

Care for Low Back Pain:
Can Health Systems Deliver?This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Bulletin of the World Health Organization 2019 (Jun 1); 97 (6): 423–433 ~ FULL TEXT
OPEN ACCESS Adrian C Traeger, Rachelle Buchbinder, Adam G Elshaug, Peter R Croft, and Chris G Mahera
Institute for Musculoskeletal Health,
University of Sydney,
PO Box M179, Missenden Road,
Camperdown NSW 2050, Australia.
Low back pain is the leading cause of years lived with disability globally. In 2018, an international working group called on the World Health Organization to increase attention on the burden of low back pain and the need to avoid excessively medical solutions. Indeed, major international clinical guidelines now recognize that many people with low back pain require little or no formal treatment. Where treatment is required the recommended approach is to discourage use of pain medication, steroid injections and spinal surgery, and instead promote physical and psychological therapies. Many health systems are not designed to support this approach.
In this paper we discuss why care for low back pain that is concordant with guidelines requires system-wide changes. We detail the key challenges of low back pain care within health systems. These include the financial interests of pharmaceutical and other companies; outdated payment systems that favour medical care over patients' self-management; and deep-rooted medical traditions and beliefs about care for back pain among physicians and the public. We give international examples of promising solutions and policies and practices for health systems facing an increasing burden of ineffective care for low back pain. We suggest policies that, by shifting resources from unnecessary care to guideline-concordant care for low back pain, could be cost-neutral and have widespread impact. Small adjustments to health policy will not work in isolation, however. Workplace systems, legal frameworks, personal beliefs, politics and the overall societal context in which we experience health, will also need to change.
From the FULL TEXT Article:
Introduction
Low back pain is the single biggest cause of years lived with disability worldwide, and a major challenge to international health systems. [1] In 2018, the Lancet Low Back Pain Series Working Group identified a global problem of mismanagement of low back pain. [2–4] The group documented the phenomenon of unnecessary care in both high- and low-income settings, whereby patients receive health services, which are discordant with international guidelines. [2–4] The articles summarized the strong evidence that unnecessary care, including complex pain medications, spinal imaging tests, spinal injections, hospitalization and surgical procedures, is hazardous for most patients with low back pain. [2–4]
Although we could not find systematic estimates for the worldwide prevalence of unnecessary care for low back pain, the CareTrack studies provide some indication of scale. Those studies estimated that 28% (95% confidence interval, CI: 19.7–38.6) of health care for low back pain in Australia (based on 164 patients receiving 6,488 care processes) [5] and 32% (95% CI: 29.5–33.6) of health care for low back pain in the United States of America (based on 489 patients receiving 4,950 care processes) [6] was discordant with clinical guidelines. The figures are likely an underestimate because they did not include diagnostic imaging tests. The upward trend in unnecessary care for low back pain is even more concerning. One meta-analysis from 2018 found that simple imaging tests were requested in one quarter of back pain consultations (415,579 of 1,675,720 consultations) and the rates of complex imaging (e.g. magnetic resonance imaging) had increased over 21 years. [7]
There is no robust evidence of benefit for spinal fusion surgery compared with non-surgical care for people with low back pain associated with spinal degeneration. [8] However, over the years 2004–2015, elective spinal fusion surgery in the United States increased by 62.3% (from 60.4 per 100,000 to 79.8 per 100,000), with hospital costs for this procedure exceeding 10 billion United States dollars (US$) in 2015. [9] In 2014, 3–4% of the adult United States population (9.6 million to 11.5 million people of 318.6 million) were prescribed long-term opioid drug therapy, in many cases because of chronic low back pain. [10] The Lancet working group called on the World Health Organization to increase attention on the burden of low back pain and “the need to avoid excessively medical solutions.” [4]
The movement away from medicalized management of low back pain is reflected in recent clinical guidelines. All six of the major international clinical guidelines released since 2016 prioritized non-medical approaches for patients with low back pain (Box 1). [11–16] Primary-care clinicians following these guidelines would manage uncomplicated cases with advice, education and reassurance. For patients at risk of developing chronic pain and disability, clinicians would, depending on which guidelines they followed, consider offering treatments such as spinal manipulation, massage, acupuncture, yoga, mindfulness, psychological therapies or multidisciplinary rehabilitation. Most health systems are not well-equipped to support this approach.
Box 1: Key messages from six international clinical guidelines for management of low back pain
Adopt a stepped or stratified approach to care of low back pain, guided by the patient’s response to previous care or the results of risk prediction tools. Recommended by 4 out of 6 guidelines. [11–14]
First step care for low back pain, which will be sufficient for many patients, is to provide advice to remain active, education on the benign nature of low back pain and reassurance about the absence of serious pathology. Recommended by all guidelines. [11–16]
Second step options for acute low back pain include physical therapies (massage, spinal manipulation, heat-wrap therapy), psychological therapies (psychologically informed physiotherapy) or complementary therapies (acupuncturea). At least one recommended by all guidelines. [11–16]
Second step options for chronic low back pain comprise physical therapies (exercise, massage, spinal manipulation), psychological therapies (cognitive behavioural therapy), complementary therapies (mindfulness-based stress reduction, yoga, acupuncture, tai chi). Recommended by 4 out of 6 guidelines. [11–13, 15]
Third step in chronic low back pain care is multidisciplinary pain management (targets physical, psychological and social aspects of low back pain and involves a team of clinicians). Recommended by 5 out of 6 guidelines. [11–15]
Care of low back pain care without medication is preferred. Recommended by all guidelines. [11–16]
If pain medication is needed, begin with a nonsteroidal anti-inflammatory drug at the lowest effective dose for the shortest time. Recommended by all guidelines. [11–16]
Avoid prescribing opioid drugs for low back pain where possible. Recommended by 3 out of 6 guidelines. [11, 14, 16]
Do not offer injectable steroid drugs to patients with chronic non-specific low back pain. Recommended by 3 out of 6 guidelines. [11, 13, 14]
Do not offer surgery for patients with non-specific low back pain outside of a randomized trial. Recommended by 3 out of 6 guidelines. [11, 13, 14]
a Acupuncture was endorsed by the United States, Danish and Australian guidelines, but discouraged by United Kingdom and German guidelines. Belgian guidelines made no recommendation.
Notes: We analysed current clinical guidelines on low back pain care from six countries (United States of America, United Kingdom of Great Britain and Northern Ireland, Australia, Germany, Belgium and Denmark) released since 2016. Some specific details of recommendations differed between the guidelines.
Discontinuing unnecessary care of low back pain is beneficial for patients. We argue that safer therapies should be offered, even though the evidence base for their effectiveness is not yet clear enough to achieve consistent endorsement across guidelines. [11–16] In this paper we expand on the policy challenge of ensuring care for low back pain is concordant with guidelines and we outline potential solutions for health systems.
Health-system challenges
Access to suitable therapies
While most people with low back pain will require little or no formal care, for those who do require extra help an immediate challenge is patients’ and clinicians’ lack of access to the recommended therapies (Box 1). For example, a German survey found that general practitioners fundamentally agreed with the content of clinical guidelines for low back pain, but almost half had no access to the recommended multidisciplinary approach to pain management. [17] A more recent qualitative study of general practitioners in the United Kingdom of Great Britain and Norther Ireland concluded the same; one general practitioner viewed recommendations to provide a course of non-pharmacological care as “lovely pie-in-the-sky plans.” [18]
People living in rural and remote areas are often unable to access multidisciplinary pain management because it is typically provided in tertiary health-care settings in cities. For example, a person with chronic low back pain living in Kununurra in rural Western Australia, which has a population of 5600, would have to travel 827 km to Darwin or 3040 km to Perth to access their nearest multidisciplinary pain management service. [19] Patients may also have limited access to recommended physical and psychological therapies, and complementary therapies such as tai chi and yoga.
Bringing together necessary health services for people with complex chronic conditions is a growing challenge for modern health systems. Australia’s chronic disease management programme was designed to coordinate services across multiple providers. The programme, however, permits too few visits for proper delivery of effective coordinated care for chronic low back pain, since it only allows for a total of five visits per annum per patient, shared across all allied health services and irrespective of the number of chronic conditions a patient has. For chronic low back pain, a programme of primary care-based cognitive behavioural therapy can consist of seven sessions [20] and a programme of mindfulness-based stress reduction can be eight sessions. [21] Exercise programmes could similarly exceed this cap; an effective yoga programme was shown to comprise 12 sessions. [22] Some patients would also have to allocate one or more of their five sessions to manage comorbidities, such as diabetes or obesity. The costs of providing this care are far lower than some of the unnecessary care options. For example, the reimbursement offered by Medicare, Australia’s publicly funded health insurance scheme, for the five visits is only 311 Australian dollars (AU$), whereas spinal fusion surgery in a New South Wales public hospital is covered up to a cost of AU$ 53,700. [23]
Lack of time and training
The new guidelines require longer, more complex consultations. General practitioners cite time pressure and lack of confidence in new approaches to care as barriers to adherence to guidelines. [24] Providing prognosis-specific care (Box 1) in a 5–minute consultation [25] is next to impossible when treating a patient with severe pain, a poor prognosis or chronic low back pain with multiple comorbidities. One survey of 6588 consultations for low back pain in Australia found that only around one fifth (21 of 100) of general practitioners performed a complete history and physical examination. [26]
Most of the recommended second-step treatment options require referral to clinicians with specific training. Some health systems, especially those in low- and middle-income countries, will not have the supply of clinicians to deliver these therapies at scale. For example, Nepal has only one physiotherapist per 20,000 people, compared with 24 per 20,000 in Australia. [27]
Furthermore, even when patients can access multidisciplinary pain management services, they may not receive care that is concordant with guidelines. [28] A patient living with chronic low back pain in West Virginia, a state with the highest opioid drug overdose rate in the United States, could receive a service that offers opioid medication, but not evidence-based care such as pain education, coping strategies or rehabilitation services. [29]
Funding arrangements
Numerous companies and individuals profit from health care for low back pain. For example, Purdue Pharma L.P., makers of the opioid painkiller OxyContin® (oxycodone), has an estimated worth of US$ 13 billion. Oxycodone is the most used drug for chronic non-cancer pain in Australia. [30] In the United Kingdom, prescriptions for opioids including oxycodone are increasing, despite evidence of poor efficacy and substantial harm to patients. [31] In 2013, prescription opioids were responsible for 44 000 drug overdose deaths in the United States. [10] A systematic review of randomized trials found that opioids were of limited benefit in chronic low back pain, even in dangerously high doses, [32] nd a recent trial found opioids led to slightly worse pain outcomes than non-opioid medication for back pain and osteoarthritis. [33] The current international low back pain guidelines provide mixed messages regarding opioids. However, the Centers for Disease Control and Prevention guideline for prescribing opioid drugs for chronic pain are clear; extended-release opioids such as oxycodone should never be used for initial management of chronic non-cancer pain. [34]
Fines for breaking the regulations around marketing of medicines to doctors may not deter wealthy companies who want to increase their market share. Even a multi-million-dollar settlement would be tiny for a major pharmaceutical company that markets pain medicines. OxyContin® is estimated to have generated approximately US$ 35 billion in revenue for its manufacturer. [35] The largest settlement that we are aware of was around US$ 2.3 billion in 2009 paid by Pfizer Inc. to settle a misleading marketing case concerning Lyrica® (pregabalin), a commonly prescribed drug for low back pain. The fine represented less than 5% of the company’s US$ 50 billion revenues that year. Regulators should keep in mind that such large companies can accept any fine as a cost of doing business in the area of pain relief.
Health-service funding arrangements too can discourage the less-is-more approach that suits most cases of uncomplicated, non-specific low back pain. Fee-for-service health systems, for example, tend to provide incentives for activity and volume of care and hence inadvertently lead to more unnecessary care. A Cochrane review of systems of payment compared health service measures in two randomized controlled trials and two controlled before–after studies of 640 primary-care physicians and 6,400 patients. The review found that fee-for-service systems had higher numbers of contacts, visits to specialists and diagnostic and curative services compared with capitation systems. [36] Capitation systems, particularly those where clinicians receive a fixed salary to provide care for those enrolled in a given location, can reduce the number of services provided. However, capitation funding may also have undesirable effects, such as encouraging clinicians to provide the most time-efficient rather than the most effective care. Systems designed to solve these issues, such as pay-for-performance systems and quality-based contingency payments, may not reward clinicians fairly for all the complexities involved in treating people with low back pain.
While knowledge of best practice for low back pain has evolved, our health systems and their funding mechanisms have not kept up. As a result, many health-care systems internationally continue to fund guideline-discordant care, such as opioid drugs, radiofrequency denervation and spinal fusion surgery (Box 1). These treatments were common practice before a robust system of assessment of best practice was in place. Current funding arrangements therefore present a challenging evidence–policy–practice paradox. For example, Australian Medicare, and many private health insurers, do not fund guideline-concordant self-care by patients, such as yoga and tai chi, and provide limited funding for supervised exercise programmes. There are two interrelated issues here. First, some of these alternative services lack a sufficiently robust evidence base that would allow them to pass an assessment, which is rightly, a prerequisite for being added to the Australian Medicare fee-for-service schedule. Second, Medicare does not have a record of funding such alternative, non-medical interventions, although this could change if the evidence base for these services were to improve and a submission was made for their inclusion in the schedule of payments.
Achieving guideline concordance

Table 1
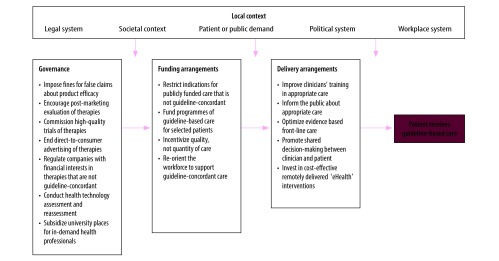
Figure 1 Some of the above challenges may appear insurmountable. However, we believe that with coordinated efforts targeting each level of the health system, change is possible. In Table 1 we provide suggestions for delivery arrangements, financial arrangements and governance of international health systems to better support guideline-concordant care for low back pain. Although most examples of initiatives in Table 1 are taken from high-income countries, we believe many could be trialled in low- and middle-countries. Figure 1 depicts a systems-level approach to achieving guideline concordance.
Change ideas about back pain
Misconceptions about management of low back pain remain common. For example, around half of patients presenting with low back pain (144 of 300) believed diagnostic imaging tests were necessary. [47] Targeting misconceptions at the population level through mass-media campaigns is an effective, but costly, approach. Campaigns such as the 1997–1999 campaign in Victoria, Australia, which encouraged people to “not take back pain lying down,” can change beliefs about low back pain and alter people’s behaviour, including the proportion of patients returning to work. [42] The growth of social media should make similar campaigns easier and cheaper to implement. Targeting young people through health information messages on social media or other channels before unhelpful beliefs become entrenched is another worthwhile approach. The Informed Health Choices initiative in Uganda, which helped primary-school children and their parents detect false treatment claims, is evidence that these programmes can succeed. [43] Whole-population education initiatives, such as these could be expanded to include information about unnecessary diagnostic tests and to target condition-specific health myths. However, those manufacturers or individuals who stand to gain financially from sales of certain therapies and who market opposing messages are a powerful force. Efforts to counteract such vested interests are likely to need sustained and coordinated support from the legislative, labour, health and government sectors (Fig. 1).
Clinicians require more training and educational support from health systems if they are to use new approaches to back pain care. Educational materials48 and workshops [49] can improve care quality. Key topics could include emphasizing the need for a history and physical examination in patients with low back pain and building skills in addressing patient concerns and requests for unnecessary care, such as imaging tests in the absence of clinical features of serious pathology. Decision-making shared with the patient can reduce unnecessary tests in other non-serious pain conditions, [50] although it remains unclear exactly how health systems can improve uptake of shared decision-making between clinicians and patient. [51]
A more immediate solution would be to borrow behavioural approaches that have shown promise in other areas of health care. Behavioural interventions, for example, can counteract cognitive biases and improve clinical decision-making. Something as simple as a letter to clinicians, noting their poor prescribing habits in comparison with their peers, can have a substantial impact. A recent randomized trial by the Australian health department involved sending peer-comparison letters to 6,649 high-prescribers of antibiotics. The outcome was a reduction in their prescriptions for inappropriate antibiotics from 109.3 to 95.8 scripts per 1000 consultations (12.3% reduction over 6 months). [52] A similar trial found sending peer comparison letters to 5,055 high-prescribers of antipsychotic drugs reduced prescriptions from 2,864 to 2,456 patient days on quetiapine per prescriber (adjusted difference, –319 days of 2864; 11.1% fewer days over 9 months). [53]
Another strategy to increase delivery of guideline-concordant care could be redesigning electronic health records. An observational study conducted in two emergency departments in Pennsylvania in the United States found that making 10 tablets the default option for prescriptions in the system was associated with a 22.8% increase in prescriptions for 10 tablets (from 20.6% to 43.3% of 3,264 prescriptions) and a 6.7% decrease in prescriptions for 20 tablets (from 22.8% to 16.1% of 3,264 prescriptions) over 4 weeks.54 Electronic health record systems are increasingly being used to collect clinical data, auto-populate risk prediction tools with relevant clinical and demographic data, and default to the most appropriate strategy for that person’s risk profile. While trials of such innovations are needed to determine their optimal design and assess their acceptability and usefulness, such approaches have the advantage that they could be implemented on a large scale and at relatively low cost.
Incentivize high-value care
Aligning funding models with best-practice care for low back pain could be difficult. As mentioned above, funding schedules already cover many non-evidence based items which remain popular with clinicians and patients and are difficult to remove funding from. [55] One example of success was the removal of the vertebroplasty procedure from the Australian Medicare funding schedule. A key factor in this achievement, however, was that vertebroplasty had only interim funding status that was contingent on trial results. When the trials did not show positive outcomes, funding for the therapy was denied, albeit with the controversy that comes with restricting or removing access. Funding is unlikely to shift from established care practices unless there is clear evidence for the superior safety, effectiveness and cost–effectiveness of the alternatives. Funding decisions are complex and rarely are such decisions influenced solely by evidence. Lobbying from vested interests, media coverage and communication around funding decisions, patient and clinician resistance to changes, as well as the current political climate, and the alignment of these factors with public opinion, also play an influential role (Fig. 1). The public needs to have a better understanding of the shortcomings of some types of established medical care. Many patients may already have such understanding (Box 2) and, in fact, patients could help drive changes to the system by lobbying for care that is evidence-based (see Local context, Fig. 1). In Oregon in the United States, patients, clinicians and policy-makers recently designed a new way to pay for appropriate care for low back pain. Oregon will be the first American state to reallocate Medicaid funds away from ineffective and potential harmful therapies for low back pain, such as long-term opioids, to evidence-based, non-medical treatments. [56]
Box 2: Examples of patient perspectives on management of chronic low back pain
Example 1: A patient with many years of chronic arthritis and back pain:“What I want and have always wanted is to stay positive, keep the pain at a comfortable level, and stay independent. Big things that have helped me were a good rapport with my boss so I could work some days from home and have my desk and seat adapted….joining in with groups for regular exercise to keep me mobile (aqua aerobics for me!)…and feeling valued so I am seen as ‘me the worker or the volunteer’ and not ‘me as the person with the pain’.
Medical treatments, including strong painkillers, have certainly helped at particular times of my life. But I really wish that, years ago, when all the pain began, there had been messages like the ones in the guidelines now. I wish there had been someone suggesting things to try for myself and to be positive about staying active and learning ways of getting on with life despite the pain. Doctors shouldn’t be nervous about suggesting people try things like heat packs and exercise first before reaching for tablets or injections – it could be a real ‘lightbulb’ moment for the patient.
But I know just how difficult this is when doctors are so busy. I was proud to be involved in a scheme for patients to help other patients with advice about simple things like public transport when they were anxious about even trying it. I have gained such a lot from doing things for myself, and I like the idea of recommending and funding more help and support for other patients with back pain to learn how to do the same – and shifting from care being all about drugs and injections.”
Example 2: A former nurse who has had back pain for many years:
“I started with back pain when I was 30 [years old] and it became so bad when I was nursing that I went from paracetamol to codeine to morphine patches. I had read about the patches and insisted my doctor prescribed them even though he was not too keen. The patches did help the pain, but they made me feel worse and I gave up after a few months. I had to give up nursing because I couldn’t move patients and that upset me. And then I decided to tackle the back pain myself.
A physiotherapist gave me some exercise sheets – 15 years later I still have them and use them. I lost weight, I stopped heavy lifting. The pain is there but I can cope with it. Not only did I get my pain under control, I felt so much better in myself. I now gauge my activity by what I feel my body can manage – [it’s] so much better than popping pills even though I still take the occasional painkiller to help when the back pain is bad. I get help from talking with other people. That’s what people need when they get back pain – someone who has time to listen, understands the pain, and helps them to find ways to stay active and engaged by way of exercise and work, rather than just giving a prescription for painkillers.”
Source: The two patients are members of the patient and public involvement panel at Keele University’s Institute of Primary and Health Care Sciences, England. They provided these thoughts after reading a draft of the paper before submission.
The optimal way to pay clinicians who treat low back pain remains unclear. Modest financial incentives for providing guideline-concordant care are unlikely to change practice. [57] There is, however, potential for episode-of-care reimbursement or risk-adjusted capitation payment models to provide incentives for complying with guidelines. Simulation models using data from 969 medical practices in the United States found that replacing fee-for-service with fixed monthly capitation payments, to cover all costs associated with providing primary care, could encourage the delivery of more care outside of consultations without financial losses from the government health-care budget. [58]
Regulate vested interests
Governments will have a key role to play in supporting new approaches to managing low back pain. Some shifts have already begun. The Australian Government scheduled codeine products as prescription-only drugs in February 2018. This change is likely to reduce the overuse of these medicines, although the broader consequences of such policies remain unclear. In 2010, in the United States, after government reduced access to a formulation of OxyContin® that was easily abused, use of the drug dropped substantially (from 35.6% to 12.8% of 2566 patients), but many patients who abused both formulations (66 of 100) simply switched to using heroin. [59] Any attempt to restrict public access to opioids should therefore be accompanied by adequate access to addiction services, social programmes and evidence-based non-pharmacological alternatives, as well as programmes to accurately monitor use of opioids.
Changes to governance arrangements will have to occur not just in health systems, but also in the complex framework in which health systems operate. Encouraging a shift away from unnecessary medical care requires support from governments, workplaces, legislative systems, consumers and professional bodies (Fig. 1).
Conclusion
Delivery of guideline-concordant care for low back pain requires system-wide changes. Strong governance at each level of the health system will be key to redefining how society views and manages low back pain. Health systems should prioritize policies that: empower clinicians and consumers to make well-informed choices; encourage clinicians to deliver the right care to those who need it most; provide financial support to evidence-based non-pharmacological treatment; and regulate the influence of those with vested interests in the current situation. Small adjustments to health policy will not work in isolation. Workplace systems, legal frameworks, personal beliefs, politics and the overall societal context in which we experience health, will also need to change. Addressing system-level barriers to guideline-based care could be cost-neutral; every year health systems waste billions of dollars on unnecessary tests and treatments for low back pain. Although disinvestment is difficult, redistributing funds to support guideline-concordant care is a promising way forward. Because current approaches to treatment often lack formal evidence, we strongly encourage careful evaluation of any new approach to funding or service delivery.
Acknowledgements
RB is also affiliated with Cabrini Institute, Malvern, Australia.
Competing interests:
Between November 2017 and February 2018 AT provided paid consultancy to a health insurance company regarding evidence-based models of physiotherapy care.
References:
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al.;
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators.
Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases
and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global
Burden of Disease Study 2017.
Lancet. 2018. November 10;392(10159):1789–858. 10.1016/S0140-6736(18)32279-7Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J et al.
What Low Back Pain Is and Why We Need to Pay Attention
Lancet. 2018 (Jun 9); 391 (10137): 2356–2367
This is the second of 4 articles in the remarkable Lancet Series on Low Back PainFoster NE, Anema JR, Cherkin D, Chou R, Cohen SP, et al.
Prevention and Treatment of Low Back Pain:
Evidence, Challenges, and Promising Directions
Lancet. 2018 (Jun 9); 391 (10137): 2368–2383
This is the third of 4 articles in the remarkable Lancet Series on Low Back PainBuchbinder R, van Tulder M, Öberg B, Costa LM, Woolf A, at al.
Low Back Pain: A Call For Action
Lancet. 2018 (Jun 9); 391 (10137): 2384–2388
This is the fourth of 4 articles in the remarkable Lancet Series on Low Back PainRunciman WB, Hunt TD, Hannaford NA, Hibbert PD, Westbrook JI, Coiera EW, et al.
CareTrack: assessing the appropriateness of health care delivery in Australia.
Med J Aust. 2012. July 16;197(2):100–5. 10.5694/mja12.10510McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al.
The quality of health care delivered to adults in the United States.
N Engl J Med. 2003. June 26;348(26):2635–45. 10.1056/NEJMsa022615Downie A, Hancock M, Jenkins H, Buchbinder R, Harris I, Underwood M, et al.
How common is imaging for low back pain in primary and emergency care?
Systematic review and meta-analysis of over 4 million imaging requests across 21 years.
Br J Sports Med. 2019. February 13;bjsports-2018-100087. 10.1136/bjsports-2018-100087Harris IA, Traeger A, Stanford R, Maher CG, Buchbinder R.
Lumbar spine fusion: what is the evidence?
Intern Med J. 2018. December;48(12):1430–4. 10.1111/imj.14120Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS.
Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases
in the United States, 2004 to 2015.
Spine. 2019. March 1;44(5):369–76. 10.1097/BRS.0000000000002822Volkow ND, McLellan AT.
Opioid abuse in chronic pain – misconceptions and mitigation strategies.
N Engl J Med. 2016. March 31;374(13):1253–63. 10.1056/NEJMra1507771National Institute for Health and Care Excellence (NICE):
Low Back Pain and Sciatica in Over 16s: Assessment and Management (PDF)
NICE Guideline, No. 59 2016 (Nov): 1–1067Van Wambeke P, Desomer A, Ailliet L et al.
Low Back Pain And Radicular Pain: Assessment And Management
Belgian Health Care Knowledge Centre, (2017).Chenot JF, Greitemann B, Kladny B, Petzke F, Pfingsten M, Schorr SG.
Non-specific low back pain.
Dtsch Arztebl Int. 2017. December 25;114(51-52):883–90NSW Agency for Clinical Innovation.
Management of People with Acute Low Back Pain
Agency for Clinical Innovation 2016 Chatswood; NSW Health (39 pages)Qaseem A, Wilt TJ, McLean RM, Forciea MA;
Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain:
A Clinical Practice Guideline From the American College of Physicians
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 514–530Stochkendahl MJ, Kjaer P, Hartvigsen J et al.
National Clinical Guidelines for Non-surgical Treatment of Patients with
Recent Onset Low Back Pain or Lumbar Radiculopathy
European Spine Journal 2018 (Jan); 27 (1): 60–75Chenot JF, Scherer M, Becker A, Donner-Banzhoff N, Baum E, Leonhardt C, et al.
Acceptance and perceived barriers of implementing a guideline for managing low back in general practice.
Implement Sci. 2008. February 7;3(1):7. 10.1186/1748-5908-3-7Bishop FL, Dima AL, Ngui J, Little P, Moss-Morris R, Foster NE, et al.
“Lovely pie in the sky plans”: a qualitative study of clinicians’ perspectives on guidelines for
managing low back pain in primary care in England.
Spine. 2015. December;40(23):1842–50. 10.1097/BRS.0000000000001215Briggs AM, Slater H, Bunzli S, Jordan JE, Davies SJ, Smith AJ, et al.
Consumers’ experiences of back pain in rural Western Australia:
access to information and services, and self-management behaviours.
BMC Health Serv Res. 2012. October 11;12(1):357. 10.1186/1472-6963-12-357Lamb SE, Hansen Z, Lall R, Castelnuovo E, Withers EJ, Nichols V, et al.;
Back Skills Training Trial investigators. Group cognitive behavioural treatment for low-back pain
in primary care: a randomised controlled trial and cost-effectiveness analysis.
Lancet. 2010. March 13;375(9718):916–23. 10.1016/S0140-6736(09)62164-4Cherkin DC, Sherman KJ, Balderson BH, Cook AJ, Anderson ML, Hawkes RJ, et al.
Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on
back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial.
JAMA. 2016. March 22-29;315(12):1240–9. 10.1001/jama.2016.2323Sherman KJ, Cherkin DC, Erro J, Miglioretti DL, Deyo RA.
Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial.
Ann Intern Med. 2005. December 20;143(12):849–56. 10.7326/0003-4819-143-12-200512200-00003Requested PBS & RPBS items processed from July 2016 to June 2017.
Medicare Australia Statistics [internet]. Canberra: Australian Government Department
of Human Services; 2019. Available from:
http://medicarestatistics.humanservices.gov.au/statistics/do.jsp?_PROGRAM=%2Fstatistics%2Fpbs_item_
standard_report&itemlst=%2702335X%27&ITEMCNT=1&LIST=2335X&VAR=BENEFIT&RPT_FMT=1&
start_dt=201607&end_dt=201706
[cited 2019 Feb 15].Slade SC, Kent P, Patel S, Bucknall T, Buchbinder R.
Barriers to primary care clinician adherence to clinical guidelines for the management of low back pain:
a systematic review and metasynthesis of qualitative studies.
Clin J Pain. 2016. September;32(9):800–16. 10.1097/AJP.0000000000000324Irving G, Neves AL, Dambha-Miller H, Oishi A, Tagashira H, Verho A, et al.
International variations in primary care physician consultation time:
a systematic review of 67 countries.
BMJ Open. 2017. November 8;7(10):e017902. 10.1136/bmjopen-2017-017902Ramanathan SA, Hibbert PD, Maher CG, Day RO, Hindmarsh DM, Hooper TD, et al.
CareTrack: toward appropriate care for low back pain.
Spine. 2017. July 1;42(13):E802–9. 10.1097/BRS.0000000000001972Sharma S, Traeger AC, Mishra SR, Sharma S, Maher CG.
Delivering the right care to people with low back pain in low- and middle-income countries:
the case of Nepal.
J Glob Health. 2019. June;9(1):010304. 10.7189/jogh.09.010304Castel LD, Freburger JK, Holmes GM, Scheinman RP, Jackman AM, Carey TS.
Spine and pain clinics serving North Carolina patients with back and neck pain:
what do they do, and are they multidisciplinary?
Spine. 2009. March 15;34(6):615–22. 10.1097/BRS.0b013e31817b8fa2Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJ, Ostelo RW, Guzman J, et al.
Multidisciplinary biopsychosocial rehabilitation for chronic low back pain:
Cochrane systematic review and meta-analysis.
BMJ. 2015. February 18;350 feb18 5:h444. 10.1136/bmj.h444Prescription strong (Schedule 8) opioid use and misuse in Australia –
options for a regulatory response. Consultation paper.
Canberra: Australian Government Department of Health; 2018. Available from:
https://www.tga.gov.au/consultation/consultation-prescription-strong-schedule-8-opioid-use
-and-misuse-australia-options-regulatory-response
[cited 2019 Feb 15].Gareth I. Opioid prescriptions rise in England despite poor efficacy and harms, finds study.
BMJ. 2018;360:k706.Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ.
Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain:
a systematic review and meta-analysis.
JAMA Intern Med. 2016. July 1;176(7):958–68. 10.1001/jamainternmed.2016.1251Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, et al.
Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain
or hip or knee osteoarthritis pain: the SPACE randomized clinical trial.
JAMA. 2018. March 6;319(9):872–82. 10.1001/jama.2018.0899Dowell D, Haegerich TM, Chou R.
CDC Guideline for Prescribing Opioids for Chronic Pain: United States, 2016
Morbidity and Mortality Weekly Report
Recommendations and Reports Vol. 65 No. 1 March 18, 2016Haffajee RL, Mello MM.
Drug companies’ liability for the opioid epidemic.
N Engl J Med. 2017. December 14;377(24):2301–5. 10.1056/NEJMp1710756Gosden T, Forland F, Kristiansen IS, Sutton M, Leese B, Giuffrida A, et al.
Capitation, salary, fee-for-service and mixed systems of payment: effects on the behaviour of
primary care physicians.
Cochrane Database Syst Rev. 2000; (3):CD002215.Helping patients make informed decisions: communicating risks and benefits [internet].
Sydney: Australian Commission on Safety and Quality in Health Care; 2018. Available from:
http://contenttest.learningseat.com/safetyandquality/index.html
[cited 2019 Feb 15].National Low Back and Radicular Pain Pathway 2017
London: National Health Service (NHS) of England; (2017)Jenkins HJ, Hancock MJ, French SD, Maher CG, Engel RM, Magnussen JS.
Effectiveness of interventions designed to reduce the use of imaging for low-back pain:
a systematic review.
CMAJ. 2015. April 7;187(6):401–8. 10.1503/cmaj.141183Fritz JM, Kim J, Dorius J.
Importance of the Type of Provider Seen to Begin Health Care
for a New Episode Low Back Pain: Associations
with Future Utilization and Costs
J Eval Clin Pract. 2016 (Apr); 22 (2): 247–252Martínez-González NA, Djalali S, Tandjung R, Huber-Geismann F, Markun S, Wensing M, et al.
Substitution of physicians by nurses in primary care: a systematic review and meta-analysis.
BMC Health Serv Res. 2014. May 12;14(1):214. 10.1186/1472-6963-14-214Buchbinder R, Jolley D.
Population based intervention to change back pain beliefs: three year follow up population survey.
BMJ. 2004. February 7;328(7435):321. 10.1136/bmj.328.7435.321Nsangi A, Semakula D, Oxman AD, Austvoll-Dahlgren A, Oxman M, Rosenbaum S, et al.
Effects of the Informed Health Choices primary school intervention on the ability of children in Uganda
to assess the reliability of claims about treatment effects: a cluster-randomised controlled trial.
Lancet. 2017. July 22;390(10092):374–88. 10.1016/S0140-6736(17)31226-6Dear BF, Titov N, Perry KN, Johnston L, Wootton BM, Terides MD, et al.
The Pain Course: a randomised controlled trial of a clinician-guided Internet-delivered cognitive behaviour
therapy programme for managing chronic pain and emotional well-being.
Pain. 2013. June;154(6):942–50. 10.1016/j.pain.2013.03.005Mechanic RE.
Mandatory Medicare bundled payment – is it ready for prime time?
N Engl J Med. 2015. October;373(14):1291–3. 10.1056/NEJMp1509155MacKean G, Noseworthy T, Elshaug AG, Leggett L, Littlejohns P, Berezanski J, et al.
Health technology reassessment: the art of the possible.
Int J Technol Assess Health Care. 2013. October;29(4):418–23. 10.1017/S0266462313000494Jenkins HJ, Hancock MJ, Maher CG, French SD, Magnussen JS.
Understanding patient beliefs regarding the use of imaging in the management of low back pain.
Eur J Pain. 2016. April;20(4):573–80. 10.1002/ejp.764Giguère A, Légaré F, Grimshaw J, Turcotte S, Fiander M, Grudniewicz A, et al.
Printed educational materials: effects on professional practice and healthcare outcomes.
Cochrane Database Syst Rev. 2012. October 17;10:CD004398.Forsetlund L, Bjørndal A, Rashidian A, Jamtvedt G, O’Brien MA, Wolf F, et al.
Continuing education meetings and workshops: effects on professional practice and health care outcomes.
Cochrane Database Syst Rev. 2009. April 15; (2):CD003030.Hess EP, Hollander JE, Schaffer JT, Kline JA, Torres CA, Diercks DB, et al.
Shared decision making in patients with low risk chest pain: prospective randomized pragmatic trial.
BMJ. 2016. December 5;355:i6165. 10.1136/bmj.i6165Légaré F, Adekpedjou R, Stacey D, Turcotte S, Kryworuchko J, Graham ID, et al.
Interventions for increasing the use of shared decision making by healthcare professionals.
Cochrane Database Syst Rev. 2018. July 19;7:CD006732Nudge vs superbugs:
a behavioural economics trial to reduce the overprescribing of antibiotics.
Canberra: Australian Government Department of Health; 2018.Sacarny A, Barnett ML, Le J, Tetkoski F, Yokum D, Agrawal S.
Effect of peer comparison letters for high-volume primary care prescribers of quetiapine in older
and disabled adults: a randomized clinical trial.
JAMA Psychiatry. 2018. October 1;75(10):1003–11. 10.1001/jamapsychiatry.2018.1867Delgado MK, Shofer FS, Patel MS, Halpern S, Edwards C, Meisel ZF, et al.
Association between electronic medical record implementation of default opioid prescription quantities
and prescribing behavior in two emergency departments.
J Gen Intern Med. 2018. April;33(4):409–11. 10.1007/s11606-017-4286-5Wulff KC, Miller FG, Pearson SD.
Can coverage be rescinded when negative trial results threaten a popular procedure?
The ongoing saga of vertebroplasty.
Health Aff (Millwood). 2011. December;30(12):2269–76. 10.1377/hlthaff.2011.0159DeBar L.
A naturalistic experiment evaluating the impact of Medicaid treatment reimbursement changes on opioid
prescribing and patient outcomes among patients with low back pain.
Washington, DC: Patient-Centered Outcomes Research Institute; 2017. Available from:
https://www.pcori.org/research-results/2017/naturalistic-experiment-evaluating-impact-medicaid-
treatment-reimbursement
[cited 2019 Feb 15].Lavergne MR.
Financial incentives for physicians to improve health care.
CMAJ. 2017. December 11;189(49):E1505–6. 10.1503/cmaj.171126Basu S, Phillips RS, Song Z, Bitton A, Landon BE.
High levels of capitation payments needed to shift primary care toward proactive team and nonvisit care.
Health Aff (Millwood). 2017. September 1;36(9):1599–605. 10.1377/hlthaff.2017.0367Cicero TJ, Ellis MS, Surratt HL.
Effect of abuse-deterrent formulation of OxyContin.
N Engl J Med. 2012. July 12;367(2):187–9. 10.1056/NEJMc1204141
Return to MEDICARE
Return to LOW BACK PAIN
Return to INITIAL PROVIDER/FIRST CONTACT
Since 3-30-2020


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |
