

An Open-label Study of Administration of EH0202,
A Health-food Additive, to Patients
With Chronic Hepatitis CThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Gastroenterol. 2004 (Sep); 39 (9): 873878 ~ FULL TEXT
Kyosuke Kaji, Satoshi Yoshida, Nobuo Nagata, Tatsuya Yamashita,
Eishiro Mizukoshi, Masao Honda, Yasuhiko Kojima, and Shuichi Kaneko
Department of Gastroenterology,
Kanazawa University Hospital,
Ishikawa, Japan
Why was this study done?
To determine the effects of a traditional Japanese (Kempo) formula on aspects of the immune system in patients with chronic hepatitis. The formula, called EH0202, includes pumpkin seeds (Cucurbita moschata), plantain seeds (Plantago asiatica), Japanese honeysuckle (Lonicera japonica), and safflower (Carthamus tinctorius).
What This Study Found
After three months of EH0202 administration, there were significant reductions in the measurement of hepatitis C virus (titers) in the blood of participants as well as significant improvements in symptoms (malaise).BACKGROUND: In this study, we examined the effect of EH0202, a mixture of four herbal extracts that are known to induce interferons, on hepatitis C virus (HCV)-RNA levels in patients with chronic hepatitis C.
METHODS: This was an open-label uncontrolled study. The study subjects ingested food containing EH0202 daily for 3 months, which was equivalent to 1 g of desiccated herbs daily. Clinical symptoms, hematology and biochemical examinations, urine, and HCV-RNA levels were examined before, during (1 month), and after the EH0202 treatment (3 months).
RESULTS: Among the 35 patients who successfully completed the study, there were improvements in malaise (seen in 6 patients before and in 2 after EH0202 treatment), bloating sensation in the abdomen (seen in 2 before and in none after treatment), and nausea and vomiting (seen in 2 before and in 1 after treatment). There were no changes in hematology or biochemical examination parameters. There was a statistically significant decrease in HCV-RNA levels in patients with high viral titers after 3 months of EH0202 administration. No serious adverse events were observed with the EH0202 treatment.
CONCLUSIONS: These findings suggest that EH0202 may be safe and useful in the treatment of patients with chronic hepatitis C. Further studies are, however, needed to obtain a definitive conclusion.
From the FULL TEXT Article:
Introduction
In recent years, the number of people who use health foods has been increasing, reflecting an increase in public awareness of diet and fitness for improving health. However, there have been few clinical studies that evaluated the safety and efficacy of these healthfood products.
EH0202 is a health-food additive that was developed by Yamanouchi Pharmaceutical Co., Ltd. (Tokyo, Japan). It is a mixture of four herbal extracts (see below) that are known to stimulate macrophage activity. Dr. Yasuhiko Kojima, [1] who was the first to describe interferon (IFN), in 1970, and who is one of the foremost experts in this field, together with his fellow researchers, has screened some 200 herbs commonly used as traditional Kampo (Chinese medicine) formulations in Japan. Based on their screening, four herbs were finally selected that were shown to be IFN inducers [2] and could be used as food additives. They were:pumpkin seeds (Cucurbita moschata),
plantain seeds (Plantago asiatica),
Japanese honeysuckle (Lonicera japonica),
and safflower (Carthamus tinctorius).EH0202 is a mixture of these four extracts.
It has been shown that EH0202 administration decreases the incidence of viral pneumonia and the mortality rate in pigs with porcine reproductive and respiratory syndrome. [3] In addition, in postmenopausal women, oral EH0202 administration for 6 months improved subjective menopausal symptoms and increased granulocyte-macrophage colony-stimulating factor (GM-CSF) levels, while decreasing follicle-stimulating hormone (FSH) levels in blood. Thus, it appears that EH0202 acts to stimulate immunological systems and may improve endocrine dysfunction. [4]
IFNs have been used to treat patients with chronic hepatitis C, and, because EH0202 has been shown to stimulate macrophages and increase IFN levels in experimental animals, we examined the effect of EH0202 on hepatitis C virus (HCV)-RNA levels, clinical symptoms, and hematological and biochemical parameters in patients with chronic hepatitis C, in an open-label uncontrolled study.
Methods
Patients
The subjects included in the study were 50 patients with chronic hepatitis C (26 men and 24 women; 2880 years old; mean ± SD, 59.0 ± 10.6 years old), who visited the Kanazawa University Hospital from February to November 2002. This study was carried out as an open-label study. Hepatitis C was diagnosed by the referring physicians, and the patients included in the study were those who agreed, in writing, to participate in the study. HCV serotypes were: group 1 (40 patients), group 2 (9 patients), and unknown (1 patient). Twenty-four patients had a history of receiving IFN therapy in the past.
After completion of the study, 15 of the 50 patients were excluded from the efficacy analysis for one of the following reasons: inappropriate dosage and/or administration (4 patients), data missing (4 patients), IFN had been administered during the 6 months prior to the commencement of administration of EH0202 (3 patients), premature discontinuation of EH0202 administration (2 patients), administration of IFN commenced during the EH0202 dosing period (1 patient), and a complication (hepatic tumor) was observed prior to administration (1 patient).
Thus, efficacy was examined based on the data of 35 evaluable subjects (17 men and 18 women; 2880 years old; mean ± SD, 60.5 ± 10.4 years). The number of patients classified based on the HCV serotype was 27 patients for group 1, 7 patients for group 2, and 1 patient, unclassified. Of the 35 evaluable patients, 15 patients had a previous history of receiving IFN therapy. The 15 patients excluded from the efficacy evaluation of EH0202, who nonetheless, took EH0202, were included in the safety analysis, together with the 35 evaluable patients.
During the EH0202 treatment period, concomitant medications for the liver conditions were used in 22 patients. Thirteen patients had received ursodeoxycholic acid prior to and during the study. During EH0202 treatment, 5 patients started to take ursodeoxycholic acid for the first time. A glycyrrhizin preparation was used in conjunction with ursodeoxycholic acid in 2 patients, and in conjunction with Kantec (malotilate; Daiichi Pharmaceutical, Tokyo, Japan) and Proheparum S (liver hydrolysate; Kaken Pharmaceutical, Tokyo, Japan) tablets in 1 patient. Taurine powder was used in conjunction with ursodeoxycholic acid in 1 patient. Aminoleban (a branchchained amino acids; Otsuka Pharmaceutical, Tokyo, Japan), Shosaiko-to (a bupleurum root-based formula; Tsumura, Tokyo, Japan), and Proheparum tablets were used as single agents in 1 patient each.
Test product
In this study, we used InterPunch (Sanwell, Tokyo, Japan), a commercially available form of EH0202, as the test product. EH0202 is a mixture of four herbal extracts: Cucurbita moschata (seeds), Plantago asiatica (mature seeds), Lonicera japonica (flowers and flower buds), and Carthamus tinctorius (dried tubular flowers). These herbs were dried and weighed and then added to water equal to ten times the total weight of the dried herbs. The mixture was heated for 30 min at 95 ± 5°C in order to prepare an extract. The extract was strained and condensed. Inactive ingredients and flavors, such as lactulose, maltitol, lactose, and starch, were added to the extract. The mixture was then formed into fine granules. Finally, the granules and Bifidobacterium longum were combined to prepare the product. Two packets of InterPunch (1.5 g X 2 packets), which is the daily dosage, contain the equivalent of 1g of the dried herbs listed above.
Test parameters
Six clinical symptoms, namely, nausea and vomiting, abdominal pain, a bloating sensation in the abdomen, hematemesis and hematochezia, pruritus, and malaise, were examined. The clinical symptoms were examined and recorded by physicians when each patient visited the hospital. The parameters in hematological examinations were: white blood cell count, red blood cell count, hemoglobin, hematocrit, platelet count, and differential white blood cell count. The parameters in the biochemical examinations were: aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, γ-guanosine triphosphate (GTP), Zincsulfate Turbidity Test (ZTT), lactic dehydrogenase (LDH), urea nitrogen, creatinine, Na, K, Cl, total bilirubin, total protein, albumin, total cholesterol, and triglycerides. Urinalysis parameters were protein, glucose, and occult blood. HCV-RNA levels were quantified, using AMPLICOR version 2.0 assay (Roche Diagnostics, Tokyo, Japan). The HCV antibody titer was measured using Lumipulse II Ortho HCV (Ortho-Clinical Diagnostics, Tokyo, Japan); the standard upper limit is 850 KIU/ml in this assay. If the measurement was above this value, HCV-RNA was remeasured following appropriate dilution of the sample. These measurements were performed prior to and 1 month and 3 months following the commencement of administration of EH0202.
Statistical analysis
Data from hematological and biochemical examinations, HCV-RNA analyses, and the HCV antibody assays were analyzed by means of Students t-test. The efficacy of concomitant medications was analyzed by means of the χ2 test.
Results
Clinical symptoms

Table 1 Malaise was observed in six patients prior to administration. At 1 month of administration, improvement was observed in four of the six patients (66.7%). Improvement was also observed in the same four patients after 3 months of administration (Table 1). Two patients had a bloating sensation in the abdomen, and improvement was observed in one of the two patients (50%) after 1 month of administration, and in both patients (100%) after 3 months of administration. Two patients had nausea and vomiting, and improvement was observed in one of the two patients (50%) after 3 months of administration.
Blood and biochemical examinations, and urinalysis

Table 2

Table 3 No change was observed in any hematological parameters, such as white blood cell (WBC) count, red blood cell count, hemoglobin, hematocrit, and platelet count (Table 2). Administration of EH0202 did not influence WBC differentials (data not shown).
In biochemical examinations, ZTT was 17.5 ± 7.6 IU/l prior to administration, while it was 18.4 ± 7.8 IU/l after 3 months of administration. The increase was significant (P < 0.05). Na was 141.5 ± 2.2mEq/l prior to administration, while it was 142.7 ± 2.0mEq/l after 1 month of administration. This increase was also significant (P < 0.01). However, serum Na concentration after 3 months of administration was similar to the value prior to administration (141.1 ± 2.2 mEq/l). Parameters that indicate the level of hepatic and/or renal function did not change during the study (Table 3). No change was observed in the hematological or biochemical indices following the cessation of EH0202 administration. No significant change was observed in urinalysis findings.
HCV-RNA quantification and HCV antibody titer
Average HCV-RNA levels for the 35 patients were 734.4 ± 716.1 KIU/ml, 605.1 ± 471.1 KIU/ml, and 578.7 ± 437.9KIU/ml prior to and 1 month and 3 months following the commencement of EH0202 administration, respectively (means ± SD). Although HCV-RNA levels tended to decrease with time, the decrease was statistically insignificant.
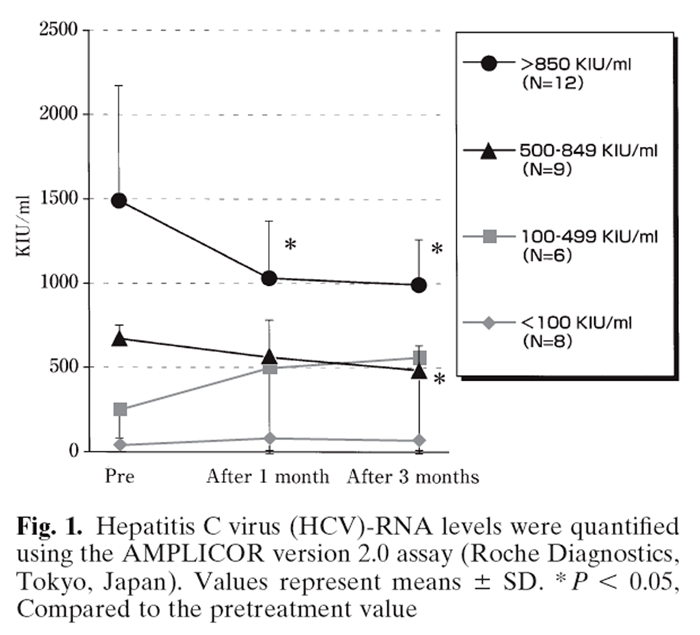
Figure 1 Further analysis was conducted by classifying patients into one of four groups, based on their baseline HCVRNA, i.e., below 100KIU/ml, 100499KIU/ml, 500 849KIU/ml, and over 850 KIU/ml. As shown in Figure 1, HCV-RNA levels had decreased significantly after 1 and 3 months of administration in patients in the over- 850-KIU/ml group (n = 12; P = 0.044 and P = 0.024, respectively). HCV-RNA had also decreased significantly after 3 months of administration in patients in the 500- to 849-KIU/ml group (n = 9; P = 0.021). In general, no significant change was observed in patients with HCV-RNA levels below 500KIU/ml, except for one patient who had a level of HCV-RNA of 1.4 KIU/ml before administration, with a level below the detection limit after 1 month of EH0202 administration. The level in this patient remained below the detection limit both at 1 month and 3 months after the cessation of the administration. We also tried to collect data on viral RNA levels after the study, but it was virtually impossible to collect useful data, because patients were treated with various drugs and regimens, including IFN.
A statistical analysis of the HCV antibody titer was carried out. Complete sets of antibody data were available only for 25 patients. The HCV antibody titer was 66.44 ± 7.78 HCV-Ab index, 67.17 ± 7.00 HCV-Ab index, and 66.83 ± 8.23 HCV-Ab index prior to and at 1 month and 3 months of administration, respectively. That is, no change was observed in the HCV antibody titer following EH0202 treatment. Further analysis by group, as specified above, did not show significant change either.
No significant correlation between HCV-RNA and AST/ALT was observed in patients whose HCV-RNA decreased significantly. In addition, we performed the χ2 test in order to analyze the difference, with respect to changes in HCV-RNA, between patients using concomitant drugs and patients not using concomitant drugs. The difference, with respect to changes in HCVRNA, between patients using ursodeoxycholic acid, which was the most commonly used concomitant drug in this study, and patients not using ursodeoxycholic acid was also analyzed. In all cases, no significant difference was observed. No particular trend was observed between improvement in clinical signs/symptoms and the amount of virus.
Adverse events
A 71-year-old female patient had mild diarrhea in the first month of the dosing period, but the diarrhea disappeared with continued use of EH0202. A 60-year-old male patient experienced a mild bloating sensation in the abdomen during the third month of the dosing period, which did not call for termination of EH0202 treatment. No other adverse events were observed.
Discussion
Currently there are 4 million patients with viral hepatitis in Japan. In addition, it is thought that there are more than 1 million carriers of HCV. [5] In the United States, it is known that 4 million people have been infected with HCV. [6] Thus, hepatitis is a major international health problem which requires significant medical attention.
The general strategies for the treatment of chronic hepatitis C are to eradicate HCV and to suppress hepatitis. IFNs are the mainstay in the treatment of hepatitis C by eradicating HCV; however, complete responses are not always obtained, and the treatment is not without side effects. More recently, a combination of IFN and ribavirin has been introduced as a better treatment of chronic hepatitis C; however, a complete response has yet to be seen, and this treatment is also associated with side effects such as granulocytopenia, thrombocytopenia, and flu-like symptoms.
The present study was an open-label, uncontrolled study of the effect of EH0202 in patients with chronic hepatitis C. In addition to the examination of clinical symptoms, quantification of hematological indices, biochemical examinations, and determination of HCVRNA levels were made prior to and 1 month and 3 months after the commencement of EH0202 treatment. The most notable finding was a significant decrease in HCV-RNA levels, which was found in patients with viral titers higher than 500KIU/ml. Namely, there was a statistically significant decrease in HCV-RNA in the 500- to 849-KIU/ml group, at 3 months, and in the over- 850-KIU/ml group at 1 month and 3 months following EH0202 commencement (P < 0.05). In addition, a few patients showed improvement in certain clinical symptoms, but this was not found in all subjects.
It has been reported that the coefficients of variation of the version 2.0 AMPLICOR test range from 18% to 39%. [7] In our study, the amount of virus in patients with a high viral load (over 850 KIU/ml) decreased to 65.7% of the baseline value (on average, from 1447KIU/ml to 951KIU/ml) following the administration of EH0202, which fell within the range of the reported variation. [7] Thus, no definitive conclusion could be made from this study, and further studies are needed to clarify this question. It has been shown in animal studies that EH0202 promotes the phagocytic activity of macrophages (monocytes) in blood when it is administered to dairy cattle. [8] It has also been reported that EH0202 promotes the phagocytic activity of peritoneal macrophages and promotes IFN production in blood in mice following oral administration. [9] These findings in experimental animals suggest that EH0202 induces IFNs within the body, which may lead to an increase in antiviral activity.
Abnormally elevated levels of AST and ALT in patients with chronic hepatitis C were not decreased or influenced by EH0202 treatment, even in patients whose HCV-RNA levels were significantly decreased. Thus, there was no overt correlation between the amount of viral load and the indices of hepatic dysfunction. It has been reported that the prognosis of hepatic diseases depends on decreases in AST and ALT. [10] In contrast, it is not yet known if a decrease in viral load is directly correlated with clinical improvement in patients with hepatitis C. Nevertheless, the use of EH0202, either concomitantly with, or without other medications, may be effective in the treatment of hepatitis C, because a complete response to IFN treatment is known to depend critically on a decrease in the viral load. In addition, because hepatitis C virus is implicated not only in the inflammatory process in hepatitis but also in the carcinogenic process leading to the development of hepatoma, [11] it appears prudent to suppress the viral titer as much and for as long as possible.
With respect to clinical symptoms, even though relatively few patients experienced symptoms at the outset of the study, the improvements were notable. At 3 months of EH0202 administration, improvements were observed in four of the six patients who experienced malaise, in two of the two patients who had a bloating sensation in the abdomen, and in one of the two patients who had nausea/vomiting. No patient experienced exacerbation of the clinical symptoms that had been observed prior to administration during or after EH0202 treatment. These findings thus suggest that EH0202 may also contribute to improvements in quality of life (QOL), and if so, EH0202 may be a useful healthfood additive with antiviral potency. However, further studies are needed to definitively prove this point, because the current study was an open-label uncontrolled study, which does not permit such conclusions.
In this study, two adverse events, both minor, were observed. One was mild diarrhea, which was observed during the first month of the dosing period. The patient recovered soon after, despite continuation of EH0202 administration, and it was therefore considered a transient reaction. Because InterPunch used in this study contains Bifidobacterium longum, the diarrhea may have been caused by this bacterium. The other adverse event was a mild bloating sensation in the abdomen, which was observed in a 60-year-old male patient during the third month of the dosing period, but it was mild and did not necessitate stopping EH0202 treatment. No other adverse event was observed in the 35 evaluable patients, or in the other 15 patients who did not qualify for evaluation of efficacy, but took EH0202 in the study. These findings suggest that oral administration of EH0202 daily for 3 months is not associated with any serious adverse event; thus, it can be considered safe.
It is known that IFNs can be toxic in high doses, as intensive IFN therapy greatly influences immunomodulation and causes adverse drug reactions that occur in the central nervous, endocrine, and/or digestive systems. In contrast to direct IFN therapy, indirect stimulation of IFN production in the body by various means has not been shown to cause any adverse events. Thus, the finding of no adverse events induced by EH0202 treatment is also consistent with the existing knowledge, and oral EH0202 treatment can be considered safe.
Our findings in this study suggest that oral EH0202 treatment may decrease the viral load in patients with chronic hepatitis C, as soon as 1 month after its administration is begun. The effect of EH0202 appears to be more dominant in patients with high viral titers than in those with a low titer. In some patients, EH0202 may also improve certain symptoms, such as malaise. That oral EH0202 treatment is safe was also shown by the lack of any serious adverse event associated with the treatment, and by the finding that parameters in the blood and biochemical examinations did not worsen. While it is clear that many more studies are necessary to clearly define the safety and the efficacy of EH0202, the preliminary findings in our present study suggest that EH0202 may be a useful health-food additive with antiviral activity to be used in the treatment of chronic hepatitis, such as hepatitis C, and it merits further investigation.
References:
Kojima Y.
Sites of interferon production in rabbits induced by bacterial endotoxin.
Kitasato Arch of Exp Med 1970;43:3544.Kojima Y. Kampo medicines and interferon inducers.
Kampo Medicine 1981;5:915.Toriumi H, Ichikawa Y, Terao T, Yamagiwa K, Shimizu K, Takeishi M, et al.
Clinical efficacy of the feed additive MACH on the incidence of respiratory diseases in piglets.
In: Proceedings of Guangzhou International Conference on the Advanced Traditional Chinese Veterinary Medicine 2000; 2000 Oct 1518;
Guangzhou, China. South China Agricultural University,
Guangzhou, China; 2000. p. 1936.Ushiroyama T, Yoshida S, Tadaki K, Ikeda A, Ueki M.
A pilot study of a Kampo formula, EH0202, with intriguing results for menopausal symptoms.
J Altern Complement Med 2004;10:3979.Satoh T.
Report on a meeting of professionals to discuss combating hepatitis.
Tokyo: Health Science Division, Ministers secretariat,
Ministry of Health, Labour, and Welfare; March 30, 2001.Alter MJ.
Epidemiology of hepatitis C.
Hepatology 1997;26: (Suppl 1):62S5S.Lee SC, Antony A, Lee N, Leibow J, Yang JQ, Soviero S, et al.
Improved version 2.0 qualitative and quantitative AMPLICOR reverse transcription-PCR tests for hepatitis C virus RNA: calibration to international units, enhanced genotype reactivity, and performance characteristics.
J Clin Microbiol 2000;38:41719.Yoshida M, Tanemura K, Wakabayashi A, Otsuka Y, Yoshida S, Onda E, et al.
Study of therapeutic effect of plant complex feed C-UP III on subclinical mastitis caused by S. aureus in dairy cows and activation of blood macrophage (in Japanese with English abstract).
In: Proceedings of Pacific Congress on Milk Quality and Mastitis Control;
2000 Nov 1316, Nagano, Japan; 2000. p. 54752.Takeishi M, Shimizu S, Tsumagari K, Kinoshita A, Yoshida S, Momotani E, et al.
Studies on efficacy of the herbal MACH on IgE, interferon and intra-peritoneal macrophage in mice.
In: Proceedings of the Fourth Advanced Traditional Chinese Veterinary Medicine Seminar;
2002 Oct 810, Guangzhou, China.
Chi Institute, Florida, USA; 2002. p. 85102.Tarao K, Rino Y, Ohkawa S, Tamai S, Miyakawa K, Takekura H, et al.
Close association between high serum alanine aminotransferase levels and multicentric hepatocarcinogenesis in patients with hepatitis C virus-associated cirrhosis.
Cancer 2002;94:178795.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, et al.
The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice.
Nat Med 1998; 4:10657
Return to SHAKLEE STUDIES
Since 12-12-2015


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |