

A Proposed Vertebral Subluxation Model
Reflecting Traditional Concepts and
Recent Advances in Health and ScienceThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Vertebral Subluxation Research 1996 (Aug); 1 (1): 1–12 ~ FULL TEXT
William R. Boone Ph.D, D.C., and Graham J. Dobson, D.C
104 Berkeley,
Irvine CA 92612
Part one of an expanded vertebral subluxation model (VSM) is presented which considers information from the traditional concept of vertebral subluxation, and other models including; the chiropractic subluxation complex, the vertebral subluxation complex, and the vertebral subluxation complex model. Other components, including health assessment and etiology, are to be introduced in the second part, and appropriate research designs for studying the expanded VSM are to be presented in the third part. All three parts discuss other models as well as classical and recent research findings which support the expanded VSM.
Key Words :Vertebral Subluxation Model (VSM), Early Vertebral Subluxation Model (EVSM),Vertebral Subluxation Complex (VSC), Chiropractic Subluxation Complex (CSC),Vertebral Subluxation Complex Model (VSCM), mental impulse, chiropractic.
From the FULL TEXT Article:
Introduction
This article proposes an expanded vertebral subluxation model (VSM) based on B.J. Palmer’s concept, contemporary models, and other information provided through research and discussion. It is anticipated that the expanded model will stimulate future research, case studies, and other reports which will impact on its veracity. This activity will insure that the concept of vertebral subluxation, and parameters associated with that condition, are readily available to the scientific community as a whole, the chiropractic profession specifically, and ultimately the lay public. Presentation of the VSM is divided into three parts, each considering pertinent aspects of other contemporary models as they relate to the VSM. This article deals with the physiological and biomechanical components which contribute to the model. The next article will elaborate the health and etiological components of the proposed VSM, and how the presence and correction of vertebral subluxation are related to these components. A third article will present a thorough discussion of current research methodolgy which permits testing of the VSM.
The development of an expanded VSM is a critical issue. As acceptance of chiropractic as a non-allopathic health care discipline broadens, it is essential to develop a model reflecting the full spectrum of components of the vertebral subluxation. This will provide a clearer depiction of the concept by incorporating those aspects of its early theory which have been evidenced, as well as recent findings which pass the test of repeatability. For instance, the concept of vertebral subluxation proposed by B.J. Palmer, [1] herein referred to as the Early Vertebral Subluxation Model (EVSM), clearly outlines four fundamental, but hypothetical components;
vertebral misalignment,
occlusion of a spinal or intervertebral foramen,
pressure on nerves, and
interference with the quantity flow of the mental impulse.
Since the literature contains considerable research which impacts positively and negatively on Palmer’s hypothesis, it would be naive to categorically accept or negate the theory based on the current state of investigation regarding its tenets.
Evolving the Early Vertebral Subluxation Model
One purpose of research is to evidence, pro or con, a given theory (hypothesis). Palmer’s EVSM provides a theory which, to date, has not been adequately tested. This has been rationalized, to some extent, by the belief that certain elements of the subluxation theory are untestable. [2, 3] Based on the counter view that virtually any theory can be developed into a testable hypothesis, it is accurate to state that, although certain elements of the subluxation theory may be untestable by a particular research approach, those same elements are testable using other approaches. The challenge is to generate questions which adequately reflect the hypothesis, and then determine the research methods most appropriate to investigate those questions. The third article in this series will describe and discuss research approaches most applicable to investigating the current VSM, which incorporates concepts from the EVSM of Palmer as well as new information.
The rationale for developing an expanded VSM stems from the observation that the profession, in regard to vertebral subluxation research, has put the cart before the horse or omitted the cart or the horse. That is, Palmer’s original concept of the vertebral subluxation has been redefined and/or remodeled in the absence of convincing research to justify such change. While these changes have been discussed by other authors, [4, 5] some examples are presented here to elaborate the rationale for developing the VSM presented in this series of articles. However, just as change to the EVSM in the absence of scientific evidence is unreasonable, it is also unreasonable to advocate, on philosophical grounds alone, that the EVSM be accepted. The intent of developing an expanded model, incorporating traditional concepts as well as new information, is to re-balance or re-set the approach utilized in investigating the vertebral subluxation.
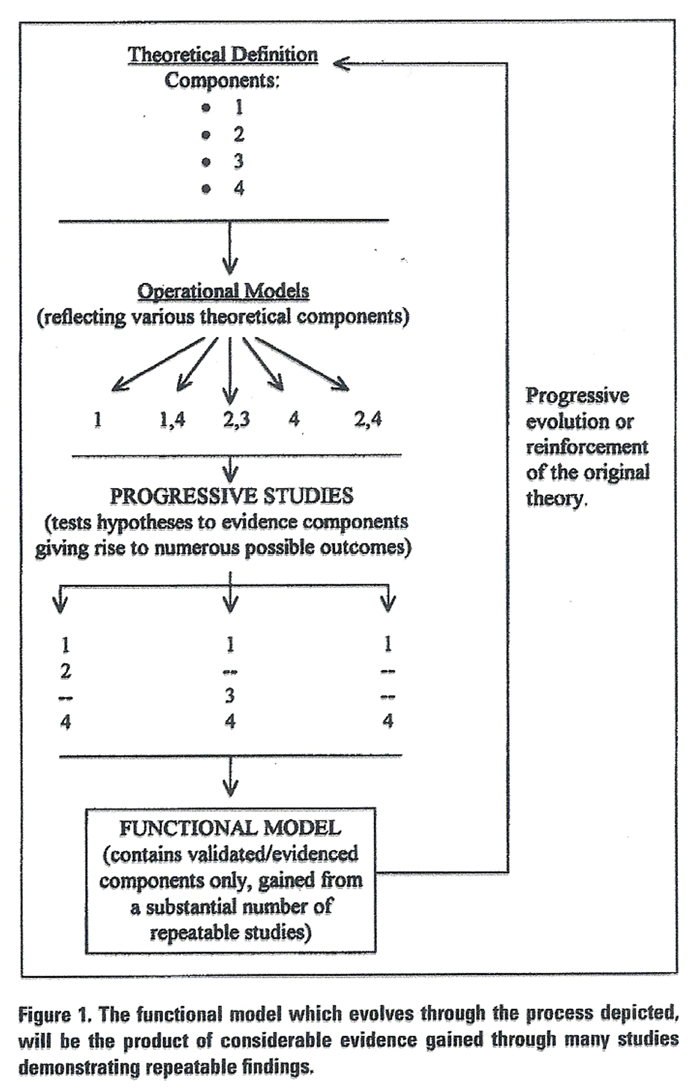
Figure 1 In order to advance the profession through appropriate study of its theoretical tenets, it is essential to establish definitions of the vertebral subluxation concept which reflect the theoretical base, not ignore it. Definitions serve the purpose of phrasing theory in terms that can be ultimately translated into quantifiable expression. The functional definition thus becomes a basis for developing operational definitions which describe the specifics of a concept. Operational definitions are usually the tool of the researcher. They define in terms of how to measure the phenomenon under investigation and usually contain a hypothesis to be tested. Figure 1 shows the continuous relationship between the formulation of a theory (which would include its definition), and the research process which provides information regarding the veracity of its components and affects its evolution.
The importance of definition has created a conundrum for the profession. Instances of rephrasing the concept of vertebral subluxation accompanied by redefinition or omission of part of its theory, have removed it from the overall concept as proposed by Palmer. This is exemplified by defining its clinical manifestations as a syndrome, or “...aggregate of signs and symptoms that relate to pathophysiology or dysfunction of spinal and pelvic motion segments or to peripheral joints.” [6] Thus, an interesting phenomenon has arisen in chiropractic. In the absence of research to justify the change, several different functional definitions have been developed which have supplanted the theoretical definition of Palmer. This is evident when reviewing the accepted definitions of subluxation by the profession’s two largest organizations. Although both are functional, neither reflects the theoretical considerations found in the EVSM of Palmer.
The first definition has been accepted by the International Chiropractor’s Association since 1987. [7]“Any alteration of the bio-mechanical and physiological dynamics of the contiguous spinal structures which can cause neuronal disturbances.”
The American Chiropractic Association has defined subluxation [8] as follows:
“An aberrant relationship between two adjacent structures that may have functional or pathological sequelae, causing an alteration in the biomechanical and/or neurophysiological reflections of these articular structures, their proximal structures, and/or body systems that may be directly affected by them.”
While no judgment is intended, it is important to draw attention to these functional definitions in terms of the respective models each frames for study. The ICA definition implies vertebral subluxation by restricting the expression of altered dynamics to the spine and contiguous structures, while the other makes no mention of the spine or its contiguous structures. However, the definition adopted by the ICA diverts from specificity by incorporating the expression “neuronal disturbances” which could of course include any disturbance. The movement away from the specificity of Palmer’s EVSM as a condition of the spine and its contiguous articulations, to any anatomical articulation is evident in the definition adopted by the ACA, thus opening the subluxation research direction to include any two adjacent surfaces. Nevertheless, both definitions recognize that involvement of the nervous system is essential to a subluxation definition, albeit the concept of interference to the mental impulse is omitted or generalized into a melange of other phenomena.
Even if altering the EVSM can be justified for the sake of framing a model for investigation, a research model reflecting either of the definitions above still remains incomplete. In both of these functional definitions, consideration of the mental impulse is absent. Consequently, the concept has virtually disappeared from contemporary subluxation research, via the definitions proposed by these organizations, or been equated with the action potential. [9] This omission has been unfortunate, and is not likely to add light to the problem of elucidating the vertebral subluxation. Morat, [10] holding this same view has stated, “...in science as elsewhere, it is always imprudent to run foul of information given by common sense, and a problem is not solved when one of the terms is omitted.”
Ironically, the transition away from the EVSM has been fostered by clarification efforts. The quality work accomplished by several chiropractors to conceive and describe the Vertebral Subluxation Complex (VSC), and to arrange it in the form of a model, has contributed immensely to descriptions of certain aspects of the EVSM and has elaborated a degeneration sequelae likely to follow if those aspects remain uncorrected. However, as will be discussed in this article, the VSC in its current conceptual and modeled form, lacks inclusion or consideration of the full spectrum of components originally associated with the term. Consequently, without a contemporary model to link current research with the EVSM of B.J. Palmer, the traditional view of vertebral subluxation is likely to disappear, not as a result of research failing to demonstrate its veracity, but as a consequence of remolding and redefining efforts to make it a more acceptable term for other disciplines.
It has been proposed that an operational definition, beyond theoretical considerations, be developed by a consensus approach to establish testable components of the subluxation theory. [11] In partial agreement with this perspective, it is apparent that operational models must be developed from which to conduct investigative studies. It is suggested, however, that the operational definitions should be derived from functional definitions reflecting the theory, not going beyond it or excluding it. Additionally, rather than develop a profession wide consensus definition, it seems more appropriate to the research paradigm for continuous operational definitions to arise as a consequence of the need to conduct scientific studies involving different investigative designs. This approach would allow for the testing of new perspectives and traditional concepts. In this regard, the current VSM is based on a functional definition which reflects the components of the original theoretical definition in a manner providing several areas for operational definitions appropriate for testing through innovative research. The VSM emerging from this process will eventually reflect a consensus of appropriately accumulated research, rather than a consensus of opinion.
In order to properly assess the EVSM, as proposed by Palmer, as well as evolving new concepts regarding the vertebral subluxation, it is necessary to investigate all pertinent parameters, theoretical and demonstrated. It is clear that the onus to conduct appropriate studies relative to this topic, rests with the institutions which advocate and educate students in regard to correction of vertebral subluxation, field practitioners who witness the health benefits of subluxation correction in their patients, and technique developers who provide innovative approaches to the detection and correction of vertebral subluxation. In an attempt to assist that process, the present and subsequent articles deal with events and information which impact specifically on the evolution of the concept of vertebral subluxation. The information is presented with the anticipation that it will contribute to a progressive depiction of this condition reflecting the latest advances in research.
Since there are several well recognized scientific methods of investigation, which will be discussed in the third part of the VSM, it is essential that several “operational” definitions which reflect the full spectrum of vertebral subluxation theory be presented for study by these methods. As the significance of this perspective, relative to the development of the proposed VSM, is presented, the insight of Keating [12] seems pertinent:“What of subluxation? Will we ever set aside the political uses of these supposed spinal boo-boos long enough to operationally define and investigate their potential role in health and illness? If we were to cease propagandizing subluxation as “the silent killer” and study it as a serious scientific phenomenon, who knows what we might find? Yet, so long as we insist on emphasizing the chiropractic lesion as our political “raison d’etre,” the real “tragedy of the subluxation” will continue to be our ignorance of it.”
The present model is designed to accept this challenge. In doing so, it becomes necessary to develop a functional definition which also encompasses parameters dealing with etiology and health and to include, for scientific investigation, those aspects of the vertebral subluxation which are likely to impact on these phenomena.
Establishing the Groundwork
A plausible vertebral subluxation model must consider findings from a combination of research approaches which, through a significant body of direct or indirect evidence, supports or negates any proposed components of vertebral subluxation. Among these are investigations, including descriptive and observational studies, which evaluate vertebral subluxation theory by testing its hypotheses; clinical research which investigates the validity, reliability, and accuracy of techniques; and protocols which affect correction of the condition. Additionally, appropriately designed and controlled clinical studies to assess changes in various types of organismal dysfunction at the biomechanical, physiological, and biochemical levels, as a function of vertebral subluxation and its correction, are essential. Also, Self-reported patient outcomes investigating the efficacy of subluxation correction care are also important in evaluating changes in health and health-related quality of life issues “before” and “during” different stages of lifetime care. A complete model representing a multi-dimensional phenomenon must also consider epidemiological studies assessing the etiology of vertebral subluxation as it relates to environmental, emotional, physical, physiological and biochemical stress. Case studies which impact on parameters of subluxation-correction care including etiology, clinical methodology, patient responses to care in terms of overall health, and reports of interesting reversals in dysfunction related to vertebral subluxation correction, are tantamount to the development of a complete VSM.
Because of the multi-dimensional nature of vertebral subluxation, special attention must be given to the methods used to assess its presence and arrest, reduce, or correct the condition. This consideration requires that a VSM include the various techniques and approaches which are specifically directed at these objectives. Since subluxation-based chiropractic care is abundantly endowed with techniques, it is incumbent upon the various developers and advocates of a particular approach to foster and conduct research which provides unbiased evaluation of the technique’s efficacy in regard to the detection and correction of vertebral subluxation. For those techniques, or other clinical approaches, which show unbiased evidence of efficacy and positive outcomes, it is then appropriate to include information which relates the technique to its purported level of intervention in the VSM.
Since there are acknowledged and documented differences in clinical objectives, protocols, and practice philosophies within the profession, [13] a single functional definition developed to encompass such a broad base would, of course, lose its specificity. The VSM presented in this article is, therefore, reflective of the following functional definition of vertebral subluxation:A vertebral subluxation is a potentially reversible and/or preventable alteration of the intervertebral relationships of one or more articulations of the spinal column or its immediate weight bearing components of the axial skeleton; accompanied by a change in the morphology of the tissue occupying the neural canal and/or intervertebral foramina; as well as an alteration of neural function sufficient to interfere with the transmission of organizing information, believed to be homologous to the mental impulse, thus contributing to negative health outcomes.
The major contribution to the development of this definition was made by a group of chiropractors and other educators, and has been modified and adopted specifically for the current model. [14] It is not intended to reflect a profession-wide consensus, but it does provide testable tenets, serving as a basis for the development of many different operational definitions. While it includes the four components of the EVSM, it does not exclude the advent of additional information, nor suggest a definitive sequence in which the components develop or are expressed. The definition includes recognition that neurological function may be altered at the nerve conduction level, but for a vertebral subluxation to be present the alteration need only be sufficient to result in an inhibition or cessation of the flow of “organizing information,” proposed to negatively affect health. Organizing information is viewed, in this definition, as being homologous with the “mental impulse.” A case is made for this position as the VSM is presented.
The Vertebral Subluxation Model
In developing the present model, it is necessary to first consider where investigation has led in regard to an understanding of the traditionally proposed components of vertebral subluxation.
The Early Vertebral Subluxation Model
From the inception of chiropractic in 1895, the groundwork was established for elucidating the significance of the “adjustment” performed by D.D. Palmer. The observation that hearing could be affected by thrusting into the spine still remains a point of controversy and inquiry. Nevertheless, following that momentous event, the work of B.J. Palmer led to the development of a definition of the concept of vertebral subluxation referred to in this article as the EVSM.
As previously described, the EVSM included four theoretical components;(1) a vertebra out of normal alignment to its corespondents above and below, which
(2) does occlude a foramen (spinal or intervertebral), which
(3) does produce pressures upon nerves, thereby
(4) interfering and interrupting the normal quantity flow of mental impulse supply between brain and body.Palmer also added that this condition thus becomes the cause of all dis-ease. The term dis-ease is described and discussed in the second article concerning the VSM. Considerable research has been conducted in a variety of disciplines which impact on the components identified in the EVSM. Other review articles have covered and evaluated many of these studies thoroughly. [15–19] The present article, therefore, refers to a select number of investigations which, in the opinion of the authors, are especially germane to the issue of the present VSM.
Vertebral Misalignment
Static and dynamic elements of vertebral misalignment are apparent at the gross level through plain film radiographic imaging, [20–22] and segmental and intersegmental movement, in general, by videofluroscopy, [23, 24] and magnetic resonance imaging. [25] Detection of change in vertebral position on static x-ray can be enhanced by biplanar radiographic analysis, [26] and dynamics associated with changes in vertebral position have been well documented for the cervical spine, described by Jirout as synkinesis, [27–29] and Burns [30] as coupling of lateral flexion and rotation.
It is important to note that measurement of aberrations in the millimeter range, or when changes are only separated by a degree, require a high level of accuracy. While reports [31, 32] vary in terms of inter-and intra-examiner reliability of various marking systems, Rochester [33] found “acceptable” to “very good” inter and intra-examiner reliability among four practitioners analyzing, and re-analyzing 10 sets of upper cervical film. Parameters which were examined included atlas, odontoid, C2 spinous, and lower angle lateralities as well as other misalignments. Although examiners used both manual and computer-assisted analysis, reliability estimates ranged from 0.83–0.96 for all measurements of laterality, and 0.54 to 0.68 for rotational misalignments. Other measurements also reflected similar ranges of reliability.
Even in consideration of the ubiquitous presence of human and mechanical error, it is evident that changes in alignment of vertebra can be determined with accuracy, precision, and reliability at the inter- and intra-examiner levels. The detection of aberrations of vertebral alignment leaves little doubt that misalignment of vertebral segments exists, and is detectable with good reliability by skilled chiropractors. It is also evident that continuing studies in this area are important for assessing quality control at the practice level and determining the best methods of detecting vertebral misalignments. Similar studies can also be used by chiropractic colleges to evaluate the efficacy of this element of the educational program.
Foraminal Occlusion (Encroachment)
The second component of vertebral subluxation, intervertebral foraminal occlusion, or encroachment, has been evidenced by Jackson, [22] Hadley, [34–36] Breig and Marion, [37] and Rosomoff and Rossman [38] spanning a period of over forty years. The classic work of Hadley in postmortem studies demonstrated the presence of foraminal encroachment throughout the spine in a number of human cadavers. Similar findings have been reported by Kovacs, [39] Sunderland, [40] and Epstein et al. [41] These studies indicate that foraminal encroachment is a well accepted phenomenon.
Palmer [42] also proposed that occlusion could involve the spinal canal as well as the IVF. In this regard, information which relates to changes in the spinal canal are considered in the next section.
Neural Pressure and Neural Dysfunction
The studies of Hadley also served to support the concept of nerve pressure associated with foraminal encroachment. Although he concluded that nerve pressure was unlikely to arise in the thoracic spine as a result of foraminal encroachment, due to the size of the foramina in that region, his studies revealed tissue damage to the dorsal nerve root epineurium supported by histological studies showing cellular damage in the cervical, thoracic, and lumbar spine of cadavers. Similar studies supported by Hadley’s findings were also performed by Lindbloom and Rexed. [43] Even though these studies are not recent, there is no current information to suggest the findings merit re-evaluation. Therefore, it is reasonable to accept these studies as support for the hypothesis that foraminal occlusion can, at least in some areas of the spine, result in nerve pressure sufficient to cause observable physical damage at the gross and cellular levels or at the level of the nerve root. The findings of these studies also suggest that pressure exerted on neural tissue at the level of the IVF is compressive in nature.
Additional information relevant to neural pressure has been provided by Breig. [44] In his survey of pathological situations responsible for histodynamic tension in the spinal cord and nerve roots, he points out that pathologically increased angulation of two or more vertebrae may be responsible for stretching or increased tension of the dura leading to neurological manifestations. This situation is further proposed to be additive since the caudal end of the dura is anchored in the sacral canal. Consequently, misaligned vertebrae in the cervical region, and possibly in the thoracic area, generating dural tension, would likely transmit this tension to the lumbar region as well. Sunderland [45] investigated the relationship between the meninges internally to the nerve roots, posterior root ganglion, and spinal nerves in the lower cervical spine of cadavers. He concluded that considerable freedom of movement was apparent for nerve roots within their respective IVF’s in the lower cervical region. However, he also recognized that the continuity of the nerve sheath with the dural sac, and the plugging action of the dural funnel at the foramen, would limit the extent of any lateral movement when abnormal traction was exerted on the spinal nerve. Other investigators suggest that nerves in the lower cervical area occupy virtually all of the IVF to percentages ranging from 10% to 50%. [46–50] Although considerable variation exists in the estimates of these investigators, it is apparent that the nerves throughout the spine, are subject to compressive or tension forces either through changes in the morphology of the IVF or the spinal canal, or through abnormal lateral traction.
An interesting finding comparing nerve roots and peripheral nerves, underlies the importance of pressure exerted at the IVF or spinal canal. Dorsal root ganglia (DRG) have been shown to be several times more sensitive to mechano-stimulation, such as generated by compressive forces, than peripheral nerves. [51] DRG often remain hyper-excited after the stimulation is ceased, whereas this phenomenon is not observed for peripheral nerves. [52] This characteristic of the DRG is likely to account for the production of pain, as well as facilitated motor responses which often accompany the pressure resulting from eccentric compression and chronic irritation or mechanical stimulation. [19 ] Compressive pressure has also been shown to damage the nerve root. Lindbloom [52] demonstrated that among 160 cadavers, ranging in age from 14 to 87 years, 60 showed nerve root compression. In another study, Lindbloom and Rexed [43] showed that nerve root compression exhibited a direct relationship to the extent of damage to the nerve root. Additionally, it was observed that most of the damage was degenerative, and primarily to the ventral root, although the dorsal root was also involved. These authors also reported that damage to the DRG was greater when compression of the nerve root was also noted.
Nerve roots, when under increasing tension, have also been shown to be more susceptible to loss of structural integrity, followed by degeneration, than peripheral nerves. [54, 55] This susceptibility is believed to be due to the parallel arrangement of less supportive collagen fibers in nerve roots as opposed to peripheral nerves. This observation, coupled with the fact that nerve roots lack the tough connective tissue coverings which add to the structural stability of peripheral nerves, suggests an explanation as to why they are more sensitive to stimulation and vulnerable to pressure and increasing tension. In consideration of the pressures which can be created at the IVF and within the neural canal from misaligned vertebrae, adhesions, space occupying lesions, and other structural, physiological, and biochemical phenomena, it is not difficult to understand the apparent biochemical (metabolic) changes and/or aberrant neurological responses which often accompany these phenomena.
Information which relates to the three components thus far discussed, and hypothesized by Palmer; vertebral misalignment, foraminal occlusion, and neural pressure have also been considered in recent models of the vertebral subluxation. Before proceeding with information relevant to the fourth component, the mental impulse, an overview of three models is presented.
The Vertebral Subluxation Complex (VSC),
Chiropractic Subluxation Complex (CSC),
and the Vertebral Subluxation Complex Model (VSCM)
In a movement to extend beyond a primarily descriptive approach of identifying individual subluxation components, the concept of vertebral subluxation as a complex integrating the biochmechanical, physiological, neurological, and biochemical components has been proposed. While the exact origin of the term “Vertebral Subluxation Complex,” is difficult to trace, it does appear in a publication by Stiga and Flesia. [56] The components of the VSC, however, have been attributed to Homewood and Janse. [18]
Stiga and Flesia [56] link considerable medical research, involving progressive degenerative kinesiopathology of the vertebral motor unit (referred to by the authors as synonymous with vertebral subluxation degeneration), to other studies resulting in neuropathophysiological states, to derive a neurokinesiological couple. This coupling is postulated to exhibit reciprocal and predictable sequential degeneration of biomechanical as well as osseous and soft tissue integrity, constituting the “vertebral subluxation complex.” These authors further propose that modeling of the VSC clarifies mechanisms, neuroanatomical impact, and therapeutic responses, with conclusions being drawn from a survey of symptomatology depicting the ultimate effects derived from the VSC.
However, the information regarding the VSC as described by Stiga and Flesia, makes no reference to which of the several definitions of vertebral subluxation it is applicable. It appears that the primary contribution of their literature study has been to connect existing findings in the medical sciences describing many forms of dysfunction such as osteoarthritic degeneration, to what would be expected to occur as a progressive consequence of such documented phenomena as vertebral misalignment, and neural encroachment; both of which are accepted components of vertebral subluxation. For example, it is proposed by these authors that the progressive phases of the VSC degenerative processes described categorically as kinesiopathology, neuropathology, myopathology, histopathology, and biochemical aberrations, are consistent with medical research findings of the same categories associated with various diseases and dysfunctional processes.
The work of Stiga and Flesia establishes a link between findings in one discipline with another. Thus the research accomplished in medicine, unrelated to the chiropractic concept of vertebral misalignment and neural encroachment, has done much to substantiate the likely (but still hypothesized) outcomes of those phenomena. Reciprocally, the chiropractic perceptions of structural misalignment and neural encroachment offer logical explanations for the etiology of degenerative processes documented by the medical profession.
A subsequent literature review by Dishman [17] regarding the static and dynamic components of what is termed the “chiropractic subluxation complex” (CSC), covers the same degeneration categories as the VSC, but are referred to as neural, kinesiopathological, muscular, cellular, and biochemical. Dishman does not consider subluxation to be the etiology of the category of degenerative processes, but rather one component of a complex or syndrome of intervertebral dyskinesia, dysarthrosis, or dysfunction in which the biochemical and histological components explain some of the pain mechanisms, tissue changes, and intervertebral fixation. However, as with the VSC, this literature study does not make reference to which definition of subluxation the CSC is applicable. The author does conclude that information gained from the literature supports the concept of a “subluxation complex” composed of the five categories related to a patho-mechanical disease cycle, which, if not corrected, leads to chronic degeneration. This, the author points out, explains the need for repeated spinal manipulations and prolonged care.
Lantz [18, 19] has contributed a vertebral subluxation complex model (VSCM) which includes the five categories of the original VSC and CSC, as well as the addition of the inflammatory response, connective tissue pathology, and vascular abnormalities. The model draws from basic science, clinical science, chiropractic, medical and osteopathic literature. The significance of this model is multifold. It provides insight into the divergent views regarding the nature of subluxation and suggests an organizational structure regarding the various components as derived from contemporary information.
The organizational structure includes cellular components involving biochemical abnormalities, histopathology, and the inflammatory response. The tissue level components involve their own respective pathologies (myopathology, vascular abnormalities, connective tissue pathology, and neuropathology) ultimately resulting in kinesiopathology which is considered to be the functional end-product of the various tissue aberrations.
Lantz elaborates the basic motion segment, fundamental to the concept of vertebral subluxation, which minimally includes two adjacent vertebrae, a disc, two posterior articulations, joint capsules, inter-transverse, and intraspinous ligaments through the term “Integrated Segmental Unit,” (ISU) referring to:“...the basic motion segment along with associated spinal structures, such as the segmental nerves, nerve roots and dorsal root ganglion, sinu-vertebral nerves, muscles, and vascular structures, such as the radicular arteries and veins. It would also include meningeal structures, such as the dural funnel, and segmental spinal circuitry and reflex arcs. It must be recognized, too, that regional differences exist in the spine. In the cervical spine, for example, the ISU would include the vertebral arteries, and the joints of Lushka, while in the thoracic spine, it would include the costal articulations, capsules and associated ligaments.”
The VSCM, proposed by Lantz, bases its central concept on immobilization degeneration. This connects the “fixation” phenomenon (viewed as a form of joint immobilization) frequently found in subluxation assessment with the documented degenerative processes of muscle, tendon, cartilage, ligaments, articular capsule, and bone which have been attributed to joint immobilization. This approach adds further substantiation to the reciprocal relationship described by Stiga and Flesia when describing the VSC and degeneration findings of conditions described by the medical community. The Lantz model of the vertebral subluxation complex also refers to trophic influences, but overlooks the significance of research in this area in regard to the EVSM. This area will be explored further as the expanded VSM is discussed.
Consideration of Current Models of the Vertebral Subluxation
The information that has been published in the context of the VSC, CSC, and the VSCM has made a major contribution to understanding three of the four components of Palmer’s subluxation model. This has been accomplished by substantiating, indirectly, the hypothesized biomechanical and neurological consequences of uncorrected vertebral subluxation. By linking this hypothesis to studies conducted in regard to medical degenerative disorders, the biomechanics and consequences of vertebral misalignment, joint immobilization, neural encroachment, and neurological dysfunctions have been described and supported by this documentation. Further research in these areas, directly linking the subluxation to specific degenerative sequelae, will be necessary to broaden our appreciation of the pathomechanics and restorative processes which occur in the presence and absence of vertebral subluxation. Nevertheless, the present evidence serves the purpose of establishing the necessary credibility to justify additional research.
While information which demonstrates the progressive degenerative processes observed in many medical diseases has been eloquently linked to degeneration sequelae hypothesized to follow uncorrected vertebral subluxation, two important points must be considered. First, as a result of clinical observations of the efficacy of detecting and affecting the correction of vertebral subluxation, it should not be presumed that all instances of this condition involve, or will lead to, the degeneration complex described in the models to date. It would not be surprising, for example, to observe little to no degenerative histopathology, myopathology, or neuropathology associated with vertebral subluxation, unless the uncorrected condition was initially severe, and/or of a relatively long duration. An analogy can be drawn from dentistry. A decayed tooth, readily observable on x-ray, is not likely to be associated with periodontal degeneration unless it has existed uncorrected for a considerable period of time. Consequently, while an appropriate model of vertebral subluxation should include the potential degenerative ramifications of its chronic presence, these potential effects are a component part of the model which may not be apparent when considering the condition relative to its acute or short term character.
Second, it is important to caution against developing a model of vertebral misalignment versus a model of vertebral subluxation. Current models have adopted the viewpoint that the fourth component of the EVSM, proposed by B.J. Palmer as the “mental impulse,” is synonymous with the action potential. [9] When this assumption is made, it follows that the neurological dysfunction associated with vertebral subluxation would be those phenomena attributable to interruption of nerve conduction via the action potential. [57] In the models discussed in this article, this assumption has led to descriptions of myopathology related to neuropathology emanating from neural conduction deficits. While it is plausible that the degenerative consequences of vertebral subluxation, or acute neural impingement as part of the subluxation, could involve conduction loss with its well documented sequelae of symptomatology, it does not appear that loss of nerve conduction via the action potential is what Palmer was describing as the primary concept of the “mental impulse.”
This caution is important to subluxation-based chiropractic. Since the vertebral subluxation models which have emerged consider only three components; misalignment of vertebra(e), occlusion or encroachment of the spinal canal or intervertebral foramen, and pressure affecting neural conduction via the action potential, then the model is not one of vertebral subluxation, but rather a “misalignment model” involving hard tissue misalignment affecting associated soft tissues. Models of this type have been in existence in the medical profession for some time. For example, it is well known that a neuropathy could arise from a cervical hard tissue lesion impinging on the brachial plexus, with predictable tissue pathology and accompanying symptoms. Consequently, a vertebral subluxation model limited to these same parameters would be nothing new, but rather a reiteration of an existing medical model involving neuropathology, myopathology, connective tissue pathology, inflammatory response, vascular response, biochemical responses, histopathology, and kinesiopathology. Such models, even in the chiropractic profession would, however, readily fall under the care regimen of spinal manipulative therapy for the correction of misalignments and the many symptomatic conditions which would be expected to accompany this aberration. It is obvious that the manipulation regimen then simply becomes another way of impacting on parameters already described and documented as a medical phenomenon.
It is apparent that contemporary depictions of the VSC, CSC, and the VSCM fall within this concept. Although the term vertebral subluxation may be applied to these models, it is argued that the application is inappropriate, as the fourth component of vertebral subluxation (mental impulse) has either not been considered, or, unnamed, it has been incorporated into the model as being synonymous with the loss of neural conduction via the action potential. While it is apparent that interference to the nerve impulse (i.e. action potential) could lead to syndromes manifesting parameters of dysfunction as previously described, it is important to include the fourth component, the mental impulse, since it has been proposed that its interference would result in loss of adaptive potential, ultimately depriving the organism of its health. [58]
Organizing or Coordinating Information and the Fourth Component
Stephenson [59] stated in 1927, that it was not fully clear what the mental impulse was. It was hypothesized however;(1) that each tissue cell requires specific mental impulses,
(2) that each adaptive change requires specific impulses,
(3) that they are constructive, used only for a particular moment for coordination,
(4) that impulses originate through the expenditure of energy, and are propelled, perhaps through a
physical movement of the cell as a contraction,
(5) that although mental impulses can be radiated, the function of nerves appears to be one of gathering
radiant energy and transforming it to a dynamic or flowing form, and
(6) that mental impulses, also called mental force (ref., p5), are not a physical or a chemical force,
nor a stimulant, and that
(7) the function of the nervous system is to transmit mental force from the brain to the tissue cell and back again.Granted, Palmer, Stephenson and their contemporaries were grappling with an advanced concept, which they admittedly did not fully understand, but nevertheless were compelled to define or describe. Regardless of their limitations, and perhaps inaccuracies, a complete VSM must evaluate this concept as to its distinctiveness from, and its relationship to, the action potential. In this regard, an immediate question arises; has any information since the writings of Palmer and Stephenson been presented which may serve to elucidate the concept of mental impulse?
Several advances and classical concepts in neuroscience provide considerable information in this regard. Physiologists have recognized the phenomenon of axoplasmic transport for over forty years. Studies have shown that various substances “flow” through the nerve cell in both directions at the same time. [60, 61] These substances include certain informational molecules such as proteins, neurotransmitters, as well as other “growth factors.” [62–65] The mechanism of propulsion behind axoplasmic flow is considered to be linked to the presence of actomyosin, a contractile protein, ubiquitous throughout the animal kingdom. [66, 67]
Other studies suggest that a number of molecules, regardless of their size, travel at the same rate of approximately 410 ± 50 mm/day independent of the size of the nerve fiber, diameter, and presence or absence of myelin. These studies suggest that molecules are exported into nerves fibers from other sites and then transported along “sliding-filaments,” in a contraction-like process. [60, 68] The bidirectional movement within the nerve fiber is referred to as anterograde and retrograde axoplasmic transport. Anterograde flow is associated with substances necessary for nerve growth and synaptic membrane maintenance, while retrograde flow is believed to reflect movement of substances, exported into the nerve fiber. [65, 69] These substances influence regulation of enzymes associated with the production of neurotransmitters, [70] thus implicating these movements as important, if not essential, to development and maintenance of the neuromuscular system. [71] It should be noted that both anterograde and retrograde axoplasmic flow are constructive in function.
Interestingly, studies have shown that axoplasmic flow can be blocked by nerve compression. [72–75] Additionally, it has been shown [50, 76, 77] that axoplasmic flow can be blocked in peripheral nerves by pressures much less than those required to block nerve conduction. If axoplasmic flow is assumed to be a subset of or synonymous with the mental impulse, then it becomes apparent that the subluxation component of interference to mental impulses could occur independent of, or in the absence of, interference to the action potential. Information which has demonstrated the increased susceptibility of nerve roots to compression, as opposed to peripheral nerves, further emphasizes the IVF as a plausible anatomical site for nerve interference not involving the action potential. Although unsupported by direct evidence of changes in nerve root morphology following acute or chronic compression, Luttges and Gerren [78] have described several areas of neural activity in the dorsal nerve root which they believed to be susceptible to compression injury, including axoplasmic transport and ephapsis. Even though peripheral nerve axoplasmic transport is more sensitive to blockage by compression pressure that nerve conduction, additional research will be necessary to affirm that axoplasmic flow in nerve roots shows a similar sensitivity.
Additional information which is pertinent to the concept of the mental impulse has been presented by Kelso, [79] who proposes that the reductionist method of explaining macroscopic events is limited since biological properties which arise at each level of complexity cannot be predicted from knowledge of component processes, particularly with regard to self-organization. For example, research has shown that neurons communicate by several modes of transmission, and therefore, have functions other than solely modulating excitatory and/or inhibitory activities between cells via the action potential. [80] In this regard, transmission has been shown to occur ephaptically. The ephapse exists where two or more axons and/or dendrites touch without forming a typical synaptic contact. [82] The ephaptic response has been observed in chronically denervated muscles, [82, 83] cerebellum, and in axon tracts affected by demyelination, [84] the hypothalamic suprachiasmatic nucleus (site of the circadian clock), [85] axon-axon interfaces in post-injury neuromas, [86] and in the pre-innervation phase of early radiculopathy. [87] This finding is particularly interesting, as it suggests that information flow to tissues can occur even in the presence of denervation (i.e.total loss of the action potential). This suggests, that ephaptic transmission is independent of the action potential, while still utilizing the nerve for conveyance. Interestingly, this is also a proposed characteristic of the mental impulse.
Ephaptic responses are not considered to be an artifact, [82] as they can be recorded and re-recorded some days later by surface electrodes. They are believed to be a late potential evoked by every stimulus or unpredictably with unstable latency periods. They have been observed following blockage of the action potential in the suprachiasmatic nucleus in which cellular integrity was monitored through continued function of the circadian clock. [85] Ephaptic responses have also been demonstrated to be evoked by the electrical activity of muscle, suggesting that muscles can elicit excitation in nerves creating a reverberating cycle affecting muscle tone. [88]
Neurons can also communicate other than through the action potential by “volume transmission.” This mode of conduction involves the flow of ionic currents and chemical signals which are transmitted or radiated from one neuron, through the fluid-filled space between cells, and received by an appropriate receptor site on another neuron. The research literature regarding this topic has been thoroughly reviewed by Agnati, et al. [89, 90] These reviews suggest that every neuron may function in a dual mode; the synaptic and the volume transmission mode. Notable is the observation that neuroendocrine release appears to only involve volume transmission, including the cerebro-spinal fluid as a medium. These reviews also point out that the chemical signals which elicit the release of neuroendocrines, involved in a wide array of coordinating processes, include the classical transmitters such as the monoamines, acetylcholine, gamma amino butyric acid, and glutamate as well as other neuropeptides.
Pert et al [91] point out that over fifty “informational substances” have been shown to modulate brain function including behavior and mood states. These authors indicate that the signal specificity resides in distinct classes of recognition molecules (receptors), rather than through the synapse. In addition, neuropeptide receptors have been shown to occur on mobile cells of the immune system. [92, 93] Black, [92] in a discussion of the emerging field of psychoneuroimmunology, has shown that the brain and the immune system interact via hormones, neurotransmitters, and other substances which travel through the blood and nervous system. Pert et al, [91] further suggest that neuropeptides and their receptors join the brain, glands, and immune system in a network of communication between the brain and the body.
It is also reasonable to assume that a nerve that does not conduct via the action potential, or conducts improperly, will likely lose its ability to relay information through the classic concept of synaptic transmission. This would involve information which contributes to numerous motor functions. Since many of these motor events can be invoked voluntarily, or expressed independent of the conscious mind, interference to the action potential must, therefore, also interfere to some extent with the transmission of the mental impulse. [94] Of interest in this regard, is the observation that deaffernated DRG can elicit action potentials. Kirk, [95] has shown that DRG spontaneously emit impulses following transection of the ventral root and spinal nerve distal to the dorsal root, thus isolating it from the periphery. The fact that afferent transmission can occur, independent of peripheral input, suggests that organizing information, either originating within the tissue cells of the DRG or at least arising there as a first level of tangible expression, is capable of being transmitted to the brain via the action potential of the nervous system or ephaptic transmission, as previously discussed. This suggests that one level of the organizing information loop can be maintained even in the presence of transection of the physical relationship between information flowing from the brain to the tissue and back again.
The impression that arises when the information from contemporary research is sorted relative to the hypothesis of the mental impulse presented by Palmer and Stephenson, leads to several insights. First, it appears that axoplasmic flow fulfills many of the criteria hypothesized as being characteristic of the mental impulse;(1) it involves the transmission of organizing (constructive) or coordinating information,
(2) substances appear to be propelled intracellularly by a contractile process,
(3) it is independent of neural conduction, and
(4) it can be interfered with at pressures lower than required to inhibit or cease neural conduction.Additionally, several other well documented modes of non-synaptic communication between cells, including; ephaptic transmission, volume transmission, field effects mediated by large extracellular currents, and weaker fields generated by axons during growth and repair, as well as peptide messengers postulated through psychoneuroimmunology, clearly demonstrate that other phenomena play an important role in the transmission of organizing information. These phenomena are also linked to the original hypothesis of the mental impulse in that the signals can be radiated between cells, and the interactions between stimulus and response is specific to the event. For example, the bidirectional chemical communication between brain and tissues is regulated by cortico-trophin releasing factor which is stimulated by specific thoughts and emotions or immune activation to influence the hypothalamic-pituitary-adrenal axis and subsequent release of endocrines which play a major role in many cellular processes. [92]
It is also plausible that organizing information derived from the tissue itself may influence the action potential, as opposed to being synonymous with it. [88] In support of such a concept, it appears possible that denervated muscles can be stimulated through ephaptic transmission. [82] Other study suggests that the muscle electrical activity itself could then act ephaptically, to induce action potentials in associated nerves. [87] In this cyclic manner, even if peripheral nerve stimulation via the action potential is lost to a muscle, its muscle character and some degree of tonus, could be retained ephaptically.
One characteristic of the mental impulse described by Stephenson, is that it is neither a physical nor chemical force. The implications of that statement are not easily interpreted relative to the other characteristics appointed to the mental impulse, which can be linked to physical and/or chemical phenomena. However, it may be that the mental impulse, as an entity, is undetectable by current technology, and that such processes as axoplasmic flow, volume transmission, the ephaptic effect, neurohumoral communication, and the action potential, all represent various aspects of the physical medium of the nervous system through which the mental impulse is conveyed. Nevertheless, substantial information attests to the necessity for a VSM to recognize that neural expression, relative to the transmission of organizing information, is not synonymous with only the action potential. This suggests that a vertebral subluxation could exist either in the absence of neural conduction pathology, and other pathological components of the vertebral subluxation complex and its model, or in the presence of this spectrum of pathologies. If the latter occurs, it is at that stage that the “misalignment models” merges with the VSM.
Summary and Conclusions
A VSM has been presented which includes the traditional concept of vertebral subluxation, as proposed by B.J. Palmer, as well as new information which reflects on that theory. This model has been proposed based on the observation that other contemporary models consider only the misalignment and nerve interference aspects of Palmer’s theory.
Other aspects of the EVSM are also included in the current VSM, such as etiology of the vertebral subluxation and its affects on health. These aspects will be covered in the second part of this series.
Information has been presented to show a variety of ways, in addition to the action potential, by which neural cells communicate allowing for a flow of information to tissues and back to the brain. In consideration of this information, it would appear to be an oversight to eliminate the concept of mental impulse from the VSM. Investigating this phenomenon will likely require techniques aimed at evidencing its presence and influence indirectly. Research in this regard may be much like the efforts of physicists to develop methods of observing the atom and subatomic particles.
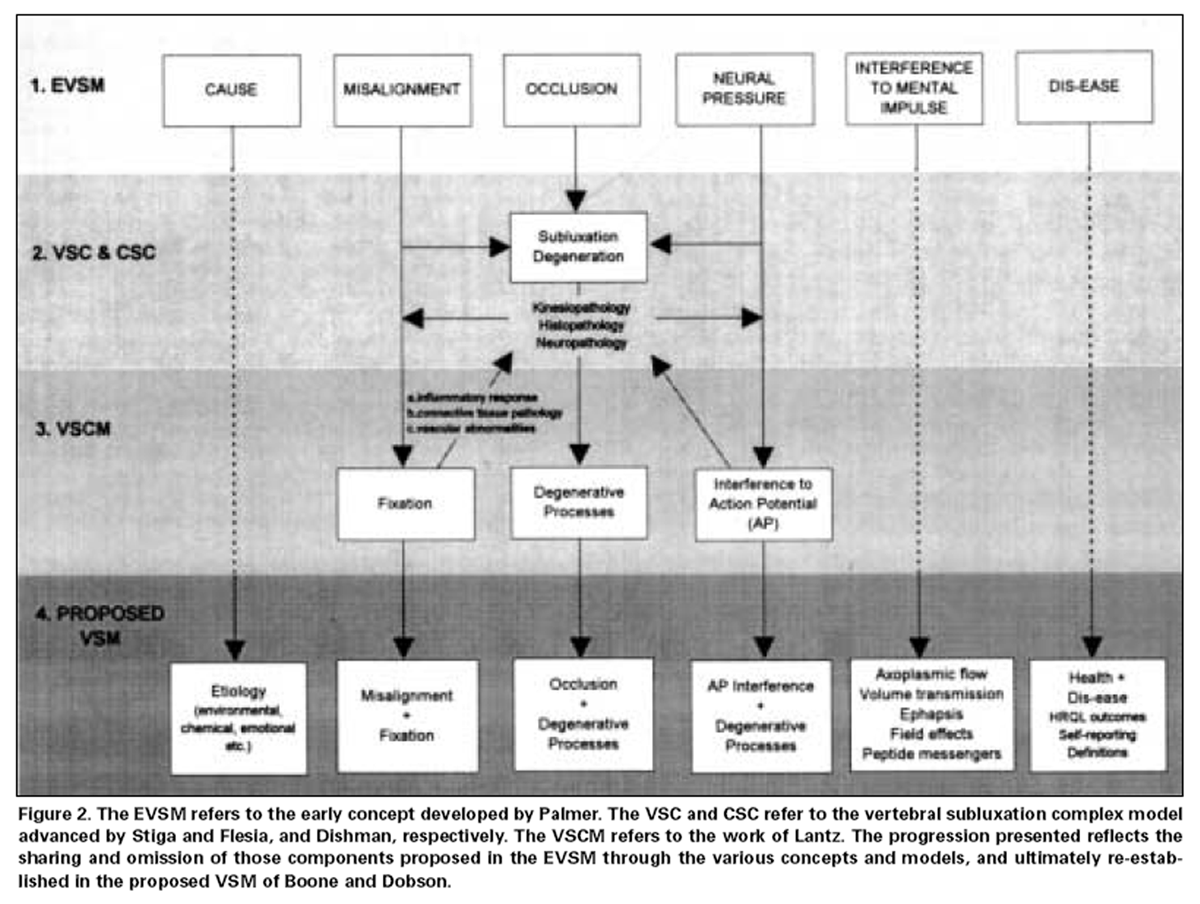
Figure 2 In order to sustain a VSM which incorporates interference to the mental impulse (coordinating information), the onus is upon researchers of the vertebral subluxation to link theorized manifestations of this interference to emerging models in neuroscience. This should be an exciting challenge in light of recent evidence regarding different levels of cell communication, as well as the ramifications of concepts including inter-relationships between the mind (CNS) and the immune system [96, 97] and studies documenting changes in quality of life measures. [98, 99] This information provides further impetus to link the outcomes of vertebral subluxation correction to increased health status. An overview is presented in Figure 2 of the evolution of the components of vertebral subluxation through the various models discussed in this article, culminating in the VSM.
A description of appropriate research models for studying the parameters of the VSM will be presented in the third article of this series. The research models will be based upon approaches which impact on all areas of vertebral subluxation including its etiology, manifestations, and consequences.
Acknowledgments
The authors would like to thank Marnie Dobson for her timely comments, critique, and assistance in the preparation of this manuscript.
REFERENCES:
Palmer BJ.
The subluxation specific-the adjustment specific.
Davenport: The Palmer School of Chiropractic, 1934 (1986 printing): 115Keating JC, Green BN, Johnson CD.
“Research” and “Science” in the first half of the chiropractic century.
J Manipulative Physiol Ther 1995; 18 (6): 357-378Keating JC.
Toward a philosophy of the science of chiropractic.
Stockton: Stockton Foundation for Chiropractic Research, 1992: 29-32Brantingham JW.
A critical look at the subluxation hypothesis.
J Manipulative Physiol Ther 1988; 11 (2): 130-132C Lantz
The Vertebral Subluxation Complex PART 2:
The Neuropathological and Myopathological Components
Chiropractic Research Journal 1990; 1 (4): 19-38Gatterman MI.
Advances in subluxation terminology and usage.
In: Lawrence DJ, et al, eds. Advances in chiropractic.Vol (2)
St. Louis: Mosby., 1995: 465International Chiropractor’s Association definition of subluxation.
November, 1987 ICA, 1901 L Street,NW, Suite 800,Washington, D.CAmerican Chiropractic Association synopsis of policies on public health and related matters.
American Chiropractic Association,
1701 Claredon Blvd., Arlington,Virginia: 18Leach RA.
The chiropractic theories. A synopsis of scientific research 2nd ed.
Baltimore:Williams and Wilkins, 1986: 27Morat JD.
Physiology of the nervous system. Chicago:WT Keener & Co., 1906,
In: Palmer BJ. The subluxation specific: 12 The adjustment specific.
Davenport: Palmer School of Chiropractic. 1934 (1986 printing): 498Keating JC.
Toward a philosophy of the science of chiropractic.
Stockton: Stockton Foundation for Chiropractic Research, 1992: 113-121.Ibidem: 49.
Chiropractic.
Seventh report to the president and congress on the status of health personnel in the united states.
Washington:U.S. Department of Health & Human Services.
Public Health Service, 1990.Committee on policy formulation.
Southern California College of Chiropractic.
Pico Rivera, CA: 1993.Stiga JP, Flesia JM.
The “vertebral subluxation complex,” research insights.
Renaissance International, S.A.Dishman RW.
Review of the Literature Supporting a Scientific Basis for the Chiropractic Subluxation Complex
J Manipulative Physiol Ther 1985 (Sep); 8 (3): 163–174Dishman RW:
Static and dynamic components of the chiropractic subluxation complex: a literature review
J Manipulative Physiol Ther. 1988 (Apr); 11 (2): 98-107C Lantz
The Vertebral Subluxation Complex PART 1:
An Introduction to the Model and Kinesiological Component
Chiropractic Research Journal 1989; 1 (3): 23-36C Lantz
The Vertebral Subluxation Complex PART 2:
An Introduction to the Model and Kinesiological Component
Chiropractic Research Journal 1990; 1 (4): 19-38Schram S, Hosek R.
Error limitations in x-ray kinematics of the spine.
J Manipulative Physiol Ther 1982; 5 (1): 5-10.Huslig EL,Howe RW.
Hyperflexion sprain of the cervical spine: a case study.
J Manipulative Physiol Ther 1986; 9: 143-145.Jackson R.
The cervical syndrome.
Springfield: Charles C.Thomas, 1977.Report of the quebec task force on spinal disorders.
Spine 1987; 12 (7): S1-S23.Tasharski C, heinze W, Pugh J.
Dynamic atlanto-axial abberation: a case study and cinefluorographic approach to diagnosis.
J Manipulative Physiol Ther 1981; 4 (2): 65-68.Shippel A, Robinson G.
Radiological and magnetic resonance imaging of cervical spine instability: a case report.
J Manipulative Physiol Ther 1987; 10 (6): 316-323.Stokes I,Wilder D, Frymoyer J, et al.
Assessment of patients with low-back pain by biplanar radiographic measurement of intervertebral motion.
Spine 1981; 6 (3): 233-240.Jirout M.
The dynamics of the craniocervical junction of the lateral inclination of the head and neck.
The Dig of Chiro Econ 1984; Jan/Feb: 141-142.Jirout M.
“Changes in the atlas-axis relations on lateral flexion of the head and neck.”
Neuroradiology 1973; 6: 215-218.Jirout J.
“Rotational synkinesis of occiput and atlas on lateral inclination.”
Neuroradiology 1981; 21: 1-4.Jirout J, Burns RE.
Author to author communication.
Dept. of Neuroradiology, Neurologic Clinic,
Charles University, 120 00 Prague 2, Katerinska 30, Czechoslovakia; July, 1980.Sigler DC, Howe JW.
Inter- and intra-examiner reliability of the upper cervical x-ray marking system.
J Manipulative Physiol Ther 1985; 8: 75-80.Jackson BL, Barker W, Bentz J, et al.
Inter- and intra-examiner reliability of the upper cervical x-ray marking system: a second look.
J Manipulative Physio Ther 1987; 10: 157-163.Rochester RP.
Inter-and intra-examiner reliability of the upper cervical x-ray marking system:
A third and expanded look.
Chiro Res J 1993; 3 (1): 1-6.Hadley LA.
Anatomico-Roentgenographic studies of the spine.
Springfield: Charles C Thomas, 1964: 172-183, 422-477.Hadley LA.
Intervertebral joint subluxation, bony impingement and foramen encroachment with nerve root changes.
Am J Rontgenol Rad Ther 1951; 65: 377-402.Hadley LA.
Constriction of the intervertebral foramen.
JAMA 1949; 140: 473-476.Breig A, Marions O.
Biomechanics of the lumbosacral nerve roots.
Acta Radiol 1962; 1: 1141-1160.Rosomoff HL, Rossman F.
Treatment of cervical spondylosis by anterior cervical diskectomy and fusion.
Arch Neurol 1966; 14: 392.Kovacs A.
Subluxation and deformation of the cervical apophyseal joints.
Acta Radiol 1955; 43: 1-15.Sunderland S.
The anatomy of the intervertebral foramen and the mechanisms of compression and stretch of nerve roots.
In Halderman S ed.
Modern developments in the principles and practice in chiropractic.
New York: Appleton-Century-Crofts, 1980: 45 -64.Epstein JA, Epstein BS, Lavine LS, et al.
Lumbar nerve root compression at the intervertebral foramina caused by arthritis of the posterior facets.
J Neurosurg 1973; 39: 362-369.Palmer BJ.
The subluxation specific-the adjustment specific.
Davenport; Palmer School of Chiropractic. 1934 (1986 printing): 87.Lindbloom K, Rexed B.
Spinal nerve injury in dorsolateral protrusions of lumbar discs.
J Neurosurg 1948; 5: 413-432.Breig A.
Adverse mechanical cord tension in the central nervous system.
New York; John Wiley & Sons, 1978: 39, 40, 123.Sunderland S.
Meningeal-neural relations in the intervertebral foramen.
J Neurosurg 1974; 40: 756-761.Garland LH.
Constriction of the Intervertebral foramen.
JAMA 1949; 140: 475.Swanberg H.
The intervertebral foramina in man.
Med Rec 1915; 87: 176-180.Hadley LA.
Roentgenographic studies of the cervical spine.
Am J Roentgen 1944; 52: 173-195.Hadley LA.
Anatomicoradiographic studies of the spine. Changes responsible for certain painful back conditions.
NY J Med 1939; 39: 969-974.Payne EE, Spillane JD.
The cervical spine.An anatomicopathological study of 70 specimens (using a special technique)
with particular reference to the problem of cervical spondylosis.
Brain 1957; 80: 571-596.Sharpless SK.
Susceptibility of spinal roots to compression block.
In: Goldstein M, ed.
The research status of spinal manipulative therapy.
Washington, DC, Government Printing Office, 1975: 155-161.Howe JF, Loeser JD, Calvin WH.
Mechanosensitivity of dorsal root ganglia and chronically injured axons:
A physiological basis for the radicular pain of nerve root compression.
Pain 1977; 3: 25-41.Lindbloom K.
Protrusions of discs and nerve compression in the lumbar region.
Acta Radiol 1944; 25: 195-212.Sunderland S.
Anatomical perivertebral influences on the intervertebral foramen.
In Goldstein M ed.
The research status of spinal manipulative therapy,
Washington, DC, Government Printing Office, 1975: 129-140.Sunderland S. Bradley KC.
Stress-strain phenomena in human spinal nerve roots.
Brain 1961; 84: 120-124.Stiga JP, Flesia JM.
The “vertebral subluxation complex,” research insights.
Colorado; Renaissance International 1982.Feeley C, Pfleger B.
The Neurophysiological Evaluation of the Subluxation Complex: Documenting the
Neurological Component with Somatosensory Evoked Potentials
Chiropractic Research Journal 1994; 3 (1): 1–4Stephenson RW.
Chiropractic Text Book.
Davenport: Palmer School of Chiropractic, 1927 (1948 edition): 2.Ibidem: 29.
Ochs S, Chan SY,Worth R.
Calcium and the mechanism of axoplasmic transport.
In Korr IM ed. The neurobiologic mechanisms in manipulative therapy.
New York, Plenum, 1978: 359-367.Ochs S.
A brief review of material transport in nerve fibers.
In Goldstein M ed.
The research status of spinal manipulative therapy.
Washington, DC, Government Printing Office, 1975: 189-196.Johnson EM, Blumberg HM, Costrini NV, et al.
Reduction by reserpine of the accumulation of retrogradely transported 125 nerve growth factor
in sympathetic neurons.
Brain Res 1979; 178: 389-401.Jessell T,Tsunoo A, Kanazawa I, et al.
Substance P: deletion in the dorsal horn of rat spinal cord after section of the peripheral
processes of primary sensory neurons.
Brain Res 1979; 168: 247-259.Bjoerklund A, Bjerre B, Steneri U.
Has nerve growth factor a role in the regeneration of central and peripheral catecholamine neurons?
In Fuxe K, Olson L, Zotterman Y eds.
Dynamic of regeneration and growth in neurons.
New York: Pergaamon, 1974: 389-409.Goodrum JF.
Axonal transport and metabolism of 3H fucose-and 35S sulfate-labeled macromolecules
in the rat visual system.
Brain Res 1979; 176: 255-272.Allen RD.
Some new insights concerning cytoplasmic transport.
Symp Soc Exp Biol 1974; 8: 15-26.Samson F.
Axonal transport. the mechanisms and their susceptibility to derangement; anterograde transport.
In Korr IM ed. The neurobiologic mechanisms in manipulative therapy.
New York, Plenum, 1978: 291-309Ochs S.
Characteristics and a model for fast axoplasmic transport in nerve.
J Neurobiol 1971; 2: 331-345.Stach RW, Stach BM, West NR.
Nerve fiber outgrowth from dorsal root ganglia: ion dependency on nerve growth factor action.
J Neurochem 1979; 33: 845-855.Lees G. Chubb I, Freeman C, Geffen L, et al.
Effect of nerve activity on transport of nerve growth factor and dopamine B-hydroxylase antibodies
in sympathetic neurons.
Brain Res 1981; 214: 186-189.Leach RA.
The chiropractic theories. A synopsis of chiropractic research.
Baltimore:Williams & Wilkins, 1986: 124-125.Kelly PT, Luttges MW.
Electrophoretic separation of nervous system proteins on exponential gradient polyacrylamide gels.
J Neurochem 1975; 24: 1077-1079.Triano JJ, Luttges MW.
Nerve irritation: a possible model of sciatic neuritis.
Spine 1982; 7: 129 -136.Luttges MW, Groswald DE.
Degenerative and regenerative characterizations in the proteins of mouse sciatic nerves.
In Suh CH ed.
Proceedings of the 7th annual biomechanics conference on the spine.
Boulder: University of Colorado, 1976: 71-81.Luttges MW, Kelly PT, Gerren RA.
Degenerative changes in mouse sciatic nerves: Electrophoretic and electrophysiologic characterization.
Exp Neurol 1970; 50: 706-733.Aguayo A, Nair CPV, Midgley R.
Experimental progressive compression neuropathy in the rabbit.
Arch neurol 1971; 24: 358-364.Rainer GW,Mayer J, Sadler TR, et al.
Effect of graded compression on nerve conduction velocity.
Arch Surg 1973; 107: 719-721.Luttges MW, Gerren RA.
Compression physiology: nerves and roots.
In Halderman S ed.
Modern developments in the principles and practice of chiropractic.
New York: Appleton-Century-Crofts, 1980: 65-92.Kelso JAS.
Dynamic Patterns: The self-organization of brain and behavior.
Cambridge;The MIT Press, 1995: 228 -229.Agnati LF, Bjelke B, Fuxe K.
Volume transmission in the brain.
American Scientist 1992; 80: 362 -373.Stedman’s Medical Dictionary.
Baltimore: Williams & Wilkins (26th ed), 1995.Roth G.
Myo-axonal ephaptic responses and their f waves in case of chronic denervation.
Electoenceph & clin Neurophysiol 1993; 89 (4): 252 -260.Roth G.
Repetitive discharge due to self-ephaptic excitation of a motor unit.
Electroenceph & Clin Neurophysiol 1994; 93 (1): 1-6.Jefferys JG.
Nonsynaptic modulation of neuronal activity in the brain; electric currents and extracellular ions.
Physiol Rev 1995; 75 (4): 689-723.Van den Pol AN, Dudek FE.
Cellular communication in the circadian clock, the suprachiasmatic nucleus.
Neurosci 1993: 56 (4): 793-811.Fried M, Govrin-Lippmann R, Devor M.
Closed apposition among neighboring axonal endings in a neuroma.
J Neurocytol 1993; 22 (8): 663-681.Colachis SC, Pease WS, Johnson EW.
Polyphasic motor unit action potentials in early radiculopathy:
their presence and ephaptic transmission as an hypothesis.
Electromyo & Neurophysiol 1992; 32 (1-2): 27-33.Biro G.
Ephaptic influence of the electrical activity of muscle on the neighboring nerve.
Electromyo & Clin Neurophysiol 1992; 32 (9): 425-434.Agnati LF, Bjelke B, Fuxe K.
Volume versus wiring transmission in the brain: a new theoretical frame for neuropsychopharmacology.
Med Res Rev 1995; 15 (1): 33-45.Agnati LF, Cortelli P, Biagini G, et al.
Different classes of volume transmission signals exist in the central nervous system and are affected
by metabolic signals, temperature gradients and pressure waves.
Neuroreport 1994; 6(1): 9-12.Pert CB, Ruff MR,Weber RJ, et al.
Neuropeptides and their receptors: A psychosomatic network.
J Immunol 1985; 135 (2 suppl): 820s-826s.Black PH.
Psychoneuroimmunology: brain and immunity.
Scientific American (Science and Medicine) 1995; Nov/Dec: 17-25.Ader R, Cohen N, Felten D.
Psychoneuroimmunology: Interactions between the nervous system and the immune system.
Lancet 1995; 354: 99-103.Stephenson RW.
Chiropractic Text Book.
Davenport: Palmer School of Chiropractic, 1927 (1948 edition): 295.Kirk EJ.
Impulses in dorsal spine nerve rootlets in cats and rabbits arising from dorsal root ganglia
isolation from the periphery.
J Comp Neurol 1975; 115: 165-175.Blalock JE.
The syntax of immune-neuroendocrine communication.
Immunology Today 1994; 15 (11): 504-511.Ballieux RE.
The mind and the immune system.
Theor Med 1994; 15: 387-395.Torrance GW.
Utility approach to measuring health-related quality of life.
J Chron Dis 1987; 40 (6): 593-600.Pavot W, Diener E.
The affective and cognitive context of self-reported measures of subjective well being.
Soc Ind Res 1993; 28: 1-20.
Return to LOCATING SUBLUXATIONS
Since 4-21-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |