

Effect of Chiropractic Intervention on Oculomotor and
Attentional Visual Outcomes in Young Adults With
Long-Term Mild Traumatic Brain Injury:
A Randomized Controlled TrialThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Manipulative Physiol Ther 2024 (Jan); 47 (1-4): 1–11 ~ FULL TEXT
OPEN ACCESS Alice E. Cade PhD • Philip R.K. Turnbull PhD
Department Optometry & Vision Science,
University of Auckland, Auckland, New Zealand;
Centre for Chiropractic Research,
New Zealand College of Chiropractic,
Auckland, New Zealand.
Objective: This study aimed to establish if chiropractic care can improve oculomotor and cognitive symptoms in individuals with persistent postconcussion syndrome (PPCS).
Methods: A single-blind, randomized controlled intervention study recorded baseline computerized eye-tracker assessment (CEA) outcomes in 40 young adults with PPCS following mild traumatic brain injury. Participants were randomly allocated to either a chiropractic or age-matched active control intervention, and the change in CEA outcomes following intervention was compared between the chiropractic and control groups. A battery of CEAs including egocentric localization, fixation stability, pursuit, saccades, Stroop, and the vestibulo-ocular reflex, were used to assess oculomotor function, visual attention/processing, and selective attention.
Results: Relative to the control group, participants receiving the chiropractic intervention scored better in the Stroop test (P < .001), had improved gaze stability during both vestibulo-ocular reflex (P < .001) and fixation stability (P = .009), and a lower vertical error in egocentric localization (P < .001). However, performance was poorer in pursuits, where they had an increased tracking error (P < .001).
Conclusion: Chiropractic care in participants with PPCS significantly improved static and dynamic gaze stability, and performance in the Stroop test, compared with a control intervention. These results suggest that chiropractic care can offer a novel avenue for alleviating certain visual and cognitive symptoms in patients with PPCS. It also adds to the growing evidence that suggests that some longstanding PPCS visual symptoms may have a spinal or proprioceptive basis.
Keywords: Brain Concussion; Chiropractic; Eye-Tracking Technology; Postconcussion Syndrome; Proprioception.
From the Full-Text Article:
Introduction
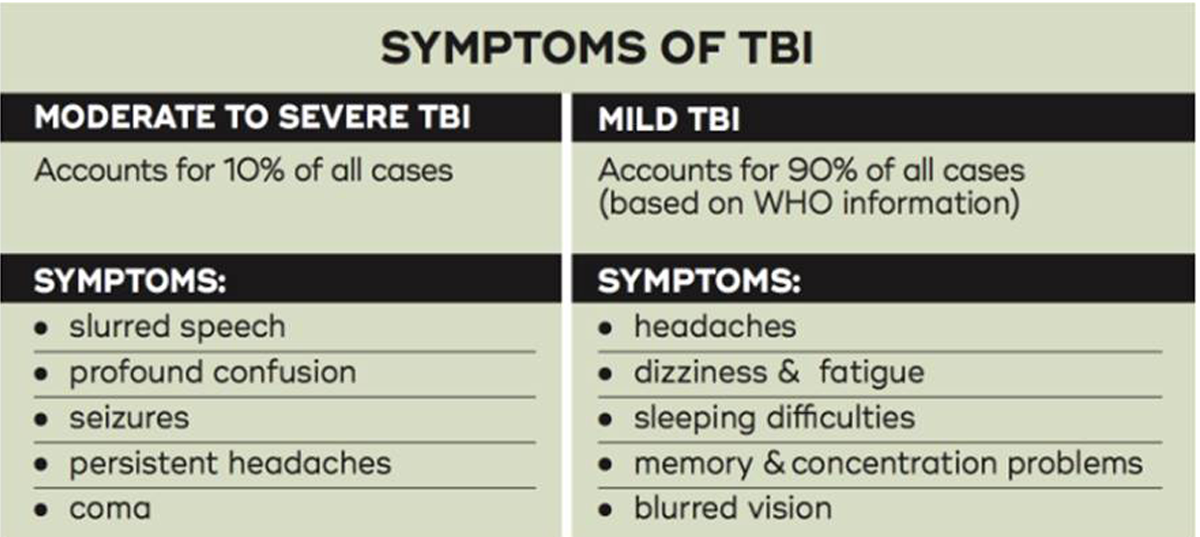
Traumatic brain injury (TBI) is a change in typical brain function that affects neurologic function after an external force to the head. [1, 2] Diagnosis and categorization of TBI severity is currently subjective, open to bias, and predicting an individual's outcome after injury is challenging. [3, 4] Although symptoms can vary depending on the neurologic area of injury, visual symptoms are common following even mild TBI (MTBI) owing to the many areas of the brain involved in processing vision [5] and controlling the eyes. Visual symptoms can include oculomotor dysfunction including disorders of convergence and accommodation, poorer fixation, slower or less accurate saccades, poorer pursuit movements, and modification of the vestibulo-ocular reflex (VOR). [6]
Other common symptoms are less specific, but impact tasks that tax attentional, inhibitive, or visuospatial processing. [7, 8] To gain accurate and objective measures of eye gaze behaviors, computerized eye-tracker assessments (CEAs) have become increasingly common. [6] Previous work using CEAs has suggested that changes in vision after mTBI may be, in addition to the primary neurologic insult, due to proprioceptive changes from cervical spine dysfunction or damage. [9, 10] This is supported by other research that shows altering proprioceptive drive with vibration changes CEA outcomes in those with mTBI. [11] Further augmenting this dual site of injury concept is those with mTBI have a higher incidence of neck pain [12, 13] and significantly worse objective measures of cervical spine function. [11]
Altering proprioceptive drive to the brain with either whole body or localized cervical spine vibration [11] has been shown to improve CEA performance in mTBI, and cognitive performance in a range of other conditions, including Alzheimer's disease, [14] Parkinson's disease, [15] and stroke, [16] and improves performance on the Stroop test in young adults. [17]
Although the exact process remains unclear, enhancing proprioceptive input to the brain is believed to aid in the integration of vestibular and sensorimotor functions, as well as improve cognitive performance. [14, 18] A drawback of vibration therapies is the transitory nature, so another proprioceptive based intervention—chiropractic care — was investigated as a potential pathway to manage visual deficits post mTBI. Chiropractic is a type of manual therapy whose aim is to manage spinal articular dysfunction and the altered neurologic component associated with it. [19]
Spinal joint dysfunction can result in altered afferent input to the central nervous system, which modifies the way it processes and integrates sensory and proprioceptive input. [20, 21] Once spinal dysfunction is corrected, the sensorimotor integration and cognitive function can improve. [22–24] Theoretically, if participants with mTBI have symptoms (as assessed by CEAs) that were caused or worsened by a related spinal injury, then managing the spinal dysfunction could, in turn, improve CEA outcomes and potentially their symptomology.
As an initial step in investigating this therapeutic intervention, this study aimed to investigate whether a chiropractic intervention intended at reducing spinal proprioceptive dysregulation can alter some of the commonly reported defects in eye-tracking function and spatial awareness that occur following mTBI.
Methods
Trial Design and Participants
This single-blinded, randomized controlled, single intervention study compared preintervention and postintervention measures from 6 CEAs between 2 age-matched groups with self-reported long-term mTBI symptoms for more than 3 months (persistent postconcussion symptoms, postconcussion syndrome [PPCS])—1 group receiving a chiropractic intervention and the other as a control group. Following measurements of baseline CEA outcomes, participants then completed the Rivermead Post-Concussion Symptoms Questionnaire (RPQ) to further assess and quantify the severity of their symptomatology. [25]
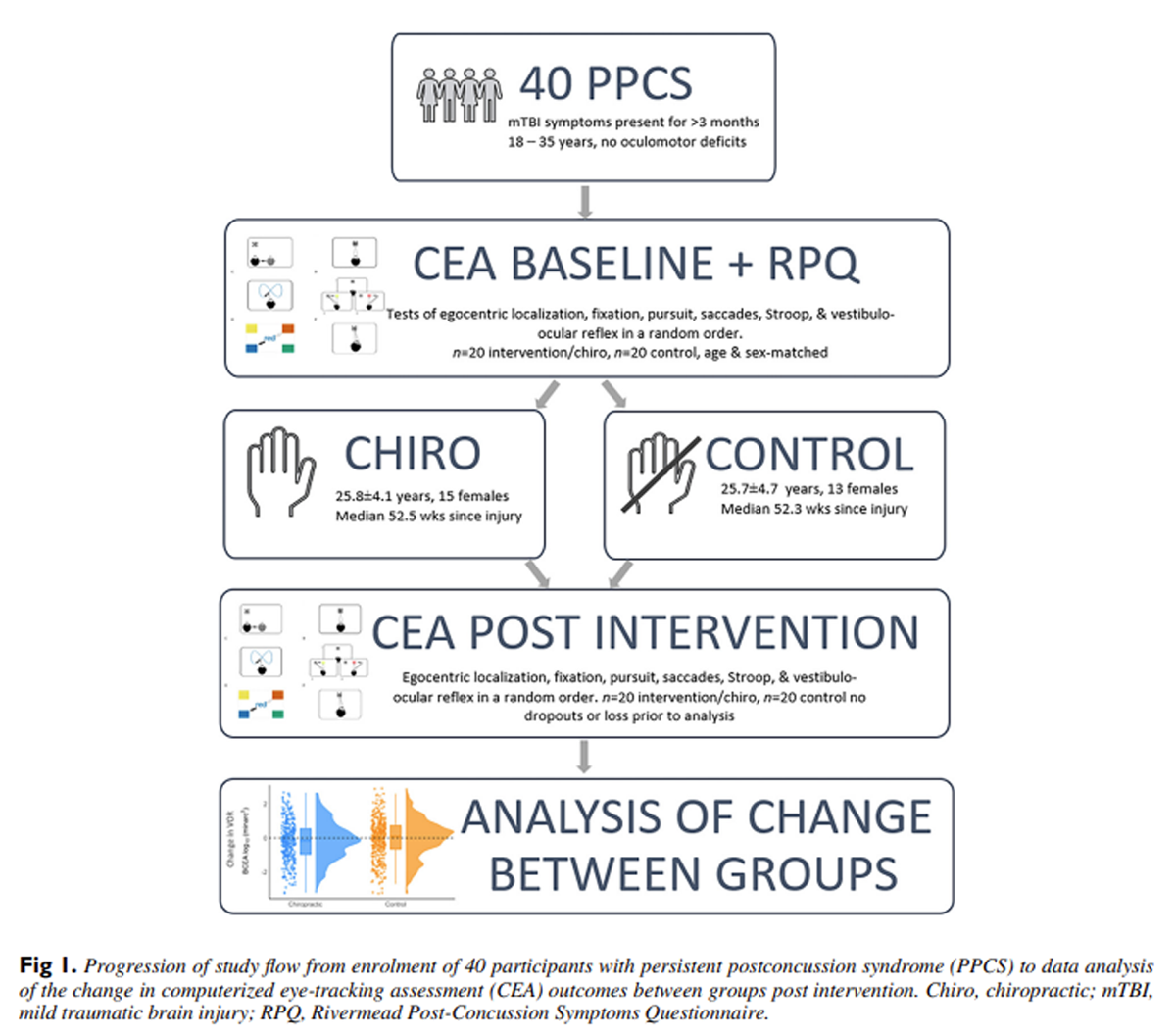
Figure 1 They then had either a chiropractic or active control intervention (detailed below), before repeating postintervention CEAs, Figure 1. Participants were randomized into an intervention group (chiropractic or control), balanced for age and gender, using a computer-generated block randomization sequence using a free, online program (QMinim [26]). The researcher that performed the randomization was the only person who knew which intervention participants were allocated to. Once randomization was completed the chiropractor was informed, outside the presence of the participant, which group the participant was assigned to, to maintain single blinding. Once allocated to either the chiropractic or control intervention participants underwent their appropriate intervention with the chiropractor.
To avoid the influence of presbyopia on the near computer task, all participants were between 18 and 35 years old, and had no known oculomotor deficits prior to their mTBI, and self-reported normal or corrected to normal vision. Persistent postconcussion symptoms were defined as symptoms of mTBI that persisted past the typical 3–month healing time. [27]
Participants were recruited by word of mouth from the New Zealand Chiropractic College using a snowball sampling method. [29] The study took place at the Centre for Chiropractic Research at the New Zealand Chiropractic College between July 2021 to April 2022 and was managed around COVID-19 related lockdowns in compliance with the government and university restrictions as required.
Sample size calculations were performed using the R software [30] package pwr. [31] Sample size calculations were based on a previous study assessing chiropractic intervention on eye tracking, where a mean improvement of 1.73 was found in the number of trials performed correctly. [32] To detect a true difference in the experimental and control means, it was calculated that 30 participants were needed to be able to reject the null hypothesis that the population means of the intervention and control groups were equal with probability (power) 0.8 and an alpha of 0.05. Bujang [33] recommends adding another 20% to 25% to participant numbers from power calculations to offset potential dropouts, population differences, or unforeseen circumstances (such as COVID-19 related challenges); therefore, a minimum of 40 participants was the recruitment aim.
Ethics
The experimental protocol and procedure were approved by the New Zealand Health and Disability Ethics Committee (HDEC 19/CEN/130, Australian New Zealand Clinical Trials Registry: ACTRN1262000407897) on August 21, 2019, and complied with the Declaration of Helsinki. [28]
Materials/Computer Setup
A laptop mounted eye tracker (Tobii 5, Tobii Group) was used to record binocular gaze (133 Hz) and head position data (33 Hz) during completion of the CEAs. Visual stimuli were presented on a laptop computer (Surface Book 2, Microsoft), and the eye tracker was calibrated using the Tobii calibration software for each participant. During testing, the participant sat approximately 70 cm from the screen, and the target stimuli consisted of a black cross-shaped target presented on a white background. [34]
Computerized Eye-Tracking Assessments
Six tests with previously established diagnostic value in differentiating mTBI, [6, 11] were used in this study.
Egocentric Localization
Participants were asked to move to align the center of their head with the target 10 times. The main outcomes were mean offset error and the mean trial completion time.
Fixation Stability
Participants maintained fixation on the target for 3 trials of 10s each. The main outcomes were the bivariate contour ellipse area (log10 minarc2), and mean gaze error.
Smooth Pursuit
Participants gaze followed a moving target as it traversed the screen in a Lissajous pattern for 4 trials of 30s. The outcomes measures were mean offset error, total gain, and the number of catch-up saccades.
Saccade Test
Saccade test was performed with 14 prosaccade and 14 antisaccade tasks interleaved and assessed simultaneously. Main outcomes were saccade latency and the number of correctly performed trials.
The Stroop Test
The Stroop test was performed following a protocol adapted from the Stroop Color-Word test. [35] Part 1 involved reading the word for 15 trials, and then part 2 required participants to match the font color for another 15 trials. Stroop outcomes included mean saccade decision-making latency, total trial time, and proportion of correct trials.
The VOR Test
Participants’ gaze was fixed on the target while they actively rotated their head left or right for 10 trials in each direction (20 trials in total). The main outcomes were fixation stability (bivariate contour ellipse area), head to eye velocity gain, and the number saccades in each trial. Eye gaze data was captured throughout the trials and analyzed after all participants had completed the study.
Intervention Procedures
Chiropractic intervention was provided by 4 different chiropractors who were asked to examine and treat each participant using best practice guidelines and the scope of chiropractic practice specified by the New Zealand Chiropractic Board. [36, 37] Each chiropractor saw between 1 and 14 participants each.
A patient history was taken from both the intervention and control groups to control for potential placebo effects from practitioner attention and time. [38, 39] Chiropractic was provided with the intention of correcting spinal dysfunction anywhere in the spine, also known as vertebral subluxations [23] using high velocity low amplitude (HVLA) adjustive thrusts to correct spinal dysfunction found. Clinical indicators were palpable restricted intersegmental range of motion, asymmetric intervertebral muscle tension, abnormal joint-play, and tenderness to palpation of the joint. [22, 40]
Control participants underwent a series of passive and active movements of the head, spine, and body. These movements were intended to act as a physiological control for possible afferent changes that may have occurred due to cutaneous, muscular, or vestibular from the passive and active movements used in preparing for spinal manipulation. [32, 41] This involved the participants being moved into spinal manipulation setup positions but without delivering actual chiropractic intervention or loading tension into any spinal joints. [42] No spinal manipulation was performed during any control session.
Statistical Analyses
Statistical analysis was performed using R software (version 4.0.3, https://www.r-project.org/) in RStudio (version 1.3.1093, Posit, PBC, https://posit.co/). A 1–way analysis of covariance to determine if there was a difference in variable change between groups (chiropractic or control) controlling for preintervention variable scores. [43] Count data used a binomial mixed effects regression model, [44] or a 0-inflated Poisson model if more than half the values were 0. [45] Statistical significance was defined as P < .05. Estimated marginal means of each group pre and post intervention were calculated using the emmeans package. [46] Pairwise differences with adjusted P values between pre and post for each group were also calculated the emmeans package.
Results
Twenty intervention (aged 25.8 ± 4.1 years; 15 females; median weeks since injury, 52.5) and 20 controls (aged 25.7 ± 4.7 years; 13 females; median weeks since injury, 52.3) with persistent postconcussion syndrome (PPCS) were recruited and completed the whole experiment, with no participants withdrawing from the trial, and no harms of any kind reported. The mean RPQ score for all participants was 30.2 ± 11.9. There were no significant differences between proportions for the 3 RPQ domains (somatic, 16.2 ± 6.6; emotional, 7.1 ± 4.4; and cognitive, 6.9 ± 2.7; χ2(2, N = 3) = 0.632; P = .729) for all participants, nor between the control or chiropractic groups (P > .999). The RPQ mean total score (30.2 ± 11.93) fell between previously recorded PPCS mean scores,47,48 revealing this study's participants reported themselves similarly affected, symptomatically, to the average person with PPCS from mTBI. For comparison, RPQ scores when administered to healthy people have a mean of ~3.5. [49]

Table 1 The type of chiropractic care was HVLA spinal adjustments; table assisted chiropractic adjustments, and instrument assisted adjustments. Table 1 summarizes the type of chiropractic care that was provided to participants and the vertebral segments adjusted. Instrument and HVLA adjustments were each provided to 5 participants, instrument only was used with 1 participant, and HVLA only approaches were used exclusively with 14 participants. There were 81 separate vertebral segments adjusted over all the participants with each participant having a mean of 4.1 ± 1.1 segments adjusted.
Most participants, 45%, sustained their mTBI from a sporting injury (χ2[5, N = 40] = 34.29; P < .001) and mechanism of injury (assault, domestic violence, falls, motor vehicle accidents, and other) were balanced between groups (P > .050).
CEA Measures

Table 2
page 6For ease of interpretation of the large number of CEA outcomes, a summary of significant differences between the chiropractic and control groups following intervention, alongside estimated marginals means, are provided in Table 2. For transparency, all CEA test (significant and nonsignificant) results are provided in a Supplementary Material table (Results for all variables).
The change in gaze stability was different between groups (F[1, 1] = 8.17; P = .005), improving after chiropractic intervention (pre, 3.26; post, 3.16 log10 minarc2) but worsening in the control group (pre, 2.94; post, 3.12 log10 minarc2). Horizontal gaze error showed a similar change (F[1, 1] = 9.35; P = .003), reducing after chiropractic (pre, 0.35; post, 0.21 degrees) but worsening after control intervention (pre, 0.20; post, 0.22 deg). There was no impact of chiropractic on the vertical gaze error (F[1, 1] = 0.19; P = .659). During the VOR test, gaze stability improved for the chiropractic group (pre, 4.00; post, 3.86 log10 minarc2; F[1, 1] = 41.41; P < .001) compared with the control group (pre, 3.94; post, 3.90 log10 minarc2). Vertical gaze error during VOR improved in both groups, but more so in the chiropractic group (pre, –0.46; post, –0.39 deg, F[1, 1] = 28.68; P < .001) compared with the control group (pre, –0.14; post, –0.34 deg). Similarly, the time taken to reach maximum gain was much poorer in the control (pre: 83.3, post: 116.6 ms) compared with the chiropractic group (pre, 83.4; post, 83.3 ms, F[1, 1] = 57.43; P < .001). There was no difference in the change in horizontal gaze error between groups during VOR (F[1, 1] = 2.00; P = .157).
For the Stroop test of selective attention, the chiropractic group showed significant improvements in the number of correct trials for part 1 (chiro pre, 14; IQR, 2; minimum, 6; post, 14; IQR, 2; minimum, 11; control pre, 15; IQR, 1; minimum, 11; post, 14; IQR, 2; minimum, 11; χ2[1, N = 40] =3508.64; P < .001), but not part 2 (χ2[1, N = 40] = 0.17; P = .678), whereas there was no difference in performance in the control group (part 1: χ2[1, N = 40] = 0.10; P = .758; part 2: χ2[1, N = 40] = 2.92; P = .087). Decision-making saccade latency for part 1 of the Stroop test increased in both groups, but slightly more in the control (pre, 733.5; post, 842.1 ms; F[1, 1] = 60.99; P < .001) than the chiropractic (pre, 716.9; post, 783.5 ms). This led to longer trial times following interventions in both groups, but more so in the control group (chiro pre, 900.0; post, 1000.0 ms; vs control pre, 917.0; post, 1067.0 ms; F[1, 1] = 68.00; P < .001). Part 2 showed no differences between groups for saccade latency (F[1, 1] = 2.48; P = .114) or total test time (F[1, 1] = 0.13; P = .721).
During saccade testing, the change in saccade latency was different between groups for both prosaccade (F[1, 1] = 14.94; P < .001) and antisaccade tests: F[1, 1] = 36.13; P < .001), improving for both groups, but more so for the chiropractic group (pro pre, 366.9; post, 317.0 ms; anti pre, 350.3; post, 317.0 ms) compared with the control group (pro pre, 366.9; post, 350.3 ms; anti pre, 367.0; post, 341.9 ms). There was no difference in the number of tests performed correctly for either group or type of saccade (P > .05). In pursuit testing, the total gaze error worsened after chiropractic intervention (F[1, 1] = 43.14; P < .001; pre, 0.20; post, 0.24 deg) compared with the slight improvement seen in the control group (pre, 0.11; post, 0.09 deg). Total gain worsened in the chiropractic group (pre, 1.02; post, 1.03; F[1, 1] = 54.97; P < .001) while staying the same for the control group (pre, 1.01; post, 1.01).
For egocentric localization, there was a difference in the vertical alignment error between groups (F[1, 1] = 52.30; P < .001), with a small improvement in the chiropractic group (pre, –37.69; post, –36.42 mm), but a much greater improvement for the control group (pre, 13.55; post, –0.37 mm). There were no changes to horizontal error after intervention (F[1, 1] = 0.72; P = .395).
Discussion
Key Findings
The results of this study show that for those with PPCS, chiropractic intervention improved gaze stability, both with a stable head (fixation) and during dynamic head movement (VOR). Chiropractic also reduced saccade latency, and improved performance in the Stroop test, but increased total gaze error during pursuits compared with the age-matched controls. These findings support the idea that vision-based mTBI symptoms involve some abnormal spinal or proprioceptive input, and that interventions aimed at reducing spinal dysfunction may potentially have some benefit in the management of mTBI.
Chiropractic Improved Gaze Stability
The chiropractic group showed improvement in gaze stability during both the fixation test and during VOR testing. Previous research has shown brain-injured people have poorer fixation than healthy controls, [50] suggesting that chronic injury may negatively affect gaze stability. Additional research also suggests that those with mTBI have concomitant neck dysfunction [13] and sensory abnormalities. [51, 52] The brain can down-weight abnormal cervical afferent information when it conflicts with vestibular and visual inputs, [53, 54] leading to less reliable input to gaze stability. Chiropractic intervention, by modifying the proprioceptive input from more functional spinal joints, could help restore this input to the brain's multisensory processing, leading to an improved internal representation of the body's spatial orientation, helping to improve fixation.
This improvement in fixation was also seen during the dynamic head movement as part of VOR testing, where additional cerebellar-vestibular pathways are involved. [55] Altered proprioception from the cervical spine, after chiropractic, may have bolstered brainstem activation or vestibulo-cerebellar components of the VOR pathway augmenting its function. [56–58] Chiropractic intervention may have also improved gaze stability via decreasing disordered proprioceptive drive from the neck and allowing the cervico-ocular reflex to function more effectively. The cervico-ocular reflex helps to steady gaze in the presence of neck movement and enhancing VOR function and further stabilizing gaze. [59]
Chiropractic Improved Attentional Reading Tasks
During the Stroop test, our PPCS participants had higher error rates [60, 61] and the time to complete a trial was much longer than that of previously found normative data. [60] This suggests that inhibitory control is affected in PPCS, perhaps more so in part 1 than part 2 of Stroop. [62] Part 1 of the Stroop test, where the required response is indicated by the color denoted by the word requires additional language processing across a wider range of neurologic areas, compared with part 2, which only requires identification of the word color before generating a response. [62]
Looking at the overall picture of Stroop results, it seems that although either intervention increased trial time or decision-making latency, only the intervention aimed at improving proprioceptive drive — chiropractic — resulted in fewer errors. There is sparse previous data for chiropractic in relation to the Stroop test, but research on a choice - reaction time test — somewhat analogous to the Stroop test — found whole body vibration used to stimulate proprioceptors reduced P300 brain wave latency. [63] The P300 wave latency is lengthened in brain injury, and a longer latency indicates slower cognition. [64] Early research supports the P300 wave having a somatosensory component but how, exactly, somatosensory stimulation affects the P300 is yet unknown. [65] Chiropractic intervention has been shown to improve proprioceptive drive, so it is possible that chiropractic care can shorten P300 wave latency by way of altering proprioceptive drive, [20, 21] and improve the ability to respond to a choice-reaction test such as the Stroop test.
Other Findings
The chiropractic intervention group showed impairments in several measures of gaze accuracy during pursuit testing. This may be because the chiropractic intervention was aimed at reducing proprioceptive, rather than vestibular dysfunction. Brainstem eye-head neurons, which help control and coordinate eye and head movements for gaze stability, are affected by vestibular inputs but not cervical proprioceptors. [66] Therefore, an intervention aimed at improving disordered proprioception, such as chiropractic, may not be expected to improve errors during pursuit. Lastly, our study suggests that chronic injury may result in an increased number of catch-up saccades during pursuits. [67]
Predictive gaze movements in pursuits stem from anticipating target movement and learning target patterns, which originate from the middle temporal area and medial superior temporal area and receive inputs from the frontal eye fields in the prefrontal cortex. [68] These are areas that can be hypo-perfused and exhibit reduced glucose metabolism after mTBI, [69] which could lead to difficulty integrating sensorimotor information. Disordered sensorimotor integration and dysfunctional cortical gaze movement areas may have led to less accurate or slower anticipatory eye movements, [70, 71] and more catch-up saccades.
Saccade latencies were both longer and showed minimal differences between prosaccade and antisaccade, compared with previous research. [72] This could be attributed to the interleaving of prosaccade and antisaccade trials, requiring participants to simultaneously consider the steps involved in both tasks. Additionally, imagining a prosaccade or antisaccade task activates the supplementary eye fields, which may inhibit frontal eye field drive. [73] Although both groups demonstrated a postintervention reduction in saccade latency, this was more pronounced in the chiropractic group. Previous research shows that chiropractic intervention can change prefrontal cortex [74] function, especially the N30 sensory evoked potential peak, which is implicated in sensorimotor processing and learning new motor skills. [75] Therefore, any improved proprioceptive drive post chiropractic intervention may have affected sensorimotor processing and assisted in learning how to perform the saccade test, resulting in a reduced decision-making latency.
Chiropractic and Its Potential Influence
How we view and interpret our visual environment is dependent on where our brains believe us to be in space. [76] The spine provides the largest amount of proprioceptive information to the brain, [77] so it follows that if the injury also causes spinal dysfunction, it could alter how we interpret visual information. [78] Chiropractic intervention is thought to activate musculature and spindles that surround spinal joints, firing 1A afferents to the brain, which are processed in the motor and prefrontal cortices. [19, 79] These inputs help to build the brain's internal map of where the joints and body is in space. [80]
Additionally, chiropractic changes motor evoked potentials, [79] and their generation time, [81] suggesting chiropractic adjustments not only affect sensory drive to the brain, but also motor outputs. [82] Although research into how chiropractic affects the brain is in its infancy, studies show that chiropractic adjustments can lead to changes in multimodal sensory integration involving visual and auditory inputs [21, 83] and motor and motor-learning outputs. [84–88] Our study results indicate that chiropractic can impact sensorimotor function and influence a range of CEA outcomes, so could be considered in those suffering from PPCS.
Strengths and Limitations
A strength of this study was the use of many different CEA-based outcome measures including attentional and inhibitive process testing, rather than focusing on just 1 aspect, such as oculomotor function, or even a single eye tracking–based test. [89] A factor which was both a strength and limitation was allowing chiropractors flexibility to make their own clinical decisions when deciding on the intervention for participants. Although the lack of intervention standardization—chiropractors were allowed to adjust where they saw fit for each participant — may have reduced internal validity, it also increased its external validity with results more likely to be consistent with what could be expected in clinical practice.
The heterogeneity of the study population, and lack of specific inclusion criteria (such as location or cause of mTBI) was also a limitation of this study. Participants varied widely in their mechanism of injury, time since injury, symptomatology, and spinal findings. These observations highlight a known issue in mTBI research — population heterogeneity and the difficulties surrounding mTBI identification, diagnosis, treatment, and chronification of the disorder. [2] The wide range of causative injuries, differing previous treatment, time since injury, and comorbid issues could have confounded or influenced the results of this study, although the randomization of groups and use of age-matched controls would have helped mitigate these factors.
Conclusion
This study found that chiropractic care can improve some aspects of visual function, particularly gaze stability, when compared with an age-matched control group. This reinforces the idea that some of the ongoing visual symptoms in persistent postconcussion syndrome (PPCS) may be due to abnormalities in the cervical spine. The study also demonstrated that a simple CEA battery can be successfully used in a clinical interventional trial in a diverse PPCS population to help provide objective markers for diagnosis and tracking the effectiveness of interventions over time.
Practical Applications
This single-blinded randomized controlled study used a chiropractic intervention to assess the difference in eye-tracking outcomes for an intervention (chiropractic) and control group.
Chiropractic care resulted in significant differences between the chiropractic and control groups for many eye-tracking outcomes, indicating that improving spinal dysfunction aids in recovery — from visual symptoms — after mTBI.
Appendix. Supplementary materials
Results for all variables (23KB docx)
Funding Sources and Conflicts of Interest
A.E.C. received a Senior Health Researcher Scholarship from the University of Auckland while completing their PhD, to which this article contributed to. No conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): A.E.C., P.K.R.T.
Design (planned the methods to generate the results): A.E.C., P.K.R.T.
Supervision (oversight, organization and implementation): P.K.R.T.
Data collection/processing (experiments, organization, or reporting data): A.E.C.
Analysis/interpretation (analysis, evaluation, presentation of results): A.E.C., P.K.R.T.
Literature search (performed the literature search): A.E.C.
Writing (responsible for writing a substantive part of the manuscript): A.E.C.
Critical review (revised manuscript for intellectual content): A.E.C., P.K.R.T.
References:
V. Feng
Mild Traumatic Brain Injury Burden in New Zealand:
A Population-Based Incidence and Short Term Outcomes Study
University of Auckland (2014)JD Cassidy, LJ Carroll, PM Peloso, et al.
Incidence, risk factors and prevention of mild traumatic brain injury:
results of the WHO Collaborating Centre Task Force on
Mild Traumatic Brain Injury
J Rehabil Med (43 suppl) (2004), pp. 28-60,
10.1080/16501960410023732BM Asken, ST DeKosky, JR Clugston, MS Jaffee, RM. Bauer
Diffusion tensor imaging (DTI) findings in adult civilian, military,
and sport-related mild traumatic brain injury (mTBI):
a systematic critical review
Brain Imaging Behav, 12 (2) (2018), pp. 585-612,
10.1007/s11682-017-9708-9A Theadom, V Parag, T Dowell, et al.
Persistent problems 1 year after mild traumatic brain injury:
a longitudinal population study in New Zealand
Br J Gen Pract, 66 (642) (2016), pp. e16-e23,
10.3399/bjgp16X683161N Merezhinskaya, RK Mallia, D Park, DW Bryden, K Mathur, FM. Barker
Visual deficits and dysfunctions associated with traumatic
brain injury: a systematic review and meta-analysis
Optom Vis Sci, 96 (8) (2019), pp. 542-555,
10.1097/OPX.0000000000001407A Cade, PR. Turnbull
Clinical testing of mild traumatic brain injury
using computerised eye-tracking tests
Clin Exp Optom, 105 (7) (2022), pp. 680-686,
10.1080/0816 4622.2021.2018915L Heinmiller, KB. Gunton
A review of the current practice in diagnosis and management of visual
complaints associated with concussion and postconcussion syndrome
Curr Opin Ophthalmol, 27 (5) (2016), pp. 407-412,
10.1097/ICU.0000000000000296MH Heitger, RD Jones, AD Macleod, DL Snell, CM Frampton, TJ. Anderson
Impaired eye movements in post-concussion syndrome indicate
suboptimal brain function beyond the influence of
depression, malingering or intellectual ability
Brain, 132 (10) (2009), pp. 2850-2870,
10.1093/brain/awp181B Willer, JJ. Leddy
Management of concussion and post-concussion syndrome
Curr Treat Options Neurol, 8 (5) (2006), pp. 415-426,
10.1007/s11940-006-0031-9O Leslie, N. Craton
Concussion: purely a brain injury?
Clin J Sport Med, 23 (5) (2013), pp. 331-332,
10.1097/JSM.0b013e318295bbb1AE. Cade
Computerised Eye Tracker Assessment in Young Adults
with Mild Traumatic Brain Injury
University of Auckland (2023)JA King, B Rodriquez, I Kim, et al.
Incidence of neck pain in patients with concussion
in a pediatric emergency department
Pediatr Emerg Care, 38 (4) (2022), pp. e1185-e1191,
10.1097/PEC.0000000000002544JA King, MA McCrea, LD. Nelson
Frequency of primary neck pain in mild
traumatic brain injury/concussion patients
Arch Phys Med Rehabil, 101 (1) (2020), pp. 89-94,
10.1016/j.apmr.2019.08.471DC Sá-Caputo, PR Da Costa, R Pacheco-Lima, et al.
Is whole body vibration exercise a viable option for
individuals with Alzheimer's disease?
Public Health Res, 4 (4) (2014), pp. 136-143,
10.5923/j.phr.20140404.05NS Pinto, MB Monteiro, P Froes Meyer, et al.
The effects of whole-body-vibration exercises in
Parkinson's disease: a short review
J Med Med Sci, 2 (1) (2010), pp. 594-600SD Santos-Filho, MOB Monteiro, DN Paiva, et al.
Possible benefits of the whole body vibration in the
treatment of complications in stroke patients
J Adv Med Med Res, 4 (7) (2014), pp. 1539-1551,
10.9734/bjmmr/2014/5562GR Regterschot, MJ Van Heuvelen, EB Zeinstra, et al.
Whole body vibration improves cognition in healthy young adults
PLoS One, 9 (6) (2014), Article e100506,
10.1371/journal.pone.0100506R Panichi, FM Botti, A Ferraresi, et al.
Self-motion perception and vestibulo-ocular reflex during whole body
yaw rotation in standing subjects: the role of head position
and neck proprioception
Hum Mov Sci, 30 (2) (2011), pp. 314-332,
10.1016/j.humov.2010.10.005H Haavik, N Kumari, K Holt, et al.
The Contemporary Model of Vertebral Column Joint Dysfunction
and Impact of High-velocity, Low-amplitude Controlled
Vertebral Thrusts on Neuromuscular Function
European J Applied Physiology 2021 (Oct); 121 (10): 2675–2720H Haavik, B. Murphy
Subclinical Neck Pain and the Effects of Cervical
Manipulation on Elbow Joint Position Sense
J Manipulative Physiol Ther. 2011 (Feb); 34 (2): 88–97KR Holt, H Haavik, AC Lee, B Murphy, CR. Elley
Effectiveness of Chiropractic Care to Improve
Sensorimotor Function Associated With Falls Risk
in Older People: A Randomized Controlled Trial
J Manipulative Physiol Ther. 2016 (May); (39) 4: 267–278CN. Henderson
The Basis for Spinal Manipulation:
Chiropractic Perspective of Indications and Theory
J Electromyography and Kinesiology 2012 (Oct); 22 (5): 632–642H Haavik Taylor, K Holt, B Murphy
Exploring the Neuromodulatory Effects
of the Vertebral Subluxation and Chiropractic Care
Chiropractic J Australia 2010 (Mar); 40 (1): 37–44KR. Holt
Effectiveness of Chiropractic Care in Improving Sensorimotor
Function Associated with Falls Risk in Older People
University of Auckland (2014)S Balalla, C Krägeloh, O Medvedev, R Siegert
Is the Rivermead Post-Concussion Symptoms Questionnaire a reliable
and valid measure to assess long-term symptoms in traumatic
brain injury and orthopedic injury patients?
A novel investigation using Rasch analysis
Neurotrauma Rep, 1 (1) (2020), pp. 63-72,
10.1089/neur.2020.0017M Saghaei, S. Saghaei
Implementation of an open-source customizable minimization program
for allocation of patients to parallel groups in clinical trials
J Biomed Sci Eng, 4 (11) (2011), pp. 734-739,
10.4236/jbise.2011. 411090Management of Concussion/mTBI Working Group
VA/DoD clinical practice guideline for management of
concussion/mild traumatic brain injury
J Rehabil Res Dev, 46 (6) (2009), pp. CP1-CP68,
10.1682/JRRD.2009.06.0076U Schmidt, A Frewer, D. Sprumont
Ethical Research : The Declaration of Helsinki, and the Past,
Present, and Future of Human Experimentation
(1st ed.), Oxford University Press (2020)AS Acharya, A Prakash, P Saxena, A. Nigam
Sampling: why and how of it?
Indian J Med Spec, 4 (2) (2013), pp. 330-333,
10.7713/ijms.2013.0032R Core Team
A language and environment for statistical computing. 2021
https://www.r-project.org/
Accessed December 10, 2017S Champely, C Ekstrom, P Dalgaard, et al.
pwr: basic functions for power analysis
https://cran.r-project.org/web/packages/pwr/index.html
Accessed March 3, 2021A Cade, K Jones, K Holt, AM Penkar, H. Haavik
The Effects of Spinal Manipulation on Oculomotor Control in Children
with Attention Deficit Hyperactivity Disorder:
A Pilot and Feasibility Study
Brain Sciences 2021 (Aug 6); 11 (8): 1047MA. Bujang
A step-by-step process on sample size determination for medical research
Malays J Med Sci, 28 (2) (2021), pp. 15-27,
10.21315/mjms2021.28.2.2L Thaler, AC Schütz, MA Goodale, KR. Gegenfurtner
What is the best fixation target? The effect of target shape
on stability of fixational eye movements
Vision Res, 76 (2013), pp. 31-42,
10.1016/j.visres.2012.10.012E Vakil, H Weisz, L Jedwab, Z Groswasser, S. Aberbuch
Stroop color-word task as a measure of selective attention:
efficiency in closed-head-injured patients
J Clin Exp Neuropsychol, 17 (3) (1995), pp. 335-342,
10.1080/01688639508405127JJ Triano, B Budgell, A Bagnulo, et al.
Review Of Methods Used By Chiropractors To Determine
The Site For Applying Manipulation
Chiropractic & Manual Therapies 2013 (Oct 21); 21 (1): 36Scope of Practice – Chiropractor
New Zealand Chiropractic Board. Accessed May 9, 2018.E Ernst, E Harkness
Spinal manipulation: a systematic review of sham-controlled,
double-blind, randomized clinical trials
J Pain Symptom Manage, 22 (4) (2001), pp. 879-889,
10.1016/s0885-3924(01)00337-2HT Vernon, JJ Triano, JK Ross, SK Tran, DM Soave, MD. Dinulos
Validation of a Novel Sham Cervical Manipulation Procedure
Spine J. 2012 (Nov); 12 (11): 1021–1028R Cooperstein, M Haneline, M. Young
Interexaminer Reliability of Thoracic Motion Palpation
Using Confidence Ratings and Continuous Analysis
J Chiropractic Medicine 2010 (Sep); 9 (3): 99–106H Haavik-Taylor, B. Murphy
Cervical Spine Manipulation Alters Sensorimotor Integration:
A Somatosensory Evoked Potential Study
Clin Neurophysiol. 2007 (Feb); 118 (2): 391–402JG Pickar, JD. Wheeler
Response of Muscle Proprioceptors to Spinal
Manipulative-like Loads in the Anesthetized Cat
J Manipulative Physiol Ther. 2001 (Jan); 24 (1): 2–11AJ Vickers, DG. Altman
Statistics notes: analysing controlled trials with
baseline and follow up measurements
BMJ, 323 (7321) (2001), pp. 1123-1124,
10.1136/bmj.323.7321.1123Bates D, Maechler M, Bolker B, Walker S.
lme4: linear mixed-effects models using “Eigen” and S4.
Accessed June 23, 2022.
https://cran.r-project.org/package=lme4A Zeileis, C Kleiber, S. Jackman
Regression models for count data in R
J Stat Softw, 27 (8) (2008), pp. 1-25,
10.18637/jss.v027.i08SR Searle, FM Speed, GA Milliken
Population marginal means in the linear model:
an alternative to least squares means
Am Stat, 34 (4) (1980), pp. 216-221,
10.1080/00031305.1980.10483031T Ingebrigtsen, K Waterloo, S Marup-Jensen, E Attner, B. Romner
Quantification of post-concussion symptoms 3 months after
minor head injury in 100 consecutive patients
J Neurol, 245 (9) (1998), pp. 609-612,
10.1007/s004150050254L Smith-Seemiller, NR Fow, R Kant, MD. Franzen
Presence of post-concussion syndrome symptoms in patients
with chronic pain vs mild traumatic brain injury
Brain Inj, 17 (3) (2003), pp. 199-206,
10.1080/0269905021000030823K Sullivan, N. Garden
A comparison of the psychometric properties of 4 postconcussion
syndrome measures in a nonclinical sample
J Head Trauma Rehabil, 26 (2) (2011), pp. 170-176,
10.1097/HTR.0b013e3181e47f95M Hunfalvay, NP Murray, FR. Carrick
Fixation stability as a biomarker for differentiating mild traumatic
brain injury from age matched controls in pediatrics
Brain Inj, 35 (2) (2021), pp. 209-214,
10.1080/02699052.2020.1865566LF Lin, TH Liou, CJ Hu, et al.
Balance function and sensory integration after
mild traumatic brain injury
Brain Inj, 29 (1) (2015), pp. 41-46,
10.3109/02699052.2014.955881PC Fino, LE Dibble, EA Wilde, et al.
Sensory phenotypes for balance dysfunction after
mild traumatic brain injury
Neurology, 99 (5) (2022), pp. e521-e535,
10.1212/WNL.0000000000200602RJ. Peterka
Sensorimotor integration in human postural control
J Neurophysiol, 88 (3) (2002), pp. 1097-1118,
10.1152/jn.2002.88.3.1097P Abedi Khoozani, G Blohm
Neck muscle spindle noise biases reaches in
a multisensory integration task
J Neurophysiol, 120 (3) (2018), pp. 893-909,
10.1152/jn.00643. 2017K Fukushima, T Yamanobe, Y Shinmei, J Fukushima, S. Kurkin
Role of the frontal eye fields in smooth-gaze tracking
Prog Brain Res, 143 (2004), pp. 391-401,
10.1016/S0079-6123(03)43037-9K Fukushima, CR. Kaneko
Vestibular integrators in the oculomotor system
Neurosci Res, 22 (3) (1995), pp. 249-258,
10.1016/0168-0102(95)00904-8K Nakamagoe, Y Iwamoto, K. Yoshida
Evidence for brainstem structures participating in oculomotor integration
Science, 288 (5467) (2000), pp. 857-859,
10.1126/science.288. 5467.857K Fujiwara, K Kunita, N. Furune
Effect of vibration stimulation to neck extensor muscles
on reaction time in various saccadic eye movements
Int J Neurosci, 119 (10) (2009), pp. 1925-1940,
10.1080/00207450802333912BK Ischebeck, J de Vries, JN Van der Geest, et al.
Eye movements in patients with whiplash associated disorders:
a systematic review
BMC Musculoskelet Disord, 17 (1) (2016), p. 441,
10.1186/s12891-016-1284-4TL Strickland, LF D'Elia, R James, R Stein
Stroop color-word performance of African Americans
Clin Neuropsychol, 11 (1) (1997), pp. 87-90,
10.1080/13854049708407034W Van der Elst, MP Van Boxtel, GJ Van Breukelen, J. Jolles
The Stroop color-word test: influence of age, sex, and education;
and normative data for a large sample across the adult age range
Assessment, 13 (1) (2006), pp. 62-79,
10.1177/1073191105283427F Scarpina, S. Tagini
The Stroop color and word test
Front Psychol, 8 (2017), p. 557,
10.3389/fpsyg.2017.00557SV Müller, AJ von Schweder, B Frank, R Dengler, TF Münte, S. Johannes
The effects of proprioceptive stimulation on cognitive processes
in patients after traumatic brain injury
Arch Phys Med Rehabil, 83 (1) (2002), pp. 115-121,
10.1053/apmr.2002.27472M Rappaport, J Leonard, S Ruiz Portillo
Somatosensory evoked potential peak latencies and amplitudes in
contralateral and ipsilateral hemispheres in normal and
severely traumatized brain-injured subjects
Brain Inj, 7 (1) (1993), pp. 3-13,
10.3109/02699059309008152S Yamaguchi, RT. Knight
P300 generation by novel somatosensory stimuli
Electroencephalogr Clin Neurophysiol, 78 (1) (1991), pp. 50-55,
10.1016/0013-4694(91)90018-yJE Roy, KE. Cullen
Brain stem pursuit pathways: dissociating visual, vestibular, and
proprioceptive inputs during combined eye-head gaze tracking
J Neurophysiol, 90 (1) (2003), pp. 271-290,
10.1152/jn.01074.2002JA. Sharpe
Neurophysiology and neuroanatomy of smooth pursuit: lesion studies
Brain Cogn, 68 (3) (2008), pp. 241-254,
10.1016/j.bandc.2008.08.015E Kowler, JF Rubinstein, EM Santos, J. Wang
Predictive smooth pursuit eye movements
Annu Rev Vis Sci, 5 (2019), pp. 223-246,
10.1146/annurev-vision-091718-014901AP Lin, HJ Liao, SK Merugumala, SP Prabhu, WP Meehan, BD. Ross
Metabolic imaging of mild traumatic brain injury
Brain Imaging Behav, 6 (2) (2012), pp. 208-223,
10.1007/s11682-012-9181-4GU Lekwuwa, GR. Barnes
Cerebral control of eye movements. I. The relationship between
cerebral lesion sites and smooth pursuit deficits
Brain, 119 (2) (1996), pp. 473-490,
10.1093/brain/119.2.473GU Lekwuwa, GR. Barnes
Cerebral control of eye movements. II. Timing of anticipatory eye movements,
predictive pursuit and phase errors in focal cerebral lesions
Brain, 119 (2) (1996), pp. 491-505,
10.1093/brain/119.2.491CA DiCesare, AW Kiefer, P Nalepka, GD. Myer
Quantification and analysis of saccadic and smooth pursuit
eye movements and fixations to detect oculomotor deficits
Behav Res Methods, 49 (1) (2017), pp. 258-266,
10.3758/s13428-015-0693-xA Kristjánsson, Y Chen, K. Nakayama
Less attention is more in the preparation of antisaccades, but not prosaccades
Nat Neurosci, 4 (10) (2001), pp. 1037-1042,
10.1038/nn723D Lelic, IK Niazi, K Holt, et al.
Manipulation of Dysfunctional Spinal Joints Affects Sensorimotor
Integration in the Prefrontal Cortex:
A Brain Source Localization Study
Neural Plast. 2016 (Mar 7); 2016: 3704964MS Navid, IK Niazi, D Lelic, et al.
Investigating the effects of chiropractic spinal manipulation
on EEG in stroke patients
Brain Sci, 10 (5) (2020), p. 253,
10.3390/brainsci10050253WV Padula, JE Capo-Aponte, WV Padula, EL Singman, J. Jenness
The consequence of spatial visual processing dysfunction
caused by traumatic brain injury (TBI)
Brain Inj, 31 (5) (2017), pp. 589-600,
10.1080/02699 052.2017.1291991B Armstrong, P McNair, D. Taylor
Head and neck position sense
Sports Med, 38 (2) (2008), pp. 101-117,
10.2165/00007256-200838020-00002C Gil, P. Decq
How similar are whiplash and mild traumatic brain injury?
A systematic review
Neurochirurgie, 67 (3) (2021), pp. 238-243,
10.1016/j.neuchi.2021.01.016JD Rollnik, S Siggelkow, M Schubert, U Schneider, R. Dengler
Muscle vibration and prefrontal repetitive transcranial magnetic stimulation
Muscle Nerve, 24 (1) (2001), pp. 112-115,
10.1002/1097-4598(200101)24:1<112::AID-MUS15>3.0.CO;2-7ER Kandel, JH Schwartz, TM. Jessell
Principles of Neural Science
Vol 4. McGraw-Hill (2000)K Miyara, S Etoh, K Kawamura, et al.
Effects of lower limb segmental muscle vibration on primary motor
cortex short-latency intracortical inhibition and
spinal excitability in healthy humans
Exp Brain Res, 240 (1) (2022), pp. 311-320,
10.1007/s00221-021-06257-8H Haavik, IK Niazi, M Jochumsen, D Sherwin, S Flavel, KS. Türker
Impact of Spinal Manipulation on Cortical Drive
to Upper and Lower Limb Muscles
Brain Sci. 2017 (Jan); 7 (1): 2B Farid, P Yielder, M Holmes, H Haavik, BA. Murphy
Association of Subclinical Neck Pain With Altered Multisensory
Integration at Baseline and 4-Week Follow-up
Relative to Asymptomatic Controls
J Manipulative Physiol Ther. 2018 (Feb); 41 (2): 81–91TL Christiansen, IK Niazi, K Holt, et al.
The Effects of a Single Session of Spinal Manipulation
on Strength and Cortical Drive in Athletes
European J Appl Physiol 2018 (Apr); 118 (4): 737-749H Haavik, MG Özyurt, IK Niazi, et al.
Chiropractic manipulation increases maximal
bite force in healthy individuals
Brain Sci, 8 (5) (2018), p. 76,
10.3390/brainsci8050076K Holt, IK Niazi, I Amjad, et al.
The Effects of 4 Weeks of Chiropractic Spinal Adjustments
on Motor Function in People with Stroke:
A Randomized Controlled Trial
Brain Sciences 2021 (May 21); 11 (6): 676H Haavik, IK Niazi, K Holt, B. Murphy
Effects of 12 Weeks of Chiropractic Care on Central
Integration of Dual Somatosensory Input in
Chronic Pain Patients: A Preliminary Study
J Manipulative Physiol Ther. 2017 (Mar); 40 (3): 127–138J Daligadu, H Haavik, PC Yielder, J Baarbe, B. Murphy
Alterations in Cortical and Cerebellar Motor Processing in
Subclinical Neck Pain Patients Following Spinal Manipulation
J Manipulative Physiol Ther. 2013 (Oct); 36 (8): 527–537MA McDonald, SJ Holdsworth, Danesh-Meyer HV
Eye movements in mild traumatic brain injury: ocular biomarkers
J Eye Mov Res, 15 (2) (2022), pp. 1-31,
10.16910/jemr. 15.2.4
Return to PEDIATRICS
Return to MILD TRAUMATIC BRAIN INJURY
Since 9-06-2025


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |