The Rapid and Progressive Degeneration of the Cervical Multifidus
in Whiplash: An MRI Study of Fatty InfiltrationThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Spine (Phila Pa 1976). 2015 (Jun 15); 40 (12): E694–700 ~ FULL TEXT
OPEN ACCESS James M. Elliott, PhD, PT, D. Mark Courtney, MD, Alfred Rademaker, PhD, Daniel Pinto, PhD, PT,
Michele M. Sterling, PhD, PT, and Todd B. Parrish, PhD
Department of Physical Therapy and Human Movement Sciences,
Feinberg School of Medicine,
Northwestern University,
Chicago, IL
STUDY DESIGN: Single-center prospective longitudinal study.
OBJECTIVE: To study the (1) temporal development of muscle fatty infiltrates (MFI) in the cervical multifidi after whiplash, (2) differences in multifidi MFI between those who recover or report milder pain-related disability and those who report moderate/severe symptoms at 3 months, and (3) predictive value of multifidi MFI outcomes.
SUMMARY OF BACKGROUND DATA: The temporal development of MFI on conventional magnetic resonance image has been shown to be associated with specific aspects of pain and psychological factors. The replication of such findings has yet to be explored longitudinally.
METHODS: Thirty-six subjects with whiplash injury were enrolled at less than 1 week postinjury and classified at 3 months using percentage scores on the Neck Disability Index as recovered/mild (0%–28%) or severe (≥30%). A fat/water magnetic resonance imaging measure, patient self-report of pain-related disability, and post-traumatic stress disorder were collected at less than 1 week, 2 weeks, and 3 months postinjury. The effects of time and group (per Neck Disability Index) and the interaction of time by group on MFI were determined. Receiver operating characteristic curve analysis was used to determine a cut-point for MFI at 2 weeks to predict outcome at 3 months.
RESULTS: There was no difference in MFI across groups at enrolment. MFI values were significantly higher in the severe group than those in the recovered/mild group at 2 weeks and 3 months. The receiver operating characteristic curve analysis indicated that MFI levels of 20.5% or above resulted in a sensitivity of 87.5% and a specificity of 92.9% for predicting outcome at 3 months.
There are more articles like this @ our:
WHIPLASH PageCONCLUSION: Consistent with previous evidence, muscle degeneration occurs soon after injury but only in those patients with poor functional recovery. This study provides further evidence that
(1) multifidi muscle fatty infiltrates (MFI) occur in tandem with known predictive risk factors (older age, pain-related disability, and post-traumatic stress disorder) and
(2) routine imaging protocols may need to be reconsidered in the vast majority of patients after whiplash.LEVEL OF EVIDENCE: 3.
From the FULL TEXT Article:
INTRODUCTION
Whiplash-Associated Disorders (WAD) from motor vehicle collisions (MVCs) afflict over 4–million Americans annually, reducing quality of life and accounting for an estimated $30 billion in medical costs. [1] Half of all those exposed to a MVC will never fully recover but have milder symptoms, [2] and 25% (~ 1 million) are expected to present with a complex clinical picture including severe-pain-related disability, [3] muscle degeneration, [4, 5] sensory and motor disturbances, [6] muscle weakness, [7] and psychological distress. [6] A number of psychosocial factors (e.g., coping, expectations, anxiety and depression) have been identified as being associated with poor functional recovery. [2] Despite the presence of and recognition for these factors, current best multimodal treatments have not substantially influenced the rate of functional recovery. [8–10] Furthermore, no structural cause of WAD has been found with available imaging technology, supporting the position that the clinical course is driven by both medical and non-medical-related factors. [11]
Despite the lack of pathological findings, a recent prospective investigation using conventional T1-weighted magnetic resonance imaging (MRI) uniquely identified neck muscle fatty infiltrates (MFI) between one- and three-months post injury in participants with more severe levels of WAD-related disability and symptoms of post-traumatic stress (PTSD). [5] Participants who recovered, or only had persistent mild symptoms, and those with chronic idiopathic neck pain [12] did not develop MFI. While the quantification of MFI in whiplash [5, 13–15] is intriguing, the mechanisms underlying its development in and their contribution towards poor functional recovery is largely unknown.
There are available [16, 17] quantitative water-fat MR techniques (3D water-fat MRI [18] and Proton-Density Fat Fraction) [19] that could help quantify rapid physiologic changes that may precede changes observed on T1-weighted sequences. [5] An earlier detection of MFI might prove crucial for identifying the early expression of MFI, its potential predictive role in the development of chronic WAD, and targets for more informed management.
While MFI has shown to occur throughout the neck muscles in WAD, larger magnitudes have been primarily observed in the multifidus. [12, 13] The purpose of this study was to1) investigate the temporal expression of MFI in the cervical multifidii,
2) investigate differences in MFI between those who fully recover or report milder
symptoms from those with moderate/severe symptoms, and
3) investigate the predictive value of MFI.We hypothesized that multifidii MFI on water-fat MRI will manifest soon following the injury event, between 1–2 weeks, and uniquely in the group with poor functional recovery.
MATERIALS AND METHODS

Table 1 A total of 89 subjects enrolled in the study. However, 53 (60%) withdrew or were deemed ineligible for the following reasons: 5 (0.05%) were too old at the time of the first visit (> 55 years); 1 (0.01%) had lodged bullet fragments in the upper torso; 1 reported being an insulin dependent diabetic; 20 (22%) consented but decided they could not commit to the 3–month study; and 26 (29%) consented in the emergency medicine department but did not show for their initial appointment. Accordingly, 36 subjects (40%) with acute WAD were followed and assessed at <1–week, 2–weeks, and 3–months post injury. The demographics and baseline measures on all participants are shown in Table 1.
The study was a single-center prospective longitudinal study with an inception cohort of 36 people with acute whiplash injury (≤1 weeks duration) after a MVC with follow-up at 2–weeks and 3–months. The local Institutional Review Board granted ethical approval and all participants provided informed written consent. Participants were recruited via an urban academic emergency medicine department with level 1 trauma designation and were eligible provided they reported neck pain resulting from a MVC and were within the Quebec Task Force Classification category of WAD Grade II. [20] Exclusion criteria were younger than 18 or older than 55 years of age, one or more previous MVC’s in their lifetime, treatment for neck pain disorders in the past ten years, any nervous system (e.g. Stroke, Parkinsons), metabolic system disorder (e.g. diabetes), or those who, by standard Emergency Medical Services’ protocols were deemed to be at risk for multi-system trauma.
One research assistant administered questionnaires on all subjects at each assessment. The MRI measures were performed at a radiology laboratory by a radiology technologist. The lead investigator, who was blind to the status of the patient in terms of questionnaire responses, measured all MFI data.
Self-reported pain and disability
Self-reported pain and disability was measured using the Neck Disability Index (NDI), which has been used extensively to quantify neck pain-related disability. [5, 9, 21] The percentage score was dichotomized into 2 groups (0–28%, mild/recovered and 30–100%, moderate to severe disability). [22]
PTSD symptoms
Symptoms of PTSD can be measured using total symptom severity score of the Posttraumatic Stress Diagnostic Scale (PDS). [23] Higher scores (out of 51) indicate more severe symptoms. A recent derivation of a clinical prediction rule (CPR) supports using the hyperarousal subscale score as predictive of long-term outcomes. [22] Accordingly, we chose to only use the hyperarousal subscale score as a way to measure emotional-mental stress.
MRI Measures and Analysis
All imaging data were collected with a 3.0T MRI scanner (Siemens, Erlangen, Germany). Each participant underwent MR examination of the cervical spine. A localizer scan and a T2-weighted sagittal turbo spin echo sequence was performed to determine the location of the water-fat scan.
3D Multi-echo Dixon water-fat MRI
A 3D multi-echo gradient echo acquisition was performed to collect the data required for the analysis of phase related to the precessional differences in fat and water for the neck multifidii muscles (C3–C7). A standard 12–channel head coil and 4–channel neck coil were used as receiver coils to improve signal-to-noise. The axial FLASH dual echo, gradient echo sequence had duration of 4:23 minutes, an in-plane resolution of 0.7mm using a rectangular field of view of 75% and thickness of 3mm and slab oversampling of 22% with 36 partitions to prevent aliasing in the 3D (superior-inferior) direction, TR/TE1/TE2 6.59/2.45/3.68 ms with a FOV of 190×320 mm. This scan covered the cephalad portion of C3 through the caudal portion of the C7 vertebral end plate. Images were co-registered across each time point using Analyze Software (v. 11.0).
Muscle water-fat quantification
Defined regions of interest (ROIs) were manually traced over each of the bilateral multifidii muscles from C3–C7 on the water-fat images. [25] The MFI (%) from 3D water-fat imaging was created from the mean pixel intensity of fat-only (Fat) and the mean pixel intensity of water-only (Water) images with the following equation:MFI (%) = Fat/(Fat+Water)*100
Statistical Analysis
Participants were classified based on NDI scores at 3–months post-injury as either recovered/mild (NDI of 0–28%) or moderate/severe (NDI ≥ 30%). These classifications have been used previously [5, 22] and supported in subsequent work. [6] Comparison of continuous measures between groups (Age, Body Mass Index (BMI), NDI, PDS, days from MVC and MFI) was done using the independent sample t-test. Comparison of gender between groups was done using Fisher’s exact test. MFI was compared across times within groups using a repeated measures linear model, with post-hoc comparisons done using the Tukey-Kramer procedure. [26] MFI was compared between groups at each time using a multiple linear regression model that adjusted for age, gender and BMI to account for their potential to influence MFI development. [14] An overall repeated measures linear model, adjusting for age, gender and BMI was used to test for a group by time interaction. Based on earlier data of group differences in MFI, [12] and a between group effect size of 1.2, 12 subjects/group were required at 80% power and p = 0.05. A post-hoc power analysis indicated that the power to detect the observed differences (assuming the observed standard deviations) at 1 week, 2 weeks and 3 months was 0.32, 0.72 and 0.99 respectively. Observed effect sizes using the larger standard deviation in the moderate/severe group ranged from 0.60 at 1 week to 2.0 at 3 months. Receiver operating characteristic curve analysis was used to determine a cut-point for MFI at 2 weeks that could predict group membership at 3 months. Statistical analyses were done using SAS (SAS Institute Inc. 2011. SAS OnlineDoc® 9.3. Cary, NC: SAS Institute Inc.)
RESULTS
MRI Findings

Table 2
Figure 1
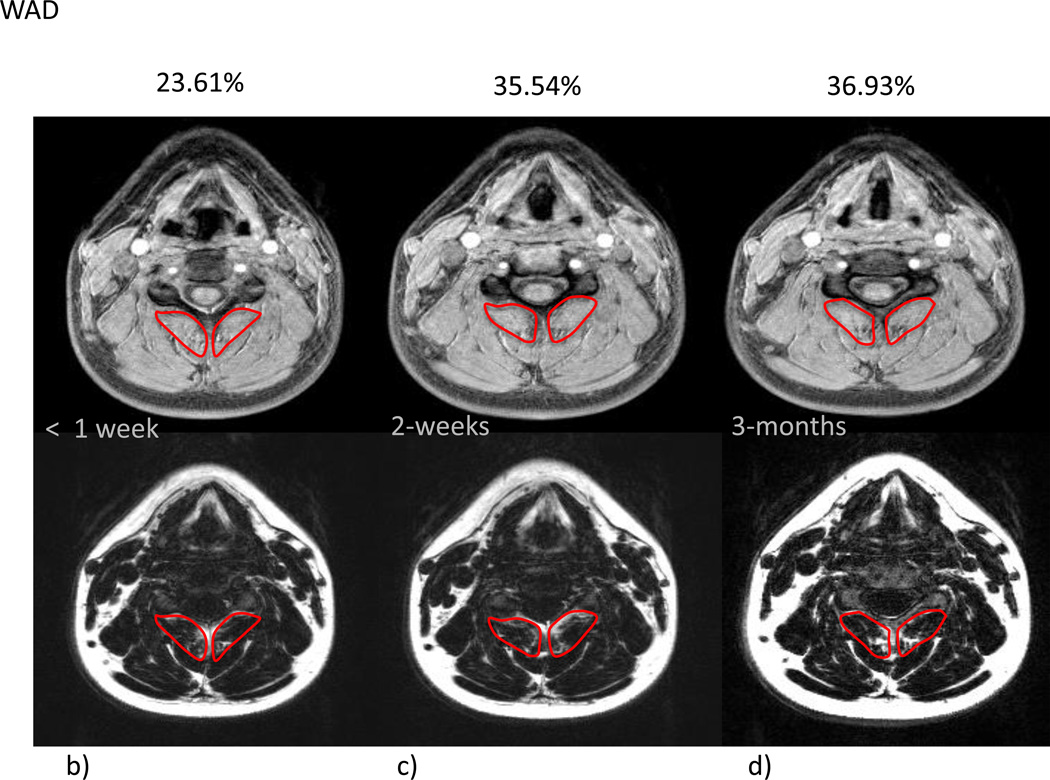
Figure 2 Mean % MFI by group and time are given in Table 2. Due to the small variation in the within group changes in the recovered group, the modest mean changes between 1 week and 3 months were statistically significant (p=0.023). In the moderate/severe disability group, mean percent MFI significantly increased across all time points (p<0.002). Comparing the recovered/mild to moderate/severe groups indicated no significant difference at 1 week (p=0.31) with significant differences at 2 weeks (p=0.0009) and at 3 months (p<0.0001). The group by time interaction (p=0.026) indicated a significantly different time course in mean % MFI between the groups (Figure 1). Figure 2a–g illustrates the temporal registration (< 1 week, 2–weeks, and 3–months from injury) of MFI at the C5 vertebral level on dual echo water- and fat-only images in a severe subject compared to a subject that nominated full-recovery.
Prediction of 3–month severity
Receiver operating characteristic curve analysis indicated that MFI levels of 20.5% or above resulted in a sensitivity of 87.5% (identification of 7/8 in the moderate severe group) and a specificity of 92.9% (identification of only 2/26 in the recovered group). Having higher baseline pain-related disability was associated with having increased MFI at 2–weeks and 3 months post-injury. MFI levels at 2 weeks may be used to predict severity based on NDI at 3 months. Since classification into the recovered versus moderate/severe group was done at 3 months, MFI levels at 2 weeks were significantly associated with this classification (p=0.0009).
DISCUSSION
This study provides further evidence for the differential development of MFI in participants with varying levels of functional recovery following whiplash. Previous work from Australia demonstrated that neck MFI on T1-weighted MRI could be observed and used to differentiate participants with varying levels of functional recovery. [5] Specifically, the group with poor functional recovery uniquely demonstrated the expression of MFI between one- and 3 months post-injury and this was related to initial pain intensity and mediated by symptoms of PTSD. [5] In a likewise manner, here we demonstrated a differential and unique expression of multifidii MFI on water-fat MRI in tandem with higher levels of initial pain-related disability and symptoms of PTSD; further supporting a biopsychological basis underlying poor functional recovery.
While both groups entered the present study within one-week post-injury and had similar initial levels of MFI, the group with poor functional recovery uniquely demonstrated large muscle changes on water-fat MRI between 1– and 2–weeks post MVC. Given that neck MFI changes have typically not been detected until at least 1 month post-injury, [5] water-fat MRI may represent a more rapid and possibly more sensitive measure for the development of MFI. [16] Although the mechanisms underlying these changes, and their influence on recovery, remain largely unknown, the rapid development, and potential predictive value of MFI occurring with established predictive factors, [22] warrant further cause/effect investigation. This is especially important when considering the available evidence suggests a lesion cannot be established in the vast majority of injured people. [27]
There are various mechanistic, but not necessarily mutually exclusive, processes that could underlie MFI, such as inflammation, denervation, disuse, altered activation of the sympathetic nervous system (SNS), and stress system dysregulation. Injury to a number of anatomical structures (facet joints, discs, ligaments, vascular tissues, and dorsal root ganglia [28–33]) could produce an inflammatory response, [34] which, similar to other chronic pain disorders, [35] could affect the functioning of the peripheral and central nervous systems [36] as well as the structure and strength of skeletal muscle. [37] While this study did not explore the role of inflammation on outcomes, a recent study investigating time-dependent changes in serum inflammatory biomarker levels in whiplash found a negative relationship between TNF-α and MFI at 3 months but no relationship with other markers (C-Reactive Protein, and IL-1β). [37] Evidence from both an animal injury model [38] and human study of pulmonary dysfunction [39] indicates that higher levels of TNF-α may influence the recovery of muscle function. On the contrary, others have demonstrated increased levels of serum TNF-α in tandem with higher levels of disability in chronic low back pain, [40] upper extremity overuse injuries, [41] and significant losses of muscle mass in patients with cancer, AIDS and chronic obstructive pulmonary disease. [42] Further study investigating the directional influence of local inflammatory factors on muscle structure and function following whiplash is warranted and well underway.
It is documented that decreased activity after a MVC increases the risk of chronic WAD, [43] but the mechanisms by which decreased activity influences skeletal muscle structure and function following whiplash are largely unknown. The effect of depriving healthy individuals from their normal daily activity (as may be expected to occur if an individual reduces normal activity after a whiplash injury) can lead to fatigue, mood swings, reductions in muscle volume and intramuscular fatty infiltration. [44–46] In addition, disuse may be, in part, a consequence of psychological factors such as fear-avoidance [47] or passive pain coping styles. [48] Regardless, the mechanisms behind disuse-induced MFI are complex and could be related to either neuropsychological origins or skeletal muscle properties as the output from both of these sources controls voluntary force production, [45] and this requires further investigation.
Support for altered activation of the SNS is also available. [49] Altered SNS activation is centrally programmed and has shown to exert a number of actions at the level of the muscle cell. [49] In a state of prolonged stress, as might be expected in some cases of whiplash, excessive sympathetic outflow could result in hypoxia and toxaemia under which, intra-myocellular oxidative stresses may affect the contractility of skeletal muscle, and possibly the MFI observed in this and another study. [5] Further research investigating SNS activity in those who develop MFI and post-traumatic stress following whiplash is important to better understand the underlying mechanisms of persistent WAD.
It is also notable that all of the subjects in the moderate/severe group (n of 8, or 22% of the study population) fit the criteria from a recent derivation clinical prediction rule (CPR), where the prediction of developing moderate/severe disability was increased in the presence of older age (> 35 years), higher initial levels of pain-related disability on the NDI (> 40%) and hyperarousal symptoms on the PDS (score of > 6). [22] While it was not the intent of this paper to validate the Australian CPR, [22] the results may be supportive, demonstrating a relationship between objective MRI findings of MFI and the CPR criteria. [22] Accordingly, this current study could be considered part of a recommended series of multi-cultural studies needed to 1) provide a preliminary validation of the CPR, and 2) replicate the findings of MFI in whiplash. [5]
While the findings are intriguing, they must be interpreted with caution. The sample sizes, albeit small, do result in sufficient power to detect group MFI differences at 3 months. It is interesting and consistent with previous investigations [5–7, 50–52] that 22% of subjects earned membership to the moderate/severe group. Furthermore, these 22% in the moderate/severe disability group, in addition to including all members of the recovered/mild group, followed recovery trajectories set forth in the Australian CPR. [22] This will need to be validated in larger scale with a different population of patients with varying levels of pain-related disability.
While the differential development MFI in subjects with chronic versus recovered/mild WAD has now been produced in two different populations (Australia [5] and the present study in the USA) with different insurance schemas and with two different fat separation methods (T1 and Dixon), they have been produced and reported by the same, albeit blinded, investigator (JE). The findings must be independently replicated before deriving more confident conclusions.
These findings may have implications for whiplash where the early assessment and management of modifiable factors (e.g. symptoms of PTSD, pain intensity, muscle degeneration [53]) may attenuate some physical aspects of the chronic condition. This and other cause and effect questions related to the origin of MFI and its predictive value in whiplash recovery requires further prospective evaluation, and this is underway. Lastly, in the case of the ‘at risk’ patients identified in this study, water-fat MRI may have implications for early assessment, characterization, and management of WAD. On the contrary, and in the vast majority of patients, the ordering and performance of early imaging applications may not be necessary.
Acknowledgements
The authors wish to thank Jill Sears and Ryan McConnell for their assistance in the recruitment of acutely injured participants from the Emergency Medicine Department at Northwestern Memorial Hospital, Chicago Illinois, USA. We also thank Marie Wasielewski for her assistance in co-ordinating follow-up visits and performing the MRI scans on all study participants.
The manuscript submitted does not contain information about medical device(s)/drug(s). The project described was supported by the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) through Grant Number KL2 RR025740 and KL2TR000107.
Relevant financial activities outside the submitted work: grants/grants pending, board membership, consultancy, other (Pain ID LLC), payment for lectures
References:
Naumann RB, Dellinger AM, Zaloshnja E, Lawrence B, Miller TR.
Incidence and Total Lifetime Costs of Motor Vehicle-Related Fatal and Nonfatal Injury by Road User Type,
United States.
Traffic Injury Prevention. 2010;11(4):353–360Carroll, LJ, Holm, LW, Hogg-Johnson, S et al.
Course and Prognostic Factors for Neck Pain in Whiplash-associated
Disorders (WAD): Results of the Bone and Joint Decade 2000-2010
Task Force on Neck Pain and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S83–92Sterling M, Hendrikz J, Kenardy J, Kristjansson E, Dumas JP, Niere K, et al.
Assessment and validation of prognostic models for poor functional recovery 12 months after whiplash injury:
a multicentre inception cohort study.
Pain. 2012;153(8):1727–1734Elliott J.
Are there implications for morphological changes in neck muscles after whiplash injury?
Spine (Phila Pa 1976) 2011;1(36(25 Suppl):S205–S210. ReviewElliott J, Pedler A, Kenardy J, Galloway G, Jull G, Sterling M.
The temporal development of Fatty infiltrates in the neck muscles following whiplash injury:
an association with pain and posttraumatic stress.
PLoS One. 2011;6(6):e21194Sterling M, Jull G, Kenardy J.
Physical and psychological factors maintain long-term predictive capacity post-whiplash injury.
Pain. 2006;122(1–2):102–108Sterling M, Jull G, Vicenzio B, Kenardy J, Darnell R.
Development of motor dysfunction following whiplash injury.
Pain. 2003;103:65–73Jull G, Kenardy J, Hendrikz J, Cohen M, Sterling M.
Management of acute whiplash: a randomized controlled trial of multidisciplinary stratified treatments.
Pain. 2013;154(9):1798–1806Lamb SE, Gates S, Williams MA, Williamson EM, Mt-Isa S, Withers EJ, et al.
Emergency department treatments and physiotherapy for acute whiplash: a pragmatic, two-step,
randomised controlled trial.
Lancet. 2013;381(9866):546–556Michaleff ZA, Maher CG, Lin CW, Rebbeck T, Jull G, Latimer J, et al.
Comprehensive physiotherapy exercise programme or advice for chronic whiplash (PROMISE):
a pragmatic randomised controlled trial.
Lancet. 2014;384(9938):133–141Dufton JA, Bruni SG, Kopec JA, Cassidy JD, Quon J.
Delayed recovery in patients with whiplash-associated disorders.
Injury. 2012;43(7):1141–1147Elliott J, Sterling M, Noteboom JT, Darnell R, Galloway G, Jull G.
Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain.
Clin Radiol. 2008;63(6):681–687Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW.
Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders:
a magnetic resonance imaging analysis.
Spine (Phila Pa 1976) 2006;31(22):E847–E855Elliott J, Sterling M, Noteboom JT, Treleaven J, Galloway G, Jull G.
The clinical presentation of chronic whiplash and the relationship to findings of MRI fatty infiltrates
in the cervical extensor musculature: a preliminary investigation.
Eur Spine J. 2009;18(9):1371–1378Elliott JM, O'Leary S, Sterling M, Hendrikz J, Pedler A, Jull G.
Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash.
Spine (Phila Pa 1976) 2010;35(9):948–954Smith AC, Parrish TB, Abbott R, Hoggarth MA, Mendoza K, Chen YF, et al.
Muscle-fat magnetic resonance imaging: 1.5 Tesla and 3.0 Tesla versus histology.
Muscle Nerve. 2014;50(2):170–176Gaeta M, Scribano E, Mileto A, Mazziotti S, Rodolico C, Toscano A, et al.
Muscle fat fraction in neuromuscular disorders: dual-echo dual-flip-angle spoiled gradient-recalled MR imaging
technique for quantification--a feasibility study.
Radiology. 2011;259(2):487–494Elliott JM, Walton DM, Rademaker A, Parrish TB.
Quantification of cervical spine muscle fat: a comparison between T1-weighted and multi-echo gradient echo
imaging using a variable projection algorithm (VARPRO)
BMC Medical Imaging. 2013;11(13):30Reeder SB, Hu HH, Sirlin CB.
Proton density fat-fraction: A standardized mr-based biomarker of tissue fat concentration.
J Magn Reson Imaging. 2012;36(5):1011–1014Spitzer W, Skovron M, Salmi L, Cassidy J, Duranceau J, Suissa S, et al.
Scientific Monograph of Quebec Task Force on Whiplash Associated Disorders:
Redefining "Whiplash" and its Management
spine. 1995;20:1–73Vernon H, Mior S.
The Neck Disability Index: A study of reliability and validity.
J Manipulative Physiol Ther. 1991;14:409–415Ritchie C, Hendrikz J, Kenardy J, Sterling M.
Derivation of a clinical prediction rule to identify both chronic moderate/severe disability
and full recovery following whiplash injury.
Pain. 2013;154(10):2198–2206Foa EB, Cashman L, Jaycox L, Perry K.
The validation of a self-report measures of posttraumatic stress disorder:
The Posttraumatic Diagnostic Scale.
Pscyhological Assessment. 1997;9:445–451. [Google Scholar]Elliott JM, Pedler AR, Jull GA, Van Wyk L, Galloway GG, O'Leary SP.
Differential Changes in Muscle Composition Exist in Traumatic and Non-Traumatic Neck Pain.
Spine (Phila Pa 1976) 2014 Jan 1;9(1):39–47Dixon W.
Simple proton spectroscopic imaging.
Radiology. 1984;153(189–194)Kramer CY.
Extension of Multiple Range Tests to Group Means with Unequal Numbers of Replications.
Biometrics. 1956;12(3):307–310Sterling M, McLean SA, Sullivan MJ, Elliott JM, Buitenhuis J, Kamper SJ.
Potential processes involved in the initiation and maintenance of whiplash-associated disorders:
discussion paper 3.
Spine (Phila Pa 1976) 2011;36(25 Suppl):S322–S329Guez M, Hildingsson C, Rosengren L, Karlsson K, Toolanen G.
Nervous tissue damage markers in cerebrospinal fluid after cervical spine injuries and whiplash trauma.
J Neurotrauma. 2003;20(9):853–858Kaneoka K, Ono K, Inami S, Hayashi K.
Motion analysis of cervical vertebrae during whiplash loading.
Spine. 1999;24(8):763–769. discussion 70Panjabi MM, Ito S, Pearson AM, Ivancic PC.
Injury mechanisms of the cervical intervertebral disc during simulated whiplash.
Spine. 2004;29(11):1217–1225Pearson AM, Ivancic PC, Ito S, Panjabi MM.
Facet joint kinematics and injury mechanisms during simulated whiplash.
Spine (Phila Pa 1976) 2004;29(4):390–397Svensson MY, Aldman B, Bostrom O, Davidsson J, Hansson HA, Lovsund P, et al.
Nerve cell damages in whiplash injuries. Animal experimental studies.
Orthopade. 1998;27(12):820–826Winkelstein BA, Nightingale RW, Richardson WJ, Myers BS.
The cervical facet capsule and its role in whiplash injury: a biomechanical investigation.
Spine. 2000 May 15;25(10):1238–1246Lee KE, Davis MB, Winkelstein BA.
Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading:
behavioral and inflammatory outcomes in a rodent model of pain.
J Neurotrauma. 2008;25(11):1383–1393DeLeo JA, Yezierski RP.
The role of neuroinflammation and neuroimmune activation in persistent pain.
Pain. 2001;90(1–2):1–6Stuve O, Zettl U.
Neuroinflammation of the central and peripheral nervous system: an update.
Clin Exp Immunol. 2014;175(3):333–335Sterling M, Elliott JM, Cabot PJ.
The course of serum inflammatory biomarkers following whiplash injury and their relationship
to sensory and muscle measures: a longitudinal cohort study.
PLoS One. 2013;8(10):e77903Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI, et al.
Physiological role of tumor necrosis factor alpha in traumatic muscle injury.
FASEB J. 2002;16(12):1630–1632Barreiro E, Schols A, Polkey M.
Cytokine profile in quadricpes muscles of patients with severe chronic obstructive pulmonary disease.
Thorax. 2008;63:100–107Wang H, Schiltenwolf M, Buchner M.
The role of TNF-alpha in patients with chronic low back pain-a prospective comparative longitudinal study.
Clinical Journal of Pain. 2008;24:273–278Carp SJ, Barbe MF, Winter KA, Amin M, Barr AE.
Inflammatory biomarkers increase with severity of upper-extremity overuse disorders.
Clin Sci (Lond) 2007;112(5):305–314Reid M, Yi-Ping L.
Tumor necrosis factor-α and muscle wasting: a cellular perspective.
Respiratory research. 2001;2:269–272Borchgrevink GE, Kaasa A, McDonagh D, Stiles TC, Haraldseth O, Lereim I.
Acute treatment of whiplash neck sprain injuries. A randomized trial of treatment during the first 14 days
after a car accident.
spine. 1998;23(1):25–31Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB.
Reduced physical activity increases intermuscular adipose tissue in healthy young adults.
Am J Clin Nutr. 2007;85(2):377–384Clark BC.
In vivo alterations in skeletal muscle form and function after disuse atrophy.
Med Sci Sports Exerc. 2009;41(10):1869–1875Glass JM, Lyden AK, Petzke F, Stein P, Whalen G, Ambrose K, et al.
The effect of brief exercise cessation on pain, fatigue, and mood symptom development in healthy,
fit individuals.
J Psychosom Res. 2004;57(4):391–398Vlaeyen JW, Linton SJ.
Fear-avoidance model of chronic musculoskeletal pain: 12 years on.
Pain. 2012;153(6):1144–1147Carroll LJ, Ferrari R, Cassidy JD, Cote P.
Coping and recovery in whiplash-associated disorders: early use of passive coping strategies is associated with
slower recovery of neck pain and pain-related disability.
Clin J Pain. 2014;30(1):1–8Passatore M, Roatta S.
Influence of sympathetic nervous system on sensorimotor function:
whiplash associated disorders (WAD) as a model.
Eur J Appl Physiol. 2006;98(5):423–449Sterling M, Jull G, Vicenzio B, Kenardy J.
sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery.
Pain. 2003;104:509–517Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R.
Physical and psychological factors predict outcome following whiplash injury.
Pain. 2005;114(1–2):141–148Sterling M, Kenardy J, Jull G, Vicenzino B.
The development of psychological changes following whiplash injury.
Pain. 2003;106:481–489O'Leary S, Jull G, Van Wyk L, Pedler A, Elliott J.
Morphological changes in the cervical muscles of women with chronic whiplash can be modified with exercise -
a pilot study.
Muscle Nerve. 2015 Feb;:20. [Epub ahead of print] [PMC free article]
Return to WHIPLASH
Return to CHRONIC NECK PAIN
Since 5-04-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |