

Chiropractic Nimmo Receptor-Tonus Technique and
McKenzie Self-Therapy Program in the Management
of Adjacent Segment Disease: A Case ReportThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Chiropractic Medicine 2020 (Dec); 19 (4): 249259 ~ FULL TEXT
OPEN ACCESS Emsal Salik, MD PhD; Ali Donat, DC; Mustafa Hulisi Agaoglu, DC
Chiropractic Program,
Health Sciences Institute,
Bahcesehir University,
Besiktas, Istanbul, Turkey
Objective: The objective of the present study objective was to describe adjacent segment disease (ASD) from a chiropractic management prospective and subsequently to stimulate further research into the chiropractic therapeutic effects on such cases and to contribute to chiropractic literature.
Clinical features: A 44year-old woman had a history of lumbar stabilization revision operation by pedicle screw fixation for spondylolisthesis. Her intractable back pain episodes, which were diagnosed as ASD, began shortly after this surgery. At presentation, she was taking pregabalin 75 mg 2 times a day for postoperative neuropathic pain without any pain relief. Clinical testing revealed myofascial tender points reproducing the pain.
Intervention and outcome: After taking the case history and performing a physical examination, the patient was managed with chiropractic Nimmo receptor-tonus technique in combination with McKenzie exercises. Nimmo was applied by manually pressing on clinically relevant points for 5 to 15 seconds in 11 visits over 3 weeks. The patient by herself did McKenzie exercises 5 to 10 times a day for 10 to 12 repetitions over 2 months. After 3 weeks of therapy, visual analog scale and Oswestry Disability Index scores were improved. Furthermore, because of the amelioration of the patient's symptoms, her neurosurgeon successfully discontinued pregabalin 75 mg 2 times a day without negative consequences to care.
There is more like this @ our:
CASE STUDIES Section and our
LOW BACK PAIN Section and our
DISC HERNIATION SectionConclusion: As far as the authors are aware, there is currently no published case of ASD care in chiropractic literature. Our rehabilitative management received a favorable response. It can be hypothesized that it offers a perspective that informs improved patient care.
Keywords: Adjacent Segment Disease; Chiropractic; McKenzie Exercises; Myofascial; Trigger Points.
From the FULL TEXT Article:
Introduction
As stated in the Handbook of Pain Management Book by Melzack and Wall, [1] surgical incision is often complicated by the division of small peripheral nerves and sometimes larger nerves, in addition to a variable amount of tissue trauma, retraction, and compression of tissues, as well as other factors.
Adjacent segment disease (ASD) is considered to be a potential complication of spinal fusion surgery. ASD is defined as a clinical phenomenon characterized by the presence of new radiculopathy or myelopathy referable to an adjacent motion segment. [25] The prevalence of ASD after lumbar fusion ranges from 8% to 30.3%. [68] Symptomatic ASD remains a significant cause of postoperative morbidity and reoperation after lumbar fusion. Patients who experience symptomatic ASD report intractable back pain with a significantly decreased quality of life. Patient age, degree of debilitation, and chronic comorbidities are just a few of the various risk factors that can influence the rate of degeneration at the adjacent level, where most surgical fusions occur (L3S1). [9] The challenge is to aid acute rehabilitation to the benefit of the patient. [1] Data suggest that chronic back pain after spinal surgery, including pain related to ASD, should be treated nonoperatively unless progressive neurologic deficits exist. [10, 11]
It is the ultimate goal of chiropractic care to treat dysfunction of the neuromusculoskeletal system. An initial course of chiropractic care typically includes 1 or more manual therapeutic procedures and exercise for pain reduction, in addition to patient education designed to reassure and instill optimal strategies for independent management. [12] Chiropractic Nimmo receptor-tonus technique, which was developed by Raymond L. Nimmo, is a soft tissue manual therapy and can be considered for pain management in ASD patients. [1319] This approach, also known as ischemic compression or trigger point therapy, has been used by chiropractors and other manual therapists for at least 90 years. [18] Home-based exercise rehabilitation, including a McKenzie self-therapy program, [2036] is generally recommended by health care professionals to nourish the tissues and to speed recovery from pain because adequate mobility is a key component of functional recovery. [20, 21] The present study aimed to define the efficacy of chiropractic Nimmo receptor-tonus technique and the McKenzie self-therapy program to manage chronic radiating pain in a patient with ASD after lumbar stabilization revision operation by pedicle screw fixation.
Case Report
On January 11, 2018, a 44year-old female customer representative presented for chiropractic rehabilitative care with intractable back pain extending from the lower back to her right buttock and then to behind the right thigh as far as her right knee. She described the pain as stabbing, annoying, sharp, and radiating. She rated it an 8 or 9 on a 10point visual analog scale (VAS), where 0 is no pain and 10 is irresistible pain. No associated paresthesia, weakness, or numbness was reported. The pain was aggravated when the patient sat or stood for more than an hour and was relieved when the patient rested or lay down. She was observed to be walking very cautiously with a cane to avoid pain.
The patient reported that she first experienced the radiating pain after the lumbar stabilization revision operation approximately 3 months ago. On September 13, 2017, she had this operation, and the spinal surgery was performed from the posterior approach; however, 2 days later she experienced an episode of severe back pain on the right side, and she was immediately taken back to second surgery. After second operation, the pain continued. She received 5, 1mg doses of corticosteroid over 3 weeks, but unfortunately she did not have any benefit. On October 16, 2017, she had a third surgery and then was discharged from the hospital, with the hospital discharge report stating the diagnosis of ASD. Her neurosurgeon prescribed 75 mg of pregabalin 2 times a day. Subsequently she rested at home in bed most of the time because of her pain. She also tried transcutaneous electrical nerve stimulation, which provided some pain relief if not complete relief from symptoms. She continued her current medication of 75 mg of pregabalin 2 times a day for 3 months, which gave her no relief. The pain prevented her from driving, sitting at work, and performing her job. She was very frustrated because of not being able to recover and work without pain, and these symptoms concerned her regarding her future, which she felt very uncertain about, particularly in terms of her inability to have a normal life like before.
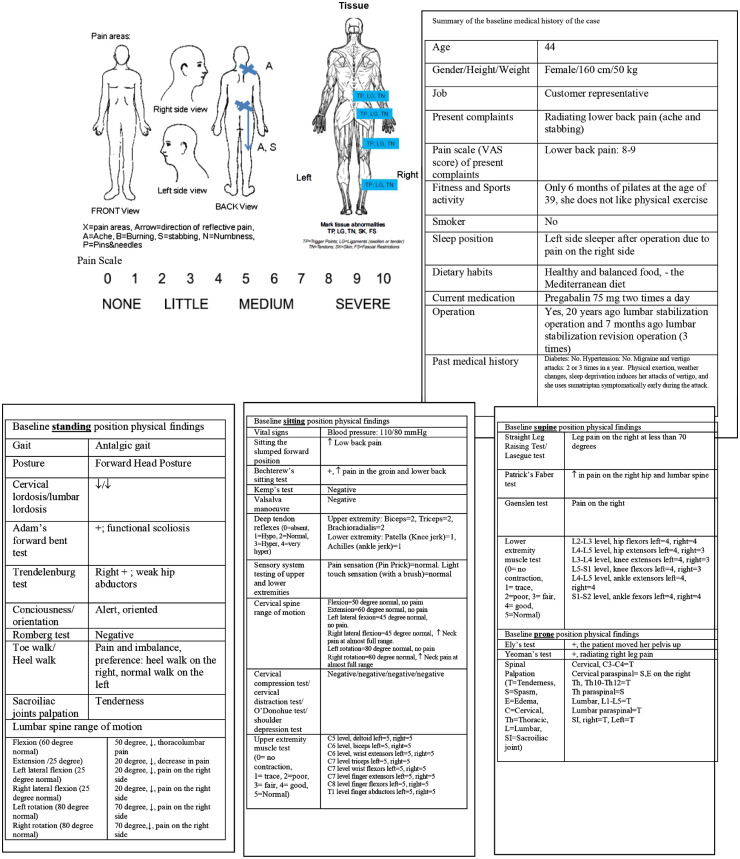
Figure 1
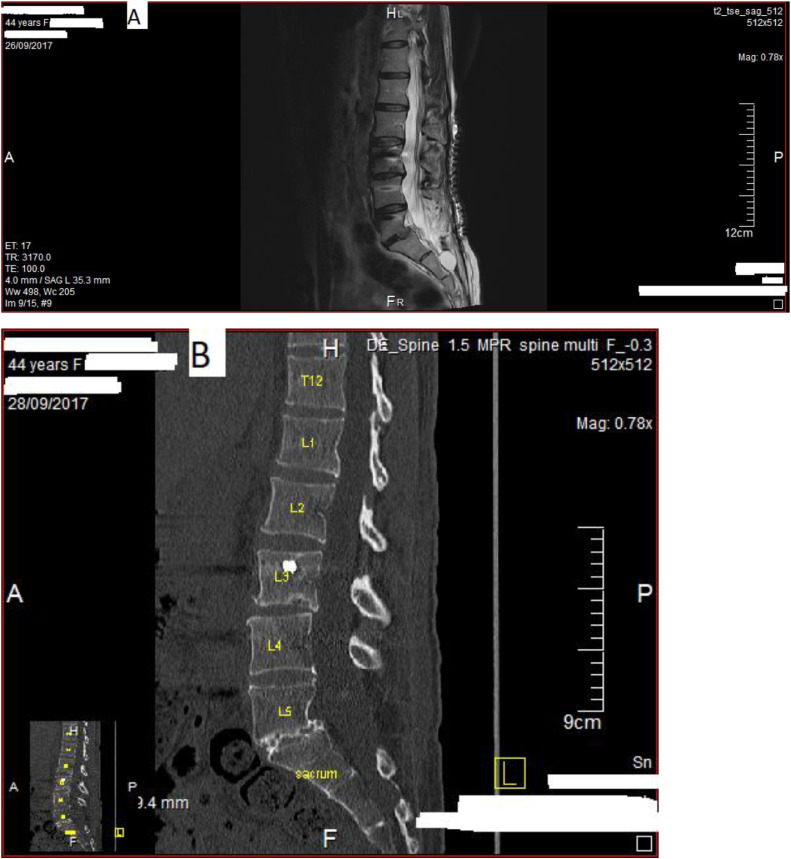
Figure 2
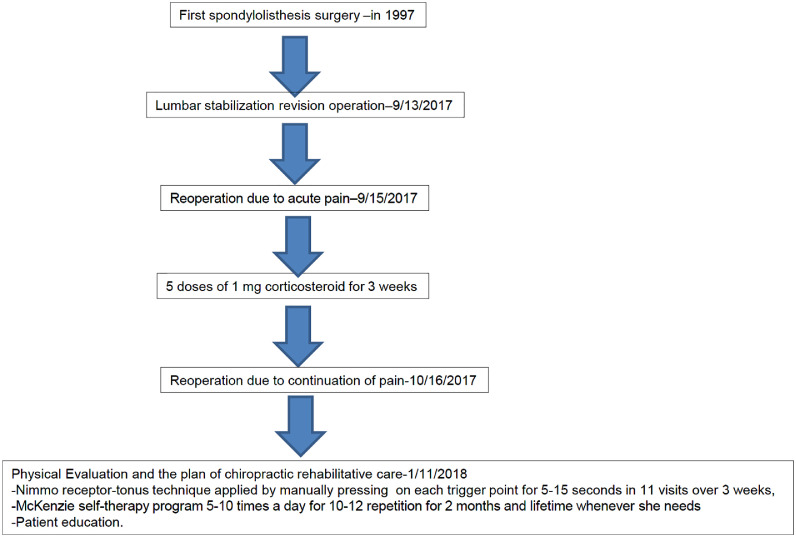
Figure 3 A detailed medical history of her current situation revealed that at age 25 (20 years ago) she was operated on by a neurosurgeon to treat what was diagnosed as advanced stage spondylolisthesis (Meyerding III); thereafter she was followed up by the same neurosurgeon every year. In the 2015, she had a low back pain. At age 43 (in 2017), she had complaints of radiculopathy and pain. Her neurosurgeon suggested revision operation including an upper vertebra because of degenerative changes in the upper region, and the patient accepted. On September 13, 2017, she had the operation and complications occurred as mentioned earlier.
The patient's fitness history data indicated that she did not like physical activity, and the only physical activity she performed for a long time was 6 months of Pilates in 2013. Sleep ergonomics indicated that because of pain the patient's sleeping position changed after the operation. She pointed out that she was a right-side sleeper before the operation, but now she is a left-side sleeper. She also mentioned that she follows a healthy and balanced diet, the Mediterranean diet, by eating primarily plant-based foods, such as fruits and vegetables; whole grains; legumes; and nuts.
Regarding her medical history, the patient stated that she did not have any chronic illness, such as diabetes and hypertension, but she had migraine and vertigo attacks 2 or 3 times a year. She explained that physical exertion, weather changes, and sleep deprivation induce her attacks, and she uses sumatriptan symptomatically early during an attack.
Chiropractic examination revealed a reduction in lumbar spine range of motion without motor-sensory deficits and myofascial tender points throughout the lumbar, pelvic, femoral, and gluteal areas on the right. A summary of the baseline chiropractic medical history and physical findings of the case are shown in Figure 1. Baseline magnetic resonance imaging and computed tomography images of lumbosacral region after spinal operation are shown in Figure 2A and 2B, respectively. A chronological timeline of the patient's operations, management, and chiropractic plan of care are seen in Figure 3.
Interventions
As stated in Clinical Practice Guideline: Chiropractic Care for Low Back Pain, [12] chiropractic-directed care includes manual treatment, self-exercise, and patient education to achieve maximum therapeutic benefit.
Manual Treatment: Nimmo Receptor-Tonus Technique
Chiropractic Nimmo receptor-tonus treatment was applied as described by Hains. [18] In general, thumb pressure was used for the identification, localization, and treatment of trigger points and tender spots within the muscles, tendons, and ligaments of the lumbar, pelvic, femoral, and gluteal areas. Because various muscles overlap, the author relied on specific skeletal reference points that are well known and easily located, namely the paraspinal muscles of the lumbar region, iliolumbar ligament, posterior superior iliac spine (PSIS), iliac crest, posterior sacroiliac line, gluteus minimus muscle, piriformis area, tensor fascia lata, iliotibial band, and tibialis anterior and soleus muscles on the right leg as explained in detail by Hains. [18] It was the authors clinical experience that only the symptomatic side should be treated.
On each treatment session, while the patient was lying in a prone position on the chiropractic table, the author examined the entire symptomatic region of the patient's low back and leg. Approximately 4 kg of pressure was applied with thumb, fingers, knuckle, or elbow depending on the size, depth, and thickness of the myofascial trigger points. The pressure was maintained for 5 to 15 seconds until the pain subsided. It was noteworthy that the pressure was sustained for more than 5 seconds on ligaments and tendons compared with muscle areas because a melting away of the trigger points needed much more time on these anatomic sites. Chiropractic Nimmo receptor-tonus treatment continued once every 2 days in 11 visits for 3 weeks.
Self-Exercise: McKenzie Program

Figure 4 Because the patient did not like physical activity throughout her life as mentioned in the medical history of the case, we provided a copy of the book 7 Steps for a Pain-Free Life by McKenzie and Kubey [20] to convince her to practice the McKenzie self-therapy program. We used the questionnaire in that book (Figure 4) [20] to take an explanatory or correlational approach. The patient answered yes on 5 out of 9 questions, meaning she could benefit from the McKenzie self-therapy program. [20]
The patient performed modified McKenzie exercises for 2 months. During the day, she performed the standing lumbar extension on wall 10 times a day (1012 repetitions slowly) and side-glide (pelvic side shift) 10 times a day (810 repetitions slowly). Each day when she woke in the morning, she did the low cobra (Sphinx) 10 times while positioned prone on her elbows. [20, 21] In total she had approximately 30 to 40 minutes of physical activity during the day.
Patient Education
The author (E.S.) discussed with the patient her current symptoms and explained the underlying pathology of ASD, including changes in intradiscal pressure, anatomic disruption, sagittal misalignment, and the natural history of degeneration. [37] The author also explained that there are 3 subunits of the stabilizing system of the spine the spinal column, the spinal muscles, and the neural control unit [3845] by using anatomic teaching spine models and illustrations to increase her understanding of spinal anatomy.
On her magnetic resonance imaging, the author showed her that the impaired paraspinal muscle morphology fat infiltration in the lumbar multifidus muscles contributed to her low back pain. [4653] The precipitating and perpetuating factors of her current pain were emphasized as repetitive spinal surgery, myofascial injury, being chronically bedridden after spinal surgery, and physical inactivity. [5458]
Outcomes
As recommended by World Health Organization (WHO), [59] we used the VAS and Oswestry disability index (ODI) [60, 61] for outcome measures of low back pain in our case.
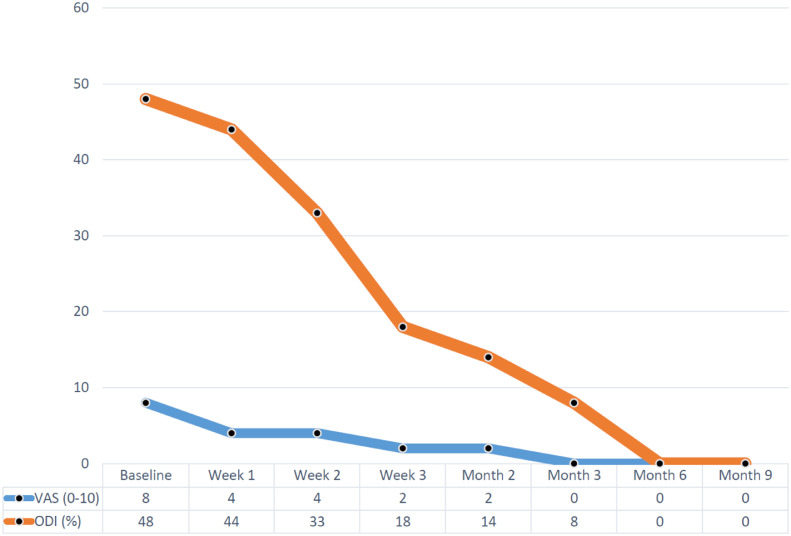
Figure 5 During treatment with the Nimmo receptor-tonus technique and McKenzie self-therapy program, the patient's VAS and ODI scores improved gradually, as seen in Figure 5. After 3 weeks, the patient reported pain of 2 out of 10 on the VAS, and her ODI scores revealed a significant improvement from being severely disabled (48%) to mild disability (18%). She reported 80% overall reduction in pain intensity and symptoms compared with baseline values. She maintained and demonstrated good compliance with McKenzie self-therapy program. After chiropractic rehabilitative care, she could drive her car again because her leg and back pain had subsided. In addition, she discontinued her medication of 75 mg of pregabalin 2 times a day under her neurosurgeon's supervision.
At the 2month follow-up appointment, improvement was noted in both the subjective report by the patient and the objective physical findings. The patient reported that her pain was significantly reduced. She also reported faithfully performing her assigned McKenzie exercises. Physical examination of the musculoskeletal system while standing revealed that active trunk range of motion appeared full in all directions except for right PSIS pain on right-side bending only. When palpating the PSIS during forward bending, the right PSIS appeared to move superiorly and anteriorly farther than the left PSIS, suggesting restricted joint play motion on the right side. On leg extension, palpation of the sacroiliac space between the PSIS and sacrum was narrower on the right side. A Faber test was performed by abducting, flexing, and laterally rotating both the left and right hip individually. Neither the low back nor the anterior hip regions were painful with the Faber test. A functional prone leg-checking protocol, the Derifield-Thompson leg check analysis, was tested to identify pelvic or cervical syndrome, [62] and a negative Derifield occurred; the reactive (shorter) right leg in the prone position stayed short in the prone extended position and became shorter in the flexed position, suggesting interiorly rotated ilium or anterior rotation of the sacrum on the right relative to the longitudinal axis of the body. Manual compression of the right sacroiliac joint was painful. Manual muscle testing in the prone position (which included both hip extensor muscles, knee flexor muscles, and hip medial and lateral rotator muscles) was normal (5 of 5). The author's clinical experience indicated that the finding of sacroiliac joint dysfunction suggested the presence of an interiorly rotated ilium on the right. A Thompson drop-table technique was performed. [63, 64]
The patient was contacted at 3, 6, and 9 months after the conclusion of formal therapy. Her ODI was 8% at 3 months, and 0% at 6 and 9 months. The patient reported that she had not experienced any recurrence of her pain and disability; she was now managing all of her work duties without restrictions or concerns, and she was regularly driving her car every day without difficulty. She reported being very satisfied with her outcome.
The patient came for a follow-up visit after 2 years. In the past year, she has been more physically active, working out in a gym 4 times per week and gardening. She also said that she was no longer in pain. Her physical examination was unremarkable; she had full functional range of motion and was pain free. The patient provided consent for her health information to be published.
Practical Applications
This report illuminates the clinical features of symptomatic ASD and those with the features of myofascial tender points reproducing the pain. This case suggests the need for more rigorous research to examine how Nimmo technique in combination with McKenzie may provide therapeutic benefit to patients with ASD.
Discussion
There is no gold standard treatment for ASD. [5] This case report illustrates an integrative perspective of management with intense and detailed care of the patient as chiropractic rehabilitation. To deal with the patient's symptoms, we focused on decreasing the pain and improving the range of motion without pain using 2 modalities the Nimmo receptor-tonus technique and McKenzie self-therapy program. Fortunately, the patient responded well. She reported a reduction of pain by 50% on VAS within 1 week and reduction of disability by 62.5% within 3 weeks, and her neurosurgeon stopped her medication, pregabalin, because he was convinced enough that adequate nonpharmacologic pain control was achieved with the help of these 2 modalities.
Instrumented fusion can produce adverse consequences on the integrity of natural biomechanical forces that can cause pain and disability for those who experience ASD. [65, 66] Lee and Langrana [65] reported that there is heightened stress at the facet joints of L34 and L45 after lumbosacral arthrodesis. Axelsson et al [66] assessed adjacent segments with the use of radiographic analysis and found hypermobility in the juxtafused segment. On a microscopic and biochemical level, there is an unresolved neuroinflammatory process behind this degenerative cascade. [67] Proinflammatory cytokines stimulate microvascular blood and nerve ingrowth, resulting in pain signaling and tissue degradation, and this may sensitize a person to chemical and/or mechanical stimuli, contributing to severe low back pain from nociceptive and/or neuropathic mechanisms. [67, 68]
The working diagnosis in our ASD case was established to be myofascial low back pain, which is a kind of dominant nociceptive pain of mechanical origin, [69] because on palpation examination, we identified tender points on paraspinal muscles of the lumbar region, iliolumbar ligament, PSIS, iliac crest, posterior sacroiliac line, gluteus minimus muscle, piriformis area, tensor fascia lata, iliotibial band, and tibialis anterior and soleus muscles on the right leg, which were treated with a chiropractic Nimmo receptor-tonus technique. One may consider why tender points examination and treatment on ligament and tendon areas apart from muscles was implemented; the answer is that myofascial pain results from irritable foci (trigger points) within skeletal muscles and their ligamentous junctions. [56, 70]
Moreover, multimodal chiropractic care of the patient also involved the McKenzie program. Although this program is primarily for centralization of pain, [20, 24, 29, 30] through a literature search we identified its efficacy as a treatment option for myofascial pain, so we tried in our case to speed recovery from this pain. [71]
Despite the fact that definite neuropathic pain requires that an objective diagnostic test confirms the lesion or disease of the somatosensory nervous system (eg, neurophysiological tests and skin biopsy), [72] our patient was taking pregabalin without a definitive diagnosis. This reality, in part, may explain the failure to obtain complete pain relief with the drug treatment alone in our case.
Our 44year-old female patient was immobile for approximately 3 months because of pain, and rapid mobilization was not achieved. It is well documented that being chronically bedridden because of pain can further complicate the situation [4547, 7375] because diurnal loading patterns of physical activity and rest are critical for maintaining health and function.45 Although not specifically on myofascial pain of ASD, lots of clinical studies and scientifically proven data have indicated the benefit of the McKenzie exercise program on different types of chronic back pain. [2135] Therefore, we considered that the McKenzie exercises helped the patient by allowing her to take a proactive approach toward her recovery and rehabilitation.
Although different disciplines use different terms for trigger point therapy, the authors preferred to use a specified term, chiropractic Nimmo receptor-tonus technique, to notice and remember Raymond Nimmo, DC (19041986), who developed an understanding of musculoskeletal pain syndromes as a soft tissue therapy in a chiropractic science that paralleled the trigger point therapy of Janet Travell (19011997). [14, 19] A previous report indicated that chiropractic Nimmo receptor-tonus technique provides improvements in function, pain, and range of motion in patients with chronic low back pain, including mechanical problems of the cervical, thoracic, and lumbar area; myofascial pain syndrome; fibromyalgia; local muscle spasm; sports injuries; shoulder problems; and degenerative arthritis. [76] Koo et al [76] reported immediate improvement on muscle elasticity, pain, and disability in participants with chronic low back pain using the Nimmo receptor-tonus technique.
Through the emergence of newer spinal instrumentation techniques and improved imaging modalities, the prevalence of lumbar arthrodesis the artificial induction of joint ossification between 2 bones by surgery has continued to increase in the past few decades. With the rising numbers of patients undergoing instrumented lumbar fusion, the spinal surgeon must be able to recognize and effectively treat postoperative sequelae such as ASD. [7779] We consider that a multidisciplinary approach with neurosurgeons and chiropractic rehabilitative care providers can be extremely beneficial to induce a more rapid recovery in patients with postoperative spinal pain, including ASD.
Last but not least, fascia has been viewed with considerable importance by practitioners, including chiropractors, physical therapists, osteopaths, and manual therapists, because the myofascial continuity of muscles, tendons, and ligaments in the body may have significant implications for health, disease, and injury. [80, 81] Anatomically the thoracolumbar fascia (lumbar fascia or thoracodorsal fascia) is the deep fascia of the back. In the lumbar region of this fascia, it is also attached to the vertebral spinous and transverse processes, [82] but in addition it forms a strong aponeurosis that is connected laterally to the flat muscles of the abdominal wall. Medially it splits into anterior, middle, and posterior layers. The first 2 layers surround the quadratus lumborum, and the last 2 form a sheath for the erector spinae and multifidus muscles. Below it is attached to the iliolumbar ligament, the iliac crest, and the sacroiliac joint. Via its extensive attachment to vertebral spines, this fascia is attached to the supraspinous and interspinous ligaments and to the capsule of the facet joints. [80] The authors think that chiropractic Nimmo receptor-tonus technique in combination with exercises, in our case McKenzie exercises, might well be helpful in restoring a degree of normality to the fascia by releasing of traumatized fascial structures of ASD.
Limitations
Case study research scientifically investigates a real-life phenomenon in depth and within its environmental context. [83] Our case report describes and analyzes the management of ASD. To our knowledge, this is the first case of ASD to describe the use of chiropractic Nimmo receptor-tonus technique and McKenzie self-therapy program in detail with reported favorable clinical outcomes. This case study is an original contribution to the literature to describe ASD from a chiropractic management prospective, subsequently to stimulate further research into the chiropractic therapeutic effects on such cases, and to contribute to the chiropractic literature. It provides preliminary findings necessary for scientists of larger-scale studies to improve the lives of ASD patients.
Conclusion
This report illuminates and informs the chiropractic management of a patient with ASD. After 3 weeks of therapy, VAS and ODI scores were improved. Furthermore, she discontinued her medication, pregabalin 75 mg 2 times a day, under her neurosurgeon's supervision because the outcomes were significant enough for the patient to discontinue her long-standing medical prescription.
Practical Applications
After 3 weeks of therapy, VAS and ODI scores were improved.
After treatment, the patient discontinued her medication (pregabalin 75 mg 2 times a day) under her neurosurgeon's supervision.
Her outcomes were improved so that the patient no longer needed her long-standing medical prescription.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): E.S., A.D., M.H.A.
Design (planned the methods to generate the results): E.S., A.D., M.H.A.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): E.S., A.D., M.H.A.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): E.S., A.D., M.H.A.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): E.S., A.D., M.H.A.
Literature search (performed the literature search): E.S., A.D., M.H.A.
Writing (responsible for writing a substantive part of the manuscript): E.S., A.D., M.H.A.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): E.S., A.D., M.H.A.
References:
Cousins M, Power I. Soft tissue, joints and bones. Acute and postoperative pain. In: Melzack R, Wall PD, editors. Handbook of Pain Management. A Clinical Companion to Wall and Melzack's Textbook of Pain. Elsevier; London, UK: 2003. pp. 1330
Trivedi NN, Wilson SM, Puchi LA, Lebl DR. Evidence-based analysis of adjacent segment degeneration and disease after LIF: a narrative review. Global Spine J. 2018;8(1):95102.
Radcliff KE, Kepler CK, Jakoi A. Adjacent segment disease in the lumbar spine following different treatment interventions. Spine J. 2013;13(10):13391349
Levin DA, Hale JJ, Bendo JA. Adjacent segment degeneration following spinal fusion for degenerative disc disease. Bull NYU Hosp Jt Dis. 2007;65(1):2936
Virk SB, Niedermeier S, Yu E, Khan SN. Adjacent segment disease. Orthopedics. 2014;37(8):547555
Bae JS, Lee SH, Kim JS, Jung B, Choi G. Adjacent segment degeneration after lumbar interbody fusion with percutaneous pedical screw fixation for adult low-grade isthmic spondylolisthesis: minimum 3 years follow-up. Neurosurgery. 2010;67:16001607
Okuda S, Yamashita T, Matsumoto T. Adjacent segment disease after posterior lumbar interbody fusion: a case series of 1000 patients. Global Spine J. 2018;8(7):722727.
Cheh G, Bridwell KH, Lenke LG. Adjacent segment disease following lumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine (Phila Pa 1976) 2007;32(20):22532257
Wohlfeld BJ, Del Monaco DC. Patient outcomes following posterior lumbar interbody fusion for adjacent segment disease using VariLift® as a standalone expandable interbody device. J Spine. 2017;6(4):384
Phillips FM, Cunningham B.
Managing chronic pain of spinal origin after lumbar surgery: the role of decompressive surgery.
Spine. 2002;27(22):25472553Coulis CM, Lisi AJ.
Chiropractic Management of Postoperative Spine Pain:
A Report of 3 Cases
J Chiropractic Medicine 2013 (Sep); 12 (3): 168175Globe, G, Farabaugh, RJ, Hawk, C et al.
Clinical Practice Guideline: Chiropractic Care for Low Back Pain
J Manipulative Physiol Ther. 2016 (Jan); 39 (1): 122Chaitow L, DeLany J, editors. Clinical Application of Neuromuscular Techniques. 2nd ed. Elsevier; London, UK: 2008. Nimmo's receptor-tonus techniques; pp. 109111
Cohen JH. Receptor-tonus technique: an overview. Chiropr Techn. 1990;2(1):1316
Vernon, H and Schneider, M.
Chiropractic Management of Myofascial Trigger Points and Myofascial Pain Syndrome:
A Systematic Review of the Literature
J Manipulative Physiol Ther. 2009 (Jan); 32 (1): 1424Chaitow L. What is NMT? J Bodyw Mov Ther. 1999;3(1):12
Cooperstein R, Gleberzon BJ. Toward a taxonomy of subluxation-equivalents. Top Clin Chiropr. 2001;8(1):4960
Hains G. Locating and treating low back pain of myofascial origin by ischemic compression. J Can Chiropr Assoc. 2002;46(4):257264
Cohen JH, Gibbons RW, Raymond L. Nimmo and the evolution of trigger point therapy, 1929-1986. J Manipulative Physiol Ther. 1998;21(3):167172
McKenzie RA, Kubey C. Penguin; New York, NY: 2000. 7 Steps for a Pain-Free Life: How to Rapidly Relieve Back and Neck Pain
Liebenson C.
McKenzie Self-treatments for Sciatica
J Bodyw Mov Ther. 2005;9:4042Elenburg JL, Foley BS, Roberts K, Bayliss AJ. Utilization of mechanical diagnosis and therapy (MDT) for the treatment of lumbar pain in the presence of known lumbar transverse process fractures: a case study. J Man Manip Ther. 2016;24(2):7479.
Saragiotto BT, Maher CG, Yamato TP. Motor control exercise for chronic non-specific low-back pain (review) Cochrane Database Syst Rev. 2016;1
De Oliveira IO, Pinto LLS, de Oliveira MA, Cera M. McKenzie method for low back pain. Rev Dor São Paulo. 2016;17(4):303306
Foster NE, Thompson KA, Baxter GD, Allen JM. Management of nonspecific low back pain by physiotherapists in Britain and Ireland. A descriptive questionnaire of current clinical practice. Spine (Phila Pa 1976) 1999;24(13):13321342
Gracey J, McDonough S, Baxter G. Physiotherapy management of low back pain. Spine (Phila Pa 1976) 2002;27(4):406411
Battie M, Cherkin D, Dunn D, Ciol M, Wheeler K. Managing low back pain: attitudes and treatment preferences of physical therapists. Phys Ther. 1994;74:219226
Long A, Donelson R, Fung T. Does it matter which exercise? A randomised control trial of exercise for low back pain. Spine (Phila Pa 1976) 2004;29(23):25932602
Petersen T, Kryger P, Ekdahl C, Olsen S, Jacobsen S. The effect of McKenzie therapy as compared with that of intensive strengthening training for the treatment of patients with subacute or chronic low back pain: a randomized controlled trial. Spine (Phila Pa 1976) 2002;27(16):17021709
Clare HA, Adams R, Maher CG. A systematic review of efficacy of McKenzie therapy for spinal pain. Aust J Physiother. 2004;50(4):209216
Moffett J, McLean S. The role of physiotherapy in the management of non-specific back pain and neck pain. Rheumatology (Oxford) 2006;45(4):371378
Werneke MW, Hart DL, Cutrone G. Association between directional preference and centralization in patients with low back pain. J Orthop Sports Phys Ther. 2011;41(1):2231
Ponte DJ, Jensen GJ, Kent BE. A preliminary report on the use of the McKenzie protocol versus Williams protocol in the treatment of low back pain. J Orthop Sports Phys Ther. 1984;6(2):130139
Dunsford A, Kumar S, Clarke S. Integrating evidence into practice: use of McKenzie-based treatment for mechanical low back pain. J Multidiscip Healthc. 2011;4:393402.
Paatelma M, Kilpikoski S, Simonen R, Heinonen A, Alen M, Videman T. Orthopaedic manual therapy, McKenzie method or advice only for low back pain in working adults: a randomized controlled trial with one year follow-up. J Rehabil Med. 2008;40(10):858863
Lee Dy, Nam CW, Sung YB, Kim K, Lee HY. Changes in rounded shoulder posture and forward head posture according to exercise methods. J Phys Ther Sci. 2017;29(10):18241827.
Saavedra-Pozo FM, Deusdara RAM, Benzel E. Adjacent segment disease perspective and review of the literature. Ochsner J. 2014;14(1):7883.
Panjabi MM. Clinical spinal instability and low back pain. J Electromyogr Kinesiol. 2003;13(4):371379
Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5(4):383389
Panjabi M, Abumi K, Duranceau J, Oxland T. Spinal stability and intersegmental muscle forces. A biomechanical model. Spine (Phila Pa 1976) 1989;14(2):194200
Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine (Phila Pa 1976) 2002;27(2):E29E36
Solomonow M, Zhou BH, Harris M, Lu Y, Baratta RV. The ligamento-muscular stabilizing system of the spine. Spine (Phila Pa 1976) 1998;23(23):25522562
Wagner H, Anders C, Puta C. Musculoskeletal support of lumbar spine stability. Pathophysiology. 2005;12(4):257265
Storheim K, Berg L, Hellum C. Fat in the lumbar multifidus muscles: predictive value and change following disc prosthesis surgery and multidisciplinary rehabilitation in patients with chronic low back pain and degenerative disc: 2-year follow-up of a randomized trial. BMC Musculoskelet Disord. 2017;18(1):145.
Bailey JF, Miller SL, Khieu K. From the international space station to the clinic: how prolonged unloading may disrupt lumbar spine stability. Spine J. 2018;18(1):714.
Kalishman L, Carmeli E, Been E. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. Biomed Res Int. 2017;2017
Kalishman L, Hodges P, Li L, Guermazi A, Hunter DJ. Changes in paraspinal muscles and their association with low back pain and spinal degeneration: CT study. Eur Spine J. 2010;19(7):11361144.
Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007;5:2.
Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, De Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9(4):266272.
Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine (Phila Pa 1976) 1994;19(2):165172
Wallwork TL, Stanton WR, Freke M, Hides JA. The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Man Ther. 2009(5):496-500. [PubMed]
Teichtahl AJ, Urquhart DM, Wang Y. Physical inactivity is associated with narrower lumbar intervertebral discs, high fat content of paraspinal muscles and low back pain and disability. Arthritis Res Ther. 2015;17(1):114.
Cooley JR, Walker BF, Ardakani EM, Kjaer P, Jensen TS, Hebert JJ. Relationships between paraspinal muscle morphology and neurocompressive conditions of the lumbar spine: a systematic review with meta-analysis. BMC Musculoskelet Disord. 2018;19(1):351.
Shapiro CM. The failed back surgery syndrome: pitfalls surrounding evaluation and treatment. Phys Med Rehabil Clin N Am. 2014;25(2):319340
Baber Z, Erdek MA. Failed back surgery syndrome: current perspectives. J Pain Res. 2016;9:979987.
Bennett R. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2007;21(3):427445
Ikezoe T, Mori N, Nakamura M, Ichihashi N. Effects of age and inactivity due to prolonged bed rest on atrophy of trunk muscles. Eur J Appl Physiol. 2012;112(1):4348
Belavý DL, Armbrecht G, Richardson CA, Felsenberg D, Hides JA. Muscle atrophy and changes in spinal morphology: is the lumbar spine vulnerable after prolonged bed-rest? Spine (Phila Pa 1976) 2011;36(2):137145
Ehrlich GE. Low back pain. Bull World Health Org. 2003;81:671676.
Fairbank J, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271273
Longo UG, Loppini M, Denaro L, Maffulli N, Denaro V. Rating scales for low back pain. Br Med Bull. 2010;94:81144
Cooperstein R. The Derifield pelvic leg check: a kinesiological interpretation. Chir Tech. 1991;3(2):6065
Thompson C. Williams Manufacturing; Elgin, IL: 1984. Thompson Technique Reference Manual. Thompson Educational Workshops
Cooperstein R. Technique system overview: Thompson technique. Chiropr Tech. 1995;7(2):6063
Lee CK, Langrana NA. Lumbosacral spinal fusion. A biomechanical study. Spine (Phila Pa 1976) 1984;9(6):574581
Axelsson P, Johnsson R, Strömqvist B, Arvidsson M, Herrlin K. Posterolateral lumbar fusion. Outcome of 71 consecutive operations after 4 (2-7) years. Acta Orthop Scand. 1994;65(3):309314
De Geer CM. Cytokine involvement in biological inflammation related to degenerative disorders of the intervertebral disc: a narrative review. J Chiropr Med. 2018;179(1):5462.
Morlion B. Pharmacotherapy of low back pain: targeting nociceptive and neuropathic pain components. Curr Med Res Opin. 2011;27(1):1133
Nijs J, Apeldoorn A, Hallegraeff H. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Phys. 2015;18(3):E333E346
Kellgren JH. A preliminary account of referred pains arising from muscle. Br Med J. 1938;1(4023):325327.
Sharan D, Rajkumar JS, Mohandoss M, Ranganathan R. Myofascial low back pain treatment. Curr Pain Headache Rep. 2014;18(9):449
Colloca L, Ludman T, Bouhassira D. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002.
Topp R, Ditmyer M, King K, Doherty K, Hornyal J. The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clin Issues. 2002;13(2):14
Bloomfield S. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29(2):197206
Parry SM, Puthucheary ZA. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem Physiol Med. 2015;4:16.
Koo TK, Cohen JH, Zheng Y. Immediate effect of Nimmo receptor tonus technique on muscle elasticity, pain perception, and disability in subjects with chronic low back pain. J Manipulative Physiol Ther. 2012;35(1):4553
Bydon M, Xu R, Santiago-Dieppa D. Adjacent-segment disease in 511 cases of posterolateral instrumented lumbar arthrodesis: floating fusion versus distal construct including the sacrum. J Neurosurg Spine. 2014;20(4):380386
Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976) 2004;29(17):19381944
Harrop JS, Youssef JA, Maltenfort M. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976) 2008;33(15):17011707
Benjamin M. The fascia of the limbs and backa review. J Anat. 2009;214(1):118.
Wilke J, Schleip R, Yucesoy CA, Banzer W. Not merely a protective packing organ? A review of fascia and its force transmission capacity. J Appl Physiol. 2018;124(1):234244
Willard FH, Vleeming A, Schuenke MD, Danneels L, Schleip R. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. 2012;221:507536.
Ridder H-G. The theory contribution of case study research designs. Bus Res. 2017;10:281305.

Return to CASE STUDIES
Return to LOW BACK PAIN
Return to DISC HERNIATION
Return to CHIROPRACTIC TECHNIQUE
Return to MYOFASCIAL TRIGGER POINTS
Since 12-11-2020


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |