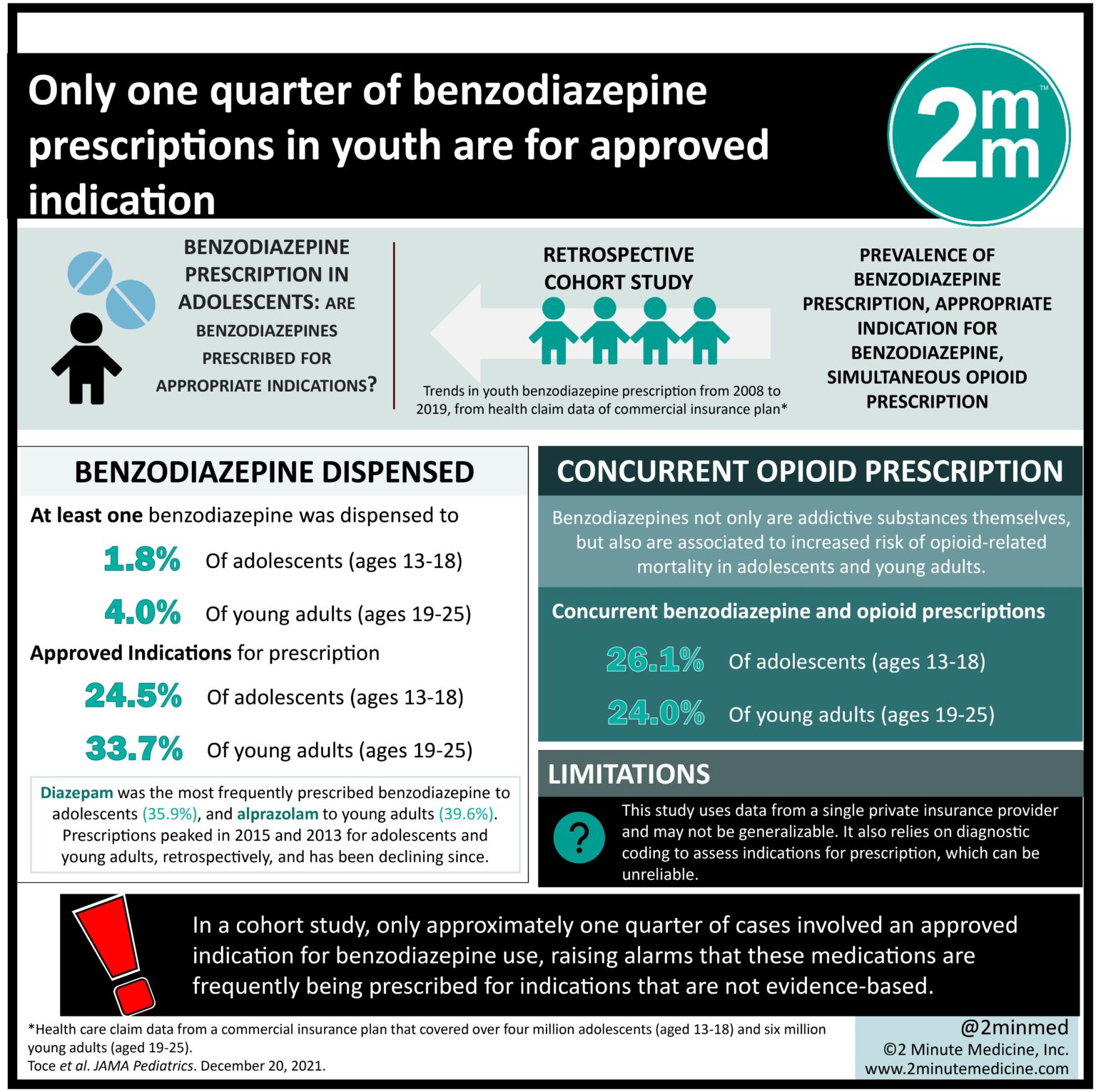
Association Between Chiropractic Spinal Manipulative Therapy
and Benzodiazepine Prescription in Patients with Radicular
Low Back Pain: A Retrospective Cohort Study Using
Real-world Data From the USAThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: BMJ Open 2022 (Jun 13); 12 (6): e058769 ~ FULL TEXT
OPEN ACCESS Robert James Trager, Zachary A Cupler, Kayla J DeLano, Jaime A Perez, Jeffery A Dusek
Connor Whole Health, University Hospitals Cleveland Medical Center,
Cleveland, Ohio, USAObjectives: Although chiropractic spinal manipulative therapy (CSMT) and prescription benzodiazepines are common treatments for radicular low back pain (rLBP), no research has examined the relationship between these interventions. We hypothesise that utilisation of CSMT for newly diagnosed rLBP is associated with reduced odds of benzodiazepine prescription through 12 months' follow-up.
Design: Retrospective cohort study.
Setting: National, multicentre 73-million-patient electronic health records-based network (TriNetX) in the USA, queried on 30 July 2021, yielding data from 2003 to the date of query.
Participants: Adults aged 18-49 with an index diagnosis of rLBP were included. Serious aetiologies of low back pain, structural deformities, alternative neurological lesions and absolute benzodiazepine contraindications were excluded. Patients were assigned to cohorts according to CSMT receipt or absence. Propensity score matching was used to control for covariates that could influence the likelihood of benzodiazepine utilisation.
Outcome measures: The number, percentage and OR of patients receiving a benzodiazepine prescription over 3, 6 and 12 months' follow-up prematching and postmatching.
Results: After matching, there were 9206 patients (mean (SD) age, 37.6 (8.3) years, 54% male) per cohort. Odds of receiving a benzodiazepine prescription were significantly lower in the CSMT cohort over all follow-up windows prematching and postmatching (p<0.0001). After matching, the OR (95% CI) of benzodiazepine prescription at 3 months was 0.56 (0.50 to 0.64), at 6 months 0.61 (0.55 to 0.68) and 12 months 0.67 (0.62 to 0.74). Sensitivity analysis suggested a patient preference to avoid prescription medications did not explain the study findings.
Conclusions: These findings suggest that receiving chiropractic spinal manipulative therapy (CSMT) for newly diagnosed radicular low back pain (rLBP) is associated with reduced odds of receiving a benzodiazepine prescription during follow-up. These results provide real-world evidence of practice guideline-concordance among patients entering this care pathway. Benzodiazepine prescription for rLBP should be further examined in a randomised trial including patients receiving chiropractic or usual medical care, to reduce residual confounding.
Keywords: back pain; complementary medicine; musculoskeletal disorders; pain management; rehabilitation medicine; spine.
From the FULL TEXT Article:
Introduction
Benzodiazepines (BZDs) are a class of psychoactive medication increasingly prescribed for patients with low back pain (LBP), [1–3] and commonly used in patients with radicular LBP (rLBP), [4] a subcategory of back pain with nerve root involvement. [5] Insufficient evidence supporting the efficacy of BZDs for LBP [6] and the risk of serious adverse events [7] has led clinical practice guidelines (CPGs) to discourage their use for this condition. [8–11] Although chiropractors frequently use non-pharmacological treatments for patients with rLBP, [12] no research has examined the association between chiropractic care and receipt of BZDs prescribed by other providers.
Previous research identified that patients receiving care with a chiropractor for incident LBP had reduced odds of receiving an opioid prescription compared with other provider types. [13–15] Like BZDs, opioids are prescribed for rLBP [4] despite CPGs recommending their limited use. [8–11] Given these parallels, the phenomenon of reduced prescription in patients receiving chiropractic care may extend to BZDs. Considering the practice guidance to limit opioid and BZD prescribing for LBP, research to identify care pathways associated with decreased prescription of these medications could inform patients’ and clinicians’ choice of initial treatment strategy.
Although chiropractors cannot prescribe opioids or BZDs within their scope of practice, [16] prescription of these medications may reflect the quality of patient care. Prescription of BZDs has been proposed as a surrogate marker for greater pain severity [17] and could serve as an indicator of provider concordance with LBP CPGs. [8–11]
Limited research has examined the association between other allied health interventions (eg, acupuncture, physical therapy) and BZD prescription. In one study, military personnel receiving at least four acupuncture treatments for back pain or other conditions over 1 year had a 14% reduction of BZD utilisation during a 60-day follow-up window. [18] A pharmacist-led opioid stewardship programme was associated with reduced opioid and BZD coprescription. [19] One study observed an increase in BZD prescription among patients receiving a physical therapy evaluation alongside an emergency department visit for back or neck pain. [20]
BZDs are sedative-hypnotic medications that act as central nervous system (CNS) depressants, and have anticonvulsant, anxiolytic and muscle relaxant properties. [21] Their primary mechanism is to potentiate the effect of γ-aminobutyric acid, the main inhibitory CNS neurotransmitter. [21, 22]
Interest in prescribing BZDs for LBP developed during the second half of the 20th century, [23–25] with a significant increase in the early 21st Century. [1–3] The number of physician visits during which BZDs was prescribed for back pain and chronic pain in the USA more than tripled from 2003 to 2015. [1] From 2008 to 2015, 11.5% of opioid-naïve adults were prescribed a BZD over a 12-month window after index LBP diagnosis. [4] In a 2018 survey, 27.0% of LBP patients reported being recommended BZDs by a medical doctor in the previous 12 months. [26]
As adjuvant analgesics, [22, 27] BZDs have been used to treat LBP-related muscle spasms [23, 28] and neuropathic pain. [24, 28] Although early research suggested BZDs had a direct analgesic effect related to central or peripheral receptor-mediated interactions, [24, 29] there has not been conclusive evidence that BZDs produce an overall analgesic effect. [22] Accordingly, researchers have proposed that benefits related to pain management could result from BZDs alleviating pain-related anxiety and/or depression. [27]
Adverse effects of BZDs include sedation, addiction [7] and increased risk of suicide. [30] Dependence occurs in 20%–100% of those taking BZDs for at least 1 month. [22] There is an increased risk of fatal, accidental overdose with concurrent use of BZDs and opioids. [7] BZDs are also a risk factor for motor vehicle collisions, falls and associated injuries, which may be explained by BZD-related psychomotor, balance and cognitive impairment. [7]
Although BZDs are increasingly prescribed for LBP, there is no strong evidence supporting their use for this condition. Recent CPGs from the National Institute for Health and Care Excellence (2020), [10] Veterans Affairs and Department of Defense (2019), [8] Global Spine Care Initiative (2018) [9] and Belgian Health Care Knowledge Centre (2017) [11] recommended against prescribing BZDs for LBP while those of the American College of Physicians (2017) concluded there was insufficient evidence for their effectiveness in acute or subacute LBP. [6]
Chiropractors are portal-of-entry providers that treat a variety of musculoskeletal conditions, the most common of which is LBP. [31] In a 2019 survey, US chiropractors reported managing radiculopathy at least once per week, and being the first provider to diagnose radiculopathy in 74% of patients. [12] The most common treatment chiropractors employ is spinal manipulative therapy (SMT), also called chiropractic SMT (CSMT). Systematic reviews have found evidence supporting this treatment for acute, [32] chronic [33] and radicular LBP, [34] while documenting its safety. [32, 33]
SMTs include hands-on and instrumented-assisted therapies applied to the spine, excluding soft tissue treatments such as massage. [35] These include high-velocity, low-amplitude manipulation involving a thrust, [36] and low-force, non-thrust or mobilisation techniques. Although other practitioners including physical therapists and osteopaths may perform SMT, chiropractors administer the majority of this therapy in the USA. [35, 37]
SMT may alleviate rLBP through several mechanisms. Specifically, SMT may relax hypertonic (abnormally tight) muscles, [38, 39] or release adhesions surrounding the lumbar disc or facet joints, [38, 40] leading to improved range of motion in those with rLBP. [41] In general, SMT has a hypoalgesic (pain-reducing) effect which may depend on patient expectations. [42] Accordingly, it is possible that rLBP relief provided by CSMT may offer an alternative option for patients who could otherwise be prescribed BZDs.
Methods
Study design
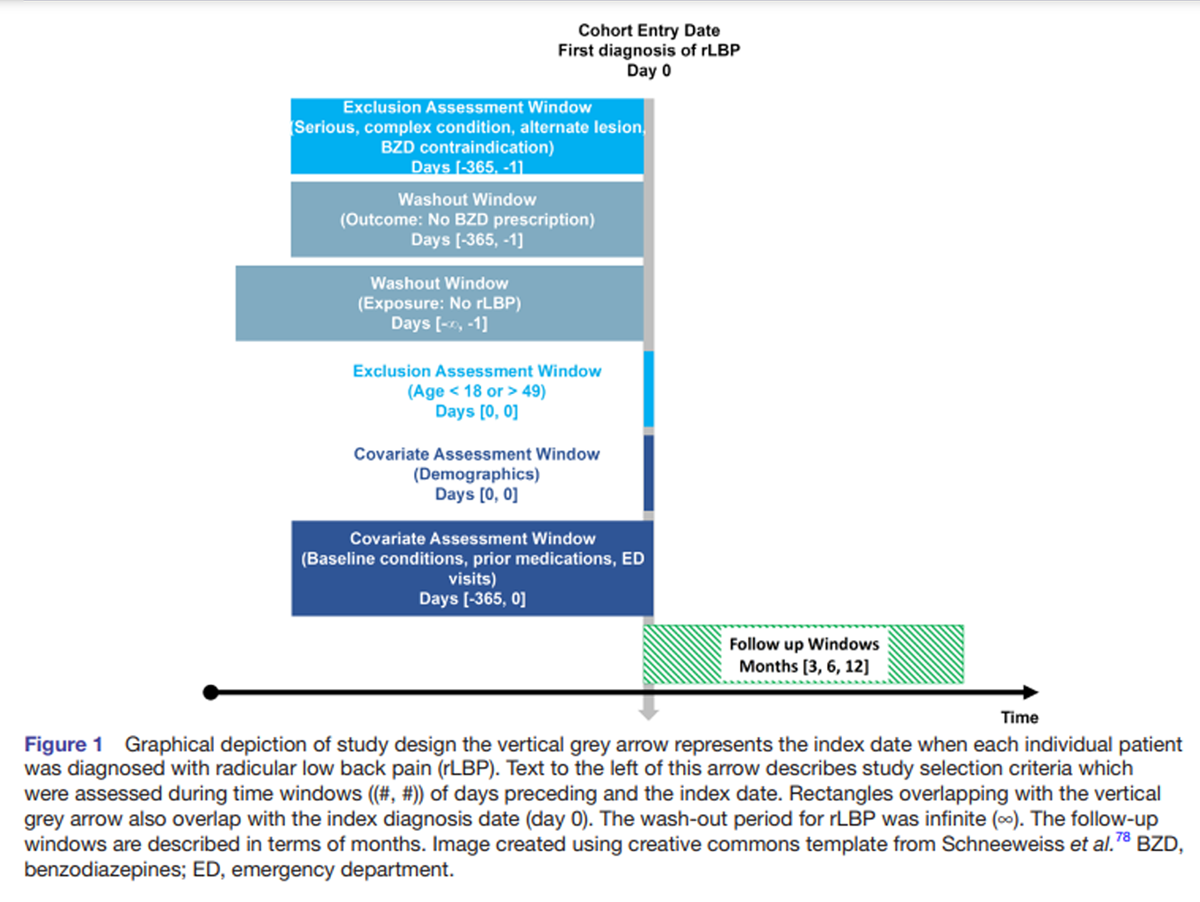
Figure 1 This study followed an a priori protocol, [43] which was modified to standardise the exclusion assessment window from days –365 to –1 (Figure 1). Having exclusion windows of different durations was not possible using TriNetX. This retrospective cohort study follows the Strengthening the Reporting of Observational Studies in Epidemiology guideline, [44] uses aggregated real-world data, and includes an active comparator, new-user design, which is recommended to reduce bias in real-world data studies. [45]
Setting and data source
This study used the TriNetX (TriNetX, Cambridge, Massachusetts, USA) national research network. [46] This network includes deidentified, aggregated electronic heath records data, from 73 million patients and 52 healthcare organisations (HCOs) at the time of sampling. Although geographical and institutional information of participating HCOs is anonymised, these are typically academically affiliated medical centres that provide outpatient, inpatient and specialty care. This data source includes patients’ treatments, problems list and diagnoses. [47]
TriNetX deidentifies data to safeguard protected health information by restricting the population to less than age 90, and rounding patient counts less than 10–10. Queries are possible using Current Procedural Terminology (CPT), Veterans Health Administration National Drug File (VANDF) and International Classification of Disease (ICD) codes. At University Hospitals of Cleveland, access to TriNetX is managed by the Clinical Research Centre. A TriNetX query on 30 July 2021 yielded data ranging from 2003 to 2021.
ParticipantsEligibility criteria
Inclusions
Adults aged 18–49 with incident rLBP were included, while serious pathology, structural deformity, prior surgery and alternate neurological lesions causing LBP were excluded. Radicular LBP is distinct from referred and axial forms of LBP, having a greater likelihood of pain radiating distal to the knee, neurologic deficits, neural tension and greater activity limitation. [48, 49] This study examined the LBP subcategory of rLBP to create uniformity between cohorts with regard to clinical features and odds of receiving a BZD prescription.
The infinite washout window for rLBP establishes new-users with a follow-up beginning at index rLBP diagnosis. The rLBP phenotype (see online supplemental table 1) included ICD-10 codes describing ‘lumbosacral radiculopathy’ or ‘nerve root disorder’ and ‘sciatica,’ a synonym for rLBP. [5]
The age range of 18–49 was chosen to narrow the population to patients with rLBP resulting from lumbar disc herniation (LDH) rather than lumbar spinal stenosis (LSS) thereby improving equipoise with respect to clinical presentation and treatment patterns. The most common cause of rLBP in patients less than 50 is LDH. [48, 50] Conversely, the prevalence of LSS increases around age 60. [51]
Lumbar intervertebral disc displacement or LDH codes were not used as inclusions, in preference for more clinically relevant rLBP codes. An LDH increases the odds of rLBP only by a small degree, as LDH can be asymptomatic, [52] or cause localised LBP, [53] which is treated differently than rLBP. [54, 55] Disc degeneration, disc bulging or spondylosis codes were not included, which do not necessarily cause radiculopathy. [53]
Patients were divided into two cohorts according to receipt of CSMT, resulting in CSMT and non-CSMT cohorts (see online supplemental table 2). This was done using the CPT codes 98940, 98941 and 98942. These codes are relatively specific to the chiropractic profession in the USA. [37] The non-CSMT cohort was considered an active-comparator as these patients were actively engaged in the healthcare system for evaluation and/or treatment of rLBP. [56]
Exclusions
Exclusions were assessed within 365 days preceding index diagnosis (see online supplemental table 3). Serious pathology causing LBP was excluded via ICD-10 codes used by previous studies for this reason: cauda equina syndrome, infection, fracture or malignancy. [13, 14, 57]
Previous lumbar surgery (postlaminectomy syndrome and arthrodesis status) was excluded which can represent a relative contraindication to CSMT, [58] while structural deformities (spondylolisthesis and scoliosis) were excluded which can increase the odds of lumbar surgery. [59]
Absolute contraindications to BZDs were excluded, which could reduce the odds of BZD prescription: closed-angle glaucoma, [21] chronic obstructive pulmonary disease, [21] myasthenia gravis, [21, 28] Parkinson’s disease, [21] porphyria21 and pregnant or breastfeeding women [21] (ICD-10: O00-O9A).
Stenosis of any spinal region (ICD-10: M48.0), together with LSS, were excluded. Cervical and thoracic stenosis could cause alternate neurological lesions, while the clinical features and management of LSS differs from rLBP related to LDH. [60] Patients with LSS are also older and have higher rates of comorbidities [61] and polypharmacy. [62] Lumbosacral plexopathy and myelopathy were excluded given these alternative neurological lesions are potentially more complex, prompting different care pathways.
Variables
Benzodiazepines
The VANDF code CN302 (benzodiazepine derivative sedatives/hypnotics) was used to identify BZD prescriptions.63 This category includes medications containing alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, lorazepam, midazolam, oxazepam, remimazolam, temazepam and triazolam.
Our 12-month BZD wash-out window adhered to a standard for defining BZD naïve patients, [64] while a 12-month follow-up window enabled comparison with previous data regarding BZD utilisation. [2, 4, 26] Additional 3-month and 6 month follow-up windows allowed examination of temporal trends in BZD prescription.
Potential confounders
Confounders present within 365 days preceding and including the index date of rLBP diagnosis were controlled for via propensity score matching (PSM) (see online supplemental table 4). These were specified a priori [43] based on evidence of an association with BZD utilisation (positive or negative):
Age, sex, race and ethnicity (positive or negative). [65, 66]
Tobacco use, [65, 66] and prescription of opioids or stimulants (positive). [66]
Prior emergency department visits (positive). [65]
Mental, behavioural and neurodevelopmental disorders including anxiety, depression [28, 67] and substance use disorders including alcohol related disorders (positive). [65, 66]
Diseases of the nervous system including movement disorders, [28] multiple sclerosis, [22] epilepsy, [28] new daily persistent headache, [22] insomnia, [21, 28] trigeminal neuralgia [28] and various pain syndromes (positive) [22, 68]; and sleep apnoea, in which BZDs are used with caution (negative). [21, 28]
Liver and renal disorders: BZDs used with caution (negative). [21]
Non-BZDs: Concurrent prescription with BZDs discouraged69 due to risk of side effects (negative). [69, 70]
Sample size
A total sample size of 357 was calculated using opioid utilisation data, given the lack of data regarding BZDs in CSMT recipients, and because opioids are also prescribed for rLBP. Calculations were made using G*Power (Universität Düsseldorf), using z-tests for logistic regression, α error of 0.05, power of 0.95, R2 of 0.9 and OR of 0.23 from a prior study, [14] assuming a normal distribution. The probability for the alternative hypothesis was 0.35, the incidence of opioid utilisation for LBP in a prior study. [4]
Statistical methods
Propensity scores for patients in each cohort were calculated using TriNetX via logistic regression. A greedy nearest-neighbour matching algorithm was used, [71] with a 1:1 ratio and calliper of 0.01 pooled SD. Baseline characteristics were compared using a Pearson χ2 test or independent-samples t-test.
Sensitivity analysis was conducted using the E-value, the minimum strength of association that unmeasured confounders would need to explain away an observational association, as measured using risk ratio (RR). [72] Variables associated with both the exposure (CSMT) and outcome (BZDs) may be considered for E-value analysis. [72] A literature search revealed that patients ‘against taking prescription drugs’ had an OR of 1.57 for self-referring to a chiropractor compared with a medical physician. [73] This was converted to an RR via square transformation, [74] to yield a value of 1.25. The current study E-values were determined by entering point estimates and confidence intervals (CIs) into an E-value calculator. [75]
Patient and public involvement
No patient or public involvement.
Results
Participants

Table 1 A large population was identified for each cohort (Table 1). Before PSM, there were 9,206 patients in the CSMT cohort and 491,187 patients in the non-SMT cohort. After PSM, there were 9,206 patients in each cohort (mean (SD) age, 37.6 (8.3) years, 54% male). Before PSM, the CSMT cohort had a higher percentage of white patients (74.7% vs 64.3%) and lower percentage of other races and individuals not Hispanic/Latino. Before matching, the CSMT cohort had a greater frequency, relative to the non-CSMT cohort, of ICD-10 categories ‘Diseases of the nervous system’ and ‘Mental, behavioural and neurodevelopmental disorders,’ as well as liver disease, prior emergency department visits, prescription of opioid analgesics, CNS stimulants and sedatives/hypnotics. Tobacco use was less frequent in the CSMT cohort. These variables were not significantly different post-PSM (p>0.05).
Descriptive data
Each cohort had a high number of average facts per patient (CSMT 2443 vs non-CSMT 905) suggesting a minimal effect of missing data. A visual diagnostic revealed that propensity scores were well matched (see online supplemental figure 1).
Key results

Table 2 The odds of receiving a BZD prescription were lower in the CSMT cohort over all follow-up windows, with statistical significance (p<0.0001) for each window (Table 2). After PSM, the OR (95% CI) was 0.56 (0.50 to 0.64) over 3 months, 0.61 (0.55 to 0.68) over 6 months and 0.67 (0.62 to 0.74) over 12 months.
Sensitivity analysis
Each of the post-PSM ORs yielded E-values (lower CI) of 2.94 (2.5) for the 3-month follow-up window, 2.66 (2.3) for 6 months and 1.64 (1.43) for 12 months. The RR for patients ‘against taking prescription drugs’ (1.25) was less than each of these E-values and lower CIs, indicating this unmeasured confounder did not account for the reduced ORs in the CSMT relative to the non-CSMT cohorts.
Discussion
To our knowledge, this is the first study to examine the association between CSMT and subsequent BZD prescription which was achieved through the use of a large, real-world database. Before PSM, recipients of CSMT had significantly reduced odds of BZD prescription for each 3-month, 6-month and 12-month follow-up window. After PSM, the magnitude of these associations was increased and maintained statistical significance. Sensitivity analysis demonstrated that a chiropractic patient preference to avoid ‘prescription drugs’ could not explain away these results.
The frequency of BZD prescription for rLBP occurring over 12 months in the non-CSMT cohort (13.3% post-PSM) is within the range of BZD utilisation in prior studies of LBP (11.5%–27.0%).2 4 26 A disproportionately large percentage of BZD prescriptions occurred during the initial 3-month window (CSMT 47.5%, non-CSMT 57.3%, post-PSM), suggesting a short-term window is needed when examining BZD prescription.
The identified post-PSM ORs had a diminishing magnitude from 3 to 12 months follow-up, indicating that the negative association between CSMT and BZD prescription is strongest in the short term. It is unclear when or if this association would diminish to the null, and a longer follow-up window would be needed to examine this question.
Reduced odds of receiving a BZD prescription among patients using CSMT for newly diagnosed rLBP is a marker of greater guideline-concordance in patients entering this treatment pathway. These results reinforce the use of CSMT as a first-line non-pharmacological option for adults with rLBP.
Although our results are consistent with prior studies identifying a reduced odds of opioid prescription in recipients of CSMT,15 the association between CSMT and prescription opioids or BZDs should be explored further with a randomised controlled trial. Retrospective studies could be conducted to replicate these findings in other LBP populations or settings, while prospective studies could reduce confounding.
Limitations
First, as an observational study, we cannot infer causality between CSMT and BZD prescriptions. In addition, prescription of BZDs is only one indicator of care and may not correspond with other markers such as patient-reported outcomes, pain severity, ability to work and avoidance of surgery.
Second, residual confounding could be present as variables associated with BZD utilisation, including education level,65 66 income,67 alcohol intake (not meeting criteria for alcohol related disorders),65 marital status, employment status and self-rated health65 were not available in TriNetX. These were also not applicable to E-value analysis given the lack of association with CSMT. Pain severity was likewise unavailable in the dataset for propensity matching, and thus could have differed between cohorts. It is possible that patients with milder pain could be more likely to trial treatments such as CSMT before moving on to BZDs. Contextual effects, such as patients’ expectations that CSMT, would have a beneficial effect could have affected our results, yet this variable was not possible to control for. Further, patient functional status and lumbar imaging findings were unavailable for propensity matching and these variables could have differed between cohorts.
Third, this study does not incorporate non-clinical variables related to the provider–patient interaction. Physician time constraints could result in pressure to prescribe BZDs.76 Conversely, chiropractors cannot yield to such pressure given they cannot prescribe BZDs.
Fourth, although we narrowed the study population and controlled for several confounders, BZDs could have been differentially prescribed for comorbid conditions. Although we controlled for substance use disorders, we were unable to directly examine illicit use of BZDs. The strength and duration of BZD prescriptions was unavailable, which would allow comparisons and risk stratification using enhanced levels of measurement such as diazepam equivalents.
Fifth, diagnoses of rLBP could be misclassified as new if patients were previously treated at a non-TriNetX facility or had incorrect data in their chart. This could not be prevented using ICD-10 codes, which lack acute/chronic designations for LBP. Misclassification could also occur if patients developed an excluded condition (eg, cauda equina syndrome) during follow-up, given exclusions were retrospective. Although we attempted to control for symptom duration with patients required to have newly diagnosed rLBP, it is possible that the mean interval between symptom onset and diagnosis varied between cohorts. We were unable to validate the rLBP phenotype accuracy as the study used multicentre, deidentified data.
Sixth, although our sensitivity analysis provides insight into whether the patients’ desire not to take prescription drugs was a factor, this question may not be specific enough. Some patients may hold attitudes and beliefs to not take potentially sedating medications such as BZDs, which may not hold true for other medication classes. Further, those patients may be more inclined to self-select to chiropractic treatment.
Finally, roughly 5.4%12 of US chiropractors are employed by HCOs in the TriNetX network. Chiropractic care pathways in these HCOs may differ from smaller private practices. Results could also be influenced by the overall care provided by chiropractors, rather than an isolated effect of CSMT. Chiropractors also educate patients, perform soft tissue therapies, exercise therapies and refer for other services when needed.12 77 Provision of such interventions could not be quantified or isolated using TriNetX. The percentage of patients receiving SMT from non-chiropractic providers (eg, osteopaths, physical therapists) could not be quantified, however, this would be a small contributor to overall SMT received.35
Conclusions
This study identified a significant reduction in odds of BZD prescription over 3-month, 6-month and 12-month follow-up windows in adults initiating care for rLBP with CSMT. These results suggest that CSMT, influences BZD utilisation, and patients entering this pathway for rLBP are more likely to receive guideline-concordant care with respect to BZD prescription. As these results derive from academically affiliated HCOs, results may not be generalisable to the broader healthcare landscape. These findings should be validated in other LBP populations and settings and examined using a randomised controlled trial.
Supplementary Data
Contributors
Concept and design: RJT, ZAC, JAD. Acquisition, analysis or interpretation of data: RJT, ZAC, KJD, JAP, JAD. Drafting of the manuscript: RJT, ZAC, JAD. Critical revision of the manuscript for important intellectual content: RJT, ZAC, KJD, JAP, JAD. Statistical analysis: JAP, JAD. Administrative, technical or material support: JAD, KJD. Supervision: JAD. Guarantor: RJT.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer
The views expressed in this article are those of the authors and do not reflect official policy or position of the Department of Veterans Affairs, or the United States Government.
Competing interests
RJT reports he has received book royalties as the author of two texts on the topic of sciatica. No other authors reported conflicts
Supplemental material
This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References:
Agarwal SD, Landon BE.
Patterns in outpatient benzodiazepine prescribing in the United States.
JAMA Netw Open 2019;2:e187399
doi:10.1001/jamanetworkopen.2018.7399
pmid:http://www.ncbi.nlm.nih.gov/pubmed/30681713Mullins PM, Merriman JG, Jaffe TA, et al.
Trends in the evaluation and management of back pain in emergency departments, United States, 2007-2016.
Pain Med 2021;22:67–74
doi:10.1093/pm/pnaa385
pmid:http://www.ncbi.nlm.nih.gov/pubmed/33338224Pourmand A, Lombardi KM, Roberson J, et al.
Patterns of benzodiazepine administration and prescribing to older adults in U.S. emergency departments.
Aging Clin Exp Res 2020;32:2621–8
doi:10.1007/s40520-020-01496-1
pmid:http://www.ncbi.nlm.nih.gov/pubmed/32056152Azad TD, Zhang Y, Stienen MN, et al.
Patterns of opioid and benzodiazepine use in Opioid-Naïve patients with newly diagnosed low back and lower extremity pain.
J Gen Intern Med 2020;35:291–7
doi:10.1007/s11606-019-05549-8
pmid:http://www.ncbi.nlm.nih.gov/pubmed/31720966Stynes S, Konstantinou K, Dunn KM.
Classification of patients with low back-related leg pain: a systematic review.
BMC Musculoskelet Disord 2016;17:226
doi:10.1186/s12891-016-1074-z
pmid:http://www.ncbi.nlm.nih.gov/pubmed/27215590Qaseem A, Wilt TJ, McLean RM, Forciea MA;
Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain:
A Clinical Practice Guideline From the American College of Physicians
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 514–530Brandt J, Leong C,
Benzodiazepines LC.
Benzodiazepines and Z-Drugs: an updated review of major adverse outcomes reported on in epidemiologic research.
Drugs R D 2017;17:493–507
doi:10.1007/s40268-017-0207-7
pmid:http://www.ncbi.nlm.nih.gov/pubmed/28865038The Diagnosis and Treatment of Low Back Pain Work Group.
VA/DoD Clinical Practice Guideline for Diagnosis and Treatment of Low Back Pain
Washington, DC: The Office of Quality, Safety and Value, VA, &
Office of Evidence Based Practice, U.S. Army Medical Command, 2017, Version 2.0.Chou R, Cote P, Randhawa K, Torres P, Yu H, Nordin M et al (2017)
The Global Spine Care Initiative: Applying Evidence-based Guidelines on the Non-invasive
Management of Back and Neck Pain to Low- and Middle-income Communities
European Spine Journal 2018 (Sep); 27 (Suppl 6): 851–860National Institute for Health and Care Excellence (NICE):
Low Back Pain and Sciatica in Over 16s: Assessment and Management
NICE Guideline, No. 59 2016 (Nov): 1–1067Van Wambeke P, Desomer A, Ailiet L.
Low back pain and radicular pain: assessment and management; 2017.Himelfarb I, Hyland J, Ouzts N, NBCE.
National board of chiropractic examiners:
Practice Analysis of Chiropractic, 2020Fritz JM, Kim J, Dorius J.
Importance of the Type of Provider Seen to Begin Health Care for a New Episode
Low Back Pain: Associations with Future Utilization and Costs
J Eval Clin Pract. 2016 (Apr); 22 (2): 247–252Kazis LE, Ameli O, Rothendler J, et al.
Observational Retrospective Study of the Association of Initial Healthcare Provider
for New-onset Low Back Pain with Early and Long-term Opioid Use
BMJ Open. 2019 (Sep 20); 9 (9): e028633Corcoran KL, Bastian LA, Gunderson CG, et al.
Association Between Chiropractic Use and Opioid Receipt Among
Patients with Spinal Pain: A Systematic Review and Meta-analysis
Pain Medicine 2020 (Feb 1); 21 (2): e139–e145Chang M.
The Chiropractic Scope of Practice in the United States:
A Cross-sectional Survey
J Manipulative Physiol Ther. 2014 (Jul); 37 (6): 363–376Park TW, Saitz R, Nelson KP, et al.
The association between benzodiazepine prescription and aberrant drug-related behaviors in primary care patients receiving opioids for chronic pain.
Subst Abus 2016;37:516–20
doi:10.1080/08897077.2016.1179242
pmid:http://www.ncbi.nlm.nih.gov/pubmed/27092738Crawford P, Penzien DB, Coeytaux R.
Reduction in pain medication prescriptions and self-reported outcomes associated with acupuncture in a military patient population.
Med Acupunct 2017;29:229–31
doi:10.1089/acu.2017.1234
pmid:http://www.ncbi.nlm.nih.gov/pubmed/28874924Tilli T, Hunchuck J, Dewhurst N, et al.
Opioid stewardship: implementing a proactive, pharmacist-led intervention for patients coprescribed opioids and benzodiazepines at an urban academic primary care centre.
BMJ Open Qual 2020;9:e000635
doi:10.1136/bmjoq-2019-000635
pmid:http://www.ncbi.nlm.nih.gov/pubmed/32269056Abstract/Kim HS, Kaplan SH, McCarthy DM, et al.
A comparison of analgesic prescribing among ED back and neck pain visits receiving physical therapy versus usual care.
Am J Emerg Med 2019;37:1322–6
doi:10.1016/j.ajem.2018.10.009
pmid:http://www.ncbi.nlm.nih.gov/pubmed/30528050Guina J, Merrill B.
Benzodiazepines I: upping the care on downers: the evidence of risks, benefits and alternatives.
J Clin Med 2018;7:17
doi:10.3390/jcm7020017
pmid:http://www.ncbi.nlm.nih.gov/pubmed/29385731Wright SL.
Limited utility for benzodiazepines in chronic pain management: a narrative review.
Adv Ther 2020;37:2604–19
doi:10.1007/s12325-020-01354-6
pmid:http://www.ncbi.nlm.nih.gov/pubmed/32378069Basmajian JV.
Cyclobenzaprine hydrochloride effect on skeletal muscle spasm in the lumbar region and neck: two double-blind controlled clinical and laboratory studies.
Arch Phys Med Rehabil 1978;59:58–63.
pmid:http://www.ncbi.nlm.nih.gov/pubmed/623512Bouckoms AJ, Litman RE.
Clonazepam in the treatment of neuralgic pain syndrome.
Psychosomatics 1985;26:933–6
doi:10.1016/S0033-3182(85)72758-2
pmid:http://www.ncbi.nlm.nih.gov/pubmed/4089130Wick JY.
The history of benzodiazepines.
Consult Pharm 2013;28:538–48
doi:10.4140/TCP.n.2013.538
pmid:http://www.ncbi.nlm.nih.gov/pubmed/24007886Goertz CM, Long CR, English C, et al.
Patient-reported physician treatment recommendations and compliance among U.S. adults with low back pain.
J Altern Complement Med 2021;27:S-99–S-105
doi:10.1089/acm.2020.0392
pmid:http://www.ncbi.nlm.nih.gov/pubmed/33788609Reddy S, Patt RB.
The benzodiazepines as adjuvant analgesics.
J Pain Symptom Manage 1994;9:510–4
doi:10.1016/0885-3924(94)90112-0
pmid:http://www.ncbi.nlm.nih.gov/pubmed/7531735Howard P, Twycross R, Shuster J, et al.
Benzodiazepines.
J Pain Symptom Manage 2014;47:955–64
doi:10.1016/j.jpainsymman.2014.03.001
pmid:http://www.ncbi.nlm.nih.gov/pubmed/24681184Munro G, Erichsen HK, Rae MG, et al.
A question of balance--positive versus negative allosteric modulation of GABA(A) receptor subtypes as a driver of analgesic efficacy in rat models of inflammatory and neuropathic pain.
Neuropharmacology 2011;61:121–32
doi:10.1016/j.neuropharm.2011.03.017
pmid:http://www.ncbi.nlm.nih.gov/pubmed/21439986Dodds TJ, Benzodiazepines P.
Prescribed benzodiazepines and suicide risk: a review of the literature.
Prim Care Companion CNS Disord 2017;19
doi:doi:10.4088/PCC.16r02037. [Epub ahead of print: 02 Mar 2017].
pmid:http://www.ncbi.nlm.nih.gov/pubmed/28257172Coulter, ID, Hurwitz, EL, Adams, AL, Genovese, BJ, Hays, R, and Shekelle, PG.
Patients Using Chiropractors in North America:
Who Are They, and Why Are They in Chiropractic Care?
SPINE (Phila Pa 1976) 2002; 27 (3) Feb 1: 291–298Paige NM, Miake-Lye IM, Booth MS, et al.
Association of Spinal Manipulative Therapy With Clinical Benefit and Harm
for Acute Low Back Pain: Systematic Review and Meta-analysis
JAMA. 2017 (Apr 11); 317 (14): 1451–1460Rubinstein SM, De Zoete A, Van Middelkoop M, Assendelft WJJ, De Boer MR, Van Tulder MW.
Benefits and Harms of Spinal Manipulative Therapy for the Treatment of
Chronic Low Back Pain: Systematic Review and Meta-analysis
of Randomised Controlled Trials
British Medical Journal 2019 (Mar 13); 364: l689Lewis RA, Williams NH, Sutton AJ, et al.
Comparative clinical effectiveness of management strategies for sciatica: systematic review and network meta-analyses.
Spine J 2015;15:1461–77
doi:10.1016/j.spinee.2013.08.049
pmid:http://www.ncbi.nlm.nih.gov/pubmed/24412033Hurwitz EL:
Epidemiology: Spinal Manipulation Utilization
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 648–654Dvorák J, Dvorák V, Gilliar W.
Definitions and Principles of Manual Medicine Diagnosis and Treatment.
In: Musculoskeletal manual medicine: diagnosis and treatment.
1st. Thieme, 2008: 4–5.Whedon JM, Haldeman S, Petersen CL, et al.
Temporal trends and geographic variations in the supply of clinicians who provide spinal manipulation to Medicare beneficiaries: a serial cross-sectional study.
J Manipulative Physiol Ther 2021;44:177–85
doi:10.1016/j.jmpt.2021.02.002
pmid:http://www.ncbi.nlm.nih.gov/pubmed/33849727Kirkaldy-Willis W, Cassidy J.
Spinal Manipulation in the Treatment of Low-back Pain
Canadian Family Physician 1985 (Mar); 31: 535–540Santilli V, Beghi E, Finucci S.
Chiropractic Manipulation in the Treatment of Acute Back Pain and Sciatica
with Disc Protrusion: A Randomized Double-blind Clinical Trial
of Active and Simulated Spinal Manipulations
Spine J. 2006 (Mar); 6 (2): 131—137Leemann S, Peterson CK, Schmid C, et al.
Outcomes of Acute and Chronic Patients With Magnetic Resonance
Imaging–Confirmed Symptomatic Lumbar Disc Herniations Receiving
High-Velocity, Low-Amplitude, Spinal Manipulative Therapy:
A Prospective Observational Cohort Study With One-Year Follow-Up
J Manipulative Physiol Ther 2014 (Mar); 37 (3): 155-163Stern PJ, Côté P, Cassidy JD.
A series of consecutive cases of low back pain with radiating leg pain treated by chiropractors.
J Manipulative Physiol Ther 1995;18:335–42
pmid:http://www.ncbi.nlm.nih.gov/pubmed/7595106Hinkeldey N, Okamoto C, Khan J.
Spinal manipulation and select manual therapies: current perspectives.
Phys Med Rehabil Clin N Am 2020;31:593–608
doi:10.1016/j.pmr.2020.07.007
pmid:http://www.ncbi.nlm.nih.gov/pubmed/32981581Trager RJ, Cupler ZA, DeLano KJ.
Association between chiropractic spinal manipulative therapy and benzodiazepine prescription in radicular low back pain: a retrospective cohort study protocol using national real-world data,
2021. osf.io/zyxgjVon Elm E, Altman DG, Egger M, et al.
The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies.
Ann Intern Med 2007;147:573–7
doi:10.7326/0003-4819-147-8-200710160-00010
pmid:http://www.ncbi.nlm.nih.gov/pubmed/17938396Gokhale M, Stürmer T, Buse JB.
Real-World evidence: the devil is in the detail.
Diabetologia 2020;63:1694–705
doi:10.1007/s00125-020-05217-1
pmid:http://www.ncbi.nlm.nih.gov/pubmed/32666226Topaloglu U, Palchuk MB.
Using a federated network of real-world data to optimize clinical trials operations.
JCO Clin Cancer Inform 2018;2:1–10
doi:10.1200/CCI.17.00067
pmid:http://www.ncbi.nlm.nih.gov/pubmed/30652541CrossRefStapff M, Stacey J.
How can real-world data support clinical trials and medical research?
Clin Res 2019;33.Konstantinou K, Dunn KM, Ogollah R, Vogel S, Hay
Characteristics of Patients with Low Back and Leg Pain
Seeking Treatment in Primary Care: Baseline Results
from the ATLAS Cohort Study
BMC Musculoskelet Disord. 2015 (Nov 4); 16: 332Kongsted A, Kent P, Albert H, Jensen TS, Manniche C.
Patients with Low Back Pain Differ From Those Who Also Have
Leg Pain or Signs of Nerve Root Involvement - A Cross-sectional Study
BMC Musculoskelet Disord. 2012 (Nov 28); 13: 236Hernández CP, Sánchez N, Navarro-Siguero A.
What are the Causes of Sciatica and Radicular Pain?
In: Laroche F, Perrot S, eds. Managing Sciatica and Radicular Pain in Primary Care Practice.
Springer Healthcare Ltd, 2013: 17–31.Miyakoshi N, Hongo M, Kasukawa Y, et al.
Prevalence, spinal alignment, and mobility of lumbar spinal stenosis with or without chronic low back pain: a community-dwelling study.
Pain Res Treat 2011;2011:1–5.
doi:10.1155/2011/340629
pmid:http://www.ncbi.nlm.nih.gov/pubmed/22110922Brinjikji W, Diehn FE, Jarvik JG, et al.
Mri findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis.
AJNR Am J Neuroradiol 2015;36:2394–9
doi:10.3174/ajnr.A4498
pmid:http://www.ncbi.nlm.nih.gov/pubmed/26359154Abstract/Suri P, Boyko EJ, Goldberg J, et al.
Longitudinal associations between incident lumbar spine MRI findings and chronic low back pain or radicular symptoms: retrospective analysis of data from the longitudinal assessment of imaging and disability of the back (LAIDBACK).
BMC Musculoskelet Disord 2014;15:152.
doi:10.1186/1471-2474-15-152
pmid:http://www.ncbi.nlm.nih.gov/pubmed/24886265CrossRefChou R, Deyo R, Friedly J et al.
Systemic Pharmacologic Therapies for Low Back Pain: A Systematic Review for an
American College of Physicians Clinical Practice Guideline
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 480–492Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, et al.
Nonpharmacologic Therapies for Low Back Pain: A Systematic Review
for an American College of Physicians Clinical Practice Guideline
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 493–505Franklin JM, Schneeweiss S.
When and how can real world data analyses substitute for randomized controlled trials?
Clin Pharmacol Ther 2017;102:924–33.
doi:10.1002/cpt.857
pmid:http://www.ncbi.nlm.nih.gov/pubmed/28836267Cherkin DC, Deyo RA, Volinn E, et al.
Use of the International classification of diseases (ICD-9-CM) to identify hospitalizations for mechanical low back problems in administrative databases.
Spine 1992;17:817–25.
doi:10.1097/00007632-199207000-00015
pmid:http://www.ncbi.nlm.nih.gov/pubmed/1386943Daniels CJ, Gliedt JA, Suri P, et al.
Management of patients with prior lumbar fusion: a cross-sectional survey of Veterans Affairs chiropractors’ attitudes, beliefs, and practices.
Chiropr Man Therap 2020;28:1–10.
doi:10.1186/s12998-020-00322-9Fukushima M, Oka H, Hara N, et al.
Prognostic factors associated with the surgical indication for lumbar spinal stenosis patients less responsive to conservative treatments: an investigator-initiated observational cohort study.
J Orthop Sci 2017;22:411–4.
doi:10.1016/j.jos.2017.01.021
pmid:http://www.ncbi.nlm.nih.gov/pubmed/28228325Tarulli AW, Raynor EM.
Lumbosacral radiculopathy.
Neurol Clin 2007;25:387–405.
doi:10.1016/j.ncl.2007.01.008
pmid:http://www.ncbi.nlm.nih.gov/pubmed/17445735Battié MC, Jones CA, Schopflocher DP, et al.
Health-related quality of life and comorbidities associated with lumbar spinal stenosis.
Spine J 2012;12:189–95.
doi:10.1016/j.spinee.2011.11.009
pmid:http://www.ncbi.nlm.nih.gov/pubmed/22193054Steinman MA, Miao Y, Boscardin WJ, et al.
Prescribing quality in older veterans: a multifocal approach.
J Gen Intern Med 2014;29:1379–86.
doi:10.1007/s11606-014-2924-8
pmid:http://www.ncbi.nlm.nih.gov/pubmed/25002159NCBO BioPortal.
Veterans health administration national drug file BENZODIAZEPINE DERIVATIVE SEDATIVES/HYPNOTICS - Classes. Available:
https://bioportal.bioontology.org/ontologies/VANDF?
p=classes&conceptid=http%3A%2F%2Fpurl.bioontology.org%
2Fontology%2FVANDF%2F4021591
[Accessed 31 May 2021].Halme AS, Beland SG, Preville M.
Uncovering the source of new benzodiazepine prescriptions in community-dwelling older adults’: Source of benzodiazepines in the elderly.
Int J Geriatr Psychiatry 2013;28:248–55.
doi:10.1002/gps.3818Blanco C, Han B, Jones CM, et al.
Prevalence and correlates of benzodiazepine use, misuse, and use disorders among adults in the United States.
J Clin Psychiatry 2018;79.
doi:doi:10.4088/JCP.18m12174. [Epub ahead of print: 16 10 2018].
pmid:http://www.ncbi.nlm.nih.gov/pubmed/30403446Maust DT, Lin LA, Blow FC.
Benzodiazepine use and misuse among adults in the United States.
Psychiatr Serv 2019;70:97–106.
doi:10.1176/appi.ps.201800321
pmid:http://www.ncbi.nlm.nih.gov/pubmed/30554562CrossRefYang H-wenK, Simoni-Wastila L, Zuckerman IH, et al.
Benzodiazepine use and expenditures for medicare beneficiaries and the implications of medicare part D exclusions.
Psychiatr Serv 2008;59:384–91.
doi:10.1176/ps.2008.59.4.384
pmid:http://www.ncbi.nlm.nih.gov/pubmed/18378837CrossRefCunningham JL, Craner JR, Evans MM, et al.
Benzodiazepine use in patients with chronic pain in an interdisciplinary pain rehabilitation program.
J Pain Res 2017;10:311–7.
doi:10.2147/JPR.S123487
pmid:http://www.ncbi.nlm.nih.gov/pubmed/28223841CrossRefSparks A, Cohen A.
Benzodiazepine and Z-Drug safety guideline, 2014. Available:
https://wa.kaiserpermanente.org/static/pdf/public/guidelines/benzo-zdrug.pdf
[Accessed 18 Jun 2021].Yu N-W, Chen P-J, Tsai H-J, et al.
Association of benzodiazepine and Z-drug use with the risk of hospitalisation for fall-related injuries among older people: a nationwide nested case-control study in Taiwan.
BMC Geriatr 2017;17:140.
doi:10.1186/s12877-017-0530-4
pmid:http://www.ncbi.nlm.nih.gov/pubmed/28693443Li Q, Lin J, Chi A, et al.
Practical considerations of utilizing propensity score methods in clinical development using real-world and historical data.
Contemp Clin Trials 2020;97:106123.
doi:10.1016/j.cct.2020.106123
pmid:http://www.ncbi.nlm.nih.gov/pubmed/32853779Haneuse S, VanderWeele TJ, Arterburn D.
Using the E-value to assess the potential effect of unmeasured confounding in observational studies.
JAMA 2019;321:602–3.
doi:10.1001/jama.2018.21554
pmid:http://www.ncbi.nlm.nih.gov/pubmed/30676631CrossRefSharma R, Haas M, Stano M.
Patient Attitudes, Insurance, and Other Determinants of
Self-referral to Medical and Chiropractic Physicians
American Journal of Public Health 2003 (Dec); 93 (12): 2111–2117VanderWeele TJ.
On a square-root transformation of the odds ratio for a common outcome.
Epidemiology 2017;28:e58–60.
doi:10.1097/EDE.0000000000000733
pmid:http://www.ncbi.nlm.nih.gov/pubmed/28816709Mathur MB, Ding P, Riddell CA, et al.
Web site and R package for computing E-values.
Epidemiology 2018;29:e45–7.
doi:10.1097/EDE.0000000000000864Sirdifield C, Anthierens S, Creupelandt H, et al.
General practitioners' experiences and perceptions of benzodiazepine prescribing: systematic review and meta-synthesis.
BMC Fam Pract 2013;14:191.
doi:10.1186/1471-2296-14-191
pmid:http://www.ncbi.nlm.nih.gov/pubmed/24330388Bronston, LJ, LE, Austin-McClellan, Lisi, AJ, Donovan, KC, and Engle, WW.
A Survey of American Chiropractic Association Members' Experiences, Attitudes,
and Perceptions of Practice in Integrated Health Care Settings
J Chiropractic Medicine 2015 (Dec); 14 (4): 227–239Schneeweiss S, Rassen JA, Brown JS, et al.
Graphical depiction of longitudinal study designs in health care databases.
Ann Intern Med 2019;170:398–406.
doi:10.7326/M18-3079
pmid:http://www.ncbi.nlm.nih.gov/pubmed/30856654
Return NON-PHARMACOLOGIC THERAPY
Since 7-06-2022


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |