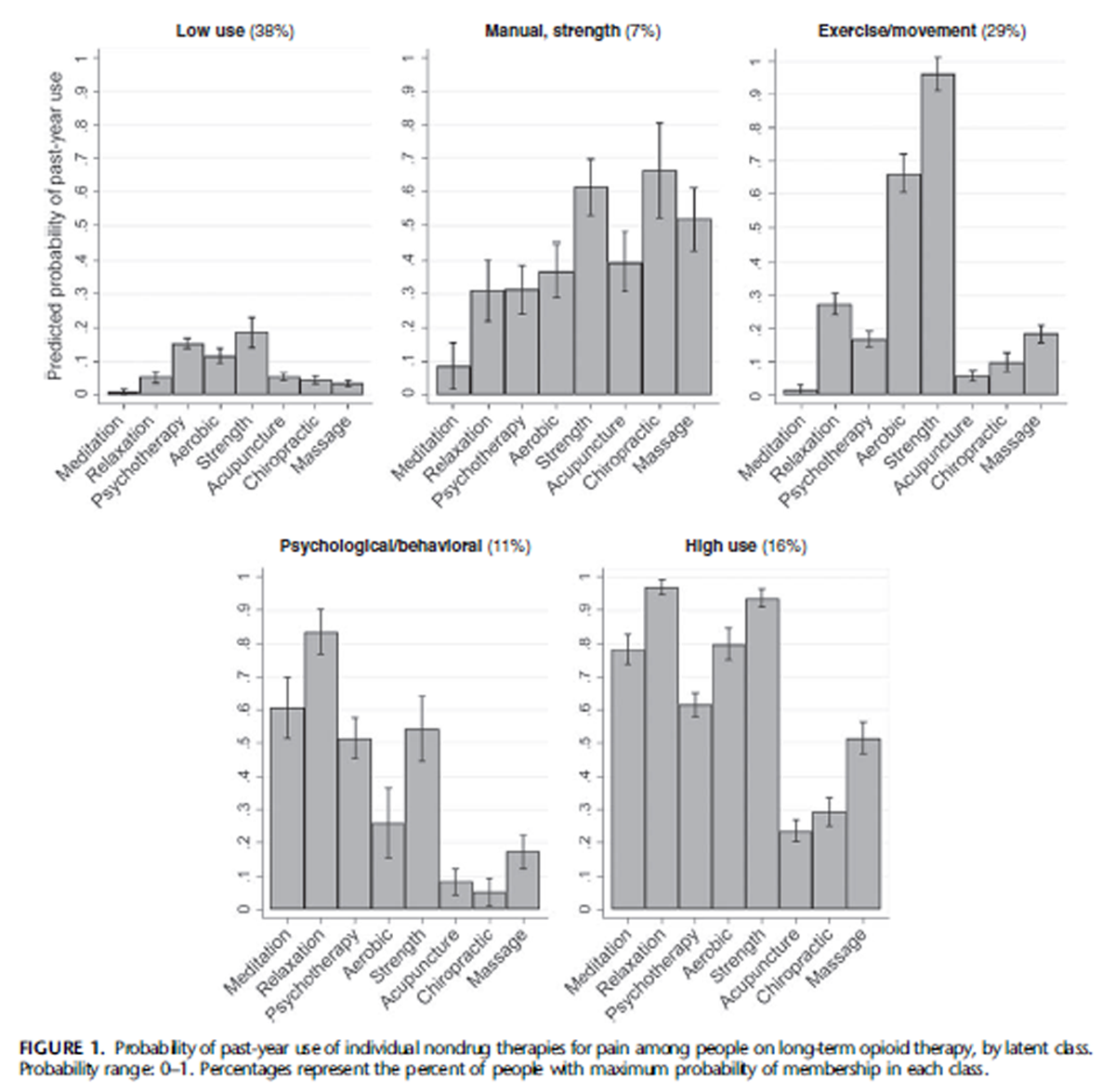
Figure 1 Figure 1 displays the probability of past-year use of each individual nondrug therapy within each latent class. Patients in the low-use class (38% of the cohort) had low likelihood of pastyear use of each nondrug therapy for pain (between 1% and 19%). The manual/strength class (7% of cohort) was characterized by higher use of acupuncture (40%) and chiropractic (66%) therapy than any other class, higher use of massage (52%) than any class except the overall high-use class, and relatively high use of stretching/strengthening (61%). The exercise/movement class (29% of cohort) was characterized by almost universal use of stretching/strengthening (96%) and high use of aerobic exercise (66%), with lower use of other therapies (2%–27%). The psychological/behavioral class (11% of cohort) was characterized by high use of relaxation (83%), meditation (61%) and psychotherapy (52%) as well as stretching/strengthening (54%), with lower use of other therapies (5%–26%). The high-use class (16% of cohort) was characterized by almost universal use of relaxation (97%) and stretching/strengthening (94%), high use of aerobic exercise (80%), meditation (78%) and psychotherapy (62%), and higher use of massage (52%) than all classes except the manual/strength class.
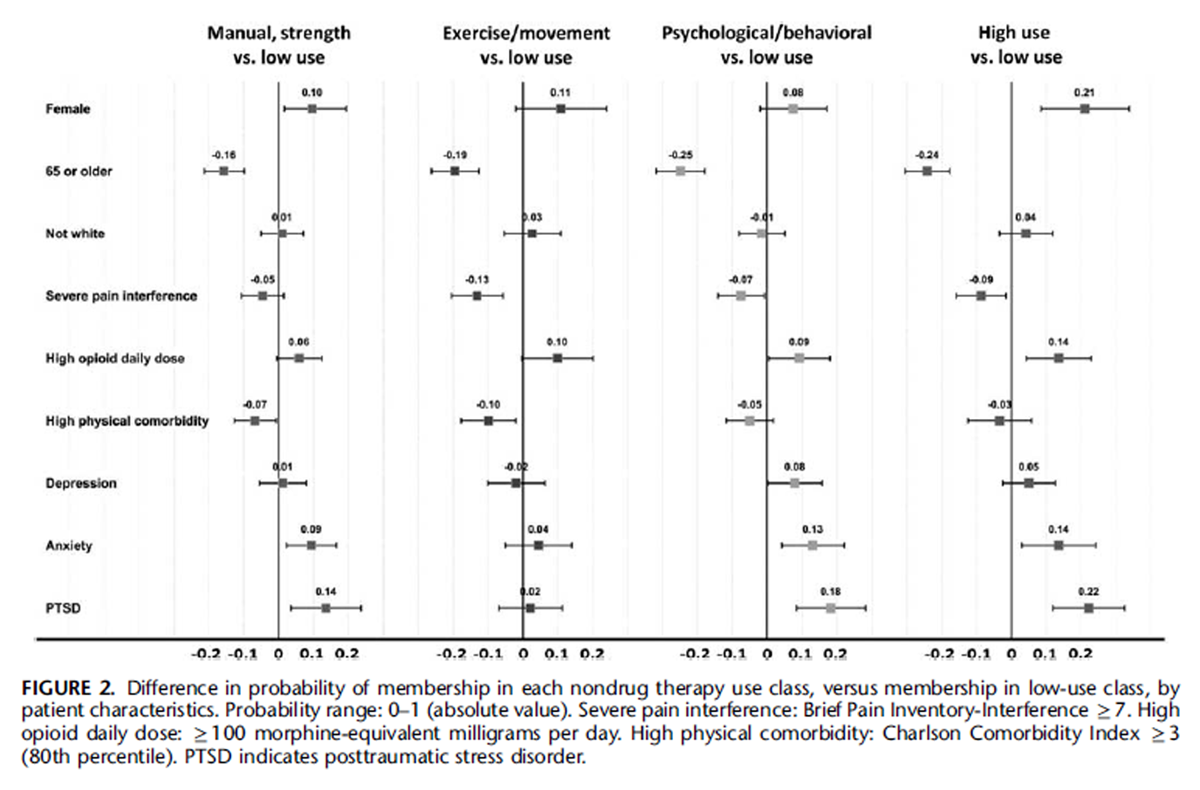
Figure 2 Figure 2 presents patient characteristics associated with differences in probability of membership in each of the 4 active classes (ie, manual/strength, exercise/movement, psychological/ behavioral, and high use) relative to the low-use class. Measures presented have an absolute value range of 0–1 and represent probability differences of membership in a nondrug therapy use class compared with membership in the low-use class. For example, being female was associated with a 0.25 (25%) greater prevalence of the high-use class than the low-use class. Being 65 or older was associated with lower probability of membership in each active class relative to the low-use class, whereas female sex and high opioid daily dose were associated with higher probability of membership in each of the active classes. Severe pain interference was associated with higher probability of membership in the lowuse class than in the exercise/movement and high-use classes. Higher medical comorbidity was associated with higher probability of membership in the low-use class than in the exercise/movement or manual/strength classes. Anxiety and PTSD were associated with higher probability of membership in the manual/strength, psychological/behavioral, and high-use classes, while depression was only associated with higher probability of psychological/behavioral class membership.
Some patient characteristics also distinguished active use classes from one another. Being female was associated with higher probability of membership in the high-use class than in every class other than exercise/movement. Severe pain interference was less associated with exercise/movement class membership than with membership in any class other than high-use, and PTSD was less associated with exercise/movement class membership than with membership in any class other than low-use. Depression was more positively associated with psychological/behavioral class membership than with membership in any class other than high-use.
DISCUSSION
We found that a large majority of US VA primary care patients on LTOT reported use of nondrug therapies for pain. Younger patients and female patients were more likely to use most nondrug therapies, and patients with severe pain interference were less likely to use exercise/movement therapies. Observed nondrug therapy use patterns derived from LCA were largely consistent with clinically relevant functional categories of psychological/behavioral, exercise/movement, and manual therapies.
Broadly, our findings regarding nondrug therapy use for pain among patients on LTOT are consistent with previous research. In 1 study of patients with a past-year musculoskeletal pain diagnosis prescribed at least 90 days of opioid therapy, 71% of 517 patients reported nondrug therapy use for pain in the preceding 6 months. [37] An earlier study of 908 primary care patients in Wisconsin with continuous pain for 3 months and prescribed opioids found that 44% reported CIH therapy use for pain in the past 12 months, with overall use more common among younger patients, women, and patients with higher pain intensity and disability. [38] To our knowledge, these are the only prior studies of nondrug therapy use patterns among people on opioid therapy for pain.
Larger observational studies that used VA administrative data to explore patterns of CIH and nondrug therapy use among people with chronic pain similarly found use to be more common among younger patients and women. [39, 40] These studies found lower nondrug therapy use prevalence than studies that assessed use via self-report, presumably because people often access nondrug therapies outside health systems. [41]
The finding that younger patients and women were more likely to use most types and combinations of nondrug and CIH therapies is consistent with past research in general US populations, [42–44] and may relate to cultural norms and patient and provider beliefs. For example, female patients in our cohort were more likely to have used yoga for pain, though yoga use was rare overall. Although 60% of yoga users perceived yoga as helpful for pain in a recent study of veterans with chronic pain on LTOT,37 other research has suggested many veterans see yoga as a therapy “for girls.” [45, 46] Engaging with patient and provider beliefs about who can and should engage in certain therapy types may increase accessibility.
A prior study conducted a LCA of nondrug and CIH therapy use (for any purpose) using data from a cohort of US Minnesota National Guard veterans. [22] Patients in that study were younger (mean age 39) and mostly without chronic pain or opioid use. Results demonstrated similar observed categories of nondrug therapy use, despite differences in study populations and reasons for CIH use.
We found that patients with worse pain interference with function were less likely to have engaged in exercise and movement therapies, particularly aerobic and stretching/strengthening exercise. Although this cross-sectional analysis cannot determine cause and effect, prior research suggests reasons for a connection between exercise and better pain-related function. Lower levels of physical activity are a risk factor for developing chronic pain. [47] Exercise interventions have been shown to prevent future pain and improve outcomes in patients with established chronic pain. [10, 14] Investigating and targeting factors that facilitate use of exercise/movement therapy among people with worse pain interference may be of particular benefit.
Mental health diagnoses were positively associated with use of psychological and behavioral therapies. This could reflect increased access to treatments often delivered by mental health clinicians, or greater comfort seeking out psychological resources. The existence of a mental health diagnosis in the chart may itself be a proxy for access to relevant therapies.
Patterns of use derived from LCA were found to reflect previously cited functional categories (ie, psychological/behavioral, exercise/movement, and manual therapies). Although several patient characteristics distinguished low-use classes from other active use classes, few differed meaningfully between active use classes, suggesting that factors unmeasured in these data may drive distinct patterns of nondrug therapy use. Differences among use patterns may represent barriers and facilitators related to nondrug therapy access and motivation for use, such as awareness of therapies, beliefs about effectiveness, and other factors at the health system, provider, and patient levels. [48, 49] Future qualitative and mixedmethods work focusing on patterns of nondrug therapy use and referral can help clarify what drives nondrug therapy use.
Our overall finding of high levels of nondrug therapy use may be a promising sign that many veterans on LTOT for chronic pain are willing to engage with nonopioid therapies. The most commonly used therapies, including exercise/movement and psychological/behavioral therapies, are relatively effective and lowcost, with potential health benefits beyond pain management that may motivate patients’ use. Our findings suggest an opportunity for health care systems to encourage and support existing interest in nondrug therapy use by people on LTOT for chronic pain.
This study has limitations. First, self-report data are subject to recall bias and misclassification. Self-report nevertheless remains the most accurate and relevant form of assessment for nondrug therapy use, as administrative data omits use of therapies sought outside the medical system or not subject to billing. [41, 50] For example, a national survey of over 3000 VA patients found that while 52% reported use of a CIH therapy in the past year, little of this care was obtained at VA. [50] Results should be considered in the context of the broader literature. Second, administrative data are also subject to misclassification, particularly with respect to mental health diagnoses. [51] Third, survey and administrative data indicators were necessarily limited in scope, and factors that we did not measure or analyze may be relevant to nondrug therapy use. Fourth, findings in this current analysis are cross-sectional and cannot consider temporality or causality. This cohort study of veterans on LTOT for chronic pain is ongoing and future analyses can explore nondrug therapy use in relation to pain and quality of life outcomes over time.
US Military Veterans on Long-term Opioids
Medical Care 2020 (Sep); 58 Supp l 2 9S: S116–S124 ~ FULL TEXT
In conclusion, our study found that US VA patients on long-term opioid therapy (LTOT) for chronic pain commonly use nondrug therapies to manage pain, that observed nondrug therapy use classes reflect clinically relevant functional groups, and that patient characteristics are associated with use of different nondrug therapies. Further exploration of factors affecting nondrug therapy access and use for specific subpopulations, such as use of exercise/movement therapy by people with high pain interference, may enable implementation of nondrug and complementary and integrative health (CIH) therapy for chronic pain and expand safe, effective pain treatment options for people prescribed LTOT.
ACKNOWLEDGMENT
The authors thank the veteran participants in the study and the members of the research team, including Sean Nugent, BA an Indulis Rutks, BS.
References:
Chou R, Turner JA, Devine EB, et al.
The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop.
Ann Intern Med. 2015;162:276–286.Bohnert AS, Valenstein M, Bair MJ, et al.
Association between opioid prescribing patterns and opioid overdose-related deaths.
JAMA. 2011;305: 1315–1321.Paulozzi LJ, Zhang K, Jones CM, et al.
Risk of adverse health outcomes with increasing duration and regularity of opioid therapy.
J Am Board Fam Med. 2014;27:329–338.Krebs EE, Gravely A, Nugent S, et al.
Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: The SPACE Randomized Clinical Trial.
JAMA. 2018;319:872–882.Wong JJ, Cote P, Sutton DA, et al.
Clinical Practice Guidelines for the Noninvasive Management of Low Back Pain: A Systematic Review
by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration
European J Pain 2017 (Feb); 21 (2): 201–216Chou R, Qaseem A, Snow V, et al.
Diagnosis and Treatment of Low Back Pain: A Joint Clinical Practice Guideline
from the American College of Physicians and the American Pain Society
Annals of Internal Medicine 2007 (Oct 2); 147 (7): 478–491Reid MC, Ong AD, Henderson CR, et al.
Why we need nonpharmacologic approaches to manage chronic low back pain in older adults.
JAMA Intern Med. 2016;176:338.Reid MC, Eccleston C, Pillemer K.
Management of chronic pain in older adults.
BMJ. 2015;350:h532.Foster NE, Anema JR, Cherkin D, Chou R, Cohen SP, Gross DP, Ferreira PH, Fritz JM.
Prevention and Treatment of Low Back Pain: Evidence, Challenges, and Promising Directions
Lancet. 2018 (Jun 9); 391 (10137): 2368–2383U.S. Department of Veterans Affairs - Office of Inspector General
VA/DoD Clinical Practice Guideline for Opioid Therapy for Chronic Pain
U.S. Department of Veterans Affairs - Department of Defense.
Version 3.0 – 2017Finestone HM, Juurlink DN, Power B, et al.
Opioid prescribing is a surrogate for inadequate pain management resources.
Can Fam Physician. 2016;62:465–468.Nahin RL, Boineau R, Khalsa PS, et al.
Evidence-based evaluation of complementary health approaches for pain management in the United States.
Mayo Clin Proc. 2016;91:1292–1306.Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, et al.
Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an
American College of Physicians Clinical Practice Guideline
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 493–505Elwy AR, Johnston JM, Bormann JE, et al.
A systematic scoping review of complementary and alternative medicine mind and body practices to improve the health of veterans and military personnel.
Med Care. 2014;52:S70–S82.Kligler B, Bair MJ, Banerjea R, et al.
Clinical Policy Recommendations from the VHA State-of-the-Art Conference
on Non-Pharmacological Approaches to Chronic Musculoskeletal Pain
J Gen Intern Med 2018 (May); 33 (Suppl 1): 16–23National Center for Complementary and Integrative Health (NCCIH).
Complementary, Alternative, or Integrative Health: What’s In a Name? 2016. Available at:
https://nccih.nih.gov/health/integrative-health
Accessed April 7, 2018.Ananth S.
2010 Complementary and Alternative Medicine Survey of Hospitals.
Alexandria, VA: Samueli Institute; 2011.Ezeji-Okoye SC, Kotar TM, Smeeding SJ, et al.
State of care: complementary and alternative medicine in Veterans Health Administration—2011 survey results.
Fed Pract. 2013;30:14–19.Kligler B, Niemtzow RC, Drake DF, et al.
The Current State of Integrative Medicine Within the U.S. Department of Veterans Affairs.
Med Acupunct. 2018;30:230–234.Krebs EE, Clothier B, Nugent S, et al.
The Evaluating Prescription Opioid Changes in Veterans (EPOCH) study: design, survey response, and baseline characteristics.
Plos One. 2020. [In press].Donaldson MT, Polusny MA, MacLehose RF, et al.
Patterns of Conventional and Complementary Non-pharmacological Health Practice
Use by US Military Veterans: A Cross-sectional Latent Class Analysis
BMC Complement Altern Med. 2018 (Sep 5); 18 (1): 246National Center for Complementary and Integrative Health.
Use of Complementary Health Approaches in the U.S.: National Health Interview Survey (NHIS); 2017.
Available at:
https://nccih.nih.gov/research/statistics/NHIS/2012/key-findings
Accessed April 7, 2018.Cleeland CS, Ryan KM.
Pain assessment: global use of the Brief Pain Inventory.
Ann Acad Med Singapore. 1994;23:129–138.Cleeland C.
The Brief Pain Inventory User Guide.
Houston, TX: University of Texas M.D.
Anderson Cancer Center; 2009.National Center for Health Statistics.
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Available at:
www.cdc.gov/nchs/icd/icd9cm.htm
Accessed April 6, 2018.National Center for Health Statistics.
International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Available at:
\www.cdc.gov/nchs/icd/icd10cm.htm#FY
Accessed April 6, 2018.Charlson ME, Pompei P, Ales KL, et al.
A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.
J Chronic Dis. 1987;40:373–383.National Center for Injury Prevention and CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors, 2018 version.
Bohnert ASB, Ilgen MA, Galea S, et al.
Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System.
Med Care. 2011;49:393–396.Dunn KM, Saunders KW, Rutter CM, et al.
Overdose and prescribed opioids: associations among chronic non-cancer pain patients.
Ann Intern Med. 2010;152:85–92.Ilgen MA, Bohnert ASB, Ganoczy D, et al.
Opioid dose and risk of suicide.
Pain. 2016;157:1079–1084.Muller CJ, Maclehose RF.
Estimating predicted probabilities from logistic regression: Different methods correspond to different target populations.
Int J Epidemiol. 2014;43:962–970.Collins LM, Lanza ST.
Latent Class and Latent Transition Analysis: Applications in the Social Behavioral, and Health Sciences Hoboken, NJ:
Wiley. Hoboken, NJ: Wiley InterScience; 2010.Masyn KE.
Latent class analysis and finite mixture modeling.
In: Little TD, ed. The Oxford Handbook of Quantitative Methods, Volume 2: Statistical Analysis.
New York, NY: Oxford University Press; 2013:551–611.StataCorp. Stata Statistical Software: Release 15; 2017.
Lozier CC, Nugent SM, Smith NX, et al.
Correlates of use and perceived effectiveness of non-pharmacologic strategies for chronic pain among patients prescribed long-term opioid therapy.
J Gen Intern Med. 2018;33:46–53.Fleming S, Rabago DP, Mundt MP, et al.
CAM therapies among primary care patients using opioid therapy for chronic pain.
BMC Complement Altern Med. 2007;7:1–7.Taylor SL, Herman PM, Marshall NJ, et al.
Use of Complementary and Integrated Health: A Retrospective Analysis of U.S. Veterans with Chronic Musculoskeletal Pain Nationally.
J Altern Complement Med. 2019;25:32–39.Evans EA, Herman PM, Washington DL, et al.
Gender Differences in Use of Complementary and Integrative Health by U.S. Military Veterans with Chronic Musculoskeletal Pain.
Womens Health Issues. 2018;28: 379–386.Healthcare Analysis & Information Group (HAIG),
Department of Veterans Affairs Veterans Health Administration.
FY 2015 VHA Complementary and Integrative Health (CIH) Services (Formerly CAM); 2015.Kessler RC, Davis RB, Foster DF, et al.
Long-term trends in the use of complementary and alternative medical therapies in the United States.
Ann Intern Med. 2001;135:262–268.Saper RB, Eisenberg DM, Davis RB, et al.
Prevalence and patterns of adult yoga use in the United States: results of a national survey.
Altern Ther Health Med. 2004;10:44–49.Tindle HA, Wolsko P, Davis RB, et al.
Factors associated with the use of mind body therapies among United States adults with musculoskeletal pain.
Complement Ther Med. 2005;13:155–164.Taylor SL, Giannitrapani KF, Yuan A, et al.
What patients and providers want to know about complementary and integrative health therapies.
J Altern Complement Med. 2018;24:85–89.Hurst S, Maiya M, Casteel D, et al.
Yoga therapy for military personnel and veterans: qualitative perspectives of yoga students and instructors.
Complement Ther Med. 2018;40:222–229.Shiri R, Falah-Hassani K.
Does leisure time physical activity protect against low back pain?
Systematic review and meta-analysis of 36 prospective cohort studies.
Br J Sports Med. 2017;51: 1410–1418.Bair MJ, Matthias MS, Nyland KA, et al.
Barriers and facilitators to chronic pain self-management: a qualitative study
of primary care patients with comorbid musculoskeletal pain and depression.
Pain Med. 2009;10:1280–1291.Becker WC, Dorflinger L, Edmond SN, et al.
Barriers and facilitators to use of non-pharmacological treatments in chronic pain.
BMC Fam Pract. 2017;18:1–8.Taylor SL, Hoggatt KJ, Kligler B.
Complementary and Integrated Health Approaches: What Do Veterans Use and Want
J Gen Intern Med. 2019 (Jul); 34 (7): 1192–1199Davis KAS, Sudlow CLM, Hotopf M.
Can mental health diagnoses in administrative data be used for research?
A systematic review of the accuracy of routinely collected diagnoses.
BMC Psychiatry. 2016;16:1–11.
Return to OPIOID EPIDEMIC
Return to INTEGRATED HEALTH CARE
Return to NON-PHARMACOLOGIC THERAPY
Return to CHIROPRACTIC CARE FOR VETERANS
Since 12-09-2020


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |