

Swiss Chiropractic Practice-based Research Network
and Musculoskeletal Pain Cohort Pilot Study:
Protocol of a Nationwide Resource to Advance
Musculoskeletal Health Services ResearchThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: BMJ Open 2022 (Jul 13); 12 (7): e059380 ~ FULL TEXT
OPEN ACCESS Rahim Lalji, Léonie Hofstetter, Alice Kongsted, Viktor von Wyl, Milo A Puhan, and Cesar A Hincapié
Department of Chiropractic Medicine,
Balgrist University Hospital and University of Zurich,
Zurich, Switzerland.
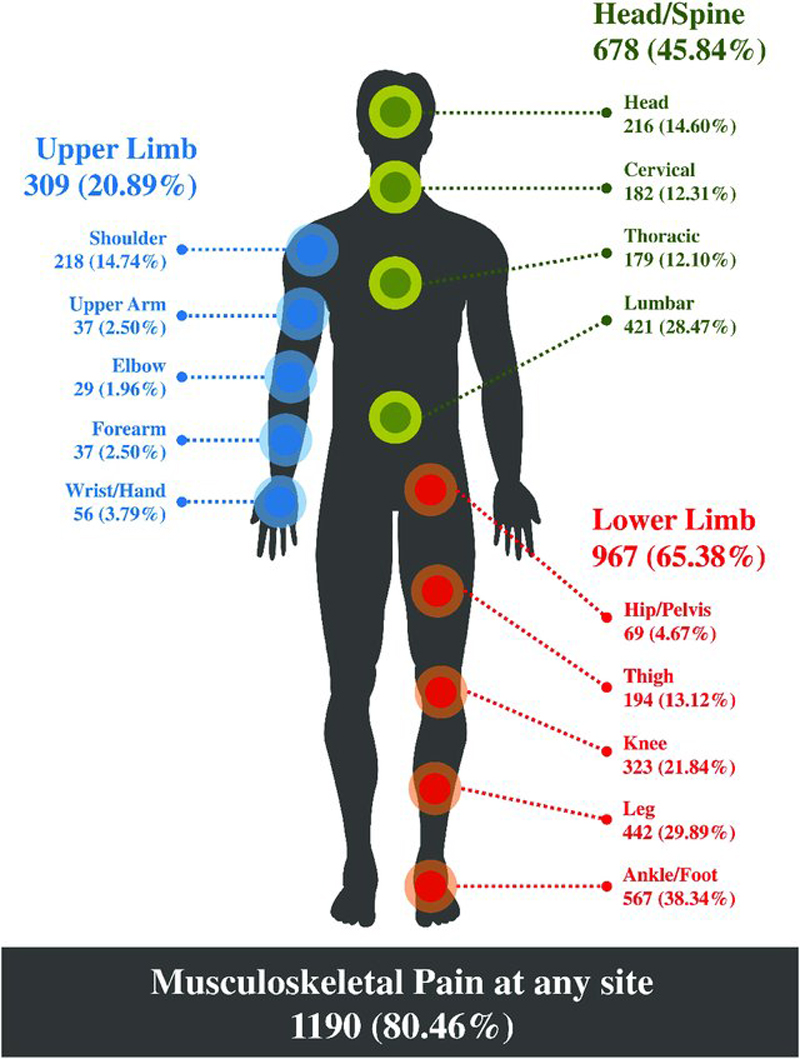
FROM: J Pain Res. 2021Introduction: Musculoskeletal (MSK) pain conditions, a leading cause of global disability, are usually first managed in primary care settings such as medical, physiotherapy, and chiropractic community-based practices. While chiropractors often treat MSK conditions, there is limited real-world evidence on the topic of health service outcomes among patients receiving this type of care. A nationwide Swiss chiropractic practice-based research network (PBRN) and MSK pain patient cohort study will have potential to monitor the epidemiological trends of MSK pain conditions and contribute to healthcare quality improvement. The primary aims of this protocol are to (1) describe the development of an MSK-focused PBRN within the Swiss chiropractic setting, and (2) describe the methodology of the first nested study to be conducted within the PBRN-an observational prospective patient cohort pilot study.
Methods and analysis: This initiative is conceptualised with two distinct phases. Phase I focuses on the development of the Swiss chiropractic PBRN, and will use a cross-sectional design to collect information from chiropractic clinicians nationwide. Phase II will recruit consecutive patients aged 18 years or older with MSK pain from community-based chiropractic practices participating in the PBRN into a prospective chiropractic cohort pilot study. All data collection will occur through electronic surveys offered in the three Swiss official languages (German, French, Italian) and English. Surveys will be provided to patients prior to their initial consultation in clinics, 1 hour after initial consultation, and at 2, 6 and 12 weeks after initial consultation.
Ethics and dissemination: Ethics approval has been obtained from the independent research ethics committee of Canton Zurich (BASEC-Nr: 2021-01479). Informed consent will be obtained electronically from all participants. Findings will be reported to stakeholders after each study phase, presented at local and international conferences, and disseminated through peer-reviewed publications.
Study pre-registration: Phase I-Swiss chiropractic PBRN (ClinicalTrials.gov identifier: NCT05046249);
Phase 2-Swiss chiropractic cohort (Swiss ChiCo) pilot study (ClinicalTrials.gov identifier: NCT05116020).
Keywords: epidemiology; musculoskeletal disorders; primary care; quality in health care.
Strengths and limitations of this study
Use of a flexible practice-based research network (PBRN) model will allow for a diverse range of nested study design types as well as the future expansion of the network.
Development of protocol methods is guided by patient and public involvement activities with key stakeholders.
Sole use of electronic data capture methods may lead to selective participation of both clinician and patient participants.
Maintenance of the PBRN and subsequent expansion of the patient cohort will depend on ongoing stakeholder support and involvement.
From the FULL TEXT Article:
Introduction
Musculoskeletal (MSK) pain conditions are the leading cause of disability worldwide, with low back pain being the largest single cause in over 160 countries, including Switzerland. [1, 2] This health burden translates to an economic cost of approximately €6.6 billion or about 2% of Switzerland’s total gross domestic product for low back pain alone. [3] Best practice recommendations and systematic reviews on MSK pain largely focus primarily on regional pain locations, such as low back pain or neck pain. [4–7] However, in the population and in primary care settings, it is common that those experiencing an MSK pain complaint also present with coexisting pain in another body region. [8–10] There is increasing evidence suggesting that these pain conditions, although localised to different regions, share similarities with respect to the course of symptoms, prognostic factors, and clinical care recommendations. [11, 12] A siloed body region focus to MSK health may create gaps in patient-centred research and difficulties with knowledge implementation in healthcare settings.
Further contributing to practice gaps is the lack of practice-based data collection in MSK healthcare research. [13] To help bridge the divide between research and practice, countries such as the UK, Denmark, Sweden, and Australia have engaged in practice-based research and worked with MSK-focused practice-based research networks (PBRNs). [14–16] A PBRN is a group of at least 15 primary care settings united under a commitment to advance the science base of clinical care. [17] These ‘real-world’ clinical research environments allow for sustained collaborations between practitioners, patients, and academicians facilitating the co-creation of relevant research questions and production of clinically applicable results. [13, 17, 18]
The chiropractic scope of practice in Switzerland includes the diagnosis and management of MSK pain conditions through manual medicine, prescription medication, and diagnostic imaging (radiography, ultrasound, CT, MRI). As of December 2021, there were approximately 326 chiropractors practising across Switzerland with the large majority providing care in community-based settings. MSK complaints such as low back pain and neck pain, which result in the largest burdens of disability, are commonly seen in chiropractic practice. [19] Chiropractic healthcare centres may serve as useful settings to further investigate MSK pain conditions, to understand what role chiropractors play in the current management of these conditions, and to identify opportunities for Swiss MSK primary healthcare quality improvement. As management of MSK conditions moves away from traditional medical and pharmacological treatments and towards more physical and preventative approaches, there is a need to describe non-pharmacological treatment options to make informed decisions on how best to use this capacity in the current healthcare system. [4, 20]
Given the high burden of MSK pain conditions, which are frequently managed by chiropractors, and limited practice-based evidence on the topic of chiropractic care for MSK conditions, particularly in Switzerland, this protocol report outlines the creation of a nationwide PBRN and subsequent nested prospective cohort (Swiss ChiCo) pilot study for chiropractic patients with MSK pain. Once established, this PBRN will provide the framework to help monitor the epidemiological trends of MSK pain in primary care settings, contribute to MSK healthcare quality improvement, and support future development and growth of practice-based MSK clinical research.
The main objectives of this protocol report are to:(1) describe the development of an MSK focused PBRN and describe the enrolment of Swiss chiropractors into the PBRN, and
(2) describe the methods of the first nested study to be conducted within the PBRN—an observational prospective patient cohort pilot study.
Methods and analysis
Study design
The Swiss chiropractic PBRN will use a substudy PBRN model, similar to that of the Australian Chiropractic Research Network. [14, 21, 22] In substudy PBRN models, data are initially collected from participating clinicians/clinical practices through self-report to first establish and describe characteristics of the PBRN. Following development, nested substudies may be performed using this PBRN framework.
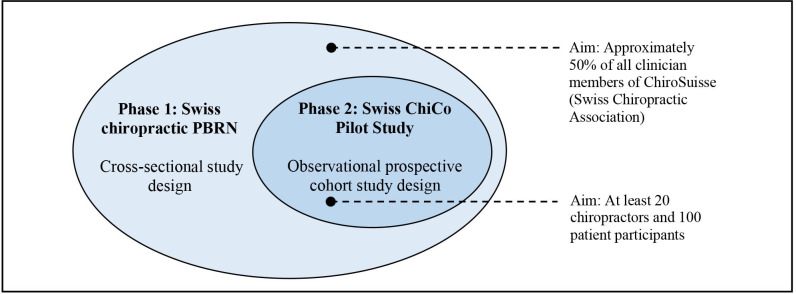
Figure 1 The current project will consist of two phases. Each project phase will have a specific aim and report on two primary feasibility and clinical outcomes related to this aim. In phase I, we aim to develop the Swiss chiropractic PBRN and describe the demographics of participating chiropractors at project initiation using a cross-sectional study design. In phase 2, we aim to launch a 12–week observational prospective Swiss chiropractic cohort (Swiss ChiCo) pilot study, which will assess the feasibility for longitudinal data collection and describe the clinical course of patients with MSK pain presenting to Swiss chiropractors. Figure 1 provides an overview of the two nested phases of this project.
Patient and public involvement
To guide development of this project, we hosted several events to gather information from key stakeholders. Key stakeholders identified include the Swiss Chiropractic Association (ChiroSuisse), the Swiss Chiropractic Patient Association (Pro Chiropractic Switzerland), Swiss chiropractors, and an international group of researchers with experience in practice-based research. Participatory engagement activities were first performed collaboratively with all stakeholders and focused on study relevance, team building, project infrastructure development, and the collaborative creation of relevant research questions. A consensus-based understanding was reached by all members, which outlined the need for more clinical MSK research within the Swiss setting and a pledge to provide support to achieve this project goal. Other recommendations included the practicality to start with a small cohort study to first test data collection methods, as well to explore both clinical and feasibility-related objectives to help drive recruitment from community-based chiropractors and patients.
Individualised one-on-one meetings were subsequently conducted to discuss specific project methods with each stakeholder group. Recommendations provided by ChiroSuisse and Pro Chiropractic Switzerland included the addition of several questions to the Swiss ChiCo pilot study patient participant questionnaires. Consequently, questions relating to patient work status, past use of chiropractic care, and use of other healthcare in MSK pain management were added. Both associations also recommended increasing patient participant recruitment weighting for the Swiss ChiCo pilot study in the French and Italian language regions of Switzerland by 5% from what was initially proposed.
One-on-one meetings with a small group of interested Swiss chiropractors were carried out for the purpose of understanding how best to integrate study processes into clinical practice settings. According to all clinician advisors, the recruitment of approximately 5–10 consecutive patients per clinical practice was feasible. Outside of clinical workflow processes, patient participant inclusion criteria were revised from new healthcare seeking for an MSK pain condition (operationalised as not having received any (patient-reported) healthcare for current MSK complaint) to new conservative healthcare seeking for an MSK complaint (not having received any (patient-reported) chiropractic, physiotherapy, osteopathy or massage therapy for current MSK complaint in the last 1 month, and not a follow-up visit). Many clinician advisors recommended this change based on the clinical profile of their patients and insurance coverage practices in Switzerland (where chiropractic care typically follows an initial visit with a primary care physician or general practitioner).
Participatory engagement is an iterative process and requires continuous reflection of previous project processes and results to inform subsequent phases (action–reflection process). [23] Following completion of each project phase, individual meetings with each stakeholder group will be scheduled to disseminate findings, discuss how best to generate future PBRN growth and explore ways to expand the MSK clinical cohort study.
Phase I: development of the Swiss chiropractic PBRN
Participants All registered active chiropractor members (fully licensed chiropractors and postgraduate assistant chiropractors) of ChiroSuisse will be eligible and invited to participate. Approximately 98% of all practicing Swiss chiropractors hold an active membership with ChiroSuisse (personal communication, 22 April 2021).
Recruitment To aid with clinician recruitment, we plan to launch the PBRN development phase on 9 September 2021 at the annual ChiroSuisse Continuing Education Convention 2021 (Lausanne, [9–11] September 2021). Clinicians will have the opportunity to ask questions directly of the project team, test electronic study methods, sign up as a clinician member of the PBRN, and provide input and feedback for the subsequent Swiss ChiCo pilot study. Those interested will be invited to join the Swiss chiropractic PBRN by scanning a quick response code and completing the linked clinician entry survey using personal mobile devices. For those who do not attend the conference, we plan to use electronic email invitations containing the Research Electronic Data Capture (REDCap) PBRN entry survey link. This invitation will be paired with an information sheet outlining project goals, good conduct procedures for the PBRN, opportunity for subsequent substudy involvement, and risks and benefits for participation. Clinician recruitment for the Swiss chiropractic PBRN will be scheduled to end on 19 December 2021. Similar to other PBRNs within the scope of chiropractic and MSK health, we hope to achieve a clinician participation proportion of approximately 50%. [21, 24]

Table 1 Data collection procedures and variables All data acquisition will occur electronically using the REDCap web application platform. [25] Clinicians participating in the Swiss chiropractic PBRN will be asked to fully complete one electronic survey of approximately 10 min duration. Clinician surveys will only be provided in English as this is the official language used for communication by ChiroSuisse. Table 1 outlines the specific data, which will be collected from clinicians for the development of the Swiss chiropractic PBRN. Online supplemental file 1 provides the data dictionary and specific response options that will be used for the Swiss chiropractic PBRN development survey.
Main outcomes and analysis The first primary clinical outcome will be practitioner self-confidence in the clinical management of patients with low back pain (as measured by the practitioner self-confidence scale (PCS)). [26] The PCS contains four items with a total score of 20. A score of 4 represents higher self-confidence in the management of patients with low back pain, while a score of 20 represents lower self-confidence. The second primary clinical outcome will be practitioner biomedical versus biopsychosocial MSK pain treatment orientation (as measured by the pain attitudes and beliefs scale, musculoskeletal version (PABS-MSK)). [27] The PABS-MSK contains two domains, with a higher score on either of the domains (each 10–items, with a score range of 10–60) representing higher biomedical and biopsychosocial MSK pain treatment orientation. The order of 20 items of the PABS-MSK will be randomised using the ‘randomizeR’ package in RStudio and administered as a single questionnaire so as to mask respondents to the specific treatment orientation domains. Both primary clinical outcomes will be reported as means and SDs, with 95% CIs calculated as appropriate.
Primary feasibility outcomes of(1) clinician participation proportion in the Swiss chiropractic PBRN will be assessed by reporting the proportion of all eligible clinicians that enrol in the PBRN development phase using raw numbers and percentages, and
(2) motivation for clinician participation in the Swiss ChiCo pilot study will be assessed using a visual analogue scale (VAS, 0–100), with higher scores reflecting higher motivation for participation.Level of motivation to participate in the Swiss ChiCo pilot study will be reported as means, SDs and with 95% CIs calculated as appropriate. Participants who score 70 or more on the pilot study motivation VAS will be conceptualised as ‘highly motivated’, and described using raw numbers, and proportions with 95% CIs.
Phase II: the Swiss chiropractic cohort (Swiss ChiCo) pilot study
Participants Patients will be eligible to participate if they are 18 years of age or older, are seeking new conservative healthcare for an MSK pain condition (new conservative healthcare seeking is operationalised as not having received (patient-reported) chiropractic care, physiotherapy, osteopathy or massage therapy for their current MSK complaint in the 1 month prior to their current initial visit to the chiropractor and not a follow-up visit); consent to chiropractic treatment, are able to respond to surveys in German, French, Italian or English, have an active email account and are willing and able to complete electronic study questionnaires. Patient participants will be excluded if they present to clinician practices with red flag symptoms (ie, saddle anaesthesia, loss of bowel and/or bladder control, history of major trauma, fracture, fever, severe or rapidly progressive neurologic deficit, sudden unexplained weight loss), and/or with a non-MSK-based pain condition based on the chiropractor’s clinical suspicion that symptoms relate to a systemic disease.
Recruitment Following the development of the Swiss chiropractic PBRN, we plan to recruit a subset of clinicians to participate in the Swiss ChiCo pilot study. Chiropractors will be recruited through general interest, VAS motivation score (≥70) on the PBRN entry questionnaire and using a purposeful sampling approach based on Swiss chiropractic clinician distribution across German, French and Italian language regions of Switzerland (55% DE, 35% FR, 10% IT). The Swiss ChiCo pilot study aims to recruit at least 20 chiropractors. Participating chiropractors will be asked to recruit new consecutive eligible patient participants from their clinical practices. We will hold pilot study training meetings with participant clinicians and clinical staff to introduce study objectives, methods and procedures prior to individual clinic pilot study launch dates, with the anticipated date for overall initiation of the patient cohort pilot study of 01 April 2022. During previous patient and public involvement work, Swiss chiropractors described the recruitment of 5–10 consecutive patients with new conservative onset MSK pain as feasible. We will aim to recruit at least 100 patient participants to enable a preliminary characterisation of the population. A stopping point for recruitment will be considered at approximately 5 to 10 patients enrolled per participating chiropractor.
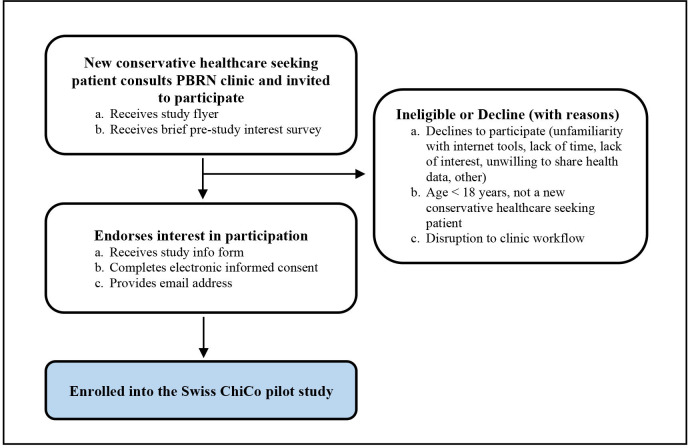
Figure 2 Potentially eligible patients visiting a participating clinician will be first provided a study flyer, which will briefly outline the study objectives and participation requirements. Patients will then be asked to indicate their interest to participate using a brief electronic survey. Those not interested will be prompted to provide reasons for non-participation. Patients expressing interest in participation will be forwarded to the full study information form and electronic informed consent procedure. This in-clinic patient participant procedure was developed in consultation with Swiss chiropractic clinicians (both women and men) across all language regions. To aid with workflow, clinicians expressed interest in asking new patients to arrive approximately 20 min prior to their appointment to complete electronic study forms. Clinicians also recommended adding ‘disruption to clinic workflow’ as a clinic-implemented response option for non-participation of an eligible patient. This survey option could be selected by clinical staff when deemed that patient participant recruitment may greatly impact clinical workflow (eg, patient was late for visit, emergency visit). Figure 2 outlines the in-clinic patient recruitment procedure.

Table 2 Data collection procedures and variables Immediately following completion of the in-clinic recruitment procedure, study participants will be forwarded to the first patient survey (previsit patient survey) on an electronic device (mobile phone or tablet). This previsit initial patient survey will collect information on clinical measures that are likely to be influenced by the first visit (ie, pain impact, MSK health status, illness perception). [25–30] The previsit patient survey will take approximately 5 min to complete and is the only survey that is completed at clinical practices. Subsequent questionnaires will take approximately 10–12 min to complete and are emailed directly to patient participants 1 hour after (postvisit patient survey) and at 2, 6 and 12 weeks following completion of the previsit survey. REDCap will be used for longitudinal data collection, with survey data transmitted automatically to the research team at Balgrist University Hospital and the University of Zurich. Similar administration procedures were performed for the Danish chiropractic low back pain cohort study. [31] Patient participant surveys will be provided in English, German, French and Italian, with patients having the ability to choose their preferred language for completion. Validated, translated versions of the patient reported outcome measures (PROMs) will be used when possible. [32–39] If not available, translation of the PROMs by a native speaker will be performed. Table 2 outlines specific outcome measures and timing of data collection for the Swiss ChiCo pilot study. Online supplemental file 2 provides the data dictionary and specific response options to be used for the Swiss ChiCo pilot study surveys.
Main outcomes and analysis The prespecified primary clinical outcomes will be:(1) change in MSK pain impact, as measured by the 3–item pain, enjoyment and general activity scale (PEG scale, score range 0–10) [28] with higher scores representing worse outcomes and
(2) change in MSK health status, as measured by the MSK health questionnaire (MSK-HQ, score range 0–56) [29] with higher scores reflecting better health status.Clinical outcomes of the PEG scale and MSK-HQ prior to initial chiropractic assessment will be reported as means, SDs and 95% CIs; and clinical course of patient pain impact and MSK health status will be reported as a mean difference with SDs and 95% CIs as appropriate.
The primary feasibility outcomes will be:
(1) the proportion of invited patients presenting to chiropractic practices who subsequently agree to participate in this study and
(2) change in patient participant follow-up and retention over 12 weeks. Invited patient participation will be reported as raw numbers and proportions.Patient participant retention will be reported as the proportion of enrolled participants who complete follow-up surveys across 12 weeks. Based on the definition of a PBRN from the Agency for Healthcare Research and Quality, [17] it will be deemed feasible to initiate the Swiss chiropractic PBRN and expand the Swiss ChiCo pilot study if at least 15 clinical practices agree to participate in the Swiss chiropractic PBRN and each recruit at least 5 patients for enrolment in the Swiss ChiCo pilot study.
Ethics and dissemination
The Swiss chiropractic PBRN and Swiss ChiCo pilot study have been reviewed and jointly approved by the independent research ethics committee of Canton Zurich (BASEC-Nr: 2021-01479). Informed consent will be obtained from both clinician and patient participants electronically on entry into the Swiss chiropractic PBRN and the Swiss ChiCo pilot study. Clinician responses for PBRN development will be stored securely and confidentially within the study REDCap database, but not anonymously due to the need of identifying clinicians to participate in future nested PBRN projects. Data collected for PBRN development and for the Swiss ChiCo pilot study will be stored as two separate projects within REDCap. Individual-level data will not be shared with study stakeholders.
The findings from the Swiss chiropractic PBRN and the Swiss ChiCo pilot study will be disseminated first to the various stakeholder groups involved in study development through individual meetings. Findings will also be presented through presentations at academic conferences and fully reported in peer-reviewed publications.
Availability of data and materials
Data from this work will be made available for research purposes. Requests, including a synopsis of the study proposal, can be addressed to the corresponding author.
Discussion
This project is designed to attract a large proportion of Swiss chiropractors into a nationwide PBRN and subsequently recruit patients from participating clinics into a longitudinal cohort pilot study. This approach combines a substudy PBRN model, with longitudinal electronic capture more readily seen in register-based approaches. The unique collaboration with clinicians, advocacy groups, and academicians — a growing trend in healthcare research — has led to the promotion of research objectives that are deemed clinically relevant and patient-centred, and a study implementation strategy supported by Swiss chiropractic primary care clinicians.
Traditional healthcare research approaches typically face challenges with regard to study relevance, patient recruitment, and knowledge translation. [13, 40] The use of a participatory research approach can help overcome such challenges by integrating the diverse knowledge, values, and preferences of non-academics into the research process. An example of a longitudinal register-based study successfully implementing this approach is the Swiss Multiple Sclerosis Registry (SMSR). [41] This project was designed in collaboration with the multiple sclerosis (MS) community in Switzerland to tackle the lack of epidemiological data and to promote patient perspectives in MS research. Participatory elements of the SMSR include a flexible approach to study involvement based on participant comfort, involvement of patients in the study design and execution, and data feedback to provide ongoing results to participants. Due to such efforts, recruitment for the SMSR exceeded expectations; with the goal of 400 participants achieved in under 20 days. [42] A second example of a participatory research approach driving recruitment is the recently established national osteopathy PBRNs of Australia and New Zealand. [24] Here, the project team engaged with both osteopathic communities for 12 months prior to clinician recruitment. Today, these two PBRNs represent the largest coverage of any voluntary health profession PBRN, with 43.5% of all registered osteopaths in Australasia. The Swiss chiropractic PBRN has followed a similar approach, with community outreach and promotion efforts lasting more than 12 months prior to clinician recruitment.
What remains unclear is if early engagement of stakeholders can overcome the unique limitations of electronic observational studies. Typically, unequal access to technology resources and lack of digital literacy can lead to a young, well-educated, and high socioeconomic status study sample. For example, participants in the SMSR who opt for physical forms are older, show increased care-seeking behaviour, and suffer from more progressive illness compared with those using electronic forms. This trend also extends to clinician participants, as our own survey on health information technology use among Swiss chiropractors found that clinicians aged 60 years and over were 74% less likely to use electronic health records when compared with the those under 40 years. [43] To limit this threat to external validity, the Swiss chiropractic PBRN will recruit clinicians through both online and in-person channels. In addition, chiropractic clinician recruitment for the Swiss ChiCo pilot study will be proportionally overweighted in French and Italian language regions. These areas showed lower health information technology use when compared with German-speaking regions of Switzerland. To recruit a diverse group of patient participants, clinicians will be asked to consecutively recruit eligible patients from private practice. Although consecutive recruitment does not eliminate the threat of self-selection bias, it ensures all eligible patients seeking chiropractic care will be invited to participate in a non-selective manner. The Swiss chiropractic PBRN and Swiss ChiCo pilot study presents a model for PBRN development and rapid engagement of a newly created clinical research network. Once complete, this PBRN will serve as a platform for answering important research questions in the field of MSK primary healthcare.
Supplementary Material
Reviewer Comments (319K, pdf)
Author's Manuscript (1.7M, pdf)Acknowledgments
The authors would like to thank members of ChiroSuisse, Pro Chiropractic Switzerland, and Swiss chiropractic clinicians involved in this project for their continued participatory engagement and support.
Contributors:
CAH conceived the project idea. CAH, RL, AK, VvW, MAP and LH contributed to the design of the protocol. RL and CAH designed, undertook and coordinated stakeholder participatory activities. RL and CAH led the drafting of the protocol manuscript. All authors gave important intellectual input and provided critical review of the protocol manuscript and approved the final version of the manuscript. CAH obtained funding. CAH and RL were the guarantors of this manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding:
This work was internally supported by the Department of Chiropractic Medicine, Faculty of Medicine, at University of Zurich and Balgrist University Hospital through funding from the Foundation for the Education of Chiropractors in Switzerland. The funder had no role in considering the research questions, study design, protocol methods or analysis, or in writing of the protocol manuscript, or the decision to submit the article for publication.
Competing interests:
None declared.
Patient and public involvement:
Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the methods and analysis sections for further details.
References
Disease GBD;
Global, Regional, and National Incidence, Prevalence, and Years Lived With
Disability for 328 Diseases and Injuries for 195 Countries, 1990-2016:
A Systematic Analysis for the Global Burden of Disease Study 2016
Lancet. 2017 (Sep 16); 390 (10100): 1211–1259Murray CJ, Vos T, Lozano R, et al.
Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21
regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010
Lancet 2013 Dec 15;380(9859):2197–223Wieser S, Horisberger B, Schmidhauser S, et al.
Cost of low back pain in Switzerland in 2005.
Eur J Health Econ 2011;12:455–67.
10.1007/s10198-010-0258-yQaseem A, Wilt TJ, McLean RM, Forciea MA;
Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain:
A Clinical Practice Guideline From the American College of Physicians
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 514–530Kreiner DS, Matz P, Bono CM, et al.
Guideline summary review: an evidence-based clinical guideline
for the diagnosis and treatment of low back pain.
Spine J 2020;20:998–1024.
10.1016/j.spinee.2020.04.006Bussières AE, Stewart G, Al-Zoubi F, et al.
Spinal Manipulative Therapy and Other Conservative Treatments
for Low Back Pain: A Guideline From the Canadian
Chiropractic Guideline Initiative
J Manipulative Physiol Ther. 2018 (May); 41 (4): 265–293Bussières, AE, Stewart, G, Al Zoubi, F et al.
The Treatment of Neck Pain-Associated Disorders and
Whiplash-Associated Disorders: Clinical Practice Guideline
J Manipulative Physiol Ther. 2016 (Oct); 39 (8): 523–564Hartvigsen J, Davidsen M, Hestbaek L, et al.
Patterns of musculoskeletal pain in the population: a latent class
analysis using a nationally representative interviewer-based
survey of 4817 Danes.
Eur J Pain 2013;17:452–60.
10.1002/j.1532-2149.2012.00225.xKamaleri Y, Natvig B, Ihlebaek CM, et al.
Localized or widespread musculoskeletal pain: does it matter?
Pain 2008;138:41–6.
10.1016/j.pain.2007.11.002Hincapié CA, Cassidy JD, Côté P, et al.
Whiplash injury is more than neck pain: a population-based
study of pain localization after traffic injury.
J Occup Environ Med 2010;52:434–40.
10.1097/JOM.0b013e3181bb806dBabatunde OO, Jordan JL, Van der Windt DA, et al.
Effective treatment options for musculoskeletal pain in
primary care: a systematic overview of current evidence.
PLoS One 2017;12:e0178621.
10.1371/journal.pone.0178621Artus M, Campbell P, Mallen CD, et al.
Generic prognostic factors for musculoskeletal pain in
primary care: a systematic review.
BMJ Open 2017;7:e012901.
10.1136/bmjopen-2016-012901Westfall JM, Mold J, Fagnan L.
Practice-based research—“blue highways” on the NIH roadmap.
JAMA 2007;297:403.
10.1001/jama.297.4.403Adams J, Steel A, Moore C, et al.
Establishing the ACORN national practitioner database: strategies
to recruit practitioners to a national practice-based
research network.
J Manipulative Physiol Ther 2016;39:594–602.
10.1016/j.jmpt.2016.08.006Kongsted A, Davies L, Axen I.
Low Back Pain Patients in Sweden, Denmark and the UK Share
Similar Characteristics and Outcomes: A Cross-National
Comparison of Prospective Cohort Studies
BMC Musculoskelet Disord. 2015 (Nov 26); 16 (1): 367Hestbaek L, Munck A, Hartvigsen L, Jarbol DE, Sondergaard J, Kongsted A.
Low Back Pain in Primary Care: A Description of 1250 Patients
with Low Back Pain in Danish General and Chiropractic Practice
Int J Family Med. 2014 (Nov 4); 2014: 106102Agency for Healthcare Research and Quality, Rockville, MD .
Primary Care Practice-based Research Networks, 2018Pearce KA, Love MM, Barron MA, et al.
How and why to study the practice content of a practice-based research network.
Ann Fam Med 2004;2:425–8.
10.1370/afm.133Humphreys BK, Peterson CK, Muehlemann D, Haueter P.
Are Swiss Chiropractors Different Than Other Chiropractors?
Results of the Job Analysis Survey 2009
J Manipulative Physiol Ther 2010 (Sep); 33 (7): 519–535Lin I, Wiles L, Waller R, Goucke R, Nagree Y, Gibberd M, et al.
What Does Best Practice Care for Musculoskeletal Pain Look Like?
Eleven Consistent Recommendations From High-quality
Clinical Practice Guidelines: Systematic Review
British J Sports Medicine 2020 (Jan); 54 (2): 79–86Adams J, Peng W, Steel A, et al.
A cross-sectional examination of the profile of chiropractors
recruited to the Australian Chiropractic Research Network
(ACORN): a sustainable resource for
future chiropractic research.
BMJ Open 2017;7:e015830.
10.1136/bmjopen-2017-015830Adams J, Steel A, Chang S, et al.
Helping address the National research and research capacity needs
of Australian chiropractic: introducing the Australian
Chiropractic Research Network (ACORN) project.
Chiropr Man Therap 2015;23:12.
10.1186/s12998-015-0057-8Lang DJ, Wiek A, Bergmann M, et al.
Transdisciplinary research in sustainability science:
practice, principles, and challenges.
Sustain Sci 2012;7:25–43.
10.1007/s11625-011-0149-xSteel A, Peng W, Sibbritt D, et al.
Introducing national osteopathy practice-based research networks
in Australia and New Zealand: an overview to
inform future osteopathic research.
Sci Rep 2020;10:846.
10.1038/s41598-020-57918-7Patridge EF, Bardyn TP.
Research electronic data capture (REDCap).
Jmla 2018;106:142–4.
10.5195/jmla.2018.319Smucker DR, Konrad TR, Curtis P, et al.
Practitioner self-confidence and patient outcomes
in acute low back pain.
Arch Fam Med 1998;7:223–8.
10.1001/archfami.7.3.223Duncan K.
The development and testing of a generic musculoskeletal version
of the pain attitudes and beliefs scale
[Doctoral thesis], 2017. Available:
https://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.726993
[Accessed 11 Oct 2021].Krebs EE, Lorenz KA, Bair MJ, et al.
Development and initial validation of the PEG, a three-item
scale assessing pain intensity and interference.
J Gen Intern Med 2009;24:733–8.
10.1007/s11606-009-0981-1Hill JC, Kang S, Benedetto E, et al.
Development and initial cohort validation of the arthritis research
UK musculoskeletal health questionnaire (MSK-HQ) for use
across musculoskeletal care pathways.
BMJ Open 2016;6:e012331.
10.1136/bmjopen-2016-012331Broadbent E, Petrie KJ, Main J, et al.
The brief illness perception questionnaire.
J Psychosom Res 2006;60:631–7.
10.1016/j.jpsychores.2005.10.020Kongsted A, Nielsen OL, Christensen HW, et al.
The Danish chiropractic low back pain cohort (ChiCo):
description and summary of an available data
source for research collaborations.
Clin Epidemiol 2020;12:1015–27.
10.2147/CLEP.S266220Glattacker M, Bengel J, Jäckel WH.
Die deutschsprachige version des illness perception questionnaire-revised:
psychometrische evaluation an patienten MIT
chronisch somatischen erkrankungen.
Zeitschrift für Gesundheitspsychologie 2009;17:158–69.Giardini A, Majani G, Pierobon A.
Contributo alla validazione italiana dell’IPQ-R.
G Ital Med Lav Erg 2007;29.Broadbent E, Wilkes C, Koschwanez H, et al.
A systematic review and meta-analysis of the brief
illness perception questionnaire.
Psychol Health 2015;30:1361–85.
10.1080/08870446.2015.1070851Karstens S, Christiansen DH, Brinkmann M.
German translation, cross-cultural adaptation and validation of
the musculoskeletal health questionnaire: a cohort study.
Eur J Phys Rehabil Med 2021;56:771–9.
10.23736/S1973-9087.20.06054-2Galeoto G, Piepoli V, Ciccone E.
Musculoskeletal health questionnaire: translation,
cultural adaptation and validation of the
Italian version (MSK-HQ-I).
Muscle Ligaments Tendons J 2019;09:295–303.
10.32098/mltj.02.2019.20Schmidt CO, Kohlmann T, Pfingsten M, et al.
Construct and predictive validity of the German Örebro questionnaire
short form for psychosocial risk factor screening of
patients with low back pain.
Eur Spine J 2016;25:325–32.
10.1007/s00586-015-4196-3Hilfiker R, Knutti IA, Raval-Roland B, et al.
Validity and responsiveness of the French version of the
Örebro musculoskeletal pain screening questionnaire
in chronic low back pain.
Eur Spine J 2016;25:2741–9.
10.1007/s00586-016-4635-9Oxford University Innovation .
Health outcomes - Musculoskeletal Health Questionnaire (MSK-HQ).
https://innovation.ox.ac.uk/outcome-measures/musculoskeletal-health-questionnaire-msk-hq/
[Accessed 16 Jul 2021].Morris ZS, Wooding S, Grant J.
The answer is 17 years, what is the question:
understanding time lags in translational research.
J R Soc Med 2011;104:510–20.
10.1258/jrsm.2011.110180Steinemann N, Kuhle J, Calabrese P, et al.
The Swiss multiple sclerosis registry (SMSR): study protocol of
a participatory, nationwide registry to promote
epidemiological and patient-centered MS research.
BMC Neurol 2018;18:111.
10.1186/s12883-018-1118-0Puhan MA, Steinemann N, Kamm CP, et al.
A digitally facilitated citizen-science driven approach
accelerates participant recruitment and increases
study population diversity.
Swiss Med Wkly 2018;148:w14623.
10.4414/smw.2018.14623Hincapié CA, Hofstetter L, Lalji R, et al.
Use of electronic patient records and encrypted email
patient communication among Swiss chiropractors:
a population-based cross-sectional study.
Med Internet Res 2022.
10.2196/40501Linton SJ, Nicholas M, MacDonald S.
Development of a short form of the Örebro musculoskeletal
pain screening questionnaire.
Spine 2011;36:1891–5.
10.1097/BRS.0b013e3181f8f775Kamper SJ, Maher CG, Mackay G.
Global rating of change scales: a review of strengths
and weaknesses and considerations for design.
J Man Manip Ther 2009;17:163–70.
10.1179/jmt.2009.17.3.163
Return LOW BACK PAIN
Return to SPINAL PAIN MANAGEMENT
Since 8-27-2022


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |