

Lithium Deficiency and the
Onset of Alzheimer's DiseaseThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Nature 2025 (Aug 6) [EPUB] ~ FULL TEXT
OPEN ACCESS Liviu Aron • Zhen Kai Ngian • Chenxi Qiu • Jaejoon Choi • Marianna Liang
Derek M Drake • Sara E Hamplova • Ella K Lacey • Perle Roche • Monlan Yuan
Saba S Hazaveh • Eunjung A Lee • David A Bennett • Bruce A Yankner
Department of Genetics,
Harvard Medical School,
Boston, MA, USA.

You may want to read this in-depth review of this study, created by PBS News.
The author interviewed the Harvard scientists, and this review is a testament to clarity and quality journalism, before you jump into reading this dense and complex study.
You will be glad that you did.The earliest molecular changes in Alzheimer's disease (AD) are poorly understood. [1-5] Here we show that endogenous lithium (Li) is dynamically regulated in the brain and contributes to cognitive preservation during ageing. Of the metals we analysed, Li was the only one that was significantly reduced in the brain in individuals with mild cognitive impairment (MCI), a precursor to AD. Li bioavailability was further reduced in AD by amyloid sequestration.
We explored the role of endogenous Li in the brain by depleting it from the diet of wild-type and AD mouse models. Reducing endogenous cortical Li by approximately 50% markedly increased the deposition of amyloid-β and the accumulation of phospho-tau, and led to pro-inflammatory microglial activation, the loss of synapses, axons and myelin, and accelerated cognitive decline.
These effects were mediated, at least in part, through activation of the kinase GSK3β. Single-nucleus RNA-seq showed that Li deficiency gives rise to transcriptome changes in multiple brain cell types that overlap with transcriptome changes in AD.
Replacement therapy with lithium orotate, which is a Li salt with reduced amyloid binding, prevents pathological changes and memory loss in AD mouse models and ageing wild-type mice.
These findings reveal physiological effects of endogenous Li in the brain and indicate that disruption of Li homeostasis may be an early event in the pathogenesis of AD. Li replacement with amyloid-evading salts is a potential approach to the prevention and treatment of AD.
From the Full-Text Article:
The identification of treatable causes of AD requires a fundamental understanding of the pathogenic processes leading to memory loss. Although substantial progress has been made in defining gene variants that confer risk for AD, the environmental factors that affect the timing of disease onset are not as well understood. [1, 6] Several factors relating to diet, lifestyle and the environment have been identified, but their contributions to AD pathogenesis are unclear. [1, 6, 7] Altered homeostasis of metals is one such factor. [7–12] These studies have focused primarily on the toxic effects of metals such as iron, copper and zinc, which can promote amyloid-β (Aβ) aggregation, tau phosphorylation or oxidative stress in model systems. [6–12] However, metals also have essential roles in brain function, and disruption of this normal physiology in AD is relatively unexplored.
Lithium deficiency in MCI and AD

Figure 1 To explore the role of metal-ion homeostasis in AD, we used inductively coupled plasma mass spectrometry (ICP–MS) to assess 27 abundant and trace metals in the brain and blood of aged individuals with no cognitive impairment (NCI) and individuals with amnestic MCI or AD. Metal levels were determined in the prefrontal cortex (PFC), which is a prominently affected region in AD, and the cerebellum, which is relatively unaffected. Of all the metals surveyed, only one, Li, showed significantly reduced levels in the PFC of individuals with both MCI and AD (Figure 1a,b and Supplementary Table 1).
The mean and median Li cortex-to-serum ratio and total cortical Li were significantly reduced in the PFC of people with MCI and AD (Fig. 1c,d), but not in the cerebellum (Extended Data Fig. 1a,b). In a second independent cohort, Li levels were also significantly reduced in the PFC of people with AD (Fig. 1e). By contrast, the mean serum Li levels in MCI and AD were not significantly different from controls (Extended Data Fig. 1c). Li levels were not significantly affected by sex or the range of postmortem intervals in this study (see Methods). The cortex-to-serum ratios of several other metals also changed in AD, but not in MCI (Fig. 1a,b and Supplementary Table 1). However, the change in Li showed the lowest adjusted P value of all the metals analysed (Fig. 1b). Together, these results indicate that endogenous Li homeostasis is perturbed in the brain in MCI and AD.
We next investigated whether endogenous Li homeostasis in the brain might be perturbed by AD pathology. Previous studies have implicated the interaction of several metals with Aβ. [8, 9] To determine whether amyloid deposition affects the distribution of Li, we performed laser absorption (LA)-ICP–MS and quantified Li in amyloid plaques compared with plaque-free regions in the frontal cortex. A highly significant concentration of Li in Aβ plaques was detected in every case of MCI and AD, which increased from MCI to AD (Fig. 1f). To complement this in situ analysis, PFC samples were subfractionated into a plaque-enriched insoluble fraction and a soluble fraction devoid of amyloid plaques (Supplementary Fig. 1). The mean and median Li levels in the PFC non-plaque fraction were significantly reduced in AD relative to control NCI cases (Fig. 1g).
Furthermore, lower Li levels in the non-plaque cortical fraction correlated with reduced cognitive test scores for episodic and semantic memory, and for a global index of cognitive function, across the entire ageing population (Supplementary Table 2). In patients with AD, lower Li levels in the non-plaque cortical fraction correlated with reduced scores for episodic memory and the index of global cognitive function (Supplementary Table 2).
To further explore the relationship of Li to Aβ, we examined the cortical distribution of endogenous Li in J20 Aβ precursor protein (App)-transgenic mice13 that exhibit widespread Aβ deposition. LA-ICP–MS showed an approximately 3–4-fold concentration of Li in cortical Aβ deposits in 12-month-old J20 mice relative to adjacent plaque-free cortical regions (Extended Data Fig. 1d). Furthermore, subfractionation of the cortex showed that Li in the non-plaque cortical fraction was significantly reduced in J20 relative to wild-type mice, consistent with Li sequestration by amyloid deposits (Extended Data Fig. 1e). By contrast, 3-month-old J20 mice before the onset of amyloid deposition did not exhibit reduced Li in the soluble cortical fraction relative to age-matched wild-type mice (Extended Data ig. 1e). Together, these results indicate that Li is sequestered by Aβ deposits, reducing its bioavailability.
Lithium deficiency in mouse models
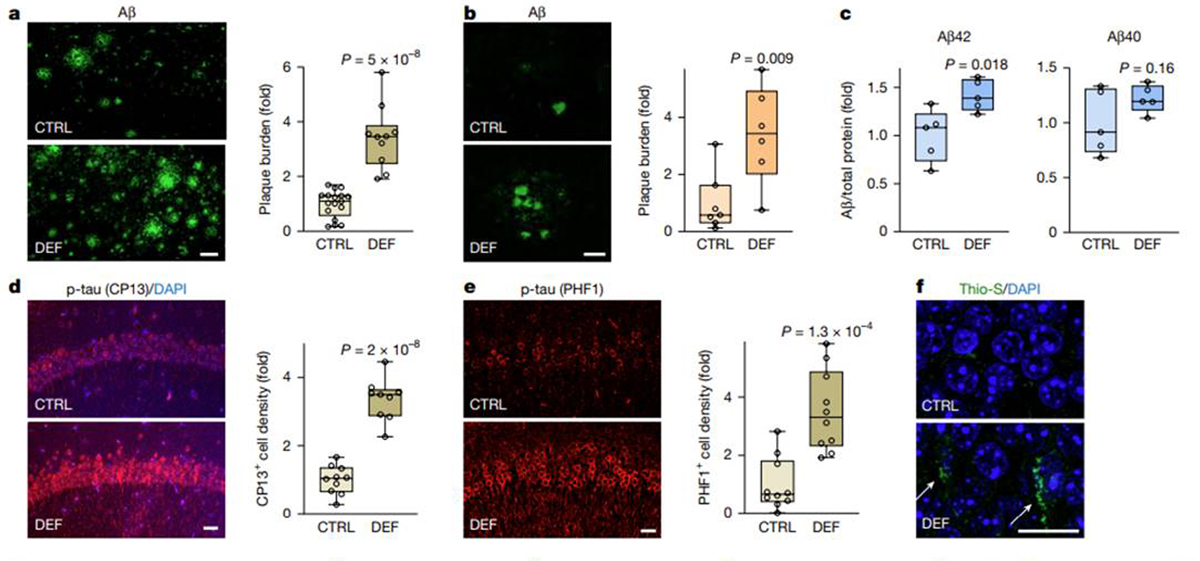
Figure 2 To explore the biology of endogenous Li, mice were maintained on a chemically defined diet that is calorically and nutritionally equivalent to the typical grain-based mouse diet, including the same Li concentration. Serum and cortical Li levels in mice on this diet were in a similar range to those in the ageing human population, and the mean values were not significantly different (see Methods section on Mouse diet). Selective removal of Li from the mouse diet (a 92% reduction) led to an 89% reduction in mean serum Li and a 47–52% reduction in mean cortical Li in the non-plaque fraction (Extended Data Fig. 2a–c and Supplementary Fig. 2).
The pathological effects of reducing endogenous Li were determined in the 3xTg AD mouse model [14], which accumulates Aβ deposits and phospho-tau, the J20 AD mouse model [13], which accumulates abundant Aβ deposits, and ageing wild-type mice without AD-type pathology. The 3xTg and J20 mice maintained on the Li-deficient diet showed a significant elevation in Aβ deposition in the hippocampus (Fig. 2a,b). An increased amyloid plaque burden was observed as early as five weeks on the Li-deficient diet and continued to increase with longer-term treatment (Fig. 2a,b and Extended Data Fig. 2d). In ageing wild-type mice, the Li-deficient diet reduced cortical Li by approximately 50% (Extended Data Fig. 2c). This resulted in a significant elevation of cortical and hippocampal Aβ42, the major pathogenic Aβ species in AD, and a trend towards an increase in Aβ40 (Fig. 2c and Extended Data Fig. 2e). Thus, Li deficiency accelerates Aβ deposition in AD mouse models and increases Aβ42 levels in ageing wild-type mice.
The role of Li homeostasis in tau pathology was explored in 3xTg mice by assessing the phospho-tau isoforms associated with early (pSer202-tau) and advanced (pSer396/Ser404-tau) stages of neurofibrillary tangle (NFT) formation in AD. Both pSer202-tau and pSer396/ Ser404-tau were increased 3–4-fold in hippocampal neurons of Li-deficient 3xTg mice (Fig. 2d,e and Extended Data Fig. 2f,g). A subset of the affected neurons showed elevated phospho-tau in thioflavin S-positive structures that resemble NFTs (Fig. 2f). As observed for Aβ, elevated phospho-tau was evident as early as five weeks into the Li-deficient diet and continued to increase with longer-term treatment (Fig. 2d,e and Extended Data Fig. 2f). These results indicate that Li deficiency promotes neuronal phospho-tau accumulation.
We next assessed whether endogenous Li affects cognitive function in the setting of AD-type pathology and during normal ageing. Administration of the Li-deficient diet to 3xTg mice significantly impaired learning (Fig. 2g) and long-term memory (Fig. 2h,i), as determined in the Morris water-maze paradigm. There were no significant changes in swimming speed, visible platform recognition or performance in the open-field test, consistent with intact visual perception, locomotor activity and exploratory behaviour (Extended Data Fig. 2h–l). Li deficiency in 3xTg mice also gave rise to significant deficits in the Y-maze and novel-object recognition tests of memory (Fig. 2j,k). Furthermore, ageing Li-deficient wild-type mice also showed significant memory loss in the Morris water maze (Fig. 2m,n) and the novel-object recognition test (Fig. 2o). These cognitive deficits in ageing wild-type mice appeared in the absence of significant changes in spatial learning (Fig. 2l), swimming speed, visible platform recognition or performance in the open-field test (Extended Data Fig. 2m–q). Thus, endogenous Li protects against memory loss in the presence of AD-type pathology, as well as during normal ageing in mice.
The transcriptome of lithium deficiency

Figure 3 We next characterized the effects of Li deficiency on the transcriptome of cell populations in the hippocampus, an early site of disease progression in MCI and AD. Single-nucleus RNA-seq (snRNA-seq) analysis of the hippocampus was done in 3xTg mice after administering the Li-deficient diet for 5 weeks. We analysed 64,772 and 54,374 high-quality nuclei from Li-deficient and control mice, respectively (Supplementary Fig. 3). Based on the expression of previously validated cell-type-specific markers ( Supplementary Fig. 4), the relative abundance of the resolved cell types was unchanged after 5 weeks of Li deficiency (Fig. 3a).
Analysis of differentially expressed genes (DEGs) showed significant transcriptome changes in excitatory, granule cell and inhibitory neurons, as well as oligodendrocytes, astrocytes, microglia and oligodendrocyte progenitor cells (OPCs) (Fig. 3b and Supplementary Tables 3 and 4). In excitatory neurons, Gene Ontology (GO) terms for synaptic signalling, organization and transmission were downregulated, whereas electron transport and pathways of neurodegeneration and AD were upregulated (Fig. 3c and Supplementary Tables 5 and 6). Downregulated synaptic genes included Homer1, Grm3, Mef2c, Lrrk2, Grik1, Grik3, Btbd9, Dlgap3 and Dlgap4 (Fig. 3d). In oligodendrocytes, GO terms for axon ensheathment and myelination, as well as neuron projection development, were strongly downregulated (Fig. 3c and Supplementary Tables 5 and 6). Downregulated myelin-related genes included Mbp, Mog, Mag, Plp1 and Opalin (Fig. 3d). In astrocytes, pathways of neurodegeneration, electron transport chain and monovalent cation transport were upregulated (Supplementary Fig. 5 and Supplementary Tables 5 and 6). Proteomic analysis of the hippocampus in Li-deficient 3xTg mice showed significantly reduced abundance of synaptic and myelin protein components and increased abundance of proteins involved in neuroinflammation, lipid metabolism and mitochondrial membrane organization (Supplementary Fig. 6 and Supplementary Table 7). These results indicate that endogenous Li broadly affects the composition of the brain transcriptome and proteome.
We next investigated whether the transcriptome of Li deficiency overlapped with that of AD by comparing our findings with a recent snRNA-seq study of cortical biopsy samples from living individuals with a range of AD pathology. [15] Samples from the human biopsy study that exhibited early stage Aβ deposition showed cell-type-specific overlap of DEGs with 3xTg mice on a Li-deficient diet (Fig. 3e). Concordant DEGs included both upregulated and downregulated genes in excitatory and inhibitory neurons, upregulated genes in microglia and OPCs, and downregulated genes in oligodendrocytes (Fig. 3e and Supplementary Table 8). Overlap with Li-deficient DEGs was more extensive in human cortical samples with both Aβ and phospho-tau pathology that were diagnosed with AD before or within one year of biopsy [15] (Fig. 3e). Concordant DEGs included both upregulated and downregulated genes in excitatory and inhibitory neurons, microglia and oligodendrocytes, and upregulated genes in astrocytes, endothelial cells and OPCs (Fig. 3e and Supplementary Table 8). Thus, the transcriptome of Li deficiency broadly overlaps with the transcriptome of AD pathology in humans.
Maintenance of synapses and myelin
Genes involved in synaptic signalling and structure were broadly downregulated by Li deficiency (Fig. 3c). Dendritic spine loss was detected by Golgi staining in Li-deficient 3xTg and wild-type mice (Fig. 3f and Extended Data Fig. 3a,b), together with reduced immunolabelling of the presynaptic and postsynaptic proteins synaptophysin and PSD-95, respectively (Fig. 3g,h). Reduced abundance of synaptic proteins was confirmed by proteomic analysis of the Li-deficient 3xTg hippocampus (Supplementary Fig. 6a,b and Supplementary Table 7). Thus, endogenous Li contributes to synapse maintenance in the ageing mouse brain.
Li deficiency downregulated the expression of myelin-related genes in oligodendrocytes and reduced the abundance of myelin-associated proteins (Fig. 3c,d and Supplementary Fig. 6b). Fluoromyelin labelling indicated a significant loss of myelin in 3xTg mice after long-term Li deficiency (Fig. 3i). This was associated with reduced numbers of oligodendrocytes, OPCs and axons in Li-deficient 3xTg and wild-type mice (Fig. 3j and Extended Data Fig. 3c,d). To assess myelin ultrastructure, transmission electron microscopy was performed on the corpus callosum in Li-deficient and control 3xTg mice (Extended Data Fig. 3e). Li-deficient mice had thinner electron-dense myelin sheaths surrounding neuronal axons and a significantly elevated g-ratio (the ratio of the inner axonal diameter to the total axon plus myelin fibre diameter), consistent with reduced axonal myelin. These results indicate that endogenous lithium contributes to the maintenance of myelin integrity.
Lithium and microglial function

Figure 4 The snRNA-seq analysis of Li deficiency showed a reduction in the number of microglia expressing the gene Cx3cr1, which encodes a homeostatic marker, and an increase in microglia expressing Apoe (Supplementary Fig. 7a), similar to the reactive microglial state observed in AD. [15] To obtain greater insight into the regulation of microglia by Li, viable microglia were isolated from the brain and analysed by deep RNA sequencing. Isolated microglia showed a high degree of purity and did not exhibit stress-related markers [16] (Supplementary Fig. 7b,c). RNA sequencing showed major transcriptome changes in microglia associated with Li deficiency in both 3xTg and wild-type mice with highly significant overlap (Supplementary Tables 9–12). Genes upregulated by Li deficiency in 3xTg and wild-type microglia were enriched in GO terms corresponding to AD and pathways of neurodegeneration, electron transport chain and respiration, regulation of amyloid fibril formation, translation and oxidative stress. Genes downregulated by Li deficiency were enriched in the GO terms DNA damage response, cellular response to stress, import into cell and protein catabolic process (Fig. 4a,b and Supplementary Table 12).
Transcriptome changes in Li-deficient wild-type microglia were enriched for many risk genes for AD identified in genome-wide association studies (GWAS) [17], including Apoe, Trem2, Bin1, Clu, Picalm, Cd33, H2-Eb1 (HLA-DRB1 orthologue), Inpp5d, Abca1, Abca7 and Adam10 (Multi-marker Analysis of GenoMic Annotation (MAGMA), false discovery rate (FDR) < 0.05). We also observed significant overlap of the transcriptome signature of Li-deficient microglia with that of microglia in AD, particularly for microglia that express glycoprotein NMB (GPNMB) (P < 10–21 for 3xTg and P < 10–14 for wild type), which expand with the progression of AD. [15] Furthermore, GPNMB expression was significantly upregulated in Li-deficient microglia (Supplementary Table 11). Thus, Li deficiency alters the microglial transcriptome in a manner that overlaps with AD.
We next examined reactive changes in microglia following Li deficiency. Immunolabelling showed that Li deficiency increased CD68-immunoreactive microglia in 3xTg mice (Fig. 4b) and elevated microglial protein expression of GPNMB and lipoprotein lipase (LPL), which are markers of microglial reactivity in AD [15] (Fig. 4c). Li deficiency also increased the density of CD68+ reactive microglia in a second transgenic AD mouse model, J20 (Supplementary Fig. 7d). To explore functional changes, isolated microglia were stimulated in culture with lipopolysaccharide (LPS). Microglia from Li-deficient wild-type mice showed elevated release of the pro-inflammatory cytokines IL-6, TNF and G-CSF, as well as the immune activating chemokines CCL3, CCL4, CCL5 and CXCL2 (Fig. 4d). Finally, microglia isolated from Li-deficient wild-type mice showed significantly reduced Aβ42 uptake and subsequent degradation of Aβ42 relative to microglia from control mice (Fig. 4e). Thus, Li deficiency leads to a reactive pro-inflammatory state and impaired Aβ clearance.
GSK3β regulation by endogenous lithium
We next identified signalling pathways that are altered by Li deficiency using Ingenuity/IPA network analysis of DEGs. Wnt–β-catenin signalling was significantly affected and was predicted to be repressed in microglia, excitatory neurons and oligodendrocytes (Extended Data Fig. 4a–c). Immunolabelling showed significantly reduced nuclear β-catenin levels in Li-deficient hippocampal CA1 neurons in 3xTg and wild-type mice (Extended Data Fig. 5a,b). Nuclear β-catenin levels were also reduced in Li-deficient oligodendrocytes and microglia (Extended Data Fig. 5c,d).
A central regulator of β-catenin signalling is the serine-threonine kinase GSK3β, which phosphorylates β-catenin, targeting it for proteasomal degradation. Another GSK3β substrate is tau, which is phosphorylated by GSK3β in AD [18–21] and showed 3–4-fold elevated phosphorylation in Li-deficient 3xTg mice (Fig. 2d,e). Li deficiency elevated total GSK3β levels in hippocampal CA1 neurons, oligodendrocytes and microglia in 3xTg mice (Fig. 4f and Extended Data Fig. 5e,f). Elevated total GSK3β protein was confirmed by proteomic analysis of the hippocampus in Li-deficient mice (Supplementary Table 7). GSK3β mRNA was also elevated by Li deficiency (Extended Data Fig. 5g), consistent with increased GSK3β expression.
GSK3β is activated by autophosphorylation of tyrosine 216, which is increased in AD. [18, 21] The levels of pTyr216-GSK3β were significantly elevated by Li deficiency in hippocampal CA1 neurons and oligodendrocytes in 3xTg and wild-type mice (Extended Data Fig. 5h,i). Phosphorylation of GSK3β at Ser9, which inhibits GSK3β activity, did not change in absolute level, but the ratio of pSer9:total GSK3β was reduced, consistent with elevated GSK3β activity (Extended Data Fig. 5j,k). By contrast, the level of inositol, which is modulated by Li at pharmacological concentrations through inositol monophosphatase [22], was not altered by endogenous Li deficiency (Extended Data Fig. 5l).
To assess the role of GSK3β in the pathogenic effects of Li deficiency, the GSK3β inhibitor CHIR99021 was administered to Li-deficient 3xTg mice. Treatment with CHIR99021 reversed the Li deficiency-associated activation of microglia (Extended Data Fig. 6a) and normalized the levels of multiple pro-inflammatory cytokines and chemokines (Extended Data Fig. 6b). Furthermore, incubation of primary microglia from Li-deficient wild-type mice with CHIR99021 resulted in the restoration of Aβ42 uptake and degradation (Fig. 4g). A similar outcome was achieved using a second GSK3β inhibitor, PF-04802367 (Fig. 4g). Moreover, treatment of Li-deficient 3xTg mice with CHIR99021 reversed the elevated Aβ deposition and tau phosphorylation and restored oligodendrocyte cell number and expression of myelin basic protein (MBP) (Extended Data Fig. 6c–e). Thus, GSK3β activation contributes to a broad range of pathology associated with Li deficiency.
Lithium replacement therapy

Figure 5
page 8The observation that Li is sequestered by amyloid deposits in MCI and AD prompted a search for therapeutic Li salts with reduced amyloid binding. We reasoned that the electrostatic interaction of the Li ion with Aβ deposits would be a function of the ionization capacity of the salt, and that Li salts with reduced ionization might show reduced amyloid sequestration. To assess ionization directly, we measured the conductivity of 16 lithium salts. Inorganic Li salts, including the clinical standard lithium carbonate (Li2CO3, hereafter LiC), showed significantly elevated conductivity, indicative of increased ionization, relative to organic Li salts (P = 8 × 10-4; Fig. 5a and Extended Data Fig. 7a). Of the organic Li salts, lithium orotate (C5H3LiN2O4, hereafter LiO) showed the lowest conductance across a broad Li concentration range (Fig. 5a and Extended Data Fig. 7a) and was therefore selected for further comparison with the clinical standard LiC.
We next investigated whether conductivity predicts Li binding to the aggregated forms of Aβ that accumulate in AD. Equilibrium dialysis binding assays demonstrated that Li binds to both fibrils and oligomers formed from synthetic human Aβ1–42 in vitro (Fig. 5b,c and Extended Data Fig. 7b). Li binding was saturable and occurred in a high-affinity range that included the physiological concentration range of Li in brain and serum (0–2 µEq l-1), as well as a lower-affinity range corresponding to clinical pharmacological doses. Notably, LiC exhibited higher binding affinity than LiO for both Aβ42 fibrils and oligomers across a broad Li concentration range (Fig. 5b,c and Supplementary Table 13).
To further explore the Li–Aβ interaction in vivo, LiO and LiC were administered to J20 and 3xTg mice at a low dose in the drinking water (4.3 µEq l-1 Li), resulting in a serum Li level in the physiological concentration range observed in ageing humans and mice (Supplementary Fig. 8a,c,d, Extended Data Fig. 1c and Methods). Serum and hippocampal Li levels were not significantly different after administration of low-dose LiO or LiC (Supplementary Fig. 8a,b). However, Li was highly concentrated in Aβ plaques after the administration of LiC, but to a much smaller extent after administration of LiO in both 3xTg and J20 mice (Extended Data Fig. 7c,d). Moreover, LiO, but not LiC, significantly elevated parenchymal Li in the non-plaque fraction in 3xTg and J20 mice (Extended Data Fig. 7e,f). Thus, LiO shows reduced amyloid sequestration relative to LiC and more effectively elevates non-plaque Li in the brain.
When LiO was administered to adult 3xTg mice, the physiological dose almost completely prevented Aβ plaque deposition and phospho-tau accumulation; by contrast, administration of LiC or the sodium orotate control had no significant effect (Extended Data Fig. 8a,b). We next investigated whether LiO could reverse more-advanced pathology in ageing mice. Administration of LiO to 3xTg mice from 9 to 18 months of age significantly reduced the Aβ plaque burden and the density of phospho-tau-positive NFT-like structures in the hippocampus, whereas LiC had no significant effect at the same dose (Fig. 5d). LiO also reduced the Aβ plaque burden by about 70% in older J20 mice with abundant and widespread Aβ deposition (Fig. 5e). Thus, LiO is highly effective at reducing Aβ deposition and phospho-tau accumulation.
LiO, but not LiC, increased the expression of the synaptic marker PSD95 (Extended Data Fig. 8c), increased myelin-associated MBP immunoreactivity and elevated oligodendrocyte cell number (Extended Data Fig. 8d,e). LiO was also more effective than LiC in suppressing microgliosis and astrogliosis in aged 3xTg mice (Extended Data Fig. 8f,g). Administration of low-dose LiO, but not LiC, also reduced total GSK3β levels in hippocampal CA1 neurons and white matter (Extended Data Fig. 9a,b), reduced the level of activated pTyr216-GSK3β (Extended Data Fig. 9c) and elevated nuclear β-catenin (Extended Data Fig. 9d).
RNA-seq analysis of the hippocampus of LiO-treated 3xTg mice showed downregulation of genes corresponding to the GO terms translation, electron transport chain, pathways of neurodegeneration, Alzheimer’s disease, β-catenin degradation and interleukin-1 signalling, and upregulation of genes corresponding to the GO terms synapse organization and signalling, neuron projection morphogenesis and learning or memory (Fig. 5f and Supplementary Tables 14 and 15). These GO terms are similar to those associated with Li deficiency but with opposite directionality of change (Figs. 3c and 4a).
The effects of LiO on genes involved in learning and memory prompted us to explore cognitive function in 3xTg mice, which show memory loss relative to wild-type mice (Fig. 5g). LiO at the lowest dose (4.3 µEq lCUP>-1) almost completely reversed the memory loss, whereas LiC and the sodium orotate control did not show significant effects (Fig. 5g). Furthermore, LiO improved learning and spatial memory in ageing J20 mice with advanced amyloid pathology (Extended Data Fig. 10a–c). LiO did not significantly affect swimming speed, visual platform localization or locomotor performance in the open-field test (Extended Data Fig. 10d–h and Supplementary Fig. 9a–f). Thus, LiO suppresses AD-type pathology, neuroinflammation and synapse loss, and restores memory.
Lithium and brain ageing
To explore the effects of Li on normal brain ageing, low-dose LiO (4.3 µEq l-1 Li) was administered to wild-type mice from 12 to 24 months of age. This resulted in a modest elevation of mean serum and cortical Li levels that overlap with the range of endogenous Li in untreated mice (Supplementary Fig. 10a). LiO almost completely prevented age-related microgliosis and astrogliosis in the hippocampus, cortex and corpus callosum (Extended Data Fig. 11a,b). Moreover, LiO reduced the age-related production of the pro-inflammatory cytokines IL-6 and IL-1β (Extended Data Fig. 12a).
Microglia isolated from aged mice showed a marked reduction in the ability to degrade Aβ42 relative to microglia from young adult mice. Treatment with LiO in vivo reversed this age-related loss of Aβ degradative capacity (Extended Data Fig. 12b). Similar results were obtained by incubating microglial BV2 cells with LiO in culture. LiO significantly elevated Aβ42 uptake and degradation in BV2 cells, whereas the control sodium orotate had no significant effect (Extended Data Fig. 12c–e). Thus, LiO reduces age-related neuroinflammatory changes and restores the ability of microglia to clear Aβ.
Synapse loss and cognitive decline are characteristic features of brain ageing in mice. Administration of LiO to wild-type mice prevented age-related loss of dendritic spines in CA1 and CA3 hippocampal neurons, whereas the control salt NaO had no significant effect (Supplementary Fig. 10b). Importantly, the decline in learning and memory associated with ageing was largely reversed by LiO, as determined by tests in the Morris water maze (Extended Data Fig. 13a,b). LiO did not affect swimming speed or localization of a visible platform (Extended Data Fig. 13c,d). LiO also prevented age-related decline in novel-object recognition memory (Extended Data Fig. 13e). Notably, long-term administration of LiO to ageing wild-type, 3xTg or J20 mice did not alter serum levels of blood urea nitrogen, creatinine or thyroid-stimulating hormone (TSH) (Supplementary Fig. 10b). Thus, low dose LiO prevents age-related pro-inflammatory changes, synapse loss and cognitive decline in mice without evidence of toxicity.
These observations prompted an examination of endogenous brain Li and cognitive resilience in normal ageing humans. Expression levels of the presynaptic proteins complexin 1 and 2, which regulate synaptic vesicle exocytosis, predict resistance to AD. [23, 24] Complexin 1 and 2 expression showed a highly significant positive correlation with Li cortex-to-serum ratio in aged individuals without MCI or AD (Extended Data Fig. 13f). Furthermore, cortical Li was positively correlated with cognitive test scores for working memory (P = 0.04) and performance on the Mini Mental State Examination (MMSE; P = 0.02). Together, these results in normal ageing mice and humans indicate that Li homeostasis may contribute to cognitive resilience.
Discussion
These observations indicate a physiological role for endogenous Li that affects brain ageing and vulnerability to AD. In humans, Li is used in psychiatry for the treatment of bipolar disorder at a dose range that raises levels in serum to approximately 1,000 times the endogenous level. We found that in normal ageing mice, low micromolar levels of endogenous Li preserve cognitive function, reduce inflammation and suppress Aβ generation. In AD mouse models, endogenous Li protects against amyloid deposition, tau hyperphosphorylation, neuroinflammation and loss of synapses, axons and myelin. These effects are mediated, at least in part, through repression of the kinase GSK3β in multiple cell types in the brain.
Metallomic profiling of the brain also uncovered alterations in other metals that have been previously observed in AD, such as increased sodium [25] and zinc [26] and reduced copper. [12] However, decreased cortical Li was the only statistically significant metal alteration observed in both MCI and AD in our dataset. This observation is consistent with a population study in Denmark that found a significant inverse correlation between Li levels in local drinking water and the incidence of dementia. [27] We were able to recapitulate the reduced Li levels observed in humans with AD in mouse models with a Li-deficient diet. snRNA-seq showed that reduced cortical Li significantly alters the transcriptome of the major brain cell types. Furthermore, DEGs associated with Li deficiency in the mouse models overlapped with DEGs previously identified in snRNA-seq of human brain biopsy samples with AD pathology. [15] Overlapping DEGs were enriched in microglia, excitatory and inhibitory neurons, OPCs, oligodendrocytes, endothelial cells and astrocytes. Consistent with these transcriptome alterations, Li deficiency resulted in deleterious effects on neurons, microglia and oligodendrocytes with loss of synapses and axons, impaired Aβ clearance and reduced myelination.
Li deficiency altered microglial expression of some of the most penetrant risk-factor genes identified in GWAS studies of AD, including Apoe, Trem2, Bin1, Picalm, Clu, Cd33 and the mouse orthologues of human MS4A6A. [17] These expression changes were associated with elevated markers of microglial activation such as CD68, as well as increased production of multiple pro-inflammatory cytokines and chemokines that are also elevated in AD28. Furthermore, Li deficiency impaired the ability of microglia to phagocytose and degrade Aβ, a phenotype that has been linked to Aβ deposition in AD. [29] These findings indicate that endogenous Li maintains microglial homeostatic function and prevents pro-inflammatory changes associated with AD.
One of the first molecular targets of Li to be characterized at pharmacological doses was the kinase GSK3β. [30] GSK3β activity is increased in AD and has been implicated in Aβ and tau pathology. [18, 20, 21, 31]Moreover, the GSK3β substrate β-catenin is reduced in AD. [18, 32, 33] The reported IC50 of the Li-GSK3β interaction in vitro is in the millimolar range [30], making this a seemingly unlikely interaction at the low micromolar concentration range of endogenous Li in the brain. However, our findings indicate that GSK3β activation and expression are elevated by endogenous Li deficiency. Moreover, GSK3β inhibitors can reverse many of the pathological consequences of Li deficiency, including Aβ deposition, phospho-tau accumulation, myelination and microglial pro-inflammatory activation, as well as restoring the ability of microglia to clear Aβ. Previous reports have shown that GSK3β activation can promote cytokine production in monocytic and microglial cells [34] and impair oligodendrocyte differentiation and myelination by inhibiting β-catenin signalling. [35] Together, our findings implicate increased GSK3β activity in the multisystem effects of Li deficiency in the brain.
Treatment with Li at pharmacologic doses has been shown to reduce Aβ [33, 36–45] and tau [37, 38, 43, 46–49] pathology in various neurodegenerative disease models. Similarly, Li prevented or reversed cognitive decline in several studies [36–39, 43, 44], but not in one short-term study. [47] More direct evidence for a therapeutic role of Li in AD emerged from several small clinical trials. In two initial trials [50, 51], Li treatment did not improve cognitive function, but in three subsequent trials, Li at lower concentrations (0.25–0.5 mEq l-1 in serum) reduced cognitive decline. [52–55] A limitation of these clinical trials might have been the use of Li salts with high levels of amyloid binding. We have characterized a Li salt, lithium orotate, with reduced binding that can bypass amyloid sequestration in AD mouse models. Treatment with LiO at a dose that maintains serum and cortical Li levels in the endogenous range prevents and reverses AD pathology, neuroinflammatory changes and memory loss in AD mouse models and ageing wild-type mice. An important limitation in the treatment of aged individuals with pharmacological doses of lithium is kidney and thyroid toxicity. [59] It is encouraging that toxicity could not be detected following long-term treatment of ageing mice with a low dose of LiO
Disruption of Li homeostasis may contribute to the long prodromal period of neuropathological changes that occur prior to the onset of clinical AD. Our findings indicate that sequestration of Li by amyloid depletes Li in affected brain regions. Li depletion can, in turn, impair microglial clearance of Aβ, which would accelerate amyloid pathology in a positive feedback loop. In parallel, Li deficiency may promote phospho-tau accumulation, inflammation and the loss of synapses, axons and myelin. The progression of this neurodegenerative process may be modulated by genetic risk variants17,57, as well as environmental factors and dietary Li intake. Li deficiency is therefore a potential common mechanism for the multisystem degeneration of the brain that leads to the onset of AD.
Methods
Human brain samples
Post-mortem human brain and serum samples were obtained in accordance with institutional guidelines and with approval from the Harvard Medical School Institutional Review Board. All procedures complied with relevant ethical regulations. All post-mortem human brain and serum samples were fully deidentified before receipt, and no identifiable private donor information was accessible to the researchers. As such, informed consent was not applicable. Frozen post-mortem samples from the prefrontal cortex (BA9/10/47) were available for all cases included in the analysis. Cerebellar tissue and the most recently collected pre-mortem serum samples were available for a subset of individuals. The primary analysis was performed on tissue samples procured from the Rush Alzheimer’s Disease Center, derived from participants in the Religious Orders Study (ROS) or Rush Memory and Aging Project (MAP) (referred to as ROSMAP). The ROSMAP is a longitudinal, clinical–pathological study of ageing, cognitive decline and AD. [58] Study participants agreed to comprehensive annual clinical and neuropsychological evaluation and brain donation at death. To assess cognitive function, 21 cognitive-function tests were used, 19 were in common and 11 were used to inform on clinical diagnoses, as previously described. [59, 60] The follow-up rate exceeded 95% and the autopsy rate exceeded 90%. All individuals who underwent autopsy were subject to a uniform structured neuropathological evaluation of AD. Informed consent, an Anatomic Gift Act and a repository consent were obtained and the studies were approved by an Institutional Review Board of Rush University Medical Center. A second set of frozen frontal cortical brain samples was obtained from brain banks at the Massachusetts General Hospital, Duke University and Washington University, and is referred to as “a second independent cohort”. Brain tissue obtained from these sources had a confirmed pathological diagnosis of AD or NCI. Samples were randomly selected by the source institutions based on tissue availability and alignment with the requested diagnostic categories (NCI, MCI and AD). Within each diagnostic group, samples were matched for age and sex to ensure group comparability.
Absolute and relative metal levels were measured by ICP–MS, with relative levels calculated as the ratio of cortical or cerebellar to serum concentrations from the same individual. Post-mortem interval had no significant effect on total or relative Li levels in this cohort. The study population comprised 40.2% male individuals and 59.8% female individuals. Within diagnostic subgroups, NCI cases comprised 40.8% male individuals and 59.2% female individuals; MCI cases, 42% male individuals and 58% female individuals; and AD cases, 36.4% male individuals and 63.6% female individuals. Individuals of both sexes were analysed, and those with MCI and AD, regardless of sex, exhibited significantly reduced cortical-to-serum Li ratios and lower total cortical Li levels. Donor sex was self-reported and provided by Rush Medical Center (ROSMAP study) and by further tissue sources, including Massachusetts General Hospital, Duke University and Washington University.
Isolation of plaque-enriched and non-plaque fractions
To fractionate brain parenchymal homogenates into amyloid plaque-enriched and non-plaque fractions, we modified a previously described protocol. [61, 62] Frozen brain samples were weighed and then Dounce-homogenized (40 strokes per sample) in 5 volumes (v/w) of ultrapure buffer containing 2% SDS (stock of ultrapure SDS 10%, ThermoFisher Scientific, 24730020) and 0.1 M β-mercaptoethanol (VWR, 97064-878) in 50 mM Tris HCl, pH 7.6 (ultrapure Tris-HCl, pH 7.5, Invitrogen, 15567-027) and water (Aristar Ultra, VWR 87003-236). The Li concentration of the complete buffer was below the detection threshold (<0.02 µg l-1). The homogenates were heated at 100 °C for 10 min and then transferred to a 15-ml Falcon tube fitted with a sieve consisting of woven mesh (polyethylene terephthalate) with a pore size of 100 µm (pluriSelect, SKU 43-10100-60). The samples were passed through the sieve by gravity and the filtrate was then centrifuged (300g for 30 min). The supernatant (soluble non-plaque fraction) was removed and stored at –80 °C. The pellet was resuspended in water at a ratio of 5 ml per gram of pellet mass and stored at –80 °C (plaque-enriched fraction). To image subfractionated Aβ and phospho-tau (Supplementary Fig. 1), 10 µl of the freshly collected plaque-enriched and non-plaque fractions was layered onto albumin-coated glass slides and allowed to dry overnight. They were then washed with ultrapure PBS (which we determined contained less than 25 parts per trillion (ppt) Li) and incubated with a rabbit monoclonal anti-Aβ antibody (Cell Signaling, 8243) and a mouse monoclonal antibody to pSer202-tau (clone CP13) overnight in 2% BSA, 0.1% Triton X-100 in PBS, followed by labelling with secondary anti-rabbit IgG coupled to Alexa Fluor 594, or anti-mouse IgG coupled to Alexa Fluor 488 (1:300 in blocking buffer). The slides were then washed three times in ultrapure PBS and mounted.
ICP–MS
For the analysis of metals in human and mouse biological samples, we modified previous protocols to optimize the detection of ultra-trace elements. We tested several protocols and found that the use of precleaned polyvinylidene difluoride (PVDF) vials fitted with perfluoroalkoxy alkane (PFA) caps, the use of ultra-trace grade reagents (nitric acid, hydrogen peroxide and water), combined with an extended sample digestion and homogenization, and a highly sensitive ICP–MS instrument (PerkinElmer NexION 2000C), allowed the robust detection of ultra-trace metals in human and mouse samples. The commercial precleaned PVDF vials (Elemental Scientific, V-14-0712-C) and PFA caps (Elemental Scientific, V-14-0309-C) were further processed by fully immersing them in 10% trace-grade nitric acid (Fisher Chemical, A509-P212) for at least 48 h, followed by abundant rinsing with double-distilled and deionized water and drying in a chemical hood for 48 h. The chemical hood was thoroughly cleaned before the experiment and was used exclusively for ICP–MS for the entire duration of the experiment to prevent contamination. We also used a protocol allowing for the simultaneous analysis of a large number of human brain samples (approximately 80–120 mg frozen brain material per region per case). First, we determined that the dry-to-wet ratio was unchanged in AD versus NCI. This was established in n = 45 NCI and n = 45 AD frozen cortical samples (100–200 mg per sample) that were weighed and then dried to a constant weight (48 h in a dry oven at 60 °C). The dry-to-wet ratios were 0.127 ± 0.048 for NCI and 0.123 ± 0.034 for AD and were not statistically different (P = 0.67), in agreement with previous work. [63]
The frozen cortical and cerebellum samples were first allowed to thaw, and were then weighed and digested in 5 volumes of nitric acid 67% (w/m, relative to wet mass; BDH Aristar Ultra, VWR, 87003-226) for 72 h with regular vortexing (20 s per vial every 12 h). The samples were fully digested after about 36 h. The serum, the brain non-plaque fractions and the aqueous solutions were digested in an equal volume of nitric acid (67%) for 48 h with regular vortexing (20 s per vial every 12 h). After digestion with nitric acid, hydrogen peroxide (30%; BDH Aristar Ultra, VWR, 87003-224) was added for 24 h with regular vortexing (20 s per vial every 12 h). We added one volume of hydrogen peroxide (w/m, relative to starting wet mass) to digested brain tissues and 0.75 volumes (relative to starting sample volume) to digested serum, non-plaque fractions and aqueous solutions. The samples were then diluted using a 2% nitric solution in ultrapure water (BDH Aristar, VWR, 87003-236). Indium was added to each solution as an internal standard (50 parts per billion; ppb). For all ICP–MS runs, we also measured freshly made solutions of element standards (0, 10 ppt, 50 ppt, 100 ppt, 1 ppb, 10 ppb and 50 ppb) using a 30-element ICP standard (Aristar, VWR, 89800-580). Each run included n = 10 digestion blanks as well as n = 20–30 blank measurements to calculate the detection limits. The samples were injected into a PerkinElmer NexION 2000C ICP–MS instrument fitted with a cross-flow nebulizer and peristaltic pump for sample introduction. The sample delay time was 30 s with a pump speed of 24 rpm. A wash solution of 2% nitric was used between analyses of samples. The human cortex, cerebellum and serum samples were each measured twice on two consecutive days (two technical replicates per sample) and the average value was obtained for each sample. The correlation coefficients between the lithium concentrations measured on day 1 and day 2 were r > 0.99 for frontal cortex, cerebellum and serum, showing that the ICP–MS measurement was highly reproducible. After each run, ICP–MS signal processing was done using GeoPro 2010 Software (Cetac Technologies).
We derived the standard curves for each element, calculated the concentration of each element in the diluted solution, and used the dilution factors to derive elemental abundance in the original samples. Li levels in the cortex and cerebellum are reported per unit of wet weight (Fig. 1d,e, Extended Data Fig. 1b and Supplementary Table 1). Limits of detection (LODs) and limits of quantification (LOQs) were calculated as follows: LOD = YB + 2tSB and LOQ = YB + 10SB, where YB is the average blank signal, t is the critical value of the one-tailed t-test (one-tailed, 95% confidence interval; for example, for 27 blank samples, df = 26 and t = 1.706) and SB is the standard deviation of a blank signal. LOD and LOQ values for all metals can be found in Supplementary Table 1. All individual Li measurements in human samples (prefrontal cortex, cerebellum and serum) were above the LOQ. In recovery experiments, wet brain samples or fluids were spiked with lithium standard added at three levels (n = 7 replicates per spiking level). The recovery of Li from spiked samples ranged from 91% to 105%. All human sample measurements were double-blinded: one lab member not involved in the study relabelled the samples and kept a file with the old and new codes. After the ICP–MS measurements, the samples were unblinded in the presence of the researchers involved in the study, as well as the lab member who was not involved in the study.
The ICP–MS findings from post-mortem human samples were replicated as follows. First, reduced Li content in the cortex of patients with AD was observed using two independent methods, after measurement of total Li levels in frozen cortical material of cases from both ROSMAP (Fig. 1d) and other sources (Fig. 1e), as well as after fractionation and removal of amyloid plaques (Fig. 1g). Second, decreased Li levels in the AD versus NCI prefrontal cortex (P = 2 × 10-3) were also independently confirmed when n = 60 NCI and AD cases were processed and analysed by ICP–MS in a different laboratory (the Spectroscopy Core Facility at the University of Nebraska, Lincoln). Third, decreased Li levels in the AD versus NCI prefrontal cortex (P = 3 × 10-3) were also confirmed when n = 48 NCI and AD cases were processed using an alternative protocol. Frozen samples were thawed and dried to a constant weight by incubating in a dry oven at 60 °C for 48 h. The dried tissue was then digested in 1 ml of 67% nitric acid using a heating block at 95 °C for 3 h. After digestion, 0.3 ml of 30% hydrogen peroxide was added and the mixture was heated for a further 3 h. Finally, the samples were diluted and analysed using ICP–MS.
Li levels measured in the PFC of ageing NCI cases (ROSMAP cases: mean 2.36 ± 1.23 ng per g, range 0.52–6.0 ng per g; non-ROSMAP cases: mean 3.50 ± 2.27 ng per g, range 0.89–9.94 ng per g; Fig. 1d,e) were similar to those measured in a previous study [64] (4.1 ± 1.7 ng per g in the prefrontal cortex of aged non-diseased cases; age, 71 ± 12 years). Similarly, Li levels measured in the cerebellum (ROSMAP cases: mean 2.90 ± 1.69 ng per g, range 0.58–8.40 ng per g; Extended Data Fig. 1b) were similar to those measured in the previous study [64] (2.9 ± 1.3 ng per g). Finally, consistent with previous studies, we observed significantly elevated levels of sodium [25] and zinc [26], along with reduced copper [12, 65] levels, in the AD cortex (Fig. 1a,b and Supplementary Table 1).
LA-ICP–MS
The Li composition in the human and mouse brains in situ was analysed using LA-ICP–MS. Frozen human and mouse brains were first embedded in OCT medium and then sectioned using a cryostat, and the resulting sections (80 µm thick) were mounted onto glass slides. Before data acquisition, the samples were placed vertically in a rack and air-dried for 1 h. The LA-ICP–MS spectrometer consisted of a laser ablation system (213 nm Nd:YAG, Cetac Technologies) connected to a Perkin Elmer NexION 2000C ICP–MS (Perkin Elmer). Using the line tool, we manually selected the area to be ablated. For human samples, we ablated a region of the prefrontal cortex. For mouse samples, we processed coronal sections where the cortex and hippocampus were readily identifiable. The analyte signal was collected using multiple parallel line scans along the entire selected area, progressing in the direction of ablation cell gas flow, using a spot size of 200 µm at 75 µm s-1. The laser operated at an energy level of 70% and a pulse repetition rate of 20 Hz. The typical run time for one sample was about 4–5 h. We also ablated parts of each section that did include brain tissue but contained embedding medium (OCT) and subtracted this background signal from the total signal. Levels of 7Li were normalized to carbon (12C) to correct for any variations in the amount of tissue ablated. Similar conclusions were reached when the analysis did not include normalization to 12C.
Matrix-matched standards were obtained by spiking homogenized samples of human or mouse tissue with three different concentrations of metal standard solution containing the analytes of interest. After homogenization, the mixtures were frozen and 80-µm sections were cut using the cryostat. The final concentrations of these standards were validated by ICP–MS. After LA-ICP–MS data acquisition, signal processing was done using Iolite Software 2018 (Iolite). A Li distribution matrix was generated computationally, using the multiple parallel line rasters. To identify the regions occupied by amyloid plaques, the section immediately adjacent to the section analysed by LA-ICP–MS was processed for Aβ immunofluorescence. In brief, the adjacent section was first fixed with 4% PFA for 2 h then washed three times with PBS. The section was then blocked for 1 h with 2% BSA, 2% fetal bovine serum, 0.1% Triton X-100 in PBS. The anti-Aβ primary antibody (Cell Signaling, 8243), diluted 1:250 in blocking buffer, was then added and the section was incubated overnight at 4 °C. The next day, the section was washed three times with PBS (in total, 30 min), and a secondary anti-rabbit Alexa 594 antibody (diluted 1:300 in blocking buffer) was added for 3 h. The section was finally washed three times with PBS (for 30 min) and mounted. We acquired multiple pictures of Aβ immunofluorescence spanning the entire section using an Olympus FV3000 confocal microscope. The images were then stitched together and imported into Iolite, where the distribution of Aβ immunofluorescence was computationally superimposed on the LA-ICP–MS Li distribution matrix. For each human or mouse sample, we manually selected multiple regions containing Aβ plaques (plaque or ‘P regions’) as well as neighbouring regions devoid of plaques (non-plaque or ‘NP regions’).
The mean Li levels in P and NP regions were determined, and after correcting for background and normalizing to 12C, the P:NP ratios were calculated. Three other isotopes were also assessed: 57Fe, 63Cu and 66Zn. All measurements in P and NP regions exceeded the LOQ, which was 0.82 ng per g for 7Li, 0.23 µg per g for 57Fe, 0.44 µg per g for 63Cu and 55.1 ng per g for 66Zn). As positive controls, 57Fe, 63Cu and 66Zn were all enriched in plaques relative to non-plaque regions in the AD brain, consistent with previous observations66.
Lithium salts
LiO was obtained from Innophos Nutrition and LiC was from Rockwood Lithium. The purity and Li content were verified by mass spectrometry and ICP–MS, respectively. Sources for other Li salts used in conductivity assays are provided in Supplementary Table 16.
Li salts were dissolved in distilled, deionized drinking water and administered ad libitum to mice. The low Li dose corresponded to 4.3 µEq l-1 (equivalent to 0.03 mg (30 µg) of elemental Li per litre). The background Li concentration in the water was minimal (0.109 µg l-1). Solutions of 4.3 µM LiO and 2.15 µM LiC were prepared to deliver equivalent amounts of elemental Li, accounting for the two Li atoms per molecule of LiC (Li2CO3). A 4.3 µM sodium orotate (NaO) solution was also prepared to assess the effects of the orotate anion in the absence of Li. Two more Li doses were also tested: 43 µEq l-1 (delivered as 43 µM LiO) and 430 µEq l-1 (delivered as 430 µM LiO or 215 µM Li2CO3). To control for the orotate anion at the high dose, a 430 µM NaO solution was also tested. Average daily water consumption was comparable across all treatment groups and the vehicle (water-only) group. To evaluate Li uptake and its biological effects in the brain, mice received the Li-containing water for defined periods. Animals were randomly assigned to treatment and control groups, with control mice receiving plain drinking water.
Conductivity measurements
To measure the conductivity of Li salts, the salts were dissolved in water to achieve Li concentrations of 4.3 mEq l-1, 430 µEq l-1, 43 µEq l-1 or 21.5 µEq l-1 in each case. Conductivity was measured using an ST300C conductivity meter (OHAUS, 83033964) equipped with a STCON7 electrode (OHAUS, 30080693) calibrated with potassium chloride conductivity standards. For each lithium salt, three independent solution replicates (n = 3) were prepared. Conductivity values are reported in µS per cm at 25 °C.
In vitro binding of lithium to Aβ
To assess the in vitro binding of Li to Aβ, both oligomeric and fibrillar forms of Aβ42 were prepared. Human Aβ1–42 peptide (1 mg) was initially dissolved in 80 µl of 1% NH4OH then diluted with PBS to a final concentration of 1 mg ml-1 (stock solution) and stored at –80 °C. Oligomeric Aβ42 was generated by resuspending the stock solution in PBS followed by overnight incubation at 4 °C. Fibrillar Aβ42 was obtained by incubating the same stock at 37 °C for 72 h. For Li binding assays, 10 µg of either oligomeric or fibrillar Aβ42 (10 µl of the 1 mg ml-1 stock) was added to 90 µl of Li-containing solutions. These solutions included varying concentrations of either LiO or LiC, matched for Li content and dissolved in ultrapure water (BDH Aristar, VWR, 87003-236). Ultrapure water alone served as the negative control.
Samples were incubated at 37 °C for 16 h. After incubation, the mixtures were transferred to dialysis membranes for 24 h to remove unbound Li (Pur-A-Lyzer mini dialysis kits were used: 6–8 kDa cut-off for oligomers and 25 kDa for fibrils). After dialysis, the samples were transferred into precleaned PVDF vials and digested by adding an equal volume of 67% nitric acid (final volume, 200 µl), followed by a 24-h digestion period. The digested samples were then diluted to 800 µl with 2% nitric acid prepared in ultrapure water. Li content was quantified using a PerkinElmer NexION 2000C ICP–MS instrument. Elemental Li standards were prepared and standard curves showed excellent linearity (r > 0.99). The bound 7Li levels were calculated across a range of Li salt concentrations, and binding curves were plotted using GraphPad Prism (v.9.4.1). Binding affinities (EC50) and 95% confidence intervals for LiO and LiC were determined using nonlinear regression analysis ([agonist] versus response–variable slope, four-parameter model; Supplementary Table 13). Binding to Aβ42 oligomers and fibrils was modelled across the full concentration range (0–500 µEq l-1) as well as within the higher-affinity subranges (0–50 µEq l-1 for oligomers and 0–30 µEq l-1 for fibrils; Supplementary Table 13).
Mice
Animal housing and experimental procedures were approved by the Institutional Animal Care and Use Committee of Harvard Medical School. All mice were housed socially (2–4 animals per cage) in a room with a 12 h:12 h light:dark cycle (lights on at 06:00), controlled for temperature (18–22 °C) and humidity (40–60%). Sentinel mice housed in each rack were tested quarterly and confirmed to be free of pathogens. All cages were individually ventilated. The standard diet 5053, as well as the chemically defined control and Li-deficient diets, were irradiated. Reverse osmosis deionized water and deionized water containing LiO, LiC or NaO was provided ad libitum in bottles that were changed at least weekly.
Wild-type mice were on a C57BL/6J background. We analysed both adult (3–6 months old) and aged (up to 26 months old) wild-type mice, treated for varying durations. The 3xTg mice14 carried APPSwe and tauP301L mutant transgenes, as well as a PS1 knock-in mutation, and were in a hybrid C57BL/6J and 129Sv/Ev background. The J20 mice13 transgenic mice expressed a mutant form of the human amyloid protein precursor bearing both the Swedish (K670N/M671L) and the Indiana (V717F) mutations (APPSwInd) in a C57BL/6J background. For breeding, 10–20 females (all litter-mates derived from the same cross) were typically mated with 8–12 males (all litter-mates derived from the same cross). Mice were identified by numbered ear tags and were randomly selected for behavioural and histological analyses.
To assess treatment effects on disease onset and progression, animals were treated either before pathology emerged (5–6 months old for 3xTg; 3 months old for J20) or after pathology was established (starting at 9 months old for 3xTg and 17 months old for J20). To investigate age-related effects in wild-type mice, chronic treatments were initiated in adulthood (10–12 months old) and continued for 10–14 months during ageing. Experiments included both sexes, and results were consistent between males and females. The number and sex of animals used in each experimental group can be found in the Source Data file. Investigators remained blinded to genotypes and treatment conditions throughout data collection and analysis. No prior sample-size calculations were done, but the number of animals used was consistent with similar studies in the field.
Mouse diet
Li levels in the cortex were comparable between human NCI cases (RUSH cohort: range 0.52–6.0 ng per g; non-RUSH cohort: range 0.89–9.94 ng per g) and mice (wild type and J20: range 1.61–4.59 ng per g). Similarly, serum Li levels in human NCI cases (range 1.53–10.41 ng ml-1) overlapped with those in mice (wild type, J20, 3xTg: range 0.75–4.50 ng ml-1), supporting the relevance of mouse models for studying the biological effects of lithium.
The regular mouse 5053 is a grain-based diet that does not allow Li levels to be manipulated experimentally. To obtain a Li-deficient diet, we used a standard, chemically defined mouse AIN-93M diet that is calorically and nutritionally equivalent to the 5053 diet and was formulated as a standard diet for laboratory rodents by the American Institute of Nutrition in 1993. We tested 5 samples of the regular mouse 5053 diet and 5 samples of the AIN-93M diet and found that the average Li content was 104.8 ng per g in the 5053 diet and 103 ng per g in the AIN-93M diet. The AIN-93M chemically defined diet was modified to exclude Li. The Li-deficient and control AIN-93M diets were formulated by Dyets. We measured Li levels in the Li-deficient diet and confirmed that Li was depleted by 92.0% relative to the chemically defined control diet. The abundances of the other 26 metals that we measured by ICP–MS were identical (data not shown). The solid diets were irradiated before administration to animals. The diets were stored in closed plastic bags that were placed inside cardboard boxes (devoid of light) at –20 °C for up to 4 months before administration to animals.
DNA extraction and genotyping by PCR
We collected about 0.5–1.0 cm of mouse tails in clean Eppendorf tubes; 500 µl of tail lysis buffer (10 mM Tris pH 8, 100 mM NaCl, 10 mM EDTA, 0.5% SDS) containing 0.4 mg ml-1 Proteinase K was added to each tube, and the tubes were incubated overnight in a 56 °C water bath. The next day, 500 µl of isopropanol was added to precipitate the DNA, and the tubes were shaken vigorously for 20 s. Tubes were centrifuged for 10 min at 18,000g and the isopropanol was carefully removed, avoiding the DNA pellet. We then added 70% ethanol and shook the tubes to wash the DNA pellet. We next centrifuged the tubes for 10 min at 18,000g. We removed the ethanol and air-dried the DNA pellet for 2–16 h. The DNA was resuspended in 100 µl acetate-EDTA buffer and placed in a 56 °C water bath overnight. To amplify DNA regions by PCR, we mixed 3 µl of DNA sample with corresponding amounts of forward and reverse PCR primers, PCR master mix and nuclease-free water, and ran the reactions in a thermocycler. Sample loading dye was added to the PCR products and the samples were run on 1–3% agarose gels (prepared by dissolving agarose in TAE buffer, to which Gel Red was added to allow DNA visualization). We also loaded a 100-bp DNA ladder. Gels were visualized using a UV transilluminator.
Quantitative RT–PCR
Total RNA was extracted from cells and tissues using TRIzol reagent (Invitrogen) followed by DNase treatment to remove genomic DNA contamination. Primers were obtained from Harvard’s PrimerBank: for mouse Gsk3b, forward 5'-TGGCAGCAAGGTAACCACAG-3' and reverse 5'-CGGTTCTTAAATCGCTTGTCCTG-3'; for mouse Gapdh, forward 5'-CTTTGTCAAGCTCATTTCCTGG-3' and reverse 5'-TCTTGCTCAGTGTCCTTGC-3'. Real-time PCR was performed for 40 cycles. The specificity and purity of PCR and RT–PCR products were confirmed by the presence of single-peak melting curves.
GSK3β inhibitor treatment
Li-deficient and control 3xTg mice 12 months old, maintained on their respective diets for three months, were treated with the GSK3β inhibitor CHIR-99021 or a vehicle control. A stock solution of CHIR-99021 was prepared in DMSO and diluted in 0.9% saline to a final concentration of 10 mg ml-1, containing 2% DMSO. The solution was warmed to 70 °C to ensure dissolution of the compound. Mice received intraperitoneal injections of CHIR99021 at a dose of 50 mg per kg body weight, once daily for 14 consecutive days. Control animals received equivalent volumes of vehicle (2% DMSO in saline). All animals tolerated the treatment without visible abnormalities and were included in the analysis.
Blood chemistry
BUN and creatinine measurements were done by IDEXX Laboratories, using mouse serum samples. TSH levels in the mouse serum were assessed by ELISA (Elabscience, E-EL-M1153).
Behavioural testingOpen field
Mice were placed in an open field box (75 cm × 75 cm) and movements were tracked in real-time using TopScan Lite software (CleverSys) coupled to a camera. Each mouse was recorded for 10 min, and the average speed and distance travelled were automatically recorded. Mice had no prior exposure to the open-field arena (spontaneous test). All behavioural experiments were performed by researchers who were blinded to the experimental conditions.
Morris water maze
To assess spatial learning and memory, we trained and tested mice in a large circular pool (1.1 m in diameter) filled with 21 °C water, which was rendered opaque by the addition of non-toxic white paint. We placed four distinct visual cues (representing different geometric shapes, patterns and colours) on each wall, to facilitate spatial orientation and the acquisition of spatial memory. Mice were given four training trials a day for 5–7 consecutive days. Each training trial lasted for 1 min. Mice were trained to remember the location of a hidden platform that was submerged 2.5 cm below the water surface. The location of the hidden platform (south-east) remained the same during the 5–7-day training period. If, after a 60-second trial, the animal failed to locate the platform, it was placed on the platform and allowed to remain on the platform for 15 s. Mice were trained four times a day and entered the pool in a randomized order of rotating entrance points (compass directions N, S, E, W, NE and SW). During each training trial, the latency to find the hidden platform was recorded. Then, 24 h after the last training trial, a probe trial was conducted. The platform was removed and mice entered the arena from the NW location (opposite from the platform). The number of entries in the target area (representing the area where the platform had been located during the training trials), the total time spent in the target area, as well as the time spent in all quadrants, and the swimming speed were recorded during the 60-s probe trial. We also conducted separate trials in which a visible platform (platform elevated above the water level, on which a small red flag had been placed) was presented. Mice were given several training sessions and the time (latency) to reach the visible platform was recorded. Mouse movements, as well as average speed, distance travelled, latency to reach a quadrant or target area and number of entries in the target area, were tracked in real time using TopScan Lite software (Clever Sys) and the different measures were automatically recorded. For measurements of learning (latency to reach the platform during the training trials), mice underwent repeated measurements (four measurements a day for 6–7 consecutive days).
Novel-object recognition
Mice were placed in the same open-field box with two novel identical objects for 10 min and allowed to freely explore the identical objects. The next day, mice were reintroduced in the open-field box and presented with a novel object, as well as one of the two objects they explored the previous day. The mice were allowed to explore the objects for 10 min and their movements were tracked in real time using TopScan Lite software (Clever Sys) coupled to a camera. The box and items were cleaned with 70% ethanol between mice. We automatically recorded the time each mouse spent exploring each object, on both day 1 (two identical objects) or day 2 (one novel object and one familiar object), and derived a novelty (discrimination) index, defined as the ratio of time spent exploring the novel object relative to the familiar one.
Y maze
Spontaneous alternation, which is a measure of spatial working memory, was assessed by allowing the mice to freely explore a Y-shaped maze for 8 min. The Y maze consisted of 3 arms (each 40 cm × 8 cm x 15 cm) at an angle of 120° from each other. Mice typically preferred to investigate a new arm of the maze, rather than returning to one that was previously visited. Using TopScanLite software, we recorded each entry in one of the three arms (A, B and C) and then derived the percentage of total correct alternations over the 8-min duration of the trial. A correct alternation (triad) is a succession of entries into three different arms (A–B–C, A–C–B, B–A–C, B–C–A, C–A–B or C–B–A).Mouse neuropathology
Mice were anaesthetized with isoflurane and carbon dioxide and then perfused with PBS at 4 °C for 20 min. Brains were rapidly removed and the two hemispheres were separated. One hemisphere was dissected into subregions (frontal cortex, temporal cortex, occipital cortex, hippocampus and cerebellum). Each subregion was placed in a separate Eppendorf tube, snap-frozen in liquid nitrogen and then stored in a freezer at –80 °C. The second hemisphere was placed in 4% paraformaldehyde for 48 h. The fixed brain was then processed for paraffin embedding, using standard procedures. Paraffin-embedded blocks were sectioned and 6-µm sections were mounted on glass slides and used for histological analyses.
Paraffin-embedded mouse brain blocks were sectioned and the sections were mounted on glass slides. We deparaffinized the sections by immersion in two xylene baths for a total of 10 min, followed by a 5-min immersion in a 50% xylene:50% ethanol solution. The sections were then rehydrated by immersion in solutions of decreasing concentrations of ethanol (95%, 90%, 70% and 50%) and then placed in water. Sections then underwent antigen retrieval using the Diva decloaker (BioCare). Sections were blocked with 3% BSA, 3% fetal bovine serum (FBS) and 0.1% Triton X-100 in PBS for 45 min at room temperature. Primary antibodies (Supplementary Table 16 has a list of antibodies used for immunolabelling) were diluted in 3% BSA, 3% FBS and 0.1% Triton X-100 in PBS. After overnight incubation at 4 °C, sections were washed three times with PBS. Secondary antibodies, diluted in 3% BSA, 3% FBS and 0.1% Triton X-100 in PBS were either biotin-coupled (1:200; Vector Labs) or coupled to Alexa fluorophores (1:300, Invitrogen). After three 10-min washes with PBS, sections were mounted with Pro-Long anti-fade mounting medium with DAPI (Invitrogen) and then imaged using confocal microscopy.
For the Aβ labelling shown in Extended Data Fig. 1e, we incubated sections with an anti-rabbit biotinylated IgG secondary antibody (VectorLabs) for 1 h, followed by three washes in PBS (1 min each) and the addition of avidin-streptavidin-HRP-coupled complex (1:200 in 2% BSA and 0.1% Triton X-100 in PBS; VectorLabs). After three washes with PBS, we added diaminobenzidine (DAB) substrate (prepared by dissolving DAB and peroxide tablets in PBS; Sigma-Aldrich) and incubated for several minutes, until a brown precipitate formed. Sections were then washed with PBS, dehydrated with increasing ethanol concentrations (50%, 70%, 90%, 95% and 100%), followed by incubation with a 50% ethanol:50% xylene solution and two immersions in 100% xylene (5 min each). Sections were mounted with a hydrophobic mounting medium (Permount). For Thioflavin S staining, after deparaffinization the brains were incubated with filtered 1% aqueous Thioflavin-S for 8 min at room temperature, then washed twice (3 min each) in 80% ethanol, once in 95% ethanol (3 min), three times in distilled water and finally mounted. For sections labelled by immunofluorescence, multiple confocal images were acquired using an Olympus Fluoview Confocal Microscope FV3000. For DAB-stained sections, we acquired pictures using a bright-field microscope coupled with a camera.
For analysis of the Aβ plaque burden, pictures of Aβ immunoreactivity (using the rabbit anti-Aβ monoclonal antibody, clone D54D2, Cell Signaling, 8243, dilution 1:250) in the hippocampus were processed using a macro developed for use with Fiji/ImageJ 2.9.0. In brief, confocal pictures were all saved in the same folder and were all automatically opened in Fiji and processed serially. For each picture, the background was subtracted (rolling ball radius was set for 25). Pictures then underwent de-noising, using a Gaussian blur filter (radius of one pixel). The images were then thresholded using the Default Fiji threshold set at 120. Particles with a minimal size of 5 µm2 were retained and their number, average size and mean fluorescence intensity were automatically recorded for each picture in an Excel file. To calculate the Aβ plaque burden, the total area occupied by Aβ plaques was divided by the area of the selection. Three coronal sections (6 µm thick) were sampled for each animal, in the rostral, intermediate and ventral hippocampus. Two 20× images were acquired per section, using an Olympus FluoView LV1000 confocal microscope. The average Aβ burden was obtained by averaging the Aβ plaque density (area occupied by Aβ plaques divided by the total area analysed) in all pictures acquired for each animal.
For analysis of tau pathology, pictures of p-Ser202 tau (CP13, dilution 1:150) or p-Ser396/Ser404 tau (PHF1, dilution 1:200) immunoreactivity in the hippocampus CA1 were processed using a macro developed for use with Fiji/ImageJ 2.9.0. In brief, confocal pictures were all saved in the same folder and were all automatically opened in Fiji and processed serially. Pictures underwent de-noising, using a Gaussian blur filter (radius of one pixel). The images were then thresholded using the Default Fiji threshold set at 150. The number of tau-positive neurons in the selected CA1 area was then manually counted for each thresholded picture and the area was measured. For each picture, we calculated the average density of tau-positive neurons (the total number of tau-positive neurons divided by the area of the region). The average tau-positive neuron densities were calculated for each animal by averaging all the pictures acquired.
Fluorescent image analysis was also performed using MetaMorph NX 2.5 (Meta Series, Molecular Devices). Mean fluorescence intensity for specific markers was quantified in each animal either in the nucleus (β-catenin) or across the entire cell body (for example, GSK3β, pSer9-GSK3β, pTyr216-GSK3β, GPNMB and LPL), based on co-labelling with cell-type-specific markers (such as MAP2, aspartoacylase and Iba1). Between 50 and 300 cells per mouse were analysed, and background signal was subtracted. Synaptophysin and PSD-95 fluorescence intensities were quantified in the CA1 region of the hippocampus, and FluoroMyelin, MBP and SMI-312 intensities were measured in the corpus callosum, with background subtraction applied in all cases. Cell densities of Iba1+, CD68+, aspartoacylase+, PDGFRα+ and GFAP+ populations were also determined in relevant brain regions by quantifying 50–500 cells per mouse.
For each measurement, multiple images were acquired at 4×, 10×, 20× or 40× magnification per animal, spanning the region of interest. Values were averaged for each animal before statistical analysis. The following primary antibodies were also used: anti-aspartoacylase [N1C3-2] (GeneTex, GTX113389; rabbit polyclonal, dilution 1:200), anti-aspartoacylase (clone D-11; Santa Cruz Biotechnology, sc-377308, mouse monoclonal, dilution 1:50), anti-β-catenin (clone E247; Abcam, ab32572; rabbit recombinant monoclonal, dilution 1:250), anti-β-catenin (clone 1B8A1; PTGlab, 66379-1-Ig, mouse monoclonal, dilution 1:200), anti-CD68 (clone KP1; Abcam, ab955; mouse monoclonal, dilution 1:200), anti-GFAP (Sigma-Aldrich, G9269; rabbit polyclonal, dilution 1:200), anti-GSK3β (clone 3D10; Novus Bio, NBP1-47470SS; mouse monoclonal, dilution 1:200), anti-pTyr216-GSK3β (Millipore Sigma, SAB4300237; rabbit polyclonal, dilution 1:100), anti-pSer9-GSK3β (Abcam, ab131097; rabbit polyclonal, dilution 1:100), anti-Iba1 (clone EPR16588; Abcam ab178846; rabbit recombinant monoclonal, dilution 1:2,000), anti-PSD-95 (clone K28/43; Biolegend, 810401; mouse monoclonal, dilution 1:250), anti-synaptophysin (clone SY38; Millipore Sigma, mouse monoclonal, MAB5258-I; dilution 1:200), anti-neurofilament marker (pan axonal marker; clone SMI-312; Biolegend, 837904; mouse monoclonal, dilution 1:200), anti-GPNMB (clone 2B10B8; PTGlab, 66926-1-Ig; mouse monoclonal, dilution 1:200), anti-LPL (Novus Bio, AF7197-SP; goat polyclonal, dilution 1:200), anti-MAP2 (Phosphosolutions, 1099-MAP2; goat polyclonal, dilution 1:500), anti-MBP (clone D8X4Q; Cell Signaling, 78896; rabbit monoclonal, dilution 1:200) and anti-PDGFRα (R&D Systems, AF1062; goat polyclonal, dilution 1:200). Secondary antibodies were used at a 1:300 dilution: donkey anti-goat Alexa 594 (Invitrogen, A-11058), donkey anti-rabbit IgG (H+L) Highly Cross-Adsorbed antibody, Alexa 488 (ThermoFisher Scientific, A21206), donkey anti-mouse IgG (H+L) Highly Cross-Adsorbed antibody, Alexa 594 (ThermoFisher Scientific, A21203), donkey anti-mouse Alexa 647 (Invitrogen, A-31571), donkey anti-rabbit Alexa 647 (Invitrogen, A-31573) and donkey anti-goat Alexa 488 (Invitrogen, A-11055).Golgi labelling
The brains were processed and stained using the FD Rapid Golgistain Kit (FD Neurotechnologies, PK401) following the manufacturer’s protocol with minor modifications. Immediately after dissection, the brains were fixed overnight in 4% PFA. After cryosectioning, free-floating sections of 100 µm were shortly (10 min) fixed in 4% PFA, then stained using the kit’s reagents and mounted using a glycerin-containing medium. Then 12 dendrites per mouse were imaged in the hippocampus or the cortex using a confocal microscope. Dendritic spine density was quantified using Fiji software v.2.9.0.
Transmission electron microscopy
The 3xTg mice were fed either a Li-deficient diet (n = 8) or a control diet (n = 8) from 6 to 12 months of age. At the end point, mice were perfused with a fixative containing 2.5% glutaraldehyde and 2.5% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.4 (Electron Microscopy Sciences, 15949). After perfusion, 1–2-mm3 brain sections were generated and post-fixed overnight at 4 °C in fresh fixative. The corpus callosum was subsequently dissected and processed for embedding in TAAB Epon resin at the Harvard Electron Microscopy Core Facility. In brief, tissue was washed in 0.1 M cacodylate buffer, post-fixed in 1% osmium tetroxide and 1.5% potassium ferrocyanide for 1 h, rinsed in distilled water and incubated in 1% aqueous uranyl acetate for 1 h. After two further water rinses, samples were dehydrated through graded ethanol (50%, 70%, 90% and twice in 100%, for 10 min each) followed by 1 h in propylene oxide. Samples were then infiltrated overnight in a 1:1 mixture of propylene oxide and TAAB Epon (Marivac), embedded in pure TAAB Epon the next day and polymerized at 60 °C for 48 h. Ultrathin sections (approximately 80 nm thick) were cut on a Reichert Ultracut-S microtome, mounted on copper grids, stained with lead citrate and imaged using either a JEOL 1200EX or a Tecnai G2 Spirit BioTWIN transmission electron microscope. Images were captured using an AMT 2k CCD camera and saved in TIFF format. Quantification of myelin sheath thickness, axon diameter and g-ratio was performed using MetaMorph NX 2.5 software (Meta Series, Molecular Devices). A total of 1,376 axons (control group) and 1,396 axons (Li-deficient group) were analysed from eight randomly selected fields per animal (×4,800 magnification) spanning the corpus callosum.Aβ detection by ELISA
Mouse endogenous Aβx–40 and Aβx–42 levels were measured using a previously established protocol67. In brief, hippocampi or cortices were homogenized in 20 volumes (v/w) of tissue lysis buffer consisting of 20 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA and 250 mM sucrose, supplemented with protease inhibitors (Roche) and 100 µM AEBSF. Soluble Aβ species were extracted from tissue homogenates by diethanolamine treatment. Mouse Aβ(x–40) and Aβx–42 were quantified using Wako ELISA kits (292-64501 and 294-62501, respectively). The LOQs were 7.44 pmol l-1 for Aβ(x–40) and 4.75 pmol l-1 for Aβx–42. Sample concentrations ranged from 26.21 to 52.98 pmol l-1 for Aβ(x–40) and from 11.72 to 22.40 pmol l-1 for Aβx–42, all above the respective LOQs.
Assessment of microglial function in vitroMicroglial purification for cell-culture assays
Wild-type Li-deficient and control mice were transcardially perfused with 1× cold PBS. The cortex and hippocampus were dissected and minced using a scalpel before transferring them to 5 ml digestion buffer (2 U ml–1 of Dispase II, 20 U ml–1 DNase I, 10 µM HEPES in HBSS without calcium or magnesium). Samples were incubated for 30 min at 37 °C on an orbital shaker. The tissue was then homogenized by successive trituration with a Pasteur pipette followed by a 1 ml pipette. An equal volume of 1× HBSS was added to the homogenate and the resulting mix was passed through a 70-µm cell strainer, then centrifuged at 300g for 10 min at 4 °C. Samples were resuspended with 40% Percoll (GE Healthcare, 17-0891-02) to remove myelin, and microglia were enriched using CD11b beads, as described above. Microglia were resuspended in pre-warmed media (DMEM/F12 with 2% FBS, 100 ng ml–1 IL-34, 50 ng ml–1 TGFβ1, 25 ng ml–1 M-CSF) and counted using a haemocytometer. Microglia were seeded into 96-well glass-bottom plates precoated with poly-l-ornithine (Sigma, P4957) at 5,000 cells per well using 100 µl of medium. A half-medium change was performed on day 2 and the downstream assay was done on day 3.
Primary microglia were also purified using an alternative protocol. After perfusion, the cortex and hippocampus were dissected, placed in 3 ml buffer (0.9% Hepes, 50 mM NaCl, pH 7.4) and minced with small scissors for 4 min. Then, 7 ml Dispase buffer (2 U ml–1 Dispase II in 0.9% Hepes, 50 mM NaCl, pH 7.4) was added and the tissue was incubated for 1 h at 37 °C on an orbital shaker. The tissue was then homogenized by gently triturating with a 10 ml pipette with a wide bore, to prevent cell shearing. The enzyme activity was halted by the 1:1 addition of 10% fetal bovine serum in PBS (10 ml) at 4 °C. The homogenate was passed through a 70-µm cell strainer to remove meninges and clumped cells. The homogenate was then spun for 10 min at 1,000g and 4 °C, and the supernatant was discarded. The pellet was resuspended in 6 ml of 75% isotonic percoll in PBS (high percoll; GE Healthcare, 17-0891-02). Then 5 ml of 35% isotonic percoll in PBS (low percoll) was added, followed by 4 ml of PBS. The resulting discontinuous gradient was allowed to settle for 15 min at 4 °C. We then centrifuged the tubes at 800g for 45 min at 4 °C. We then aspirated the top (PBS-containing) layer and part of the upper percoll layer. The band containing microglia (approximately 1.5 ml), situated at the interface between the 35% percoll and 75% percoll layers, was gently collected. Then 50 ml of PBS was gently added and the tube was inverted 20 times. The microglia were then centrifuged at 1,000g for 10 min. The supernatant was discarded and the pellet was resuspended in a pre-warmed (at 37 °C) buffer containing 2% fetal bovine serum, 50 U ml–1 penicillin and 50 µg ml–1 streptomycin in RPMI medium.
The BV2 microglial cell line has been maintained in the Yankner laboratory for more than 20 years and stored long-term in liquid nitrogen at –180 °C. After revival, the BV2 cells were authenticated based on their characteristic microglial morphology (small, round to slightly elongated shape, clear cytoplasm and occasional short processes) as well as positive immunolabelling for the microglial markers CD11b and Iba1. Mycoplasma contamination testing was not done.
Microglial Aβ uptake and degradation assays
Microglial Aβ uptake and degradation assays were done as previously described68. Human amyloid Aβ1–42 was purchased from Anaspec (AS-20276). Next, 1 mg of Aβ1–42 peptide was dissolved in 80 µl 1% NH4OH, followed by dilution with PBS to 1 mg ml–1 (stock) and storage at –80 °C. Oligomeric Aβ1–42 was prepared by resuspending the stock solution in DMEM/F12 to 500 µg ml–1 (100 µM) and overnight incubation at 4 °C. On day 3, the medium was replaced with DMEM/F12 containing 2% FBS and Aβ42 oligomers diluted to a final concentration of 2 µg ml–1 (0.4 µM). To assess Aβ42 uptake, cells were incubated for 3 h at 37 °C, followed by three washes with 1× PBS and fixation with 4% PFA for 15 min. To assess microglial Aβ42 degradation, cells incubated with Aβ42 oligomers for 3 h were first washed three times with warm medium. The cells were then incubated with warm medium devoid of Aβ42 for an extra 3 h. They were then washed with 1× PBS and fixed with 4% PFA for 15 min. The fixed cells were washed twice with PBS and blocked with 2% BSA, 2% FBS, 0.1% Triton X-100 in PBS for 1 h. Anti-Iba1 and anti-Aβ (6E10) antibodies, diluted 1:500 in the blocking buffer, were then added and incubated overnight at 4 °C. The next day, the cells were washed three times with PBS 1× and incubated with secondary antibodies (1:300 in blocking buffer) at room temperature for 2 h. Cells were washed three times with 1× PBS, then mounted and analysed by confocal microscopy. We also assessed Aβ42 uptake and clearance by microglia using fluorescently labelled (HiLyte Fluor 555) human Aβ1–42 (AnaSpec, AS-60480). The fluorescently labelled Aβ42 was directly added to the medium containing 2% FBS to reach a concentration of 2 ng µl–1, and the uptake and degradation assays were conducted as detailed above.
Microglial stimulation and treatment with GSK3β inhibitors
To assess cytokine release, primary microglia isolated from control and Li-deficient mice were treated with 50 ng ml–1 LPS on day 2 for 16 h followed by supernatant collection. Inflammatory cytokines were detected and measured using a mouse cytokine array kit (R&D Systems, ARY006). To assess the effects of GSK3β inhibitors on microglial function, microglia were pretreated with 3 µM CHIR99021 or 1 µM of PF-04802367 on day 2 for 24 h before Aβ42 uptake and clearance or cytokine-detection assays.snRNA-seq
Sample preparation
We performed snRNA-seq on the hippocampus of 12-month-old 3xTg mice that were fed a Li-deficient (n = 5 mice) or chemically defined control (n = 4 mice) diet for five weeks. Mice were transcardially perfused with ice-cold PBS at a speed of 6 ml min–1 for 8 min to repress the transcriptional response during the brain dissection and sample preparation69,70. Hippocampal tissue was dissected on ice and flash frozen in liquid nitrogen. Both frozen hippocampal tissues were subsequently thawed together in 500 µl HB buffer (0.25 M sucrose, 25 mM KCl, 5 mM MgCl2, 20 mM Tricine-KOH, pH 7.8, 1 mM DTT, 0.15 mM spermine and 0.5 mM spermidine) and homogenized with the tight pestle of a dounce homogenizer in the same HB buffer with the addition of 0.32% of IGEPAL (Sigma) (average of 25–30 times per sample) on ice. Subsequently, single nuclei were diluted to 9 ml in HB buffer, passed through a 40-µm filter and separated from debris and multinuclei by iodixanol gradient centrifugation. Specifically, we prepared a 50% iodixanol solution by diluting 60% iodixanol (Optiprep density gradient medium, Sigma D1556) with diluent (150 mM KCl, 30 mM MgCl2, 120 mM Tricine-KOH, pH7.8), and subsequently diluted them with HB buffer and supplemented with 0.04% BSA and 64 U ml–1 RNasin Plus ribonuclease inhibitor (Promega, N2611) to prepare 40% iodixanol and 30% iodixanol. We layered 1 ml of 40% iodixanol in the bottom, 1 ml of 30% iodixanol in the middle and gently layered 9 ml of the diluted nuclei suspension on top of the 30% iodixanol layer. The three layers were visually confirmed to be distinct and were subjected to 18 min of 10,000g centrifugation. Single nuclei were carefully recovered from the 30% iodixanol layer in between the 30% and 40% interface. An aliquot was taken for trypan blue staining and visual inspection of nucleic morphology under a microscope, which showed a homogeneous size distribution and absence of major debris or doublets. The numbers of nuclei were determined initially by haemocytometer and subsequently confirmed with an automated counter. The remainder of the nucleic suspension was diluted for nuclei encapsulation and sequencing library preparation at the Harvard Single Cell Core, according to the 10X Genomics manual. The size and quality of the prepared libraries were confirmed on Agilent high-sensitivity TapeStation and the library was independently quantified by qPCR. The prepared libraries were sequenced by Nova-Seq S4 at the Harvard Biopolymers Facility, at an average coverage of 32,897 reads per nucleus (Supplementary Table 3). Sequencing data and individual animal metadata have been deposited at the NCBI Gene Expression Omnibus GSE272344 and linked to BioProject PRJNA1136488.
Single-nucleus RNA-seq quality control
We aligned the demultiplexed raw sequencing reads to the mouse genome (mm10 from 10X Genomics) using Cell Ranger (v.6.1.2)71, with the –include-introns option, to account for nuclear pre-mRNAs. The generated counts table was loaded to Seurat (v.4)72 to generate Seurat objects. Cells with more than 10% of reads being attributed to mitochondrial transcripts were filtered out. Cells that expressed fewer than 200 features (low-quality cells) or more than 8,000 features (apparent doublets) were also filtered out. These thresholds were determined by visual inspection of the distribution of features among cells (Seurat, VlnPlot) and are generally consistent with previous reports57,73. The cells that passed quality controls were log-normalized using the NormalizeData function from Seurat with a scale factor of 10,000. Variable features were identified using the FindVariableFeatures function from Seurat with the vst selection method and 2,000 features. Data were scaled using ScaleData and principal component analysis (PCA) was performed using RunPCA with the identified variable features using Seurat. Nearest neighbours were found using FindNeighbors with dimension 1:30, which was determined by ElbowPlot following the Seurat manual. The number of clusters was determined by the FindClusters function using Seurat v.4. UMAP and TSNE were performed using RunUMAP and RunTSNE using Seurat with dimensions 1:30 and do.fast=TRUE parameter. Potential doublets were removed using DoubletFinder (v.3)74 following the default parameters. After filtration, cluster-specific markers were determined using the FindAllMarkers function (Seurat v.4), with parameters only.pos = F, min.pct = 0.25 and max.cell.per.ident = 500.
Cell-type-specific annotation and differential gene expression analyses
Cell types were identified by cross-referencing the transcriptome of each individual cell to the Mouse Cell Atlas75 and independently validated by confirming the expression of established cell-type-specific markers on a cluster-to-cluster basis. Violin and heat scatter plots to demonstrate the expression distribution of selected established markers were plotted using the VlnPlot and FeaturePlot functions of Seurat. Examples of cell-type markers include Slc17a7 (excitatory neurons), Prox1 (granule cells), Gad1 and Gad2 (inhibitory neurons), Mbp (oligodendrocytes), Aldoc, Aqp4 (astrocytes), Pdgfra (OPCs), Vtn (pericytes), Cx3cr1 and Tgfbr1 (microglia), Cldn5 and Flt1 (endothelial cells), Prlr and Folr1 (choroid plexus cells; Supplementary Fig. 4). Cell type-specific abundance and differential gene expression analyses were performed on the main cell types (excitatory neurons, inhibitory neurons, granule cells, microglia, astrocytes, oligodendrocytes, OPCs and endothelial cells). Clusters of the same cell types were combined to increase the statistical power, as described previously69,70,76, and clusters with mixed cell types (less than 80% homogenous) were removed for cell-type-specific analyses. The relative abundance of each cell type was computed by dividing the number of cells of the particular cell type by the total number of cells. Two-tailed unpaired Student’s t-tests were done to determine whether there were significant differences in the relative abundance of each cell type between the control and Li-deficient conditions. DEGs were computed by the MAST77 test in Seurat. Genes expressed in fewer than 1% of the cells in each cell type were filtered out. All the DEGs (FDR < 0.05) are reported in Supplementary Table 4. DEGs with FDR < 0.05 and |log2fold change| > 0.1 were used for Gene Ontology enrichment analyses using Metascape78 v.3.5.20240101. The heat scatter plot for DEGs (Fig. 3d) was generated using the FeaturePlot function of Seurat.Purified microglia RNA-seq
Purification of microglia
Li-deficient or control wild-type and 3xTg mice were perfused transcardially using PBS at 4 °C. The cortex and hippocampus were quickly dissected and pooled, then minced using a scalpel, before transferring to 5 ml dissection buffer (HBSS without calcium and magnesium) and Protector RNase inhibitor (Sigma) at 4 °C in a dounce homogenizer. Brain samples were dounced 20 times with a loose pestle and 10 times with a tight pestle. The cell suspension was passed through a pre-wetted 70-µm cell strainer into a pre-chilled 15 ml tube. Cells were then spun down at 300g for 10 min at 4 °C. Cell pellets were resuspended in 10 ml ice-cold 40% Percoll and centrifuged at 800g for 20 min at 4 °C. Myelin debris was removed by vacuum suction and the cell pellet was washed with 5 ml ice-cold HBSS and spun again for 5 min at 300g at 4 °C. The pellets were resuspended in 180 µl ice-cold MACs buffer (0.5% BSA, 2 mM EDTA, Protector RNase inhibitor in PBS) with 20 µl of CD11b microbeads (Miltenyi Biotec, 130-049-601) and incubated on ice for 15 min. After incubation, 1 ml of MACs buffer was added to samples and cells were centrifuged for 5 min at 300g and 4 °C. Microglia were then isolated using LS columns with QuadroMACS Separator following the manufacturer’s instructions. In brief, LS columns were pre-washed three times with 3 ml MACs buffer. Samples were resuspended in 500 µl MACs buffer and transferred to LS columns, followed by three more washes with 3 ml MACs buffer. Finally, microglia were released with 5 ml MACs buffer (without EDTA), then used for RNA extraction.
Microglial RNA sequencing quality control and analysis
Total microglial RNA was extracted from MACs-purified microglia using RNAzol RT (Sigma, R4533). RNA extracted from each microglial preparation was quantified using an Agilent Tapestation 4200 instrument, with a corresponding Agilent Tapestation High Sensitivity RNA assay (5067-5579). The samples were normalized to 4 ng of input in 9.5 µl, and the polyadenylated mRNA was selected for using 3' SMART-Seq CDS Primer II A as part of the Takara SMART-Seq v.4 Ultra Low Input RNA (634894) workflow, which generated cDNA. From there, an Agilent Bioanalyzer High Sensitivity DNA assay (5067-4626) was used to quantify the cDNA concentration. Libraries were obtained using the Illumina NexteraXT kit (FC-131-1096). Adapter ligation, indexing and amplification were done subsequently as part of the same workflow. After amplification, residual primers were eluted away using KAPA Pure Beads (07983298001) in a 0.6× SPRI-based clean-up. The resulting purified libraries were run on an Agilent 4200 Tapestation instrument, with a corresponding Agilent D5000 ScreenTape assay (5067-5588 and 5067-5589) to visualize the libraries and check the size and concentration of each library. Molarity values obtained from this assay were used to normalize all samples in equimolar ratio for one final pool. The pooled library was denatured and loaded onto a single lane of an Illumina NovaSeq 6000 S4 flow cell to generate 100-bp paired-end reads. The pool was loaded at 200 pM (normalized to 1 nM pre-denaturation), with 1% PhiX spiked in as a sequencing control. The base-call files were demultiplexed through the Harvard BPF Genomics Core pipeline and the resulting fastq files were used in subsequent analysis. Raw RNA-sequencing data in FASTQ format were subjected to quality assessment using FastQC (v.0.11.9) and sequencing reads were aligned to mouse genome (mm10) using a STAR aligner79 with the following options: --outFilterMismatchNmax 999 --outFilterMismatchNoverLmax 0.04 --alignSJDBoverhangMin 1 --alignSJoverhangMin 8 --outFilterMultimapNmax 20 --outFilterType BySJout --alignIntronMin 20 --alignIntronMax 1000000 --alignMatesGapMax 1000000. Microglia RNA-seq yielded an average of 100 million uniquely mapped reads for each sample, and gene expression levels were quantified using htseq-count80. To reduce the computational burden and focus on biologically relevant genes, we initially prefiltered the count data. Genes were retained if they had at least five counts in at least three samples. To validate the purity of the isolated microglia, we determined that microglial markers (Csf1r, P2ry12 and Tmem119) were strongly enriched, whereas neuronal (Map2 and Nsg2), astrocytic (Gfap and Aldh1l1) and oligodendrocyte (Olig2 and Mog) marker genes were negligibly expressed (Supplementary Fig. 7b,c). We also verified that markers of ex vivo microglia activation81 (Fos, Jun, Hspa1a and Zfp36) were minimally expressed in our samples (Supplementary Fig. 7b,c). Differential gene expression analysis was done using DESeq2 (ref. 82) to identify DEGs between Li-deficient and control microglia, with an adjusted P value cut-off of 0.05 (Supplementary Tables 10 and 11). Upregulated and downregulated DEGs from Li-deficient wild-type and 3xTg microglia were further analysed for overlapping DEGs, and the overlapping DEGs were subjected to Gene Ontology enrichment analysis using Metascape78 v.3.5.20240101. Results are summarized in Fig. 4a and provided in Supplementary Table 12.
Ingenuity Pathway Analysis
Signalling pathway and molecular network analyses were done on DEGs identified from the snRNA-seq and microglia RNA-seq datasets (FDR < 0.05) using Ingenuity Pathway Analysis (IPA)83. Significantly enriched pathways and disease or function annotations were identified and ranked based on the FDR, calculated using a one-sided Fisher’s exact test followed by a Benjamini–Hochberg correction for multiple comparisons. To visualize the results, the top pathway-enriched DEGs were integrated into a signalling network using IPA’s build and overlay function (Extended Data Fig. 4b,c).RNA-seq of hippocampus from 3xTg mice treated with LiO
RNA extraction
Twelve-month-old 3xTg mice that were treated with 4.3 µM LiO (n = 9 females) or vehicle (water; n = 9 females) from 6 to 12 months of age were transcardially perfused with cold PBS 1× and the hippocampi were rapidly dissected and snap frozen. The total hippocampal RNA was extracted using Trizol reagent (Ambion, 15596018) and purified using a Direct-zol RNA Mini Prep kit (Zymo Research, R2050) according to the manufacturer’s instructions. RNA integrity and concentration were assayed using an Agilent 2100 Bioanalyzer instrument. All RNA samples had an RNA integrity number of more than 8.2.
RNA library preparation and sequencing
Libraries were prepared using Illumina TruSeq Stranded mRNA sample-preparation kits from 500 ng of purified total RNA according to the manufacturer’s protocol. The finished dsDNA libraries were quantified using a Qubit fluorometer, Agilent TapeStation 2200, and RT–qPCR using a Kapa Biosystems library quantification kit according to the manufacturer’s protocols. Uniquely indexed libraries were pooled and sequenced on an Illumina NextSeq 500 instrument with paired-end 75-bp reads by the Dana-Farber Cancer Institute Molecular Biology Core Facilities. Samples were pooled with multiple samples per lane and sequenced. There were two sequencing batches (batch 1, n = 5 mice per group; batch 2, n = 4 mice per group).
RNA sequencing quality control and quantification of gene expression
Quality control of sequencing reads (Supplementary Table 14) was done using FastQC v.0.11.5 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Reads were aligned to the Mouse GRCm38 genome with GENCODE M21 gene models using STAR79 v.2.7.0f with options --outSAMunmapped Within --alignSJDBoverhangMin 1 --alignSJoverhangMin 8 --outFilterMultimapNmax 20 --outFilterType BySJout --alignIntronMin 20 --alignIntronMax 5000000 --alignMatesGapMax 5000000 --twopassMode Basic. The expression of genes was quantified as gene counts using STAR at the same time as alignment with option --quantMode GeneCounts.
Gene-expression normalization and covariate adjustment
Gene counts were input to edgeR. Genes were deemed expressed if at least n = 9 samples (where n is the group size) had more than one count per million (CPM). Genes not satisfying these criteria were removed, keeping the original library sizes. This filtering retained n = 14,862 expressed genes out of 55,536 annotated genes for subsequent analyses. Counts were then normalized using the TMM method in edgeR. Finally, log(CPM) values were calculated for analyses other than differential expression.
To adjust gene expression for covariates, we fit the linear regression model for each gene and cohort separately using lm() in R: gene expression ~ group + covariates, where gene expression is log(CPM), and using the group and covariates: factor, two levels: LiO and water (reference level), covariates (sequencing batch (factor, two levels)) and one RUV with residuals (RUVr) covariate (continuous). The final normalized and adjusted gene-expression values were derived from adding the regression residuals to the estimated effect of the group level to preserve the effect of the group on expression. These normalized and adjusted gene-expression values were used to perform gene–gene regression analysis and gene–gene group regression analysis, and to visualize gene expression.
To adjust for technical variation, we used the RUVr method84 implemented in the RUVSeq v.1.18.0 Bioconductor package. We performed a first pass edgeR analysis, up to and including the glmFit() step with the covariates listed above, excluding the RUVr covariates. Then we used residuals() with argument type = ‘deviance’ to obtain a matrix of deviance residuals. The specified number of unwanted factors (RUVr covariates) used in final analyses were then estimated by the RUVr function using log(CPM) expression values and the residuals. The number of unwanted factors was selected based on separation of groups in PC plots using normalized and adjusted gene-expression values and checking that histograms of differential expression P values showed a uniform or anti-conservative pattern.
Differential expression and gene set enrichment analysis
Differential expression analysis between groups with covariate adjustment using the covariates listed above was performed for expressed genes using edgeR (estimateDisp, glmFit and glmLRT with default arguments) in R. Genes were considered differentially expressed if FDR < 0.05. Gene Ontology enrichment analysis was done separately for upregulated genes and downregulated genes using Metascape v.3.5.20240101 and is summarized in Supplementary Table 15 and Fig. 5f.GWAS-DEG enrichment analysis
Before doing the GWAS-DEG enrichment analysis, we converted the mouse gene symbols to their human orthologues, using a two-step process. First, we used the alias2SymbolTable function in the Limma R package85 v.3.58.1 to map any gene aliases to their corresponding main symbols. Subsequently, the resulting gene symbols were converted to human orthologues using the MGI orthologue table86. If there were multiple mapping candidates, all possible conversions were applied. For example, if a mouse gene had multiple human orthologues, records with all the relevant human gene symbols were generated. This standardized gene nomenclature enabled cross-species comparisons in subsequent analyses.
To do the GWAS-DEG enrichment analysis for microglia isolated from Li-deficient mice, we used MAGMA87 v.1.10. The gene set of DEGs identified by microglia bulk RNA-seq analysis was used and the summary statistics from the GWAS catalogue AD (accession ID: MONDO_0004975)88 were obtained and formatted for MAGMA input. The GWAS catalogue AD contains GWAS records from multiple studies. If multiple records were found for the same variant, we retained the entry with the lowest P value. Gene-set analysis was conducted using the default MAGMA settings, with multiple testing correction applied to account for the number of gene sets tested. Enrichment results were considered significant at a false discovery rate (FDR) of 0.05.
Overlap of mouse and human DEGs
To assess the overlap between DEGs derived from our transcriptomic analyses and DEGs derived from the analysis of human brain samples with varying degrees of AD pathology15 (Fig. 3d), we first converted mouse gene symbols to their human orthologues, as described above. We matched the cell types analysed in our mouse studies with those analysed in humans15. To assess the statistical significance of the overlap between the two DEG sets, we did a Fisher’s exact test. To control for multiple comparisons arising from the analysis of different cell types and DEG directions (upregulated and downregulated), we adjusted the P values using the Benjamini–Hochberg procedure. Two sets of adjusted P values were calculated (Fig. 3d and Supplementary Table 8): one set for the overlap of genes upregulated in both datasets (indicated in red), and another for the overlap of genes downregulated in both datasets (indicated in blue).
Proteome analysis by mass spectrometry
The proteomic analysis was done at the Harvard Center for Mass Spectrometry. Hippocampal homogenates from Li-deficient and control 3xTg mice containing an equal amount (100 µg) of protein were reduced with 200 mM tris[2-carboxyethyl] phosphine (TCEP) at 55 °C for 1 h, then alkylated with 375 mM iodoacetamide at room temperature for 30 min in the dark. Proteins were precipitated using the methanol/chloroform/water precipitation method and then digested with trypsin overnight at 37 °C. TMT labelling of digested samples was done according to the manufacturer’s instructions (ThermoFisher). In brief, TMT labelling reagents were dissolved with 41 µl anhydrous acetonitrile, and an equal volume of TMT reagent mix was added to each sample. After incubation for 1 h at room temperature, the reaction was quenched with 8 µl of 5% hydroxylamine. Equal amounts of peptides from each sample were combined and dried in a SpeedVac. The peptides were then separated using an Agilent 1200 HPLC system with a PolyWAX LP column (PolyLC), 200 × 2.1 mm, 5 µm and 300 A running under electrostatic repulsion–hydrophilic interaction chromatography (ERLIC) mode conditions. Peptides were separated across a 90-minute gradient from 0% buffer A (90% acetonitrile, 0.1% acetic acid) to 75% buffer B (30% acetonitrile, 0.1% formic acid) with 20 fractions collected by time. Each fraction was dried in a SpeedVac and resuspended in 0.1% formic acid solution before analysis by mass spectrometry. Each ERLIC fraction was submitted for a single liquid chromatography–tandem mass spectrometry (LC-MS/MS) experiment that was done on a Q Exactive HF-X High Resolution Orbitrap (Thermo Fisher) coupled with an Ultimate 3000 nanoLC (Thermo Fisher) at the Harvard Center for Mass Spectrometry. Peptides were first isolated on a trapping cartridge (300 µm × 5 mm PepMap Neo C18 trap cartridge, Thermo Scientific) before separation on an analytical column (µPAC, C18 pillar surface, 50-cm bed, Thermo Scientific). The LC gradient was as follows: 2–27% in mobile phase B (0.1% formic acid in acetonitrile) for 70 min and increased to 98% mobile phase B for 15 min at a flow rate of 300 nl min–1. The mass spectrometer operated in data-dependent mode for all analyses. Electrospray-positive ionization was enabled with a voltage of 2.1 kV. A full scan ranging from 400 to 1,600 m/z was done with a mass resolution of 12 × 104 and an automated gain control (AGC) target set to 1 × 106.Proteomics quality control
The top three most intensive precursor ions from each scan were used for MS2 fragmentation (normalized collision energy of 32) at a mass resolution of 3.0 × 104 and an AGC of 1 × 105. The dynamic exclusion was set at 50 s with a precursor isolation window of 1.2 m/z. Raw data were submitted for analysis in Proteome Discoverer 3.0 software (Thermo Scientific). The MS/MS data were searched against the UniProt reviewed Mus musculus (mouse) database along with known contaminants, such as human keratins and common lab contaminants. Sequest HT searches were performed using the following guidelines: a 10-ppm MS tolerance and 0.02-Da MS/MS tolerance; trypsin digestion with up to two missed cleavages; carbamidomethylation (+57.021 Da) on cysteine, TMT 6-plex tags on peptide amino termini and lysine residue (+229.163 Da) were set as static modification; oxidation (+15.995 Da) of methionine was set as variable modification; minimum required peptide length was set to ≥6 amino acids. At least one unique peptide per protein group was required to identify proteins. Of 13,404 proteins identified, only n = 3,392 proteins were identified with high confidence (MS2 spectra assignment, FDR < 0.01 on both protein and peptide level by applying the target-decoy database search by Percolator) and were included in the statistical analysis, as per the standard procedures of the Harvard Center for Mass Spectrometry89,90,91. The sample labels were control (samples 1, 3, 5 and 7) and Li-deficient (samples 2, 4, 6 and 8) and were all 3xTg homozygous females, aged 15 months (treatment from 6 to 15 months of age). An ANOVA followed by Tukey’s post-hoc test was used to assess differences in protein abundance between Li-deficient and control samples. P values were adjusted for multiple comparisons using the Benjamini–Hochberg method to control the FDR. Proteins with an adjusted P < 0.05 were considered differentially abundant (Supplementary Table 7). The proteins identified with high confidence and included in the statistical analysis are listed in Supplementary Table 7. All other proteins, identified with lower confidence, can be accessed from the files deposited at the ProteomeXchange Consortium through the PRIDE partner repository with the dataset identifier PXD063039. This represents 21 files, including one .msf file (containing all search results: peptide-spectrum matches, peptide groups, protein groups, modifications, scores, FDR and metadata) and 20 .raw files (containing MS1/2 spectra and metadata), one for each of the 20 fractions analysed by mass spectrometry.Statistics and reproducibility
Statistical analysis was done using GraphPad software v.10.3.0 (507). The statistical tests used are noted in the figure legends. Throughout the paper, all tests are two sided and unpaired unless stated otherwise. A significance level of 0.05 was used to reject the null hypothesis. The sample size, age and sex of experimental animals, as well as the summary of each statistical test (including degrees of freedom, confidence intervals and P values) can be found in the Source Data file. All animal experiments were done once per condition using biologically independent samples (individual animals), with group sizes indicated in the corresponding figure legends. Representative immunolabelling images shown in the figures are from one animal per group, selected from multiple animals that consistently showed similar results.
Supplementary information
Supplementary Figures This file contains Supplementary Figs. 1–11. [PDF]
Reporting Summary Software and code for: Statistics, Data collection, etc. [PDF 8 pages]
Supplementary Table 1 Metallomic analysis of NCI, MCI and AD. [EXCEL .XLSX]
Supplementary Table 2 Correlations between cortical Li in the non-plaque fraction
and cognitive and pathological indices in the ageing population.
[EXCEL .XLSX]
Supplementary Table 3 snRNA-seq analysis of the hippocampus in 3xTg mice:
summary of quality control and DEGs.
[EXCEL .XLSX]
Supplementary Table 4 DEGs in cell populations of Li-deficient 3xTg hippocampus
analysed by snRNA-seq.
[EXCEL .XLSX]
Supplementary Table 5 GO analysis of downregulated pathways identified by snRNA-seq
analysis of the Li-deficient 3xTg hippocampus.
[EXCEL .XLSX]
Supplementary Table 6 GO analysis of upregulated pathways identified by snRNA-seq
analysis of the Li-deficient 3xTg hippocampus.
[EXCEL .XLSX]
Supplementary Table 7 Proteomic analysis of the Li-deficient 3xTg hippocampus.
[EXCEL .XLSX]
Supplementary Table 8 Overlap between snRNA-seq DEGs and AD DEGs.
[EXCEL .XLSX]
Supplementary Table 9 Purified microglia RNA-seq: alignment and mapping statistics.
[EXCEL .XLSX]
Supplementary Table 10 DEG list and GO analysis of Li-deficient 3xTg microglia.
[EXCEL .XLSX]
Supplementary Table 11 DEG list and GO analysis of Li-deficient wild-type microglia.
[EXCEL .XLSX]
Supplementary Table 12 DEGs shared by Li-deficient 3xTg and wild-type microglia and GO analysis.
[EXCEL .XLSX]
Supplementary Table 13 Binding characteristics of LiO and LiC to Aβ42 oligomers and fibrils.
[EXCEL .XLSX]
Supplementary Table 14 RNA-seq of LiO-treated 3xTg hippocampus: alignment and mapping statistics.
[EXCEL .XLSX]
Supplementary Table 15 RNA-seq of LiO-treated 3xTg hippocampus: DEGs and GO analysis.
[EXCEL .XLSX]
Supplementary Table 16 Antibodies and reagents.
[EXCEL .XLSX]
Supplementary Data Source Data file for Supplementary Figs. 2 and 5–11.
[EXCEL .XLSX]
Peer Review File Lithium Deficiency and the Onset of Alzheimer’s Disease
[PDF]
Supplementary Figures This file contains Supplementary Figs. 1–11. [PDF]
Peer Review File Lithium Deficiency and the Onset of Alzheimer’s Disease
[PDF]
Extended data figures and tablesExtended Data Fig. 1 Analysis of brain and serum lithium levels.
[.HTML]
Extended Data Fig. 2 Lithium deficiency does not impair exploratory
behavior or motor function in mice.
[.HTML]
Extended Data Fig. 3 Li deficiency results in loss of dendritic spines,
axons and oligodendrocytes.
[.HTML]
Extended Data Fig. 4 Lithium deficiency and Wnt/β-catenin
[.HTML]
Extended Data Fig. 5 Regulation of GSK3β and β-catenin
by endogenous lithium.
[.HTML]
Extended Data Fig. 6 Inhibition of GSK3β rescues Li deficiency.
[.HTML]
Extended Data Fig. 7 Lithium orotate exhibits reduced conductivity
and Aβ binding compared to lithium carbonate.
[.HTML]
Extended Data Fig. 8 Suppression of AD patholog by lithium orotate.
[.HTML]
Extended Data Fig. 9 Lithium orotate suppresses GSK3β activity.
[.HTML]
Extended Data Fig. 10 Lithium orotate restores spatial memory in
J20 mice with advanced amyloid pathology.
[.HTML]
Extended Data Fig. 11 Lithium orotate prevents age-related neuroinflammation.
[.HTML]
Extended Data Fig. 12 Lithium orotate promotes microglial clearance of Aβ.
[.HTML]
Extended Data Fig. 13 Lithium and cognitive resilience during aging.
[.HTML]Acknowledgements
We thank A. Johnston, A. Stebbins and J. Seravalli for assistance with ICP–MS; M. Chen for assistance with mass spectrometry; S. Valentin and A. Thompson for assistance with tissue processing; G. Klein for assistance with sample selection; and S. Nadarajan for technical assistance. This work was supported by NIH grants RO1AG046174 and RO1AG069042 to B.A.Y.; K01AG051791 and DP2AG072437 to E.A.L.; P30AG10161, P30AG72975, R01AG15819, R01AG17917, U01AG46152 and U01AG61356 to D.A.B.; and grants from the Ludwig Family Foundation, the Glenn Foundation for Medical Research and the Aging Mind Foundation to B.A.Y.
Contributions
L.A. performed metallomic analysis of human and mouse brain by ICP–MS, with assistance from M.L., analysed human and mouse brain by quantitative immunohistochemistry, did cell-culture studies with BV2 cells and carried out mouse behavioural studies with assistance from M.L., S.E.H., E.K.L., P.R., M.Y. and S.S.H.
Z.K.N. isolated and characterized primary microglia, did RNA sequencing and analysis of cytokine production and Aβ clearance, and did Li-Aβ equilibrium binding assays.
C.Q. prepared single-nucleus libraries and did RNA sequencing.
J.C., Z.K.N., C.Q., D.M.D., L.A. and B.A.Y. did computational and statistical analyses, with input from E.A.L.
D.A.B. provided human brain samples and phenotypic data from the ROSMAP study.
L.A. and B.A.Y. wrote the manuscript with input from all authors.
B.A.Y. supervised all aspects of the work.
Competing interests
The authors declare no competing interests.
Peer review information
Nature thanks Ashley Bush, Junmin Peng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
References:
James, B. D. & Bennett, D. A. Causes and patterns of dementia: an update in the era of redefining Alzheimer’s disease. Annu. Rev. Public Health 40, 65–84 (2019).
Sperling, R., Mormino, E. & Johnson, K. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron 84, 608–622 (2014).
De Strooper, B. & Karran, E. The cellular phase of Alzheimer’s disease. Cell 164, 603–615 (2016).
Murdock, M. H. & Tsai, L.-H. Insights into Alzheimer’s disease from single-cell genomic approaches. Nat. Neurosci. 26, 181–195 (2023).
Mathys, H. et al. Single-cell multiregion dissection of Alzheimer’s disease. Nature https://doi.org/10.1038/s41586-024-07606-7 (2024).
Migliore, L. & Coppedč, F. Gene–environment interactions in Alzheimer disease: the emerging role of epigenetics. Nat. Rev. Neurol. 18, 643–660 (2022).
Killin, L. O. J., Starr, J. M., Shiue, I. J. & Russ, T. C. Environmental risk factors for dementia: a systematic review. BMC Geriatr. 16, 175 (2016).
Kawahara, M., Kato-Negishi, M. & Tanaka, K. Cross talk between neurometals and amyloidogenic proteins at the synapse and the pathogenesis of neurodegenerative diseases. Metallomics 9, 619–633 (2017).
Huat, T. J. et al. Metal toxicity links to Alzheimer’s disease and neuroinflammation. J. Mol. Biol. 431, 1843–1868 (2019).
Bakulski, K. M. et al. Heavy metals exposure and Alzheimer’s disease and related dementias. J. Alzheimers Dis. 76, 1215–1242 (2020).
Bush, A. I. et al. Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science 265, 1464–1467 (1994).
James, S. A. et al. Elevated labile Cu is associated with oxidative pathology in Alzheimer disease. Free Radic. Biol. Med. 52, 298–302 (2012).
Mucke, L. et al. High-level neuronal expression of Aβ1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 (2000).
Oddo, S. et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39, 409–421 (2003).
Gazestani, V. et al. Early Alzheimer’s disease pathology in human cortex involves transient cell states. Cell 186, 4438–4453 (2023).
Herron, S., Delpech, J.-C., Madore, C. & Ikezu, T. Using mechanical homogenization to isolate microglia from mouse brain tissue to preserve transcriptomic integrity. STAR Protoc. 3, 101670 (2022).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51, 414–430 (2019).
Folke, J., Pakkenberg, B. & Brudek, T. Impaired Wnt signaling in the prefrontal cortex of Alzheimer’s disease. Mol. Neurobiol. 56, 873–891 (2019).
Lauretti, E., Dincer, O. & Praticň, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell. Res. 1867, 118664 (2020).
Leroy, K. et al. The active form of glycogen synthase kinase-3β is associated with granulovacuolar degeneration in neurons in Alzheimer’s disease. Acta Neuropathol. 103, 91–99 (2002).
Leroy, K., Yilmaz, Z. & Brion, J.-P. Increased level of active GSK-3β in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 33, 43–55 (2007).
Guilliot, S., Gauthier, S., Touchon, J. & Soto, M. E. Lithium, a treatment option for Alzheimer’s disease? A review of existing evidence and discussion on future perspectives. J. Alzheimers Dis. 96, 473–482 (2023).
Yu, L. et al. Cortical proteins associated with cognitive resilience in community-dwelling older persons. JAMA Psychiatry 77, 1172–1180 (2020).
Ramos-Miguel, A. et al. Presynaptic proteins complexin-I and complexin-II differentially influence cognitive function in early and late stages of Alzheimer’s disease. Acta Neuropathol. 133, 395–407 (2017).
Graham, S. F. et al. Quantitative measurement of [Na+] and [K+] in postmortem human brain tissue indicates disturbances in subjects with Alzheimer’s disease and dementia with Lewy bodies. J. Alzheimers Dis. 44, 851–857 (2015).
Religa, D. et al. Elevated cortical zinc in Alzheimer disease. Neurology 67, 69–75 (2006).
Kessing, L. V. et al. Association of lithium in drinking water with the incidence of dementia. JAMA Psychiatry 74, 1005–1010 (2017).
Heneka, M. T. et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405 (2015).
Romero-Molina, C., Garretti, F., Andrews, S. J., Marcora, E. & Goate, A. M. Microglial efferocytosis: diving into the Alzheimer’s disease gene pool. Neuron 110, 3513–3533 (2022).
Klein, P. S. & Melton, D. A. A molecular mechanism for the effect of lithium on development. Proc. Natl Acad. Sci. USA 93, 8455–8459 (1996).
Sofola, O. et al. Inhibition of GSK-3 ameliorates Aβ pathology in an adult-onset Drosophila model of Alzheimer’s disease. PLoS Genet. 6, e1001087 (2010).
Aghaizu, N. D., Jin, H. & Whiting, P. J. Dysregulated Wnt signalling in the Alzheimer’s brain. Brain Sci. 10, 902 (2020).
Liu, C.-C. et al. Deficiency in LRP6-mediated Wnt signaling contributes to synaptic abnormalities and amyloid pathology in Alzheimer’s disease. Neuron 84, 63–77 (2014).
Martin, M., Rehani, K., Jope, R. S. & Michalek, S. M. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 6, 777–784 (2005).
Azim, K. & Butt, A. M. GSK3β negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia 59, 540–553 (2011).
Nunes, M. A. et al. Chronic microdose lithium treatment prevented memory loss and neurohistopathological changes in a transgenic mouse model of Alzheimer’s disease. PLoS ONE 10, e0142267 (2015).
Zhao, L. et al. Beneficial synergistic effects of microdose lithium with pyrroloquinoline quinone in an Alzheimer’s disease mouse model. Neurobiol Aging 35, 2736–2745 (2014).
Trujillo-Estrada, L. et al. In vivo modification of Abeta plaque toxicity as a novel neuroprotective lithium-mediated therapy for Alzheimer’s disease pathology. Acta Neuropathol. Commun. 1, 73 (2013).
Zhang, X. et al. Long-term treatment with lithium alleviates memory deficits and reduces amyloid-β production in an aged Alzheimer’s disease transgenic mouse model. J. Alzheimers Dis. 24, 739–749 (2011).
Fiorentini, A., Rosi, M. C., Grossi, C., Luccarini, I. & Casamenti, F. Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS ONE 5, e14382 (2010).
Phiel, C. J., Wilson, C. A., Lee, V. M.-Y. & Klein, P. S. GSK-3? regulates production of Alzheimer’s disease amyloid-β peptides. Nature 423, 435–439 (2003).
Su, Y. et al. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-β precursor protein processing. Biochemistry 43, 6899–6908 (2004).
Rockenstein, E. et al. Neuroprotective effects of regulators of the glycogen synthase kinase-3β signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J. Neurosci. 27, 1981–1991 (2007).
Wilson, E. N. et al. NP03, a microdose lithium formulation, blunts early amyloid post-plaque neuropathology in McGill-R-Thy1-APP Alzheimer-like transgenic rats. J. Alzheimers Dis. 73, 723–739 (2020).
Sofola-Adesakin, O. et al. Lithium suppresses Aβ pathology by inhibiting translation in an adult Drosophila model of Alzheimer’s disease. Front. Aging Neurosci. 6, 190 (2014).
Leroy, K. et al. Lithium treatment arrests the development of neurofibrillary tangles in mutant tau transgenic mice with advanced neurofibrillary pathology. J. Alzheimers Dis. 19, 705–719 (2010).
Caccamo, A., Oddo, S., Tran, L. X. & LaFerla, F. M. Lithium reduces tau phosphorylation but not Aβ or working memory deficits in a transgenic model with both plaques and tangles. Am. J. Pathol. 170, 1669–1675 (2007).
Nakashima, H. et al. Chronic lithium treatment decreases tau lesions by promoting ubiquitination in a mouse model of tauopathies. Acta Neuropathol. 110, 547–556 (2005).
Noble, W. et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl Acad. Sci. USA 102, 6990–6995 (2005).
Hampel, H. et al. Lithium trial in Alzheimer’s disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J. Clin. Psychiatry 70, 922–931 (2009).
Macdonald, A. et al. A feasibility and tolerability study of lithium in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 23, 704–711 (2008).
Forlenza, O. V. et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br. J. Psychiatry 198, 351–356 (2011).
Nunes, M. A., Viel, T. A. & Buck, H. S. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr. Alzheimer Res. 10, 104–107 (2013).
Forlenza, O. V., Radanovic, M., Talib, L. L. & Gattaz, W. F. Clinical and biological effects of long-term lithium treatment in older adults with amnestic mild cognitive impairment: randomised clinical trial. Br. J. Psychiatry 215, 668–674 (2019).
Damiano, R. F. et al. Revisiting global cognitive and functional state 13 years after a clinical trial of lithium for mild cognitive impairment. Braz. J .Psychiatry 45, 46–49 (2023).
Kakhki, S. & Ahmadi-Soleimani, S. M. Experimental data on lithium salts: From neuroprotection to multi-organ complications. Life Sci. 306, 120811 (2022).
Haney, M. S. et al. APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia. Nature 628, 154–161 (2024).
Bennett, D. A. et al. Religious Orders Study and Rush Memory and Aging Project. J. Alzheimers Dis. 64, S161–S189 (2018).
Bennett, D. A. et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 27, 169–176 (2006).
Bennett, D. A. et al. Natural history of mild cognitive impairment in older persons. Neurology 59, 198–205 (2002).
Ihara, Y., Abraham, C. & Selkoe, D. J. Antibodies to paired helical filaments in Alzheimer’s disease do not recognize normal brain proteins. Nature 304, 727–730 (1983).
Selkoe, D. J., Abraham, C. R., Podlisny, M. B. & Duffy, L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer’s disease. J. Neurochem. 46, 1820–1834 (1986).
Xu, J. et al. Evidence for widespread, severe brain copper deficiency in Alzheimer’s dementia. Metallomics 9, 1106–1119 (2017).
Ramos, P. et al. Alkali metals levels in the human brain tissue: anatomical region differences and age-related changes. J. Trace Elem. Med. Biol. 38, 174–182 (2016).
Squitti, R. et al. Copper imbalance in Alzheimer’s disease: meta-analysis of serum, plasma, and brain specimens, and replication study evaluating ATP7B gene variants. Biomolecules 11, 960 (2021).
Hutchinson, R. W. et al. Imaging and spatial distribution of β-amyloid peptide and metal ions in Alzheimer’s plaques by laser ablation-inductively coupled plasma-mass spectrometry. Anal. Biochem. 346, 225–233 (2005).
Teich, A. F., Patel, M. & Arancio, O. A reliable way to detect endogenous murine β-amyloid. PLoS ONE 8, e55647 (2013).
Griciuc, A. et al. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78, 631–643 (2013).
Kalish, B. T. et al. Single-cell transcriptomics of the developing lateral geniculate nucleus reveals insights into circuit assembly and refinement. Proc. Natl Acad. Sci. USA 115, E1051–E1060 (2018).
Qiu, C. et al. Cis P-tau underlies vascular contribution to cognitive impairment and dementia and can be effectively targeted by immunotherapy in mice. Sci. Transl. Med. 13, eaaz7615 (2021).
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Yang, A. C. et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 603, 885–892 (2022).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337 (2019).
Sun, H., Zhou, Y., Fei, L., Chen, H. & Guo, G. scMCA: a tool to define mouse cell types based on single-cell digital expression. Methods Mol. Biol. 1935, 91–96 (2019).
Pálovics, R. et al. Molecular hallmarks of heterochronic parabiosis at single-cell resolution. Nature 603, 309–314 (2022).
Finak, G. et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278 (2015).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Marsh, S. E. et al. Dissection of artifactual and confounding glial signatures by single-cell sequencing of mouse and human brain. Nat. Neurosci. 25, 306–316 (2022).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Krämer, A., Green, J., Pollard, J. Jr & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530 (2014).
Risso, D., Ngai, J., Speed, T. P. & Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 32, 896–902 (2014).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Baldarelli, R. M. et al. Mouse Genome Informatics: an integrated knowledgebase system for the laboratory mouse. Genetics 227, iyae031 (2024).
de Leeuw, C. A., Neale, B. M., Heskes, T. & Posthuma, D. The statistical properties of gene-set analysis. Nat. Rev. Genet. 17, 353–364 (2016).
Sollis, E. et al. The NHGRI-EBI GWAS Catalog: knowledgebase and deposition resource. Nucleic Acids Res. 51, D977–D985 (2023).
Elias, J. E. & Gygi, S. P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 (2007).
Chen, M. et al. Common proteomic profiles of induced pluripotent stem cell-derived three-dimensional neurons and brain tissue from Alzheimer patients. J. Proteomics 182, 21–33 (2018).
Lee, H.-K. et al. Three dimensional human neuro-spheroid model of Alzheimer’s disease based on differentiated induced pluripotent stem cells. PLoS ONE 11, e0163072 (2016).
Perez-Riverol, Y. et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 53, D543–D553 (2025).

Return to ALZHEIMER's
Since 8-28-2025


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |