

Spinal Manipulative Therapy Has an Immediate Effect
on Thermal Pain Sensitivity in People With
Low Back Pain: A Randomized Controlled TrialThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Phys Ther. 2009 (Dec); 89 (12): 1292–1303 ~ FULL TEXT
OPEN ACCESS Parvaneh Mohammadian, PhD, Antonio Gonsalves, DC, Chris Tsai, DC
Thomas Hummel, MD, Thomas Carpenter, DC
Department of Physical Therapy,
University of Florida,
Gainesville, FL 32610-0154, USA.
BACKGROUND: Current evidence suggests that spinal manipulative therapy (SMT) is effective in the treatment of people with low back pain (LBP); however, the corresponding mechanisms are unknown. Hypoalgesia is associated with SMT and is suggestive of specific mechanisms.
OBJECTIVE: The primary purpose of this study was to assess the immediate effects of SMT on thermal pain perception in people with LBP. A secondary purpose was to determine whether the resulting hypoalgesia was a local effect and whether psychological influences were associated with changes in pain perception.
DESIGN: This study was a randomized controlled trial.
SETTING: A sample of convenience was recruited from community and outpatient clinics.
PARTICIPANTS: Thirty-six people (10 men, 26 women) currently experiencing LBP participated in the study. The average age of the participants was 32.39 (SD=12.63) years, and the average duration of LBP was 221.79 (SD=365.37) weeks.
INTERVENTION AND MEASUREMENTS: Baseline demographic and psychological measurements were obtained, followed by quantitative sensory testing to assess temporal summation and Adelta fiber-mediated pain perception. Next, participants were randomly assigned to ride a stationary bicycle, perform low back extension exercises, or receive SMT. Finally, the same quantitative sensory testing protocol was reassessed to determine the immediate effects of each intervention on thermal pain sensitivity.
RESULTS: Hypoalgesia to A-delta fiber-mediated pain perception was not observed. Group-dependent hypoalgesia of temporal summation specific to the lumbar innervated region was observed. Pair-wise comparisons indicated significant hypoalgesia in participants who received SMT, but not in those who rode a stationary bicycle or performed low back extension exercises. Psychological factors did not significantly correlate with changes in temporal summation in participants who received SMT.
LIMITATIONS: Only immediate effects of SMT were measured, so the authors are unable to comment on whether the inhibition of temporal summation is a lasting effect. Furthermore, the authors are unable to comment on the relationship between their findings and changes in clinical pain.
CONCLUSIONS: Inhibition of A-delta fiber-mediated pain perception was similar for all groups. However, inhibition of temporal summation was observed only in participants receiving SMT, suggesting a modulation of dorsal horn excitability that was observed primarily in the lumbar innervated area.
TRIAL REGISTRATION: ClinicalTrials.gov NCT00922220
From the Full-Text Article:
Background
Injury will increase the neuronal excitability within the spinal cord, leading to the activation of pain fibers with less provocation and a heightened pain response. [1] This occurrence, known as central sensitization, is characterized by allodynia (pain perception to a previously nonpainful stimulus) and hyperalgesia (increased magnitude of pain intensity in response to a previously painful stimulus) and is implicated in the progression of acute pain to chronic pain and in the maintenance of chronic pain. [1–3] Subsequently, acute pain resulting from peripheral input may provoke neuroplastic changes within the nervous system and a subsequent shift toward a centrally maintained mechanism of pain. [1, 2] Evidence for a centrally maintained mechanism of pain is observed in a number of musculoskeletal conditions such as fibromyalgia, [4, 5] temporomandibular joint disorder, [6, 7] and whiplash-associated disorder. [8, 9] For example, compared with people who are healthy, individuals with whiplash-associated disorder and temporomandibular joint disorder may have lower pain thresholds at sites distal from the region of injury. [7, 9] The corresponding general hypersensitivity to pain is indicative of a central pain mechanism and suggests neuroplastic changes in pain perception.
Mounting evidence also suggests chronic low back pain (LBP) is characterized by central sensitization. [10–12] For example, individuals experiencing chronic LBP may report greater pain intensity in response to a standard pressure pain stimulus applied to the thumbnail compared with individuals who are pain-free. [12] Consequently, the pain associated with chronic LBP is potentially maintained by centrally mediated mechanisms, and interventions effective in the management of LBP either may prevent the progression of acute pain from a peripheral to a centrally mediated mechanism or directly affect a central mechanism of pain. Consequently, interventions that favorably alter central sensitization may be desirable in the treatment of individuals experiencing LBP.
Current evidence suggests spinal manipulative therapy (SMT) is effective in the treatment of people with LBP. [13–17] Despite SMT's clinical effectiveness, its corresponding mechanisms are undetermined. Boal and Gillette [18] suggested that SMT may provide a novel counter-irritant, resulting in inhibition of neuroplastic changes associated with central sensitization at the dorsal horn of the spinal cord. Prior studies have shown immediate hypoalgesia (decreased magnitude of pain intensity in response to a standard stimulus) associated with SMT [19–23] and support such a mechanism. For example, Fernández-Carnero et al [19] observed increased pain pressure threshold and pain-free grip in response to SMT applied to the cervical spine.
Temporal summation is a specific behavioral measure of dorsal horn cell central sensitization mediated by the C fiber afferents in which a painful stimulus of unchanging magnitude provided at an interpulse interval frequency of less than 3 seconds is perceived as increasingly painful. [4, 24] We have previously observed inhibition of temporal summation following SMT in people who were pain-free, which was not observed following other interventions for LBP. [25] Conversely, hypoalgesia to Aδ fiber–mediated pain perception was not unique to SMT. [25]
Collectively, these studies support a mechanism of SMT related to the alteration of neuroplastic changes associated with pain that may be specific to inhibition of temporal summation. Although these effects have been observed following SMT in people who were healthy, they have not been replicated in a sample of people experiencing LBP.
The purpose of this study was threefold and parallel to a previous study of individuals who were healthy. [25] First, we compared immediate changes in Aδ and C fiber (temporal summation)–mediated pain perception across 3 interventions (SMT, low back extension exercises, and use of a stationary bicycle) for individuals experiencing LBP. Similar to the prior study, [25] we hypothesized hypoalgesia to Aδ fiber–mediated pain over time regardless of group assignment, with temporal summation inhibition greater in participants receiving SMT. Second, we wanted to determine whether hypoalgesia following SMT was a local effect (occurring in the lumbar innervated region) or a general effect (also occurring in the cervical innervated region). We hypothesized that, similar to our prior study, [25] hypoalgesia to thermal stimuli would be localized to the lumbar innervated region. Third, we investigated the association between psychological factors related to pain and hypoalgesia to thermal stimuli. Psychological factors have an association with clinical LBP [26–29] and with thermal pain perception in individuals with LBP. [30] We hypothesized a similar relationship would be evident in changes in thermal pain perception following SMT in participants experiencing LBP.
Method
Participants
A sample of convenience was recruited from the University of Florida Health Science Center community and affiliated outpatient clinics by flyer and word of mouth. Potential participants were introduced to the study method and screened for eligibility by a study representative. Individuals wanting to participate then signed an informed consent form. Inclusion criteria were being 18 to 60 years of age and currently experiencing LBP. Individuals with concomitant lower-extremity complaints were eligible for study participation. Exclusion criteria were being non–English speaking, systemic medical conditions (eg, diabetes, hypertension), current use of psychiatric medications, pregnancy, signs and symptoms indicative of nerve root compression (reflex change, myotomal weakness, or sensation change), and a history of surgery to the low back.
Procedure
Demographic information, psychological questionnaires, and measurements of baseline thermal pain sensitivity were collected prior to random assignment of participants to treatment groups. The interventions were each applied for 5 minutes to standardize measurement time of experimental pain testing from baseline to immediately after intervention. The examiner was not blinded to group assignment.
MeasuresNumeric rating scales. Numeric rating scales (NRSs) were used to measure pain perception. Participants were asked to quantify the pain experienced during experimental pain testing using a numeric rating scale with anchors of 0 (“no pain at all”) and 100 (“worst pain imaginable”). The NRS is frequently used as a measure of both clinical and experimental pain and has demonstrated sound psychometric properties in previous studies. [31–34]
Psychological questionnaires. Psychological measures known to influence experimental pain [35–38] and LBP [26–29] were chosen and are described below.
The Fear of Pain Questionnaire–III (FPQ-III) [39] is 30-item questionnaire, with individual items scored from 1 to 5 to measure fear of normally painful situations. Higher scores indicate greater pain-related fear. The FPQ has demonstrated sound psychometric properties in both experimental and clinical pain studies. [37, 40]
The Tampa Scale of Kinesiophobia (TSK) is an 11-item questionnaire, with individual items scored from 1 to 4. The questionnaire was developed to quantify the fear of movement and of injury or reinjury for individuals currently experiencing pain. Higher TSK scores indicate greater fear of movement and of injury or reinjury due to pain. The TSK has demonstrated acceptable psychometric properties. [41]
The Coping Strategies Questionnaire (CSQ-R), commonly used in the assessment of pain, uses a 27-item, 7-point rating scale to measure the frequency of use for common pain coping strategies. [42] Consistent with the previous study involving participants who were healthy, [25] we included only the catastrophizing subscale using the currently recommended scoring system (CSQ-R). [43] The validity of the catastrophizing subscale of the CSQ-R has been supported in prior studies. [42–45]
The State-Trait Anxiety Inventory (STAI) is commonly used to assess anxiety. It uses a 40-item, 4-point rating scale to assess dispositional (trait) and situational (state) anxiety symptoms. [46] We reported the state portion of the STAI, as this construct better matched the purposes of this study.
The Anxiety Sensitivity Index (ASI) uses a 16-item, 4-point rating scale to assess anxiety sensitivity or the perception of harm from experiencing symptoms of anxiety. The ASI is commonly used in pain studies and has demonstrated sound psychometric properties. [47, 48]
Assessment of thermal pain sensitivity. The same quantitative sensory testing (QST) protocol from our previous study [25] also was used in this study. The QST was performed using the Medoc Neurosensory Analyzer (TSA 2001*) with handheld peltier element-based stimulator. Participants first underwent a practice session in order to familiarize themselves with the pain testing protocol.Briefly, the practice session included a continuous heat pulse delivered to the volar part of the dominant forearm starting at 35°C and increased at a rate of 0.5°C/s. Participants were instructed to indicate when the stimulus first changed from warmth to pain and the heat pulse was terminated at this point. This procedure was performed twice, with the mean of the 2 temperatures at which the participant reported pain serving as a measure of pain threshold. Participants then underwent separate protocols to measure Aδ fiber–mediated pain sensitivity and temporal summation [4, 24] in both the upper and lower extremities. These sites were chosen to observe the treatment response in a dermatome specific to the application site of the SMT (lower extremity) and a dermatome separate from the application site of the SMT (upper extremity). Participants were blinded to the temperature of the thermal stimuli, as well as to whether the Aδ fiber–mediated pain sensitivity or temporal summation protocol was being applied.
Aδ fiber–mediated pain sensitivity was assessed on the volar part of the nondominant forearm and the nondominant calf of each participant through the application of heat stimuli of 3 seconds’ duration. The thermode was applied with a baseline temperature of 35°C, which rose rapidly (10°C/s) to a peak of 45°, 47°, 49°, or 50°C. The sequence of testing the extremity (ie, calf and forearm) and the sequence of application of the finishing temperatures were determined randomly to prevent an order effect. Participants were asked to rate their “first” pain intensity using a 0 to 100 NRS. These ratings are believed to be mediated primarily by the Aδ fibers. [4, 24] The protocol was performed 2 times at each temperature at each extremity, with the average rating of each temperature analyzed. The thermode was moved slightly, and the researcher waited 60 seconds between trials in order to prevent habituation.
Temporal summation was assessed on the plantar surface of the nondominant foot and the palmar surface of the nondominant hand using a train of 10 consecutive heat pulses of less than 1 second duration at an inter-stimulus frequency of 0.33 Hz. The baseline temperature of each pulse was 35°C, and temperature peaked at 51°C. Participants were asked to rate their delayed (second) pain for each of the 10 pulses using an NRS. The sequence of testing was counterbalanced to prevent an order effect.
Interventions
Participants were instructed, as part of the informed consent process, that they would be randomly assigned to receive 1 of 3 interventions commonly used in the management of LBP. [49] Randomization was computer generated, with group assignments maintained in sealed, opaque, sequentially numbered envelopes. The envelopes were opened in sequential order based on entry in the study and after all baseline measures were completed for the participant. We elected to not incorporate a true control group in this study because we wanted all participants to have some form of intervention for their LBP. Furthermore, changes in temporal summation did not seem biologically plausible in a true control group, but was biologically plausible from performing other activity. All interventions were performed under the supervision of research staff to ensure adherence to the described parameters.Stationary bicycle. Hypoalgesia has been reported in response to general exercise. [50, 51] Participants rode a stationary bicycle and served as the nonspecific activity comparison group. The treatment dosage was 5 minutes at 60 to 70 rpm and 1 KPa.
Lumbar extension exercise. Participants performed a prone low back extension exercise and served as a specific activity comparison group. The exercise has been described previously in the literature for treatment of people with LBP, [52, 53] and several studies have demonstrated favorable outcomes in participants with LBP after performing this exercise. [54–58] Although not specific to this exercise, hypoalgesia has been reported in response to specific exercise in the cervical spine. [59] The treatment dosage was 3 sets of 15 repetitions within a 5-minute period.
Spinal manipulative therapy. This technique has been well described in the literature [15, 17] and was used in our prior studies of the immediate effects of SMT. [25, 60] Spinal manipulative therapy has been shown to be effective in the management of LBP in participants meeting a clinical prediction rule. [15, 17] The treatment dosage was performance of the SMT 2 times on each side of the pelvis, for a total of 4 manipulations in the 5-minute period, regardless of whether cavitation was experienced. Immediately following the assigned intervention, the previously described QST protocol was repeated.Sample Size Estimates
This study, to our knowledge, was the first to use thermal pain sensitivity as an outcome measure for SMT for participants with LBP. Therefore, limited data were available for a priori sample size calculation. We set a minimum recruitment threshold of 30 participants based on observed effect sizes from the previous study of participants who were healthy. [25]
Data Analysis
All statistical analysis was performed using SPSS for Windows version 16.0.† Significance was set at P≤.05 for all analyses because we were attempting to confirm an observation made in prior studies. [25, 60]
Descriptive statistics were generated for continuous and categorical measures. Univariate analysis of variance (ANOVA) was used to assess post-randomization differences in continuous variables of demographic, psychological, and baseline thermal testing measures. Chi-square analysis was used to assess for post-randomization differences in categorical demographic variables.
Our primary outcome of interest was change in thermal pain sensitivity over time (prior to and immediately following the assigned intervention). We used separate repeated-measures ANOVAs with group assignment as the between-subject factor and preintervention and postintervention measures of Aδ fiber–mediated pain sensitivity and temporal summation as the within-subject factor to assess for group differences in pain sensitivity. We included the ratings from only the 47°C and 49°C temperatures from the Aδ fiber–mediated pain to match the parameters of our previous study. Furthermore, 45°C is not a suprathreshold stimulus for all participants, and 50°C tends to be at tolerance, causing concerns for floor and ceiling effects, respectively. We performed this analysis in both the upper and lower extremities in order to observe for local and general effects of SMT. We used pair-wise comparisons, as indicated by the repeated-measures ANOVA results, to explore specific group changes.
Next, we wanted to observe for significant associations between psychological factors and changes in pain perception. Pearson correlation coefficients were calculated to observe for significant associations among psychological factors, measures of clinical pain, and changes in temporal summation.
Role of the Funding Source
The project was supported by grant AT002796 from the National Institutes of Health, National Center for Complementary and Alternative Medicine, awarded to Dr Bishop, Dr Robinson, and Dr George (principal investigator). Dr Bialosky also received support from grant AT002796 and from the National Institutes of Health T-32 Neural Plasticity Research Training Fellowship (T32HD043730). This work also was supported with resources from the Brain Rehabilitation Research Center, a VA Rehabilitation Research and Development Center of Excellence at the Malcom Randall VA Medical Center in Gainesville, Florida.
Results
Figure 1

Table 1
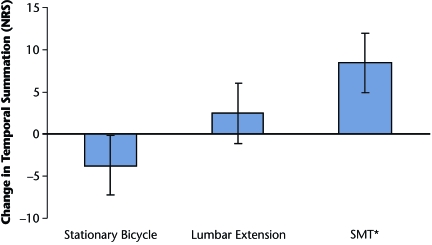
Figure 2

Table 2 Thirty-six individuals met the criteria for the study and agreed to participate, and all individuals completed the study (Figure 1). Number of excluded individuals and reasons for study exclusion were not tracked as part of this study. Group comparisons of baseline questionnaire and demographic information are presented in Table 1. Significant group differences were observed for sex and fear in baseline measures. Further exploration for covariate consideration indicated fear and sex were not significantly correlated with change in Aδ fiber–mediated pain sensitivity or temporal summation in either the upper or lower extremity (P>.05). Subsequently, we elected to exclude fear and sex as covariates in the subsequent analyses, as neither variable met the assumptions for covariance analysis.
Pain Response in Lumbar Innervated Region (Local Response)Aδ fiber–mediated pain sensitivity. The intervention groups did not differ in Aδ fiber–mediated pain sensitivity in the lower extremity to heat pulses of 47°C (P=.73) and 49°C (P=.96). Additionally, a main effect for time was not observed at 47°C (P=.31) or 49°C (P=.94), suggesting no change in Aδ fiber–mediated pain sensitivity occurred over time.
Temporal summation. A significant group (randomization) × time (preintervention to postintervention) interaction was observed for pain sensitivity to the temporal summation protocol in the lower extremity (F2,33=3.41, P=.05, partial η2=0.17), suggesting changes in temporal summation differed by group assignment (Table 2, Figure 2). Pair-wise comparisons indicated a significant hypoalgesic effect of temporal summation in the lower extremity of participants who received the SMT (mean decrease in pain perception=–8.5, SD=11.8; P=.03, Cohen d=0.37). Inhibition of temporal summation was not observed in those riding a stationary bicycle (mean increase in pain perception=3.7, SD=9.2; P=.19, Cohen d=0.16) or performing lumbar extension exercises (mean decrease in pain perception=–2.5, SD=13.1; P=.52, Cohen d=0.08).Pain Response in Cervical Innervated Region (General Response)
Aδ fiber–mediated pain sensitivity. The intervention groups did not differ in Aδ fiber–mediated pain sensitivity in the upper extremity to heat pulses of 47°C (P=.37) and 49°C (P=.53). Additionally, a main treatment effect for time was not observed at 47°C (P=.26) or 49°C (P=.49), suggesting no change in Aδ fiber–mediated pain sensitivity occurred over time.
Temporal summation. A significant group (randomization) × time (preintervention to postintervention) interaction (P=.40) was not observed in the upper extremity, indicating the lack of a group-dependent change in temporal summation. Instead, a main treatment effect for time (F1,31=6.78, P=.01, partial η2=0.18) was observed for temporal summation in the upper extremity (mean decrease in pain perception=–6.1, SD=13.5; Cohen d=0.22), suggesting all participants had a decrease in temporal summation.Association Between Psychological Factors and Changes in Thermal Pain Sensitivity

Table 3 Both pain catastrophizing (r=–.67, P=.02) and state anxiety (r=–.62, P=.04) were significantly associated with changes in Aδ fiber–mediated pain sensitivity in the lower extremity in participants who received SMT (Table 3). Psychological variables were not correlated with the change in temporal summation in the lower extremity observed in participants who received the SMT (P>.05). The largest association was a small to medium association with pain catastrophizing (r=.32).
Discussion
Spinal manipulative therapy is hypothesized to inhibit pain at the dorsal horn of the spinal cord through the alterations of neuroplastic changes consistent with central sensitization. [18] We have previously observed inhibition of temporal summation following SMT in participants who were healthy. [25, 60] We investigated the same phenomenon in the current study in participants experiencing LBP to determine the relevance of this observation in clinical populations. Similar to our prior study in participants who were healthy, [25] we observed inhibition of temporal summation in participants with LBP associated with SMT that was greater than the changes observed in response to riding a stationary bicycle or performing lumbar extension exercises. Inhibition of temporal summation appeared to be a local effect, as it was observed primarily in lumbar innervated areas (lower extremity) and was not strongly correlated with psychological variables. Furthermore, this effect did not appear to be a general blunted response to the QST protocol because it was not observed in the Aδ fiber–mediated pain perception.
This is the first study, to our knowledge, to observe SMT-associated alterations in temporal summation in a clinical sample. Recent rehabilitation interventions for individuals experiencing neurological impairments, such as those observed following a stroke or spinal cord injury, have attempted to influence neuroplasticity of the central nervous system to regain motor function. [61–34] For example, rather than prioritizing dysfunctions such as specific strength (force-generating capacity) and range-of-motion deficits, some rehabilitation protocols now attempt to promote neuroplastic changes in motor control through repetitive or forced use of an involved extremity. [62, 64, 65] Similarly, the observed inhibition of temporal summation suggests SMT may work through a neurophysiological mechanism specific to the alteration of neuroplastic changes associated with pain. Clinically, SMT frequently is applied in response to a biomechanical dysfunction such as decreased range of motion or a hypomobile joint. For example, an evaluative process is used to localize a dysfunctional vertebral segment followed by the application of a specific SMT technique to correct the noted problem. Despite this focus, the evaluative process for SMT generally is unreliable, [66, 67] and prior studies have documented a poor relationship between physical factors such as strength and range of motion and LBP-related outcomes. [68–70] Subsequently, clinical application of SMT may be better guided by the goal of decreasing pain than by the correction of a biomechanical lesion. Our findings of temporal summation inhibition specific to SMT (in comparison with riding a stationary bicycle and performing press-up exercises) suggest SMT may provide a novel stimulus effective in altering the neuroplastic changes associated with central sensitization and support current hypotheses about the counter-irritant properties of SMT. [18] Consequently, our findings provide preliminary support for the clinical use of SMT as a means to inhibit neuroplastic changes (eg, central sensitization) associated with LBP. The potential specificity of SMT's effect is corroborated by a lack of inhibition for Aδ fiber–mediated pain perception for participants with LBP.
Our findings of a local effect of SMT further suggest a focused neurophysiological response. Similar to our prior study, [25] we observed a localized hypoalgesic effect for SMT in participants experiencing LBP in which inhibition of temporal summation occurred in the dermatomes related to the area of SMT (leg) and not to an unrelated dermatome (upper extremity). Similarly, Dishman et al [71] observed changes in lumbar motoneuron pool excitability following SMT to the low back but not the cervical spine. Collectively, these studies suggest a local effect of SMT specific to the region of application. The current findings in a clinical sample and prior observations in participants who were healthy [25] suggest the neurophysiological responses associated with SMT to the low back are specific to the lumbar innervated regions.
Our findings of hypoalgesia to C fiber–mediated pain through the assessment of temporal summation suggest a spinal cord–mediated effect of SMT. Psychological factors are known to influence both outcomes associated with LBP [26–29] and response to experimental pain. [30, 37, 38] Subsequently, we wanted to observe the association of these factors to our outcome measure. Significant correlations between psychological factors and inhibition of temporal summation could suggest a descending, supraspinal mechanism of SMT related to fear, catastrophizing, or negative cognitions. Psychological associations with Aδ fiber–mediated pain perception were expected and parallel a previous study of thermal pain and LBP. [30] However, changes in temporal summation did not significantly correlate to any of the psychological measures, and all associations were relatively small, the largest being pain catastrophizing (r=.32). We have not observed a significant correlation between psychological factors and SMT-related changes in temporal summation in participants who were healthy [25] and now participants experiencing LBP. Collectively, these findings suggest the immediate inhibition of temporal summation related to SMT is independent of descending inhibition related to psychological factors or only minimally influenced by these factors.
Limitations
The current study represented our first investigation into the effects of SMT on temporal summation in a clinical sample and has several limitations. First, we made no attempt to blind the examiner to the intervention received by the individual participant. As a result, we cannot be certain that examiner bias did not play a role in our findings.
As this study was exploratory, we incorporated a sample of convenience and obtained a sample of participants with chronic LBP with lower pain intensity ratings than those typically enrolled in clinical trials. As a result, our findings may not be applicable to individuals with acute LBP or to those with a higher intensity of LBP.
We used temporal summation as an indirect measure of central sensitization. Animal studies have directly observed activity at the dorsal horn of the spinal cord with similar methods to induce temporal summation. [72–74] However, we currently are unable to directly visualize the spinal cord in human participants. Consequently, we cannot be certain that the associated findings of the current study were due to a direct effect mediated by the dorsal horn of the spinal cord. Additionally, pain is a multifaceted sensation relying on the peripheral nervous system in combination with the spinal cord and supraspinal centers, and the effect of SMT on pain likely results from an interaction of sites across the nervous system. [75] Spinal manipulative therapy has been suggested to influence pain through mechanisms related to the peripheral nervous system [76, 77] and the supraspinal centers. [78–80] Although our findings of diminished temporal summation suggest a spinal cord–mediated effect, other than assessing commonly reported psychological factors, we did not account for potential peripheral and supraspinal influences on our findings.
Our primary outcome in the current study was inhibition of temporal summation, and we did not assess clinical pain ratings following treatment. The study was intended to investigate a specific mechanism of SMT, and the benefit of studying an experimental pain model was the ability to control the magnitude of the painful stimulus. The study was not intended to measure immediate changes in clinical pain intensity because we did not plan to track outcomes past immediate follow-up. Our protocol provided an indirect measure of a particular pain pathway believed to be important in the development of chronic pain conditions, but we are unable to comment upon the relationship between our findings and changes in clinical pain, as we did not include clinical pain ratings as an outcome measure.
Future Studies
The current study focused on a potential spinal cord–mediated mechanism of SMT. The mechanisms through which SMT influence musculoskeletal pain are likely related to multiple interactions throughout the nervous system. [75] Subsequently, future studies should attempt to replicate the current findings while controlling for or manipulating additional potential factors such as those related to peripheral mechanisms and descending inhibition from supraspinal levels. Researchers in future studies also may want to include multiple sessions in order to assess dose response to SMT and to observe for longitudinal relationships between changes in temporal summation and clinical pain. Prior reviews [13, 14] suggest SMT is effective in the treatment of people with LBP; however, the evidence is stronger when homogeneous samples are included based on signs and symptoms suggesting a positive response. [15–17] In future studies, researchers may want to include a sample of participants meeting a clinical prediction rule suggesting the likelihood of a positive clinical response.
Conclusions
There were no differences in inhibition of Aδ fiber–mediated pain sensitivity for SMT in comparison with lumbar exercise and riding a stationary bicycle. The current study is the first to report that SMT specifically inhibits temporal summation in individuals with LBP. Additionally, this inhibition appears to be local, as it was observed only in the lower extremity and psychological factors were not strongly associated with resultant inhibition of temporal summation. These findings suggest that inhibition of temporal summation is a potential mechanism for pain relief following SMT for individuals with LBP.
The Bottom Line
The Bottom Line is a clinical summary that translates study findings for application to practice. It is not intended to substitute for a critical reading of the research article. Summaries are written by invited writers.
The Bottom Line Review
Footnotes
Dr Bialosky, Dr Bishop, Dr Robinson, and Dr George provided concept/idea/research design and writing. Dr Bialosky and Mr Zeppieri provided data collection. Dr Bialosky provided data analysis. Dr George provided fund procurement. All authors provided consultation (including review of manuscript before submission).
This study was approved by the Institutional Review Board of the University of Florida.
A platform presentation of this research was given at the Combined Sections Meeting of the American Physical Therapy Association; February 6–9, 2008; Nashville, Tennessee.
The project was supported by grant AT002796 from the National Institutes of Health, National Center for Complementary and Alternative Medicine, awarded to Dr Bishop, Dr Robinson, and Dr George (principal investigator). Dr Bialosky also received support from grant AT002796 and from the National Institutes of Health T-32 Neural Plasticity Research Training Fellowship (T32HD043730). This work also was supported with resources from the Brain Rehabilitation Research Center, a VA Rehabilitation Research and Development Center of Excellence at the Malcom Randall VA Medical Center in Gainesville, Florida.
This study was registered at www.clinicaltrials.gov under the identifier of NCT00922220.
*Medoc Ltd, Ha'dekel St, Ramat Yishai, Israel 30095.
†SPSS Inc, 233 S Wacker Dr, Chicago, IL 60606.
References:
Winkelstein BA.
Mechanisms of central sensitization, neuroimmunology, and injury biomechanics
in persistent pain: implications for musculoskeletal disorders.
J Electromyogr Kinesiol 2004;14:87–93Rygh LJ, Svendsen F, Fiska A, et al.
Long-term potentiation in spinal nociceptive systems: how acute pain may become chronic.
Psychoneuroendocrinology 2005;30:959–964Staud R, Domingo M.
Evidence for abnormal pain processing in fibromyalgia syndrome.
Pain Med 2001;2:208–215Staud R, Vierck CJ, Cannon RL, et al.
Abnormal sensitization and temporal summation of second pain (wind-up)
in patients with fibromyalgia syndrome.
Pain 2001;91:165–175Staud R, Robinson ME, Price DD.
Temporal summation of second pain and its maintenance are useful
for characterizing widespread central sensitization of fibromyalgia patients.
J Pain 2007;8:893–901Ayesh EE, Jensen TS, Svensson P.
Hypersensitivity to mechanical and intra-articular electrical stimuli in persons
with painful temporomandibular joints.
J Dent Res 2007;86:1187–1192Mohn C, Vassend O, Knardahl S.
Experimental pain sensitivity in women with temporomandibular disorders and
pain-free controls: the relationship to orofacial muscular contraction
and cardiovascular responses.
Clin J Pain 2008;24:343–352Sterling M, Jull G, Vicenzino B, Kenardy J.
Characterization of Acute Whiplash-associated Disorders
Spine (Phila Pa 1976). 2004 (Jan 15); 29 (2): 182–188Curatolo M, Petersen-Felix S, Arendt-Nielsen L, et al.
Central hypersensitivity in chronic pain after whiplash injury.
Clin J Pain 2001;17:306–315Diers M, Koeppe C, Diesch E, et al.
Central processing of acute muscle pain in chronic low back pain patients:
an EEG mapping study.
J Clin Neurophysiol 2007;24:76–83O'Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L.
Generalized deep-tissue hyperalgesia in patients with chronic low-back pain.
Eur J Pain 2007;11:415–420Giesecke T, Gracely RH, Grant MA, et al.
Evidence of augmented central pain processing in idiopathic chronic low back pain.
Arthritis Rheum 2004;50:613–623Bronfort G Haas M Evans RL et al.
Efficacy of Spinal Manipulation and Mobilization for Low Back Pain and Neck Pain:
A Systematic Review and Best Evidence Synthesis
Spine J (N American Spine Soc) 2004 (May); 4 (3): 335–356Koes BW, Assendelft WJ, van der Heijden GJ, Bouter LM.
Spinal manipulation for low back pain: an updated systematic review
of randomized clinical trials.
Spine 1996;21:2860–2871Childs JD, Fritz JM, Flynn TW, Irrgang JJ, Johnson KK, Majkowski GR, et al.
A Clinical Prediction Rule To Identify Patients With Low Back Pain Most Likely To Benefit
from Spinal Manipulation: A Validation Study
Annals of Internal Medicine 2004 (Dec 21); 141 (12): 920–928Cleland JA, Fritz JM, Whitman JM, et al.
The use of a lumbar spine manipulation technique by physical therapists in
patients who satisfy a clinical prediction rule: a case series.
J Orthop Sports Phys Ther 2006;36:209–214Flynn T, Fritz JM, Whitman JM, et al.
A Clinical Prediction Rule for Classifying Patients with Low Back Pain who
Demonstrate Short-term Improvement with Spinal Manipulation
Spine (Phila Pa 1976). 2002 (Dec 15); 27 (24): 2835–2843Boal RW, Gillette RG.
Central neuronal plasticity, low back pain and spinal manipulative therapy.
J Manipulative Physiol Ther 2004;27:314–326Fernández-Carnero J, Fernández-de-Las-Peñas C, Cleland JA.
Immediate hypoalgesic and motor effects after a single cervical spine manipulation
in subjects with lateral epicondylalgia.
J Manipulative Physiol Ther 2008;31:675–681Fernández-de-Las-Peñas C, Alonso-Blanco C, Cleland JA, et al.
Changes in Pressure Pain Thresholds Over C5-C6 Zygapophyseal Joint After a
Cervicothoracic Junction Manipulation in Healthy Subjects
J Manipulative Physiol Ther. 2008 (Jun); 31 (5): 332–337Mohammadian P, Gonsalves A, Tsai C, Hummel T, Carpenter T.
Areas of Capsaicin-Induced Secondary Hyperalgesia and Allodynia Are Reduced by a
Single Chiropractic Adjustment: A Preliminary Study
J Manipulative Physiol Ther. 2004 (Jul); 27 (6): 381–387Vernon, H.
Qualitative Review of Studies of Manipulation-induced Hypoalgesia
J Manipulative Physiol Ther 2000 (Feb); 23 (2): 134–138Vernon HT, Aker P, Burns S, et al.
Pressure pain threshold evaluation of the effect of spinal manipulation
in the treatment of chronic neck pain: a pilot study.
J Manipulative Physiol Ther 1990;13:13–16Price DD, Staud R, Robinson ME, et al.
Enhanced temporal summation of second pain and its central modulation
in fibromyalgia patients.
Pain 2002;99:49–59George SZ, Bishop MD, Bialosky JE, et al.
Immediate effects of spinal manipulation on thermal pain sensitivity:
an experimental study.
BMC Musculoskelet Disord 2006;7:68.Hill JC, Dunn KM, Lewis M, et al.
A Primary Care Back Pain Screening Tool: Identifying Patient Subgroups For Initial Treatment
(The STarT Back Screening Tool)
Arthritis Rheum. 2008 (May 15); 59 (5): 632–641Fritz JM, George SZ, Delitto A.
The role of fear-avoidance beliefs in acute low back pain:
relationships with current and future disability and work status.
Pain 2001;94:7–15Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA.
Reduction of pain catastrophizing mediates the outcome of both physical and
cognitive-behavioral treatment in chronic low back pain.
J Pain 2006;7:261–271Spinhoven P, Ter KM, Kole-Snijders AM, et al.
Catastrophizing and internal pain control as mediators of outcome
in the multidisciplinary treatment of chronic low back pain.
Eur J Pain 2004;8:211–219George SZ, Wittmer VT, Fillingim RB, Robinson ME.
Sex and pain-related psychological variables are associated with thermal pain
sensitivity for patients with chronic low back pain.
J Pain 2007;8:2–10Bolton JE, Wilkinson RC.
Responsiveness of pain scales: a comparison of three pain intensity measures
in chiropractic patients.
J Manipulative Physiol Ther 1998;21:1–7DeLoach LJ, Higgins MS, Caplan AB, Stiff JL.
The visual analog scale in the immediate postoperative period:
intrasubject variability and correlation with a numeric scale.
Anesth Analg 1998;86:102–106Hartrick CT, Kovan JP, Shapiro S.
The numeric rating scale for clinical pain measurement: a ratio measure?
Pain Pract 2003;3:310–316Jensen MP, Karoly P, Braver S.
The measurement of clinical pain intensity: a comparison of six methods.
Pain 1986;27:117–126George SZ, Dannecker EA, Robinson ME.
Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither
factor predicts tolerance or blood pressure reactivity: an experimental
investigation in pain-free individuals.
Eur J Pain 2006;10:457–465Osman A, Barrios FX, Gutierrez PM, et al.
The Pain Catastrophizing Scale: further psychometric evaluation with adult samples.
J Behav Med 2000;23:351–365Osman A, Breitenstein JL, Barrios FX, et al.
The Fear of Pain Questionnaire–III: further reliability and validity
with nonclinical samples.
J Behav Med 2002;25:155–173Schmidt NB, Cook JH.
Effects of anxiety sensitivity on anxiety and pain during a cold pressor
challenge in patients with panic disorder.
Behav Res Ther 1999;37:313–323McNeil DW, Rainwater AJ., III
Development of the Fear of Pain Questionnaire–III.
J Behav Med 1998;21:389–410Roelofs J, Peters ML, Deutz J, et al.
The Fear of Pain Questionnaire (FPQ): further psychometric examination
in a non-clinical sample.
Pain 2005;116:339–346Woby SR, Roach NK, Urmston M, Watson PJ.
Psychometric properties of the TSK-11: a shortened version of the
Tampa Scale for Kinesiophobia.
Pain 2005;117:137–144Rosenstiel AK, Keefe FJ.
The use of coping strategies in chronic low back pain patients:
relationship to patient characteristics and current adjustment.
Pain 1983;17:33–44Robinson ME, Riley JL, III, Myers CD, et al.
The Coping Strategies Questionnaire: a large-sample, item-level factor analysis.
Clin J Pain 1997;13:43–49Keefe FJ, Brown GK, Wallston KA, Caldwell DS.
Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy.
Pain 1989;37:51–56Stewart MW, Harvey ST, Evans IM.
Coping and catastrophizing in chronic pain: a psychometric analysis
and comparison of two measures.
J Clin Psychol 2001;57:131–138Spielberger CD, Gorsuch RL, Lushene RE, et al.
Manual for the State and Trait Anxiety Inventory (Form Y)
Palo Alto, CA: Consulting Psychologists Press; ;1983Schmidt NB, Joiner TE.
Structure of the Anxiety Sensitivity Index psychometrics and factor
structure in a community sample.
J Anxiety Disord 2002;16:33–49Dehon C, Weems CF, Stickle TR, et al.
A cross-sectional evaluation of the factorial invariance of anxiety
sensitivity in adolescents and young adults.
Behav Res Ther 2005;43:799–810Jette AM, Delitto A.
Physical therapy treatment choices for musculoskeletal impairments.
Phys Ther 1997;77:145–154Kemppainen P, Pertovaara A, Huopaniemi T, et al.
Modification of dental pain and cutaneous thermal sensitivity
by physical exercise in man.
Brain Res 1985;360:33–40Droste C, Greenlee MW, Schreck M, Roskamm H.
Experimental pain thresholds and plasma beta-endorphin levels during exercise.
Med Sci Sports Exerc 1991;23:334–342Delitto A, Erhard RE, Bowling RW.
A treatment-based classification approach to low back syndrome:
identifying and staging patients for conservative treatment.
Phys Ther 1995;75:470–485McKenzie RA.
The Lumbar Spine: Mechanical Diagnosis and Treatment
Waikanaie, New Zealand: Spinal Publications Ltd; ;1989Donelson R, Grant W, Kamps C, Medcalf R.
Pain response to sagittal end-range spinal motion: a prospective,
randomized, multicentered trial.
Spine 1991;16(6 suppl):S206–S212Donelson R, Silva G, Murphy K.
Centralization phenomenon: its usefulness in evaluating and treating referred pain.
Spine 1990;15:211–213Werneke M, Hart DL.
Centralization phenomenon as a prognostic factor for
chronic low back pain and disability.
Spine 2001;26:758–764Werneke M, Hart DL, Cook D.
A descriptive study of the centralization phenomenon: a prospective analysis.
Spine 1999;24:676–683Werneke MW, Hart DL.
Categorizing patients with occupational low back pain by use of the
Quebec Task Force Classification System versus pain pattern classification
procedures: discriminant and predictive validity.
Phys Ther 2004;84:243–254O'Leary S, Falla D, Hodges PW, et al.
Specific therapeutic exercise of the neck induces immediate local hypoalgesia.
J Pain 2007;8:832–839Bialosky JE, Bishop MD, Robinson ME, et al.
The Influence of Expectation on Spinal Manipulation Induced Hypoalgesia:
An Experimental Study in Normal Subjects
BMC Musculoskelet Disord. 2008 (Feb 11); 9: 19Barriere G, Leblond H, Provencher J, Rossignol S.
Prominent role of the spinal central pattern generator in the recovery
of locomotion after partial spinal cord injuries.
J Neurosci 2008;28:3976–3987Behrman AL, Bowden MG, Nair PM.
Neuroplasticity after spinal cord injury and training: an emerging
paradigm shift in rehabilitation and walking recovery.
Phys Ther 2006;86:1406–1425Mark VW, Taub E, Morris DM.
Neuroplasticity and constraint-induced movement therapy.
Eura Medicophys 2006;42:269–284Gauthier LV, Taub E, Perkins C, et al.
Remodeling the brain: plastic structural brain changes produced by
different motor therapies after stroke.
Stroke 2008;39:1520–1525Behrman AL, Harkema SJ.
Physical rehabilitation as an agent for recovery after spinal cord injury.
Phys Med Rehabil Clin N Am 2007;18:183–202Seffinger MA, Najm WI, Mishra SI, et al.
Reliability of spinal palpation for diagnosis of back and neck pain:
a systematic review of the literature.
Spine 2004;29:E413–E425Troyanovich SJ, Harrison DD, Harrison DE.
Motion palpation: it's time to accept the evidence.
J Manipulative Physiol Ther 1998;21:568–571Poitras S, Loisel P, Prince F, Lemaire J.
Disability measurement in persons with back pain: a validity study of
spinal range of motion and velocity.
Arch Phys Med Rehabil 2000;81:1394–1400Nattrass CL, Nitschke JE, Disler PB, et al.
Lumbar spine range of motion as a measure of physical and functional
impairment: an investigation of validity.
Clin Rehabil 1999;13:211–218Gross DP, Battié MC.
The prognostic value of functional capacity evaluation in patients
with chronic low back pain, part 2: sustained recovery.
Spine 2004;29:920–924Dishman JD, Cunningham BM, Burke J.
Comparison of tibial nerve H-reflex excitability after cervical and
lumbar spine manipulation.
J Manipulative Physiol Ther 2002 ;25:318–325Craig AD, Andrew D.
Responses of spinothalamic lamina I neurons to repeated brief contact
heat stimulation in the cat.
J Neurophysiol 2002;87:1902–1914Duggan AW, Hope PJ, Jarrott B, et al.
Release, spread and persistence of immunoreactive neurokinin A in the
dorsal horn of the cat following noxious cutaneous stimulation:
studies with antibody microprobes.
Neuroscience 1990;35:195–202Jeftinija S, Urban L.
Repetitive stimulation induced potentiation of excitatory transmission
in the rat dorsal horn: an in vitro study.
J Neurophysiol 1994;71:216–228Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ.
The Mechanisms of Manual Therapy in the Treatment of Musculoskeletal Pain:
A Comprehensive Model
Man Ther. 2009 (Oct); 14 (5): 531–538Degenhardt BF, Darmani NA, Johnson JC, et al.
Role of osteopathic manipulative treatment in altering pain biomarkers:
a pilot study.
J Am Osteopath Assoc 2007;107:387–400McPartland JM, Giuffrida A, King J, et al.
Cannabimimetic effects of osteopathic manipulative treatment.
J Am Osteopath Assoc 2005;105:283–291Wright A.
Hypoalgesia post-manipulative therapy: a review of a potential
neurophysiological mechanism.
Man Ther 1995;1:11–16Sterling M, Jull G, Wright A.
Cervical mobilisation: concurrent effects on pain, sympathetic nervous system
activity and motor activity.
Man Ther 2001;6:72–81Vicenzino B, Collins D, Benson H, Wright A.
An investigation of the interrelationship between manipulative therapy-induced
hypoalgesia and sympathoexcitation.
J Manipulative Physiol Ther 1998;21:448–453
Return to SUBLUXATION
Return to SPINAL PAIN MANAGEMENT
Since 4–30–2017


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |