

Painful Diabetic Neuropathy:
An UpdateThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Ann Neurosci. 2011 (Oct); 18 (4): 168–175
Sharonjeet Kaur, Promila Pandhi, and Pinaki Dutta, DM,
Department of Pharmacology Endocrinology,
Post Graduate Institute of Medical Education
and Research (PGIMER),
Chandigarh, INDIA-160012Diabetes, a silent killer, is a leading cause of neuropathy. Around 50% of diabetic patients develop peripheral neuropathy in 25 years. Painful diabetic neuropathy manifests as burning, excruciating, stabbing or intractable type of pain or presents with tingling or numbness. The pathophysiology of this condition is due to primarily metabolic and vascular factors. There is increase in sorbitol and fructose, glycated endproducts, reactive oxygen species and activation of protein kinase C in the diabetic state. All these factors lead to direct damage to the nerves. The first step in the management of painful diabetic neuropathy is a tight glycaemic control. Currently there is no drug which can halt or reverse the progression of the disease. Most of the therapies prevalent aim at providing symptomatic relief. Antidepressants like tricyclic antidepressants (TCAs) and selective norepinephrine reuptake inhibitors (SNRIs) have good efficacy in controlling the symptoms. Selective serotonin reuptake inhibitors have not shown the same consistent results. Anticonvulsants including pregabalin, gabapentin and lamotrigine have shown good results in the control of symptoms whereas same was not found with carbamazepine, oxcarbazepine and topiramate. Topical agents (capsaicin, topical nitrates and topical TCAs) and local anaesthetics have also been used with good results. Use of opioids and non steroidal anti-inflammatory drugs although common but is not preferable. The newer therapies under studies are NMDA antagonists, aldose reductase inhibitors, neurotropic factors, vascular endothelial growth factor, Gamma linolenic acid, protein kinase C beta inhibitors, immune therapy, hyperbaric oxygen and alpha lipoic acid.
From the FULL TEXT Article:
Introduction
Diabetes mellitus is a leading cause of diabetic neuropathy, resulting in great morbidity, mortality and deteriorates ones quality of life, and poses a huge financial burden for patient and patient’s caregivers. [1] Diabetic neuropathy is very broad and heterogeneous term which encompasses a number of mono and polyneuropathies as well as plexopathies and radiculopathies. It was first described by Marchel de Calvi in 1864, who stated neuropathy as a consequence rather than a cause of diabetes. [2] This article primarily discusses about painful diabetic neuropathy (PDN).
Definition
An international meeting on the diagnosis and management of diabetes produced a consensus statement defining diabetic peripheral neuropathy as “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes.” [3] Other causes of neuropathy such as hereditary, inflammatory, and other metabolic neuropathies should be actively excluded.
Clinical manifestations
Patients with painful diabetic neuropathy characteristically present with tingling sensation, numbness, burning, excruciating stabbing type of pain, sometimes intractable and may be associated with paraesthesia and hyperesthesia coupled with deep aching in feet or hands. This is typically a distal symmetrical sensorimotor type of neuropathy. The other clinical characteristics are due to involvement of both small and large (mixed sensorimotor) fibres. Initially, the most distal parts of the extremities are affected, leading to typical gloves and stocking pattern of sensory loss, indicating the involvement of longest nerve fibres. This is followed by involvement of distal upper limbs, the anterior aspect of trunk and subsequently the vertex of the head. Overall there occurs a disturbance of light touch sensation, sensitivity to pressure and vibration, and joint position sense. It typically affects at night and over all it affects the individual’s quality of life including mobility, work, sleep, mood, self worth, recreation and social activities. [4]
Epidemiology
Poor glycaemic control is a major risk factor for development of diabetic neuropathy. A direct relationship has been found between duration of poor glycaemic control and diabetic neuropathy. It has been observed that an estimated 50% of patients develop peripheral neuropathy 25 years after the initial diagnosis of diabetes mellitus. The prevalence of PDN ranges from 10% to 20% of patients with diabetes and in those with diabetic neuropathy it ranges from 40% to 50%. [5-7] Hyperglycemia, as causative factor in neuropathy, was established from randomised prospective trial namely Diabetes Control and Complication Trial. This landmark trial demonstrated that a tight glycaemic control leads to significant reduction in development and progression of clinical neuropathy by 64%. [8, 9] Other comorbid factors associated with diabetic neuropathy are hyperlipidemia, hypertension, cigarette smoking, consumption of alcohol, and obesity.
Classification
There are many types of neuropathy with varying clinical presentations. Peripheral neuropathy can manifest either with painful or painless symptoms or both. The two most common types of diabetic neuropathies associated with pain are acute sensory neuropathy and chronic sensorimotor neuropathy. Acute sensory type neuropathy presents with either acute or subacute onset characterized by severe sensory symptoms, usually with a few, if any, clinical signs. It is usually associated with hyperglycemia or intensification of glycemic control and may gradually lessen as euglycemia is obtained. Chronic sensory-motor neuropathy is the most common form of DPN, associated with symptomatic pain and clinical signs of neuropathy.
Pathophysiology
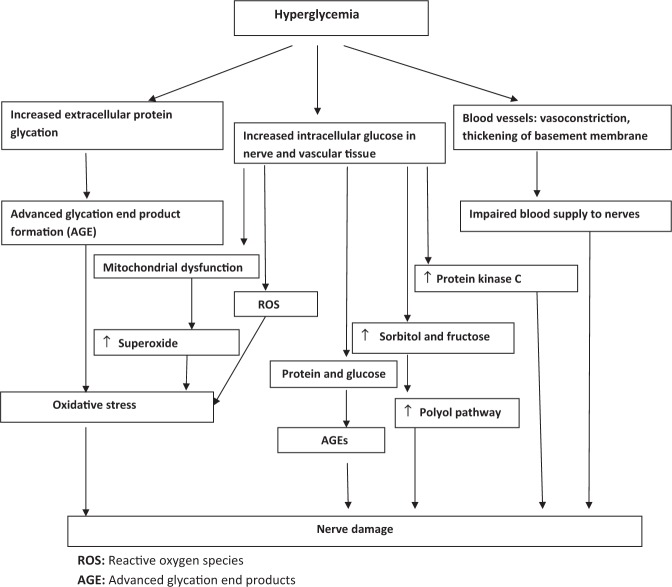
Figure 1 Pathophysiology of diabetic neuropathy involves both metabolic and vascular factors. Hyperglycemia is central to pathogenesis which results in the following (Figure 1):
Increased sorbitol and fructose
Hyperglycemia leads to increased activity of the enzyme aldose reductase (rate limiting step in polyol pathway) which leads to the accumulation of the sorbitol and fructose and a decrease in free nerve myoinositol (competition for myoinositol uptake into tissue). It also causes imbalance in the nicotinamide adenine dinucleotide phosphate and its reduced form. Reduced NADPH is a cofactor for the enzyme nitric oxide synthase which reduces nitric oxide formation, which is a major vasodilator. All these factors leads to impairment of the blood supply to the nerves. [10]
Advanced glycation end products
Hyperglycemia leads to incorporation of glucose into the proteins nonenzymatically by an unregulated glycation reaction. [11] The different proteins that get glycated include haemoglobin, plasma albumin, lipoproteins, fibrin and collagen. All these glycated end products gradually form fluorescent cross linked protein products called advanced glycation end products. They are responsible for causing the tissue damage because of their reactivity and protein cross linking.
Reactive oxygen species
Auto-oxidation of the excess glucose leads to formation of reactive oxygen species. These oxygen free radicals cause damage of the nerves by a direct toxic effect or by inhibiting the nitric oxide production by the endothelium thereby leading to ischemia of nerves. Hence oxidative stress is also predominantly implicated in neuropathy
Inappropriate activation of protein kinase C and others:
Other factors contributing to neuropathy are inappropriate activation of protein kinase C, decrease in the nerve growth factor (responsible for the regeneration of the nerves), biochemical abnormalities like decreased gamma-linolenic acid, which is precursor of a prostanoid including prostacyclin. Prostacyclin is a potent vasodilator, and if reduced blood flow it leads to reduced blood flow.
Assessment of neuropathy
A careful history regarding pain, discomfort, or numbness in the legs should be conducted. Epidemiological studies have confirmed that nerve conduction studies are the most valuable tool for evaluation of nerve conduction. [12] A careful clinical examination should be conducted along with specified tests. Use of monofilament to evaluate the touch sensitivity and tactile circumference discrimination are the two commonly used methods. [13-16] Other instruments like neuropathy symptom score and neuropathy symptom profile are used to assess the symptom quantitation. [17, 18] Vibration test by tuning fork is done by placing it on the bony prominence at the dorsum of the great toe. Patient is then asked to state when they first feel the vibration and when it ceases. This test should be repeated twice on each foot. Apart from this, patients’ mobility, gait, and balance should also be assessed.
Mechanism of pain generation in painful diabetic neuropathy
Sensitization
The underlying mechanism for generation of pain is hyperexcitability in primary afferent nociceptors (peripheral sensitization), which occurs due to damage to peripheral nerves. This in turn leads to hyperexcitability in central neurons (central sensitization) and generation of spontaneous impulses within the axon as well as the dorsal root ganglion of these peripheral nerves. Sensitization refers to a lowered activation threshold, increased response to a given stimulus, and abnormal spontaneous activity. It is a self resolving process except if in chronic disease such as diabetes with ongoing damage, spontaneous symptoms continues to be generated due to continued sensitization and altered processes in nociceptors. [19]
Sympathetic nervous system
The role of sympathetically mediated pain in PDN has been demonstrated in different studies. In one of the study, impaired sympathetically mediated vasoconstriction in patients with PDN was demonstrated suggestive of inappropriate local blood flow regulation in these patients. [20, 21]
Role of sodium channels
At the site of injury and along the length of axon, there occurs accumulation of sodium channels. This phenomenon leads to ecotopic electric discharge and hyperexictability leading to the increased bursts of electrical impulses to the dorsal horn, alteration in gating mechanism and substance P expression. [22]
Long term potentiation
Peripheral excitation leads to central excitation, which further leads to activation of N-methyl D-asparate (NMDA) receptors, which are located post synpatically in dorsal horn. Activation of NMDA receptors leads to glutamate release, causing synaptic potentiation, which are much larger postsynaptic potentials known as long term potentiation. This phenomenon has been observed in various pain states. [19, 22]
Treatment of neuropathy
Control of hyperglycaemia
It has already been documented that the optimal control of hyperglycaemia by intensive therapy in type 1 diabetes reduces the appearance of neuropathy by 60% over a 5 year period.8 Adequate glycaemic control is achieved by either oral hypoglycaemic, euglycaemic agents or insulin therapy.
Specific therapy
An ideal agent should prevent or arrest the progressive loss of nerve function and improves symptoms with minimal side effects. A large number of agents have been tried and tested in number of randomized controlled trials which includes antidepressants, anticonvulsants, opioids, N-Methyl-D-Asparate (NMDA) receptor antagonists, and antiarrhythmics. Topical agents and aldose reductase inhibitors (Alrestatin/Sorbinil/Tolrestat/Zepolrestat), Non steroidal antinflammatory drugs (NSAIDS), protein kinase C inhibitors, gamma-linolenic acid, nerve growth factor (recombinant human nerve growth factor/ Insulin like growth factor-1), vascular endothelial growth factors, immune therapy, acupuncture, hyperbaric oxygen, and physical therapies also have been being tested.
Antidepressants

Table 1 Amitriptyline was introduced in 1977 while others include imipramine, nortriptyline and desipramine which are the metabolites of amitriptyline and imipramine, respectively. Duloxetine is the only antidepressant approved by FDA for diabetic neuropathic pain. SSRIs that have been studied include venlafaxine, citalopram and paroxetine and fluoxetine for diabetic neuropathy. (Table 1)
Mechanism of action
Serotonin and norepinephrine, together with endogenous opioids and gama amino butyric acid modulate actions of the nociceptive pathways. Presynaptic reuptake inhibition of serotonin and norepinephrine increases the levels of these amines in the synaptic clefts which causes increased pain suppression induced by the descending inhibitory pathways. Hence, the central mechanism by which antidepressants act is by diffuse noxious inhibitory control by inhibition of presynaptic reuptake of serotonin and norepinephrine. TCAs also block a-adrenegic, H1-histamine, muscarinic cholinergic, and N-methyl-D-aspartate receptors. [23] TCA also act on peripheral nociceptors, descending inhibitory pathways, central sensitization and brain areas involved in pain and emotional processing. SSRIs work by the inhibition of presynaptic reuptake of serotonin but not norepinephrine.
Clinical studies
Among the tricyclic antidepressants (TCA), amitriptyline is most commonly prescribed and has demonstrated significant benefits in burning, aching, sharp, throbbing or stinging type of pain. [24] Selective serotonin reuptake inhibitors has been studied and studies have not shown great efficacy. There is some evidence from small controlled studies, to support the use of paroxetine and citalopram in dosages of up to 40 mg/day in diabetic neuropathic pain. Selective Norepinephrine reuptake inhibitors (SNRIs) such as venlafaxine has been found to be equivalent in efficacy to TCAs. [25] Raskin et al. evaluated the effect of duloxetine in doses of 60 and 120 mg in 348 patients with diabetic neuropathy for 12 weeks and reported it to be effective and well tolerated. [26] In one of the cross over trial, while comparing duloxetine with amitryptyline, it was found that duloxetine was preferred by patients although efficacy of both the drugs was similar. [27] There is a limited data on efficacy of, selective serotonin reuptake inhibitors, hence they have not been approved for the treatment of neuropathic pain.
Anticonvulsants
Mechanism of action

Table 2 Anticonvulsants (Table 2) act by different mechanism which includes sodium channel blockade, potentiation of G-amino butyric acid activity, calcium channel blockade, antagonism of glutamate at N-Methyl-D-Aspartate receptors. Pregabalin has been FDA approved for diabetic neuropathy. It has anti-epileptic, analgesic and anxiolytic activity. Although mechanism of action is unclear, it is postulated that it reduces excitatory neurotransmitter release by binding to the a-2-d protein subunit of voltagegated calcium channels. It is known to interact with L-amino acid transporters and also binds with high affinity to a-2-d subunit of calcium channels. Valporate has been studied in different placebo controlled trials and has demonstrated efficacy in neuropathic pain. [28, 29] Lacosamide is a novel anticonvulsant that selectively enhances the slow inactivation of voltage-dependent sodium channels, and interacts with a neuronal cytosolic protein, the collapsin response mediator protein 2 (crmp-2), which plays an important role in nerve sprouting and excitotoxicity.
Clinical studies
Pregabalin is a second agent approved for diabetic neuropathic pain. In one of the trial comparing pregabalin (300 mg/day) with placebo in patients with peripheral diabetic neuropathy, pregabalin showed a significant improvement in the mean pain scores, mean sleep interference, mood disturbance, and tension–anxiety during week 1, which remained significant throughout the study, but were associated with dizziness and somnolence compared with placebo. [30] A multicentric double blind study has demonstrated the beneficial effect of gabapentin in both type 1 and type 2 diabetic patients. [31] Recently lamotrigine, an antiepileptic displayed beneficial effects in neuropathic pain. A randomized double blind, cross over study in 59 patients supported the use of lamotrigine as compared to placebo at different doses (200, 300 and 400 mg/day). [32] Similar results were depicted when lamotrigine was compared to amitriptyline. [33] However, it is advisable to monitor drug therapy with these agents as they are associated with various adverse drug reactions. There is limited data regarding the efficacy of carbamazepine, oxcarbazapine and topiramate. Lacosamide has been tried in clinical trials of diabetic neuropathy but still more rigorous studies are needed to establish its efficacy.
Topical agents
Capsaicin
Topical preparation of capsaicin, an extract from chilli pepper, and leads to depletion of substance P, which is the most commonly, implicated neurotransmitter in the pain transmission pathway. If applied, 0.075% multiple times a day is effective, but is associated with burning sensation that may persist for several weeks. [34]
Topical nitrate
It has been observed in experimental models that impaired nitric oxide generation induces hyperalgesia. A randomized trial reported local application of topical nitrate resulted in significant reduction in pain and burning sensation over 4 weeks in diabetic patient. [35]
Topical TCAs
have demonstrated a noticeable decrease in the pain scores (6.22 to 5.04; p< 0.010) as compared to placebo which showed increase in the pain scores (6.49 to 6.91). [36]
Local anaesthetics and antiarrthymics
Mechanism of action
Lidocaine by virtue of its sodium channel blocking property, blocks both peripheral nociceptor sensitization and central nervous system hyperexcitability. Local anaesthetics remain in the skin and have an excellent safety profile. Intravenous lidocaines has shown to ameliorate intractable painful diabetic neuropathy in those who have failed to respond to or are intolerant to available conventional therapies. [37] Mexilitine is a class 1B antiarrhythmic agent and a structural analog of lignocaine. Mexilitine has been found to be effective in reducing nocturnal pain and sleep disruption in neuropathic pain patients. [38] It is not used because of its side effects and the need for regular electrocardiogram monitoring.
Opioids Analgesics
The use of opioids for neuropathic pain remains controversial. Opioids such as morphine, methadone and time released formulations of oxycodone have been found to be effective in relieving neuropathic pain. Morphine in combination with gabapentin has lead to a greater reduction in the total score on the Short-Form McGill Pain Questionnaire at lower doses of each drug than either drug used as a single agent, although constipation, sedation, and dry mouth were frequent adverse effects in patients with diabetic neuropathy. [39] Oxycodone has advantages over morphine which includes its higher bioavailability, more potency and lesser adverse drug reactions. Oxycodone has demonstrated beneficial effect on mean daily pain (both steady pain and brief pain) as well as on total pain and disability in one of the placebo controlled trial. [40] Tramadol, a opioid-like, centrally acting synthetic narcotic analgesic has been confirmed to be efficacious in a neuropathic pain with added advantages of no abuse potential. A multicentric, double blind, randomized controlled trial demonstrated significant improvement in pain and physical and social functioning, but no benefit on sleep was observed. Significant side effects were somnolence, nausea, constipation, and headache. [41] Caution should be advised in patients with substance abuse while prescribing opioids analgesics. Opioids analgesics and tramadol have demonstrated efficacy in treating patients having coexisting osteoarthirits responsible for non-neuropathic pain.
Nonsteriodal antiinflammatory drugs (NSAIDS)
NSAIDS act by decreasing pain and inflammation by inhibiting cycloxygenase which decreases prostaglandin synthesis. A single blind 24 week placebo controlled study demonstrated statistically significant reduction in paraesthesia scores by both Ibuprofen (600 mg four times a day) and sulindac (200 mg twice a day) in 18 veterans with diabetic neuropathy. [42] They have a beneficial effect in acute phase of neuropathic pain especially the gnawing type of pain. Caution is advised regarding their propensity to impair renal function and cause gastrointestinal bleeding.
Physical and surgical therapy
Acupuncture has found to be associated with reduction in the use of other analgesics and benefits lasts for 6 months. [43] However, controlled studies are needed to confirm these results. Transcutaneous electrical nerve stimulation has been tried with modest success Other less rigorously tested methods include surgical sympathectomy, spinal cord stimulation, central neuraxial blockade, static magnetic field therapy, low-intensive laser therapy and monochromatic infrared light.
NMDA antagonists
Newer agents have been tried include ketamine, memantine (20-55 mg per day) and dextromethorphan (380 mg per day). A small study conducted on diabetic neuropathic patients dextromethorphan demonstrated a reduction in pain by 24% in comparison to placebo. [44] In one of the cross over trial comparing dextromethorphan plus memantine with placebo, no statistically significant response for pain reduction was found, although in 10 dextromethorphan responders, showed a significant dose–response effect on pain intensity. [45]
Aldose reductase inhibitor
Aldose reductase inhibitors interfere with the polyol pathway, which in turn prevent the conversion of glucose to sorbitol. Drugs which have shown such effect in human and animal models are tolerestat, zopolrestat, eoalrestat, alreastat. Human studies of these drugs have shown inconsistent result either due to small sample size or due to insufficient duration of trial. They also produced unacceptable toxicity. [46, 47]
Neurotropic factors
Animal studies have demonstrated the role of neurotropic factors in the regeneration of nerves. Examples of neurotropic factors are nerve growth factors, brain derived neurotropic factors, neurotropins, insulin like growth factors and glial cell derive neurotropic factor. TX14 a prosaposin derived neurotrophic peptide has shown efficacy in two different rat models. [48] On the other hand these benefits are not seen in clinical trials in humans. A large phase 3 study of recombinant human nerve growth factor in patients with diabetic polyneuropathy did not reveal any significant benefit. [49]
Vascular endothelial factors (VEGF)
The role of VEGF has generated a lot of interest in the therapy of neuropathy. Its potential role in the diabetic neuropathy has been documented in rat and rabbit models of diabetes. VEGF gene administration has shown to restore nerve conduction velocity, nerve blood flow and nerve vessel number to normal in diabetic neuropathy. [50, 51]
Gamma linolenic acid
Gamma linolenic acid is an important constituent of the neuronal membrane and has shown to preserve nerve blood flow. One of the trial demonstrated improvement in clinical assessment of the condition and electrophysiological tests. [52]
Protein kinase C beta inhibitors
One of the postulated factors in the development of diabetic neuropathy is neural vascular insufficiency. Protein Kinase C (PKC) activation is a critical step in the pathway to diabetic microvascular complications. Ruboxistaurin mesylate (LY333531) is a protein kinase Cß inhibitor. A multinational, randomized, phase-2, double-blind, placebo controlled trial to test the efficacy and tolerability of ruboxistaurin in patients with diabetic neuropathy showed a statistically significant improvement in symptoms in ruboxistaurin-treated neuropathy groups. [53]
Immune therapy
Various forms like chronic inflammatory demyelinating polyneuropathy, multiple motor polyneuropathy, vasculitis and monoclonal gammopathy of unknown significance (MGUS) in diabetes has been shown to improve with immune therapy. [54, 55]
Hyperbaric Oxygen
Its use is still debatable as the pathogenesis in diabetic foot is impaired oxygen delivery to the tissue, so this therapy might be effective in neuropathy by promoting oxygen delivery from plasma to the affected tissues. [56]
Alpha Lipoic acid
Alpha lipoic acid is a antioxidant, with free radical scavenging property. It has shown efficacy in modifying natural history of peripheral diabetic neuropathy and improving symptoms in diabetic neuropathy. [57, 58]
Miscellaneous agents
Pentoxiphylline, clonidine, reiki therapy and vitamins have been tried with inconsistent results. Angiotensin converting enzyme inhibitors (ACE inhibitors) have shown effectiveness in neuropathy due to unclear mechanism. [59]

Table 3

Table 4 Table 3 describes the drug of choice for different types of pain
Conclusion
Diabetic neuropathy is associated with long term diabetes. Tight glycaemic control along with preventive management helps to prevent neuropathic complications. Newer advances still in developmental stage includes glutamate antagonists, cytokine inhibitors, vanilloid- receptor agonists, catecholamine modulators, ion-channel blockers, anticonvulsants, opioids, cannabinoids, COX inhibitors, acetylcholine modulators, adenosine receptor agonists. Pharmacotherapeutic strategies are the cornerstone of neuropathy treatment but still results are inconclusive, hence vigorous research should be directed towards these aspects to find a better counterparts.
Competing interests
None
Source of Funding
None
References:
Holzer SE, Camerota A, Martens L. et al. Costs and duration of care for lower extremity ulcers in patients with diabetes. Clin Ther. 1998;20:169–181
Boulton AJM, Malik RA. Diabetic Neuropathy. Medical Clinics of North America. 1998;82:909–929
Boulton AJ, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med. 1998;15:508–514
Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: Epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123–134
Veves A, Manes C, Murray HJ. et al. Painful neuropathy and foot ulceration in diabetic patients. Diabetes Care. 1993;16:1187–1189
Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: Epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123–128
Gordois A, Scuffham P, Shearer A. et al. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26:1790–1795
Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986
DCCT Research Group.The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med. 1995;122:561–588
Cameron NE, Cotter MA. The relationship of vascular changes to metabolic factors in diabetes mellitus and their role in the development of peripheral nerve complications. Diabetes Metab Res. 1994;10:189–224
Bhadada SK, Sahay RK, Jyotsna VP. et al. Diabetic Neuropathy: Current Concepts. Journal of Indian Academy of Clinical Medicine. 2001;2:304–318.
Vinik AI, Kong X, Megerian JT. et al. Diabetic nerve conduction abnormalities in the primary care setting. Diabetes Technol Ther. 2006;8:654–662
Armstrong DG, Lavery LA, Vela SA. et al. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med. 1998;76:68–71
Maser RE, Laudadio C, Lenhard MJ. et al. A cross-sectional study comparing two quantitative sensory testing devices in individuals with diabetes. Diabetes Care. 1997;20:179–181
Rodriguez M, Trinajstic E, Munoz P. The tactile circumferential discriminator: an instrument for detecting patients at risk of foot ulceration. Diabetes Care. 1997;20:1799
Vileikyte L, Hutchings G, Hollis S. et al. The tactile circumferential discriminator. A new, simple screening device to identify diabetic patients at risk of foot ulceration. Diabetes Care. 1997;20:623–626
Dyck PJ. Quantitating severity of neuropathy. In: Dyck PJ, Thomas PK, Griffin JW, editors. Peripheral neuropathy. 3rd ed. Philadelphia: WB Saunders; 1993. pp. 686–697.
Dyck PJ, Karnes J, OBrien PC. et al. Neuropathy symptom profile in health, motor neuron disease, diabetic neuropathy, and amyloidosis. Neurology. 1986;36:1300–1308
Baron R. Peripheral neuropathic pain: From mechanisms to symptoms. Clin J Pain. 2000;16:S12–S20
Scadding JW. Development of ongoing activity, mechanosensitivity, and adrenaline sensitivity in severed peripheral nerve axons. Exp Neurol. 1981;73:345–364
Tsigos C, Reed P, Weinkove C. et al. Plasma norepinephrine in sensory diabetic polyneuropathy. Diabetes Care. 1993;16:722–727
Spruce MC, Potter J, Coppini DV. The pathogenesis and management of painful diabetic neuropathy: A review. Diabet Med. 2003;20:88–98
Sindrup SH, Otto M, Finnerup NB. et al. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96:399–409
Max MB, Culnane M, Schafer SC. et al. Amitryptiline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987;37:589
Sindrup SH, Bach FW, Madsen C. et al. Venlafaxine versus imipramine in painful diabetic polyneuropathy: A randomized, controlled trial. Neurology. 2003;60:1284–1289
Raskin J, Pritchett JL, Wang F. et al. A double blind, multicenter trial comparing duloxetine with placebo in the treatment of diabetic peripheral neuropathic pain [abstract]. Diabetes. 2005;54:A126.
Kaur H, 100 D, Bhansali A. et al. A comparative trial to evaluate Amitryptyline and Duloxetine in painful diabetic neuropathy. Diabetes Care. 2011
Kochar DK, Jain N, Agarwal RP. et al. Sodium valproate in the management of painful neuropathy in type 2 diabetes: a randomized placebo controlled study. Acta Neurol Scand. 2002;106:248–252
Kochar DK, Rawat N, Agrawal RP. et al. Sodium valproate for painful diabetic neuropathy: a randomized double-blind placebo-controlled study. QJM. 2004;97:33–38
Rosenstock J, Tuchman M, La Moreaux L. et al. Pregabalin for the treatment of painful diabetic peripheral neuropathy: A double-blind, placebo-controlled trial. Pain. 2004;110:628–638
Rosenberg JM, Harrell C, Risitic H. et al. The effect of gabapentine on neuropathic pain. Clin J Pain. 1997;13:251–255
Vinik AI, Tuchman M, Safirstein B. et al. Lamotrigine for treatment of pain associated with diabetic neuropathy: Results of two randomized, double-blind, placebo-controlled studies. Pain. 2007;128:169–179
Jose VM, Bhansali A, Hota D. et al. Randomized double-blind study comparing the efficacy and safety of lamotrigine and amitriptyline in painful diabetic neuropathy. Diabet. Med. 2007;24:377–383
The capsaicin study group.Treatment of painful diabetic neuropathy with topical capsaicin: a multicenter, double-blind, vehicle controlled study. Arch Intern Med. 1991;151:2225–2229
Yuen KC, Baker NR, Rayman G. Treatment of chronic painful diabetic neuropathy with isosorbide dinitrate spray: A double-blind placebo controlled crossover study. Diabetes Care. 2002;25:1699–1703
McCleane GJ. Topical doxepin hydrochloride reduces neuropathic pain: a randomized, double blind, placebo controlled study. Pain Clin. 1999;12:47–50.
Viola V, Newnham HH, Simpson RW. Treatment of intractable painful diabetic neuropathy with intravenous lignocaine. J Diabetes Complication. 2006;20(1):34–39
Oskarsson P, Iiunggren J-G, Lins P-E. Efficacy and safety of mexilitine in the treatment of painful diabetic neuropathy. The mexilitetine study group. Diabetes Care. 1997;20:1594–1597
Gilron I, Bailey JM, Tu D. et al. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352:1324–1334
Watson CP, Moulin D, Watt-Watson J. et al. Controlled-release oxycodone relieves neuropathic pain: A randomized controlled trial in painful diabetic neuropathy. Pain. 2003;105:71–78
Harati Y, Gooch C, Swenson M. et al. Double blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998;50:1842–1846
Cohen KL, Susanne H. Efficacy and safety of nonsteroidal anti-inflammatory drugs in the therapy of diabetic neuropathy. Arch Intern Med. 1987;147:1442–14444
Abuaisha BB, Constanzi JB, Boulton AJM. Acupuncture for the treatment of chronic painful diabetic neuropathy: A longterm study. Diabetes Res Clin Pract. 1998;39:115–121
Nelson KA, Park KM, Robinovitz E. et al. High-dose oral dextromethorphan versus placebo in painful diabetic neuropathy and post herpetic neuralgia. Neurology. 1997;48:1212–1218
Sang CN, Booher S, Gilron I. et al. Dextromethorphan and memantine in painful diabetic neuropathy and postherpetic neuralgia: Efficacy and dose–response trials. Anesthesiology. 2002;96:1053–1061
Pfeifer MA, Schumer MP, Gelber DA. Aldose reductase inhibitors: the end of an era or the need for different trial designs? Diabetes. 1997;46(Suppl. z2):S82–S89
Bril V. Status of current clinical trials in diabetic polyneuropathy. Can J Neurol Sci. 2001;28:191–198
Mizisin AP, Steinhardt RC, O’Brien JS. et al. TX14(A), a prosapos in derived peptide, reverses established nerve disorders in streptozotocin diabetic rats and prevents them in galactose-fed rats. J Neuropathol Exp Neurol. 2001;60:953–960
Apfel SC, Schwartz S, Adornato BT. et al. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: a randomized controlled trial. JAMA. 2000;284:2215–2221
Veves A, King GL. Can VEGF reverse diabetic neuropathy in human subjects? J Clin Invest. 2001;107:1215–1218. [PMC free article] [PubMed]
Schratzberger P, Walter DH, Rittig K. et al. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J Clin Invest. 2001;107:1083–1092. [PMC free article] [PubMed]
Keen H, Paxan J, Allawi J. et al. Treatment of diabetic neuropathy with gamma - linoleic acid. Diabetes Care. 1993;16:8–15
Vinik A, Bril V, Kempler P. et al. for the MBBQ Study. Treatment of symptomatic diabetic peripheral neuropathy with protein kinase CB inhibitor ruboxistaurin mesylate during a 1-year randomized, placebo-controlled, doubleblind clinical trial. Clin Therapeut. 2005;27:1164–1180
Krendel DA, Costigan DA, Hopkins LC. Successful treatment of neuropathies in patients with diabetes mellitus. Arch Neurol. 1995;52:1053–1061
Barada A, Reljanovic M, Milicevic Z. et al. Proximal diabetic neuropathy: response to immunotherapy [abstract]. Diabetes. 1999;48(Suppl 1):A148.
Bloomgarden ZT. Diabetic Neuropathy. Daibetes Care. 2008;31:616–621
Reljanovic M, Reichel G, Rett K. et al. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): A two year multicentre randomized double blind placebocontrolled trial (ALADIN II). Free Radic Res. 1999;31:171–179
Ametov AS, Barinov A, Dyck PJ. et al. SYDNEY Trial Study Group. The sensory symptoms of diabetic polyneuropathy are improved with alphalipoic acid: The SYDNEY trial. Diabetes Care. 2003;26:770–776
Malik RA, Williamson S, Abbott C. et al. Effect of angiotensin-converting- enzyme (ACE) inhibitor trandolapril on human diabetic neuropathy: randomised double-blind controlled trial. Lancet. 1998;352(9145):1978–1981
Bril V, England J, Franklin GM. Evidence based guideline: Treatment of painful diabetic neuropathy. Apr 11, 2011. www.neurology.org. www.neurology.org

Return to the GAMMA-LINOLENIC ACID Page
Since 1-27-2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |